Abstract
Background:
Ventriculitis usually occurs as the result of infection and results in the inflammation of the ependymal lining of the ventricular system. Mortality rates remain high despite treatment.
Case Description:
We present the case of a 66-year-old man who presented with altered mental status and progressively became comatose. He was found to have fulminant ventriculitis due to a ruptured intracranial abscess. He was treated with bilateral IRRAflow® catheter (IRRAS, Stockholm, Sweden) placement through which continuous irrigation with vancomycin was initiated.
Conclusion:
This treatment was safe and led to improvement in the patient’s neurologic examination, imaging findings, and cerebrospinal fluid profiles.
Keywords: IRRAflow® system, Technology, Ventriculitis

BACKGROUND
Ventriculitis is defined as inflammation of the ependymal lining of the ventricular system and usually occurs as the result of infection. Ways in which infection can seed the ependymal system include meningitis, rupture of intracranial abscesses, trauma, and catheter-associated spread.[12,13] The mortality associated with ventriculitis caused by intraventricular rupture of an intracranial abscess tends to be high, with rates from 26% to 72.7% reported in various case series depending on the route of infection and the organism responsible.[10,14] Intraventricular antibiotic administration has been posed as a potential treatment of these aggressive infections with some positive results.[5]
The advent of self-irrigating catheter systems, such as the IRRAflow® (IRRAS, Stockholm, Sweden), has expanded interest in applications for this technology with early reports of use in the treatment of chronic subdural hematomas and intraventricular hemorrhage.[2,7] In addition to the standard “drain-only” option available on a typical ventricular drain, the IRRAflow® can be set to irrigate small volumes at a fixed rate through its dual lumen catheter. Typically, normal saline is used as the irrigation solution, although in theory this technology could be used as a drug delivery system. We present a case report of a patient treated with continuous intraventricular vancomycin for ventriculitis using the IRRAflow® system for this purpose.
CASE DESCRIPTION
The patient was a 66-year-old man with a medical history of hepatitis C and benign prostatic hypertrophy who presented originally to our institution with altered mental status following 1 week of headaches. He attended a religious event and after arriving home was found in front of his computer, attempting to use it even though it was turned off. His examination on arrival was remarkable for disorientation, a right homonymous hemianopia, and nonsensical speech. Computed tomography (CT) of the head demonstrated a left occipital lesion concerning for intracranial abscess with intraventricular extension [Figure 1]. This was confirmed on contrast-enhanced magnetic resonance imaging (MRI) that also demonstrated diffuse enhancement of the lateral and third ventricles [Figure 2a and b].
Figure 1:
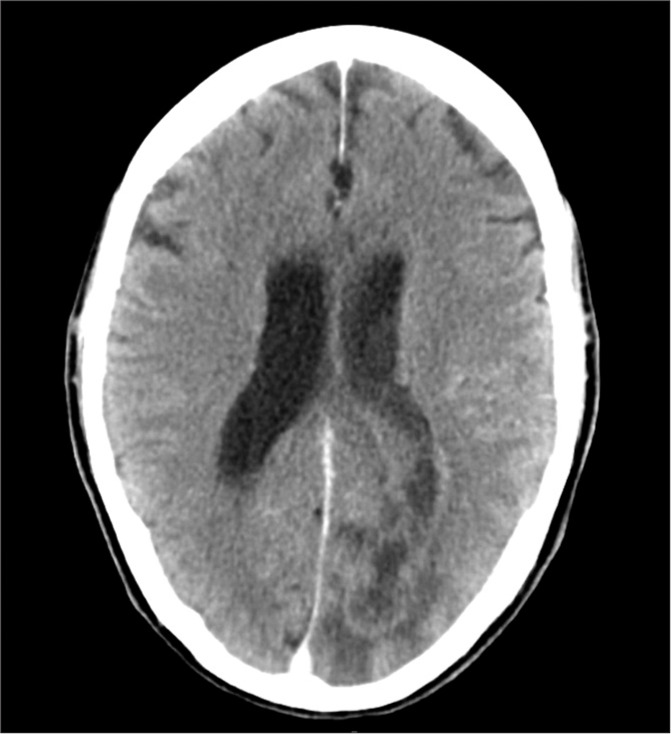
Computed tomography of the head demonstrating a mixed density left occipital lesion with the presence of hypodense material extending into the lateral ventricle.
