Abstract
AIM
To report the etiologies, risk factors, treatments, and outcomes of infectious keratitis (IK) at a major Vietnamese eye hospital.
METHODS
This is a retrospective review of all cases of IK at Vietnam National Eye Hospital (VNEH) in Hanoi, Vietnam. Medical histories, demographics, clinical features, microbiological results, and treatment outcomes were reviewed.
RESULTS
IK was diagnosed in 1974 eyes of 1952 patients, with ocular trauma being the greatest risk factor for IK (34.2%), frequently resulting from an agriculture-related injury (53.3%). The mean duration between symptom onset and presentation to VNEH was 19.3±14.4d, and 98.7% of patients had been treated with topical antibiotic and/or antifungal agents prior to evaluation at VNEH. Based on smear results of 1706 samples, the most common organisms identified were bacteria (n=1107, 64.9%) and fungi (n=1092, 64.0%), with identification of both bacteria and fungi in 614 (36.0%) eyes. Fifty-five of 374 bacterial cultures (14.7%) and 426 of 838 fungal cultures (50.8%) were positive, with the most commonly cultured pathogens being Pseudomonas aeruginosa, Streptococcus pneumonia, Fusarium spp., and Aspergillus spp. Corneal perforation and descemetocele developed in 391 (19.8%) and 93 (4.7%) eyes, respectively. Medical treatment was successful in resolving IK in 50.4% eyes, while 337 (17.1%) eyes underwent penetrating or anterior lamellar keratoplasty. Evisceration was performed in 7.1% of eyes, most commonly in the setting of fungal keratitis.
CONCLUSION
Ocular trauma is a major risk factor for IK in Vietnam, which is diagnosed in almost 400 patients each year at VNEH. Given this, and as approximately one quarter of the eyes that develop IK require corneal transplantation or evisceration, greater emphasis should be placed on the development of prevention and treatment programs for IK in Vietnam.
Keywords: infectious keratitis, bacterial keratitis, fungal keratitis, microsporidial keratitis, Acanthamoeba keratitis, herpes simplex virus keratitis, Vietnam
INTRODUCTION
Infectious keratitis (IK) is a vision-threatening corneal condition that is a major public health concern in tropical and developing countries, particularly within South, Southeast, and East Asia[1]–[2]. Even with optimal treatment regimens, some cases of severe keratitis can result in permanent blindness or loss of the eye. IK is clinically characterized by the presence of corneal ulceration with or without stromal infiltration, and frequently leads to corneal scarring, the fourth leading cause of blindness worldwide[3]. Complications of IK, including corneal perforation, persistent infection, and corneal scarring, are leading indications for corneal transplantation in many developing countries in Asia[4]–[5].
It is difficult to determine a precise estimate of the global burden of IK, other than to suggest that it disproportionately affects poor, rural populations. Epidemiological studies have reported the incidence of IK to range from 3.6[6] to 52.1[7] per 100 000 people in North American and European countries versus 6.3[8] to 799[9] per 100 000 people in Asian countries. However, the true incidence of IK in Asia may be higher, particularly in economically less developed countries where data is currently lacking.
The demographic profile and clinical features of IK vary significantly based on geographic region[10]. The Asia Cornea Society Infectious Keratitis Study (ASCIKS) surveyed IK in senven Asian countries (India, China, Japan, South Korea, Thailand, the Philippines, and Singapore) and reported demographics, risk factors, organisms, and outcomes of IK in each of these countries[10]. Though the ASCIKS has provided important insights into IK in Asia, it includes a minority of Asian countries, and thus data from other countries in Asia, especially those that are historically underrepresented in such multinational studies, is needed. Currently, there is no published data regarding IK in Vietnam. Therefore, the purpose of this study is to report the risk factors for, organisms associated with, treatments for and outcomes of nearly 2000 cases of IK managed at the Vietnam National Eye Hospital (VNEH) during a 5-year period.
SUBJECTS AND METHODS
Ethical Approval
This is a retrospective study approved by the VNEH Research and Ethics Committee. Medical records of all patients who were treated at VNEH from January 1, 2013 to December 31, 2017 and diagnosed with any type of IK, including bacterial, fungal, viral, microsporidial, and Acanthamoeba keratitis, were reviewed.
