Abstract
Background
Urinary tract infection (UTI) is a common bacterial infection that can lead to significant morbidity including stricture, abscess formation, fistula, bacteraemia, sepsis, pyelonephritis and kidney dysfunction. Mortality rates are reported to be as high as 1% in men and 3% in women due to development of pyelonephritis. Because probiotic therapy is readily available without a prescription, a review of their efficacy in the prevention of UTI may aid consumers in making informed decisions about potential prophylactic therapy. Institutions and caregivers also need evidence‐based synopses of current evidence to make informed patient care decisions.
Objectives
Compared to placebo or no therapy, did probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality, when used to prevent UTI in susceptible patient populations?
Compared to other prophylactic interventions, including drug and non‐drug measures (e.g. continuous antibiotic prophylaxis, topical oestrogen, cranberry juice), did probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality when used to prevent UTIs in susceptible patient populations?
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 21 September 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) of susceptible patients (e.g. past history of UTI) or healthy people in which any strain, formulation, dose or frequency of probiotic was compared to placebo or active comparators were included.
Data collection and analysis
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at comparing probiotics to no therapy, placebo, or other prophylactic interventions were included. Summary estimates of effect were obtained using a random‐effects model, and results were expressed as risk ratios (RR) and their 95% confidence intervals (CI) for dichotomous outcomes.
Main results
We included nine studies that involved 735 people in this review. Four studies compared probiotic with placebo, two compared probiotic with no treatment, two compared probiotics with antibiotics in patients with UTI, and one study compared probiotic with placebo in healthy women. All studies aimed to measure differences in rates of recurrent UTI.
Our risk of bias assessment found that most studies had small sample sizes and reported insufficient methodological detail to enable robust assessment. Overall, there was a high risk of bias in the included studies which lead to inability to draw firm conclusions and suggesting that any reported treatment effects may be misleading or represent overestimates.
We found no significant reduction in the risk of recurrent symptomatic bacterial UTI between patients treated with probiotics and placebo (6 studies, 352 participants: RR 0.82, 95% CI 0.60 to 1.12; I2 = 23%) with wide confidence intervals, and statistical heterogeneity was low. No significant reduction in the risk of recurrent symptomatic bacterial UTI was found between probiotic and antibiotic treated patients (1 study, 223 participants: RR 1.12, 95% CI 0.95 to 1.33).
The most commonly reported adverse effects were diarrhoea, nausea, vomiting, constipation and vaginal symptoms. None of the included studies reported numbers of participants with at least one asymptomatic bacterial UTI, all‐cause mortality or those with at least one confirmed case of bacteraemia or fungaemia. Two studies reported study withdrawal due to adverse events and the number of participants who experienced at least one adverse event. One study reported withdrawal occurred in six probiotic participants (5.2%), 15 antibiotic participants (12.2%), while the second study noted one placebo group participant discontinued treatment due to an adverse event.
Authors' conclusions
No significant benefit was demonstrated for probiotics compared with placebo or no treatment, but a benefit cannot be ruled out as the data were few, and derived from small studies with poor methodological reporting.
There was limited information on harm and mortality with probiotics and no evidence on the impact of probiotics on serious adverse events. Current evidence cannot rule out a reduction or increase in recurrent UTI in women with recurrent UTI who use prophylactic probiotics. There was insufficient evidence from one RCT to comment on the effect of probiotics versus antibiotics.
Plain language summary
Probiotics for preventing urinary tract infections in adults and children
Background
Urinary tract infections (UTIs) occur in kidneys, ureters, urethra or bladder. UTIs are one of the most common bacterial infections and can lead to other health problems.
Probiotics (live micro‐organisms which, when administered in adequate amounts, confer a health benefit on the host) are thought to work by preventing other infectious bacteria from climbing up the urinary tract and causing infection. We were interested in studying any form of probiotics (bacteria used to change balance of bacteria) compared with no treatment, antibiotics, hormone therapy, cranberry juice or other interventions in people at risk of UTI. To assess if probiotics were effective, we planned to measure how many people had recurrent UTIs.
Study characteristics
We conducted a literature search up to September 2015 and nine studies were eligible for inclusion according to our selection criteria. The nine studies reported data on 735 participants and investigated probiotics for preventing UTI: seven studies involved women or girls with recurrent UTIs, one looked at children with abnormal urinary tracts, and one investigated UTI in healthy women.
Key results
Generally, studies were poor quality with high risk of bias. Aside from the different populations, there were also many different species of probiotics used, different dosage forms such as vaginal and oral, and probiotics were given for varying lengths of time. All of these factors may have affected our results.
Most studies did not collect information on adverse effects so we were unable to estimate any harms associated with probiotic therapies. We found no significant reduction in the risk of recurrent symptomatic bacterial UTI between patients treated with probiotics and placebo and no significant reduction in the risk of recurrent symptomatic bacterial UTI was found between probiotic and patients treated with antibiotics.
Quality of the evidence
The currently available evidence shows no reduction in UTI using probiotics.
Summary of findings
for the main comparison.
| Probiotics compared with placebo or antibiotics for urinary tract infections (UTI) | ||||||
|
Patient or population: adults and children at risk of UTI Settings: outpatient Intervention: probiotics Comparison: placebo or antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Probiotics | |||||
|
Symptomatic bacterial UTI in adults and children in patients with and without recurrent UTI Probiotics versus placebo (follow‐up) |
395 per 1000 | 296 per 1000 (197 to 446) | RR 0.75 (0.50, 1.13) | 352 (6) | ⊕⊕⊝⊝ low | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
|
Symptomatic bacterial UTI in adults and children with recurrent UTI Probiotics versus placebo (follow‐up) |
421 per 1000 | 315 per 1000 (227 to 425) | RR 0.74 (0.54, 1.01) | 275 (4) | ⊕⊕⊝⊝ low | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus placebo |
|
Symptomatic bacterial UTI in women with recent UTI Probiotics versus antibiotics (follow‐up) |
666 per 1000 | 745 per 1000 (632 to 885) | RR 1.12 (0.95, 1.33) | 223 (1) | ⊕⊕⊝⊝ low | Risk of bias was assessed at unclear or high in most domains and suggest that results are imprecise or overestimate probiotic effects versus antibiotics. Imprecision also due to small sample from only one RCT |
|
Symptomatic bacterial UTI in children with VUR Probiotics versus placebo (follow‐up) |
270 per 1000 | 145 per 1000 (64 to 332) | RR 0.54 (0.24, 1.23) | 96 (1) | ⊕⊕⊝⊝ low | Risk of bias was assessed at unclear or high in most domains of and suggest that results are imprecise or overestimate probiotic effects versus placebo. Imprecision also due to small sample from only one RCT |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
UTi ‐ urinary tract infection
Background
Description of the condition
Urinary tract infections (UTIs) are defined as infections of kidneys, ureters, urethra, or bladder due to bacterial colonisation. UTIs are one of the most common bacterial infections and can lead to significant morbidity including strictures, abscess formation, fistulas, bacteraemia, sepsis, pyelonephritis, and kidney dysfunction. Mortality rates are reported to be as high as 1% in men and 3% in women due to development of pyelonephritis. One in two women experience UTI at some point in their lifetime. UTI incidence in men is related to age (1.1% to 1.6% in the first 10 years of life, 5 to 8 infections/year/10,000 men up to age 50 years, and higher after age 50 due to prostate enlargement and subsequent complications) (Foxman 2003, Howes 2009; Howes 2010). Elderly people are more susceptible to asymptomatic UTI; prevalence is 30% in women and 10% in men per year in women and men (Richards 2004).
Several interventions have been studied for preventing UTI. Mixed results have been seen for intravaginal hormonal therapy for women and management of incontinence (Perrotta 2008; Ouslander 1995; Schnelle 1995). Improved urinary catheter technology and catheter management strategies have demonstrated efficacy in reducing UTI incidence (CDC 2000; Christensen 2001; Maki 2001; Richards 2001; Saint 2000). A systematic review of randomised controlled trials (RCTs) concluded that there is some evidence that cranberry juice reduces the incidence of UTIs in women (Jepson 2012). Prophylactic antibiotics have been shown to reduce the incidence of UTIs in non‐pregnant women with recurrent UTIs (Albert 2004) and may reduce asymptomatic UTIs in children (Williams 2011).
