Abstract
AIM
To assess the effectiveness of core vitrectomy-phacoemulsification-intraocular lens (IOL) implantation-capsulo-hyaloidotomy in treating phakic eye at least 1mo after the onset of malignant glaucoma.
METHODS
A retrospective analysis were performed on malignant glaucoma patients treated in Zhongshan Ophthalmic Center between 2016 and 2018. Demographic and clinical data were described. The preoperative and postoperative visual acuity (VA), intraocular pressure (IOP), number of IOP-lowering medications used, and anterior chamber depth (ACD) of the case series were compared by Wilcoxon signed-rank test.
RESULTS
Thirteen phakic eyes with long time intervals between onset and surgery were identified in this case series. Core vitrectomy-phacoemulsification-IOL implantation-capsulo-hyaloidotomy reduced the IOP (P=0.046) and the number of IOP-lowering medications used (P=0.004), deepened the ACD (P=0.005). Complete success was achieved in 38.5% of the eyes, and anatomical success was achieved in 100% of the eyes without any recurrence. The only postoperative complication observed is corneal endothelial decompensation. It occurred in two cases.
CONCLUSION
Core vitrectomy-phacoemulsification-IOL implantation-capsulo-hyaloidotomy is safe and effective for treatment of long onset phakic malignant glaucoma.
Keywords: phakic, malignant glaucoma, surgical management, long time interval
INTRODUCTION
Malignant glaucoma, also referred to as aqueous misdirection syndrome, is a severe disease characterized by a uniform flattening or shallowing of the central and peripheral anterior chamber in the absence of suprachoroidal effusion, hemorrhage, or pupillary block[1]–[4]. The mechanism of malignant glaucoma is not fully understood[5]–[9]. For phakic eyes, it is thought that cilio-lenticular block hampers forward movement of the aqueous humor; moreover, the backward movement of the aqueous humor increases the cilio-lenticular block, thereby creating a vicious cycle[6],[10].
Surgery for malignant glaucoma is still a challenge. Various studies have assessed the surgical outcomes of malignant glaucoma after the failure of conservative treatment[3],[11]–[16], and most studies included eyes treated surgically after a short time interval between onset and surgery[17]. However, little research has been conducted on the prognoses of surgery for phakic eyes with long time intervals between malignant glaucoma onset and surgery. This study aimed to provide additional data on cases of phakic eyes characterized by long time intervals between malignant glaucoma onset and surgery and to assess the therapeutic safety and efficacy of surgery.
SUBJECTS AND METHODS
Ethical Approval
The present study was approved by the Institutional Review Board/Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University (Guangzhou, China) and adhered to the tenets of the Declaration of Helsinki. Informed written consent was obtained from the participants.
Study Design and Patients Selection
This retrospective study included consecutive patients who underwent core vitrectomy-phacoemulsification-intraocular lens (IOL) implantation-capsulo-hyaloidotomy at least 1mo after the onset of malignant glaucoma between 2016 and 2018 in Zhongshan Ophthalmic Center. Malignant glaucoma was defined as uniform shallowing to flattening of the central and peripheral anterior chambers, and with intraocular pressure (IOP) over 22 mm Hg. Patients were excluded if they had: 1) a suprachoroidal effusion or hemorrhage, 2) pupillary block, or 3) lens subluxation.
Age, sex, preexisting glaucoma type, previous surgery type, and preoperative and postoperative information, including onset to surgery interval (months), axial length (AL), corneal endothelium density (/mm2), number of IOP-lowering medications used, best-corrected visual acuity (BCVA), IOP, anterior chamber depth (ACD), follow-up duration (months), and complications were obtained from a review of the medical records. The time interval between onset and surgery is from the diagnosis time of the patients at the other hospitals to their surgeries in our hospital. The IOP was measured by Goldmann applanation tonometry. The BCVA was measured using a Snellen chart and then converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analyses. The ACD was measured from the central inner corneal surface to the most anteriorly visible part of the lens (preoperative) or IOL (postoperative) by ultrasound biomicroscopy (UBM) as previously described[18]. The corneal endothelium density was counted by specular microscopy according to manufacturer's instruction[19].
