Abstract
Individuals who get involved in the disinfection of public settings using sodium hypochlorite might suffer adverse health effects. However, scarce information is available on the potential oxidative stress damage caused at low concentrations typically used for disinfection. We aimed to assess whether exposure to sodium hypochlorite during the COVID-19 pandemic causes oxidative stress damage in workers engaged in disinfection tasks. 75 operators engaged in the disinfection of public places were recruited as the case group, and 60 individuals who were not exposed to disinfectant were chosen as the control group. Spot urine samples were collected before (BE) and after exposure (AE) to disinfectants in the case group. Likewise, controls provided two spot urine samples in the same way as the case group. Urinary malondialdehyde (MDA) levels were quantified by forming thiobarbituric acid reactive substances in the urine. In addition, the concentration of 8-hydroxy-2′-deoxyguanosine (8-OHdG) in the urine was determined using an ELISA kit. Results showed significant differences in the urinary levels of oxidative stress markers, where median 8-OHdG (AE case: 3.84 ± 2.89 μg/g creatinine vs AE control 2.54 ± 1.21 μg/g creatinine) and MDA (AE case: 169 ± 89 μg/g creatinine vs AE control 121 ± 47 μg/g creatinine) levels in case group AE samples were 1.55 and 1.35-times higher than the control group AE samples (P < 0.05), respectively. Besides, urinary levels of oxidative stress markers in AE samples of the case group were significantly higher than in BE samples (8-OHdG BE 3.40 ± 1.95 μg/g creatinine, MDA BE 136 ± 51.3 μg/g creatinine, P < 0.05). Our results indicated that exposure to even low levels of sodium hypochlorite used in disinfection practices might cause oxidative stress related damage. With this in mind, implementing robust protective measures, such as specific respirators, is crucial to reduce the health burdens of exposure to disinfectants.
Keywords: Biomonitoring, COVID-19, Disinfectants, Oxidative stress damage, Urinary biomarkers
Graphical abstract
1. Introduction
COVID-19, a disease caused by the severe acute respiratory syndrome 2 (SARS-CoV-2) virus, was first reported in late 2019 in Wuhan, China, and then rapidly spread to other countries worldwide. The new coronavirus showed higher transmissibility potential than SARS-CoV and MERS-CoV (Zhu et al., 2020). With this in mind and because of its rapid spread, the World Health Organization (WHO) declared this viral outbreak a pandemic on March 11, 2020 (WHO, 2020). In addition, according to the WHO COVID-19 dashboard, there have been over 249 million confirmed cases and over five million deaths reported around the world, with almost six million confirmed cases and over 127,400 deaths in Iran (as of November 8, 2021) (WHO, 2020).
To date, studies reported that SARS-CoV-2 could be transmitted to humans through person-to-person contact, fomites, aerosol, and droplets (Chan et al., 2020; Peng et al., 2020; Tang et al., 2020). Contamination of frequently touched surfaces in public places is one of the potential sources of SARS-CoV-2 transmission. With this in mind, WHO recommends consistent and proper environmental cleaning and disinfection procedures to ensure that frequently touched surfaces are free from SARS-CoV-2 (WHO, 2020). Studies recently reported the effectiveness of various disinfectants such as ethanol (78–95%), isopropanol (70–100%), formaldehyde (0.7–1%) and other available disinfectants against the inactivation of SARS-CoV-2 (Kampf, 2020; Pradhan et al., 2020). However, various disinfectants require different concentrations to be effective against SARS-CoV-2. For instance, hydrogen peroxide could inactivate SARS-CoV-2 at a concentration of 0.5%, while other disinfectants like sodium hypochlorite need a concentration of at least 0.21% to inactivate SARS-CoV-2 (Kampf, 2020).
Individuals who get involved in public place disinfection procedures can be potentially exposed to significant levels of disinfectant chemicals through inhalation and dermal contact, which could cause adverse effects on their health. For example, adverse health effects such as asthma, inflammatory reactions in the airways, decreased lung function, and eye irritation have been correlated with exposure to disinfectants and cleaning products (Clausen et al., 2020). In addition, Vizcaya et al. (2015) found a significant correlation between the use of cleaning sprays and lower forced expiratory volume (FEV1) in cleaning workers. To date, studies published in disinfectant exposure assessment have mostly focused on pulmonary health consequences, and there is a lack of scientific-based evidence related to other potential health effects resulting from disinfectant exposure. The COVID-19 pandemic has resulted in massive increases in the usage of disinfectants worldwide, which represents an opportunity to advance our understanding of a wide range of health effects related to disinfectant use. One potential adverse health effect resulting from disinfectant exposure could be triggering oxidative stress, an important mechanism in cell cytotoxicity and the development of various health endpoints. Disinfectant exposure has been associated with increased levels of reactive oxygen species (ROS), membrane damage, ROS-mediated DNA damage, and increased stress response in bacterial communities (da Cruz Nizer et al., 2020; Zhang et al., 2021b) and mice (Li et al., 2021). Sodium hypochlorite is a potent oxidant (Hawkins and Davies, 1998) that reacts with a large number of biomolecules, including proteins, lipids, and DNA (Strempel et al., 2017), and hence with the ability to produce oxidative stress damage. Whilst some studies have assessed health effects related to oxidative stress in human cellular models (Hidalgo et al., 2002; Uğur Aydin et al., 2018), scarce information is available on the potential oxidative stress damage caused at low concentrations typically used for disinfection from human exposure studies. Considering that sodium hypochlorite is a potent oxidant, it can be hypothesised that exposure to sodium hypochlorite at the concentrations used in disinfection activities might result in oxidative stress damage in humans.
Human biomonitoring (HBM) is a reliable complementary approach to exposure assessment and has been widely used for many years to characterize environmental and occupational exposure to different contaminants in the general population (Hoseini et al., 2018; Rafiee et al., 2020) and various occupational settings (Hoseini et al., 2018; Longo et al., 2021; Rafiee et al., 2018; Rafiee et al., 2019; Rafiee et al., 2020). HBM has also been successfully used to assess health effects associated with oxidative stress damage (Rafiee et al., 2021; Rafiee et al., 2022). With this in mind, this study was aimed to evaluate whether exposure to sodium hypochlorite at the concentrations used in disinfection activities produces oxidative stress damage in humans. We employed a biomonitoring approach to assess the health effects of disinfectant exposure among individuals involved in the disinfection against SARS-CoV-2 by measuring oxidative stress markers, including malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG).
