Introduction
Chronic pain is often undertreated or neglected in youth leading to long-term consequences across the lifespan[16]. While the causes of chronic pain are multifactorial, it is increasingly recognized that some chronic pain arises from pathologic mechanisms related to how the central nervous system (CNS) interprets and modulates sensory information, also known as nociplastic pain[23; 32]. It is possible that this pathophysiology is initiated early in life, as many adult chronic pain patients report first experiencing pain in childhood[24]. This raises a critical question for pain management: are we missing a critical window in childhood where the lifelong course of chronic pain could be altered or even arrested?
One out of four children are affected by chronic pain[44] and a large proportion of these children will experience chronic pain in adulthood[6; 24]. In children, headache, abdominal pain and musculoskeletal pain are among the most commonly reported conditions[44]. When a child experiences pain in multiple body sites, for example headache and musculoskeletal pain, without peripheral signs of injury, it is likely that CNS sensitization is involved[23]. In adults, functional magnetic resonance imaging (fMRI) has demonstrated that nociplastic pain conditions are often characterized by amplification of ascending pain signals, a failure of descending pain inhibition, and enhanced integration of sensory, default mode and affective brain networks[2; 29; 31; 41]. The few studies examining CNS alterations in children with nociplastic pain also indicate increased nociceptive activity and decreased descending inhibition[40; 55]. However, there are essentially no data addressing whether such changes precede, correlate with, or are consequences of chronic pain. Further, while some studies have addressed CNS predictors of pain persistence, in these cases all of the participants had acute pain at baseline[3; 27]. Examining the neurobiological underpinnings of the natural history of pain in children has the potential to identify critical vulnerabilities and targets for prevention and early treatment[15].
The Adolescent Brain and Cognitive Development (ABCD) study is the largest prospective longitudinal study of brain development and child health in the U.S.[4], and thus provides an unprecedented opportunity to examine whether differences in neural activity frequently reported in adults with chronic pain also exist in children before the onset of pain.
Here we examined a subset of children from the first two assessments of the ABCD study who were pain free at baseline and then exhibit new multisite pain one year later. We chose to use new multisite pain as the outcome of interest because multisite pain is more likely to reflect a vulnerability mediated by aberrations in CNS processing of pain rather than a transient peripheral injury or pain restricted to one body area[23]. We hypothesized that children who develop multisite pain would have increased activity and functional connectivity within brain regions associated with pain processing, specifically the insula, primary somatosensory cortex and anterior cingulate, at baseline relative to matched controls. We also hypothesized that children who develop pain would have decreased functional connectivity in the periaqueductal gray, a key antinociceptive brain region. In exploratory analyses, we examined if structural changes were also present before the onset of multisite pain.
Methods
Participants:
4,951 children (aged 9–11 years old) with baseline and 1-year follow-up data were available for this analysis. The Institutional Review Board of each of the 21 participating centers of the ABCD study from which children were recruited approved the procedures, and all participants provided written informed consent (parent) or informed assent (child) in accordance with the Declaration of Helsinki.
Pain Assessment:
Details of the physical health assessment battery are described elsewhere [4]. The primary outcome of interest was the development of new multisite pain one year after reporting a pain-free baseline assessment. The presence of pain was captured in the Child Behavior Checklist (CBCL) [1], which was completed by the parent(s) about their child at baseline and the 1-year assessment. Parents were asked if the child currently or within the past 6 months suffered from the following physical problems without known medical cause: 1) aches or pains (not stomach or headaches), 2) headaches, and 3) stomachaches. Responses were categorized as 0 = “not true”, 1 = “somewhat or sometimes true”, and 2 = “very true or often true”. We operationalized new multisite pain as parental endorsement of at least two of the three pain items at the 1-year assessment (either “sometimes,” or “often”), when no pain was reported at baseline.
Matched controls did not have pain at either time point and were matched to new multisite pain cases based on sex, pubertal status, race/ethnicity and handedness. These variables were collected as described elsewhere [4]. The matching procedure was performed using the ‘optmatch’ package for the R programming language, version 3.6.1.
Sample Selection:
Participants were excluded (n = 1,821) if they had missing clinical or imaging data, or if the neuroimaging data did not pass ABCD’s quality control metrics (see Imaging Instruments Release Notes https://nda.nih.gov/study.html?id=721). To exclude sources of dependence, if multiple children from one family participated in the study, we used only the first enrollee. Participants were also excluded (n = 1,342) if they had any pain at baseline, resulting in 1,788 remaining participants. From these, 115 participants had pain in at least 2 locations at the 1-year assessment. Because there was a large pool of available controls, we matched controls to new multisite pain cases at a ratio of 2:1 (n = 230) to arrive at more precise estimates of between group differences. Six participants (1 pain case, 5 matched controls) were missing functional images which resulted in a total of 114 children with new multisite pain and 225 matched controls for fMRI analyses (Supplementary Figure 1). Demographics are shown in Supplementary Table 1.
Neuroimaging:
Neuroimaging sessions were completed on 3T MRI scanners (Siemens Prisma, General Electric and Philips). Detailed protocol and imaging parameters have been described elsewhere [9]. Briefly, a high-resolution structural MRI and four functional resting state scans (5 minutes each) were acquired in each participant. The minimally processed structural and resting state fMRI data were downloaded and initial preprocessing was performed using fMRIPrep 1.1.8 [18].
