Abstract
Objectives
Serological assays for SARS-CoV-2 have a critical role not only in diagnosis of COVID-19, but also in assessing the degree and duration of response of specific antibodies against the virus obtained through infection or vaccination. We present the results obtained with a competitive immunoenzymatic method (Chorus SARS-CoV-2 “Neutralizing” Ab) for quantitative determination of total neutralizing anti-S1 SARS-CoV-2 antibodies (IgG, IgM, and IgA) in human serum obtained on a disposable device with the Chorus TRIO instrument using a recombinant strong neutralizing antibody as tracer.
Methods
A total of 694 sera were evaluated for SARS-CoV-2 neutralizing antibodies: 407 uninfected, 201 symptomatic subjects, 37 post-infection patients, and 49 vaccinated. Sixty-eight of the previous sera were used to compare the Chorus SARS-CoV-2 “Neutralizing” Ab results with those obtained with micro-neutralization of the Alpha and original variants. A set of 74 positive sera for other respiratory infections were analyzed to evaluate the possible cross reaction to SARS-CoV-2 virus.
Results
Of the 694 samples, only 3 had discordant results between micro-neutralization and values measured by Chorus SARS-CoV-2 “Neutralizing” Ab: 1 false negative and 2 false positives. Values of sensitivity and specificity were very high: percent positive agreement (sensitivity) 99.6% (95% CI: 97.7 – 99.9) and percent negative agreement (specificity) 99.6% (95% CI: 98.0 -99.9). Concordance was high with a Gwet's Ac1 of 0.992. No significant differences were observed between the alpha and original variants.
Conclusions
The Chorus SARS-CoV-2 “Neutralizing” Ab test was highly sensitive and specific, and varies from most other currently available tests since it analyzes only antibodies with viral-neutralizing capacity.
Keywords: SARS-CoV-2, COVID-19, Neutralizing antibodies, Diagnostic assay, Immunoassay
1. Introduction
Antibodies against SARS-CoV-2 play a central role in clearing the virus from infected patients. To prevent COVID-19, antibodies should be able to engage the S1 subunit of SARS-CoV-2 spike protein, which contains the receptor binding domain (RBD) to angiotensin-converting enzyme (ACE) 2, and neutralize the virus [1]. It has been extensively reported that the degree of the antibody response correlates with the severity of COVID-19 and that the quantity of neutralizing antibodies declines rather rapidly with time [2], [3], [4].
Serological assays for SARS-CoV-2 play a role in diagnosis of COVID-19, in understanding viral epidemiology and screening convalescent sera for therapeutic and prophylactic purposes, to better understand the immune response to the virus, and to assess the degree and duration of the response of specific antibodies [5], [6], [7]. Moreover, serological assays are of major importance in monitoring immunization status in those previously infected with COVID-19 and in individuals vaccinated against SARS-CoV-2 over time. Tests designed to measure antibodies to SARS-CoV-2 antigens have been developed, but most have not been adequately evaluated and validated [8]. Commercially available SARS-CoV-2 serological assays based on different viral antigens have been approved for the qualitative determination of anti-SARS-CoV-2 antibodies, but there are limited data correlating the results from commercial assays that measure neutralizing antibodies [9, 10].
Diesse Diagnostica Senese SpA (Siena, Italy), in collaboration with the Spallanzani Institute (Rome, Italy), has developed serological diagnostic kits to rapidly detect IgM, IgG, and IgA antibodies using indirect ELISA. Unlike other commercial serological tests based on specific recombinant viral antigens, such as N or S proteins, these assays are based on whole inactivated virus from crude extracts of SARS-CoV-2 with the aim of increasing the possibility of specific antibody detection [11].
Among the many neutralizing antibodies from COVID-19 survivors, it has been shown that the most potent recognize the spike protein RBD, followed by antibodies that recognize the S1 domain, spike protein trimer, and S2 subunit [12]. Monitoring the total anti-S1 antibody titer can thus serve as an indication of immune protection for post-COVID-19 patients or for vaccinated individuals. The ability to monitor neutralizing antibody titer is especially relevant given the emerging viral variants as neutralizing antibodies from both prior infection or vaccines may be less amenable to bind the spike protein in variants [13].
Herein, we present the results obtained with an automated diagnostic kit applied to the Chorus TRIO Diesse instrument based on a competitive enzyme immunoassay for the quantitative determination of anti-S1 SARS-CoV-2 total neutralizing antibodies. Data comparing the accuracy of the Chorus SARS-CoV-2 “Neutralizing” Ab test with the micro-neutralization assay versus the original and Alpha variants are also presented.