2. Materials and methods
2.1. Study area and selection of the participants
We collected urine samples from two groups of subjects according to their known occupational exposure to disinfectants. The exposed group were individuals involved on a daily basis in the disinfection process of various big grocery stores across Tehran, Iran's capital, with a population of over 10 million inhabitants (Rafiee et al., 2022). Disinfection procedures were routinely performed in the workplace in the morning and afternoon. The Ethical aspects of the present study were approved by the National Institute for Medical Research Development (NIMAD) of Iran, under ethic no. IR.NIMAD.REC.1399.085.
Since this is the first study to evaluate oxidative damage related to disinfectant exposure, no power calculation could be done to determine the number of samples. Instead, scientific judgement and professional expertise were used to decide an optimal group size of 75 men who were healthy and engaged in public places' disinfection to be recruited as the case group. Besides, the non-exposed group consisted of 60 healthy men who were not exposed to disinfectant exposure, either from occupational or environmental exposure.
Several inclusion criteria were set for both the case and control subjects. Recruitment of the case participants into the study was subjected to the following inclusion criteria:
-
1.
Individual must have engaged in the public places disinfection process as an operator;
-
2.
Participation in the study should be voluntary;
-
3.
Participation was restricted to non-smokers, to capture the effect of exposure to disinfectant only;
-
4.
Healthy individuals with no background disease;
-
5.
No occupational exposure to disinfectants; and
-
6.
Individuals not exposed to high levels of occupational stressors, such as high temperature and humidity, and higher levels of chemical pollutants in their working environment.
Subjects who could not satisfy the case inclusion criteria mentioned above were excluded from the study.
In addition, the inclusion criteria for subjects in the control group were:
-
1.
Individuals not occupationally exposed to disinfectants.
-
2.
Participation in the study should be voluntary;
-
3.
Not using sodium hypochlorite for disinfecting their living environment;
-
4.
Healthy individuals with no background disease;
Any subjects who could not satisfy the inclusion criteria for the control group mentioned above were excluded from the study. Subjects in the control group were asked to minimize their hand sanitizer usage and wash their hands with soap instead. In addition, all subjects filled out a questionnaire, including socio-demographic information and information about respiratory and cardiovascular impairments such as asthma and high blood pressure. All subjects in the case group used sodium hypochlorite as the disinfectant in the course of performing their operations.
2.2. Urine sampling
Spot urine samples from subjects in the case group were collected twice: the first urine sample was collected in the morning (7:30–7:45 a.m.) before the disinfection process, which is considered the before exposure (BE) sample hereafter. The sampling process started at 8 a.m. and lasted for 2 h. The second urine sample was collected 6 h after the disinfection process completion (4:15–4:45 p.m.), considered the after exposure (AE) sample. We requested managers to assign staff involved in the disinfection process to light work once the disinfection procedure was completed on the sampling day to minimize their exposure to other occupational stressors that could potentially affect oxidative stress levels in their bodies. Likewise, participants in the control group provided two spot urine samples in the same way as the case group. In total, 270 urine samples were collected, including 150 samples from the case group and 120 samples from the control subjects. Urine samples were collected into 60 mL polypropylene vials, labelled, and transferred to the laboratory using a portable fridge at 4 °C.
2.3. Identification of urinary MDA and 8-OHdG
The urinary MDA levels were quantified by the formation of thiobarbituric acid reactive substances in the urine based on the method described elsewhere (Chatziargyriou and Dailianis, 2010). Briefly, 500 μL of urine were exposed to phorbol-myristate acetate (PMA) (10 μg mL−1). In the next step, samples were centrifuged at 1200 rpm for 10 min at 4 °C and the supernatant was collected. After vortexing for 5 s, butylated hydroxytoluene (BHT) was added at a concentration of 0.02% to prevent further lipid peroxidation. Finally, samples were incubated at 90–100 °C for 15 min, cooled at room temperature, centrifuged at 10,000 rpm for 10 min, and finally measured spectrophotometrically at a wavelength of 535 nm.
ELISA assay kit (Zell Bio, GmbH., Germany) was used to determine the concentration of 8-OHdG in the urine based on the method described elsewhere (Ściskalska et al., 2014). In brief, 100 μL of conjugate 8-OHdG/bovine serum albumin (BSA) was added to each of the 96-well plates of the ELISA kit and incubated overnight at 4 °C and washed with water, followed by 200 μL blocking buffer and incubated for 1 h at room temperature. 50 μL of samples of 8-OHdG standards were added, and after 10 min of incubation, 100 μL of monoclonal anti-8-OHdG was added and incubated for 1 h at room temperature, then washed three times by the addition of secondary antibody conjugated to 100 μL of horseradish peroxidase, followed by 1-h incubation at room temperature. Next, 100 μL of substrate for peroxidase was added to the plate and incubated for 20 min. Then, 100 μL of reaction stop solution was added. Absorbance was spectrophotometrically measured at a wavelength of 450 nm. The amount of 8-OHdG was calculated by comparison with a standard curve determined from standards treated similarly to the samples. In addition, both MDA and 8-OHdG were corrected for creatinine, as determined by the Jaffé reaction method (Butler, 1975).
2.4. Statistical approach
In the present study, SPSS 21.0 package software (SPSS Inc., Chicago, IL) and GraphPad Prism software 8.0 were used to perform statistical analysis on oxidative stress markers in urine samples. The normality of the data distribution was checked using the Kolmogorov–Smirnov test. Since data was not normally distributed, the Mann–Whitney U test was employed to assess differences in urinary levels of MDA and 8-OHdG among the studied groups. In addition, multiple linear regression analysis was applied to evaluate the association between concentrations of oxidative stress biomarkers, including MDA and 8-OHdG and variables describing the use of disinfectants and personal protective equipment. We have also included age and body mass index (BMI) as covariate factors in the regression model to assess their effects on the urinary oxidative stress markers. In addition, the Spearman correlation test was used to assess the collinearity between variables prior to being introduced into the regression model. We used a pairwise correlation coefficient (r) of <0.5 as the indicator for introducing variables into the model.