Treatment Protocol and Surgical Techniques
Medical treatment was attempted using 1% atropine sulfate, topical steroid, and topical and systemic IOP-lowing medications. If reformation of the anterior chamber was not achieved in 7d, surgery was performed using the following techniques. A 23-gauge vitrectomy probe was inserted through the pars plana, 3.5 mm posterior to the limbus, using a trocar under peribulbar anesthesia. A limited core vitrectomy was performed (2500-5000 cuts/min, 0-500 mm Hg vacuum) to debulk the vitreous body and soften the eye under microscope illumination. A temporal, self-sealing, transparent corneal incision was made to avoid the conjunctival bleb. An ophthalmic viscosurgical device was then injected to deepen the anterior chamber. Hydrodissection and hydrodelineation were performed after continuous curvilinear capsulorhexis. The lens was extracted using standard phacoemulsification and irrigation/aspiration. A foldable IOL was then implanted in the capsular bag. Subsequently, posterior capsulotomy and anterior vitrectomy were performed using a 23-gauge vitrectomy probe with irrigation of the corneal incision. The transconjunctival pars plana entry site was sutured with 7-0 absorbable sutures.
Tobramycin and dexamethasone solution (Tobradex; Alcon, Fort Worth, TX, USA) was administered every 2h during week 1, and four times per day during weeks 2-4 after surgery. Tobramycin and dexamethasone ointment (Tobradex; Alcon, Fort Worth, TX, USA) was used every night during the first month. IOP-lowering medications were used if the postoperative IOP was higher than 21 mm Hg.
Outcome Measures
Anatomical success
Anatomical success was the primary outcome measure, which was defined as deepening of the anterior chamber without iridocorneal touch.
Complete success
Complete success was the secondary outcome measure, which was defined as the reformation of the anterior chamber with IOP lower than 21 mm Hg, and BCVA improvement in the absence of any IOP-lowering medication. Improvement of BCVA was defined as an increase of at least two lines after surgery compared to the preoperative BCVA.
Qualified success
Qualified success was defined as the reformation of the anterior chamber with IOP lower than 21 mm Hg, and with a reduction in the number of IOP-lowering medications.
Recurrence
Recurrence was defined as a postoperatively shallow or flat anterior chamber with or without medication.
Data Analysis
Demographic and clinical data were analyzed by descriptive statistics. Continuous variables are described by the mean, median and range. Categorical variables are described by the rate or ratio. The preoperative and postoperative visual acuity (VA), IOP, number of IOP-lowering medications used, and ACD were compared by Wilcoxon signed-rank test.
RESULTS
This retrospective study reviewed 13 consecutive malignant glaucoma patients who underwent core vitrectomy, phacoemulsification, IOL implantation, and capsulo-hyaloidotomy. The demographic and clinical data are shown in Tables 1 and 2. All eyes had been diagnosed with primary angle-closure glaucoma (PACG); 11 eyes (86.4%) developed the condition after trabeculectomy. The mean onset to surgery interval was 6.9mo. Significant preoperative and postoperative differences were detected in the IOP (P=0.046), number of IOP-lowering medications used (P=0.004), and ACD (P=0.005). Complete success, qualified success, and anatomical success was achieved in 38.5%, 77%, and 100% of the eyes, respectively.
Table 1. Demographic and clinical data of case series (n=13 eyes).