Functional Data Preprocessing
Functional data preprocessing conducted through fMRIPrep included co-registration to structural T1 (bbregister), realignment (mcflirt, FSL 5.0.9), normalization to MNI standard space (ANTs 2.2.0), and resampling to 2mm isometric voxels. No slice-timing correction was performed. Further details of specific algorithms used in fMRIPrep can be found elsewhere [18]. The preprocessed fMRIPrep output were then entered into the CONN Toolbox (v18.a; https://www.nitrc.org/projects/conn/) and the functional data were smoothed with a 6mm kernel. For each participant, functional outliers where head motion exceeded 0.5 mm and a global signal threshold of Z=3 were flagged using the Artifact Detection Tool (www.nitrc.org/projects/artifact_detect/). The CONN Toolbox concatenated the resting state scans within individual participants and treated them as a continuous session in future steps. Next, denoising was performed simultaneously and included the following steps: linear detrending, outlier censoring, motion regression with six subject-specific motion parameters and their first order derivatives, and aCompCor to remove six principal components of noise based on subject-specific white matter and CSF mask [5]. Finally, a bandpass filter of 0.01 – 0.1 Hz was applied to focus on low-frequency fluctuations. After denoising, data quality procedures were performed to assess the distribution of functional connectivity values and whether functional connectivity was correlated with motion across participants. Functional connectivity data were checked for correlations between mean motion and functional connectivity and between mean motion and functional connectivity distance dependence. See supplementary methods and supplementary Figures 2, 3, and 4.
Local neural activity:
Local spontaneous brain activity was determined for low frequency fluctuations of the blood-oxygen-level-dependent (BOLD) signal using fractional Amplitude of Low Frequency Fluctuations (fALFF) algorithm implemented in the CONN Toolbox. fALFF is a measure of spontaneous BOLD activity in the resting brain and a surrogate measure of resting neural activity [56]. fALFF was determined in the frequency domain, as the ratio of the root mean square of BOLD activity in the 0.01–0.1Hz frequency band relative to the total frequency content of the signal [56]. Group level analyses were performed to examine differences in fALFF using a two-sample t-test. Scanner manufacturers were entered as covariates of no interest as opposed to study site due to a small number of children represented at some study sites, and because a recent study using ABCD data found that scanner type had a larger impact on resting state functional connectivity reproducibility than study site [37]. A voxel-level threshold of p < 0.001 was applied to all contrasts and results were deemed significant at the cluster level p < 0.05 FDR corrected for multiple comparisons.
Independent Component Analysis (ICA):
Group ICA [8] was performed using the CONN Toolbox. Three subnetworks, previously shown to be involved in chronic pain in adults [2; 25; 41], were identified through the ICA analyses, including the default mode (DMN), salience (SLN), and sensorimotor networks (SMN). Networks were confirmed by visual inspection and spatial correlation between component maps and the default resting state network template maps in the CONN Toolbox.
Seed to whole brain connectivity analysis:
Next, we used a region-of-interest (ROI) based approach to examine functional connectivity in brain regions relevant to pain processing. For functional ROIs (bilateral anterior, mid and posterior insulae, dorsal, perigenual, and subgenual anterior cingulate cortex (ACC), medial prefrontal cortex, periaqueductal gray, and the nucleus accumbens) spheres were created with 5mm radius centered around the peak coordinates from previous studies (see Supplementary Table 2 for coordinates) [3; 35; 50; 52]. Structural ROIs (thalamus, amygdala) were generated using the WFU Pickatlas (https://www.nitrc.org/projects/wfu_pickatlas/). After denoising in the CONN toolbox, seed-to-whole brain functional connectivity Fisher-transformed correlation maps for each participant were calculated in first level analyses.
Group level analyses contrasting the connectivity between children with new multisite pain and matched controls were conducted with independent sample t-test’s using a general linear model in the CONN toolbox. Scanner manufacturers were entered as nuisance covariates. A voxel-level threshold of p < 0.001 was applied to all contrasts and results were deemed significant at the cluster level p < 0.05 FDR corrected for multiple comparisons. The Fisher-transformed correlation values were extracted using MarsBaR software (http://marsbar.sourceforge.net) and post-hoc analyses performed in SPSS v26.
Conditional Logistic Regression Models:
To formally address the relationship between identified differences in neuroimaging measures at baseline and new multisite pain, we conducted additional analyses that account for the matched nature of the data. We used conditional logistic regression (CLR) models through the ‘clogit’ function in the ‘survival’ package for the R programming language, version 3.6.1 [21]. Extracted connectivity and fALFF values were standardized for ease of interpretation and then used as predictors of new multisite pain. Because the differences were first identified at FDR-corrected levels of significance, we use the confidence intervals from these models, rather than p-values, to determine significance.
Structural Data Preprocessing
Within the fMRIPrep pipeline, the T1-weighted image was non-uniformity corrected, skull-stripped, and brain surfaces were reconstructed using recon-all (FreeSurfer 6.0.1 [14]) to assess cortical thickness. We also measured gray matter volume using voxel based morphometry in SPM12. See supplementary methods for details on structural preprocessing and analysis procedures.
Results
Increased neural activity in somatosensory and motor cortices precedes new multisite pain
At baseline, children who develop new multisite pain had increased neural activity in the left superior parietal lobule/primary somatosensory cortex (S1; p = 0.007 FDR; Figure 1A) and left primary motor cortex (M1)/S1 (p = 0.017 FDR; Figure 1B), and a trend towards decreased neural activity in the medial prefrontal cortex (mPFC; p = 0.079 FDR; Figure 1C and Table 1) compared to control children who do not develop new pain.
Figure 1. Altered neural activity in children before the onset of multisite pain.