Description of the intervention
Probiotics are defined as "a preparation of, or a product containing viable, defined micro‐organisms in sufficient numbers, which alter the microflora (by implantation or colonisation) in a compartment of the host and by that exert beneficial health effects in this host" (Schrezenmeir 2001). There are a number of species and strains of probiotics available that are used in many formulations administered via several different routes.
How the intervention might work
Probiotic organisms (e.g. lactobacillus) are thought to establish a barrier against infectious pathogens ascending the urinary tract, colonising, and subsequently causing infection. The protective effects thought to be exerted by probiotics are thought to include reducing pathogen adherence, growth and colonisation, and modulating host defences (Bruce 1988; Hawthorn 1990; Heineman 2000; Osset 2001; Velraeds 1998).
Why it is important to do this review
A 2006 systematic review concluded that carefully selected strains of probiotics when tested in case‐control studies and RCTs had mixed effects in terms of UTI prophylaxis (Falagas 2006). The authors concluded that there was some in vitro and in vivo evidence that probiotics restore normal vaginal flora and prevent recurrent UTI in women (Falagas 2006).
Probiotic therapy is readily available without prescription. A review of their efficacy in preventing UTIs may aid consumers and healthcare providers to make informed decisions about potential prophylactic therapy.
Objectives
Our review aimed to assess:
Compared to placebo or no therapy, do probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality, when used to prevent UTIs in susceptible patient populations?
Compared to other prophylactic interventions, including drug and non‐drug measures (e.g. continuous antibiotic prophylaxis, topical oestrogen, cranberry juice), do probiotics (any formulation) provide a therapeutic advantage in terms of morbidity and mortality when used to prevent UTIs in susceptible patient populations?
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at comparing probiotics to no therapy, placebo, or other prophylactic interventions were included.
Types of participants
Men, women, and children with histories of recurrent bacterial UTI (two episodes within the last two months)
Men and women over the age of 60 years
Pregnant women
Men, women and children with an indwelling catheter or requiring intermittent catheterisation
Men, women and children with an abnormal urinary tract (for example vesicoureteric reflux, urinary obstruction, dysfunctional voiding)
Men and women resident in residential and long‐term care facilities
Men and women with asymptomatic bacteriuria.
Studies exclusively involving critically ill or immunosuppressed patients were excluded. Applicable patient data were extracted from studies with mixed populations.
Types of interventions
All available probiotics in any formulation including tablets, capsules, food products (i.e. shakes, yogurt) for preventing UTIs in adults and children.
Any study in which probiotics were used for the treatment (versus prevention) of suspected or proven bacterial UTI was excluded.
Studies investigating prophylaxis with probiotics in combination with antibiotics were not included. These topics were beyond the scope of this review.
Types of outcome measures
Primary outcomes
Numbers of patients with at least one symptomatic bacterial UTI in each group (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen and defined as > 105 CFU/mL, or as defined by authors).
Secondary outcomes
Numbers with at least one asymptomatic bacterial UTI (confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen)
Withdrawal due to adverse events
Total adverse events
All‐cause mortality
Numbers with at least one non‐fatal serious adverse events
Numbers with at least one confirmed case of bacteraemia or fungaemia.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 21 September 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that potentially included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and where necessary, the full text of these studies to determine which satisfied inclusion criteria. There were no language restrictions.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancies among published versions was planned to be highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes results were expressed as risk ratio (RR) with 95% confidence intervals (CI). All prespecified outcomes were dichotomous; therefore no analysis of continuous outcome data was necessary.
Unit of analysis issues
Data from all patients individually randomised to each intervention were included in the analyses. Care was taken to identify situations in which data had been censored or excluded or if data presented were the total number of events or the total number of patients with a first event. Authors were contacted for clarification if necessary. The rates of each outcome in the probiotic groups group were compared to the rate of that outcome in control groups to calculate risk differences. If the rates for an outcome were not provided, a narrative summary of data was presented. UTI rates were extracted for numbers of patients experiencing at least one UTI, not the number of UTIs in a treatment group.
Dealing with missing data
In general if there were missing data, the authors of the study were contacted for clarification to determine if details were available. If not, or if authors did not respond to requests, the worst outcome was imputed for all missing data points in the experimental treatment group (i.e. worst case scenario). A sensitivity analysis was performed to see if the effect size for any particular outcome was sensitive to conducting the worst case scenario with imputed data versus ignoring the missing data (i.e. using only the available data).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
A funnel plot was not created because of the few included studies; the resulting analysis would likely be underpowered to detect possible publication bias (Higgins 2011).
Data synthesis
Data were pooled using relative risks with the random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were conducted for studies comparing probiotics with placebo or active comparators. In addition, a post‐hoc subgroup analysis was conducted for different patient characteristics: adult women; children, and children with vesicoureteral reflux.
Sensitivity analysis
Sensitivity analysis was performed to test for robustness of the results. Analysis of the following categories was undertaken separately.
Studies without proper randomisation or concealment of allocation compared to those without these characteristics.
Studies performed without intention‐to‐treat (ITT) analysis compared to those with an ITT analysis.
Unblinded studies versus blinded studies.
Studies using different probiotic formulations.
The effects of probiotics when there is missing data for patients receiving probiotics, these patients are assumed to have had the worst possible outcome.
Results
Description of studies
Results of the search
We identified 389 records. Following assessment of titles and abstracts, 28 full‐text records were screened. Of these, nine studies (14 records) were included and eight studies (10 records) were excluded. Two ongoing studies were identified (NCT00781625; ProSCIUTTU Study 2014), one study is awaiting translation (Skerk 2010), and one study was identified prior to publication ( Reid 1995). These four studies and will be assessed in a future update of this review (Figure 1).
1.
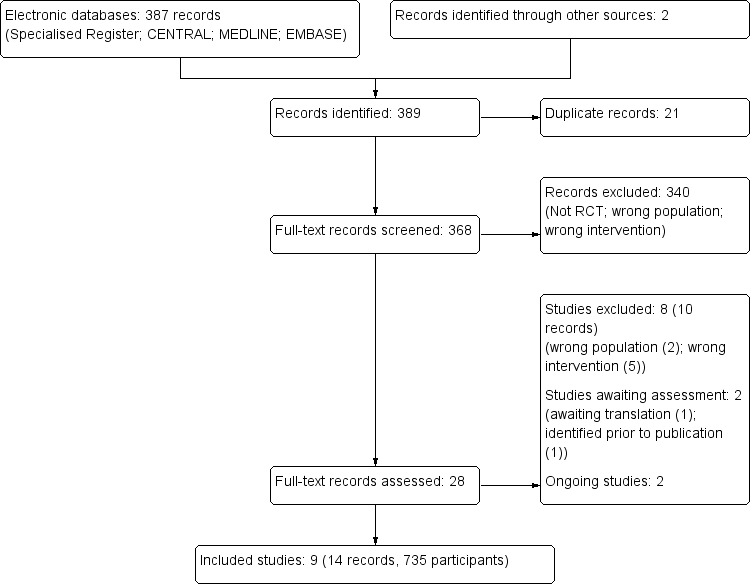
Study flow diagram
Included studies
We included nine studies in this review (Baerheim 1994; Czaja 2007; Ferrara 2009; Kontiokari 2001; Lee 2007a; NAPRUTI Study II 2006; Reid 1992; Reid 2003; Stapleton 2011).
Six studies compared probiotics with placebo (Baerheim 1994; Czaja 2007; Reid 1992; Stapleton 2011) or no comparator (Ferrara 2009; Kontiokari 2001); two studies compared probiotics with antibiotic prophylaxis in patients with UTI (one in adults (NAPRUTI Study II 2006) and one in children with VUR (Lee 2007a)); and one study compared probiotics with placebo in healthy women (Reid 2003).
Design
The included studies were parallel RCTs with a mix of active comparators, placebo or no comparators. Efficacy of the probiotics in placebo‐controlled studies (Baerheim 1994; Czaja 2007; Ferrara 2009; Kontiokari 2001; Reid 1992; Stapleton 2011) could not be compared to studies that used effective prophylactic measures such as antibiotics (NAPRUTI Study II 2006). Placebo‐controlled studies were therefore analysed separately from active comparator studies. The 'no comparator' arms of the Kontiokari 2001 and Ferrara 2009 studies were used to include them with the four studies comparing probiotics with placebo.