| Items | Mean±SD | Median, range |
| Age, y | 48.7±15.7 | 48, 17-72 |
| Onset to surgery interval, mo | 6.9±13 | 1, 1-48 |
| AL, mm | 19.7±2.7 | 19.7, 15.7-22.8 |
| Corneal endothelium densitya, /mm2 | 947.2±1148.4 | 320.5, 0-2780 |
| Preoperative VA, logMAR | 2.1±0.9 | 2.7, 0.5-2.8 |
| Postoperative VA logMAR | 1.6±0.8 | 1.7, 0.2-2.8 |
| Preoperative IOPb, mm Hg | 32.7±14.1 | 27.7, 14-60 |
| Postoperative IOPb, mm Hg | 20.4±11.6 | 16.4, 7-47 |
| Preoperative number of IOP-lowering medications used | 2.9±1.2 | 3, 1-5 |
| Postoperative number of IOP-lowering medications used | 1±1.5 | 0, 0-4 |
| Preoperative ACDc, mm | 0.45±0.49 | 0.22, 0-1.25 |
| Postoperative ACDd, mm | 2.77±0.66 | 2.86, 1.59-3.71 |
| Follow-up, mo | 14.3±9.2 | 13.5, 3-37 |
| Sex (male:female) | 4:9 | |
| Eye (OD:OS) | 8:5 | |
| Diagnosis | PACG | |
| Previous treatment, % | ||
| Trabeculectomy | 84.6 | |
| Diode laser cyclodestruction | 7.7 | |
| Pilocarpine | 7.7 | |
| Rate, % | ||
| Anatomical success | 100 | |
| Complete success | 38.5 | |
| Qualified success | 77 | |
| Recurrence | 0 | |
| Complication, n | ||
| Corneal endothelial decompensation | 2 |
AL: Axial length; VA: Visual acuity; IOP: Intraocular pressure; ACD: Anterior chamber depth. aCorneal endothelium density was not measured in one eye; bThe IOP was measured after IOP-lowering medications used if any; cPreoperative ACD was not measured in one eye of which the anterior chamber was recorded as flat; dPostoperative ACD was not measured in two eyes of which the anterior chamber was recorded as deep.
Table 2. Preoperative and postoperative clinical data of case series.
| Case | Age (y) | Gender | Laterity | Previous treatment | Preoperative |
Follow-up (mo) | Postoperative |
||||||||||||||
| Onset to surgery interval (mo) | BCVA logMAR | IOP (mm Hg) | No. of medications used | ACD (mm) | AL (mm) | Corneal endothelium density (/mm2) | No. of medications used | BCVA logMAR | IOP (mm Hg) | ACD (mm) | Anatomical success | Complete success | Qualified success | Recurrence | Complication | ||||||
| 1 | 48 | F | OD | Trabeculectomy | 48 | 2.7 | 14 | 3 | 0 | 19.7 | 0 | 13.5 | 0 | 2.7 | 21 | 3.04 | 1 | 0 | 1 | 0 | CED |
| 2 | 55 | M | OD | Trabeculectomy | 3 | 2.7 | 27 | 2 | 0.86 | 22.8 | 0 | 12 | 0 | 1.5 | 13 | 2.86 | 1 | 1 | 1 | 0 | CED |
| 3 | 49 | F | OS | Trabeculectomy | 1 | 2.6 | 44 | 3 | 0.25 | 19 | 0 | 21 | 0 | 1.4 | 13 | 2.14 | 1 | 1 | 1 | 0 | |
| 4 | 70 | F | OD | Trabeculectomy | 12 | 2.7 | 24 | 3 | 0 | 22.8 | 0 | 15 | 0 | 1.7 | 7 | 2.84 | 1 | 1 | 1 | 0 | |
| 5 | 48 | F | OS | Trabeculectomy | 6 | 2.7 | 33 | 1 | 0 | 17.4 | NA | 17 | 1 | 1.7 | 19 | 1.59 | 1 | 0 | 0 | 0 | |
| 6 | 29 | M | OS | Trabeculectomy | 12 | 1 | 27.7 | 3 | 0.19 | 17.9 | 2529 | 6 | 0 | 1.5 | 15 | 3.28 | 1 | 0 | 1 | 0 | |
| 7 | 48 | F | OD | Trabeculectomy | 1 | 1.7 | 26 | 4 | 0 | 15.7 | 0 | 7 | 4 | 2.8 | 47 | 2.14 | 1 | 0 | 0 | 0 | |
| 8 | 32 | F | OS | Trabeculectomy | 1 | 2.7 | 52 | 4 | 1.24 | 15.8 | 2780 | 9 | 0 | 1.7 | 14.3 | 2.22 | 1 | 1 | 1 | 0 | |
| 9 | 63 | F | OD | Trabeculectomy | 2 | 2.7 | 34.6 | 2 | 0.78 | 21 | 641 | 6 | 1 | 1.7 | 16 | Deep | 1 | 0 | 1 | 0 | |
| 10 | 56 | F | OD | Trabeculectomy | 1 | 0.5 | 22 | 4 | 1.25 | 22.3 | 2363 | 24 | 2 | 0.2 | 16.4 | 3.71 | 1 | 0 | 1 | 0 | |
| 11 | 72 | F | OD | Pilocarpine | 1 | 2.8 | 60 | 3 | Flat | 22.7 | 0 | 37 | 0 | 1.1 | 19.3 | 3.23 | 1 | 1 | 1 | 0 | |
| 12 | 17 | M | OS | Trabeculectomy | 1 | 1.5 | 15 | 1 | 0.05 | 17.7 | 1030 | 16 | 1 | 2.8 | 21 | Deep | 1 | 0 | 0 | 0 | |
| 13 | 46 | M | OD | Trabeculectomy 4y, diode laser cyclodestruction 1mo | 1 | 0.52 | 46 | 5 | 0.72 | 21.7 | 2023 | 3 | 4 | 0.3 | 42.8 | 3.45 | 1 | 0 | 1 | 0 | |
BCVA: Best corrected visual acuity; IOP: Intraocular pressure; ACD: Anterior chamber depth; AL: Axial length; CED: Corneal endothelial decompensation; M: Male; F: Female; OD: Right eye; OS: Left eye; NA: Not available.