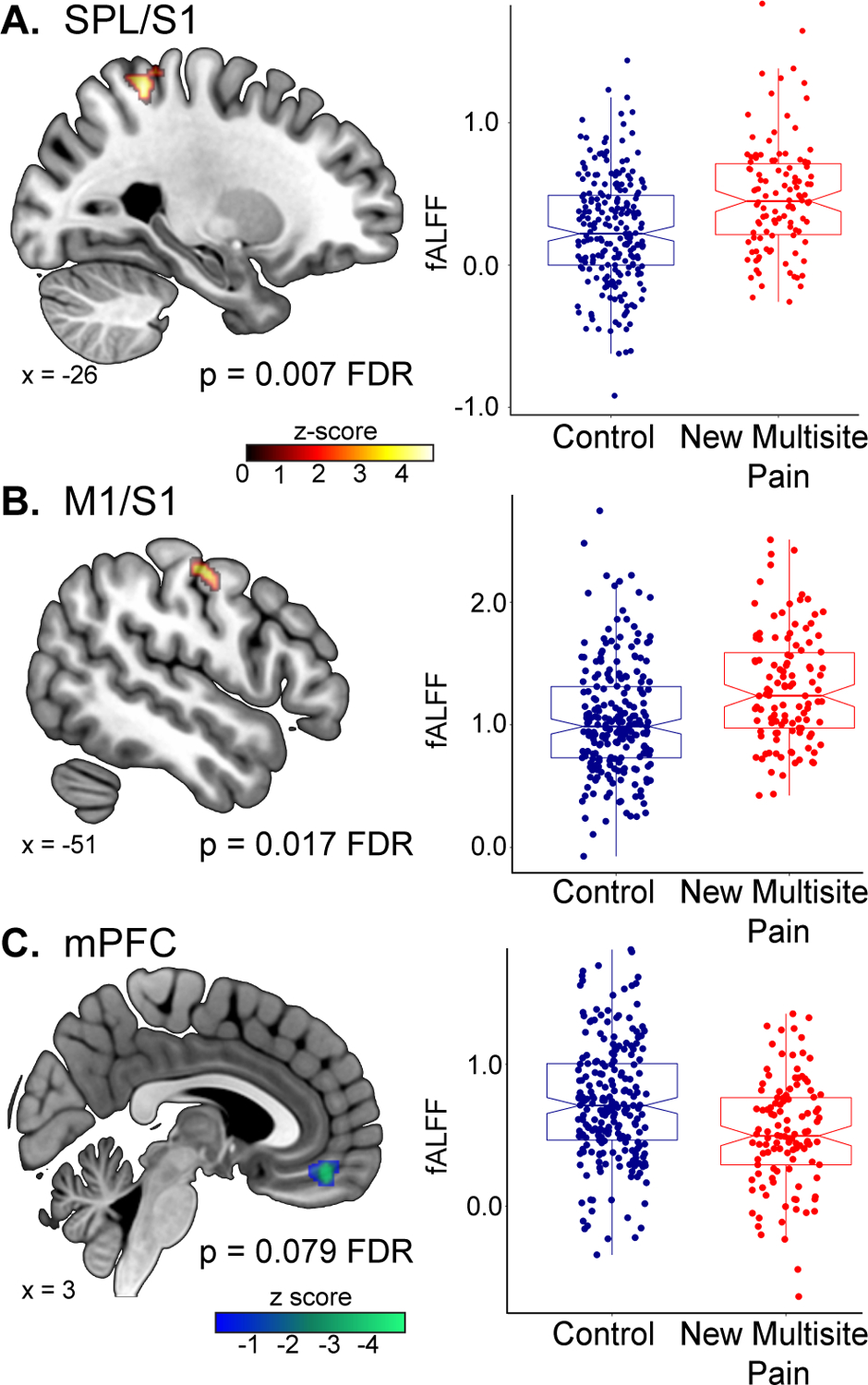
At baseline, children who develop multisite pain had increased spontaneous brain activity, as measured by fALFF, in the (A) left superior parietal lobule (SPL)/primary somatosensory cortex (S1) and (B) the left primary motor cortex (M1)/S1 relative to matched controls. (C) Additionally, children who develop multisite pain had a marginal decrease in neural activity in the medial prefrontal cortex (mPFC) compared to controls.
Table 1:
Changes in neural activity and functional connectivity precede the development of new multisite pain one year later
| fALFF | ||||
|---|---|---|---|---|
| New multisite pain > matched controls | MNI coordinates (x, y, z) | Z score | Cluster size (voxels) | p-value, FDR |
| L Superior parietal lobule/S1 | −28, −46, 58 | 4.62 | 120 | 0.007 |
| L M1/S1 | −58, −8, 46 | 4.26 | 88 | 0.017 |
| New multisite pain < matched controls | ||||
| mPFC | 2 46 −16 | 4.91 | 78 | 0.079† |
| ICA | ||||
| New multisite pain > matched controls | MNI coordinates (x, y, z) | Z score | Cluster size (voxels) | p-value, FDR |
| SLN - L M1/S1 | −28 −22 66 | 4.17 | 185 | 0.016 |
| SLN - R Angular Gyrus/MTG | 50 −54 10 | 4.19 | 133 | 0.036 |
| Seed-to-whole-brain | ||||
| New multisite pain > matched controls | MNI coordinates (x, y, z) | Z score | Cluster size (voxels) | p-value, FDR |
| R Posterior Insula Seed | ||||
| Bilateral M1/mid-cingulate | 2 −20 50 | 4.38 | 198 | 0.012 |
| R Angular Gyrus/MTG | 50, −48, 14 | 3.80 | 105 | 0.071† |
| L Mid Insula Seed | ||||
| R M1/S1 | 40 −18 52 | 4.35 | 230 | 0.003 |
| L Posterior Insula Seed | ||||
| L Angular Gyrus/MTG | −48 −70 22 | 3.82 | 114 | 0.096† |
| New multisite pain < matched controls | ||||
| R Thalamus Seed | ||||
| R Occipital Pole | 26 −92 4 | 4.38 | 314 | 0.002 |
| L Occipital Pole | −26 −90 −8 | 4.22 | 232 | 0.006 |
| L Occipital Pole | −26, −90, 8 | 3.91 | 135 | 0.037 |
n.s. at p < 0.05 FDR
Increased connectivity to the salience network in children who develop multisite pain
Children who develop multisite pain had increased SLN – M1/S1 (p = 0.016 FDR; Table 1 and Figure 2A) and SLN – angular gyrus/middle temporal gyrus (MTG; p = 0.036 FDR; Figure 2B) connectivity at baseline compared to matched controls. There were no significant findings related to DMN or SMN seeds.