3. Results and discussion
3.1. General characteristics of the participants
Table 1 represents the socio-demographic characteristics and health status of the participants. No significant differences were observed between cases and controls in terms of age and BMI (P > 0.05). There was a significant difference between studied groups regarding education (P < 0.05). Subjects in the case group had used significantly higher hand sanitizers than controls (P < 0.05). In addition, subjects in the case group used significantly higher volumes of disinfectants than controls (P < 0.05). According to information obtained by the questionnaire and personal interviews, almost half of the subjects in the case group reported skin and eye irritation after using disinfectants, a frequency significantly higher than reported by participants in the control group (P < 0.05).
Table 1.
Socio-demographic characteristics and health status of the participants.
| Variables | Case (n = 75) | Control (n = 60) |
|---|---|---|
| Age (years) | 39 ± 9 | 41 ± 10 |
| Height (cm) | 177 ± 5 | 175 ± 4 |
| Weight (kg) | 78 ± 11 | 75 ± 9 |
| BMI (kg/m2) | 27 ± 4 | 25 ± 5 |
| Education (%) | ||
| High school Diploma | 55 | 10 |
| Bachelor | 45 | 50 |
| Master | – | 40 |
| Traffic situation near the place of residence (%) | ||
| Low | 25 | 35 |
| Medium | 50 | 55 |
| High | 25 | 10 |
| Using hand sanitizers per day (%) | ||
| Once | 7 | 38 |
| 1–3 | 35 | 55 |
| 3–6 | 27 | 7 |
| More than 6 | 31 | – |
| Volume of disinfectants usage per month (%) | ||
| Less than 50 cm3 | – | 55 |
| 51–100 cm3 | 29 | 35 |
| 101–200 cm3 | 38 | 10 |
| More than 200 cm3 | 33 | – |
| Using disinfectants to disinfect surfaces per day (%) | ||
| Once | 5 | 70 |
| 1–3 | 33 | 30 |
| 3–6 | 35 | – |
| More than 6 | 27 | – |
| Skin irritation after using disinfectants (%) | ||
| Yes | 45 | 36 |
| No | 55 | 64 |
| Eyes irritation after using disinfectants (%) | ||
| Yes | 48 | 25 |
| No | 52 | 75 |
3.2. Urinary profile of MDA and 8-OHdG
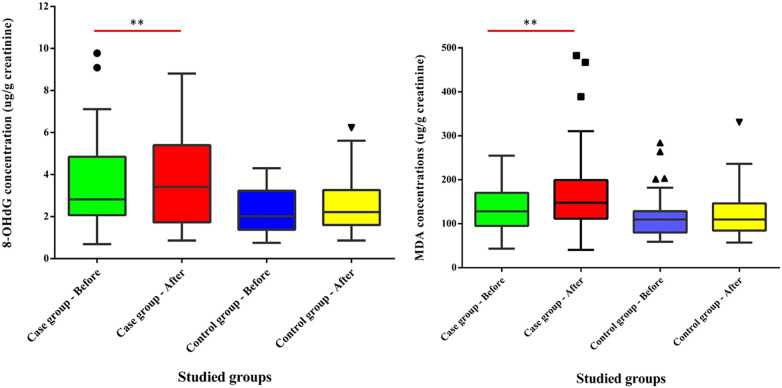
Results of oxidative stress urinary biomarkers are presented in Fig. 1 and Table S1. Concentrations of 8-OHdG in the present study (arithmetic means ranging between 2.27 and 3.84 μg/g creatinine) are similar to those reported (3.28 and 3.65 μg/g creatinine) for garage and waste collection workers exposed to traffic emissions in Finland (Harri et al., 2005) and China (Zheng et al., 2018), as well as for workers exposed to TiO2, SiO2 and indium tin oxide nanomaterials in Taiwan (Liou et al., 2017). Similar concentrations (2.86–4.10 μg/g creatinine) were measured in participants of the control group in occupational exposure studies in China (Ke et al., 2016; Zheng et al., 2018), Taiwan (Liu et al., 2008; Pan et al., 2018) and Finland (Harri et al., 2005). Very few occupational exposure studies reported lower concentrations than those measured in the present study, such as those reported for farmers exposed to pesticides (Lee et al., 2017) in South Korea and concentrations in the control group in Taiwan (Liou et al., 2017; Liu et al., 2009), which range from 0.9 to 1.84 μg/g creatinine. On the contrary, most occupational exposure studies report 8-OHdG concentrations higher than those measured in the present study, between 15 and 30 μg/g creatinine (Chang et al., 2011; Huang et al., 2012; Wang et al., 2011). Workers occupationally exposed to heavy metals present larger concentrations (5.0–7.8 μg/g creatinine) than those measured in this study (Liu et al., 2009; Pan et al., 2018; Samir and Rashed, 2018), as do workers exposed to combustion emissions (7.56–14.47 μg/g creatinine) (Ke et al., 2016; Miglani et al., 2019; Zhao et al., 2018).
Fig. 1.
Urinary MDA and 8-OHdG levels in studied groups.
The concentrations of MDA in workers involved in disinfectant jobs to eliminate SARS-CoV-2 from surfaces in Iran (169 ± 89 μg/g creatinine) are similar to concentrations measured in cooks in Taiwan exposed to cooking fumes (Ke et al., 2016; Pan et al., 2008), electroplating workers exposed to hexavalent chromium (Huang et al., 1999; Pan et al., 2018) and miners exposed to elemental mercury (Kobal et al., 2003), with concentrations of MDA ranging between 152 and 199 μg/g creatinine. Similarly, the MDA concentrations measured in the control groups are within the same range of concentrations (102–135 μg/g creatinine) as those measured in the control group in the present study (129 ± 52 μg/g creatinine). On the other hand, lower MDA concentrations were reported for wildland firefighters exposed to woodsmoke (68.4 ± 21.6 μg/g creatinine) (Adetona et al., 2013), rural populations in the north of China exposed to e-waste PAH emissions (ranging from 44.2 to 132 μg/g creatinine) (Yang et al., 2015) or farmers exposed to pesticide (9.58 ± 5.04 μg/g creatinine) (Lee et al., 2017).