All specimens for smear and culture were obtained by scraping the edge of a corneal infiltrate using a small sterilized curette or Kimura spatula at the slit lamp. If there was no infiltrate, a corneal biopsy or anterior chamber (AC) tap was performed to obtain a specimen. If a keratoplasty was performed following the diagnosis of IK, the excised corneal tissue was used as a specimen for smear and culture. Specimens were prepared using Gram's staining and 10% potassium hydroxide. Subsequent scrapings were inoculated directly onto various culture media for isolation of bacteria (blood and chocolate agar) and fungi (sabouraud dextrose agar). All bacterial media were incubated in 5% CO2, whereas fungal media were stored in a 25°C-30°C incubator. Herpes simplex virus (HSV) keratitis was diagnosed based on the medical history (i.e., prior history of HSV infection) and exam (decreased corneal sensation, dendritic epithelial lesions, geographic ulceration, etc). Cytology and polymerase chain reaction (PCR) were performed in selected cases.
For each patient, the following data were obtained from medical records: demographics, duration of symptoms, details of past and concurrent ocular and systemic diseases, history of ocular trauma, contact lens wear, and prior ocular medications. Clinical exam findings evaluated included visual acuity (VA), infiltrate characteristics (appearance, location, dimensions, depth), presence of a hypopyon, and associated anterior segment findings. Intraocular pressure and lens and fundus exam results were included when available.
Etiologies of IK were determined based on microbiology results and treatment outcomes. If smears and/or cultures from an eye demonstrated a bacterium and another microorganism (i.e., fungus, virus, Acanthamoeba or microsporidia), the other organism was considered to be the pathogenic organism for classification purposes. In cases of negative or lack of microbiologic results, patients were treated empirically, and the causative agent of the patient's IK was identified based on response to therapy or clinical impression.
Bacterial keratitis was treated with a topical antibiotic, typically a fluoroquinolone. In severe cases of bacterial infection, patients were initially treated with combined fortified cefazolin (33 mg/mL) and gentamycin (14 mg/mL) eye drops after corneal scraping. The treatment regimen was subject to modification according to the patient's clinical response and microbiological results. Natamycin 5% or amphotericin B 0.15% was used for patients with suspected or culture-proven fungal keratitis and acyclovir 3% ointment was prescribed for patients with suspected or confirmed HSV keratitis. In the case of Acanthamoeba or microsporidial keratitis, both a fluoroquinolone antibiotic eye drop and an antifungal eye drop were prescribed. For keratitis complicated by impending corneal perforation, actual perforation, and/or scleral or intraocular extension, systemic antibiotics were commonly prescribed. Therapeutic keratoplasty was performed if the IK was unresponsive to at least 2mo of medical treatment, in the setting of an impending perforation (descemetocele) and following development of a corneal perforation. Evisceration was performed following corneal perforation if donor corneal tissue was unavailable, following spread of infection to the vitreous cavity or sclera, and following extrusion of the intraocular contents.
Statistical Analysis
Statistical analyses were performed using SPSS v, 20.0 software (IBM Corp., Armonk, NY, USA), with P<0.05 considered to be statistically significant.
RESULTS
Demographics
IK was diagnosed in 1974 eyes of 1952 patients, twenty-two of whom presented with bilateral IK. Of the 1952 patients diagnosed with IK, significantly more were male (1056, 54.1%) than female (896 patients, 45.9%; P<0.05). The mean age was 54.3±16.6y (range 2-98y), with the majority of patients (61.2%) between 18 and 60y. The majority of patients lived in rural regions (84.1%) and were farmers (66.3%). The mean time between onset of symptoms and presentation to the hospital was 19.3±14.4d. More than half of patients (52.7%) presented to the hospital within 14d after symptom onset, whereas 15.4% of patients presented to the hospital more than 30d after symptom onset (Table 1).
Table 1. Characteristics of patients with infectious keratitis at VNEH.
