Abstract
AD is a neurodegenerative disease, and its frequency is often reported to be higher for women than men: almost two-thirds of patients with AD are women. One prevailing view is that women live longer than men on average of 4.5 years, plus there are more women aged 85 years or older than men in most global subpopulations; and older age is the greatest risk factor for AD. However, the differences in the actual risk of developing AD for men and women of the same age is difficult to assess, and the findings have been mixed. An increasing body of evidence from preclinical and clinical studies as well as the complications in estimating incidence support the sex-specific biological mechanisms in diverging AD risk as an important adjunct explanation to the epidemiologic perspective. Although some of the sex differences in AD prevalence are due to differences in longevity, other distinct biological mechanisms increase the risk and progression of AD in women. These risk factors include (1) deviations in brain structure and biomarkers, (2) psychosocial stress responses, (3) pregnancy, menopause, and sex hormones, (4) genetic background (i.e., APOE), (5) inflammation, gliosis, and immune module (i.e., TREM2), and (6) vascular disorders. More studies focusing on the underlying biological mechanisms for this phenomenon are needed to better understand AD. This review presents the most recent data in sex differences in AD—the gateway to precision medicine, therefore, shaping expert perspectives, inspiring researchers to go in new directions, and driving development of future diagnostic tools and treatments for AD in a more customized way.
Keywords: Aging, Dementia, Gender difference, Cognition, Hormones, Estrogen, Menopause
Introduction
As an irreversible neurodegenerative disease, Alzheimer’s disease (AD) is the most common form of dementia, and there is about 50 million people currently have AD or ADRD worldwide [1]. The worst part is that this number will increase by about 70% when it reaches middle century [2]. The clinical pathologies of AD include neurofibrillary tangles (NFTs), amyloid-β (Aβ) plaque deposition, gliosis, and brain atrophy, often accompanied by inflammation, synaptic alterations, and neurovascular amyloidosis and breakdown [3]. In early stage, AD patient may show memory impairment, difficulties executing speech and routine daily activities, and depression. In late stage, AD patient may show severe memory loss, disorientation, hallucination, and lack self-sufficiency [1, 2].
Aging and AD
Aging is the greatest risk factor for late-onset AD (LOAD). The prevalence of AD for people over age of 65 is about 10%, and increases significantly with age, reaching about 32% for 85 old [1, 4, 5]. AD is a type of abnormal aging where neurons are undergoing genomic destabilization, DNA damage/oxidation, epigenetic alterations, telomere shortening, reductions and alterations in metabolic pathways, and mitochondria dysfunctions [3]. Aged glia and their activation also contribute to the AD pathogenesis through oxidative stress, inflammation and immunomodulation [4, 5].
Epidemiologic evidence for sex differences in AD
The frequency of AD is often reported to be higher for women than men [6–14]. Almost two-thirds of Americans with AD are women. One prevailing reason that has been stated is that women live longer than men on average of 4.5 years, and there are more women aged 85 years or older than men in most global subpopulations, while older age is the greatest risk factor for AD. However, the differences in the actual risk of developing AD for men and women of the same age is difficult to assess, and the findings have been mixed [6–32]. Many studies reported higher age-adjusted numbers for women and/or accelerated progression in women while other studies failed to find an association between incidence, progression, and sex, or remained inconclusive. It is also important to point out there even if there is not a difference, it doesn’t necessarily mean that there aren’t sex differences in mechanisms. There can be the same prevalence of a disease, but different ways to get there for a man or women.
There are many factors could attribute to the inconsistent findings among those different studies, including (1) differences in inclusion/exclusion criteria, (2) sample size and statistical power, (3) study design and type—retrospective cohort, prospective cohort, or cross-sectional analysis, (4) cultural differences affecting diet, exercise, and stress response strategies, and (5) the historical different classification and diagnoses methods on AD cases [14, 19, 29]. The classification bias as an issue in determining the real incidence of AD in men and women was of particular important as a definitive diagnosis of AD could only be obtained at autopsy historically, and many different criteria had been used across studies. However, now with advances in amyloid PET, and CSF amyloid and tau for clinical use, it is possible to definitively determine AD dementia. Therefore, the discrepancies in the incidence of AD between men and women across studies now can be minimized [19].
Data from the Framingham Heart Study found that the estimated lifetime risk for AD at age 45 was about one in five (20%) for women and one in 10 (10%) for men, and the risks for both sexes were slightly higher at age 65 (Fig. 1) [1, 17]. A recent meta-analysis including 22 studies on sex differences reported all estimates of incidence and prevalence were higher for women than for men despite that the differences were not statistically different [33]. Other studies such as the Cache County Memory Study also confirmed a higher incidence among women, and longevity remains an important reason for more female with AD than male [25, 34, 35]. Taken together, the increasing body of evidence from preclinical and clinical studies as well as the complications in estimating incidence support the sex-specific biological mechanisms in diverging AD risk as an important adjunct explanation to the epidemiologic perspective, which deserves more careful and closer investigations in future studies [19].
Fig. 1.
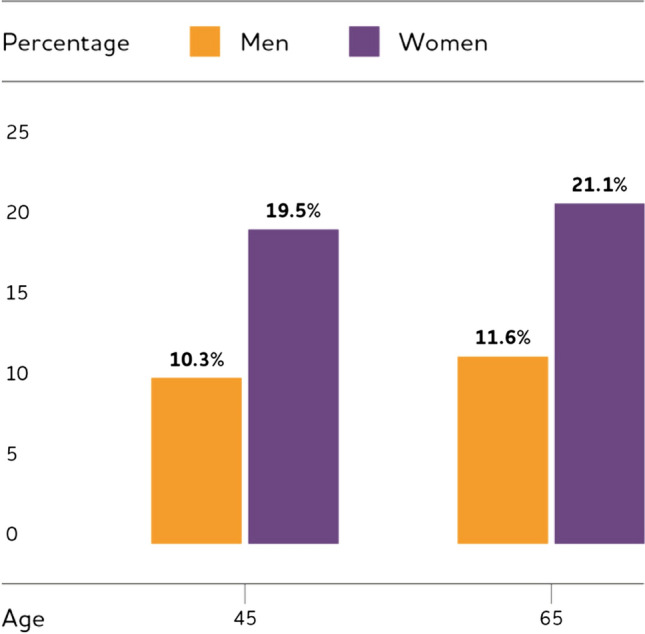
Estimated Lifetime Risk for AD by Sex at Ages 45 and 65. (Reprinted/adapted with permission from [1])
Mechanisms attributing to sex differences in AD
If there is a difference in the risk of AD between male and female, there are a number of potential social and biological explanations. One possible reason is that the lower educational attainment in women than in men born in the first half of the twentieth century could account for some of the elevated risk, as limited formal education is a risk factor for dementia [36–39]. Interestingly, some investigations using Framingham Heart Study data suggested that men appear to have a lower risk for dementia due to “survival bias” [17]. Hence, the men included in the study were the ones with a healthier cardiovascular risk profile and survived beyond age 65 (men have a higher rate of death from cardiovascular disease in middle age than women) and thus a lower risk for dementia [40, 41]. Certainly, more large-scale studies with larger number of enrollment and different educational, ethical background are needed to support these interpretations.
Although some of the sex differences in AD prevalence are due to differences in longevity, it is very plausible that other socioeconomic risk factors as well as distinct biological mechanisms increase the risk and progression of AD in women [42]. The socioeconomic risk factors are often referred as gender differences in AD [8]. Women usually have a lower income and lower education than men in most cultures, and they are the primary informal caregivers in their families. The caregiving burden is associated with higher rates of unemployment and an increased psychological risk factors in AD including depression and sleep disorders. In fact, 75% of unpaid caregivers for people with chronic debilitating disease like AD are females [43, 44].
The sexual dimorphism in AD can also be explained by many distinct biological risk factors, including: (1) deviations in brain structure, (2) depression, sleep disorders, and psychosocial stress responses, (3) pregnancy, menopause, and sex hormones, (4) genetic background (e.g., APOE), (5) inflammation, gliosis, and immune module (e.g., TREM2), (6) vascular risk factors, and (7) sex differences at single cell level [8, 14, 19, 45].