Based on Jepson 2012, it appears that cranberry juice cannot be recommended for the prevention of UTI due to small effect sizes and studies with significant biases that limit the reliability of the data. There is also no identified evidence that lingonberry juice alone or in combination with cranberry juice has proven efficacy or safety versus placebo for the prevention of UTI. It is for this reason that probiotics were not compared versus cranberry juice (Ferrara 2009) or cranberry‐lingonberry juice (Kontiokari 2001).
Sample sizes
The smallest study included 30 participants (Czaja 2007) and the largest 252 participants (NAPRUTI Study II 2006). Most studies (60%) included 100 participants or fewer (Baerheim 1994; Czaja 2007; Ferrara 2009; Reid 1992; Reid 2003; Stapleton 2011).
Setting
All nine studies took place in outpatient settings.
Participants
Patient populations differed in terms of time since the last acute UTI and previous use of prophylactic antibiotics. Only Reid 2003 included exclusively healthy women. Three studies required participants with acute UTI at inclusion to be treated before commencing the study (Ferrara 2009; Kontiokari 2001; Stapleton 2011), Reid 2003 randomised women to acute antibiotic therapy before randomising them to prophylaxis; and three studies listed acute UTI or recent antibiotic use as exclusion criteria (Baerheim 1994; Czaja 2007; NAPRUTI Study II 2006). The study in children with VUR included children with persistent primary VUR following 12 months of antibiotic prophylaxis (Lee 2007a).
Interventions
The species and mode of administration of the probiotic intervention varied widely among studies, as did duration of therapy (eight weeks to 12 months). Details of formulations and species of probiotics in the studies are presented in Characteristics of included studies.
Outcomes
Only Reid 2003 did not report our primary outcome of UTI, although definitions varied by study (Characteristics of included studies). Few studies reported on our prespecified secondary outcomes. Four studies reported on adverse events (Czaja 2007; Reid 1992; Reid 2003; Stapleton 2011), and only two considered serious adverse events (Czaja 2007; NAPRUTI Study II 2006).
Excluded studies
Seven studies were excluded (Characteristics of excluded studies). Dani 2002 was conducted in a neonatal population; Colodner 2003 did not include an arm where participants did not receive a probiotic; Molander 1990 did not include investigation of a probiotic; and NCT00900653 studied a combination of probiotic and hormonal therapies. Manley 2007 and Pushkarev 2005 studied treatment rather than prophylaxis; Ranganathan 2009 enrolled people with chronic kidney disease (CKD) to determine if probiotics exerted renoprotective effects and reduced uraemia symptoms.
Risk of bias in included studies
NAPRUTI Study II 2006 was large and methods and events were adequately reported; however, most studies had small sample sizes and reported insufficient methodological detail to enable robust assessment (Figure 2; Figure 3).
2.
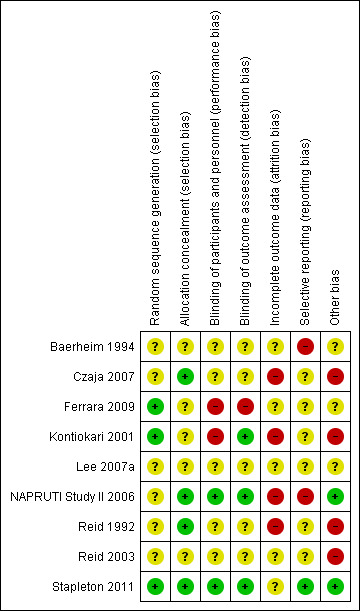
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
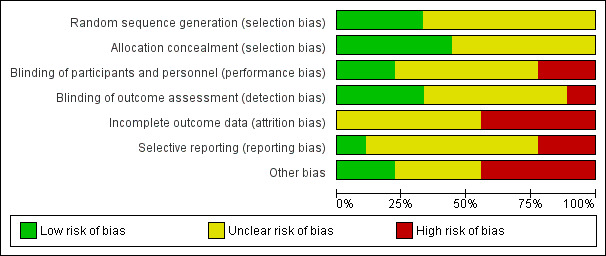
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Overall, there was a high risk of bias among the included studies. This meant that we were unable to draw firm conclusions or determine if any reported treatment effects may be misleading or represent overestimates.
Allocation
Three studies (Ferrara 2009; Kontiokari 2001; Stapleton 2011) reported on adequate methods of sequence generation and the other six studies (Baerheim 1994; Czaja 2007; Lee 2007a; NAPRUTI Study II 2006; Reid 1992; Reid 2003) did not describe sequence generation methods.
Four studies reported adequate allocation concealment (Czaja 2007; NAPRUTI Study II 2006; Reid 1992; Stapleton 2011). Allocation concealment was unclear for the remaining five studies.
Blinding
Two included studies were adequately described as double blind (NAPRUTI Study II 2006; Stapleton 2011), two were open label (Ferrara 2009; Kontiokari 2001) and there were insufficient data to assess blinding for the remaining studies; blinding was either not reported or lacked sufficient detail to determine who was blinded.
Incomplete outcome data
Satisfactory explanation was provided for changes in the number of participants for only one of the studies assessed (Czaja 2007). Reid 2003 had a significant proportion of their small study population excluded from analysis; attrition was significant and not explained satisfactorily in three studies (Kontiokari 2001; NAPRUTI Study II 2006, Stapleton 2011). Remaining studies lacked sufficient information to determine if attrition was likely to result in significant bias. Due to this incomplete follow‐up, worst case scenarios were undertaken for both the probiotics in comparison to placebo analysis and the probiotics in comparison to antibiotics analysis.
Selective reporting
Many of the included studies either did not report secondary outcomes, or lacked sufficient detail in reporting secondary outcomes; many of our prespecified secondary outcomes were not addressed, including all‐cause mortality and number with at least one confirmed case of bacteraemia or fungaemia.
We searched for published protocols for the included studies. Protocols were found for NAPRUTI Study II 2006 and Stapleton 2011. The outcomes reported in Stapleton 2011 aligned completely with the published protocol. The published report of the NAPRUTI Study II 2006 included several outcomes that were not prespecified in the protocol: mean number of antibiotic prescriptions for UTI treatment; and a subgroup analysis of mean number of clinical recurrences in women with complicated versus uncomplicated UTI.
Other potential sources of bias
Several studies were funded by manufacturing companies (Czaja 2007; Reid 1992), and one had an issue with supply that resulted in treatment duration inequality between the study arms (Kontiokari 2001). UTI definitions were fairly consistent among studies, although microbiological criteria ranged from at least 103 CFU/mL to 105 CFU/mL and clinical criteria were more stringent in some studies compared with others.
Effects of interventions
See: Table 1
Primary outcome
The primary outcome was to assess numbers of participants with at least one symptomatic bacterial UTI in each group (as confirmed by a catheter specimen of urine, midstream urine specimen if possible, or a clean catch specimen, and defined as > 105 CFU/mL or as defined by triallists).
Analyses were conducted according to probiotics in women and probiotics in children with VUR. Placebo‐controlled studies were subdivided into studies that enrolled women and children who had recently been treated with antibiotics for UTI (Ferrara 2009; Kontiokari 2001; Reid 1992; Stapleton 2011) and studies enrolling participants who had previous UTIs (Baerheim 1994; Czaja 2007). We decided to first determine if probiotics exerted a positive effect versus placebo before analysing studies versus active comparators.
A meta‐analysis of six studies that involved 352 randomised women and children demonstrated no significant reduction in the risk of recurrent symptomatic bacterial UTI between probiotics and placebo (Analysis 1.1 (6 studies, 352 participants): RR 0.82, 95% CI 0.60 to 1.12; I2 = 23%), heterogeneity was low. The confidence interval for all studies suggests a range that includes a 15.8 % absolute decrease to a 4.7% absolute increase in the risk of recurrent bacterial UTI with probiotics versus placebo given that the placebo rate of recurrence was 39.5% over 8 to 52 weeks.
1.1. Analysis.
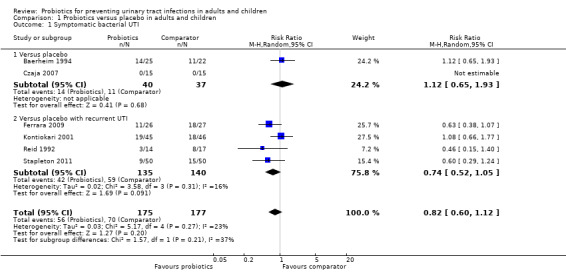
Comparison 1 Probiotics versus placebo in adults and children, Outcome 1 Symptomatic bacterial UTI.