| Patients | FK | BK | HK | MK | AK | All |
| Occupation | n=1111 | n=486 | n=211 | n=78 | n=66 | n=1952 |
| Farmer | 779 (70.1) | 300 (61.7) | 120 (56.9) | 54 (69.2) | 38 (57.6) | 1294 (66.3) |
| Industrial worker | 109 (9.8) | 58 (11.9) | 19 (9.0) | 0 | 7 (10.6) | 191 (9.8) |
| Businessperson | 44 (4.0) | 57 (11.7) | 11 (5.2) | 0 | 19 (28.8) | 131 (6.7) |
| Other | 179 (16.1) | 71 (14.6) | 61 (28.9) | 24 (30.8) | 2 (3.0) | 336 (17.2) |
| Risk factors (eyes) | n=1115 | n=486 | n=226 | n=81 | n=66 | n=1974 |
| Trauma | 436 (39.1) | 174 (35.8) | 18 (8.0) | 22 (27.2) | 26 (39.4) | 676 (34.2) |
| Corneal disease | 45 (4.0) | 22 (4.5) | 45 (19.9) | 19 (23.2) | 4 (6.1) | 135 (6.8) |
| Diabetes | 37 (3.3) | 8 (1.6) | 7 (3.1) | 4 (4.9) | 0 | 57 (2.9) |
| Topical corticosteroid use | 3 (0.3) | 1 (0.2) | 54 (23.9) | 2 (2.5) | 0 | 60 (3.0) |
| Entropion, trichiasis, and/or lagophthalmos | 21 (1.9) | 30 (6.2) | 3 (1.3) | 2 (2.5) | 1 (1.5) | 57 (2.9) |
| Contact lens wear | 0 | 3 (0.6) | 1 (0.4) | 0 | 24 (36.4) | 28 (1.4) |
| Prior corneal surgery | 9 (0.8) | 7 (1.4) | 6 (2.7) | 2 (2.5) | 1 (1.5) | 25 (1.3) |
| Graves disease | 11 (1.0) | 1 (0.2) | 0 | 0 | 12 (0.6) | |
| Other systemic diseases (arthritis, bronchitis, etc.) | 16 (1.4) | 4 (0.8) | 5 (2.2) | 0 | 0 | 25 (1.3) |
| Unreported | 541 (48.5) | 234 (48.1) | 87 (38.5) | 30 (37.0) | 9 (13.6) | 902 (45.7) |
| Prior treatment (eyes) | n=1115 | n=486 | n=226 | n=81 | n=66 | n=1974 |
| Antibiotic (topical, oral or both) | 305 (27.4) | 198 (40.7) | 63 (27.9) | 24 (29.6) | 19 (28.8) | 609 (30.9) |
| Antifungal (topical, oral or both) | 331 (29.7) | 18 (3.7) | 13 (5.8) | 39 (48.1) | 5 (7.6) | 406 (20.6) |
| Antiviral (topical, oral or both) | 17 (1.5) | 9 (1.9) | 72 (31.9) | 9 (11.1) | 4 (6.1) | 111 (5.6) |
| Corticosteroid | 28 (2.5) | 9 (1.9) | 6 (2.7) | 2 (2.5) | 1 (1.5) | 46 (2.3) |
| Unknown | 513 (46.0) | 270 (55.6) | 74 (32.7) | 30 (37.0) | 36 (54.5) | 923 (46.8) |
| None | 12 (1.1) | 4 (0.8) | 7 (3.1) | 1 (1.2) | 1 (1.5) | 25 (1.3) |
FK: Fungal keratitis; BK: Bacterial keratitis; HK: Herpes simplex viral keratitis; MK: Microsporidial keratitis; AK: Acanthamoeba keratitis; IK: Infectious keratitis.
n (%)
Risk Factors
The 1070 of the 1974 eyes (54.2%) had one or more predisposing risk factor(s) for IK (Table 1). The most frequently identified risk factor was ocular trauma (676 cases, 34.2%), which included trauma secondary to agricultural (360 cases, 53.3%), industrial (128 cases, 18.9%) and housework-related (188 cases, 27.8%) injuries.
Prior Treatment
One eye might be treated with more than one kind of eye drop. A total of 1126 eyes had been treated with topical and/or systemic antimicrobial therapy prior to referral to VNEH, most commonly antibiotic (30.9%) and antifungal (20.6%) drugs, while 46.8% of eyes were treated with an unknown medication before evaluation at VNEH (Table 1).
Microbiologic Profile
Corneal scraping and smear were performed in 1706 (86.4%) eyes. Bacteria (n=1107, 64.9%) and fungi (n=1092, 64.0%) were the most commonly identified pathogens on smear. In 640 (37.5%) eyes, two different classes of microbes were isolated from a single smear, most commonly a bacterium and a fungus [614 (36.0%) eyes; Table 2].