Brain structure
It is possible that the sex differences in AD risk arise from the divergent changes in brain structures that male and female have exposed to risk factors. Men usually have a larger brain volume on average, and thus are less sensitive to pathological agents for AD and suffer less or slower structural loss as compared to their women counterparts [2]. Numerous brain imaging studies showed that annual atrophy rates were slower in male MCI and AD patients [30, 46]. Moreover, men with MCI had less atrophy in numerous brain regions and a slower decline in certain cognitive tasks as compared to women, as well as less atrophy in numerous regions once an AD diagnosis had been made [47, 48]. However, women have greater cortical thickness than men throughout the lifespan, which would be protective. Quantitative proteomics studies further revealed more alterations in the white matter and mitochondrial proteomes, redox proteins, ATP synthase and cytochrome oxidase in women [49]. These pathophysiological increases in women suggest women are subjected to more rapid neurodegeneration than men once it starts. In addition, women could be more sensitive to those AD pathologic biomarkers than men. This is in consistent with a recent study, reporting that women had higher CSF total tau and Aβ42 levels, more rapid cognitive decline and hippocampal atrophy, indicative of worse pathologic changes than men [10]. It is also important to consider sex differences in resilience, as adverse life events can lead to stable changes in brain structure and function and are considered primary sources of risk for post-traumatic stress disorder, depression and other neuropsychiatric disorders [50]. Stress during the prenatal period and early postnatal life could lead to a higher risk of developing diseases involving socialization for male offspring while stress during puberty has a stronger proximal effect on girls, including higher risk of developing depression, anxiety, and posttraumatic stress disorder [51]. Although evidence showed sex differences regarding to the divergent responses in structural integrity to pathologic insults in AD, the underlying molecular mechanism remain elusive.
Depression
Depression is a risk factor for AD. It is a global mental health concern, and disproportionally affects women with twice more prevalence than men [52]. There are higher levels of inflammatory, neurotrophic, and serotonergic markers and a stronger correlation between levels of some inflammatory and neurotrophic factors and the severity of depressive symptoms in women [53]. Given sex differences in both AD and depression, it is important to examine whether there are sex differences in their association. Interestingly, moderate/severe depressive symptoms were associated with a twofold higher risk of an MCI in women but not in men though mild symptoms were associated with a twofold higher risk of an MCI in men but not in women [54, 55]. In addition, inconsistent findings regarding sex differences in the association between depression and AD were reported in the literature [56]. The fact that depression is more prevalent and severe among females warrants a more careful investigation in the future with more longitudinal studies in this area using consistent approaches to recruitment and measurement.
Sleep disorders
The development and progression of AD pathology has been linked to sleep disorders. The production and clearance of Aβ are correlated with the wake/sleep cycles as Aβ production mainly occurs when awake while its clearance occurs mainly during the sleep stage [57, 58]. Older people tend to have poor quality sleep which leads to reduced Aβ clearance and increased Aβ accumulation in the brain as well as the increased risks of developing AD [59]. Sleep may also selectively attenuate synaptic strength between neurons, decreases cellular stress, and restores brain energy reserves [60]. Sex differences in risks of developing sleep disorders are well-established with women having more sleep problem and reaching a peak during menopause [14]. However, men develop obstructive sleep apnea at earlier ages and have a higher prevalence than women until later ages. Although women are generally more prone to present sleep disorders, further longitudinal study with consistent approaches to recruitment and measurement is needed to understand the mechanistic association between sleep and the sex-specific risk of developing AD.
Stress
Stress and increased stress hormone (e.g., cortisol) levels have been associated with cognitive impairment and AD [61]. Changes in stress hormone signaling have been found in AD patients. In AD patients, the levels of corticotrophin releasing factor 1 (CRF1) were found much higher in hippocampal brain regions, which may alter the hypothalamus–pituitary–adrenal axis signaling, resulting in hippocampal atrophy [62, 63]. In general, women are more vulnerable to stress-related disorders, and levels of cortisol in women with mild-to-moderate AD were found much higher than in men. Preclinical animal studies also confirmed that high level of CRF1 signaling is linked to increased AD pathologies including elevated amyloidosis and tauopathy [62, 63]. More investigations combining molecular/cellular, animal model, and human subjects are required to fully understand the stress-related sex dimorphism in AD pathogenesis.
Sex differences at single cell level
A recent comprehensive study on single-cell transcriptomics of AD showed robust sex differences in the association of AD pathology between cells from female versus male individuals [45]. AD-pathology-associated cell subpopulations were enriched with female cells, but not with male cells, and the marker genes in females had higher expression. It was suggested that the differences might stem from a sex-specific differential transcriptional response to AD pathology [45]. Moreover, transcriptional responses between sexes were contrasting and qualitatively distinct, particularly in oligodendrocytes and neurons. The progression of AD pathology in males was correlated with a global transcriptional activation in oligodendrocytes, but not in females. On the other hand, increased pathology was correlated with a global gene downregulation in both excitatory neurons and inhibitory neurons in females; while only excitatory neurons showed a qualitatively similar but much less pronounced response in males. These observations on reduced transcriptional response, particularly in oligodendrocytes, in females with AD pathology support an underlying sex bias that there is a significant association between the volume of white-matter lesions and lower cognition in females, but not in males. This evidence highlights myelination-related processes in AD pathogenesis, and supports sex differences in AD that manifests at the single cell transcriptomic level [45].
There are certainly other possible biological risk factor attributing to the sex dimorphism in AD, including but not limited to obesity, diabetes mellitus, metabolic syndrome, and thyroid dysfunction which have been reviewed elsewhere. Next, we put more emphases on sex hormones, APOE, TREM2, and vascular risk factors in the following sections.
Sex hormone in AD
Endocrinological factors especially sex steroid hormones have been found associated with onset and progression of AD, and their changes during lifetime are risk for AD [24, 27, 64]. The age-related depletion of estrogens in women and androgens in men, resulting in a loss of neuroprotective hormone effects, is viewed as a potential risk factor for AD development. It is also known as the sex hormone “activational effects” which are the more transient actions of sex hormones in the adult [27, 65]. In addition, emerging evidence suggests that developmental effects of sex hormones responsible for sexual differentiation of the brain may yield a female brain that is inherently more vulnerable to AD pathogenesis. This is also known as “organizational effects” which are the long-lasting or permanent roles of hormones in sexual development and differentiation [27, 65]. Sex steroid hormones including estrogens, progestogens, and androgens exert a variety of activational effects in the brain that increase neural health and resistance to MCI and AD through regulations of amyloidosis, tauopathy, and gliosis [27, 66, 67]. Moreover, sex hormones can act more generally to increase brain function and resilience.
Estrogens
Sex differences in AD are often significantly linked to the estrogen in women, which has attracted the most attentions in preclinical and clinical studies [68–71]. Estrogen is mainly produced in the ovaries, but a significant amount is also synthesized in the brain, especially the primary female hormone estrogen 17β-estradiol (E2). Evidence showed that E2 can regulate synaptic plasticity, promote neural survival, and mediate sex-specific behaviors [72]. Therefore, the depletion in endogenous estrogen levels during menopause was proposed as a trigger for the development of AD in women. In agreement, some but not all investigations showed that estrogen reductions in adulthood are linked to higher risk of AD in women [68–70]. Lifetime estrogen exposure suggest that dementia risk is associated with reduced estrogen. Childbearing women showed higher risk of cognitive impairment and dementia than nulliparous women as pregnancies lead to an overall decrease in women’s lifetime exposure to estrogen [27, 73]. On the other hand, one study suggested that longer lifetime estrogen exposure may increase risk of dementia [74]. The correlative relationship between low estrogen and AD is also supported by surgical menopause-induced estrogen depletion [75–77]. Surgical menopause conducted prior to natural menopause, not after, disrupted the normal cyclic production of sex steroid hormones, resulted in faster cognitive decline and more tauopathy [76]. Preclinical studies also showed increased soluble Aβ levels in the brains of wild-type mice after ovariectomy (OVX) [66, 78]. In agreement, OVX in various AD transgenic female mice led to significant acceleration of Aβ pathology and worsening of behavioral performance when compared with gonadally intact controls, and estrogen replacement therapy blocked this effect [66, 68, 71, 79]. However, in some other AD transgenic strains, OVX or estrogen therapy did not change the brain Aβ level significantly, suggesting that differences in transgenes or strains with different neurosteroidogenesis might mediate the relative impact of estrogen in these models [80, 81].