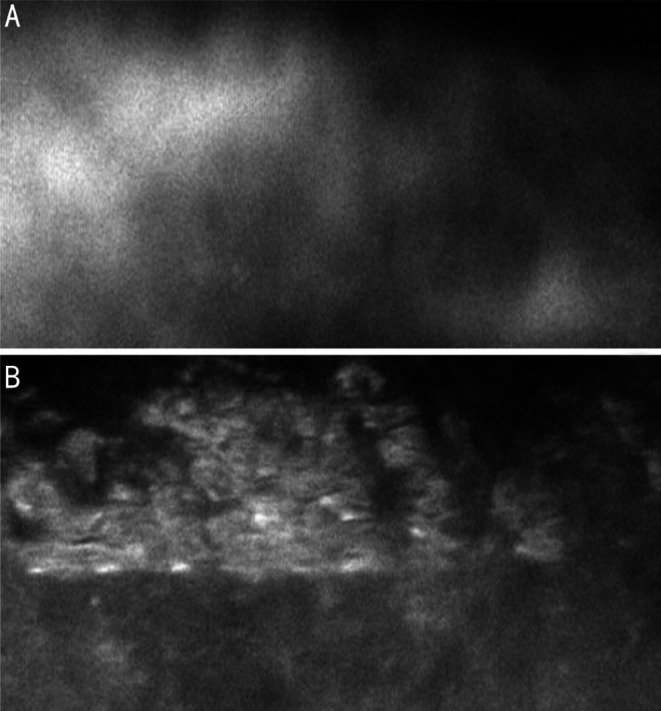
Corneal endothelial decompensation was postoperatively observed in two cases. The onset to surgery interval was 4y in case 1, and 3mo in case 2. No normal corneal endothelium could be identified in either eye (Figure 1). The preoperative and postoperative appearances of the anterior segments of cases 1 and 2 are shown in Figure 2. Diode cyclophotocoagulation was required to control the IOP in another two cases (cases 8 and 13).
Figure 1. The corneal endothelium of the two cases with postoperative corneal endothelial decompensation.
A: The corneal endothelium of case 1; B: The corneal endothelium of case 2.
Figure 2. Preoperative and postoperative anterior segment of the two cases with postoperative corneal endothelial decompensation.