Figure 2:
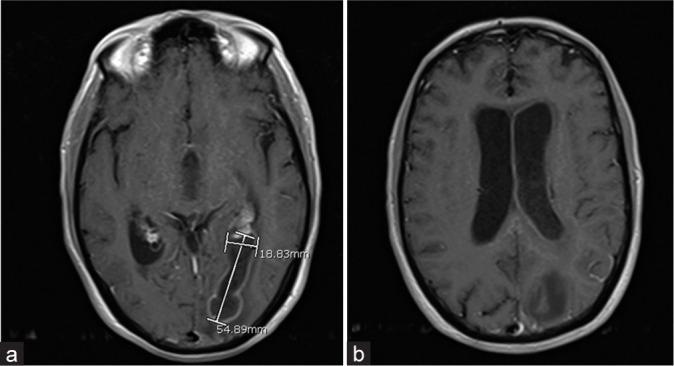
Magnetic resonance images (a and b) of the brain showing a ring-enhancing left occipital lesion with intraventricular enhancement consistent with intraventricular rupture of an intracranial abscess.
Soon the patient began to have a seizure, becoming unresponsive with right gaze deviation and hypertonicity in his extremities. Despite treatment with several milligrams of intravenous (IV) lorazepam and 1000 mg of fosphenytoin, he continued to seize requiring intubation and heavy sedation with a continuous infusion of propofol. To treat the hydrocephalus associated with his ventriculitis, a left-sided Kocher’s point IRRAflow® catheter system was placed and set to drain-only mode [Figure 3]. Blood and cerebrospinal fluid (CSF) cultures were submitted for pathologic analysis, and the patient was admitted to the intensive care unit. IV antibiotic therapy was initiated consisting of vancomycin (1250 mg every 12 h), ceftriaxone (2 g every 12 h), and metronidazole (500 mg every 8 h). Continuous electroencephalography (EEG) was used to target a pattern of burst suppression. Overnight, the IRRAflow® catheter became clogged with thick pus, and a second right-sided IRRAflow® catheter was placed [Figure 4]. Gradually, his sedation was weaned off. Despite discontinuation of the sedation, his neurologic examination findings were extremely poor. His pupils were reactive, but he had weak corneal reflexes and minimal motor response to noxious stimuli. EEG was continued to monitor for any seizure activity, although none was captured.
Figure 3:
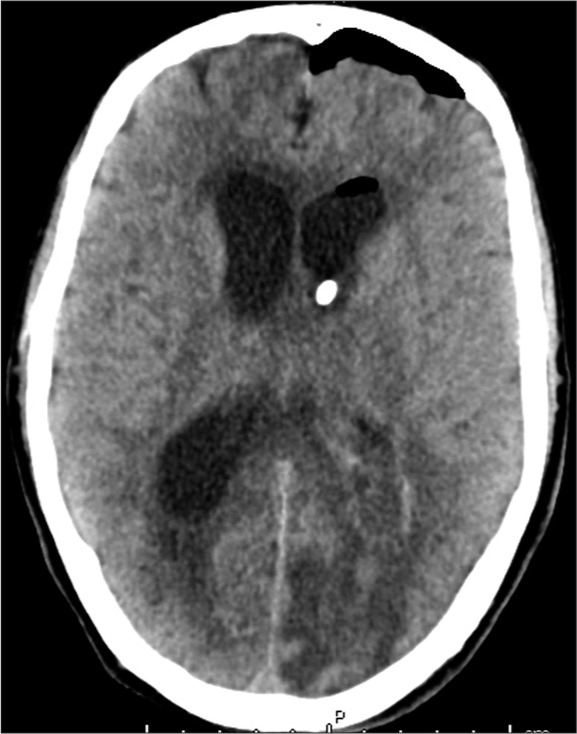
Computed tomography of the head used to confirm placement of the IRRAflow® catheter within the left lateral ventricle.