2. Materials and methods
2.1. Chorus SARS-CoV-2 “neutralizing” AB test
The Chorus SARS-CoV-2 "Neutralizing" Ab test was performed on Diesse Diagnostica Senese Chorus system, a fully automated instrument capable of processing 30 samples in about 90 min. The format is a monotest device containing all the reagents necessary to carry out the test, which is processed automatically by the instrument by reading a bar code. No operator intervention is required except to place the sample in the device well.
The Chorus SARS-CoV-2 "Neutralizing" Ab assay is a competitive binding immunoenzymatic assay. SARS-CoV-2 anti-S1 total antibodies present in the test sample (IgG, IgM and IgA) compete with the tracer (peroxidase-conjugated SARS-CoV-2 anti-S1 therapeutic recombinant antibody) to occupy binding sites, available in limited numbers, of the antigen fixed on the solid phase. Details of antigen production have been previously described [11].
The recombinant tracer used is a human therapeutic antibody (J08; resulting from collaboration with the Monoclonal Antibody Discovery of Fondazione Toscana Life Sciences – Siena, Italy) directed against the receptor-binding domain of the S1 subunit of the Spike protein of original SARS-CoV-2 virus that shows strong neutralizing activity against the virus and its variants (Alpha, Beta, and Gamma) [14]. The higher the concentration of antibodies in the test sample, the lower the amount of conjugated antibodies those bind. The unbound components are eliminated by washing and the bound enzymatic activity is evaluated colorimetrically by transformation of a chromogenic substrate. The intensity of the color developed is inversely proportional to the concentration of antibodies in the sample under examination. The results are expressed in binding antibody units (BAU/ml) calculated with reference to the first international standard WHO 20/136 for anti-SARS-CoV-2 [15]. The test on the sera examined was interpreted as follows: positive when > 50.0 BAU/ml; negative when < 20.0 BAU/ml (20.0 BAU/ml is the limit of detection); equivocal for values between 20.0–50.0 BAU/ml.
2.2. Cell culture
The cell line VERO E6 (ATCC—CRL 1586; kidney from the African green monkey Cercopithecus aethiops) was obtained from American Type Culture Collection (Manassas, Virginia USA). Cells were grown in Dulbecco's modified Eagle's medium (DMEM Bio-Concept, Salem New Hampshire, USA) containing 10% fetal bovine serum (FBS; Gibco™, Thermo Fisher, Waltham, MA, US); 96-well plates were used for titration and neutralization tests.
2.3. Virus and titration
The SARS-CoV-2 original virus "2019-nCov/Italy-INMI1-strain" from Spallanzani 125 Institute (Rome, Italy) and the Alpha variant "HUMAN NCOV19 ISOLATE/ ENGLAND/ MIG457/2020″ were purchased through the European Virus Archive Global (EVAg - Marseille, France). The same culture and titration protocol was followed for both variants. Virus was titrated in dilutions to obtain a 50% tissue culture infective dose (TCID 50). Plates were observed daily for 3 days to evaluate the presence of a cytopathic effect (CPE). End-point titers were calculated using the method of Reed & Muench with 8 replicates for each titration.
2.4. Viral growth in cell culture
Vero E6 cells were seeded in T175 flasks at a density of 40,000/cm2, and grown in DMEM with 10% FBS and 100 IU/ml penicillin-streptomycin (Sigma-Aldrich, St. Louis, Missouri, USA). After 4–7 days of growth, cells were infected with 12–14 ml of DMEM with 5% FBS containing virus at a multiplicity of infection (MOI) of 0.05. After 1 h of incubation at 37 °C, 70 ml of DMEM containing 5% FBS was added. Flasks were monitored daily and virus was harvested when a CPE of 80–90% was seen, and aliquoted and stored at −80 °C.
2.5. Micro-neutralization assay (live-CPE)
This protocol can be used to assess whether the antibodies present in serum are able to neutralize SARS-CoV-2, both the original and Alpha variants, in vitro. Serum samples were incubated for 30 min at 56 °C; two-fold serial dilutions, starting from 1:10, were then mixed with an equal volume of viral solution containing 100 TCID 50 of SARS-CoV-2. The mixture was incubated 1 h at 37 °C, after which 100 µl was added to semi-confluent VERO E6 cultures in duplicate and incubated for 3 days at 37 °C. The following controls were also included in the test: 1) back titration: virus + cells to confirm charge 100 TCID 50; 2) white: control of uninoculated cells to avoid cytopathic affection; and 3) known samples: high positive, medium positive and negative serum. Plates were then analyzed with an inverted optical microscope and the highest serum dilution that could protect >90% of cells from CPE were considered as the neutralization titer.