NAPRUTI Study II 2006 reported no significant difference in the rate of recurrent symptomatic bacterial UTI in women between probiotics and antibiotics (Analysis 2.1 (1 study, 223 women): RR 1.12, 95% CI 0.95 to 1.33).
2.1. Analysis.
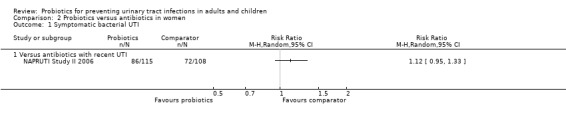
Comparison 2 Probiotics versus antibiotics in women, Outcome 1 Symptomatic bacterial UTI.
Lee 2007a included children with VUR. There was no significant difference in the rate of recurrent symptomatic bacterial UTI between probiotics and antibiotics (Analysis 3.1 (1 study, 96 children): RR 0.54, 95% CI 0.24 to 1.23).
3.1. Analysis.
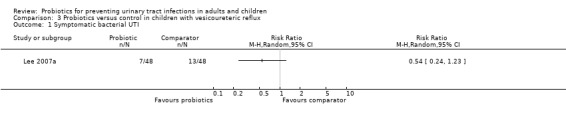
Comparison 3 Probiotics versus control in children with vesicoureteric reflux, Outcome 1 Symptomatic bacterial UTI.
We analysed six month recurrent UTI data from Kontiokari 2001. However, the authors also reported recurrent UTI at 12 months; a sensitivity analysis demonstrated that the results of the meta‐analysis did not change meaningfully if 12 month data were used. Three studies did not report on this outcome and this may have improved the precision of the effect size if the data were available. Stapleton 2011 reported 17 and 13 recurrent symptomatic bacterial UTIs for probiotics and placebo respectively. However, correspondence with the lead author indicated that two women in each arm of the study had symptomatic UTI but these were not confirmed with positive cultures; therefore, these events were excluded. We included these unconfirmed UTI in our analysis. In a sensitivity analysis, the results of the meta‐analysis did not change meaningfully if only culture‐confirmed UTI data were used.
Secondary outcomes
None of the included studies reported numbers of participants with at least one asymptomatic bacterial UTI, all‐cause mortality or those with at least one confirmed case of bacteraemia or fungaemia. The only secondary outcomes of interest reported by the included studies were withdrawal due to adverse events, total adverse events and numbers of participants with at least one non‐fatal serious adverse event.
Withdrawal due to adverse events was reported by NAPRUTI Study II 2006 and Stapleton 2011. NAPRUTI Study II 2006 reported that withdrawals due to adverse events occurred in six probiotic participants (5.2%) versus 15 antibiotic participants (12.2%). Stapleton 2011 noted that one placebo group participant discontinued treatment due to an adverse event.
Ferrara 2009, Kontiokari 2001, and Reid 1992 did not report adverse events. NAPRUTI Study II 2006 and Stapleton 2011 reported participants who experienced at least one adverse event but data were not meta‐analysed because participants in the NAPRUTI Study II 2006 control group received an antibiotic, and Stapleton 2011 provided a placebo to control group participants. NAPRUTI Study II 2006 reported that 66 probiotic (57.4%) and 72 antibiotic participants (58.5%) respectively experienced at least one adverse event (the most commonly reported adverse effects in both groups were diarrhoea, nausea, vomiting, constipation and vaginal symptoms). Reid 2003 identified adverse events through a questionnaire that was sent to patients. They reported that no probiotic patients reported an adverse event however it is unclear how many comparator group patients experienced at least one adverse event; they only reported that 2 comparator patients reported yeast infections.
Stapleton 2011 reported that 28 probiotic (56%) and 25 placebo participants (50%) experienced at least one adverse event (the most common were vaginal discharge, itching and moderate abdominal discomfort).
Baerheim 1994 stated that treatment was well tolerated in both groups; four participants from the probiotic arm and one from placebo complained of discharge, with no other adverse effects noted.
Czaja 2007 documented a range of self‐reported adverse effects including abnormal vaginal discharge, external genital irritation, vaginal candidiasis, vaginal odour, abdominal pain and dysuria. Abnormal vaginal discharge occurred in about half of all participants, but the overall frequency of adverse effects was low.
NAPRUTI Study II 2006 and Czaja 2007 reported serious adverse events. In NAPRUTI Study II 2006, no significant difference in serious adverse events was noted; 17 probiotic (14.8%) and 14 antibiotic participants (11.4%) experienced at least one serious adverse event.
Subgroup analyses
In a post‐hoc subgroup analysis, there was no significant difference in recurrence of UTI between the subgroups of women without a UTI prior to enrolment compared (Analysis 1.1.1 (2 studies, 77 participants): RR 1.12, 95% CI 0.65 to 1.93; I2 = 0%) with those with UTI being treated with antimicrobials at enrolment (Analysis 1.1.2 (4 studies, 275 participants): RR 0.74, 95% CI 0.52 to 1.05; I2 = 16%). The overall pooled estimate for all studies in both subgroups was not significantly different from the pooled estimate of each subgroup (Test for subgroup differences: Chi2 = 1.57, df = 1 (P = 0.21), I2 = 36.5%).
Sensitivity analyses
Sensitivity analyses were planned to determine if treatment effects on recurrent symptomatic bacterial UTI rates differed in studies based on a number of variables. Removal of studies that were open label or that had unclear allocation concealment did not change the effect of probiotics on recurrent symptomatic bacterial UTI however only few studies had unclear allocation concealment (Baerheim 1994; Ferrara 2009; Kontiokari 2001) or were open label (Ferrara 2009; Kontiokari 2001) and it was felt that sensitivity analyses would not be informative and maybe misleading.
Sensitivity analysis was also conducted for symptomatic bacterial UTI involving Czaja 2007, NAPRUTI Study II 2006 and Stapleton 2011 as these three studies suggested that not all randomised patients were included in the UTI analysis; hence we wanted to see the impact of imputing data for a worst case scenario. In this analysis all missing patients in one group were assumed to have had UTI, and those missing from the other group were assumed to not have had UTI. For the comparison of probiotics versus placebo, the effect did not change using a worst case scenario for probiotics (Analysis 1.2: RR 0.94, 95% CI 0.63 to 1.39; I2 = 49%). For the comparison of probiotics versus antibiotics, a worst case scenario for antibiotics of the NAPRUTI Study II 2006 now demonstrated that fewer probiotic patients experienced a recurrent symptomatic bacterial UTI versus antibiotics (25 antibiotic patients, Analysis 2.3; RR 0.83, 95% CI 0.74 to 0.94). When the worst case scenario analysis for probiotics was conducted, more probiotic patients had recurrent symptomatic bacterial UTI (5 probiotic patients, Analysis 2.2; RR 1.19, 95% CI 1.01 to 1.40).
1.2. Analysis.
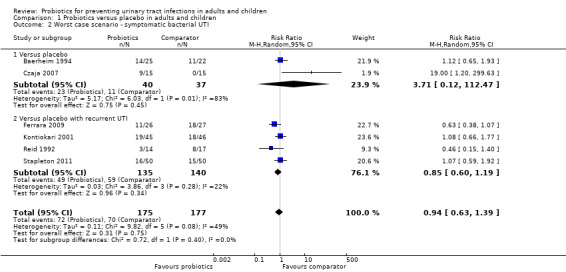
Comparison 1 Probiotics versus placebo in adults and children, Outcome 2 Worst case scenario ‐ symptomatic bacterial UTI.
2.3. Analysis.
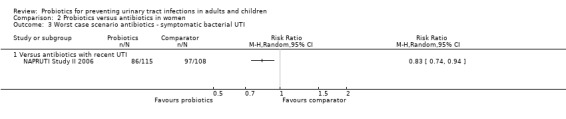
Comparison 2 Probiotics versus antibiotics in women, Outcome 3 Worst case scenario antibiotics ‐ symptomatic bacterial UTI.