Androgens
In parallel to the association between low estrogen in women and higher risk of AD, reduced testosterone levels may increase the risk of AD in men. This is supported by several lines of evidence [82, 83]. There are low serum levels of testosterone in men with AD, and low serum testosterone and AD risk is apparent at least ten years before dementia diagnosis clinically. Moreover, significant reduction in brain testosterone levels was observed in men with early and late stages of AD neuropathology than age-matched normal controls, and there was a reverse correlation between brain testosterone levels and brain soluble Aβ levels in early AD [84]. Lastly, androgen-deprivation therapy for patients with prostate cancer increased serum Aβ levels and the risk of AD significantly [64]. In line with this, preclinical studies suggested that decrease in brain androgen level was closely associated with increase in soluble Aβ in rats. Endogenous testosterone depletion by orchiectomy (ORX) resulted in increased level of soluble Aβ in brain, and could be reversed by androgen therapy but not estrogen [85]. Similar results were observed in 3 × Tg-AD and APP23 male mice that testosterone is a negative regulator of Aβ [86]. Collectively, available evidence is prone to suggests that decreased testosterone in men but not in women increases the risk of AD in men, and more closer investigations using better animal models and human studies are needed to fully illustrate androgen’s role in AD risk.
Estrogen replacement therapy (ERT)
If it is true that estrogen depletion due to menopause in women plays a critical role in increased risk of MCI and AD as compared to age-matched men, then ERT should help reverse or alleviate such sex-specific AD pathology. However, clinical studies resulted in inconclusive data, with some even showing adverse effect on cognition and AD risk [35, 69]. It is surprising to see these clinical results, but it is possible that ERT confers other risks like cardiovascular problems that interact with AD pathogenesis to worsen cognition. It is also not clear whether estrogen plus progesterone or progesterone alone could have different outcomes as compared to those postmenopausal women enrolled in ERT. Interestingly, preclinical studies have demonstrated that ERT can reduce risk of AD during certain “critical windows”—ERT had a higher likelihood of neuroprotective in the perimenopause period but not postmenopause period [19, 27]. This is in consistence with the data from several clinical trials including the Cache County Memory Study [35], the WHIMS-Young study [87] and the Kronos Early Estrogen Prevention Study (KEEPS) [88]. Therefore, the “Window of Opportunity” hypothesis emerged arguing that neuroprotective effect of estrogen depends on the age and stage of menopause, and ERT started during postmenopause when a new hormone–receptor equilibrium is already achieved may disturb the established balance, resulting in adverse effect on cognition and AD. Moreover, there is another “Healthy Cell Bias of Estrogen Action” hypothesis which suggests that the benefits of ERT decline over one’s lifetime as cognitive health declines during aging [89–91]. Hence, the effects of estrogen on cognitive function may progress from beneficial to neutral or deleterious over time. This explains why estrogen administered later in life (postmenopause) had neutral or adverse effects on cognition. In this scenario, estrogen is protective only if neurons are healthy at time of its administration while is negative if neuronal health is compromised since neurons during perimenopause are more likely healthier than at postmenopause stage. Although there are merits in these two inter-related hypotheses, critical underlying cellular and molecular mechanisms remain elusive.
Sex and APOE in AD
ApoE is a lipoprotein responsible for transport of cholesterol and phospholipids, and there are three genetic variants—APOE2, APOE3, and APOE4 [14]. The APOE4 has been established as the strongest genetic risk factor for LOAD and related dementia whereas APOE2 is somewhat neuroprotective [5, 13, 15, 20, 23, 29, 31, 92]. In addition, ApoE has Aβ-binding motif and is the best characterized Aβ chaperone which can facilitate Aβ degradation via trafficking of amyloid to lysosomes [31]. Evidence showed that APOE-ε4 may increase Aβ oligomer formation, Aβ deposition as well as upregulation of β-site APP cleavage enzyme (BACE1) [23]. APOE4 was showed to accelerate BACE1 by indirectly modulating cholesterol quantities and increased APP recycling. Recent studies also demonstrated that ApoE regulates tau pathogenesis independent of Aβ pathology, and ApoE4 leads to a gain of toxic effect on tau pathologies [93].
APOE-ε4 increases one’s risk of having AD while ε2 form decreases one’s risk when compared with having the ε3 form. People with one copy of ε4 may have threefold higher risk of AD as compared to those with two copies of ε3 form, and the risk hikes to (8–12)-fold higher if having two copies of ε4 form. Moreover, people with the APOE-ε4 form are shown to have amyloidosis and dementia at a younger age than those having ε2 or ε3. Among all AD patients in US, up to 65% had at least one copy of APOE-ε4 gene [1, 5, 29].
Although APOE4 is the biggest genetic risk factor for AD development, women with at least one copy of APOE-ε4 often exhibited more risk and faster cognitive decline and deterioration than counterpart men. APOE4-associated risk of AD and MCI peaked earlier in women than in men, giving significant elevation between the ages of 65 and 75 for AD (4.37 times increased risk in women as compared to 3.14 in men) and 55 and 70 for MCI (1.43 in women versus 1.07 in men) while the APOE4-associated risk for AD or MCI among men peaked in the 75- to 85-year-old group [94]. In agreement, women with APOE4 have more risk of converting from MCI into AD as compared to non-APOE4 carrier women or men with any APOE isoform [95]. The sex dimorphism is somehow most significant among APOE3 and APOE4 heterozygotes and are most apparent in females with menopause [5, 20, 29, 94]. These findings indicate that estrogen and APOE4 interaction may play a key role in elevated risks of AD development in women as compared to men.
Although estrogen decline in women due to menopause is implicated in cognitive vulnerability in AD, clinical ERT showed that females with APOE4 background receiving estrogen treatment had more cognitive decline than those APOE4 females without ERT [96–99]. Interestingly, ERT led to cognitive improvement for women with APOE2 or APOE3 isoforms [100]. This contradicting result could be explained by the positive loop feedback mediated by estrogen receptors alpha and beta (ERα and ERβ), which are involved in upregulation or downregulation of ApoE protein expression, respectively [101]. Thus, APOE4 carries receiving ERT may suffer an exacerbated ApoE4 overexpression stemming from ERα stimulation by estrogen whereas APOE2/3 carriers with the same treatment may benefit from neuroprotective ApoE2/3 overexpression [101]. Moreover, another explanation is related to the effect of estrogen on metabolic pathways in the brain [102, 103]. Previous studies showed that estrogen promotes glucogenic metabolic pathways but inhibits ketogenic pathways for ATP in female brains. In AD patients with APOE4 form, there is a trend of metabolic shift from glucogenic pathway to a more ketogenic system that relies on utilizing white matter for fuel in the brain. Since APOE4 brains rely on a dual metabolic system of both glucose and keto, estrogen therapy could suppress ketogenic pathway, leading to a compromised ATP production in brain [14]. In contrast, promoting glucogenic pathway and mitigating ketogenic pathway in APOE2/3 carriers by estrogen can lead to a more positive and conducive bioenergetic profile for ATP production because APOE2/3 brains are more tune to using glucose as the primary metabolic pathway [102, 103].