Culture of SARS-CoV-2 and micro-neutralization were performed by trained personnel in a Biosafety Level 3 Laboratory (BLS-3) located in Diesse. The analyses were carried out in double blind. Data were analyzed by a Diesse researcher who did not take part in the laboratory tests.
2.6. Serum samples
Serum samples were obtained from 694 subjects, comprising 407 (58.6%) from serum of individuals from the pre-pandemic period collected in 2017 (Uninfected), 201 (29.0%) from subjects with SARS-CoV-2-like symptoms (fever, cough, fatigue, and dyspnea) and resulting positive by a molecular swab (Symptomatic), 37 (5.3%) from persons infected with SARS-CoV-2 at 2–4 months post-infection, with previous positive molecular swab (Post-infection), and 49 (7.1%) from individuals vaccinated against COVID-19 with an mRNA vaccine at one week after the second dose (Vaccinated). All samples were obtained from those that were left over following routine analyses. Among these, a group of 68 sera (randomly selected from the various groups excluding the uninfected group) were used to perform a micro-neutralization test with the Alpha and original variants and the results were compared to those obtained with the Chorus SARS-CoV-2 “Neutralizing” Ab test.
A second group of 74 sera were analyzed with the Chorus SARS-CoV-2 “Neutralizing” Ab to evaluate possible cross-reaction with SARS-CoV-2. These samples came from the Diesse archive for the production of kits for other respiratory diseases or were specially purchased from external companies (AbBaltis, Sittingbourn, UK and Biomex Gmbh, Heidelberg, DE) and were positive for antibodies against various infections including other Coronavirus (HKU1, OC43, NL63, 229E), influenza viruses and other respiratory diseases (Mycoplasma pneumoniae, Chlamydophila pneumoniae, Adenovirus, Epstein-Barr virus).
3. Statistical analysis
All analyses were performed using R v4.1.0. Qualitative variables were summarized with absolute frequencies and percentages, while quantitative ones with median and interquartile range (IQR). Kruskal-Wallis and the post hoc Dunn's test were used to compare the Chorus SARS-CoV-2 “Neutralizing” Ab method with clinical status. To compare the two methods, Spearman's correlation coefficient and Bland-Altman analyses were performed. Gwet's agreement coefficient (Ac1) was used to estimate the degree of concordance. Sensitivity, specificity, positive predictive value, and negative predictive value were estimated with 95% confidence interval (CI). A p value < 0.05 was considered statistically significant.
4. Results
4.1. Assay results with negative and positive sera
A total of 694 sera were evaluated using the assay for SARS-CoV-2 neutralizing antibodies. The samples were distributed as follows: Uninfected, Symptomatic, Post-infection and Vaccinated. Fig. 1 shows the results with the Chorus SARS-CoV-2 “Neutralizing” Ab test (BAU/ml) according to clinical status. As expected, all SARS-CoV-2 “Uninfected” sera yielded a < 20 BAU/ml. The highest values were obtained with sera from “Vaccinated” individuals (median 1241.5, IQR 370.9), while lower values were obtained with “Post-infection” (median 273.5, IQR 236.4) and “Symptomatic” (median 214.0, IQR 504.3) sera. Kruskal-Wallis test and post-hoc analysis showed that the differences between all groups were significant (p < 0.001) with the exception of “Post-infection”. Fig. 2 shows that higher micro-neutralization titers corresponded to higher values of BAU/ml. This was also confirmed with Spearman's correlation coefficient which showed near perfect correlation between the two methods (r = 0.980, p < 0.001).
Fig. 1.
Distribution of Chorus SARS-CoV-2 “Neutralizing” Ab results (BAU/ml) in the groups obtained according to clinical status, i.e. uninfected (N = 407), symptomatic (N = 201), post-infection (N = 37), and vaccinated (N = 49).
Fig. 2.
Correlation between Chorus SARS-CoV-2 “Neutralizing” Ab results (BAU/ml) with the micro-neutralization assay.
Of 694 samples, 3 had discordant results between micro-neutralization and Chorus SARS-CoV-2 “Neutralizing” Ab values: 1 false negative and 2 false positives (Table 1 ). The values of sensitivity and specificity in reference to the micro-neutralization assay were very high: 99.6% (95% CI: 97.7 – 99.9) and 99.6% (95% CI: 98.0 −99.9), respectively.. The high degree of agreement between methods was also confirmed by a Gwet's Ac1 of 0.992.
Table 1.