Table 2. Pathogens isolated from smears of eyes diagnosed with infectious keratitis at VNEH.
| Pathogens | No. of positive smears (n) | % of smears |
| Bacteria | ||
| GPC | 268 | 15.7 |
| GNC | 11 | 0.6 |
| GPR | 6 | 0.4 |
| GNR | 165 | 9.7 |
| GPC & GNR | 15 | 0.9 |
| GPR & GNR | 2 | 0.1 |
| Fungi | ||
| Filamentous | 478 | 28 |
| Yeast | 0 | 0 |
| Fungus & bacterium | 614 | 36 |
| Acanthamoeba | 54 | 3.2 |
| Acanthamoeba & bacterium | 12 | 0.7 |
| Microsporidium | 67 | 3.9 |
| Microsporidium & bacterium | 14 | 0.8 |
GPC: Gram positive cocci; GNC: Gram negative cocci; GPR: Gram positive rod; GNR: Gram negative rod.
n=1706
Cultures were performed for 1212 of the 1974 eyes diagnosed with IK, with positive results in 481 (39.7%). A significantly higher percentage of fungal cultures were positive compared to bacterial cultures [50.8% (426/838) vs 14.7% (55/374); P<0.05], with Fusarium being the most commonly cultured organism (Table 3).
Table 3. Pathogens isolated from cultures of eyes diagnosed with infectious keratitis at VNEH.
| Pathogen | No. of positive cultures (n) | % of positive bacterial or fungal cultures |
| Bacteria | n=55 | |
| Pseudomonas aeruginosa | 21 | 38.2 |
| Streptococcus pneumonia | 16 | 29.1 |
| Klebsiella pneumonia | 4 | 7.3 |
| Corynebacterium | 3 | 5.5 |
| Nocardia | 3 | 5.5 |
| Staphylococcus aureus | 2 | 3.6 |
| Staphylococcus epidermis | 2 | 3.6 |
| Bacillus spp | 2 | 3.6 |
| Moraxella | 2 | 3.6 |
| Fungi | n=426 | |
| Fusarium | 169 | 39.7 |
| Aspergillus | 94 | 22.1 |
| Cylindrocarpon | 27 | 6.3 |
| Cephalosporium | 21 | 4.9 |
| Curvularia | 16 | 3.8 |
| Penicillium | 2 | 0.5 |
| Unidentified filamentous fungi | 97 | 22.8 |
Clinical Diagnosis of Infectious Keratitis
In 42 cases of IK, microbiologic results were negative and thus patients were treated empirically. Based on clinical features and response to therapy, 23 of these cases were diagnosed as fungal and 19 were diagnosed as bacterial.
Final Diagnosis of Infectious Keratitis
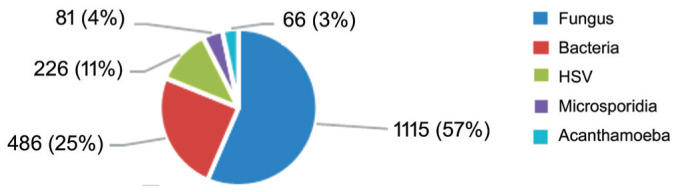
Based on the results of the corneal smears, cultures, PCR testing, and clinical diagnosis, fungal keratitis was the most common type of IK, accounting for 57% of cases, followed by bacterial keratitis (25%), HSV keratitis (11%), microsporidial keratitis (4%) and Acanthamoeba keratitis (3%, P<0.05; Figure 1).
Figure 1. Distribution of types of infectious keratitis at Vietnam National Eye Hospital.
Clinical Features
The majority of cases involved unilateral ulcers (98.9%), whereas 22 (1.1%) cases involved bilateral ulcers. In the setting of bilateral ulceration, viral keratitis was the most common etiology, identified in 11 (50%) patients. On presentation, the majority of affected eyes had VA less than count fingers (CF) at 3 m (1838 eyes; 93.1%), with only 34 (1.7%) eyes having VA better than 20/80. The area of corneal involvement was 4 to 6 mm in diameter in 820 (42.1%) eyes and greater than 6 mm in diameter in 929 (47.1%) eyes. The 4.7% (93/1974) of eyes progressed to descemetocele formation while 19.8% (391/1974) of eyes progressed to corneal perforation, which developed most frequently in eyes with fungal keratitis (22.4%) and least frequently in eyes with Acanthamoeba keratitis (4.5%).