Figure 2. Increased functional connectivity in children before the onset of multisite pain.
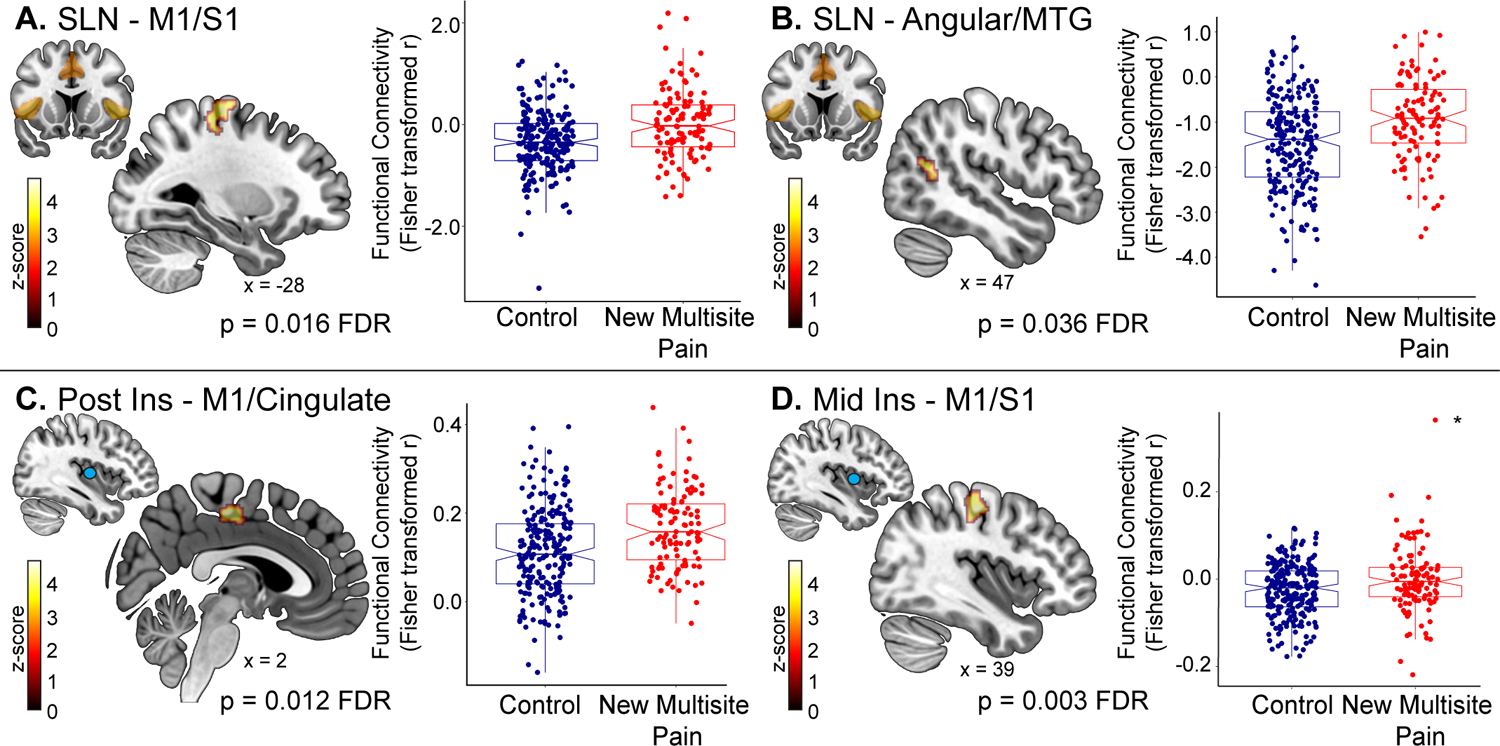
Independent component analysis (ICA) was performed to assess functional connectivity of resting state networks. There was increased connectivity at baseline between the salience network (SLN) and (A) left primary motor/somatosensory cortices (M1/S1) and (B) right angular/middle temporal gyrus (MTG) in children who develop new multisite pain 1-year later, relative to matched pain-free controls. Next, seeds were placed in regions previously shown to be associated with chronic pain in adults to examine functional connectivity to the rest of the brain. Increased connectivity between the (C) right posterior insula and bilateral M1/mid-cingulate, (D) left mid insula and right M1/S1 at baseline preceded the development of multisite pain in children 1-year later. *denotes outlier according to the Grubb’s test. The left mid insula seed analysis was performed again after removing the outlier and the result remained significant (p = 0.034 FDR).
