Abstract
Objective:
To evaluate the effects of fish oil (FO), a source of the omega-3 polyunsaturated fatty acids (n-3 PUFA) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), on emotion-generated corticolimbic functional connectivity in depressed youth at high risk for developing bipolar I disorder.
Methods:
Thirty-nine antidepressant-free youth with a current depressive disorder diagnosis and a biological parent with bipolar I disorder were randomized to 12-week double-blind treatment with FO or placebo. At baseline and endpoint, fMRI (4 Tesla) scans were obtained while performing a continuous performance task with emotional and neutral distractors (CPT-END). Seed-to-voxel functional connectivity analyses were performed using bilateral orbitofrontal cortex (OFC) and amygdala (AMY) seeds. Measures of depression, mania, global symptom severity, and erythrocyte fatty acids were obtained.
Results:
Erythrocyte EPA+DHA composition increased significantly in the FO group (+47%, p≤0.0001) but not in the placebo group (−10%, p=0.11). Significant group by time interactions were found for functional connectivity between the left OFC and the left superior temporal gyrus (STG), and between the right AMY and right inferior temporal gyrus (ITG). OFC-STG connectivity increased in the FO group (p=0.0001) and decreased in the placebo group (p=0.0019), and AMY-ITG connectivity decreased in the FO group (p=0.0014) and increased in the placebo group (p<0.0001). In the FO group, but not placebo group, the decrease in AMY-ITG functional connectivity correlated with decreases in Childhood Depression Rating Scale-Revised and Clinical Global Impression-Severity Scale scores.
Conclusions:
In depressed high-risk youth FO supplementation alters emotion-generated corticolimbic functional connectivity which correlates with changes in symptom severity ratings.
Keywords: Bipolar disorder, Familiar risk, High-risk, Adolescent, Omega-3 polyunsaturated fatty acids
1. INTRODUCTION
The first episode of mania, and by definition bipolar I disorder, frequently occurs during the late childhood and adolescent period,1 and is commonly preceded by several years of depressive symptoms.2–5 In typically developing youth, this developmental period is associated with progressive reductions in regional prefrontal cortex (PFC) synaptic density and cortical thickness,6–11 and changes in amygdala (AMY) volume.12–14 These dynamic structural changes are associated with progressive increases in negative PFC-AMY coupling and decreased AMY activation in response to emotional stimuli.15,16 Deviations from these maturational trajectories are associated with elevated ratings of depression and anxiety in typically developing youth.17,18 Moreover, adolescent and adult patients with mood disorders, including bipolar I disorder and major depressive disorder (MDD), exhibit corticolimbic structural and functional abnormalities and AMY hyperactivation.19–30 Although these findings suggest that a delay or disruption in typical adolescent corticolimbic maturation are relevant to the etiology of mood dysregulation, the underlying developmental risk and resilience mechanisms remain poorly understood.
Converging evidence suggests that the pathophysiology and potentially etiology of mood disorders is associated with a deficiency in omega-3 polyunsaturated fatty acids (n-3 PUFA), including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).31 Cross-national and cross-sectional studies suggest that lower intake of fish and seafood, primary dietary sources of n-3 PUFA, is associated with higher lifetime prevalence rates of MDD32–34 and bipolar disorders.35 Consistent with these observations, meta-analyses have found that patients with MDD36 or bipolar I disorder37 exhibit lower blood EPA and/or DHA levels compared with healthy subjects. Erythrocyte (red blood cell) membrane EPA+DHA deficits coincide with, and likely precede, the initial onset of mania,38 and are observed in adolescents at elevated risk for bipolar I disorder.39 Although controversial, meta-analyses of placebo-controlled trials suggest that fish oil (FO) supplementation, which increases erythrocyte n-3 PUFA levels, is superior to placebo for reducing depressive symptoms in adults with MDD40–42 and bipolar disorder.43 Importantly, in typically developing youth cortical levels of DHA, the most abundant n-3 PUFA in the mammalian brain, increase rapidly during childhood and adolescence,44 and animal studies have found that developmental deficits in brain DHA accrual disrupt synaptic plasticity and connectivity.45–49 These and other findings50 suggest that developmental n-3 PUFA insufficiency may represent a plausible and modifiable risk factor for corticolimbic structural and functional maturation abnormalities observed in youth with mood disorders.
The present study investigated the effects of 12-week FO supplementation on corticolimbic functional connectivity in adolescents at elevated risk for mood dysregulation and progression i.e., they have depressive symptoms and a biological parent with bipolar I disorder, in the context of a randomized placebo-controlled treatment trial.51 fMRI scans were acquired while patients performed a continuous performance task with emotional and neutral distractors (CPT-END) which engages prefrontal and limbic brain regions.52 Based on the evidence reviewed above, we selected the bilateral OFC (BA 11/47) and AMY as seed regions and performed seed-to-voxel based functional connectivity analyses. The primary objectives were to determine whether FO supplementation would alter emotion-generated corticolimbic network functional connectivity, and whether these connectivity changes correlate with changes in symptom ratings.
2. Method
2.1. Study design
This was a 12-week randomized, double blind, parallel group, placebo-controlled fixed-dose trial. Patients, and if ≤18 years their legal guardians, provided written informed assent and consent, respectively after study procedures were explained. During the 12-week treatment phase, patients participated in weekly visits to perform symptom ratings, and fMRI scans were performed at baseline and Week 12. Efficacy and safety results from this trial have been reported previously.51 This study was approved by the Institutional Review Board of University of Cincinnati and was registered at clinicaltrials.gov with identifier NCT00917501. The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2. Study participants
This study enrolled youth (ages 9–21 years) with a current DSM-IV-TR (APA, 2000) diagnosis of MDD or Depressive Disorder NOS (operationalized as 4 of 5 criteria for a major depressive episode or meeting all MDD criteria except duration), determined by the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia, WASH-U-KSADS,53 a Childhood Depression Rating Scale-Revised Version (CDRS-R)54,55 score of ≥40, and at least one biological parent with bipolar disorder, type I, as determined by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV).56 Diagnostic instruments were administered by a trained psychiatrist or qualified clinician with established diagnostic reliability (κ>0.9). Inclusion and exclusion criteria are detailed in the parent study.51 Additionally, subjects were excluded by left-hand dominance or a contraindication to MRI scans.