2.2. Analysis.
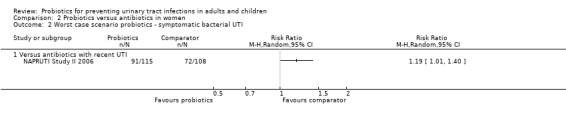
Comparison 2 Probiotics versus antibiotics in women, Outcome 2 Worst case scenario probiotics ‐ symptomatic bacterial UTI.
Discussion
Summary of main results
This review included nine studies involving a total of 735 participants. No significant difference in risk of recurrent UTI was seen for probiotics in comparison to placebo or antibiotic prophylaxis in either women or children. There was no significant difference found between probiotics and either placebo or antibiotic prophylaxis for harms.
The studies included in this review were generally small and of poor quality with inconsistent and limited reporting of harm, and as such the data are insufficient to exclude either a benefit or harm from probiotics versus either placebo or antibiotic prophylaxis.
Only NAPRUTI Study II 2006 compared probiotics and antibiotics; no significant difference in the rate of recurrent symptomatic bacterial UTI or harm was found between groups.
Adverse events, when reported, were poorly described with insufficient data to perform statistical evaluation. Overall the frequency of reported side effects was low and mild in nature (e.g. vaginal discomfort).
We suggest caution when interpreting the lack of a subgroup difference in Analysis 1.1, as there were too few studies to be able to confidently conclude the presence or absence of subgroup differences.
There was insufficient evidence to comment on the differences in effects of probiotics in children and women as only one study included only children (Ferrara 2009) and a subgroup analysis may be misleading.
Overall completeness and applicability of evidence
We included nine studies in this review ranging from 30 to 252 participants totalling a relatively small overall sample of 735 participants. No statistically significant difference was seen in recurrent symptomatic bacterial UTI. Given the low overall quality and quantity of data available a decrease or increase in recurrent symptomatic UTI cannot be ruled out.
There was some reporting bias in that several studies did not report on symptomatic bacterial UTI, and very limited information on harm. For example, Reid 2003 did not report symptomatic bacterial UTI recurrence; these data would have been valuable because the meta‐analysis of studies that did report this outcome did not rule out a clinically important increased or decreased risk. These studies also included different patient populations resulting in the analysis of separate small groups of studies instead of the evidence base as a whole.
There was insufficient evidence to determine if probiotics provide a therapeutic advantage over placebo for susceptible patient populations (e.g. previous history of UTI, women, school‐aged girls, men with enlarged prostates, and the elderly). Included studies randomised primarily women and young girls with no studies enrolling men with enlarged prostates or the elderly.
Quality of the evidence
Our assessments suggested an unclear or high risk of bias (Figure 2; Figure 3, Table 1). As such, evidence has been downgraded to low for all outcomes listed in the Table 1. This suggested that treatment effects were likely overestimated and that better methodological control is required in future research. Future studies should model methods from Stapleton 2011. Adequate allocation concealment was described in four of the eight included studies; only two studies were double blinded. Attrition bias was of concern in all but one included study.
The available evidence varied in terms of probiotic used, route of delivery and duration of therapy. These differences make drawing specific conclusions difficult and likely contribute to the heterogeneity seen in the pooled estimates. Most included studies did not systematically collect adverse event information, thus we could not draw conclusions regarding the potential harms associated with these therapies.
Potential biases in the review process
Our literature search included several international databases and search criteria were intentionally broad to identify as many potentially relevant articles as possible. We did not exclude studies published in languages other than English. We contacted study authors for missing information.
Agreements and disagreements with other studies or reviews
Grin 2013 is the most recent systematic review on this topic. Grin 2013 included five RCTs, against nine included in this review. Our assessment is that Grin 2013 limited their search to include only studies that included premenopausal women with history of UTI. Our inclusion criteria did not limit to a particular population nor did it exclude studies in healthy people (e.g. Reid 2003 enrolled healthy women and met our inclusion criteria). Grin 2013 concluded that it was possible that certain strains lactobacillus‐containing suppositories could prevent recurrent UTI in premenopausal women. Grin 2013 suggested that more RCTs were required to be certain of the effect on recurrent UTI. In addition, Grin 2013 suggested that current RCTs did not enable definitive conclusions to be made about the safety of probiotics.
In general, our conclusions are similar to Grin 2013 in that more RCTs are required to determine the net health impact of probiotics, although our conclusions apply to a broader patient population.
Authors' conclusions
Implications for practice.
There was insufficient evidence to determine whether or not probiotics reduce the risk of further UTIs in susceptible patient populations (e.g. previous history of UTI, women, school‐aged girls, men with enlarged prostates, and the elderly) compared with either placebo or antibiotic prophylaxis. This conclusion is limited by the generally high risk of bias in the small number of studies available, with limited reporting on harm, mortality and serious adverse events.
Implications for research.
Larger well‐designed RCTs are necessary and should include recurrent symptomatic UTI as primary outcome. The potential for probiotics to reduce recurrent UTI in women and children with recently‐treated UTI compared to those without a recently‐treated UTI should be explored. Optimal probiotic agents, dosing and duration of therapy also remain to be determined. Studies should be placebo‐controlled or contain both a placebo and active antibiotic control group. Emphasis should be placed on the measurement of harm as well as development of recurrent UTI during follow‐up. We feel that until there is sufficient evidence that probiotics provide a therapeutic advantage over placebo, future studies should not focus on active comparators alone.
Acknowledgements
Peter Jewesson for his help refining our research question and assistance in writing the protocol
We wish to thank the referees for their comments and feedback during the preparation of review
Stephen Adams for help in retrieving studies
Drs Stapleton and Beerepoot for providing data.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Probiotics versus placebo in adults and children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic bacterial UTI | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.60, 1.12] |
| 1.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.65, 1.93] |
| 1.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.52, 1.05] |
| 2 Worst case scenario ‐ symptomatic bacterial UTI | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 2.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.12, 112.47] |
| 2.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.19] |
Comparison 2. Probiotics versus antibiotics in women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic bacterial UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Worst case scenario probiotics ‐ symptomatic bacterial UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Worst case scenario antibiotics ‐ symptomatic bacterial UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Probiotics versus control in children with vesicoureteric reflux.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic bacterial UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Worst case scenario imputation ‐ symptomatic bacterial UTI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Placebo comparison | 6 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.63, 1.39] |
| 1.1 Versus placebo | 2 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.12, 112.47] |
| 1.2 Versus placebo with recurrent UTI | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.19] |
| 2 Antibiotic comparison ‐ worst case probiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Antibiotic comparison ‐ worst case antibiotics | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Versus antibiotics with recent UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
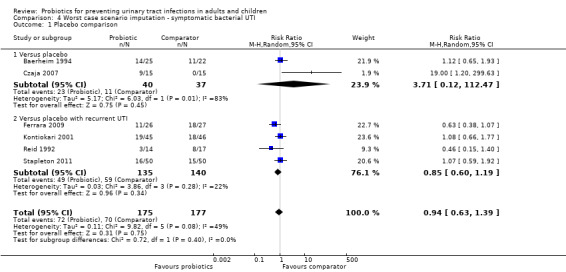
Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 1 Placebo comparison.
4.2. Analysis.
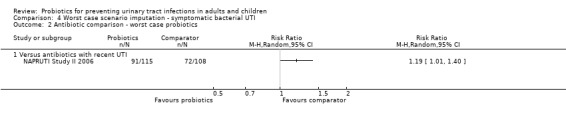
Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 2 Antibiotic comparison ‐ worst case probiotics.