3.3. Effects of exposure to sodium hypochlorite-based disinfectants on oxidative stress
Significant differences were observed in urinary 8-OHdG levels between the studied groups, where the median 8-OHdG levels in case group AE samples were 1.55-time higher than control group AE samples (P < 0.05). Likewise, the urinary median 8-OHdG level in case group AE samples was 1.21-fold higher than in BE samples (P < 0.05).
In terms of MDA, significant differences were observed between studied groups, where the median urinary MDA levels of case group AE samples were 1.15-fold higher than BE samples (P < 0.05). Likewise, the median MDA levels in case group AE samples were 1.35-time higher than the corresponding values in control group AE samples (P < 0.05).
The range of 8-OHdG and MDA concentrations in the case participants is larger than in the control participants. This might be associated with the fact that case participants are exposed to sodium hypochlorite-based disinfectants everyday in their working routines. On the other hand, despite the BE range of 8-OHdG and MDA concentrations being larger for the case than for the control participants, no significant differences were observed in median urinary 8-OHdG concentrations, nor for MDA levels between case group BE samples and control group BE samples in the non-parametric tests (P > 0.05). In addition, there were no significant differences for median urinary 8-OHdG or MDA levels between case group BE samples and control group AE samples (P > 0.05).
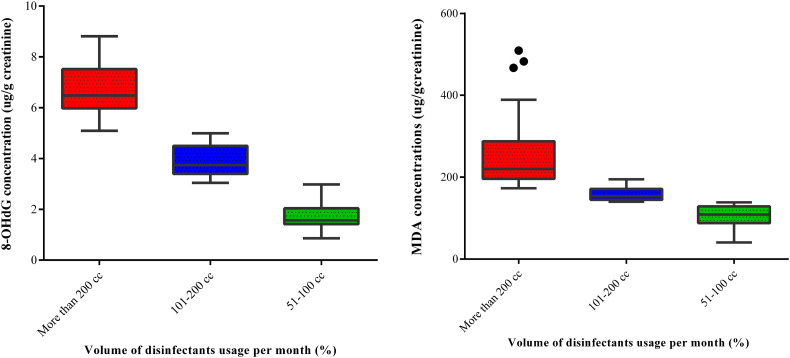
The concentrations of MDA and 8-OHdG of operators in the case group exposed to different levels of disinfectants during their work were also investigated to provide more insight into how exposure to various volumes of disinfectants affected oxidative stress status in the body after exposure. Subjects in the case group were sub-grouped by the volume of disinfectant used in one month as follows: (1) operators who used 51–100 cm3 of sodium hypochlorite; (2) operators who used 101–200 cm3 of sodium hypochlorite; and (3) operators who used over 200 cm3 of sodium hypochlorite for the disinfection of the studied public places. The results (Fig. 2 ) showed significant differences between the studied groups in a dose-response, i.e. increasing use of sodium hypochlorite (hence exposure exposure), produces an increasing concentration of urinary oxidative stress markers. The findings indicate significant differences between studied sub-groups in terms of urinary oxidative stress biomarkers (MDA and 8-OHdG) (P < 0.05). Both MDA and 8-OHdG levels measured in the urine of the case subjects who used over 200 cm3 of sodium hypochlorite for disinfection purposes were significantly higher than the corresponding values in the urine of operators who used 101–200 cm3 and those who used 51–100 cm3 (P < 0.05). In addition, MDA and 8-OHdG levels in the urine of the operators who used 101–200 cm3 of sodium hypochlorite for disinfection were significantly higher than the concentrations in the urine of subjects who used 51–100 cm3 of the disinfectant. It is worthwhile to note that disinfection operators who used over 200 cm3 of sodium hypochlorite per month were responsible for the disinfection of larger areas and thus exposed to higher levels of disinfectant for a longer time.
Fig. 2.
The effect of the volume of disinfectant usage on MDA and 8-OHdG concentrations.
The comparative analysis results between the DNA and lipid oxidative damage markers suggest that the use of sodium hypochlorite as a disinfectant produces oxidative damage in the workers. This is consistent with information extracted from the Hazardous Substances Data Bank (HSDB), which reports that sodium hypochlorite is a strong oxidizing agent (HSDB, 2021; Lewis, 1993). Hawkins and Davies (1998) reported that hypochlorite damages proteins by reaction with amino acid side-chains or backbone cleavage producing high- and low-molecular-mass nitrogen-centred protein-derived radicals. In their experiment, these radicals reacted with ascorbate, glutathione, and synthetic vitamin E (Hawkins and Davies, 1998). The results of an in vitro study on human peripheral blood cells have also shown that exposure to sodium hypochlorite increases the chromosomal aberration rate in human lymphocyte cells (Gül et al., 2009). These findings suggest that hypochlorite exposure may produce hypochlorite-derived radicals, leading to oxidative stress, which could overwhelm the antioxidant capacity, leading to oxidative DNA and lipid damage. In addition, exposure to disinfection by-products in chlorinated water has also been associated with oxidative stress, with increased concentrations of urinary biomarkers of oxidative stress [8-hydroxy-2-deoxyguanosine and 8-isoprostaglandin F2α] (Zhang et al., 2021a). It is worthwhile to note that hypochlorite can also directly react with numerous biomolecules, including proteins, lipids, and DNA (Gray et al., 2013; Klebanoff, 2005; Rosen et al., 1990; Strempel et al., 2017), which could also explain the increase in DNA and lipid oxidative damage markers measured in the exposed workers.
3.4. Potential routes of exposure
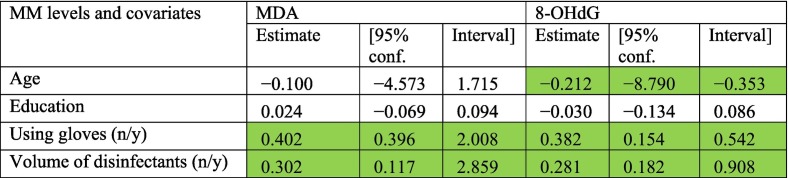
Results of the multivariate regression analysis are provided in Table 2 . Using gloves and the volume of disinfectants used were highlighted as the predictor factors affecting the urinary concentrations of MDA and 8-OHdG in the studied groups. The fact that the volume of disinfectant used is a predictor of the concentration of MDA and 8-OHdG urinary concentrations strengthens the suggestion that exposure to sodium hypochlorite is associated with DNA and lipid oxidative damage.