Stromal infiltration that progressed to involve more than two-thirds of the corneal thickness was observed in 74.9% of cases and was significantly more common in eyes with fungal keratitis than keratitis associated with non-fungal pathogens (82.2% vs 63.7%, P<0.001). Hypopyon was observed in 740 (40.6%) eyes, most commonly in eyes affected with fungal (47.1%) and bacterial (39.1%) keratitis and least commonly in eyes affected with Acanthamoeba keratitis (6.1%).
Treatment and Outcomes
The mean duration of inpatient treatment at VNEH following the diagnosis of infectious keratitis was 22.9±13.5d (range 7-98d), with the shortest duration for bacterial keratitis (21.4±12.8d) and the longest for Acanthamoeba keratitis (26.0±15.2d). Half of the IK cases were successfully treated with medical therapy alone (995/1974 eyes, 50.4%). The rate of medical treatment failure was highest in microsporidial keratitis (38.2%), followed by fungal keratitis (28.1%), and viral keratitis (11.9%). Corneal debridement was performed in 23% of affected eyes, most commonly in those affected with Acanthamoeba keratitis (39.4%). AC washout, indicated if a thick exudate was present posterior to the cornea or for diagnostic purposes, was most commonly performed for eyes with fungal keratitis (28.1%) and least commonly for eyes with Acanthamoeba keratitis (4.5%; Table 4).
Table 4. Treatments used for infectious keratitis at VNEH.
| Treatment modalities | FK (n=1115) | BK (n=486) | HK (n=226) | MK (n=81) | AK (n=66) | All IK (n=1974) |
| Medical only | 427 (38.3) | 336 (69.1) | 156 (69.0) | 36 (44.4) | 40 (60.6) | 995 (50.4) |
| Keratectomy | 327 (29.3) | 69 (14.2) | 15 (6.6) | 17 (21.0) | 26 (39.4) | 454 (23.0) |
| AC washout | 313 (28.1) | 83 (17.1) | 17 (7.5) | 9 (11.1) | 3 (4.5) | 425 (21.5) |
| AMT | 17 (1.5) | 34 (7.0) | 45 (19.9) | 3 (3.7) | 2 (3.0) | 101 (5.1) |
| Lid surgery | 13 (1.2) | 7 (1.4) | 2 (0.9) | 0 | 1 (1.5) | 23 (1.2) |
| Tarsorrhaphy | 27 (2.4) | 12 (2.5) | 5 (2.2) | 1 (1.2) | 0 | 45 (2.3) |
| PK | 192 (17.2) | 60 (12.3) | 20 (8.8) | 29 (35.8) | 6 (9.1) | 307 (15.6) |
| ALK | 6 (0.5) | 12 (2.5) | 12 (5.3) | 0 | 0 | 30 (1.5) |
| Evisceration | 112 (10.0) | 22 (4.5) | 3 (1.3) | 2 (2.5) | 2 (3.0) | 141 (7.1) |
FK: Fungal keratitis; BK: Bacterial keratitis; HK: Herpes simplex viral keratitis; MK: Microsporidial keratitis; AK: Acanthamoeba keratitis; IK: Infectious keratitis; AC: Anterior chamber; AMT: Amniotic membrane transplant; PK: Penetrating keratoplasty; ALK: Anterior lamellar keratoplasty.
n (%)
Amniotic membrane transplantation (AMT) was typically performed in the setting of a persistent epithelial defect, most commonly in cases of viral ulceration (19.9%) and least commonly in cases of fungal ulceration (1.5%). A total of 337 eyes underwent corneal transplantation: penetrating keratoplasty (PK) in 307 (15.6%) eyes and lamellar keratoplasty (LK) in 30 (1.5%) eyes. Microsporidial keratitis was associated with the highest percentage of infections requiring PK (29/81 cases, 35.8%) and HSV keratitis was associated with the highest percentage of infections requiring LK (12/226 cases, 5.3%). Evisceration was most commonly performed for fungal keratitis, which was also associated with the highest percentage of infections requiring evisceration (112/1115 eyes, 10.0%).
On the day of discharge, 1689 (87.5%) eyes had VA of CF at less than 3 m. Only 52 (2.6%) eyes had VA 20/80 or better, and 141 (7.1%) eyes were no light perception following evisceration.