4.3. Analysis.
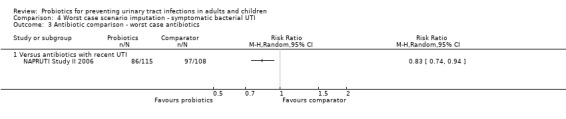
Comparison 4 Worst case scenario imputation ‐ symptomatic bacterial UTI, Outcome 3 Antibiotic comparison ‐ worst case antibiotics.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baerheim 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Double‐blind..." Page 240 ‐ Materials and methods. Not described further |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "One was later excluded on her own request before she started to use the vaginal suppositories." Page 240 ‐ Results 1/48 lost prior to initiation of therapy. Authors did not report to which arm the patient had been randomised |
| Selective reporting (reporting bias) | High risk | Main outcome reported with non‐significant difference between groups. Two outcomes described in the methods (compliance and causative pathogen) were not reported and no explanation was given. No protocol published or in a clinical trial registry |
| Other bias | Unclear risk | Organon A/S, Oslo, Norway provided drug and funding support Limited reporting of harm. Control group had a significant increase in Lactobacilli, intervention group did not ‐ explained as regression to mean. Page 240 ‐ Results |
Czaja 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment Group
Control Group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Pre‐packaged by manufacturer according to randomisation schedule and supplied to the study site sequentially labelled with subject number |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In a double‐blind fashion..." "L. crispatus CTV‐05 and placebo suppositories were similar in appearance..." Page 2 ‐ Study design. Authors did not specify who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In a double‐blind fashion..." Page 2 ‐ Study design. Authors did not specify who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low risk at four weeks ‐ 30/30 women completed the baseline, 1‐ and 4‐week visits. High risk at six months ‐ 9/15 intervention and 10/15 control patients completed the 6‐month phone call ‐ figure 1, page 3 Two intervention patients completed treatment over 6 days instead of 5 and one control patient used 6 suppositories over 6 days ‐ page 2 results |
| Selective reporting (reporting bias) | Unclear risk | All outcomes appear to be reported, adverse drug reactions reporting may have been subjective. There was no published protocol or clinical trial registry protocol available |
| Other bias | High risk | Product supplied by the manufacturer |
Ferrara 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised into three groups using random number tables" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four children dropped out of the study (5%) for poor compliance to the protocol (one from cranberry juice arm, one from Lactobacillus GG arm and two from control arm). Unclear how data was collected or accounted for in these patients. |
| Selective reporting (reporting bias) | Unclear risk | No reporting on any of the secondary outcomes of this review. No protocol was published or in a clinical trial registry |
| Other bias | Unclear risk | Patients had previously treated UTIs Funding unclear Described 5/27 probiotic intervention patients and 7/29 control patients requiring "antimicrobial prophylaxis" Page 371 ‐ Results. Not further described |
Kontiokari 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly allocated into three groups by using tables of random numbers and a block technique..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement Quote: "...a block technique using a block size of 6." Page 1 ‐ Study population and design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...laboratory staff were unaware as to which of the treatment groups participants belonged." Page 2 ‐ Study population and design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/50 cranberry patients, 4/50 lactobacillus patients and 5/50 control patients dropped out of the study Page 2 – Results “One subject in the lactobacillus group who was taking postcoital antimicrobials was excluded from the analysis” Page 2 “Only cultures with > 105 CFU/mL were accepted and recorded as events. A urine sample with no bacterial growth was required between two episodes before they were regarded as separate events. Women who had three or more episodes in six months were offered antimicrobial prophylaxis” Page 2 – Each woman contributed days at risk until she dropped out, became pregnant, or started antibiotic prophylaxis Page 3 – "We also did an analysis based on the assumption that women who dropped out of the intervention groups subsequently had a UTI whereas those left in the control group did not, but the differences in the occurrence of the first urinary tract infection remained significant (P = 0.046 at 12 months). We also did an analysis based on the assumptions that women who dropped out of the intervention groups subsequently had a UTI whereas those who left the control group did not, but the differences in the occurrence of the first UTI remained significant (P = 0.046 at 12 months)" |
| Selective reporting (reporting bias) | Unclear risk | Outcome appears to be completely reported including some additional outcomes that had not been pre‐specified. No published protocol or clinical registry protocol available. |
| Other bias | High risk | "The dosing frequencies and the duration of the prophylaxis were based on the availability of the products from our suppliers.” Page 1 Lactobacillus given for one year but juice given for six months Page 1 |
Lee 2007a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...prospectively randomised..." "...stratified randomisation..." Page 1316 ‐ Patients and methods Not described further |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/60 in the probiotic group and 1/60 in the antibiotic group were noncompliant (< 80%) Pg1316 ‐ Patients and methods May have gone to outpatient clinics, therefore may have missed some cases of UTI – Page 1317 No loss to follow up described |
| Selective reporting (reporting bias) | Unclear risk | Outcome appears to be completely reported. Causative organism was not pre‐specified but was reported. No published or clinical trial registry protocol available |
| Other bias | Unclear risk | No placebo comparator Unclear funding source |
NAPRUTI Study II 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control Group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The coordinating centre prepared drug randomisation lists for each study site in advance." Page 705 ‐ Intervention No further description |
| Allocation concealment (selection bias) | Low risk | "Concealed randomisation was ensured using computer‐aided block randomisation (block size remained masked), with prestratification by centre and presence (yes/no) of complicating host factors" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, double dummy. Asked patients to guess which arm they had been in after the study ended |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Analysis on main outcome measures was performed before breaking the treatment code." Page 706 ‐ Statistical Analysis |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "...performed an intention‐to‐treat analysis among participants who took at least one dose of study medication." Page 706 ‐ Statistical Analysis 12 TMP/SMX patients and 2 Lactobacilli patients were not included in the analysis as they withdrew consent prior to receiving the assigned medication Two additional TMP/SMX patients were lost to follow up as were six Lactobacilli patients. Complete follow up data for six months: TMP/SMX 100/115, Lactobacilli 98/123 Complete follow up data for 12 months: TMP/SMX 90/115, Lactobacilli 84/123 Complete follow up data for 15 months: TMP/SMX 88/115, Lactobacilli 79/123 |
| Selective reporting (reporting bias) | High risk | The registered protocol states Primary outcomes
Secondary outcomes
However, the published report states the following outcomes were measured (and these were not mentioned in the protocol): Mean number of antibiotic prescriptions for treatment of UTIs and subgroup analysis of mean number of clinical recurrences in women with complicated versus uncomplicated UTIs |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Reid 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...each patient randomly received one capsule..." Page 12 ‐ Study design |
| Allocation concealment (selection bias) | Low risk | Allocation was blinded randomly by hospital pharmacists |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Six patients decided not to take suppositories...” Page 13 ‐ Results “...31 of the original 41 patients complied well...” Page 13 ‐ Results “...nine did not return for long‐term follow up so could not be included in recurrent UTI analysis” Table Page 13 |
| Selective reporting (reporting bias) | Unclear risk | All outcomes appear to be reported in full. No published protocol cited or available. |
| Other bias | High risk | Sponsored by Merck Frosst Used skim milk placebo – unknown effect on promotion or inhibition of UTI or lactobacillus growth/colonisation |
Reid 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomly allotted to receive either a freeze dried capsule containing the L. rhamnosus GR‐1 and L. fermentum RC‐14 or calcium carbonate placebo by mouth once daily for 60 days" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The subjects and investigators were blinded to the therapy." No mention of double‐dummy methods or details of how people were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered or published |
| Other bias | High risk | Funded by Procter and Gamble |
Stapleton 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study participants were randomly assigned to Lactin‐V or placebo by use of a computer‐generated randomised number system in blocked assignments to achieve equal sample sizes in both groups" |
| Allocation concealment (selection bias) | Low risk | "The assigned intervention substance (Lactin‐V or placebo) was packaged in identically appearing packets according to assignment and sequential study number" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Confirmed with author (telephone, 10 January 2013) that everyone associated with patient care, data analysis, study interventions or outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Confirmed with author (telephone, 10 January 2013) that everyone associated with patient care, data analysis, study interventions or outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two people in each group were not included in the final analysis for UTI. Reason submitted by study team (email communication 10 January 2013): "These women had symptomatic episodes for which they were treated empirically, without a urine culture result. They were excluded due to the lack of culture confirmation for a treated symptomatic episode during their follow‐up." In the flow sheet, a few more people did not complete the 10 weeks follow‐up (43 versus 50 probiotic patients and 44 versus 50 control patients) "They did not attend the last scheduled follow‐up visits. Some had recurrences before they were LTFU. Those who did not have recurrences recorded were assumed to have been negative for a recurrence in the main ITT analysis, since our clinic is their main health care provider. Per protocol analyses was also performed and yielded similar results" |
| Selective reporting (reporting bias) | Low risk | Registered protocol aligns with published report |
| Other bias | Low risk | Grant funded |
CFU ‐ colony forming units; CI ‐ confidence intervals; HIV ‐ human immunodeficiency virus; HPF ‐ high power field; ITT ‐ intention‐to‐treat; LTFU ‐ loss to follow‐up; PI ‐ primary investigator; RCT ‐ randomised controlled trial; SAE ‐ serious adverse event; SD ‐ standard deviation; STI ‐ sexually transmitted infection; TMP/SMX ‐ trimethoprim/sulphamethoxazole; UTI ‐ urinary tract infection; VUR ‐ vesicoureteric reflux
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Colodner 2003 | Same product used as intervention in both study arms |
| Dani 2002 | Neonatal population |
| Manley 2007 | Probiotic was used for treatment, not prophylaxis |
| Mohseni 2013 | Not probiotic monotherapy (combined with antibiotics) |
| Molander 1990 | Intervention used was a hormone product, not a probiotic |
| NCT00900653 | Intervention was a combination of low dose oestriol and Lactobacilli |
| Pushkarev 2005 | Probiotic was used for treatment, not prophylaxis |
| Ranganathan 2009 | Enrolled patients had CKD. Probiotics were tested for renoprotective effects and to relieve symptoms of uraemia. Patients were not selected for their susceptibility for UTI |
CKD ‐ chronic kidney disease; UTI ‐ urinary tract infection
Characteristics of studies awaiting assessment [ordered by study ID]
Reid 1995.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Full‐text article currently not available |
Skerk 2010.