2.3. Intervention
Patients took three placebo (olive oil) or FO capsules (Inflammation Research Foundation, Marblehead, MA USA) daily which were identical in size, shape, and color to protect the blind. Each FO capsule contained 450 mg EPA, 40 mg DPA, and 260 mg DHA for a total daily dose of 2,130 mg EPA+DHA (1.7:1 EPA/DHA ratio) or 2,250 mg n-3 PUFAs (EPA+DPA+DHA). At baseline and endpoint, erythrocyte membrane fatty acid composition (mg fatty acid/100 mg fatty acids) was determined by gas chromatography (Shimadzu GC-2010, Shimadzu Scientific Instruments Inc., Columbia MD USA). Primary measures of interest were EPA+DHA, arachidonic acid (AA, 20:4n-6), and the AA/(EPA+DHA) ratio.39
2.4. Symptom ratings
Depression symptom severity was determined using the CDRS-R,54,55 manic symptom severity was determined using the Young Mania Rating Scale (YMRS),57 and the Clinical Global Impression-Severity Scale (CGI-S) was used to assess global change in illness severity.58 All clinician ratings were administered by a child and adolescent psychiatrist or psychologist with established inter-rater reliabilities (kappa >0.9) and who was blinded to treatment assignment.
2.5. fMRI
2.5.1. fMRI image acquisition
Patients were scanned using a 4.0 Tesla Varian Unity INOVA Whole Body MRI/MRS system (Varian Inc., Palo Alto, CA). Anatomical T1-weighted, 3-D brain scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) sequence (TMD = 1.1 s, TR = 13 ms, TE = 6 ms, FOV = 256 × 256 × 192 mm, matrix 256 × 256 × 192 pixels, flip angle = 20 degrees). A midsagittal localizer scan was obtained to place 40 contiguous 4 mm axial slices that extend from the inferior cerebellum to encompass the entire brain. Subjects then completed a fMRI session while performing the Continuous Performance Task with Emotional and Neutral Distracters (CPT-END)52 using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence (TR/TE = 2000/30 ms, FOV = 256 × 256 mm, matrix 64 × 64 pixels, slice-thickness = 4 mm, flip angle = 75 degrees. All images were selected from the International Affective Picture System. Visual stimuli were presented using high-resolution video goggles (Resonance Technologies, Inc., Northridge, CA).
2.5.2. fMRI data analyses
Preprocessing of the fMRI data was performed using Statistical Parametric Mapping (SPM12, Wellcome Center for Human Neuroimaging, London, UK) and SPM-based Conn Toolbox 2018b (McGovern Institute for Brain Research, MIT, Cambridge, MA),59 running in MATLAB (The Mathworks Inc.; MA, USA) platform. The steps included realignment, slice-timing correction, co-registration to structural T1 scan, spatial normalization to Montreal Neurological Institute coordinates (MNI) space, spatial smoothing (8-mm Gaussian kernel) and band-pass filtering (0.009 < f < 0.08 Hz). The T1 scans were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) tissue classes. The component-based noise correction known as a CompCor (White matter and CSF ROIs, 5 components each).60 The Artifact Detection Tools (ADT, http://www.nitrc.org/projects/artifact_detect) was used to detect outlier head motion parameters and artifacts. For each scan, time points were taken as outliers if the global mean intensity exceeded 3 SDs from the mean or if scan-to-scan motion deviation was greater than 0.5 mm. The motion parameters (12 regressors: 6 motion parameters plus 6 first-order temporal derivatives) and outliers detected by the ADT were included as regressors in the first-level general linear model for control of physiological and movement confounds.
Seed-to-voxel functional connectivity analyses were performed using Harvard-Oxford Atlas-based OFC (BA 11/47) and the AMY seed regions. The boundaries of the seed regions were determined using the built-in anatomic atlas of Conn Toolbox in the MNI space. The seed-based FC map was generated by computing the condition-specific Pearson’s correlation coefficients between the seed BOLD timeseries and each voxel BOLD timeseries using general linear model (GLM). Condition-specific weights are defined from the hemodynamic response function convolved events for each condition. The connectivity difference between emotion and square/neutral conditions was obtained by subtracting the seed-based connectivity during square/neutral condition from the emotion condition. Connectivity maps of all combination of laterality, seeds, and condition contrasts were analyzed by random-effects analysis of variance (ANOVA). The pre-to-post changes were compared between the FO and placebo groups. Sex, age and IQ were treated as the nuisance variables in the voxel-wise statistical models. Regions showing a group difference during processing of emotional images were reported if they survived a stringent cluster-extent threshold at family-wise error (FWE)-corrected p<0.05 and voxel-height threshold at uncorrected p<0.001. The effect size of strength of pre-to-post FC changes obtained from the ANOVA test was extracted from the statistical map for each condition contrast and participant. Post hoc paired t-tests were then conducted within each group.
2.5.3. Morphological analyses
We analyzed group differences in morphological changes using CAT 12 toolbox (http://www.neuro.uni-jena.de/cat/). Please refer to the supplemental material for additional details.
2.5.4. Statistical analyses
Statistical analyses were performed using the Statistical Analysis System (SAS Institute, Cary, NC, USA). For longitudinal assessments of CDRS-R and CGI-S scores, a mixed linear regression model on post-baseline scores was performed. Correlational analyses controlled for age, sex and IQ and were performed using R (Version 3.6.0). We used GLM to characterize the relationships between sex, age and changes in FC. Interaction terms were also calculated to test for treatment differences in the relationships between connectivity measures and symptom ratings.61
3. RESULTS
3.1. Subject characteristics
A total of 56 patients met study criteria and were randomized to placebo (n=29) or FO (n=27), a total of 42 patients completed the 12-week trial (placebo n=21; FO n=21), and a total of 39 patients had usable data from both baseline and endpoint fMRI scans (placebo n=18; FO n=21). There were no significant differences in demographic or clinical characteristics of patients randomized to placebo and FO (Table 1). A majority of participants were girls (79%) and White (64%), and the overall mean age of participants was 14.4±2.8 years, and the mean baseline CDRS-R score was 47.6±7.5.
Table 1.