Recent multi-omics analyses also unraveled a profound impact of age, APOE, and sex on metabolism, supporting AD as a metabolic disorder as well [104]. It is well documented that age-dependent differences existed in levels of amino acids, sphingomyelins (SMs), acylcarnitines, phosphatidylcholines (PCs), and ceramides [105, 106]. APOE genetic variants are associated with the differential cholesterol and lipid metabolisms as well as various levels of different SMs [107]. Similar to age and APOE, sex affects blood levels of a variety of metabolites. For example, there are higher levels of most lipids but lower levels of most amino acids in females [108, 109]. A recent systematical study on group-specific metabolic alterations of 139 serum metabolites in 1,517 individuals from the AD Neuroimaging Initiative (ADNI) with AD biomarkers revealed substantial sex differences in effects of 15 metabolites with partially overlapping differences for APOE4 status groups [104]. There are subgroup-specific metabolic effects limited to APOE4 females, indicating greater impairment of mitochondrial energy production in females than males.
Clearly, APOE plays an important role in AD development and treatment responses with major differences between APOE isoforms and between men and women. More studies, such as large-scale single-cell-level analyses and additional experimental follow-up studies, are needed to illustrate the underlying mechanisms of this sex differences in AD pathogenesis with different APOE background.
Sex and immune modulation in AD
Neuroinflammation and neuro-immune response is another important risk factor for AD development, which manifests in gliosis, characterized by proliferation and activation of microglia and astrocytes. Aβ and tau can induce gliosis and neuroinflammation while glial cells and inflammation can regulate Aβ and tau pathogenesis reciprocally. Several key genetic polymorphisms are indicated in the immune modules of AD pathogenesis including TREM2, CD33, and CR1 [14, 19]. Other diseases and environmental factors like viral infection, obesity, TBI, Ca2+/Mg2+-dyshomeostasis may also increase the inflammation. Neuroinflammation involves the activation of reactive glial cells including microglia and astrocytes, leading to higher levels of pro-inflammatory cytokines and Aβ accumulation. Non-steroid anti-inflammatory drugs were found to reduce the risk of AD progression in people over 55 age.
Sex differences in immune systems including neuro-immune modulation of memory and cognitive function also exist. Women usually have stronger immune responses to stimulations than men involving different pathways and immune cells, correlating to higher susceptibility to infections in male whereas higher incidence of autoimmune disorders in females [110]. One possible reason is the differences between sex hormones and sex chromosomes in men and women [110]. Sex differences in dysregulation of glial cell-mediated neuro-immune responses have been implicated in AD and TBI [111]. However, sex-specific cellular and signaling mechanisms in neuro-immune modulation is still largely unclear.
A recent preclinical study demonstrated that a gene module of “immune response” was significantly upregulated in aged mice, and this module was led by the top hub genes including TREM2 and TYROBP [5]. Trem2 is the key regulator of microglial functions in the brain, and the R47H risk variant of the TREM2 gene is a genetic risk factor of AD [112, 113]. Interestingly, in tau mice, female expressing an AD risk variant of R47H-TREM2 exhibited a stepped-up microglial reaction and had worse memory while the same TREM2 mutation did no such thing in male mice [112–114]. The maladaptive microglia are a likely culprit behind the greater memory loss in females. Microglia in both sexes expressed the disease-associated microglia (DAM) signature of genes. However, expression of certain DAM genes shifted only in females with R47H-Trem2 form. The R47H increased the expression of many pro-inflammatory cytokines of the DAM signature and lessened expression of a suite of neuronal genes in female mice while R47H variant exerted no overt change on the DAM signature in male mice [114]. This sexual dimorphism seemed not due to more tau pathology in females, but to an altered microglial response to that burden. Multiple lines of evidence suggest that microglia respond differently between the sexes in some instances, but the driving force, underlying molecular mechanisms, and how they ultimately affect the course of disease, remain elusive.
Sex and vascular risk factors in AD
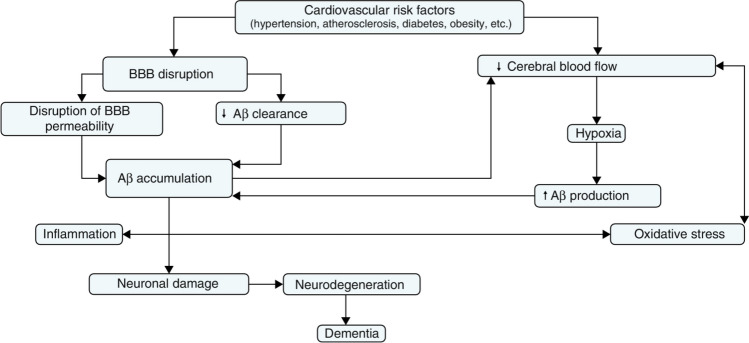
It is well established that vasculature plays a critical role in the progression of AD as a main comorbidity contributing to AD pathogenesis. More than 50% of all patients with vascular cognitive impairment (VCI) will advance to dementia [115]. The vascular hypothesis as an alternative to amyloid hypothesis argues that cardiovascular risk factors including hypertension, atherosclerosis, diabetes, obesity, and other microvessel pathology lead to reduced cerebral blood flow, BBB disruption, hypoxia, impaired Aβ clearance, elevated oxidative stress/inflammation, and ultimately neurodegeneration and dementia (Fig. 2) [116]. In agreement, a recent study showed that BBB breakdown is an early event in the aging human brain in MCI patients and early clinical stages of AD [117]. Vascular abnormality may act in synergy with brain Aβ levels through a positive feedback loop where vascular risk factors promotes Aβ accumulation in the brain parenchyma, and Aβ accumulation in turn aggravates vascular dysfunctions. Accumulating evidence suggest there is also a link between vascular dysfunction, neuronal damage, and inflammation in AD where cerebral vasculature-involved inflammation may precede Aβ deposition which in turn promotes the inflammatory responses.
Fig. 2.
Vascular hypothesis explaining the link between vascular dysfunction and AD. (Reprinted/adapted with permission from [116])
APOE4 also plays an important role in acceleration of BBB breakdown and brain capillary pericytes degeneration. Latest evidence from human studies suggest that people with at least one APOE-ε4 are distinguished from those with APOE-ε3 by BBB breakdown in medial temporal lobe and hippocampus, apparent in APOE4 carriers without cognitive impairment and more severe in those with cognitive impairment [118]. Surprisingly, this APOE4-mediated BBB breakdown is not related to amyloid or tau pathology [118]. These findings suggest that APOE4 leads to BBB dysfunction predicting cognitive decline independently of AD pathology.
The sex differences in vascular risk factors for AD development are emerging. Men have a much higher incidence of coronary artery disease (CAD) than women in all ages, which is linked to cognitive decline with brain microvascular lesions [119]. One possible reason is the protective effects of estrogen against inflammation, oxidative stress, and atherosclerosis in females [120]. While some studies showed that men have a higher incidence of stroke than women and at much earlier ages, other studies found women have a higher risk of stoke due to their longer lifespan [121–123]. Some unique vascular risk factors to women are hypertensive pregnancy disorders, including preeclampsia, eclampsia, and chronic gestational hypertension, which are directly associated with rain lesions and cognitive impairment [124, 125]. Women at age of ~ 60 with prior history of hypertensive pregnancy disorders had more brain atrophies after pregnancies as compared to those with normotensive pregnancies [124].
Recent investigations unraveled some sex differences on cerebral small vessel diseases (SVD) which may also play a role in AD. SVD refers to conditions where damage to arterioles and capillaries is predominant, leading to reduced, or interrupted perfusion of the affected brain regions, particularly the white matter [126]. Thus, it could be a major etiologic cause in dementia and AD. Sex differences were observed in blood lipids and SVD in 817 neurologically healthy men and women over 50 years of age. HDL-C and apoA-1 were inversely associated with the severity of white matter lesions in women but not in men [127]. In another study with 668 individuals aged 60–90 years, women had more marked progression of subcortical white matter lesions and incident lacunar infarcts compared with men. It is suggested that besides higher age, cigarette smoking, elevated blood pressure, and baseline lesion load, female sex was also associated with SVD progression [128].