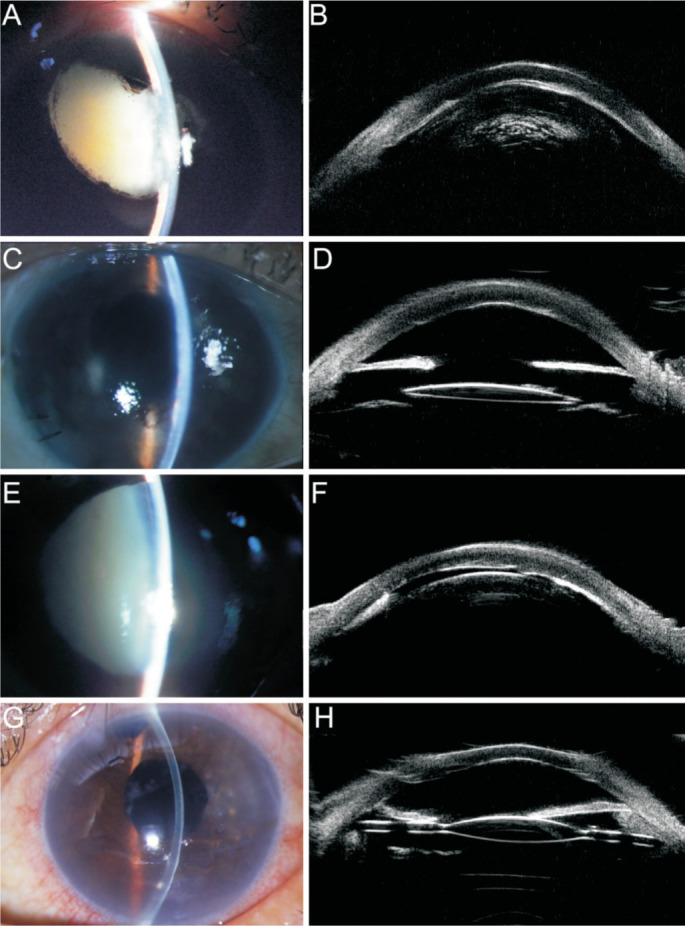
A: The preoperative anterior segment of case 1; B: The preoperative UBM results of case 1; C: The postoperative anterior segment of case 1; D: The postoperative UBM results of case 1; E: The preoperative anterior segment of case 2; F: The preoperative UBM results of case 2; G: The postoperative anterior segment of case 2; H: The postoperative UBM results of case 2.
Illustrative Case
A 56-year-old female with a prior history of PACG underwent trabeculectomy in both eyes at another hospital. After trabeculectomy, the IOP was controlled by 0.5% timolol, 1% brinzolamide, and 0.2% brimonidine tartrate twice daily. She had decreased VA in her right eye at two years after surgery. A diagnosis of malignant glaucoma was made. Topical and systemic medical treatments (intravenous mannitol, 50 mg oral methazolamide twice daily, 1% atropine sulfate twice daily, 1% prednisolone acetate every two hours, 0.5% timolol, 1% brinzolamide, and 0.2% brimonidine tartrate twice daily) were used.
At presentation at our clinic 1mo after the diagnosis of malignant glaucoma, the parameters of the patient's right eye were as follows: logMAR BCVA: 0.5; IOP range: 22-36 mm Hg; cup-to-disc ratio: 0.8; AL: 22.3 mm; and ACD: 1.25 mm (Figure 3A, 3B). Core vitrectomy-phacoemulsification-IOL implantation-capsulo-hyaloidotomy were performed. At 1y postoperatively, she continued to use 0.5% timolol and 1% brinzolamide twice daily in the right eye, in which the logMAR BCVA was 0.2, the IOP was 16.4 mm Hg, and the ACD was 3.71 mm (Figure 3C, 3D).
Figure 3. Preoperative and postoperative anterior segment of the illustrative case.
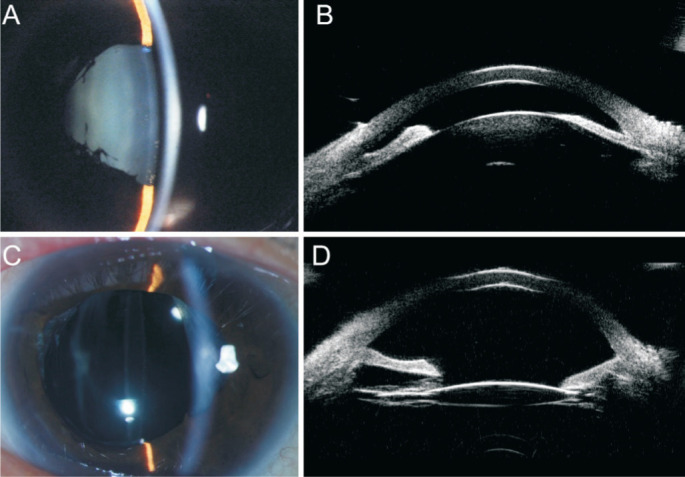
A: The preoperative anterior segment; B: The preoperative UBM results; C: The postoperative anterior segment; D:The postoperative UBM results.