Table 2.
Association between oxidative stress biomarkers and exposure to disinfectants and other potential confounders.
Green cells represent regression coefficients with P-value < 0.05.
The critical evaluation of information extracted from Tables 1 and 2 provides insights into the possible routes of exposure to sodium hypochlorite.
3.4.1. Dermal contact
Exposed subjects report a higher frequency of skin irritation (Table 1). In addition, according to Table 2, those who did not use gloves reported concentrations of MDA 0.402 μmol/mol creatinine (95% CI: 0.396, 2.008 μmol/mol creatinine) higher and concentrations of 8-OHdG 0.382 ng/mmol creatinine (95% CI: 0.154, 0.542 ng/mmol creatinine) higher than those who do use gloves. These results are highly suggestive that dermal contact might be an important route of exposure to sodium hypochlorite, leading to increased levels of DNA and lipid oxidative damage in the workers in charge of disinfecting surfaces with sodium hypochlorite as a preventative measure against COVID-19 transmission.
3.4.2. Inhalation
Likewise, Table 1 shows that workers using sodium hypochlorite to disinfect surfaces reported eye irritation more frequently (P < 0.05). This suggests that vapours associated with the use of sodium hypochlorite might cause eye irritation. However, under normal conditions, chlorine gas is not released by bleach solutions and hence inhalation of sodium hypochlorite vapours is very rare (PHE, 2015). On the other hand, mixing bleach with acids, like acidic cleaning agents, releases highly irritant gases (PHE, 2015; Racioppi et al., 1994). The large frequency of workers reporting eye irritation suggests that mixing sodium hypochlorite with other acidic cleaning agents might have been a common practice undertaken during their job chores in the population under study. In addition, exposure to disinfectant through inhalation could cause pulmonary impairments in the exposed subjects. For example, previous studies reported a significant association between occupational exposure to disinfectants and an increased risk of chronic obstructive pulmonary disease (COPD) among nurses who had worked in different hospitals across the United States (Dumas et al., 2019). Although the mechanisms by which exposure to disinfectants affects human health are not fully understood, some mechanistic pathways have been highlighted, particularly relating to pulmonary effects. For example, it has been revealed that chronic exposure to corrosive chemicals, commonly found in disinfectants, through inhalation could damage the respiratory mucosa, resulting in inflammation responses and consequently pulmonary impairments such as asthma (Clausen et al., 2020; Siracusa et al., 2013)
3.5. Strengths and limitations
The main limitation of the study is that no information is available on other sources of exposure that could have led to increased levels of DNA and lipid oxidative damage in the population under study. Oxidative stress damage could have also been associated with exposure to traffic emissions (Graille et al., 2020; Lai et al., 2005; Rafiee et al., 2022), cooking fumes (Kamal et al., 2016; Ke et al., 2009; Ke et al., 2016), per- and polyfluoroalkyl substances (Bonato et al., 2020; Lin et al., 2020), heavy metals (Bortey-Sam et al., 2018; Rafiee et al., 2021; Yang et al., 2020), VOCs (Chang et al., 2011; Fenga et al., 2017) and pesticides (Lee et al., 2017; Tope and Panemangalore, 2007).
On the other hand, this study has adequately controlled for the possible oxidation damage to DNA and lipid oxidation related to smoking by recruiting non-smokers only. Equally, subjects recruited have similar anthropometric measures (age, height, weight, BMI), and were restricted to one sex (males), controlling for factors which also may affect oxidative biomarker concentrations (Loft et al., 1992; Maehira et al., 2004; Mizoue et al., 2007; Oba et al., 2019; Varghese et al., 2020).
4. Conclusions
To the best of our knowledge, this is the first study to examine the association between the use of sodium hypochlorite as a disinfectant against SARS-CoV-2 and the health effects associated with oxidative damage, using biomarkers of DNA and lipid peroxidation.
A large proportion of workers involved in disinfection activities with sodium hypochlorite frequently reported eye irritation. The results also show that exposure to sodium hypochlorite at the concentrations used in disinfection activities produces oxidative stress damage in humans, both DNA and lipid peroxidation, in a dose-response manner.
A simple protective measure, such as the use of gloves, was identified as an effective way of reducing exposure to sodium hypochlorite and its associated oxidative stress effects on DNA and lipid oxidation damage. Since sodium hypochlorite is extensively used to clean surfaces as a preventative measure against COVID-19 transmission and other disinfection tasks, it is recommended that workers handling sodium hypochlorite use gloves, suitable respirators, safety goggles to reduce health effects associated with its handling. Employers should also review shift-work schedules to reduce exposure to disinfectants.
The following is the supplementary data related to this article.
Urinary levels (μmol/mol creatinine) of DNA oxidative damage markers in the studied groups.
CRediT authorship contribution statement
Ata Rafiee: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Juana Maria Delgado-Saborit: Writing – original draft, Writing – review & editing. Peter D. Sly: Writing – review & editing. Hoda Amiri: Data curation. Shamim Mosalaei: Investigation. Mohammad Hoseini: Funding acquisition, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The present study has received financial support from the National Institute for Medical Research Development (NIMAD) of Iran, under grant no. 993682. JMDS is funded by the Generalitat Valenciana - Regional Ministry of Education, Research, Culture and Sport under the Talented Researcher Support Programme - Plan GenT (CIDEGENT/2019/064). Authors also wanted to thank all the subjects who voluntary participated in this study.