The effects of APOE genotype on sex differences for vascular risks have also been explored. There was a significantly higher rate of CVD for men APOE4 carriers (18.6%) than counterpart women APOE4 carriers (9.9%) as shown by a Framingham Heart Study with 3413 participants [129]. The APOE-ε2 seemed protective for female (4.9%), but not for male (18.2%), suggesting ε2 or ε4 is associated to higher risk for CVD in male. The mortality due to cardiovascular disease in men was sixfold higher than in women at ages between 45 to 54 according to another Framingham Heart Study with 7901 participants, and this difference was still evident even comparing the lowest risk group of men (i.e. ε3/ε3) to the highest risk group of women (i.e. ε4/ε4) [17]. Thus, it is speculated that lower risk of AD in men older than 65 years is due to this so called “survivor hypothesis,” which is supported by the evidence that there is no differences between male and female for midlife risk of AD while significantly higher risk for female after midlife, and postmenopause from age of 65 years and older [29].
Evidence from a meta-analysis showed the association of APOE4 with severe cerebral amyloid angiopathy (CAA) [130]. APOE4 is also linked to higher risk of cognitive decline and vascular cognitive impairment during normal aging [131]. A recent investigation examined the effects of APOE, sex, and pathological components on CAA in 428 AD subjects [31]. Overall CAA scores were found to be higher in males than females, and APOE4 was associated with higher overall CAA scores. It is suggested that APOE genotypes and sex differentially affect the presence and severity of CAA in AD, likely through the interaction and aggregation of Aβ40 and apoE [31].
Gap between clinical and preclinical studies
Although sex differences are implicated in the heterogeneities of AD prevalence and clinical manifestations by clinical and preclinical studies, there is always a gap regarding behavior and cognitive performance, disease course and prognosis, and pathology between human and animal studies. For example, a recent study by comparing human brain aging with mouse models revealed major gaps in our understanding of sex differences across species on APOE and sex interactions [132]. Aging male APOE4 carriers had sevenfold more microbleeds than women, while it was opposite to male EFAD mice that had sevenfold fewer. However, the EFAD mice showed modest female excess in prevalence of Aβ-positive vessels or CAA, consistent with some postmortem human studies. This supports the notion that AD is a uniquely human condition.
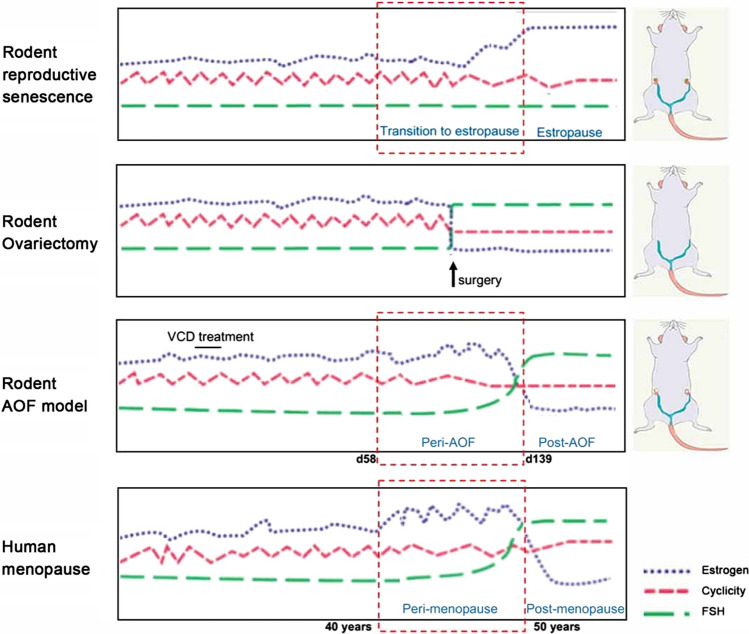
To explore the role of estrogen and menopause in AD, mice models have been constructed to replicate different elements of human menopause including natural aging, ovariectomy (OVX) and hormone replacement [76, 133]. However, they often fall short to recapitulate the human menopause process adequately [91]. The intact aging mice model fails to achieve very low levels of estrogen while the mice OVX model lacks perimenopause stage. To bridge the gap, a better mice model mimicking the human menopause should be used, and the innovative accelerated ovarian failure (AOF) using the chemical 4-vinylcyclohexene diepoxide (VCD) could be used (Fig. 3) [91]. It uniquely recapitulates hormonal changes that occur during human menopause, including estrous acyclicity and fluctuation (as in human perimenopause), followed by undetectable, estrogen levels (as in human postmenopause). This model also allows for the dissociation of the effects of aging from the effects of hormone levels in young mice [91, 134].
Fig. 3.
Models of menopause in rodents and human. (Reprinted/adapted with permission from [91])
Preclinical animal models of AD are critical to gaining a better understanding of pathogenesis and to assess the potential of novel therapeutic approaches. The fact that AD clinical trials so far has very limited success could be, at least partially, related to the premature translation of high successes in animal models that mirror only limited aspects of AD pathology to humans. Animal models are advantageous in offering the option to conduct preclinical testing in vivo [135]. New knock-in mouse models are potentially more representative of physiological models of AD. Nonhuman primates may provide more genetic similarity to humans and a more physiological relevant pathogenesis of AD, but are limited by availability, costs, time, and inconsistency [135]. To enhance the success of translation from preclinical studies to patients, a better understanding of the strengths and weakness of different preclinical models and the use of more than one model could be helpful.
Conclusion
There is mounting evidence to support sex and gender differences on the risk of AD development [6–14, 21, 24, 25, 48, 95, 110, 112, 121]. The underlying mechanisms for the apparent differences still remain elusive, but intrinsic biological risk factors are increasing prominent besides the longevity for women. Among them, sexual dimorphism in CNS structures, changes in sex hormone signaling, risk genes and sex interactions, immune responses, and vascular diseases are considered important determinants. Biological sex and common co-morbidities for AD should be considered in both preclinical and clinical studies. Providing that there are currently no effective treatments available for AD, it is critical that we understand how to mitigate risk factors for this devastating disease in both sexes.
To fully answer this question on sex differences in AD, more large-scale comprehensive AD risk assessments, biomarker collection, and genetic stratification are needed [8]. Better disease models therefore could be generated on the basis of big data analyses including individual variability like sex, phenotypic, genetic, epigenetic, biomarker, lifestyle, and psychosocial characteristics. These data-driven large-scale studies will stimulate a paradigm shift towards precision medicine and precision pharmacology in AD, highlighting the need for tailored interventions considering specific biological make-up including sex for each individual.