DISCUSSION
As a rare and intractable disease, large-scale studies and randomized clinical trials of malignant glaucoma are still lacking[20]–[23]. The present study observed the treatment safety and efficacy of surgery for long onset phakic malignant glaucoma in a case series. Overall, the VA, IOP, number of IOP-lowering medications used, and ACD improved after surgery. Significant preoperative and postoperative differences were detected in the IOP, number of IOP-lowering medications used, and ACD. High success rate and low complication rate were achieved.
The patients included in the present case series had the following characteristics: 1) All had been diagnosed with PACG; 2) Short axial length (mean 19.7 mm, range 15.7-22.8 mm); 3) Low corneal endothelium density (mean 947.2/mm2, range 0-2780/mm2); 4) Long time interval between onset and surgery (mean 6.9mo, range 1-48mo). Except for two cases with long time intervals because of well-controlled of malignant glaucoma via medications in Krėpštė et al[24], all eyes in the previously reported studies had short time intervals. In contrast, the present study included patients with long time intervals. The reason for the 7mo between diagnosis and surgical treatment in the present study is poor follow-up compliance. The time of malignant glaucoma diagnosis is from the medical records of the patients diagnosed at the other hospitals. Therefore, the time interval between onset and surgery is from their diagnosis time at the other hospitals to their surgeries in our hospital. In contrast to the long-time interval of follow-ups, all the 13 cases included in the present study were treated with medical treatment including 1% atropine sulfate, topical steroid, and topical and systemic IOP-lowing medications once they were diagnosed at our hospital. If reformation of the anterior chamber was not achieved in 7d, surgery was performed. Patients with the above characteristics are commonly considered to have a very poor prognosis with minimal benefit expected from surgery because that the degree of optic disc damage caused by high IOP and corneal endothelial dysfunction caused by physical lens-cornea touch might be different according to disease duration. However, in our case series, the improvements in VA, IOP, number of IOP-lowering medications used, and ACD were not inferior to those of previous reports which included patients with short time intervals. A possible explanation for the good surgical outcome of the present study is the surgical technique used. Previous studies reported that oxidative stress in eyes with glaucoma eyes might promote vitreous liquefaction[25], starting from the central vitreous and progressing to the cortex of the vitreous body[26]. Therefore, effective technique might involve creating a channel connecting the vitreous cavity and the anterior chamber, thus breaking the vicious circle of aqueous misdirection. Compared with most of the previous studies[24],[27]–[30] which performed zonulo-capsulo-hyaloidotomy creating the channel through the peripheral iris, we applied capsulo-hyaloidotomy creating the channel through the pupil and the central posterior capsule. Another possible reason for the difference in prognoses between the present and previous study is that the area of vitrectomy might have been larger in out study due to the better visualization afforded by central capsulo-hyaloidotomy. Further methodologically sound examinations and sufficiently sized clinical trials are needed to validate the mechanism.
Longstanding iridocorneal touch resulted in a decrease in corneal endothelium density, with a mean of 947.2/mm2; 50% of the eyes had a corneal endothelium density of 0. Only two of the six patients with a corneal endothelium density of 0 (counted by endothelioscopy) eventually developed postoperative corneal endothelial decompensation. Their VAs were restored to different degrees after Descemet membrane endothelial keratoplasty (DMEK) surgeries (all clinical data of these two eyes were obtained from the period before DMEK was performed). The above observation suggests that the postoperative corneal endothelial decompensation might not be occurred even though the preoperative endothelioscopy test result is poor. A possible explanation is that endothelioscopy might underestimate the corneal endothelium density in the eyes without anterior chamber. For these eyes, the corneal edema might recover with a certain period of time after operation.
There was one case (case 11) developed anterior chamber flattening, IOP elevation after merely used pilocarpine. Since there was severe ACD disparity in both eyes, the PACG was excluded. Additionally, the patient did not have ocular trauma history, and we did not observe intumescent cataract, iridodonesis, zonules laxity or rupture, and other signs related to secondary glaucoma during surgery. Therefore, secondary glaucoma was also excluded. The patient was diagnosed as aqueous misdirection. The possible mechanism of pilocarpine induced aqueous misdirection is that pilocarpine induces ciliary muscle contraction causing narrowing of ciliary body ring diameter, laxity of lens zonules, forward movement of the lens-iris diaphragm, ciliolenticular block, and finally leading to aqueous misdirection.