| Methods | RCT |
| Participants | N = 117. Probiotic = 56; control = 61 Inclusion criteria: lower UTI (dysuria, polyuria, urgency) symptoms, identical microbiological findings or cervical swab and urinary culture, leucocyturia, ultrasound ruling out urinary tract abnormalities Exclusion criteria were not reported |
| Interventions | All participants received antimicrobial therapy for seven days then Acidophilus probiotic orally for three months and vaginally for seven days simultaneously or no treatment |
| Outcomes | Recurrent cystitis |
| Notes | Abstract only available in English and the manuscript is yet to be translated (Croatian) |
UTI ‐ urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
NCT00781625.
| Trial name or title | Probiotics/Lactobacillus as a Prophylactic Aid in Recurrent Bacterial Cystitis in Women. A randomised, Prospective, Double‐Blinded, Placebo Controlled, Multi‐Center Study |
| Methods | Double‐blind, randomised, parallel efficacy study |
| Participants | Women aged 18 to 70 years with more than three UTIs in the previous year, without urinary tract abnormalities. For complete Inclusion and exclusion criteria see http://www.clinicaltrials.gov/ct2/show/NCT00781625 |
| Interventions | Oral UREX‐cap‐5 Vaginal UREX‐cap‐5 Oral placebo Y cap G‐3 Vaginal placebo Y cap G‐3 |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | October 2008 |
| Contact information | Caroline Ursin Skagemo, MD Gunn Iren Meling, PhD, MD |
| Notes | Email from author 26 March 2013. No data to report |
ProSCIUTTU Study 2014.
| Trial name or title | A multicenter randomised double‐blind, double‐dummy placebo‐controlled study to assess the efficacy, safety and cost‐utility of Probiotic prophylaxis of spinal cord injury Urinary Tract Infection. A Therapeutic Trial (ProSCIUTTU) |
| Methods | Double‐blind, multicenter, double‐dummy, randomised, placebo‐controlled study |
| Participants | Men or women with spinal cord injury suffering from recurrent UTI resulting from multi‐resistant organisms. Men or women with stable multiple sclerosis or cerebral vascular disease, with documented neurogenic bladder on video urodynamic assessment, who also suffer from recurrent UTI resulting from multi‐resistant organisms. For complete inclusion and exclusion criteria see http://www.anzctr.org.au/ACTRN12610000512022.aspx |
| Interventions | Oral probiotic, two lactobacillus/Bifidobacterium capsules daily for six months Oral placebo, two capsules daily for six months |
| Outcomes | Primary
Secondary
|
| Starting date | August 2010 |
| Contact information | Dr Bon San Bonne Lee Spinal Injuries Unit, Prince of Wales Hospital, Randwick, Australia |
| Notes | Email sent 26 March 2013 requesting study publication or an update |
UTI ‐ urinary tract infection
Differences between protocol and review
The protocol specified that men, women and children who were at risk of recurrent UTI would be included in the review. We intended this to exclude children aged under one year old, and did so during the selection of studies, although this was not made clear in the protocol.
In addition, we had planned to conduct subgroup analysis of studies comparing probiotics with placebo or active comparators. However, there was only one study with an active comparator (antibiotics) known to have efficacy versus placebo (NAPRUTI Study II 2006). We chose to analyse this separately rather than as a subgroup. Ferrara 2009 and Kontiokari 2001 used comparators with no known efficacy or safety advantage versus placebo and we did not feel it would be appropriate to compare these with probiotics. No subgroup analyses were conducted for these two studies.
We conducted post‐hoc subgroup analyses for studies that enrolled participants who had recent UTI compared with studies that enrolled patients who had not had a recent UTI (Analysis 1.1).
Contributions of authors
Draft the protocol: ES, AT, PL
Study selection: ES, AT
Extract data from studies: ES, AT
Enter data into RevMan: ES, AT
Carry out the analysis: ES, AT, PL
Interpret the analysis: AT, PL
Draft the final review: ES, AT, PL
Disagreement resolution: PL
Update the review: ES, AT, PL
Declarations of interest
Erin M Schwenger: None known
Aaron M Tejani: None known
Peter S Loewen: None known
New
References
References to studies included in this review
Baerheim 1994 {published data only}
- Baerheim A, Larsen E, Digranes A. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scandinavian Journal of Primary Health Care 1994;12(4):239‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Czaja 2007 {published data only}
- Czaja CA, Stapleton AE, Yarova‐Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infectious Diseases in Obstetrics & Gynecology 2007;2007:35387. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrara 2009 {published data only}
- Ferrara P, Romaniello L, Vitelli O, Gatto A, Serva M, Cataldi L. Cranberry juice for the prevention of recurrent urinary tract infections: a randomized controlled trial in children. Scandinavian Journal of Urology & Nephrology 2009;43(5):369‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kontiokari 2001 {published data only}
- Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M. Randomised trial of cranberry‐lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 2001;322(7302):1‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2007a {published data only}
- Lee SJ, Kim HJ, Shim YH, Lee JW. The effect of probiotic prophylaxis for preventing recurrent urinary tract infection in children with persistent primary vesicoureteral reflux [abstract no: COD. PP 81]. Pediatric Nephrology 2006;21(10):1542. [CENTRAL: CN‐00724966] [Google Scholar]
- Lee SJ, Shim YH, Cho SJ, Lee JW. Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatric Nephrology 2007;22(9):1315‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NAPRUTI Study II 2006 {published data only}
- Beerepoot MA, Stobberingh EE, Geerlings SE. A study of non‐antibiotic versus antibiotic prophylaxis for recurrent urinary‐tract infections in women (the NAPRUTI study) [Onderzoek naar niet‐antibiotische versus antibiotische profylaxe bij vrouwen met recidiverende urineweginfecties (de NAPRUTI‐studie)]. Nederlands Tijdschrift voor Geneeskunde 2006;150(10):574‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Beerepoot MA, Ter Riet G, Nys S, Wal WM, Borgie CAJM, Reijke TM, et al. Lactobacilli versus antibiotics to prevent urinary tract infections: A randomized, double‐blind, noninferiority trial in postmenopausal women [Lactobacillen versus antibiotica ter preventie van urineweginfecties: 'Non‐in feriority'‐studie bij post men opauzale vrouwen]. Nederlands Tijdschrift voor Geneeskunde 2013;157(10):A5674. [EMBASE: 2013248979] [Google Scholar]
- Beerepoot MA, Ter Riet G, Nys S, Wal WM, Borgie CA, Reijke TM, et al. Lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double‐blind, noninferiority trial in postmenopausal women. Archives of Internal Medicine 2012;172(9):704‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beerepoot MA, Heijer CD, Penders J, Prins JM, Stobberingh EE, Geerlings SE. Predictive value of Escherichia coli susceptibility in strains causing asymptomatic bacteriuria for women with recurrent symptomatic urinary tract infections receiving prophylaxis. Clinical Microbiology & Infection 2012;18(4):E84‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Beerepoot MA, Wal WM, Nys S. Lactobacillus rhamnosus gr‐1 and l. Reuteri rc‐14 versus trimethoprim‐sulfamethoxazole (TMP/SMX) in the prevention of recurrent urinary tract infections (RUTIs) in postmenopausal women: a randomized double‐blind non‐inferiority trial [abstract no: L1‐1656a]. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2012 Sep 12‐15; San Francisco (CA). 2012.