Figure 4:
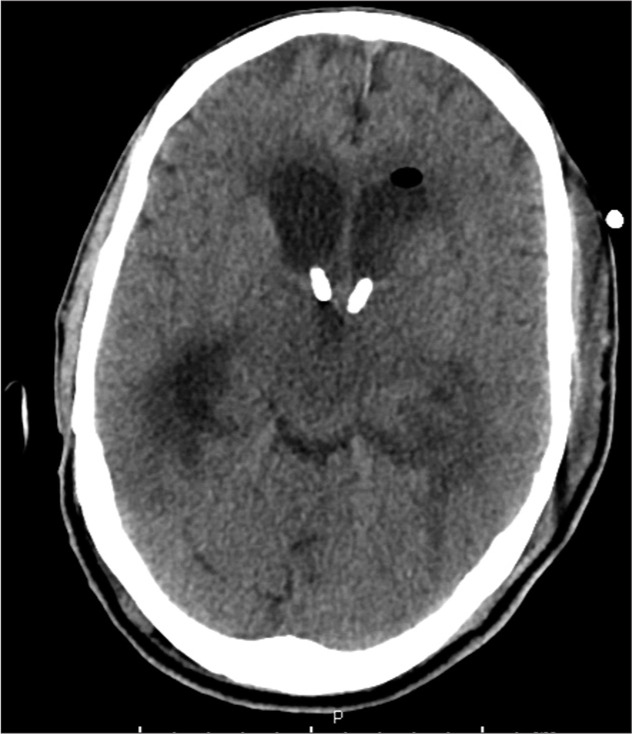
Computed tomography of the head demonstrating placement of the second IRRAflow® catheter within the right lateral ventricle.
On hospital day 2, irrigation with normal saline was started through the IRRAflow® system bilaterally at a rate of 40 mL/h. A single dose of intraventricular vancomycin (10 mg) was given through the system on hospital day 3, and irrigation was resumed following a 1-h clamp of the system to allow distribution of the antibiotic therapy. On hospital day 4, the normal saline irrigation solution was replaced with 50 mg of vancomycin diluted in 1 L of normal saline. This solution was continuously irrigated through the system at 60 ml/h bilaterally. In addition, 4 mg of IV dexamethasone was administered every 6 h.
Over the subsequent days, the patient’s neurologic condition began to improve. By hospital day 6, he was extubated. He was conversant and following commands. In the interim, blood and CSF cultures were positive for Streptococcus intermedius. The IV ceftriaxone and metronidazole, as well as the intraventricular vancomycin were continued at the previous doses. An extensive workup was conducted to explore the source of the infection, including CT of the abdomen, pelvis, face, and sinuses. These were all negative. A human immunodeficiency virus test was negative. No source was able to be identified.
During the patient’s hospital course, CSF profiles obtained on a daily basis showed continued improvement [Table 1]. Inflammatory marker tests were also regularly obtained, and the values down trended. Even the drainage from the IRRAflow® system began to clear, going from thick, and proteinaceous pus to a thin straw-like fluid. Repeat MRI completed approximately 1 week after the patient’s presentation demonstrated decrease in the abscess burden and ventricular enhancement [Figure 5].
Table 1:
Cerebrospinal fluid profiles.