The small sample size is the major limitation of this case series; this may explain the lack of a significant difference between the preoperative and postoperative VA, which might in turn have led to underestimation of the efficacy of the surgical techniques for treating malignant glaucoma.
In conclusion, the present study indicated that core vitrectomy-phacoemulsification-IOL implantation-capsulo-hyaloidotomy is safe and effective treatment for malignant glaucoma patients with long time intervals between malignant glaucoma onset and surgery, achieved a high rate of anatomical success and low rate of postoperative complications. The surgeries significantly reduced the IOP and the number of IOP-lowering medications used, deepened the ACD.
Acknowledgments
Foundation: Supported by the National Natural Science Foundation of China (No.81700812).
Conflicts of Interest: Wang QW, None; Zuo CG, None; Li J, None; Lin XS, None; Chen W, None; Zhu QL, None; Zhou FQ, None; Lin HT, None; Chen WR, None.
REFERENCES
- 1.Luntz MH, Rosenblatt M. Malignant glaucoma. Surv Ophthalmol. 1987;32(2):73–93. doi: 10.1016/0039-6257(87)90101-9. [DOI] [PubMed] [Google Scholar]
- 2.Trope GE, Pavlin CJ, Bau A, Baumal CR, Foster FS. Malignant glaucoma. Clinical and ultrasound biomicroscopic features. Ophthalmology. 1994;101(6):1030–1035. doi: 10.1016/s0161-6420(94)31222-x. [DOI] [PubMed] [Google Scholar]
- 3.Dave P, Senthil S, Rao HL, Garudadri CS. Treatment outcomes in malignant glaucoma. Ophthalmology. 2013;120(5):984–990. doi: 10.1016/j.ophtha.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Shahid H, Salmon JF. Malignant glaucoma: a review of the modern literature. J Ophthalmol. 2012;2012:852659. doi: 10.1155/2012/852659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003;12(2):167–180. doi: 10.1097/00061198-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZH, Huang JJ, Lin JL, Liang XW, Cai XY, Ge J. Quantitative measurements of the ciliary body in eyes with malignant glaucoma after trabeculectomy using ultrasound biomicroscopy. Ophthalmology. 2014;121(4):862–869. doi: 10.1016/j.ophtha.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Teichmann K. Aqueous misdirection syndrome is not actually caused by aqueous misdirection. J Glaucoma. 2003;12(2):184; author reply 184; author reply 185. doi: 10.1097/00061198-200304000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Chen XX, Guo XX, Xu XY, Xiao H, Liu X. Is thicker choroid a risk factor for malignant glaucoma? Ophthalmic Res. 2018;60(3):161–168. doi: 10.1159/000490914. [DOI] [PubMed] [Google Scholar]
- 9.Prata TS, Dorairaj S, De Moraes CG, Mehta S, Sbeity Z, Tello C, Liebmann J, Ritch R. Is preoperative ciliary body and iris anatomical configuration a predictor of malignant glaucoma development? Clin Exp Ophthalmol. 2013;41(6):541–545. doi: 10.1111/ceo.12057. [DOI] [PubMed] [Google Scholar]
- 10.Hodes BL. Malignant glaucoma after laser iridotomy. Ophthalmology. 1992;99(11):1641–1642. doi: 10.1016/s0161-6420(92)38526-4. [DOI] [PubMed] [Google Scholar]
- 11.He F, Qian Z, Lu L, Jiang J, Fan X, Wang Z, Xu X. Clinical efficacy of modified partial pars plana vitrectomy combined with phacoemulsification for malignant glaucoma. Eye (Lond) 2016;30(8):1094–1100. doi: 10.1038/eye.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dervenis N, Mikropoulou AM, Dervenis P, Lewis A. Anterior segment approach for the surgical management of aqueous misdirection syndrome. J Cataract Refract Surg. 2017;43(7):877–878. doi: 10.1016/j.jcrs.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Qian S, Yao J, Tang Y, Qian J, Lu Y, Xu G, Sun X. Clinical analysis of 50 Chinese patients with aqueous misdirection syndrome: a retrospective hospital-based study. J Int Med Res. 2012;40(4):1568–1579. doi: 10.1177/147323001204000437. [DOI] [PubMed] [Google Scholar]