Funding
This work was supported by National Institutes of Health [Grant number R01AG064798, R01HL140562, R01AG061288, R03AG063287, R21AG066090, R01NS110687]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s disease facts and figures (2020) Alzheimers Dement 16(3):391–460
- 2.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease. Mol Neurodegener. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tublin JM, Adelstein JM, del Monte F, Combs CK, Wold LE. Getting to the heart of Alzheimer disease. Circ Res. 2019;124(1):142–149. doi: 10.1161/CIRCRESAHA.118.313563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao N, Ren Y, Yamazaki Y, Qiao W, Li F, Felton LM, Mahmoudiandehkordi S, Kueider-Paisley A, Sonoustoun B, Arnold M. Alzheimer’s risk factors age, APOE genotype, and sex drive distinct molecular pathways. Neuron. 2020;106:727–742. doi: 10.1016/j.neuron.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke SL, Hu T, Fava NM, Li T, Rodriguez MJ, Schuldiner KL, Burgess A, Laird A. Sex differences in the development of mild cognitive impairment and probable Alzheimer's disease as predicted by hippocampal volume or white matter hyperintensities. J Women Aging. 2019;31(2):140–164. doi: 10.1080/08952841.2018.1419476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Bush WS, Gifford KA, Chibnik LB, Mukherjee S, De Jager PL. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain. 2019;142(9):2581–2589. doi: 10.1093/brain/awz206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Dimech AS, Chadha AS, Baracchi F, Girouard H, Misoch S, Giacobini E. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 9.Gur E, Fertan E, Kosel F, Wong AA, Balci F, Brown RE. Sex differences in the timing behavior performance of 3xTg-AD and wild-type mice in the peak interval procedure. Behav Brain Res. 2019;360:235–243. doi: 10.1016/j.bbr.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 10.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205–213. doi: 10.1007/s11682-016-9523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laws KR, Irvine K, Gale TM. Sex differences in Alzheimer’s disease. Curr Opin Psychiatry. 2018;31(2):133–139. doi: 10.1097/YCO.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 12.Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Varsavsky I, Osorio RS, Pupi A, Vallabhajosula S, Isaacson RS. Sex differences in Alzheimer risk: brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382–1390. doi: 10.1212/WNL.0000000000004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tensil M, Hessler JB, Gutsmiedl M, Riedl L, Grimmer T, Diehl-Schmid J. Sex differences in neuropsychological test performance in Alzheimer’s disease and the influence of the ApoE genotype. Alzheimer Dis Assoc Disord. 2018;32(2):145–149. doi: 10.1097/WAD.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 14.Toro CA, Zhang L, Cao J, Cai D. Sex differences in Alzheimer’s disease: understanding the molecular impact. Brain Res. 2019;1719:194–207. doi: 10.1016/j.brainres.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold M, Nho K, Kueider-Paisley A, Massaro T, Huynh K, Brauner B, MahmoudianDehkordi S, Louie G, Moseley MA, Thompson JW. Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-14959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bundy JL, Vied C, Badger C, Nowakowski RS. Sex-biased hippocampal pathology in the 5XFAD mouse model of Alzheimer's disease: a multi-omic analysis. J Comp Neurol. 2019;527(2):462–475. doi: 10.1002/cne.24551. [DOI] [PubMed] [Google Scholar]
- 17.Chene G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310–320. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan CC, Banks SJ, Thompson WK, Chen C-H, McEvoy LK, Tan CH, Kukull W, Bennett DA, Farrer LA, Mayeux R. Sex-dependent autosomal effects on clinical progression of Alzheimer’s disease. Brain. 2020;143(7):2272–2280. doi: 10.1093/brain/awaa164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher DW, Bennett DA, Dong HX. Sexual dimorphism in predisposition to Alzheimer's disease. Neurobiol Aging. 2018;70:308–324. doi: 10.1016/j.neurobiolaging.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamache J, Yun Y, Chiba-Falek O. Sex-dependent effect of APOE on Alzheimer’s disease and other age-related neurodegenerative disorders. Dis Model Mech. 2020;13(8):dmm045211. doi: 10.1242/dmm.045211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int. 2019;127:38–55. doi: 10.1016/j.neuint.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Giacobini E, Pepeu G. Sex and gender differences in the brain cholinergic system and in the response to therapy of Alzheimer disease with cholinesterase inhibitors. Curr Alzheimer Res. 2018;15(11):1077–1084. doi: 10.2174/1567205015666180613111504. [DOI] [PubMed] [Google Scholar]
- 23.Hou X, Adeosun SO, Zhang Q, Barlow B, Brents M, Zheng B, Wang J. Differential contributions of ApoE4 and female sex to BACE1 activity and expression mediate Aβ deposition and learning and memory in mouse models of Alzheimer’s disease. Front Aging Neurosci. 2015;7:207. doi: 10.3389/fnagi.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li RN, Singh M. Sex differences in cognitive impairment and Alzheimer's disease. Front Neuroendocrin. 2014;35(3):385–403. doi: 10.1016/j.yfrne.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matyi J, Tschanz JT, Rattinger GB, Sanders C, Vernon EK, Corcoran C, Kauwe JSK, Buhusi M. Sex differences in risk for Alzheimer’s disease related to neurotrophin gene polymorphisms: the Cache County Memory Study. J Gerontol a-Biol. 2017;72(12):1607–1613. doi: 10.1093/gerona/glx092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paranjpe MD, Belonwu S, Wang JK, Oskotsky T, Gupta A, Taubes A, Zalocusky K, Paranjpe I, Glicksberg BS, Huang Y (2020) Sex-specific cross tissue meta-analysis identifies immune dysregulation in women with Alzheimer’s disease. bioRxiv [DOI] [PMC free article] [PubMed]
- 27.Pike CJ. Sex and the development of Alzheimer's disease. J Neurosci Res. 2017;95(1–2):671–680. doi: 10.1002/jnr.23827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: triad of risk of Alzheimer’s disease. J Steroid Biochem. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampedro F, Vilaplana E, de Leon MJ, Alcolea D, Pegueroles J, Montal V, Carmona-Iragui M, Sala I, Sanchez-Saudinos MB, Anton-Aguirre S, Morenas-Rodriguez E, Camacho V, Falcon C, Pavia J, Ros D, Clarimon J, Blesa R, Lleo A, Fortea J. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6(29):26663–26674. doi: 10.18632/oncotarget.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shinohara M, Murray ME, Frank RD, Shinohara M, DeTure M, Yamazaki Y, Tachibana M, Atagi Y, Davis MD, Liu CC, Zhao N, Painter MM, Petersen RC, Fryer JD, Crook JE, Dickson DW, Bu G, Kanekiyo T. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. 2016;132(2):225–234. doi: 10.1007/s00401-016-1580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Touma C, Ambree O, Gortz N, Keyvani K, Lewejohann L, Palme R, Paulus W, Schwarze-Eicker K, Sachser N. Age- and sex-dependent development of adrenocortical hyperactivity in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2004;25(7):893–904. doi: 10.1016/j.neurobiolaging.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Fiest KM, Roberts JI, Maxwell CJ, Hogan DB, Smith EE, Frolkis A, Cohen A, Kirk A, Pearson D, Pringsheim T, Venegas-Torres A, Jette N. The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can J Neurol Sci. 2016;43:S51–S82. doi: 10.1017/cjn.2016.36. [DOI] [PubMed] [Google Scholar]
- 34.Miech RA, Breitner JCS, Zandi PP, Khachaturian AS, Anthony JC, Mayer L, Grp CCS. Incidence of AD may decline in the early 90s for men, later for women—the Cache County study. Neurology. 2002;58(2):209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 35.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS, Investig CCMS. Hormone replacement therapy and incidence of Alzheimer disease in older women—the Cache County Study. Jama J Am Med Assoc. 2002;288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick AL, Kuller LH, Ives DG, Lopez OL, Jagust W, Breitner JCS, Jones B, Lyketsos C, Dulberg C. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 37.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence—a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- 38.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sando SB, Melquist S, Cannon A, Hutton M, Sletvold O, Saltvedt I, White LR, Lydersen S, Aasly J. Risk-reducing effect of education in Alzheimer's disease. Int J Geriatr Psych. 2008;23(11):1156–1162. doi: 10.1002/gps.2043. [DOI] [PubMed] [Google Scholar]
- 40.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the united states: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer's disease greater for women than for men? Am J Epidemiol. 2001;153(2):132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 42.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 43.Pusswald G, Lehrner J, Hagmann M, Dal-Bianco P, Benke T, Loitfelder M, Marksteiner J, Mosbacher J, Ransmayr G, Sanin G, Schmidt R, Grp PS. Gender-specific differences in cognitive profiles of patients with Alzheimer’s disease: results of the prospective dementia registry Austria (PRODEM-Austria) J Alzheimers Dis. 2015;46(3):631–637. doi: 10.3233/JAD-150188. [DOI] [PubMed] [Google Scholar]