Baseline Demographic and Clinical Characteristics
| Placebo | Fish Oil | ||
|---|---|---|---|
| Variable1 | (n=18) | (n=21) | P-value2 |
| Age, years | 14.1 ± 2.8 | 14.7 ± 2.9 | 0.6 |
| Gender, n (%) female | 13 (72) | 17 (81) | 0.1 |
| Race, n (%) White | 11 (61) | 14 (67) | 0.4 |
| Prepubescent, n (%) | 2 (11) | 2 (10) | 1.0 |
| BMI, kg/m2 | 26.1 ± 8.5 | 26.4 ± 8.5 | 0.9 |
| WASI | 103.0 ± 13.0 | 100.3 ± 10.9 | 0.5 |
| Diagnosis, n (%) | |||
| MDD | 13 (72) | 16 (76) | 0.5 |
| Depressive Disorder NOS | 5 (28) | 5 (24) | 0.5 |
| CDRS-R Total Score | 49.1 ± 7.9 | 46.4 ± 7.1 | 0.3 |
| CGI-S | 4.6 ± 0.5 | 4.5 ± 0.6 | 0.5 |
| Erythrocyte Fatty acids (wt % TTL) | |||
| EPA+DHA | 3.3 ± 0.7 | 3.3 ± 0.6 | 0.8 |
| AA | 17.9 ± 1.2 | 17.3 ± 1.7 | 0.2 |
| AA/EPA+DHA | 5.5 ± 1.0 | 5.4 ± 1.0 | 0.7 |
Values are group mean ± S.D. or number of subjects (n) and percent (%).
t-tests or X2
3.2. Erythrocyte fatty acids
At baseline the overall mean erythrocyte EPA+DHA composition was 3.3±0.6%, and there were no significant group differences for EPA+DHA (p=0.82), arachidonic acid (AA, p=0.19), or the AA/(EPA+DHA) ratio (p=0.74)(Table 1). The treatment group by time (baseline, week 12) interaction was significant for EPA+DHA (p≤0.0001), arachidonic acid (AA) (p=0.02), and the AA/(EPA+DHA) ratio (p≤0.0001)(Fig. 1). At week 12, EPA+DHA composition increased significantly from baseline in the FO group (+47%, p≤0.0001) but not in the placebo group (−10%, p=0.11), AA decreased in the FO group (−9%, p=0.004) but not in the placebo group (+2%, p=0.60), and the AA/(EPA+DHA) ratio decreased in the FO group (−49%, p≤0.0001) but not in the placebo group (+9%, p=0.08).
Figure 1.
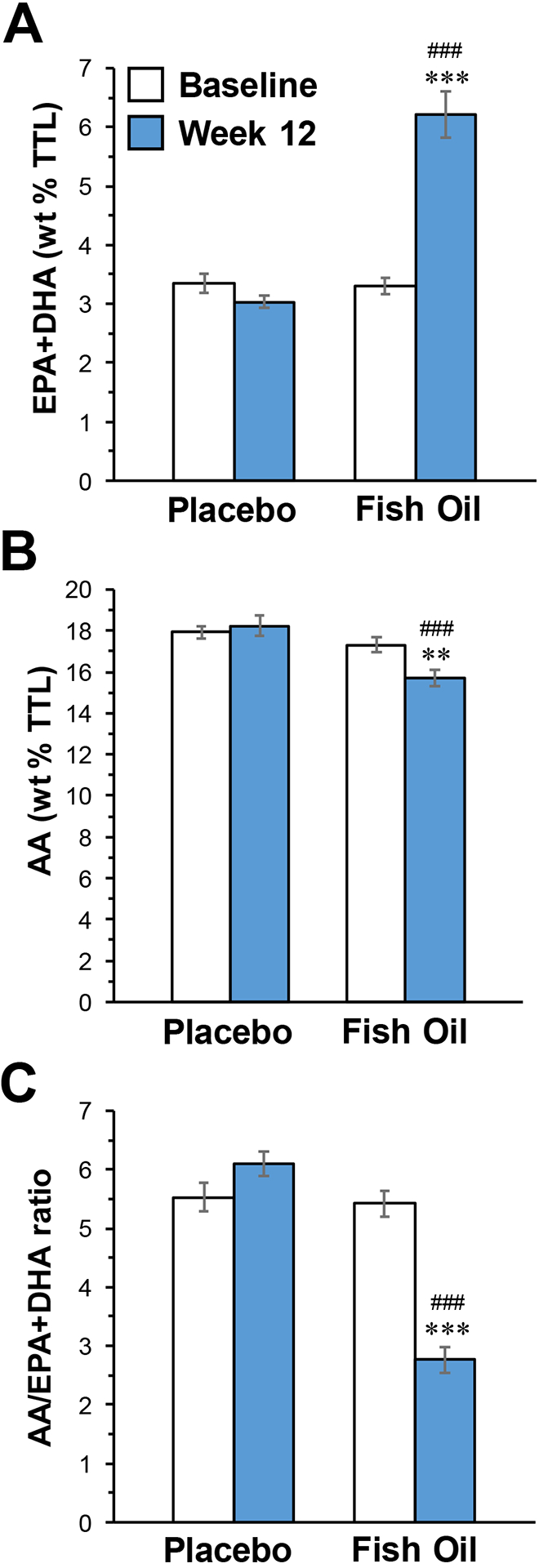
Erythrocyte EPA+DHA (A) and arachidonic acid (AA)(B) composition, and the AA/(EPA+DHA) ratio (C), at baseline and following 12-week supplementation with FO or placebo. Data are expressed as group mean fatty acid composition (mg fatty acid/100 mg fatty acids) or ratio ± S.E.M. **P≤0.001, ***P≤0.0001 vs. Baseline, ###P≤0.0001 vs. Placebo week 12.
3.3. Symptom ratings
Efficacy results of this trial are detailed elsewhere,51 and only baseline and endpoint data are presented. In brief, there were no significant treatment group by time interactions for CDRS-R total score (p=0.414)(baseline-endpoint decreases: PBO: −41%, p≤0.0001; FO: −45%, p≤0.0001)(Fig. 2A) or YMRS total score (p=0.53)(baseline-endpoint decrease: PBO: −59.6%, ≤0.0001; FO: −66.8%, p≤0.0001). However, a significant group by time interaction was observed for CGI-S scores (p=0.015) (PBO: −30%, p≤0.0001; FO: −44%, p≤0.0001)(Fig. 2B).
Figure 2.