Stronger connectivity between pain-related brain regions precedes new multisite pain
Compared to matched controls, children who develop new multisite pain had increased functional connectivity between the right posterior insula and bilateral medial M1/mid cingulate (p = 0.012 FDR; Figure 2C; Table 1) at baseline. They also had increased connectivity between the left mid insula and right M1/S1 (p = 0.003 FDR; Figure 2D). There were trends towards increased baseline functional connectivity between the posterior insulae and the bilateral angular gyri extending into the middle temporal gyri (right posterior insula – right angular/MTG p = 0.071 FDR; left posterior insula – left angular/MTG p = 0.096 FDR). Lastly, children who develop pain had decreased functional connectivity between the right thalamus and right occipital pole (p = 0.002 FDR), and two regions in the left occipital pole (p = 0.006 FDR and p = 0.037 FDR). There were no significant findings in remaining a priori seed regions.
Conditional Logistic Regression
Each of the neuroimaging metrics identified above were significant predictors of new multisite pain in the CLR framework. The strongest association was for activation of the superior parietal lobule/S1 (OR: 2.00, 95% CI: 1.50, 2.65) and the weakest was for connectivity between the L mid-insula and M1/S1 (OR: 1.60, 95% CI: 1.21, 2.12). Table 2 displays all model parameters. We did not observe any clear evidence of sex effects on the prediction of multisite pain using the extracted neuroimaging values (data not shown).
Table 2:
Results of conditional logistic regression models. Neuroimaging metrics are standardized for ease of interpretation.
| Metric | Odds Ratio | 95% CI LL | UL | Likelihood Ratio Test statistic | Likelihood ratio test p value |
|---|---|---|---|---|---|
| fALFF | |||||
| Superior parietal lobule/S1 | 1.996 | 1.500 | 2.654 | 28.041 | <.001 |
| L M1/S1 | 1.800 | 1.389 | 2.332 | 23.221 | <.001 |
| mPFC | .569 | .439 | .738 | 20.731 | <.001 |
| Functional Connectivity | |||||
| SLN – L M1/S1 | 1.892 | 1.430 | 2.502 | 24.531 | <.001 |
| SLN – R Angular/MTG | 1.737 | 1.347 | 2.240 | 20.391 | <.001 |
| R posterior insula – Bilateral M1/mid-cingulate | 1.736 | 1.350 | 2.232 | 21.051 | <.001 |
| R posterior insula – R Angular/MTG | 1.646 | 1.283 | 2.111 | 16.891 | <.001 |
| L mid insula – R M1/S1 | 1.604 | 1.214 | 2.121 | 12.921 | <.001 |
| L posterior insula – L Angular/MTG | 1.625 | 1.275 | 2.071 | 16.931 | <.001 |
| R thalamus – R occipital pole | .608 | .477 | .774 | 18.241 | <.001 |
| R thalamus – L occipital pole | .555 | .428 | .718 | 23.071 | <.001 |
| R thalamus – L occipital pole | .578 | .449 | .745 | 20.471 | <.001 |
Gray Matter Volume and Cortical Thickness
No significant differences in brain gray matter volume or cortical thickness were detected between children who did or did not develop multisite pain.
Discussion
To our knowledge, this is the first study to report that altered brain signatures precede the development of new multisite pain in children who were pain free at baseline. We found that increased activity in sensorimotor regions and increased functional connectivity between the insula, sensorimotor and DMN regions predicted new-onset multisite pain one year later. Many of the results presented here are consistent with those observed in adults with chronic nociplastic pain conditions which are characterized by widespread pain, as well as the ‘pain vulnerable’ brain networks hypothesized by Denk et al. to set the stage for abnormal pain processing later in life [15].
The role of the somatosensory cortex in pain perception has long been appreciated [7], and altered activity and connectivity in S1 is frequently reported in various chronic pain conditions [53]. M1 shares many connections with the sensory nuclei of the thalamus and other pain processing regions and may therefore be an important modulator of pain perception by virtue of its connectivity patterns [10]. We report an increase in S1 and M1 neural activity before multisite pain symptoms manifest. We also found stronger baseline functional connectivity between the mid insula and M1/S1, between the posterior insula and bilateral M1/mid-cingulate, and between the SLN and M1/S1. This latter result partially overlaps with findings in adults with chronic multisite pain [31] (Figure 3), and suggests that this connectivity may precede the development of chronic pain.
Figure 3. SLN - M1/S1 result overlaps with previous study of adults with established multisite pain.