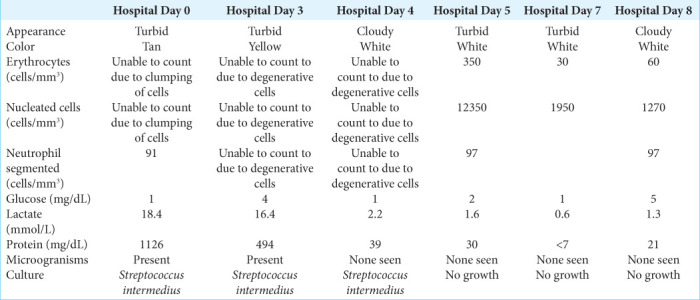
Figure 5:
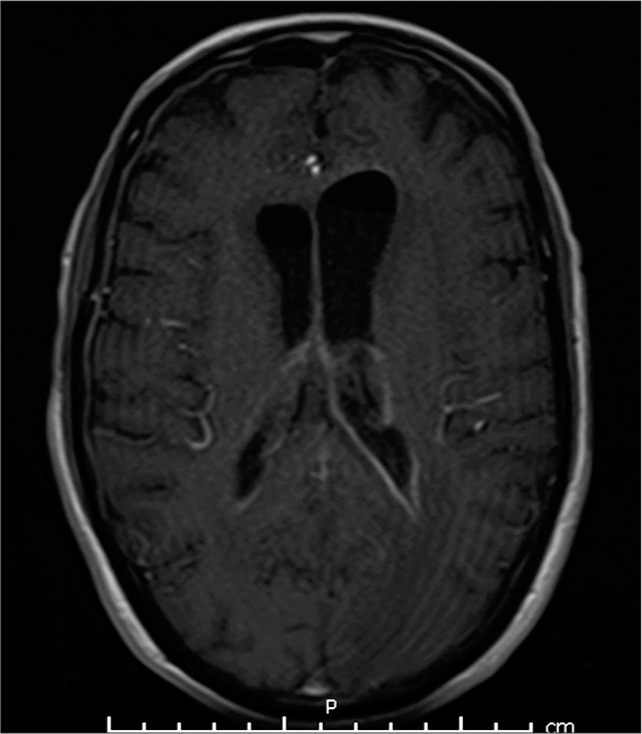
Magnetic resonance imaging of the brain following IRRAflow® treatment showing decreased ventricular abscess burden.
Despite improvement in the patient’s clinical status following treatment, he was significantly debilitated. Discussions were had with the patient’s family who emphasized that the patient’s previous state of function was excellent, and he would not want aggressive measures if his function would not return exactly to its previous state. As such, the patient’s family proceed with comfort care measures, and the patient subsequently died several weeks later.
DISCUSSION
Spontaneous intraventricular rupture of an intracranial abscess is a devastating complication with a mortality rate approaching 80% in one series.[16] The high rate of mortality is due to acute hydrocephalus and rapid dissemination of infectious materials throughout the CSF space leading to fulminant meningoencephalitis.[16] In those who are fortunate enough to survive the acute phase, the ongoing inflammatory process leads to scarring of the ependymal surface ultimately leading to septations and loculated hydrocephalus.[9] Treatment of septations, once they have already formed, can be accomplished through a neuroendoscopic procedure in which the septations are broken up and the inflammatory debris drained.[6]
One factor that influences the treatment of ventriculitis is the ability to achieve adequate penetration of antimicrobial agents into the brain due to significant purulence.[4] To combat this, intrathecal antibiotics have been proposed.[8] In their case series, Isono et al. report that early ventricular drainage, along with intraventricular antibiotics, dramatically improved outcomes in patients with intraventricular rupture of an intracranial abscess.[3] In addition, they found that early, aggressive ventricular irrigation can prevent formation of septations and improve prognosis. Similarly, Remeš et al. demonstrated the rapid sterilization of CSF following intrathecal antibiotics administered to 34 post-neurosurgical patients with meningitis or ventriculitis, with nearly 50% of patients demonstrating sterilization within 24 h.[8] Only 3 of the 34 patients demonstrated adverse drug reactions, which supports the safety of this intervention.