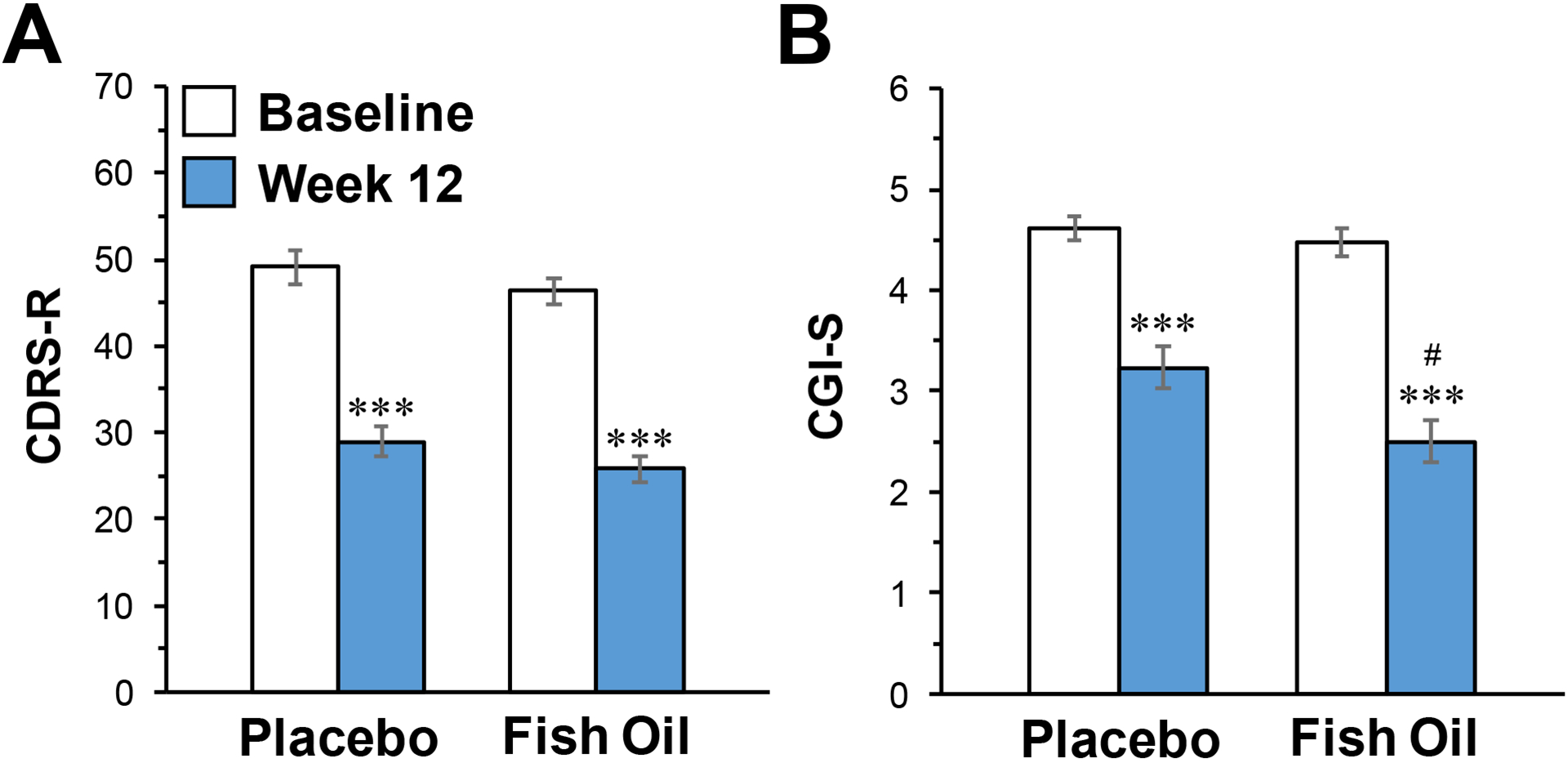
CDRS-R (A) and CGI-S (B) total scores at baseline and following 12-week supplementation with FO or placebo. Data are expressed as group mean ± S.E.M. ***P≤0.0001 vs. Baseline, #P≤0.01 vs. Placebo week 12.
3.4. fMRI functional connectivity
There were no significant group differences in CPT-END performance measures at baseline, and no group by time interactions were observed (all p>0.05). For the emotion–square contrast, a significant group by time interaction was observed for functional connectivity between the left OFC (seed) and left superior temporal gyrus (STG), encompassing three subpeaks in the left middle temporal gyrus, the left supramarginal gyrus and left superior temporal cortex (Fig 3A and Table 2). Post-hoc tests found that left OFC to left STG connectivity increased significantly in the FO group (t = 4.72, p=0.0001) and decreased significantly in the placebo group (t = 3.66, p=0.0019)(Fig. 3C). A significant group by time interaction was also found for functional connectivity between the right AMY (seed) and right inferior temporal gyrus (ITG), encompassing two subpeaks at right fusiform gyrus and right inferior temporal gyrus (Fig. 3B and Table 2). Post-hoc tests found that right AMY to right ITG connectivity decreased significantly in the FO group (t = 3.72, p=0.0014) and increased significantly in the placebo group (t = 7.02, p<0.0001)(Fig. 3D). Among all participants OFC-STG and AMY-ITG functional connectivity were inversely correlated at baseline (r = −0.34, p=0.033) and endpoint (r = −0.32, p=0.05). There were no significant effects of age or sex on OFC-STG or AMY-ITG FC changes (Fig. S1 and Fig. S2).
Figure 3.