Comparison of results between Chorus SARS-CoV-2 "Neutralization" Ab (Test results) and micro-neutralization results (Predicate). Of 694 samples analyzed, 236 were concordant positives; 455 were concordant negative; 1 false negative and 2 false positives were detected with the Chorus SARS-CoV-2 “Neutralizing” Ab test.
Overall Percent Agreement = 99.6 95% CI = 98.7 99.8.
Percent Positive Agreement = 99.6 95% CI = 97.7 99.9.
Percent Negative Agreement = 99.6 95% CI = 98.4 99.9.
All 74 samples analyzed for possible cross reaction in Chorus SARS-CoV-2 “Neutralizing” Ab with other respiratory infectious diseases were negative, confirming the high specificity obtained from the results on the 407 “Uninfected” samples.
4.2. Assay results with neutralizing sera on the original and alpha variants
Sixty-eight sera were used to compare the micro-neutralization capacity in live-CPE between the Alpha and original variants. Micro-neutralization results were then compared with the Chorus SARS-CoV-2 “Neutralizing” Ab test. Concordance based on Gwet's Ac1 is shown in Table 2 .
Table 2.
Comparison of results with the 68 samples analyzed by micro-neutralization on the original and Alpha variants with that of the Chorus SARS-CoV-2 “Neutralizing” Ab test (BAU/ml).
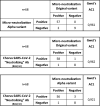 |
Bland-Altman plots revealed no significant differences between micro-neutralization on the alpha and original variants (Fig. 3 ). The mean difference was 31.5 with upper and lower limits of 371.0 and −308.0, respectively. Two outliers were seen with a difference between the two variants of >371.0. However, these two samples did not show substantial differences from a clinical point of view as they were strongly positive.
Fig. 3.
Bland-Altman plot showing differences between the test sera and the Alpha and original SARS-CoV-2 variants.
5. Discussion
Among the many tests developed, the Chorus SARS-CoV-2 “Neutralizing” Ab has the advantage of detecting total neutralizing anti-S1 SARS-CoV-2 antibodies (IgG, IgM, and IgA), in accordance with the first international standard WHO 20/136 for anti-SARS-CoV-2, using a disposable device. Another advantage of the Chorus SARS-CoV-2 “Neutralizing” Ab is that it is simple to perform with minimal operator intervention and can be carried out in any standard laboratory. Moreover, the test developed showed near perfect correlation with BAU/ml and micro-neutralization titer with Spearman's correlation coefficient. Only two samples predicted by micro-neutralization to be positive were negative, and only one predicted to be negative was positive. The reason for the false negative samples may be due to low antibody concentrations that are below the limits of detection along with differences in individual immune responses and time after infection, while the false positive could be due to allosteric interaction of non-neutralizing antibodies. The ability to detect variants was also high, and the Chorus SARS-CoV-2 “Neutralizing” Ab test had a very high correlation with the micro-neutralization assay, which is the gold standard to evaluate the ability of a sample to neutralize the virus.
In addition to a potential diagnostic role, serological tests may help to better understand the relevance of immunity in those recovering from COVID-19 or in individuals who have been vaccinated. It is possible that serologic tests may have a greater role in the future in this regard. Compared to some of the currently available commercial serological tests that are based on recombinant viral antigens (such as N or S proteins), the Chorus SARS-CoV-2 “Neutralizing” Ab assay relies on crude extracts from SARS-CoV-2 whole virus, which has the potential to increase the detection of specific antibodies [11]. The results of the assay clearly demonstrate that immunological status correlated well with the expected results, which would also allow identification of subjects who were previously infected with SARS-CoV-2. Indeed, serological assays can aid in defining those with previous exposure to SARS-CoV-2 and investigate the level of neutralizing antibodies [16]. Clearly, the test does not confirm the presence of virus, but provides only immunological evidence of infection. Given that the test detects IgG, IgM, and IgA, it could possibly find applications in early diagnosis and monitoring during treatment.
Currently, serological tests for SARS-CoV-2 antibodies have a very broad range of sensitivities and specificities. For example, in a recent systematic review and meta-analysis 5106 studies were identified, and 40 were included in the analysis [17]. Sensitivity ranged from 69.9% to 98.9% with specificities ranging from 96.6% to 99.7%. Moreover, in general the sensitivity was higher at least three weeks after onset of symptoms of COVID-19. Other studies have reported that sensitivity is highest at >14 days after the onset of symptoms [9].