Despite the positive outcomes reported by Isono et al. and Remeš et al.,[3,8] large case series and randomized controlled trials are lacking. In addition, there is no clear consensus on the use of intrathecal antibiotics for ventriculitis within the neurosurgical community.[15] von Spreckelsen et al. queried 121 neurosurgical programs across Germany regarding their treatment of ventriculitis.[15] Of the 33 respondents, 30% of programs did not utilize intrathecal antibiotics, 36% considered intrathecal antibiotics on a case-by-case basis, and 21% utilized intraventricular antibiotics routinely. Introducing antibiotics directly into the intraventricular space is thought to improve the bioavailability of antibiotics in the central nervous system (CNS). The utilization of irrigation may decrease the risk of seizures or aseptic meningitis associated with intraventricular antibiotic administration.[11]
Given the high mortality of intraventricular rupture of an intracranial abscess and the lack of evidence-based treatment protocols, we elected to mimic the promising protocol reported by Isono et al.[3] The use of the IRRAflow® system allowed us to expand on this by providing continuous irrigation of the ventricular system and constant cycling of antibiotics directly into the CNS. Our hope was that aggressive irrigation would help to clear the inflammatory byproducts of the abscess, thus preventing the scarring of the ependymal surface that can occur.[9] We also felt continuous antibiotic irrigation was needed to promote penetration of the drug through the proteinaceous material within the ventricle. We used dexamethasone in the patient described in this case because steroid use may be beneficial in controlling vasogenic edema in patients with intracranial abscesses.[1] We also felt that there may be some benefit in preventing inflammatory scarring of the ependyma but that has not been proven.
Following the implementation of our therapy, the patient had dramatic improvement in his neurologic status within 3 days. He began to follow commands and was able to have a conversation. Repeat MRI demonstrated a notable improvement in the size of the patient’s abscess and intraventricular pus burden. Finally, the CSF profiles also showed remarkable improvement. Given his family’s wishes to pursue palliative options, it is difficult to say whether this novel treatment would have benefited the patient in the long term or prevented the need for shunting. In retrospect, aggressive irrigation with the IRRAflow® system on initial placement may have prevented the need for a second device. We were hesitant to begin irrigation until the patient’s intracranial pressure (ICP) stabilized because he did not have a neurologic examination to follow due to requiring heavy sedation. Despite our initial concerns, ICPs began to normalize after irrigation was started. This is an important learning point for future use of this device as only a single IRRAflow® is likely needed as long as it remains patent. Based on our experience, we advocate for aggressive irrigation to promote drainage of viscous materials, such as pus or clotted blood, to reduce ICP.
At present, several ventricular catheters are available, such as the Codman and Integra systems. To the best of our knowledge, the IRRAflow® system is the only self-irrigating ventricular catheter system on the market. Comparison of the IRRAflow® with other available devices is somewhat limited because of this, although we noted some advantages and disadvantages during this case. Continuous infusion is not possible with conventional external ventricular drainage systems; therefore, a benefit of the IRRAflow® system was that medications could be administered into the ventricular system without halting drainage. Clamping of the system to allow for proper distribution of intraventricular medications, such as vancomycin or tissue plasminogen activator, can be associated with elevations in ICPs. Although manual irrigation through the Codman and Integra systems can be performed, a clear advantage of the IRRAflow® is that this process is automatic, occurring on a minute-by-minute basis.
It is unclear whether this confers a benefit in the treatment of the underlying disease process; however, it certainly simplifies patient care.
Disadvantages of the IRRAflow® system are mainly centered on the lack of familiarity with the system. As noted in the case discussion, the IRRAflow® was used without irrigation when it was initially placed because of concern for elevation of the patient’s ICPs, whereas the standard Codman system available at our institution would typically have been manually irrigated to promote drainage of the intraventricular pus. As our experience with the device has increased, we have become more comfortable initiating aggressive drainage early on. Similarly, the initial rate of irrigation was based on our comfort level and not an evidence-based protocol. Given that the IRRAflow® is a new device, these protocols will need to be developed as neurosurgeons familiarize themselves with the device. Finally, determining the reliability of ICP measurements using waveforms from a pressure transducer is not currently available with the IRRAflow® system. At our institution, a transducer is typically attached to the buretrol, which is leveled at the tragus. Waveforms from the transducer are referenced when we examine ICP to ensure the recordings are accurate. Lack of a waveform was one of the factors contributing to our hesitancy to initiate irrigation earlier. We did attach a transducer directly to the IRRAflow® catheter outflow port later in the patient’s clinical course to obtain pressure waveforms. Although the expected three-peak waveform was present, the transducer needed to manually be leveled each time the pressures were checked.