- 14.Al Bin Ali GY, Al-Mahmood AM, Khandekar R, Abboud EB, Edward DP, Kozak I. Outcomes of pars plana vitrectomy in the management of refractory aqueous misdirection syndrome. Retina. 2017;37(10):1916–1922. doi: 10.1097/IAE.0000000000001430. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof B. The contribution of vitreoretinal surgery to the management of refractory glaucomas. Curr Opin Ophthalmol. 1999;10(2):117–120. doi: 10.1097/00055735-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhry NA, Flynn HW, Jr, Murray TG, Nicholson D, Palmberg PF. Pars plana vitrectomy during cataract surgery for prevention of aqueous misdirection in high-risk fellow eyes. Am J Ophthalmol. 2000;129(3):387–388. doi: 10.1016/s0002-9394(99)00405-5. [DOI] [PubMed] [Google Scholar]
- 17.Foreman-Larkin J, Netland PA, Salim S. Clinical management of malignant glaucoma. J Ophthalmol. 2015;2015:283707. doi: 10.1155/2015/283707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao YE, Gong XH, Zhu XN, Li HM, Tu MJ, Coursey TG, Pflugfelder SC, Gu F, Chen D. Long-term outcomes of ciliary sulcus versus capsular bag fixation of intraocular lenses in children: an ultrasound biomicroscopy study. PLoS One. 2017;12(3):e0172979. doi: 10.1371/journal.pone.0172979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garza-Leon M. Corneal endothelial cell analysis using two non-contact specular microscopes in healthy subjects. Int Ophthalmol. 2016;36(4):453–461. doi: 10.1007/s10792-015-0133-z. [DOI] [PubMed] [Google Scholar]
- 20.Teichmann K, Quigley HA. Malignant glaucoma, an enigma solved? J Glaucoma. 2003;12(5):446–447. author reply 447–448. doi: 10.1097/00061198-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kaplowitz K, Yung E, Flynn R, Tsai JC. Current concepts in the treatment of vitreous block, also known as aqueous misdirection. Surv Ophthalmol. 2015;60(3):229–241. doi: 10.1016/j.survophthal.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Grzybowski A, Kanclerz P. Acute and chronic fluid misdirection syndrome: pathophysiology and treatment. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):135–154. doi: 10.1007/s00417-017-3837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mardelli PG, Mardelli ME. Slit-lamp needling of the anterior capsule for aqueous misdirection after hyaloido-zonulectomy and iridectomy. J Glaucoma. 2018;27(4):e77–e79. doi: 10.1097/IJG.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 24.Krėpštė L, Žemaitienė R, Miliauskas A. Clinical characteristics and outcomes of malignant glaucoma after different procedures treated with pars plana vitrectomy: a 10-year retrospective study. Medicina (Kaunas) 2018;54(4):E65. doi: 10.3390/medicina54040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab C, Glatz W, Schmidt B, Lindner E, Oettl K, Riedl R, Wedrich A, Ivastinovic D, Velikay-Parel M, Mossboeck G. Prevalence of posterior vitreous detachment in glaucoma patients and controls. Acta Ophthalmol. 2017;95(3):276–280. doi: 10.1111/aos.13339. [DOI] [PubMed] [Google Scholar]
- 26.Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987;225(2):89–93. doi: 10.1007/BF02160337. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Sii F, Shah P, Kirkby GR. Vitrectomy-phacoemulsification-vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology. 2006;113(11):1968–1973. doi: 10.1016/j.ophtha.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Debrouwere V, Stalmans P, van Calster J, Spileers W, Zeyen T, Stalmans I. Outcomes of different management options for malignant glaucoma: a retrospective study. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):131–141. doi: 10.1007/s00417-011-1763-0. [DOI] [PubMed] [Google Scholar]
- 29.Tang JF, Du EG, Li XY. Combined surgical techniques for the management of malignant glaucoma. J Ophthalmol. 2018;2018:9189585. doi: 10.1155/2018/9189585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balekudaru S, Choudhari NS, Rewri P, George R, Bhende PS, Bhende M, Lingam V, Lingam G. Surgical management of malignant glaucoma: a retrospective analysis of fifty eight eyes. Eye (Lond) 2017;31(6):947–955. doi: 10.1038/eye.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]