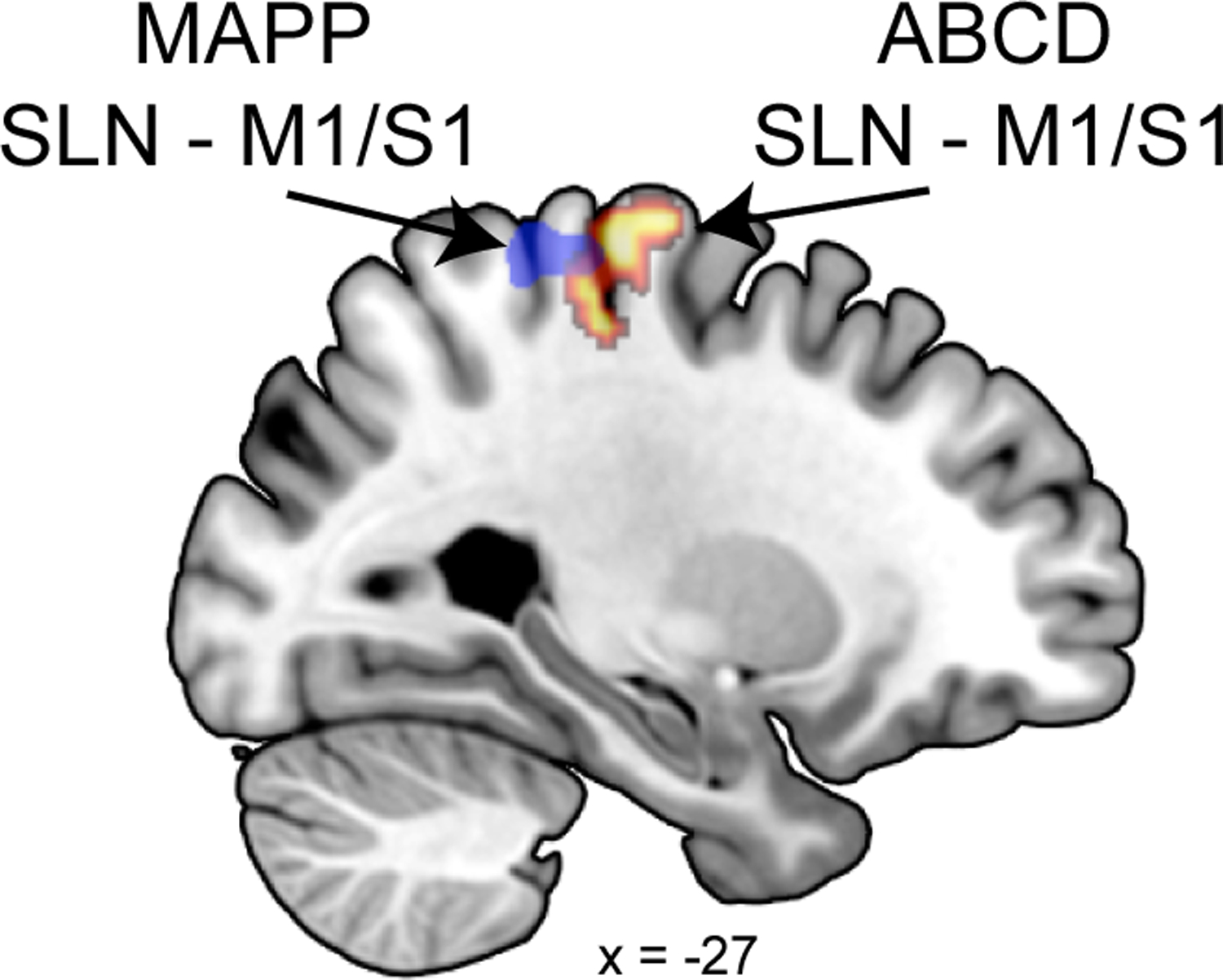
Children who develop multisite pain had increased SLN – M1/S1 (p = 0.016 FDR) functional connectivity at baseline compared to matched controls. This result partially overlaps with findings in adults with established multisite pain as part of the Multi-Disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network [31].
The insula is a key node of the SLN and an important region for pain perception and cross-modal sensory integration [12]. The insula plays a critical role in converting salient physiological inputs into higher level cognitive states or emotions [49], and acts as causal switch for attentional control by coordinating activity in task positive (e.g. frontoparietal) and task negative (e.g. DMN) brain networks [39]. The DMN, comprised of the posterior cingulate, precuneus, inferior parietal lobule (which includes the angular gyrus), lateral temporal cortex and mPFC, is active at rest and engaged during self-referential thought [45]. In healthy participants, the DMN and SLN are anti-correlated at rest [20]. In children who develop multisite pain, we found increased connectivity between the SLN and angular gyrus/MTG and trends towards increased connectivity between the bilateral posterior insulae and bilateral angular gyri/MTG. These findings are remarkably consistent with neuroimaging studies in adults with chronic pain that have found increased connectivity between the insula and DMN [11; 41].
One major, unanswered question in adult studies is whether increased connectivity among SLN, SMN and DMN regions is a cause or consequence of chronic pain. Our results provide evidence that such changes precede symptomatic, multisite pain. One hypothesis is that increased insula – DMN functional connectivity may prime an individual to be more sensitive to sensory inputs, an idea consistent with the insula’s role in directing attention and adjusting the gain on incoming stimuli [39]. This hypothesis is further supported by the finding of increased M1/S1 neural activity and a trend towards decreased mPFC in children who develop pain. Heightened insula/SLN to DMN connectivity in pain-free children may be a subclinical marker of neural vulnerability for developing multisite pain later in life.
It is possible that brain circuits have been primed by innate (genetic) or acquired (early life stress or environmental exposure) factors [15] in children who develop multisite pain, resulting in brain regions being hyperactive and/or more strongly connected to each other. Indeed, animal models have shown that activity in the insula, amygdala, cingulate, and S1 is altered in adult rats that have experienced early life stress [28].
Interestingly, altered corticostriatal circuitry was not a significant predictor of new onset pain. Previous work has implicated this pathway in the transition from acute to chronic pain in adults [3]. We suspect that the children examined here are too early in the stages of pain development to show this alteration, and that the involvement of these regions may become apparent at future timepoints in children whose pain is unresolved.
There were no significant baseline differences in cortical thickness or gray matter volume in children who developed multisite pain, suggesting that brain functional changes precede pain onset, but structural changes do not. Rodriguez-Raecke and colleagues hypothesized that gray matter changes are a consequence rather than the cause of chronic pain (i.e. represent neuroplasticity) since they noted that gray matter abnormalities resolved after successful hip replacement surgery in osteoarthritis patients [48], a finding that has been since replicated in chronic pediatric pain [17].