The limitations of the research presented here include its retrospective nature and inclusion of a single patient. Larger retrospective and prospective studies examining a wide variety of neurosurgical pathologies are needed to determine the benefit of the IRRAflow® system and create evidence-based treatment protocols.
CONCLUSION
The use of an IRRAflow® catheter for continuous delivery of intraventricular irrigation with vancomycin is a technically feasible and safe treatment for ventriculitis. In our case, it led to marked improvement in neurologic status, imaging findings, and CSF profiles.
Acknowledgements
The authors thank Paul H. Dressel BFA for formatting the images and Debra J. Zimmer for editorial assistance.
Footnotes
How to cite this article: Hess RM, Khan A, Edwards M, Siddiqui AH, Levy EI. Continuous intraventricular vancomycin for treatment of ventriculitis using IRRAflow®: A case report. Surg Neurol Int 2021;12:583.
Contributor Information
Ryan M. Hess, Email: rhess@ubns.com.
Asham Khan, Email: akhan@ubns.com.
Mallory Edwards, Email: medwards@kaleidahealth.org.
Adnan H. Siddiqui, Email: asiddiqui@ubns.com.
Elad I. Levy, Email: elevy@ubns.com.
Declaration of patient consent
The authors certify that they have obtained the appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
RMH, AK, and ME: Nothing to disclose AHS—Consulting fees: Amnis Therapeutics, Apellis Pharmaceuticals, Inc., Boston Scientific, Canon Medical Systems USA, Inc., Cardinal Health 200, LLC, Cerebrotech Medical Systems, Inc., Cerenovus, Cerevatech Medical, Inc., Cordis, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Inc., Penumbra, Q’Apel Medical, Inc., Rapid Medical, Serenity Medical, Inc., Silk Road Medical, StimMed, LLC, Stryker Neurovascular, Three Rivers Medical, Inc., VasSol, Viz.ai, Inc., W.L. Gore and Associates. Leadership or fiduciary role in other board, society, committee or advocacy group: Secretary of the Board of the Society of NeuroInterventional Surgery, Chair of the Cerebrovascular Section of the AANS/ CNS. Stock or stock options: Adona Medical, Inc., Amnis Therapeutics, Bend IT Technologies, Ltd., BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc, Cerevatech Medical, Inc., Cognition Medical, CVAID Ltd., E8, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Instylla, Inc., International Medical Distribution Partners, Launch NY, Inc., NeuroRadial Technologies, Inc., Neurotechnology Investors, Neurovascular Diagnostics, Inc., PerFlow Medical, Ltd., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Adona Medical, Inc., Amnis Therapeutics, Bend IT Technologies, Ltd., BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc, Cerevatech Medical, Inc., Cognition Medical, CVAID Ltd., E8, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Instylla, Inc., International Medical Distribution Partners, Launch NY, Inc., NeuroRadial Technologies, Inc., Neurotechnology Investors, Neurovascular Diagnostics, Inc., PerFlow Medical, Ltd., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, SongBird Therapy, Spinnaker Medical, Inc., StimMed, LLC, Synchron, Inc., Three Rivers Medical, Inc., Truvic Medical, Inc., Tulavi Therapeutics, Inc., Vastrax, LLC, VICIS, Inc., Viseon, Inc. Other financial or non-financial interests: National PI/Steering Committees: Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE and SWIFT DIRECT Trials; MicroVention FRED Trial and CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; InspireMD C-GUARDIANS IDE Pivotal Trial. EIL--Consulting fees: Claret Medical, GLG Consulting, Guidepoint Global, Imperial Care, Medtronic, Rebound, StimMed, Misionix, Mosiac, Clarion, IRRAS. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Medtronic; Payment for expert testimony: for rendering medical/legal opinions as an expert. Support for attending meetings and/or travel: Reimbursement for travel and food for some meetings with the CNS and ABNS. Stock or stock options: NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, Three Rivers Medical.