DISCUSSION
We report the first large retrospective study evaluating the etiology, organisms, and management of IK in Vietnam. Many of the results that we report are consistent with those in published series of IK from other Asian countries[10]. In regard to patient demographics, the higher incidence of IK in men in Vietnam is consistent with prior reports on higher occurrences of eye injuries among men compared to women[11]. Similarly, the findings that more than half of the individuals with IK in Vietnam were between 18 and 60 years old, the age range of the working population, and that the majority of affected individuals were farmers and lived in rural regions, are consistent with demographics reported in other studies of IK in Asia[1],[12]. Additionally, our finding that ocular trauma, primarily associated with agricultural exposures, was the most common risk factor for IK in Vietnam is consistent with what was has been reported in South India (92.2%)[13], China (66.8%)[10], and Australia (43.9%)[14].
Based on smear results in our study, bacteria and fungi were the most common microbes isolated in polymicrobial infections. There are several explanations for this finding: the resident bacterial flora may be simultaneously isolated during specimen collection; given the ubiquity of fungi and bacteria in the agricultural environment, both microbes may be introduced into the corneal stroma through ocular trauma; prolonged use of topical corticosteroids in certain patients may suppress the local immune response, potentially facilitating opportunistic infections; and two organisms may establish synergistic interactions, such that the primary organism establishes a “niche” for the secondary organism to become pathogenic[15].
The relatively low percentage of bacterial cultures that were positive (14.7%), despite a clear clinical presentation of bacterial keratitis, is consistent with percentages previously reported in the United States (23.7%)[16] and Thailand (25.6%)[15]. The low percentage of positive bacterial cultures may be due to a variety of reasons, including culturing after the initiation of topical antibiotics and difficulty obtaining a sufficient corneal specimen in the setting of a corneal perforation or impending perforation. Our finding of Pseudomonas aeruginosa as the most common bacterium isolated, followed by Streptococcus pneumoniae, is consistent with prior series, not only from Asia but also from around the globe[1],[10].
Our finding that fungal keratitis is the most common type of IK in Vietnam, with a 2.3:1 ratio of fungal to bacterial keratitis, is comparable to reports from several other tropical Asian countries, including China and India[10]. Mycotic ocular infections largely predominate in regions with large agricultural and manual labor workforces, as in Vietnam, especially among individuals exposed to ocular trauma from plant material[1]. In our study, the higher incidence of fungal keratitis may also be due in part to increased referral of these cases to VNEH, a tertiary eye-care center with access to antifungal therapy, as antifungal agents are largely unavailable at provincial and district hospitals. Natamycin, which is not always commercially available in Vietnam, may debatably have limited effectiveness in treating deeper keratitis due to poor corneal penetration[17]. Given the fact that more than 80% of cases of fungal keratitis involved more than two-thirds of the corneal thickness on presentation to VNEH, the primary treatment for fungal keratitis at our hospital is topical amphotericin B 0.15% compounded in house.
The most frequently isolated fungal pathogen in this study was Fusarium species, followed by Aspergillus species. Of note, yeast-associated keratitis was not identified in our study. The ACSIKS also reported Fusarium species to be the most common cause of IK in China (30.9%) and in India (25.7%)[10], which have comparable climates and exposure risks to Vietnam. Filamentous fungi such as Fusarium and Aspergillus thrive in tropical regions like Vietnam, whereas yeast such as Candida are more common in temperate areas and are more likely to affect patients with preexisting ocular surface disease and/or predisposing systemic diseases[1],[18]–[19].
While microsporidial keratitis accounted for only 4% of IK cases in this study, the number of cases has been increasing in Vietnam since it was initially reported in 2015[5] and based on this study is currently the fourth most common cause of IK. It is necessary to raise awareness of this pathogen among ophthalmologists to facilitate earlier diagnosis and surgical intervention. If a definitive diagnosis of microsporidial keratitis can be made early in the course of disease, timely management may prevent progression of keratitis and decrease the need for therapeutic keratoplasty. The lower incidence of Acanthamoeba keratitis (3.0%) identified in our study population is consistent with that reported in other studies[20], and is likely associated with the low percentage (1.4%) of keratitis cases associated with contact lens wear in this series[21].
Half of the IK cases were successfully treated with medical therapy, consistent with the 55.0% of IK cases successfully treated with medical therapy in the ACSIKS[10]. The percentage of cases successfully treated medically was lowest for fungal keratitis, indicative of the more recalcitrant nature of fungal keratitis. The outcomes of fungal keratitis have been reported to be worse than those of bacterial keratitis, due in part to the limited efficacy of topical and systemic antifungal therapy[22].