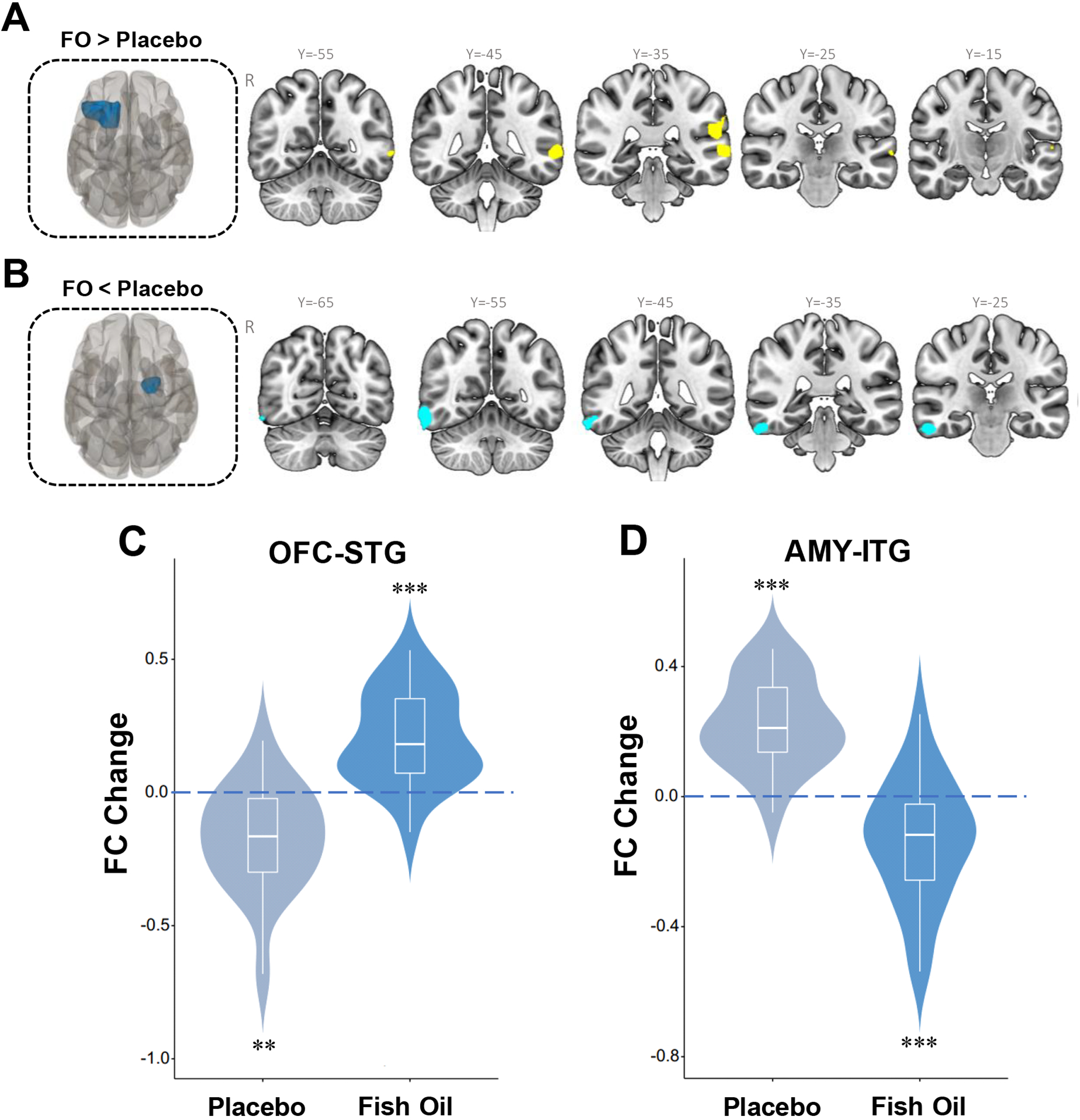
Significant group by time interactions for change in functional connectivity between the left OFC (seed, red) and the left superior temporal gyrus (STG) (cluster size = 563 voxels, p<0.001, FWE corrected)(A), and between the right AMY (seed, red) and right inferior temporal gyrus (ITG) (cluster size = 573 voxels, p<0.001, FEW corrected)(B). Significant bidirectional baseline-endpoint changes in left OFC-STG (C) and right AMY-ITG (D) functional connectivity within both the placebo and FO groups. **P≤0.001, ***P≤0.0001 within group baseline vs. endpoint.
Table 2.
Group-Wise Differences in Functional Connectivity Changes
| Seeds | Contrasts | Clusters | Cluster size (voxels) | Cluster FWE-corrected p-value | Peak coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left OFG | FO > Placebo | Left STG | 563 | 0.005 | −66 | −42 | 2 |
| Left SMG | −58 | −36 | 20 | ||||
| Left PAC | −62 | −18 | 6 | ||||
| Right AMY | FO < Placebo | Right ITG | 573 | 0.004 | 62 | −56 | −16 |
| Right ITG | 58 | −30 | −22 | ||||
Coordinates are in Montreal Neurological institute (MNI) space.
FWE, family-wise error; ITG, inferior temporal gyrus; STF, superior temporal gyrus; STG; SMG, supramarginal gyms; PAC, primary auditory cortex.
3.5. Associations with symptom ratings
Among all participants at baseline, left OFC-STG and right AMY-ITG functional connectivity were not significantly correlated with symptom ratings. Baseline left OFC-STG and right AMY-ITG functional connectivity were not significantly correlated with baseline-endpoint changes in symptom ratings in either treatment group, and there were no significant interactions. Baseline-endpoint change in left OFC-STG functional connectivity was not correlated with changes in symptom ratings in either treatment group. The decrease in right AMY-ITG functional connectivity was correlated with decreases in CDRS-R scores in the FO group (r = +0.44, p=0.04) but not the placebo group (r = +0.04, p=0.88), and the interaction was not statistically significant (z = −1.24, p=0.216) (Fig. 4A). The decrease in right AMY-ITG functional connectivity was not correlated with decreases in YMRS scores in either treatment group and the interaction was not significant (p>0.05). The decrease in right AMY-ITG functional connectivity was correlated and decreases in CGI-S scores in the FO group (r = +0.54, p=0.011) but not the placebo group (r = −0.39, p=0.11), and the interaction was significant (z = −2.91, p=0.0037) (Fig. 4B).
Figure 4.