REFERENCES
- 1.Hakan T. Management of bacterial brain abscesses. Neurosurg Focus. 2008;24:E4. doi: 10.3171/FOC/2008/24/6/E4. [DOI] [PubMed] [Google Scholar]
- 2.Hess RM, OConnor TE, Khan A, Siddiqui AH, Davies J. Minimally invasive approach to subdural hematoma treatment using IRRAflow catheter and middle meningeal artery embolization. Cureus. 2021;13:e13979. doi: 10.7759/cureus.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isono M, Wakabayashi Y, Nakano T, Fujiki M, Mori T, Hori S. Treatment of brain abscess associated with ventricular rupture--three case reports. Neurol Med Chir (Tokyo) 1997;37:630–6. doi: 10.2176/nmc.37.630. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan K. Brain abscess. Med Clin North Am. 1985;69:345–60. [PubMed] [Google Scholar]
- 5.Lewin JJ III, Cook AM, Gonzales C, Merola D, Neyens R, Peppard WJ, et al. Current practices of intraventricular antibiotic therapy in the treatment of meningitis and ventriculitis: Results from a multicenter retrospective cohort study. Neurocrit Care. 2019;30:609–16. doi: 10.1007/s12028-018-0647-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishizaki T, Ikeda N, Nakano S, Sakakura T, Abiko M, Okamura T. Successful neuroendoscopic treatment of intraventricular brain abscess rupture. Clin Pract. 2011;1:e52. doi: 10.4081/cp.2011.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajjoub K, Hess RM, O’Connor TE, Khan A, Siddiqui AH, Levy EI. Drainage, irrigation, and fibrinolytic therapy (DRIFT) for adult intraventricular hemorrhage using IRRAflow® self-irrigating catheter. Cureus. 2021;13:e15167. doi: 10.7759/cureus.15167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remes F, Tomas R, Jindrak V, Vanis V, Setlik M. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J Neurosurg. 2013;119:1596–602. doi: 10.3171/2013.6.JNS122126. [DOI] [PubMed] [Google Scholar]
- 9.Schultz P, Leeds NE. Intraventricular septations complicating neonatal meningitis. J Neurosurg. 1973;38:620–6. doi: 10.3171/jns.1973.38.5.0620. [DOI] [PubMed] [Google Scholar]
- 10.Shang F, Xu Y, Wang N, Cheng W, Chen W, Duan W. Diagnosis and treatment of severe neurosurgical patients with pyogenic ventriculitis caused by gram-negative bacteria. Neurol Sci. 2018;39:79–84. doi: 10.1007/s10072-017-3146-8. [DOI] [PubMed] [Google Scholar]
- 11.Shofty B, Neuberger A, Naffaa ME, Binawi T, Babitch T, Rappaport ZH, et al. Intrathecal or intraventricular therapy for post-neurosurgical Gram-negative meningitis: Matched cohort study. Clin Microbiol Infect. 2016;22:66–70. doi: 10.1016/j.cmi.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267–84. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 13.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, et al. 2017 Infectious diseases society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64:e34–65. doi: 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuon FF, Penteado-Filho SR, Amarante D, Andrade MA, Borba LA. Mortality rate in patients with nosocomial acinetobacter meningitis from a Brazilian hospital. Braz J Infect Dis. 2010;14:437–40. [PubMed] [Google Scholar]
- 15.von Spreckelsen N, Jung N, Telentschak S, Hampl J, Goldbrunner R, Grau S. Current treatment concepts for iatrogenic ventriculitis: A nationwide survey in Germany. Acta Neurochir (Wien) 2018;160:505–8. doi: 10.1007/s00701-017-3393-8. [DOI] [PubMed] [Google Scholar]
- 16.Zeidman SM, Geisler FH, Olivi A. Intraventricular rupture of a purulent brain abscess: Case report. Neurosurgery. 1995;36:189–93. doi: 10.1227/00006123-199501000-00026. [DOI] [PubMed] [Google Scholar]