- 44.Vemuri P, Knopman DS, Lesnick TG, Przybelski SA, Mielke MM, Graff-Radford J, Murray ME, Roberts RO, Vassilaki M, Lowe VJ, Machulda M, Jones DT, Petersen RC, Jack CR. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. 2017;74(6):718–726. doi: 10.1001/jamaneurol.2017.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570(7761):332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, Dufouil C. Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med. 2015;45(9):1931–1944. doi: 10.1017/S0033291714003055. [DOI] [PubMed] [Google Scholar]
- 47.Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Weiner MW, Thompson PM, A.s.D.N. Initi Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol Aging. 2010;31(8):1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. J Alzheimers Dis. 2016;50(3):847–857. doi: 10.3233/JAD-150780. [DOI] [PubMed] [Google Scholar]
- 49.Gallart-Palau X, Lee BST, Adav SS, Qian JR, Serra A, Park JE, Lai MKP, Chen CP, Kalaria RN, Sze SK. Gender differences in white matter pathology and mitochondrial dysfunction in Alzheimer's disease with cerebrovascular disease. Mol Brain. 2016;9:27. doi: 10.1186/s13041-016-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fallon IP, Tanner MK, Greenwood BN, Baratta MV. Sex differences in resilience: experiential factors and their mechanisms. Eur J Neurosci. 2020;52(1):2530–2547. doi: 10.1111/ejn.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 2019;86(6):421–432. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eid RS, Gobinath AR, Galea LAM. Sex differences in depression: insights from clinical and preclinical studies. Prog Neurobiol. 2019;176:86–102. doi: 10.1016/j.pneurobio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Labaka A, Goni-Balentziaga O, Lebena A, Perez-Tejada J. Biological sex differences in depression: a systematic review. Biol Res Nurs. 2018;20(4):383–392. doi: 10.1177/1099800418776082. [DOI] [PubMed] [Google Scholar]
- 54.Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM. Understanding the impact of sex and gender in Alzheimer's disease: a call to action. Alzheimers Dement. 2018;14(9):1171–1183. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goveas JS, Hogan PE, Kotchen JM, Smoller JW, Denburg NL, Manson JE, Tummala A, Mysiw WJ, Ockene JK, Woods NF, Espeland MA, Wassertheil-Smoller S. Depressive symptoms, antidepressant use, and future cognitive health in postmenopausal women: the Women’s Health Initiative Memory Study. Int Psychogeriatr. 2012;24(8):1252–1264. doi: 10.1017/S1041610211002778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Underwood EA, Davidson HP, Azam AB, Tierney MC. Sex differences in depression as a risk factor for Alzheimer’s disease: a systematic review. Innov Aging. 2019;3(2):igz015. doi: 10.1093/geroni/igz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep–wake disruptions and Alzheimer's disease. Sleep Med Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanderheyden WM, Lim MM, Musiek ES, Gerstner JR. Alzheimer’s disease and sleep-wake disturbances: amyloid astrocytes, and animal models. J Neurosci. 2018;38(12):2901–2910. doi: 10.1523/JNEUROSCI.1135-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloret MA, Cervera-Ferri A, Nepomuceno M, Monllor P, Esteve D, Lloret A. Is sleep disruption a cause or consequence of Alzheimer’s disease? Reviewing its possible role as a biomarker. Int J Mol Sci. 2020;21(3):1168. doi: 10.3390/ijms21031168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Csernansky JG, Dong HX, Fagan AM, Wang L, Xiong CJ, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am J Psychiat. 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, Desouza EB. Displacement of corticotropin-releasing factor from its binding-protein as a possible treatment for Alzheimers-disease. Nature. 1995;378(6554):284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 63.DeSouza EB. Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrino. 1995;20(8):789–819. doi: 10.1016/0306-4530(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 64.Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32(4):604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poling MC, Kauffman AS. Organizational and activational effects of sex steroids on kisspeptin neuron development. Front Neuroendocrin. 2013;34(1):3–17. doi: 10.1016/j.yfrne.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, Murphy MP, Beckett TL, Finch CE, Brinton RD, Pike CJ. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153(11):5467–5479. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosario ER, Pike CJ. Androgen regulation of beta-amyloid protein and the risk of Alzheimer's disease. Brain Res Rev. 2008;57(2):444–453. doi: 10.1016/j.brainresrev.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27(48):13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song YJ, Li SR, Li XW, Chen X, Wei ZX, Liu QS, Cheng Y. The effect of estrogen replacement therapy on Alzheimer’s disease and parkinson's disease in postmenopausal women: a meta-analysis. Front Neurosci. 2020;14:157. doi: 10.3389/fnins.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uddin MS, Rahman MM, Jakaria M, Rahman MS, Hossain MS, Islam A, Ahmed M, Mathew B, Omar UM, Barreto GE, Ashraf GM. Estrogen signaling in Alzheimer's disease: molecular insights and therapeutic targets for Alzheimer’s dementia. Mol Neurobiol. 2020;57:2654–2670. doi: 10.1007/s12035-020-01911-8. [DOI] [PubMed] [Google Scholar]
- 71.Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80(1):191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 72.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fox M, Berzuini C, Knapp LA, Glynn LM. Women’s pregnancy life history and Alzheimer's risk: can immunoregulation explain the link? Am J Alzheimers Dis. 2018;33(8):516–526. doi: 10.1177/1533317518786447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geerlings MI, Ruitenberg A, Witteman JCM, van Swieten JC, Hofman A, van Duijn CM, Breteler MMB, Launer LJ. Reproductive period and risk of dementia in postmenopausal women. JAMA J Am Med Assoc. 2001;285(11):1475–1481. doi: 10.1001/jama.285.11.1475. [DOI] [PubMed] [Google Scholar]
- 75.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82(3):222–229. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rocca WA. Surgical menopause and increased risk of cognitive impairment and dementia—a protective role for estrogen? Biol Reprod. 2011;85:97. [Google Scholar]
- 77.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69(11):1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 78.Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp Gerontol. 2000;35(9–10):1317–1325. doi: 10.1016/s0531-5565(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 79.Levin-Allerhand JA, Lominska CE, Wang J, Smith JD. 17Alpha-estradiol and 17beta-estradiol treatments are effective in lowering cerebral amyloid-beta levels in AbetaPPSWE transgenic mice. J Alzheimers Dis. 2002;4(6):449–457. doi: 10.3233/jad-2002-4601. [DOI] [PubMed] [Google Scholar]
- 80.Golub MS, Germann SL, Mercer M, Gordon MN, Morgan DG, Mayer LP, Hoyer PB. Behavioral consequences of ovarian atrophy and estrogen replacement in the APPswe mouse. Neurobiol Aging. 2008;29(10):1512–1523. doi: 10.1016/j.neurobiolaging.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Green PS, Bales K, Paul S, Bu GJ. Estrogen therapy fails to alter amyloid deposition in the PDAPP model of Alzheimer’s disease. Endocrinology. 2005;146(6):2774–2781. doi: 10.1210/en.2004-1433. [DOI] [PubMed] [Google Scholar]
- 82.Gillett MJ, Martins RN, Clarnette RM, Chubb SAP, Bruce DG, Yeap BB. Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis. 2003;5(4):267–269. doi: 10.3233/jad-2003-5401. [DOI] [PubMed] [Google Scholar]
- 83.Paoletti AM, Congia S, Lello S, Tedde D, Orru M, Pistis M, Pilloni M, Zedda P, Loddo A, Melis GB. Low androgenization index in elderly women and elderly men with Alzheimer’s disease. Neurology. 2004;62(2):301–303. doi: 10.1212/01.wnl.0000094199.60829.f5. [DOI] [PubMed] [Google Scholar]