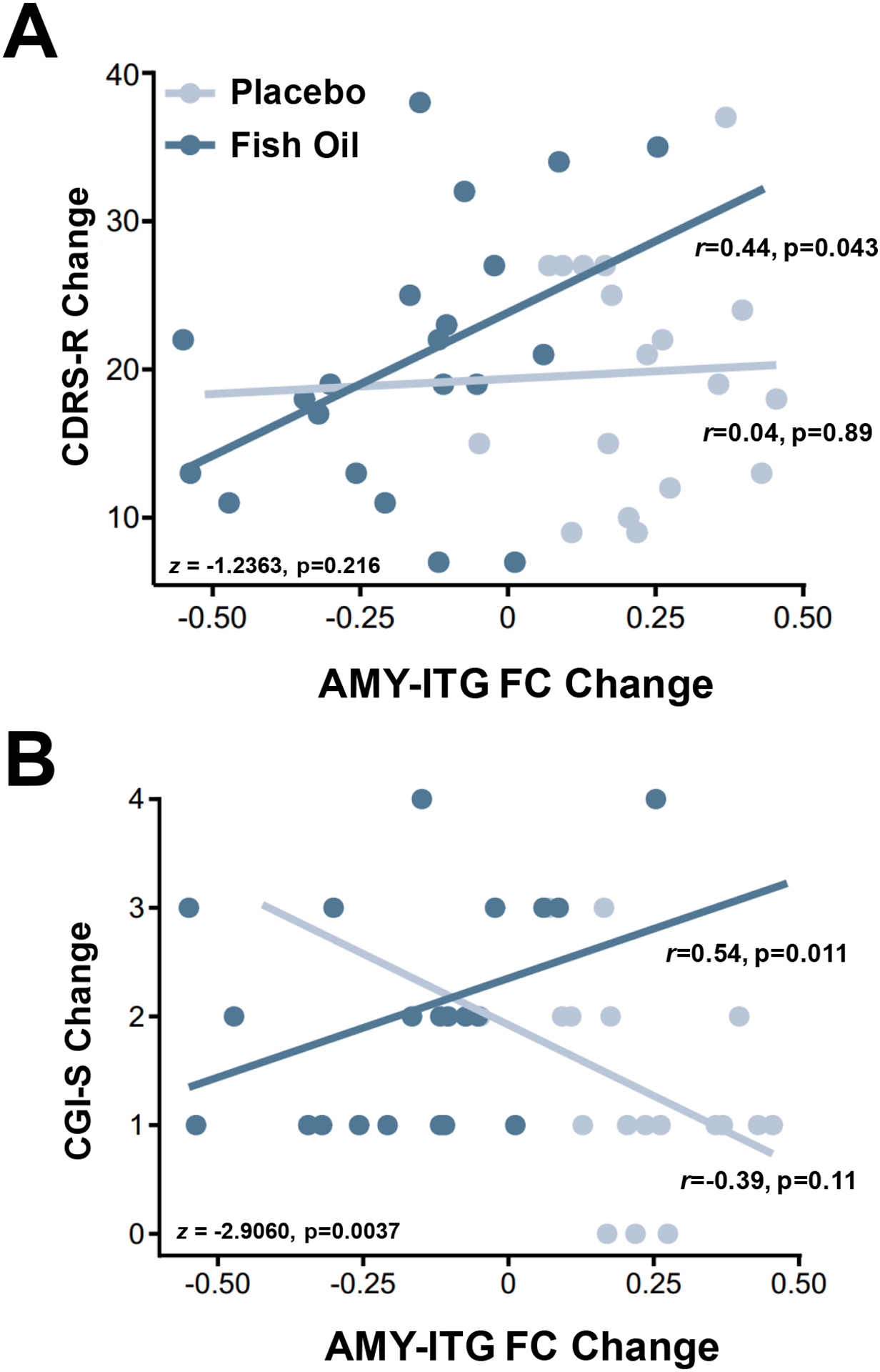
Correlations between baseline-endpoint change in AMY-ITG functional connectivity and baseline-endpoint change in CDRS-R (A) and CGI-S (B) total scores in placebo and FO groups. Within group correlations and group interaction terms are presented.
4. DISCUSSION
This study investigated whether FO supplementation altered emotion-generated corticolimbic network functional connectivity in depressed youth at high risk of developing bipolar disorder. The primary findings were that OFC-STG connectivity increased in the FO group and decreased in the placebo group, and AMY-ITG connectivity decreased in the FO group and increased in the placebo group. Moreover, among patients randomized to FO, but not to placebo, the baseline-endpoint decrease in AMY-ITG functional connectivity correlated with decreases in CDRS-R and CGI-S scores. Together, these results provide novel evidence that increasing n-3 PUFA biostatus can alter emotion-generated corticolimbic functional connectivity in depressed high-risk youth, and that FO-induced alterations in AMY-ITG functional connectivity are associated with changes in symptom severity ratings.
A primary finding was that FO supplementation produced robust bidirectional changes in emotion-generated functional connectivity, significantly increasing left OFC-STG functional connectivity and significantly decreasing right AMY-ITG functional connectivity. Importantly, non-human primate studies provide evidence for both AMY-ITG62,63 and OFC-STG64,65 structural connectivity. Human AMY-ITG connectivity is mediated by the superior longitudinal fasciculus66 and OFC-STG connectivity by the uncinate fasciculus67 white matter tracts. OFC-STG and AMY-ITG functional connectivity were inversely correlated at baseline and endpoint, suggesting these two systems have opposing effects on emotional processing. The bidirectional change observed following FO supplementation is consistent with an increase in ‘top-down’ (OFC-STG), and a decrease in ‘bottom-up’ (AMY-ITG), neural systems involved in the perception and appraisal of emotional stimuli, respectively.68 Furthermore, the integration and storage of these emotional representations may occur in associated subregions of the temporal cortex.69 Changes in OFC-STG and AMY-ITG functional connectivity were left and right lateralized, respectively, which is congruent with prior studies finding that emotion-generated corticolimbic functional connectivity is lateralized.15,16 Increased OFC-STG functional connectivity following FO supplementation is generally consistent with the observation that feeding FO-fortified diet during development increased resting-state functional connectivity between the anterior insula (seed) and superior temporal sulcus compared with a n-3 PUFA deficient diet in non-human primates.49 It is also notable that a meta-analysis found that adolescents with MDD exhibit decreased resting-state functional connectivity in the right AMY-ITG compared with healthy adolescents as well as adult MDD patients.26 Additional studies to evaluate the effects of FO supplementation on resting-state functional connectivity in these networks are warranted.
An unanticipated finding was that the placebo group exhibited baseline-endpoint changes in OFC-STG and AMY-ITG functional connectivity that were opposite to those observed in the FO group. One potential explanation for this is that fatty acids in the olive oil placebo, primarily saturated fatty acids (stearic acid, palmitic acid) and the monounsaturated fatty acid oleic acid, had neurophysiological effects.70–72 For example, a preliminary resting-state MRI study found that feeding a diet enriched with saturated fatty acids, but not a diet enriched with monounsaturated fatty acids, for 12 weeks decreased hippocampal and inferior parietal cortex activity in healthy human subjects.73 Although supplementation with olive oil did not significantly alter any erythrocyte fatty acid, including saturated and monosaturated fatty acids,51 the direction of the changes in EPA+DHA and the AA/(EPA+DHA) ratio were opposite to those observed in the FO group. The present findings therefore provide additional evidence that dietary enrichment with saturated and monounsaturated fatty acids may significantly alters emotional-generated corticolimbic functional connectivity, and that the pattern of change is opposite to that produced by FO supplementation. However, it is also possible that the changes observed in the placebo group were due to test-retest effects including habituation to the task.