The 17.1% of eyes that required keratoplasty in this series is similar to the percentages reported by the ACSIKS in other Asian countries: 19.6% (China), 14.9% (Philippines) and 9.5% (India)[10]. In Vietnam, China, and India, IK is the main indication for PK[2],[10],[23]–[25]. Unfortunately, these countries have limited access to corneal tissue despite high demand, with an estimated 2 million people in China and 7 million people in India currently waiting for a corneal transplantation[26]. In these situations, surgeons may opt to use lower grade corneal tissue than those used for optical transplants, perhaps contributing to the high rates of graft failure and poor visual outcomes after corneal transplantation. The insufficient supply of donor corneal tissue is a contributing factor to the higher rate of evisceration in the majority of developing Asian countries such as Vietnam, the Philippines, and Thailand compared with more developed Asian countries and regions such as Japan, Singapore, and Taiwan, China[10].
In cases of IK that did not require surgical intervention, nearly 90% of patients had a VA in the affected eye of less than CF at 3 m on the day of discharge, and less than 5% had a VA ≥20/80. Unfortunately, follow-up after discharge was limited for many of the patients, and thus long-term visual outcomes are unknown. The long-term visual outcomes in eyes requiring surgical intervention are likely even more guarded, even without inclusion of the eyes that required evisceration. Given poor short-term visual outcomes and higher rate of evisceration following IK in Vietnam than in more developed countries, there is a need to develop more effective measures to reduce corneal blindness through prevention. For example, eye protection may reduce the risk of ocular trauma, the primary cause of corneal infection in Vietnam and other developing Asian countries[10]. Antibiotic or antiseptic prophylaxis following traumatic corneal abrasions in a village setting may also be effective in preventing both bacterial and fungal keratitis[27]–[28].
Limitations of this study include an inability to accurately assess the distribution of types of IK in the general population of Vietnam, given the disproportionately large number of fungal keratitis cases referred to VNEH, a tertiary referral hospital that receives severe cases of IK that may not be representative of all cases of IK in the population. Additionally, given the retrospective nature of the study, diagnostic and treatment protocols were not standardized, and the outcome of treatment following discharge from VNEH was not systematically collected. Further studies are needed to characterize long-term outcomes of IK in Vietnam.
In conclusion, the present study found that in Vietnam, ocular trauma is the most common risk factor for IK, which is most commonly caused by fungi. The relatively high frequency of evisceration, the poor early visual outcomes and the shortage of donor corneal tissue for severe IK requiring keratoplasty indicate a need for greater emphasis on prevention and early treatment of IK in Vietnam. As part of this effort, we believe that it is imperative to increase awareness of the importance of wearing eye protection during work and other activities that are associated with an increased risk of eye injury, especially among susceptible populations.
Acknowledgments
Conflicts of Interest: Dong PN, None; Hang DTT, None; Duong NTN, None; Lien MT, None; Chen AC, None; Aldave AJ, None.
REFERENCES
- 1.Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64(3):255–271. doi: 10.1016/j.survophthal.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran TM, Duong H, Bonnet C, Kashanchi A, Buckshey A, Aldave AJ. Corneal blindness in Asia: a systematic review and meta-analysis to identify challenges and opportunities. Cornea. 2020;39(9):1196–1205. doi: 10.1097/ICO.0000000000002374. [DOI] [PubMed] [Google Scholar]
- 3.Flaxman SR, Bourne RRA, Resnikoff S, et al. Vision Loss Expert Group of the Global Burden of Disease Study Global causes of blindness and distance vision impairment 1990-2020:a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 4.Gao H, Huang T, Pan ZQ, et al. Survey report on keratoplasty in China: a 5-year review from 2014 to 2018. PLoS One. 2020;15(10):e0239939. doi: 10.1371/journal.pone.0239939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong PN, Tue DT, Thu TT. Microsporidia: the pathogen of stromal keratitis was firstly discovered in Vietnam. Journal of Military Pharmaco–Medicine. 2015;40(8) [Google Scholar]