Editor: Daqiang Yin
References
- Adetona O., Zhang J., Hall D.B., Wang J.-S., Vena J.E., Naeher L.P. Occupational exposure to woodsmoke and oxidative stress in wildland firefighters. Sci. Total Environ. 2013;449:269–275. doi: 10.1016/j.scitotenv.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Bonato M., Corrà F., Bellio M., Guidolin L., Tallandini L., Irato P., et al. PFAS environmental pollution and antioxidant responses: an overview of the impact on human field. Int. J. Environ. Res. Public Health. 2020;17:8020. doi: 10.3390/ijerph17218020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortey-Sam N., Ikenaka Y., Akoto O., Nakayama S.M.M., Asante K.A., Baidoo E., et al. Association between human exposure to heavy metals/metalloid and occurrences of respiratory diseases, lipid peroxidation and DNA damage in Kumasi, Ghana. Environ. Pollut. 2018;235:163–170. doi: 10.1016/j.envpol.2017.12.005. [DOI] [PubMed] [Google Scholar]
- Butler A.R. The jaffe reaction. Identification of the coloured species. Clin. Chim. Acta. 1975;59:227–232. doi: 10.1016/0009-8981(75)90033-9. [DOI] [PubMed] [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To KK-W. Chu H., Yang J., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F.K., Mao I.F., Chen M.L., Cheng S.F. Urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to ethylbenzene. Ann. Occup. Hyg. 2011;55:519–525. doi: 10.1093/annhyg/mer010. [DOI] [PubMed] [Google Scholar]
- Chatziargyriou V., Dailianis S. The role of selenium-dependent glutathione peroxidase (Se-GPx) against oxidative and genotoxic effects of mercury in haemocytes of mussel Mytilus galloprovincialis (Lmk.) Toxicol. in Vitro. 2010;24:1363–1372. doi: 10.1016/j.tiv.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Clausen P.A., Frederiksen M., Sejbæk C.S., Sørli J.B., Hougaard K.S., Frydendall K.B., et al. Chemicals inhaled from spray cleaning and disinfection products and their respiratory effects. A comprehensive review. Int. J. Hyg. Environ. Health. 2020;229 doi: 10.1016/j.ijheh.2020.113592. [DOI] [PubMed] [Google Scholar]
- da Cruz Nizer W.S., Inkovskiy V., Overhage J. Surviving reactive chlorine stress: responses of gram-negative bacteria to hypochlorous acid. Microorganisms. 2020;8:1220. doi: 10.3390/microorganisms8081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas O., Varraso R., Boggs K.M., Quinot C., Zock J.-P., Henneberger P.K., et al. Association of occupational exposure to disinfectants with incidence of chronic obstructive pulmonary disease among US female nurses. JAMA Netw. Open. 2019;2:e1913563. doi: 10.1001/jamanetworkopen.2019.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenga C., Gangemi S., Teodoro M., Rapisarda V., Golokhvast K., Anisimov N.Y., et al. 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to low-dose benzene. Toxicol. Rep. 2017;4:291–295. doi: 10.1016/j.toxrep.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graille M., Wild P., Sauvain J.-J., Hemmendinger M., Guseva Canu I., Hopf N.B. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21113743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.J., Wholey W.-Y., Jakob U. Bacterial responses to reactive chlorine species. Annu. Rev. Microbiol. 2013;67:141–160. doi: 10.1146/annurev-micro-102912-142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gül S., Savsar A., Tayfa Z. Cytotoxic and genotoxic effects of sodium hypochlorite on human peripheral lymphocytes in vitro. Cytotechnology. 2009;59:113–119. doi: 10.1007/s10616-009-9201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harri M., Svoboda P., Mori T., Mutanen P., Kasai H., Savela K. Analysis of 8-hydroxydeoxyguanosine among workers exposed to diesel particulate exhaust: comparison with urinary metabolites and PAH air monitoring. Free Radic. Res. 2005;39:963–972. doi: 10.1080/10715760500190115. [DOI] [PubMed] [Google Scholar]
- Hawkins C.L., Davies M.J. Hypochlorite-induced damage to proteins: formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem. J. 1998;332(Pt 3):617–625. doi: 10.1042/bj3320617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Bartolome R., Dominguez C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem. Biol. Interact. 2002;139:265–282. doi: 10.1016/s0009-2797(02)00003-0. [DOI] [PubMed] [Google Scholar]
- Hoseini M., Nabizadeh R., Delgado-Saborit J.M., Rafiee A., Yaghmaeian K., Parmy S., et al. Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a middle eastern area. Environ. Pollut. 2018;240:781–792. doi: 10.1016/j.envpol.2018.04.077. [DOI] [PubMed] [Google Scholar]
- HSDB . 2021. Hazardous Substances Data Bank (HSDB). Sodium hypochlorite. [Google Scholar]
- Huang Y.L., Chen C.Y., Sheu J.Y., Chuang I.C., Pan J.H., Lin T.H. Lipid peroxidation in workers exposed to hexavalent chromium. J. Toxicol. Environ. Health, Part A. 1999;56:235–247. doi: 10.1080/009841099158088. [DOI] [PubMed] [Google Scholar]
- Huang Y.W., Jian L., Zhang M.B., Zhou Q., Yan X.F., Hua X.D., et al. An investigation of oxidative DNA damage in pharmacy technicians exposed to antineoplastic drugs in two Chinese hospitals using the urinary 8-OHdG assay. Biomed. Environ. Sci. 2012;25:109–116. doi: 10.3967/0895-3988.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Kamal A., Cincinelli A., Martellini T., Malik R.N. Biomarkers of PAH exposure and hematologic effects in subjects exposed to combustion emission during residential (and professional) cooking practices in Pakistan. Environ. Sci. Pollut. Res. 2016;23:1284–1299. doi: 10.1007/s11356-015-5297-6. [DOI] [PubMed] [Google Scholar]
- Kampf G. Infection Prevention in Practice. Vol. 2. 2020. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y.B., Cheng J.Q., Zhang Z.C., Zhang R.L., Zhang Z.Z., Shuai Z.H., et al. Increased levels of oxidative DNA damage attributable to cooking-oil fumes exposure among cooks. Inhal. Toxicol. 2009;21:682–687. doi: 10.1080/08958370802474728. [DOI] [PubMed] [Google Scholar]