- 84.Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62(2):188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 85.Ramsden M, Nyborg AC, Murphy MP, Chang L, Stanczyk FZ, Golde TE, Pike CJ. Androgens modulate beta-amyloid levels in male rat brain. J Neurochem. 2003;87(4):1052–1055. doi: 10.1046/j.1471-4159.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 86.McAllister C, Long JG, Bowers A, Walker A, Cao P, Honda SI, Harada N, Staufenbiel M, Shen Y, Li RN. Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci. 2010;30(21):7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Craig MC, Maki PM, Murphy DGM. The women's health initiative memory study: findings and implications for treatment. Lancet Neurol. 2005;4(3):190–194. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- 88.Miller VM, Naftolin F, Asthana S, Black DM, Brinton EA, Budoff MJ, Cedars MI, Dowling NM, Gleason CE, Hodis HN, Jayachandran M, Kantarci K, Lobo RA, Manson JE, Pal L, Santoro NF, Taylor HS, Harman SM. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause. 2019;26(9):1071–1084. doi: 10.1097/GME.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marongiu R. Accelerated ovarian failure as a unique model to study peri-menopause influence on Alzheimer’s disease. Front Aging Neurosci. 2019;11:242. doi: 10.3389/fnagi.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vermunt L, Sikkes SA, Van Den Hout A, Handels R, Bos I, Van Der Flier WM, Kern S, Ousset PJ, Maruff P, Skoog I. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888–898. doi: 10.1016/j.jalz.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao LZ, Luo WJ, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Oh JR, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, Mccue L, Xiong CJ, Del-Aguila JL, Morris JC, Cruchaga C, Fagan AM, Miller BL, Boxer AL, Seeley WW, Butovsky O, Barres BA, Paul SM, Holtzman DM. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang L-S, Romero K, Arneric SP, Redolfi A. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O'Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33(4):720–731.e4. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD—results of a randomized study. Neurology. 2001;57(4):605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- 97.Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women—randomized, double-blind, placebo-controlled trial. Neurology. 2000;54(2):295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 98.Mulnard RI, Cotman CW, Kawas C, van Dyck CH, Sano H, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ, St ADC. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease—a randomized controlled trial. JAMA J Am Med Assoc. 2000;283(8):1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 99.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Investigators W. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women—the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA J Am Med Assoc. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 100.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 101.Wang JM, Irwin RW, Brinton RD. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc Natl Acad Sci USA. 2006;103(45):16983–16988. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yao J, Irwin R, Chen SH, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiol Aging. 2012;33(8):1507–1521. doi: 10.1016/j.neurobiolaging.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reiman EM, Chen KW, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon 4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102(23):8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arnold M, Nho K, Kueider-Paisley A, Massaro T, Huynh K, Brauner B, MahmoudianDehkordi S, Louie G, Moseley MA, Thompson JW, John-Williams LS, Tenenbaum JD, Blach C, Chang R, Brinton RD, Baillie R, Han X, Trojanowski JQ, Shaw LM, Martins R, Weiner MW, Trushina E, Toledo JB, Meikle PJ, Bennett DA, Krumsiek J, Doraiswamy PM, Saykin AJ, Kaddurah-Daouk R, Kastenmuller G. Sex and APOE epsilon4 genotype modify the Alzheimer's disease serum metabolome. Nat Commun. 2020;11(1):1148. doi: 10.1038/s41467-020-14959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez-Covarrubias V, Beekman M, Uh HW, Dane A, Troost J, Paliukhovich I, van der Kloet FM, Houwing-Duistermaat J, Vreeken RJ, Hankemeier T, Slagboom EP. Lipidomics of familial longevity. Aging Cell. 2013;12(3):426–434. doi: 10.1111/acel.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Z, Zhai G, Singmann P, He Y, Xu T, Prehn C, Romisch-Margl W, Lattka E, Gieger C, Soranzo N, Heinrich J, Standl M, Thiering E, Mittelstrass K, Wichmann HE, Peters A, Suhre K, Li Y, Adamski J, Spector TD, Illig T, Wang-Sattler R. Human serum metabolic profiles are age dependent. Aging Cell. 2012;11(6):960–967. doi: 10.1111/j.1474-9726.2012.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long T, Hicks M, Yu HC, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, Brewerton S, Turpaz Y, Perkins BA, Evans AM, Miller LA, Guo L, Caskey CT, Schork NJ, Garner C, Spector TD, Venter JC, Telenti A. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49(4):568–578. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 108.Krumsiek J, Mittelstrass K, Do KT, Stuckler F, Ried J, Adamski J, Peters A, Illig T, Kronenberg F, Friedrich N, Nauck M, Pietzner M, Mook-Kanamori DO, Suhre K, Gieger C, Grallert H, Theis FJ, Kastenmuller G. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11(6):1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 2011;7(8):e1002215. doi: 10.1371/journal.pgen.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tronson NC, Collette KM. (Putative) Sex differences in neuroimmune modulation of memory. J Neurosci Res. 2017;95(1–2):472–486. doi: 10.1002/jnr.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dzamba D, Harantova L, Butenko O, Anderova M. Glial cells—the key elements of Alzheimer’s disease. Curr Alzheimer Res. 2016;13(8):894–911. doi: 10.2174/1567205013666160129095924. [DOI] [PubMed] [Google Scholar]
- 112.Kodama L, Gan L. Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends Mol Med. 2019;25(9):741–749. doi: 10.1016/j.molmed.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kodama L, Guzman E, Etchegaray JI, Li YQ, Sayed FA, Zhou L, Zhou YG, Zhan LH, Le D, Udeochu JC, Clelland CD, Cheng ZL, Yu GQ, Li QY, Kosik KS, Gan L. Microglial microRNAs mediate sex-specific responses to tau pathology. Nat Neurosci. 2020;23(2):167. doi: 10.1038/s41593-019-0560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joint Keystone Symposia: Neurodegenerative Diseases: New Insights and Therapeutic Opportunities and Neural Environment in Disease: Glial Responses and Neuroinflammation. https://www.alzforum.org/news/conference-coverage/down-sex-boy-and-girl-microglia-respond-differently
- 115.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia. J Am Coll Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rius-Perez S, Tormos AM, Perez S, Talens-Visconti R. Vascular pathology: cause or effect in Alzheimer disease? Neurologia. 2018;33(2):112–120. doi: 10.1016/j.nrl.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, Pachicano M, Joe E, Nelson AR, D'Orazio LM, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Reiman EM, Caselli RJ, Chui HLC, Julia TCW, Chen YN, Pa J, Conti PS, Law M, Toga AW, Zlokovic BV. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):70. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kivipelto M, Helkala EL, Hanninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment—a population-based study. Neurology. 2001;56(12):1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 120.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 121.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 122.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 123.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke—estimates from the Framingham Study. Stroke. 2006;37(2):345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 124.Mielke MM, Milic NM, Weissgerber TL, White WM, Kantarci K, Mosley TH, Windham BG, Simpson BN, Turner ST, Garovic VD. Impaired cognition and brain atrophy decades after hypertensive pregnancy disorders. Circ Cardiovasc Qual. 2016;9(2):S70–S76. doi: 10.1161/CIRCOUTCOMES.115.002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Postma IR, Bouma A, Ankersmit IF, Zeeman GG. Neurocognitive functioning following preeclampsia and eclampsia: a long-term follow-up study. Am J Obstet Gynecol. 2014;211(1):37.e1–37.e9. doi: 10.1016/j.ajog.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 126.Hakim AM. Small vessel disease. Front Neurol. 2019;10:1020. doi: 10.3389/fneur.2019.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yin ZG, Wang QS, Yu K, Wang WW, Lin H, Yang ZH. Sex differences in associations between blood lipids and cerebral small vessel disease. Nutr Metab Cardiovasc Dis. 2018;28(1):28–34. doi: 10.1016/j.numecd.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 128.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39(10):2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 129.Lahoz C, Schaefer EJ, Cupples LA, Wilson PWF, Levy D, Osgood D, Parpos S, Pedro-Botet J, Daly JA, Ordovas JM. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154(3):529–537. doi: 10.1016/s0021-9150(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 130.Rannikmae K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N, Al-Shahi Salman R, Sudlow CL. APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(3):300–305. doi: 10.1136/jnnp-2013-306485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cacciottolo M, Christensen A, Moser A, Liu JH, Pike CJ, Smith C, Ladu MJ, Sullivan PM, Morgan TE, Dolzhenko E, Charidimou A, Wahlund LO, Wiberg MK, Shams S, Chiang GCY, Finch CE. The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol Aging. 2016;37:47–57. doi: 10.1016/j.neurobiolaging.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas. 2016;87:5–17. doi: 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drummond E, Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133(2):155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]