- 6.Seal DV, Kirkness CM, Bennett HG, Peterson M, Keratitis Study Group Population-based cohort study of microbial keratitis in Scotland: incidence and features. Cont Lens Anterior Eye. 1999;22(2):49–57. doi: 10.1016/s1367-0484(99)80003-4. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 8.Lam DS, Houang E, Fan DS, Lyon D, Seal D, Wong E, Hong Kong Microbial Keratitis Study Group Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye (Lond) 2002;16(5):608–618. doi: 10.1038/sj.eye.6700151. [DOI] [PubMed] [Google Scholar]
- 9.Upadhyay MP, Karmacharya PC, Koirala S, Shah DN, Shakya S, Shrestha JK, Bajracharya H, Gurung CK, Whitcher JP. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol. 2001;85(4):388–392. doi: 10.1136/bjo.85.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khor WB, Prajna VN, Garg P, et al. ACSIKS Group The Asia cornea society infectious keratitis study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018;195:161–170. doi: 10.1016/j.ajo.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 11.Riccò M, Vezzosi L, Mezzoiuso AG. Occupational Eye Injuries in the agricultural settings: a retrospective study from North-Eastern Italy. Acta Biomed. 2020;90(4):457–467. doi: 10.23750/abm.v90i4.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh YY, Sun CC, Hsiao CH. Epidemiology and the estimated burden of microbial keratitis on the health care system in Taiwan: a 14-year population-based study. Am J Ophthalmol. 2020;220:152–159. doi: 10.1016/j.ajo.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol. 1996;3(3):159–166. doi: 10.3109/09286589609080122. [DOI] [PubMed] [Google Scholar]
- 14.Chew R, Woods ML. Epidemiology of fungal keratitis in Queensland, Australia. Clin Exp Ophthalmol. 2019;47(1):26–32. doi: 10.1111/ceo.13346. [DOI] [PubMed] [Google Scholar]
- 15.Brogden KA, Guthmiller JM. Polymicrobial diseases: current and future research. 2002. [DOI] [PubMed]
- 16.Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San francisco. Cornea. 2018;37(1):84–87. doi: 10.1097/ICO.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khames A, Khaleel MA, El-Badawy MF, El-Nezhawy AOH. Natamycin solid lipid nanoparticles–sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: preparation and optimization. Int J Nanomed. 2019;14:2515–2531. doi: 10.2147/IJN.S190502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao GL, Ling J, Wong T, Yeung SN, Iovieno A. Candida keratitis: epidemiology, management, and clinical outcomes. Cornea. 2020;39(7):801–805. doi: 10.1097/ICO.0000000000002306. [DOI] [PubMed] [Google Scholar]
- 19.Chen CA, Hsu SL, Hsiao CH, Ma DH, Sun CC, Yu HJ, Fang PC, Kuo MT. Comparison of fungal and bacterial keratitis between tropical and subtropical Taiwan: a prospective cohort study. Ann Clin Microbiol Antimicrob. 2020;19(1):11. doi: 10.1186/s12941-020-00353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting DSJ, Ho CS, Deshmukh R, Said DG, Dua HS. Correction to: infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye (Lond) 2021;35(10):2908. doi: 10.1038/s41433-021-01568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg P, Kalra P, Joseph J. Non-contact lens related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65(11):1079–1086. doi: 10.4103/ijo.IJO_826_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678–1689. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong PN, Han TN, Aldave AJ, Chau HT. Indications for and techniques of keratoplasty at Vietnam national institute of ophthalmology. Int J Ophthalmol. 2016;9(3):379–383. doi: 10.18240/ijo.2016.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie LX, Song ZH, Zhao J, Shi WY, Wang FH. Indications for penetrating keratoplasty in North China. Cornea. 2007;26(9):1070–1073. doi: 10.1097/ICO.0b013e318093de07. [DOI] [PubMed] [Google Scholar]
- 25.Sony P, Sharma N, Sen S, Vajpayee RB. Indications of penetrating keratoplasty in northern India. Cornea. 2005;24(8):989–991. doi: 10.1097/01.ico.0000157406.34662.0f. [DOI] [PubMed] [Google Scholar]
- 26.Gain P, Jullienne R, He ZG, Aldossary M, Acquart S, Cognasse F, Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–173. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 27.Maung N, Thant CC, Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalakshmi R, Whitcher JP. Corneal ulceration in South East Asia. II: a strategy for the prevention of fungal keratitis at the village level in Burma. Br J Ophthalmol. 2006;90(8):968–970. doi: 10.1136/bjo.2006.094706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Getshen K. Corneal ulceration in South East Asia. I: a model for the prevention of bacterial ulcers at the village level in rural Bhutan. Br J Ophthalmol. 2006;90(3):276–278. doi: 10.1136/bjo.2005.076083. [DOI] [PMC free article] [PubMed] [Google Scholar]