Of note, we found no sex differences in the neural activity or functional connectivity findings that preceded new multisite pain. This may initially seem inconsistent with the established sex differences observed in pain sensitivity and the prevalence of many chronic pain conditions, but it is actually consistent with indications that sex differences in chronic pain emerge in mid-late puberty, and the majority of participants in this study were pre-pubertal or in early puberty. Before puberty, boys and girls have a similar prevalence of chronic pain [34]. However, this prevalence changes dramatically during puberty as significantly more girls develop chronic pain than boys [33; 34; 36; 38; 51]. This largely remains constant throughout the lifespan: adult females are about twice as likely to report multisite pain than males [19]. Puberty is characterized by striking hormonal, physical and behavioral changes that are often sex-specific, and is also a period of substantial neural reorganization and development particularly in cortical and limbic brain regions [26; 54]. Sex differences in brain function and structure coincide with pubertal development [30], and appear to be partially mediated by hormones [42; 43]. Sex-specific hormonal changes occurring during puberty may differentially affect pain processing and vulnerability [38], and therefore, it is likely that sex differences in brain function and pain will emerge as the ABCD sample reaches mid-late puberty.
Our study represents the largest pediatric pain neuroimaging study to date. However, it has limitations. We relied on parental report of their children’s pain as there are no self-report measures of pain in the ABCD timepoints examined here. At this phase of the ABCD study, only parents have completed the CBCL about their child, but previous studies have examined parent-child correspondence on the CBCL (parent report) and the Youth Self Report (YSR; an age-appropriate self-report version of the CBCL) [22; 46; 47]. Although associations between the pain questions in the CBCL and YSR tend to be low-to-moderate (aches and pains r = 0.19, headaches r = .36, stomachaches r = .28)[47], parents generally underreport pain in their children [22; 46; 47]. Thus, it is unlikely that our findings were inflated by parental report, and instead, reflect conservative estimates. Moreover, parental report of pain in children still appears to have predictive power for important outcomes like pain chronification. In at least one study of which we are aware, parental report of pain was associated with pain chronicity 24 months later, whereas child-reported pain was not [13]. While the forms of pain assessed by the CBCL represent some of the most common in pediatric populations, they are not comprehensive. We also did not control for the different types and combinations of pain present across individuals. Future analyses should examine if the neurobiological predictors are generalizable or are unique to specific body regions. Further, the duration, severity and cause (i.e. injury or disease) of pain is unknown. Importantly, at this stage we do not know if the pain reported here will become chronic. The ABCD study presents an opportunity to follow these children over time and examine the risk factors that facilitate chronification and/or the protective factors that confer resilience to pain persistence.
In summary, we demonstrated that increases in activity and functional connectivity between pain processing brain regions exist before the onset of multisite pain. Although our findings were based on parental report of their child’s pain and should be interpreted with caution, these results may represent the neural underpinnings of pain vulnerability and highlights the need for more research on the developmental origins of pain. Future studies should examine how the brain changes during pain chronification, and the moderating impacts of environmental, psychosocial, and genetic factors. If these results are confirmed, they may represent targets for early interventions that prevent pain chronification.
Supplementary Material
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
C.M.K. was supported by the University of Michigan Anesthesiology Post-Doctoral Research Training Program (NIGMS; 5 T32 GM103730-08).
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest relevant to this work.
References
- [1].Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 2000;21(8):265–271. [DOI] [PubMed] [Google Scholar]
- [2].Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. PLoS One 2014;9(9):e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15(8):1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D, Alia-Klein N, Potter AS, Paulus MP, Prouty D, Zucker RA, Sher KJ. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci 2018;32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007;37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brattberg G Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain 2004;8(3):187–199. [DOI] [PubMed] [Google Scholar]
- [7].Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A 1999;96(14):7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 2001;14(3):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ Jr., Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM, Workgroup AIA. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 2018;32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Castillo Saavedra L, Mendonca M, Fregni F. Role of the primary motor cortex in the maintenance and treatment of pain in fibromyalgia. Med Hypotheses 2014;83(3):332–336. [DOI] [PubMed] [Google Scholar]
- [11].Ceko M, Frangos E, Gracely J, Richards E, Wang B, Schweinhardt P, Catherine Bushnell M. Default mode network changes in fibromyalgia patients are largely dependent on current clinical pain. Neuroimage 2020;216:116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3(8):655–666. [DOI] [PubMed] [Google Scholar]
- [13].Czyzewski DI, Self MM, Williams AE, Weidler EM, Blatz AM, Shulman RJ. Maintenance of Pain in Children With Functional Abdominal Pain. J Pediatr Gastroenterol Nutr 2016;62(3):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9(2):179–194. [DOI] [PubMed] [Google Scholar]
- [15].Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 2014;17(2):192–200. [DOI] [PubMed] [Google Scholar]
- [16].Eccleston C, Fisher E, Howard RF, Slater R, Forgeron P, Palermo TM, Birnie KA, Anderson BJ, Chambers CT, Crombez G, Ljungman G, Jordan I, Jordan Z, Roberts C, Schechter N, Sieberg CB, Tibboel D, Walker SM, Wilkinson D, Wood C. Delivering transformative action in paediatric pain: a Lancet Child & Adolescent Health Commission. Lancet Child Adolesc Health 2021;5(1):47–87. [DOI] [PubMed] [Google Scholar]