A second objective was to assess whether changes in emotion-generated network connectivity following FO supplementation correlated with changes in symptom severity ratings. No significant associations were found between changes in OFC-STG connectivity and symptom ratings in either treatment group. We did however observe significant associations between changes in AMY-ITG functional connectivity and CDRS-R and CGI-S ratings within the FO group. Interestingly, a prior study found that lower resting-state AMY-ITG functional connectivity was associated with greater depression symptom severity (BDI scores) in adult MDD patients.23 Although we did not observe significant correlations within the placebo group, a significant group interaction was observed for associations with changes in CGI-S ratings, which decreased to a greater extent in the FO group. However, the present findings suggest that the opposite changes in emotion-generated AMY-ITG and OFC-STG functional connectivity observed in placebo and FO groups were both associated with similar and significant reductions in CDRS-R scores. Therefore, additional studies will be required to better understand how the directionality of these changes impact symptom ratings.
This study has several notable limitations. First, the sample size was relatively small and larger studies to replicate the current findings are warranted. Second, healthy subjects were not included to evaluate whether the observed changes were in the direction of typically developing youth. Third, the duration of FO supplementation was relatively short (12 weeks), and more robust changes in functional connectivity in other regions may emerge following longer treatment. Fourth, as discussed, the placebo oil contained fatty acids that have neurophysiological effects,73 and future imaging studies should employ a fatty acid-free placebo. Fifth, individual differences in arousal elicited by the emotional images were not measured the present study. Study strengths include a well-characterized cohort of antidepressant-free youth with depression and a familial risk for bipolar disorder, the randomized double-blind placebo-controlled study design, and fMRI seed-to-voxel assessment of emotion-generated corticolimbic functional connectivity.
We present novel evidence that increasing n-3 PUFA biostatus through FO supplementation alters emotion-generated functional connectivity within corticolimbic networks of antidepressant-free high-risk youth with depression. The results further indicate that the placebo oil induced changes in OFC-STG and AMY-ITG functional connectivity that were opposite to those observed in the FO group. Additionally, decreases in AMY-ITG functional connectivity following FO supplementation correlated with decreases in symptom severity ratings. These preliminary findings encourage additional research into whether FO supplementation initiated prior to the onset of mood symptoms can abrogate abnormalities in corticolimbic functional connectivity as well as progressive mood dysregulation in high-risk youth.
Supplementary Material
Acknowledgement
This trial was supported in part by R34 NIH/NIMH grant MH083924 to R.K.M and M.P.D (Co-PIs); NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors thank the Inflammation Research Foundation, Marblehead, MA USA for providing the fish oil and placebo capsules.
Disclosures
R.K.M. has received research support from Martek Biosciences Inc, Royal DSM Nutritional Products, LLC, Inflammation Research Foundation, Ortho-McNeil Janssen, AstraZeneca, Eli Lilly, NARSAD, and national institutes of health (NIH), and previously served on the scientific advisory board of the Inflammation Research Foundation. J.R.S. has received research support from Edgemont, Shire, Neuronetics, Otsuka, Allergan and NIH and received material support from and served as a consultant to Assurex/Genesight. He receives royalties from Springer Publishing and UpToDate and has received honoraria from CMEology and Current Psychiatry. M.P.D. receives research support from NIH, PCORI, Acadia, Allergan, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, and Sunovion. She is also a consultant, on the advisory board, or has received honoraria for speaking for Alkermes, Allergan, Assurex, CMEology, Janssen, Johnson and Johnson, Lundbeck, Myriad, Neuronetics, Otsuka, Pfizer, Sunovion, and Supernus. LRP receives research support from ACAAP, PCORI, Acadia, Allergan, Janssen, Johnson and Johnson, Lundbeck, Otsuka, Pfizer, and Sunovion. The remaining authors do not have disclosures.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Perlis RH, Dennehy EB, Miklowitz DJ, Delbello MP, Ostacher M, Calabrese JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, Sachs G. Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord. 2009;11:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correll CU, Hauser M, Penzner JB, Auther AM, Kafantaris V, Saito E, Olvet D, Carrión RE, Birmaher B, Chang KD, DelBello MP, Singh MK, Pavuluri M, Cornblatt BA. Type and duration of subsyndromal symptoms in youth with bipolar I disorder prior to their first manic episode. Bipolar Disord. 2014;16:478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000;39:1245–1252. [DOI] [PubMed] [Google Scholar]
- 4.Howes OD, Lim S, Theologos G, Yung AR, Goodwin GM, McGuire P. A comprehensive review and model of putative prodromal features of bipolar affective disorder. Psychol Med. 2011;41:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010;126:1–13. [DOI] [PubMed] [Google Scholar]
- 6.Gerber AJ, Peterson BS, Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. [DOI] [PubMed] [Google Scholar]
- 9.Petanjek Z, Judaš M, Šimic G, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–954. [DOI] [PubMed] [Google Scholar]
- 11.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albaugh MD, Nguyen TV, Ducharme S, Collins DL, Botteron KN, D’Alberto N, Evans AC, Karama S, Hudziak JJ. Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol. 2017;124:133–140. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. [DOI] [PubMed] [Google Scholar]
- 15.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M, Kujawa A, Lu LH, Fitzgerald DA, Klumpp H, Fitzgerald KD, Monk CS, Phan KL. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum Brain Mapp. 2016;37:1684–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducharme S, Albaugh MD, Hudziak JJ, et al. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albaugh MD, Nguyen TV, Ducharme S, Collins DL, Botteron KN, D’Alberto N, Evans AC, Karama S, Hudziak JJ. Age-related volumetric change of limbic structures and subclinical anxious/depressed symptomatology in typically developing children and adolescents. Biol Psychol. 2017;124:133–140. [DOI] [PubMed] [Google Scholar]
- 19.Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. [DOI] [PubMed] [Google Scholar]
- 20.Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, Simmons AN, Yang TT. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 20171;207:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng W, Rolls ET, Qiu J, Xie X, Lyu W, Li Y, Huang CC, Yang AC, Tsai SJ, Lyu F, Zhuang K, Lin CP, Xie P, Feng J. Functional connectivity of the human amygdala in health and in depression. Soc Cogn Affect Neurosci. 2018;13:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Pu W, Wu G, Zhao J, Xue Z. Abnormal resting-state cerebral-limbic functional connectivity in bipolar depression and unipolar depression. BMC Neurosci. 2019;20:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang S, Lu L, Zhang L, Hu X, Bu X, Li H, Hu X, Gao Y, Zeng Z, Gong Q, Huang X. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine. 2018;36:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. [DOI] [PubMed] [Google Scholar]