- Ke Y.B., Huang L.H., Xia J.J., Xu X.Y., Liu H.H., Li Y.R. Comparative study of oxidative stress biomarkers in urine of cooks exposed to three types of cooking-related particles. Toxicol. Lett. 2016;255:36–42. doi: 10.1016/j.toxlet.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Kobal A.B., Horvat M., Prezelj M., Briski A.S., Krsnik M., Dizdarevic T., et al. The impact of long-term past exposure to elemental mercury on antioxidative capacity and lipid peroxidation in mercury miners. J. Trace Elem. Med. Biol. 2003;17:261–274. doi: 10.1016/S0946-672X(04)80028-2. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Liou S.H., Lin H.C., Shih T.S., Tsai P.J., Chen J.S., et al. Exposure to traffic exhausts and oxidative DNA damage. Occup. Environ. Med. 2005;62:216–222. doi: 10.1136/oem.2004.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Park S.-Y., Lee K., Oh S.-S., Ko S.B. Pesticide metabolite and oxidative stress in male farmers exposed to pesticide. Ann. Occup. Environ. Med. 2017;29:5. doi: 10.1186/s40557-017-0162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R.J., Sr. 12th edition. Van Nostrand Rheinhold Co; New York, NY: 1993. Hawley’s Condensed Chemical Dictionary. [Google Scholar]
- Li S.-W., Chang M.-H., Zhao W.-J., Li H.-L., Sun H.-J., Hong H.-C., et al. Oxidative stress and lipid peroxidation with exposure of emerging disinfection byproduct 2,6-dichlorobenzoquinone in mice. Res. Square. 2021 doi: 10.21203/rs.3.rs-672041/v1. [DOI] [Google Scholar]
- Lin C.Y., Lee H.L., Hwang Y.T., Su T.C. The association between total serum isomers of per- and polyfluoroalkyl substances, lipid profiles, and the DNA oxidative/nitrative stress biomarkers in middle-aged Taiwanese adults. Environ. Res. 2020;182 doi: 10.1016/j.envres.2019.109064. [DOI] [PubMed] [Google Scholar]
- Liou S.H., Wu W.T., Liao H.Y., Chen C.Y., Tsai C.Y., Jung W.T., et al. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017;331:329–335. doi: 10.1016/j.jhazmat.2017.02.042. [DOI] [PubMed] [Google Scholar]
- Liu H.-H., Lin M.-H., Liu P.-C., Chan C.-I., Chen H.-L. Health risk assessment by measuring plasma malondialdehyde (MDA), urinary 8-hydroxydeoxyguanosine (8-OH-dG) and DNA strand breakage following metal exposure in foundry workers. J. Hazard. Mater. 2009;170:699–704. doi: 10.1016/j.jhazmat.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Liu H.H., Shih T.S., Chen I.J., Chen H.L. Lipid peroxidation and oxidative status compared in workers at a bottom ash recovery plant and fly ash treatment plants. J. Occup. Health. 2008;50:492–497. doi: 10.1539/joh.l8062. [DOI] [PubMed] [Google Scholar]
- Loft S., Vistisen K., Ewertz M., Tjønneland A., Overvad K., Poulsen H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- Longo V., Forleo A., Giampetruzzi L., Siciliano P., Capone S. Human biomonitoring of environmental and occupational exposures by GC-MS and gas sensor systems: a systematic review. Int. J. Environ. Res. Public Health. 2021;18:10236. doi: 10.3390/ijerph181910236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehira F., Nakama Y., Ohmine N., Miyagi I. Age-related changes in the oxidation–reduction characteristics and the 8-OHdG accumulation in liver, lung, brain of SAMP1 and SAM R1. Int. Congr. Ser. 2004;1260:259–262. [Google Scholar]
- Miglani K., Kumar S., Yadav A., Aggarwal N., Ahmad I., Gupta R. A multibiomarker approach to evaluate the effect of polyaromatic hydrocarbon exposure on oxidative and genotoxic damage in tandoor workers. Toxicol. Ind. Health. 2019;35:486–496. doi: 10.1177/0748233719862728. [DOI] [PubMed] [Google Scholar]
- Mizoue T., Tokunaga S., Kasai H., Kawai K., Sato M., Kubo T. Body mass index and oxidative DNA damage: a longitudinal study. Cancer Sci. 2007;98:1254–1258. doi: 10.1111/j.1349-7006.2007.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba S., Inaba Y., Shibuya T., Oshima J., Seyama K., Kobayashi T., et al. Changes in oxidative stress levels during two weeks of smoking cessation treatment and their association with nutritional characteristics in Japanese smokers. Exp. Ther. Med. 2019;17:2757–2764. doi: 10.3892/etm.2019.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.H., Chan C.C., Huang Y.L., Wu K.Y. Urinary 1-hydroxypyrene and malondialdehyde in male workers in Chinese restaurants. Occup. Environ. Med. 2008;65:732–735. doi: 10.1136/oem.2007.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.H., Jeng H.A., Lai C.H. Biomarkers of oxidative stress in electroplating workers exposed to hexavalent chromium. J. Expo. Sci. Environ. Epidemiol. 2018;28:76–83. doi: 10.1038/jes.2016.85. [DOI] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12:1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHE . In: Compendium of Chemical Hazards: Sodium Hypochlorite. Toxicological Overview. England P.H., editor. PHE publications; 2015. gateway number: 2014790. [Google Scholar]
- Pradhan D., Biswasroy P., Ghosh G., Rath G. A review of current interventions for COVID-19 prevention. Arch. Med. Res. 2020;51(5):363–374. doi: 10.1016/j.arcmed.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi F., Daskaleros P.A., Besbelli N., Borges A., Deraemaeker C., Magalini S.I., et al. Household bleaches based on sodium hypochlorite: review of acute toxicology and poison control center experience. Food Chem. Toxicol. 1994;32:845–861. doi: 10.1016/0278-6915(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Rafiee A., Delgado-Saborit J.M., Aquilina N.J., Amiri H., Hoseini M. Assessing oxidative stress resulting from environmental exposure to metals (Oids) in a middle eastern population. Environ. Geochem. Health. 2021:1–20. doi: 10.1007/s10653-021-01065-z. [DOI] [PubMed] [Google Scholar]
- Rafiee A., Delgado-Saborit J.M., Gordi E., Quémerais B., Moghadam V.K., Lu W., et al. Use of urinary biomarkers to characterize occupational exposure to BTEX in healthcare waste autoclave operators. Sci. Total Environ. 2018;631:857–865. doi: 10.1016/j.scitotenv.2018.03.090. [DOI] [PubMed] [Google Scholar]