- [17].Erpelding N, Simons L, Lebel A, Serrano P, Pielech M, Prabhu S, Becerra L, Borsook D. Rapid treatment-induced brain changes in pediatric CRPS. Brain Struct Funct 2016;221(2):1095–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 2019;16(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd., Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gail MH, Lubin JH, Rubinstein LV. Likelihood Calculations for Matched Case-Control Studies and Survival Studies with Tied Death Times. Biometrika 1981;68(3):703–707. [Google Scholar]
- [22].Hart SL, Hodgkinson SC, Belcher HM, Hyman C, Cooley-Strickland M. Somatic symptoms, peer and school stress, and family and community violence exposure among urban elementary school children. J Behav Med 2013;36(5):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. Journal of Applied Biobehavioral Research 2018;23(2):e12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hassett AL, Hilliard PE, Goesling J, Clauw DJ, Harte SE, Brummett CM. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. J Pain 2013;14(11):1390–1397. [DOI] [PubMed] [Google Scholar]
- [25].Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct 2016;221(8):4203–4219. [DOI] [PubMed] [Google Scholar]
- [26].Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol 2014;35(1):89–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain 2017;158(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holschneider DP, Guo Y, Mayer EA, Wang Z. Early life stress elicits visceral hyperalgesia and functional reorganization of pain circuits in adult rats. Neurobiol Stress 2016;3:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain 2012;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 2019;44(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kutch JJ, Ichesco E, Hampson JP, Labus JS, Farmer MA, Martucci KT, Ness TJ, Deutsch G, Apkarian AV, Mackey SC, Klumpp DJ, Schaeffer AJ, Rodriguez LV, Kreder KJ, Buchwald D, Andriole GL, Lai HH, Mullins C, Kusek JW, Landis JR, Mayer EA, Clemens JQ, Clauw DJ, Harris RE, Network MR. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain 2017;158(10):1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10(9):895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].LeResche L Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med 1997;8(3):291–305. [DOI] [PubMed] [Google Scholar]
- [34].LeResche L, Mancl LA, Drangsholt MT, Saunders K, Von Korff M. Relationship of pain and symptoms to pubertal development in adolescents. Pain 2005;118(1–2):201–209. [DOI] [PubMed] [Google Scholar]
- [35].Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 2012;60(1):505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646–657. [DOI] [PubMed] [Google Scholar]
- [37].Marek S, Tervo-Clemmens B, Nielsen AN, Wheelock MD, Miller RL, Laumann TO, Earl E, Foran WW, Cordova M, Doyle O, Perrone A, Miranda-Dominguez O, Feczko E, Sturgeon D, Graham A, Hermosillo R, Snider K, Galassi A, Nagel BJ, Ewing SWF, Eggebrecht AT, Garavan H, Dale AM, Greene DJ, Barch DM, Fair DA, Luna B, Dosenbach NUF. Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Dev Cogn Neurosci 2019;40:100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Melchior M, Poisbeau P, Gaumond I, Marchand S. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience 2016;338:63–80. [DOI] [PubMed] [Google Scholar]
- [39].Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Molina J, Amaro E Jr., da Rocha LGS, Jorge L, Santos FH, Len CA. Functional resonance magnetic imaging (fMRI) in adolescents with idiopathic musculoskeletal pain: a paradigm of experimental pain. Pediatr Rheumatol Online J 2017;15(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62(8):2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience 2011;191:28–37. [DOI] [PubMed] [Google Scholar]
- [43].Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 2011;36(8):1101–1113. [DOI] [PubMed] [Google Scholar]
- [44].Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, van der Wouden JC. Pain in children and adolescents: a common experience. Pain 2000;87(1):51–58. [DOI] [PubMed] [Google Scholar]
- [45].Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98(2):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rescorla LA, Ginzburg S, Achenbach TM, Ivanova MY, Almqvist F, Begovac I, Bilenberg N, Bird H, Chahed M, Dobrean A, Dopfner M, Erol N, Hannesdottir H, Kanbayashi Y, Lambert MC, Leung PW, Minaei A, Novik TS, Oh KJ, Petot D, Petot JM, Pomalima R, Rudan V, Sawyer M, Simsek Z, Steinhausen HC, Valverde J, Ende J, Weintraub S, Metzke CW, Wolanczyk T, Zhang EY, Zukauskiene R, Verhulst FC. Cross-informant agreement between parent-reported and adolescent self-reported problems in 25 societies. J Clin Child Adolesc Psychol 2013;42(2):262–273. [DOI] [PubMed] [Google Scholar]
- [47].Rey JM, Schrader E, Morris-Yates A. Parent-child agreement on children’s behaviours reported by the Child Behaviour Checklist (CBCL). J Adolesc 1992;15(3):219–230. [DOI] [PubMed] [Google Scholar]
- [48].Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci 2009;29(44):13746–13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Satpute AB, Kang J, Bickart KC, Yardley H, Wager TD, Barrett LF. Involvement of Sensory Regions in Affective Experience: A Meta-Analysis. Front Psychol 2015;6:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmidt-Wilcke T, Ichesco E, Hampson JP, Kairys A, Peltier S, Harte S, Clauw DJ, Harris RE. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin 2014;6:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992;267(1):64–69. [PubMed] [Google Scholar]
- [52].Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 2009;30(9):2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].van der Miesen MM, Lindquist MA, Wager TD. Neuroimaging-based biomarkers for pain: state of the field and current directions. Pain Rep 2019;4(4):e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH. Puberty and the human brain: Insights into adolescent development. Neurosci Biobehav Rev 2018;92:417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Youssef AM, Ludwick A, Wilcox SL, Lebel A, Peng K, Colon E, Danehy A, Burstein R, Becerra L, Borsook D. In child and adult migraineurs the somatosensory cortex stands out … again: An arterial spin labeling investigation. Hum Brain Mapp 2017;38(8):4078–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008;172(1):137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