- 29.Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, Lee JH, Welge JA, DelBello MP, Fleck DE, Adler CM. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messamore E, Almeida DM, Jandacek RJ, McNamara RK. Polyunsaturated fatty acids and recurrent mood disorders: Phenomenology, mechanisms, and clinical application. Prog Lipid Res. 2017;66:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak A, Galvano F. Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies. J Affect Disord. 2016;205:269–281. [DOI] [PubMed] [Google Scholar]
- 33.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213. [DOI] [PubMed] [Google Scholar]
- 34.Peet M. International variations in the outcome of schizophrenia and the prevalence of depression in relation to national dietary practices: an ecological analysis. Br J Psychiatry. 2004;184:404–408. [DOI] [PubMed] [Google Scholar]
- 35.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160:2222–2227. [DOI] [PubMed] [Google Scholar]
- 36.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. [DOI] [PubMed] [Google Scholar]
- 37.McNamara RK, Welge JA. Meta-analysis of erythrocyte polyunsaturated fatty acid biostatus in bipolar disorder. Bipolar Disord. 2016;18:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara RK, Jandacek R, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, DelBello MP, Strakowski SM. First-episode bipolar disorder is associated with erythrocyte membrane docosahexaenoic acid deficits: Dissociation from clinical response to lithium or quetiapine. Psychiatry Res. 2015;230:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNamara RK, Jandacek R, Tso P, Blom TJ, Welge JA, Strawn JR, Adler CM, Strakowski SM, DelBello MP. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: a candidate prodromal risk biomarker. Early Interv Psychiatry. 2016;10:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 20147;9:e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhé HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. 2016;6:e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. [DOI] [PubMed] [Google Scholar]
- 44.Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull. 2001;56:79–85. [DOI] [PubMed] [Google Scholar]
- 45.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbone BE, Abouleish M, Watters KE, Vogel S, Ribic A, Schroeder OH, Bader BM, Biederer T. Synaptic connectivity and cortical maturation are promoted by the ω-3 fatty acid docosahexaenoic acid. Cereb Cortex. 2020;30:226–240. [DOI] [PubMed] [Google Scholar]
- 47.de Velasco PC, Mendonça HR, Borba JM, Andrade da Costa BL, Guedes RC, Navarro DM, Santos GK, Faria-Melibeu Ada C, Campello Costa P, Serfaty CA. Nutritional restriction of omega-3 fatty acids alters topographical fine tuning and leads to a delay in the critical period in the rodent visual system. Exp Neurol. 2012;234:220–229. [DOI] [PubMed] [Google Scholar]
- 48.Moreira JD, Knorr L, Ganzella M, Thomazi AP, de Souza CG, de Souza DG, Pitta CF, Mello e Souza T, Wofchuk S, Elisabetsky E, Vinadé L, Perry ML, Souza DO. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochem Int. 2010;56:753–759. [DOI] [PubMed] [Google Scholar]
- 49.Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci. 2014;34:2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNamara RK, Almeida DM. Omega-3 polyunsaturated fatty acid deficiency and progressive neuropathology in psychiatric disorders: A review of translational evidence and candidate mechanisms. Harv Rev Psychiatry. 2019;27:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNamara RK, Strawn JR, Tallman MJ, Welge JA, Patino LR, Blom T, DelBello MP. Effects of fish oil monotherapy on depression and prefrontal neurochemistry in adolescents at high risk for bipolar I disorder: A 12-week placebo-controlled 1H MRS trial. J Child Adolesc Psychopharmacol. 2020;30:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C: Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry 40:450–455, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Poznanski EO, Cook SC, Carroll BJ: A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 55.Poznanski EO, Cook SC, Carroll BJ, Corzo H: Use of the Children’s Depression Rating Scale in an inpatient psychiatric population. J Clin Psychiatry. 1983;44:200–203. [PubMed] [Google Scholar]
- 56.First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV). New York State, 1996. [Google Scholar]
- 57.Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 58.Guy W: National Institute of Mental Health. In: ECDEU Assessment Manual for Psychopharmacology. Revised 12-1-12-6, 1970. [Google Scholar]
- 59.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. 2012;2:125–141. [DOI] [PubMed] [Google Scholar]
- 60.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. NeuroImage. 2014;96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diedenhofen B, Musch JA. Comprehensive solution for the statistical comparison of correlations. PLoS ONE. 2015;10:e0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iwai E, Yukie M, Suyama H, Shirakawa S. Amygdalar connections with middle and inferior temporal gyri of the monkey. Neurosci Lett. 1987;83:25–29. [DOI] [PubMed] [Google Scholar]
- 63.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984;230:465–496. [DOI] [PubMed] [Google Scholar]
- 64.Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. [DOI] [PubMed] [Google Scholar]
- 65.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. [DOI] [PubMed] [Google Scholar]
- 66.Rizzo G, Milardi D, Bertino S, Basile GA, Di Mauro D, Calamuneri A, Chillemi G, Silvestri G, Anastasi G, Bramanti A, Cacciola A. The limbic and sensorimotor pathways of the human amygdala: A structural connectivity study. Neuroscience. 2018;385:166–180. [DOI] [PubMed] [Google Scholar]
- 67.Bhatia K, Henderson L, Yim M, Hsu E, Dhaliwal R. Diffusion tensor imaging investigation of uncinate fasciculus anatomy in healthy controls: Description of a subgenual stem. Neuropsychobiology. 2017;75:132–140. [DOI] [PubMed] [Google Scholar]
- 68.Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gabrieli JD, Gross JJ. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. 2013;8:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dumas JA, Bunn JY, Nickerson J, Crain KI, Ebenstein DB, Tarleton EK, Makarewicz J, Poynter ME, Kien CL. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism. 2016;65:1582–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kien CL, Bunn JY, Tompkins CL, Dumas JA, Crain KI, Ebenstein DB, Koves TR, Muoio DM. Substituting dietary monounsaturated fat for saturated fat is associated with increased daily physical activity and resting energy expenditure and with changes in mood. Am J Clin Nutr. 2013;97:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kien CL, Bunn JY, Stevens R, Bain J, Ikayeva O, Crain K, Koves TR, Muoio DM. Dietary intake of palmitate and oleate has broad impact on systemic and tissue lipid profiles in humans. Am J Clin Nutr. 2014;99:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sartorius T, Ketterer C, Kullmann S, Balzer M, Rotermund C, Binder S, Hallschmid M, Machann J, Schick F, Somoza V, Preissl H, Fritsche A, Häring HU, Hennige AM. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes. 2012;61:1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