- Rafiee A., Delgado-Saborit J.M., Sly P.D., Amiri H., Hoseini M. Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci. Total Environ. 2019;656:540–546. doi: 10.1016/j.scitotenv.2018.11.398. [DOI] [PubMed] [Google Scholar]
- Rafiee A., Delgado-Saborit J.M., Sly P.D., Amiri H., Hoseini M. Exploring urinary biomarkers to assess oxidative DNA damage resulting from BTEX exposure in street children. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111725. [DOI] [PubMed] [Google Scholar]
- Rafiee A., Delgado-Saborit J.M., Sly P.D., Quémerais B., Hashemi F., Akbari S., et al. Environmental chronic exposure to metals and effects on attention and executive function in the general population. Sci. Total Environ. 2020;705 doi: 10.1016/j.scitotenv.2019.135911. [DOI] [PubMed] [Google Scholar]
- Rosen H., Orman J., Rakita R.M., Michel B.R., VanDevanter D.R. Loss of DNA-membrane interactions and cessation of DNA synthesis in myeloperoxidase-treated Escherichia coli. Proc. Natl. Acad. Sci. 1990;87:10048–10052. doi: 10.1073/pnas.87.24.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samir A.M., Rashed L.A. Effects of occupational exposure to aluminium on some oxidative stress and DNA damage parameters. Hum. Exp. Toxicol. 2018;37:901–908. doi: 10.1177/0960327117747024. [DOI] [PubMed] [Google Scholar]
- Ściskalska M., Zalewska M., Grzelak A., Milnerowicz H. The influence of the occupational exposure to heavy metals and tobacco smoke on the selected oxidative stress markers in smelters. Biol. Trace Elem. Res. 2014;159:59–68. doi: 10.1007/s12011-014-9984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa A., De Blay F., Folletti I., Moscato G., Olivieri M., Quirce S., et al. Asthma and exposure to cleaning products–a European Academy of allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68:1532–1545. doi: 10.1111/all.12279. [DOI] [PubMed] [Google Scholar]
- Strempel N., Nusser M., Neidig A., Brenner-Weiss G., Overhage J. The oxidative stress agent hypochlorite stimulates c-di-GMP synthesis and biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Mao Y., Jones R.M., Tan Q., Ji J.S., Li N., et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020;144 doi: 10.1016/j.envint.2020.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tope A.M., Panemangalore M. Assessment of oxidative stress due to exposure to pesticides in plasma and urine of traditional limited-resource farm workers: formation of the DNA-adduct 8-hydroxy-2-deoxy-guanosine (8-OHdG) J. Environ. Sci. Health B. 2007;42:151–155. doi: 10.1080/03601230601123276. [DOI] [PubMed] [Google Scholar]
- Uğur Aydin Z., Akpinar K.E., Hepokur C., Erdönmez D. Assessment of toxicity and oxidative DNA damage of sodium hypochlorite, chitosan and propolis on fibroblast cells. Braz. Oral Res. 2018;32 doi: 10.1590/1807-3107bor-2018.vol32.0119. [DOI] [PubMed] [Google Scholar]
- Varghese J., Bhat V., Chianeh Y.R., Kamath V., Al-Haj Husain N., Özcan M. Salivary 8-hydroxyguanosine levels in smokers and non-smokers with chronic periodontitis. Odontology. 2020;108:569–577. doi: 10.1007/s10266-020-00496-x. [DOI] [PubMed] [Google Scholar]
- Vizcaya D., Mirabelli M.C., Gimeno D., Antó J.-M., Delclos G.L., Rivera M., et al. Cleaning products and short-term respiratory effects among female cleaners with asthma. Occup. Environ. Med. 2015;72:757–763. doi: 10.1136/oemed-2013-102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang L., Chen X., Rao K.M., Lu S.Y., Ma S.T., et al. Increased urinary 8-hydroxy-2 '-deoxyguanosine levels in workers exposed to di-(2-ethylhexyl) phthalate in a waste plastic recycling site in China. Environ. Sci. Pollut. Res. 2011;18:987–996. doi: 10.1007/s11356-010-0420-1. [DOI] [PubMed] [Google Scholar]
- WHO . Vol. 82. 2020. Coronavirus disease 2019 (COVID-19): situation report. [Google Scholar]
- Yang D.F., Liu Y.L., Liu S., Li C., Zhao Y., Li L., et al. Exposure to heavy metals and its association with DNA oxidative damage in municipal waste incinerator workers in Shenzhen, China. Chemosphere. 2020;250 doi: 10.1016/j.chemosphere.2020.126289. [DOI] [PubMed] [Google Scholar]
- Yang Q., Qiu X., Li R., Ma J., Li K., Li G. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China plain. Environ. Sci. Pollut. Res. Int. 2015;22:1760–1769. doi: 10.1007/s11356-014-3284-y. [DOI] [PubMed] [Google Scholar]
- Zhang M., Liu C., Cui F.-P., Chen P.-P., Deng Y.-L., Luo Q., et al. The role of oxidative stress in association between disinfection by-products exposure and semen quality: a mediation analysis among men from an infertility clinic. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.128856. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang Y., Lu J., Yu Z., Song H., Bond P.L., et al. Chlorine disinfection facilitates natural transformation through ROS-mediated oxidative stress. ISME J. 2021;15:2969–2985. doi: 10.1038/s41396-021-00980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.J., Shou Y.P., Mao T.Y., Guo L.Q., Li P.H., Yi X.L., et al. PAHs exposure assessment for highway toll station workers through personal particulate sampling and urinary biomonitoring in Tianjin, China. Polycycl. Aromat. Compd. 2018;38:379–388. [Google Scholar]
- Zheng J.H., Zheng W.W., Zhou Y., Jiang S.H., Spencer P., Ye W.M., et al. Heavy exposure of waste collectors to polycyclic aromatic hydrocarbons in a poor rural area of middle China. Environ. Sci. Technol. 2018;52:8866–8875. doi: 10.1021/acs.est.8b02024. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Lian X., Su X., Wu W., Marraro G.A., Zeng Y. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir. Res. 2020;21:1–14. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Urinary levels (μmol/mol creatinine) of DNA oxidative damage markers in the studied groups.