To further confirm the specificity obtained from experimentation, we analyzed a series of positive samples for other respiratory infections (other coronaviruses, influenza, and respiratory pathogens) and found no cross reactivity with SARS-COV-2. Individuals infected with SARS-CoV-2 develop a large repertoire of antibodies against multiple proteins and epitopes of the virus, but only some have neutralizing properties. For this reason, the Chorus SARS-CoV-2 “Neutralizing” Ab test is different from most of the currently available tests, because it analyzes only antibodies with viral-neutralizing capacity. It is likely that the excellent results obtained with the Chorus SARS-CoV-2 “Neutralizing” Ab test are related to the use of a strongly neutralizing antibody, carefully selected for its therapeutic potential, which allows identification not only of individuals who have had COVID-19 or who have been vaccinated, but which also provides the possibility to identify the actual neutralizing capacity of the sample analyzed.
Author contributions
All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Declaration of Competing Interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: HC, TB, MC, VR, ST, AB, GT, CS, ST, AB are employees of Diesse Diagnostica Senese SpA. AC declares that there is no conflict of interest.
Acknowledgements
The authors thank “Fondazione Toscana Life Sciences” and in particular the “MAD (Monoclonal Antibody Discovery) LAB” group who have provided the Neutralizing recombinant antibody used in our research. Alessandra Cartocci performed the statistical analysis.
Editorial and medical writing support was provided by Ma.CRO.
References
- 1.Pecetta S., Pizza M., Sala C., Andreano E., Pileri P., Troisi M., et al. Antibodies, epicenter of SARS-CoV-2 immunology. Cell Death Differ. 2021;28 doi: 10.1038/s41418-020-00711-w. 821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annen K., Morrison T.E., DomBourian M.G., McCarthy M.K., Huey L., Merkel P.A., et al. Presence and short-term persistence of SARS-CoV-2 neutralizing antibodies in COVID-19 convalescent plasma donors. Transfusion. 2021;61 doi: 10.1111/trf.16261. 1148-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L., Zhang H., Zhan M., Jiang J., Yin H., Dauphars D.J., et al. Antibody response and therapy in COVID-19 patients: what can be learned for vaccine development? Sci. China Life Sci. 2020;63 doi: 10.1007/s11427-020-1859-y. 1833-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y., Jiang X., Yang L., Chen L., Zeng X., Liu G., et al. SARS-CoV-2-specific immune response in COVID-19 convalescent individuals. Signal Transduct. Target Ther. 2021;6:256. doi: 10.1038/s41392-021-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020;383 doi: 10.1056/NEJMoa2026116. 1724-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F., Simon V. Serology assays to manage COVID-19. Science. 2020;368 doi: 10.1126/science.abc1227. 1060-1. [DOI] [PubMed] [Google Scholar]
- 7.Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26 doi: 10.1038/s41591-020-0897-1. 845-8. [DOI] [PubMed] [Google Scholar]
- 8.Cheng M.P., Yansouni C.P., Basta N.E., Desjardins M., Kanjilal S., Paquette K., et al. Serodiagnostics for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann. Intern. Med. 2020;173:450–460. doi: 10.7326/M20-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicol T., Lefeuvre C., Serri O., Pivert A., Joubaud F., Dubee V., et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech) J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weidner L., Gansdorfer S., Unterweger S., Weseslindtner L., Drexler C., Farcet M., et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerutti H., Ricci V., Tesi G., Soldatini C., Castria M., Vaccaro M.N., et al. Large scale production and characterization of SARS-CoV-2 whole antigen for serological test development. J. Clin. Lab. Anal. 2021;35 doi: 10.1002/jcla.23735. e23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreano E., Nicastri E., Paciello I., Pileri P., Manganaro N., Piccini G., et al. Extremely potent human monoclonal antibodies from COVID-19 convalescent patients. Cell. 2021;184 doi: 10.1016/j.cell.2021.02.035. 1821-35 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J.W., Tambyah P.A., Hui D.S. Emergence of a new SARS-CoV-2 variant in the UK. J. Infect. 2021;82 doi: 10.1016/j.jinf.2020.12.024. e27-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreano E., Piccini G., Licastro D., Casalino L., Johnson N.V., Paciello I., et al. SARS-CoV-2 escape from a highly neutralizing COVID-19 convalescent plasma. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2103154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Available at: https://www.who.int/news-room/feature-stories/detail/standardization-of-vaccines-for-coronavirus-disease-covid-19. Accessed 3 Sep, 2021.
- 16.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26 doi: 10.1038/s41591-020-0913-5. 1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisboa Bastos M., Tavaziva G., Abidi S.K., Campbell J.R., Haraoui L.P., Johnston J.C., et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2516. m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]