Reid 1992 {published data only}
- Reid G, Bruce AW, Taylor M. Influence of three‐day antimicrobial therapy and lactobacillus vaginal suppositories on recurrence of urinary tract infections. Clinical Therapeutics 1992;14(1):11‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Reid 2003 {published data only}
- Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR‐1 and L. fermentum RC‐14 significantly alters vaginal flora: randomized, placebo‐controlled trial in 64 healthy women. FEMS Immunology & Medical Microbiology 2003;35(2):131‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stapleton 2011 {published and unpublished data}
- Stapleton AE, Au‐Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, et al. Randomized, placebo‐controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clinical Infectious Diseases 2011;52(10):1212‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Colodner 2003 {published data only}
- Colodner R, Edelstein H, Chazan B, Raz R. Vaginal colonization by orally administered Lactobacillus rhamnosus GG. Israel Medical Association Journal: Imaj 2003;5(11):767‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Dani 2002 {published data only}
- Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double‐blind study. Biology of the Neonate 2002;82(2):103‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manley 2007 {published data only}
- Manley K, Fraenkel M, Mayall B, Power D. Yoghurt and VRE: Can a probiotic make a difference? [abstract no: 007]. Journal of Renal Nutrition 2008;18(3 Suppl 1):S3. [Google Scholar]
- Manley KJ, Fraenkel MB, Mayall BC, Power DA. Probiotic treatment of vancomycin‐resistant enterococci: a randomised controlled trial. Medical Journal of Australia 2007;186(9):454‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mohseni 2013 {published data only}
- Mohseni MJ, Aryan Z, Emamzadeh‐Fard S, Paydary K, Mofid V, Joudaki H, et al. Combination of probiotics and antibiotics in the prevention of recurrent urinary tract infection in children. Iranian Journal of Pediatrics 2013;23(4):430‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Molander 1990 {published data only}
- Molander U, Milsom I, Ekelund P, Mellstrom D, Eriksson O. Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post‐menopause. Maturitas 1990;12(2):113‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT00900653 {published data only}
- NCT00900653. Low dose estriol with lactobacilli (Gynoflor) treatment for preventing recurrent urinary tract infection in postmenopausal women: a randomized, open, parallel‐group study. www.clinicaltrials.gov/ct2/show/NCT00900653 (accessed 21 September 2015).
Pushkarev 2005 {published data only}
- Pushkarev AM. Efficacy of probiotic bactisporin in therapy of intrahospital urinary infection. Urologiia (Moscow, Russia) 2005, (4):48‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Ranganathan 2009 {published data only}
- Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6‐month pilot scale trial in Canada. Current Medical Research & Opinion 2009;25(8):1919‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Advances in Therapy 2010;27(9):634‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Reid 1995 {published data only}
- Reid G, Bruce AW, Taylor M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecology & Therapy 1995;23:32‐45. [Google Scholar]
Skerk 2010 {published data only}
- Skerk V, Ferincevic R, Markotic A, Andrasevic S, Milosevic V, Vargovic M, et al. Research on the efficacy of prophylactic use of Acidosalus probiotic in women with recurrent cystitis [Ispitivanje djelotvornosti profilakticke primjene probiotika Acidosalus u zena s rekurentnim cistitisom]. Infektoloski Glasnik 2010;30(1):21‐5. [EMBASE: 2010599396] [Google Scholar]
References to ongoing studies
NCT00781625 {published data only}
- NCT00781625. Probiotics/lactobacillus as a prophylactic aid in recurrent bacterial cystitis in women. a randomized, prospective, double‐blinded, placebo controlled, multi‐center study. www.clinicaltrials.gov/ct2/show/NCT00781625 (accessed 21 September 2015).
ProSCIUTTU Study 2014 {published data only}
- Toh SL, Lee BS, Ryan S, Simpson J. Prophylaxis of spinal cord injury urinary tract infection therapeutic trial (ProSCIUTTU): protocol [abstract]. 53rd ISCoS Annual Scientific Meeting; 2014 Sept 2‐4; Maastricht, Netherlands. 2014.
Additional references
Albert 2004
- Albert X, Huertas I, Pereiró I, Sanfélix J, Gosalbes V, Perrotta C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruce 1988
- Bruce AW, Reid G. Intravaginal instillation of lactobacilli for the prevention of recurrent urinary tract infections. Canadian Journal of Microbiology 1988;34(3):339‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CDC 2000
- Centers for Disease Control and Prevention (CDC). Monitoring hospital‐acquired infections to promote patient safety‐‐United States, 1990‐1999.[Erratum appears in MMWR Morb Mortal Wkly Rep 2000 Mar 10;49(9):189‐90]. MMWR ‐ Morbidity & Mortality Weekly Report 2000;49(8):149‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Christensen 2001
- Christensen J, Jepsen OB. Reduced rates of hospital acquired UTI in medical patients. Prevalence surveys indicate effect of active infection control programmes. Journal of Hospital Infection 2001;47(1):36‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Falagas 2006
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. Journal of Antimicrobial Chemotherapy 2006;58(2):266‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foxman 2003
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Disease‐a‐Month 2003;49(2):53‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grin 2013
- Grin PM, Kowalewska PM, Alahazanni W, Fox‐Robichaud AE. Lactobacillus for preventing recurrent urinary tract infections in women: meta‐analysis. Canadian Journal of Urology 2013;20(1):6607‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Hawthorn 1990
- Hawthorn LA, Reid G. Exclusion of uropathogen adhesion to polymer surfaces by Lactobacillus acidophilus. Journal of Biomedical Materials Research 1990;24(1):39‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heineman 2000
- Heinemann C, Hylckama Vlieg JE, Janssen DB, Busscher HJ, Mei HC, Reid G. Purification and characterization of a surface‐binding protein from Lactobacillus fermentum RC‐14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiology Letters 2000;190(1):177‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howes 2009
- Howes DS. Urinary tract infections, female. emedicine.medscape.com/article/778670‐overview (accessed August 2010).
Howes 2010
- Howes DS, Pillow MT. Urinary tract infections, male. emedicine.medscape.com/article/778578‐overview (accessed August 2010).
Jepson 2012
- Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001321.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maki 2001
- Maki D, Tambyah PA. Engineering out the risk of infection with urinary catheters. Emerging Infectious Diseases 2001;7(2):342‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osset 2001
- Osset J, Bartolome RM, Garcia E, Andreu A. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. Journal of Infectious Diseases 2001;183(3):485‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ouslander 1995
- Ouslander JG, Schnelle JF, Uman G, Fingold S, Nigam JG, Tuico E, et al. Predictors of successful prompted voiding among incontinent nursing home residents. JAMA 1995;273(17):1366‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Perrotta 2008
- Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005131.pub2] [DOI] [PubMed] [Google Scholar]
Richards 2001
- Richards C, Emori TG, Peavy G, Gaynes RP. Promoting quality through measurement of performance and response: prevention success stories. Emerging Infectious Diseases 2001;7(2):299‐301. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Richards 2004
- Richards CL. Urinary tract infections in the frail elderly: Issues for diagnosis, treatment and prevention. International Urology & Nephrology 2004;36(3):457‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saint 2000
- Saint S, Wiese J, Amory JK, Bernstein ML, Patel UD, Zemencuk JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters?. American Journal of Medicine 2000;109(6):476‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schnelle 1995
- Schnelle JF, McNees P, Crooks V, Ouslander JG. The use of a computer‐based model to implement an incontinence management program. Gerontologist 1995;35(5):656‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrezenmeir 2001
- Schrezenmeir J, Vrese M. Probiotics, prebiotics, and synbiotics ‐ approaching a definition. American Journal of Clinical Nutrition 2001;73(2 Suppl):361S‐364S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Velraeds 1998
- Velraeds MM, Belt‐Gritter B, Mei HC, Reid G, Busscher HJ. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. Journal of Medical Microbiology 1998;47(12):1081‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 2011
- Williams G, Craig JC. Long‐term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001534.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schwenger 2010
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008772] [DOI] [PMC free article] [PubMed] [Google Scholar]


