Abstract
Background:
Brain abscess in children is a neurosurgical emergency with potentially catastrophic outcome despite the advances made in neuroimaging techniques and antibiotic therapy. Symptoms are nonspecific and may vary with the child’s age, location, size, numbers and stage of abscess, and the primary source of infection. Treatment is usually with broad-spectrum antibiotics in combination and surgical evacuation in most cases or antibiotics alone in selected cases with clear-cut indications. This study was to document clinical characteristics, etiological factors, and spectrum of bacteriologic agents responsible for pediatric brain abscess in an African city, the challenges and management outcome over the study period.
Methods:
This was a retrospective study over an 11-year period involving 89 children who presented with brain abscess. Information of interest was extracted from the medical records of each participant. The results from data analysis were presented in charts and tables.
Results:
Eighty-nine children aged 0.85–15.7 years (median age of 6.4 years) met the inclusion criteria. The male-to-female ratio was 1.8:1. Headache (80%), fever (78%), and hemiparesis (78%) were the most common symptoms. Brain imaging deployed was CT scan in 56 (63%), MRI in 9 (10%), and transfontanel ultrasound scan in 24 (27%) children. Seventy-one (80%) children had antibiotics with surgical evacuation while 18 (20%) children received only antibiotics. In 19 (27%) children, the culture of the abscess was negative. In 53 (75%) children, Gram-positive aerobic organisms were isolated. A total of 75 patients (84%) had a favorable outcome.
Conclusion:
Pediatric brain abscess still poses significant public health challenge, especially in resource-limited regions. Successful management of brain abscess requires high index of suspicion for early diagnosis, referral, and intervention.
Keywords: Africa, Antibiotic, Brain abscess, Children, Gram-positive aerobes

INTRODUCTION
Despite the significant decline in mortality rate with advancements in diagnostic imaging and neurosurgical techniques, brain abscesses remain a potentially fatal central nervous system infection. Published literature reveals that approximately 25% of all brain abscesses are seen in childhood, mostly in the age group of 4–7 years.[28,36,45] The decline in the mortality rate of brain abscess from 30% to 60% in the 1970s and 1980s to 4–24% in recent years has been attributed to improvement of hygiene and vaccination in pediatric population, advances in diagnostic imaging leading to early diagnosis, improved and rapid neurosurgical intervention techniques, and broad-spectrum antibiotics covering both aerobic and anaerobic organisms.[7,11,22-24,28,33,35,36,44,45]
Brain abscess may be asymptomatic at the early stage. Clinical findings may be unspecific, mild or severe, and may be influenced by the patient’s age, the stage, size and location of abscess, presence of meningitis, and patient’s immune status. [12,14,15,18,28,36,45] The classic triad of headache, fever, and focal neurologic deficit is seen in only 9–28% of children.[28,36,45] Most commonly, these patients present with some degree of altered level of consciousness.[3,10,14,28,33,36,45] The majority of brain abscesses are associated with congenital heart defects, infections in the head-and-neck region, and dental and neurosurgical procedures.[3,13,15] The presence of immunosuppressive illness increases the risk of formation of brain abscess.[3,5,11,28,33,36,45]
The main microorganisms responsible for brain abscess are aerobic and anaerobic streptococci and staphylococci. Others are Bacteroides species, Proteus species, Haemophilus influenzae, Escherichia coli,Citrobacter group, Nocardia,Aspergillus, and Corynebacterium species and Mycobacterium tuberculosis.[3,10,14,33,36,41,45] Sterile cultures were in ranges of 10–56% in published literatures.[5,12,18,28,33,36,41,45]
A combination of broad-spectrum or organism-specific antimicrobial therapy and surgical drainage is the preferred therapeutic method in most cases. Antimicrobial therapy alone is used for small-sized lesions usually <2.5 cm, multiple abscesses, and deep-seated lesions.[12,14,27,28,34,36,45]
The objective of this study was to determine the clinical characteristics, predisposing factors, and management challenges and outcome of brain abscess in children treated in an urban African city over an 11-year period.
MATERIALS AND METHODS
This retrospective study included children with cerebral abscess treated in three facilities between January 2006 and December 2016. Lagos state is the most densely populated state in Nigeria with multiethnic and multinational representations. It is the economic capital of Nigeria with a population of over 18 million. Ethical clearance was obtained from the relevant authority (Reference No ADM/ DCST/ HREC/APP/1977).
Brain abscess was defined as a single or multifocal lesion located in the cerebrum, cerebellum, or midbrain, identifiable on computerized tomography (CT), magnetic resonance imaging (MRI) scan, or transfontanel ultrasound scan (TFUSS) which met at least one of the following criteria:
Neuroradiological findings suggesting brain abscess with clinical response to antimicrobial therapy
Purulent material within the defined lesion at surgery
A positive culture of intracerebral material
Microscopic or histologic features of an abscess.
The age, gender, clinical features at presentation, the locations and the causes of the abscesses where identified, and laboratory findings were recorded. Patients were either treated with antibiotics alone if the sizes were <2.5 cm or deep seated or antibiotics combined with surgical drainage if abscesses were large and readily accessible. In those with multiple abscesses, the large collections were drained and antibiotics continued. Surgical drainage was by craniotomy, burr hole, or percutaneous transfontanel aspiration. All aspirates were sent for Gram staining, culture, and sensitivity. Percutaneous aspiration was followed by CT scan or TFUSS and if the abscess increased in size postaspiration, it was repeated.
The results of microscopy, culture, and sensitivity of aspirates and abscess materials were documented. The treatment modalities, including surgical techniques, type and duration of antibiotic therapy, complications, and outcomes, were documented. The Glasgow Outcome Scale was used to determine favorable and unfavorable outcome.
RESULTS
Demographics
Eighty-nine children were included in this study, age 0.85–15.7 years (median age of 6.4 years). The average admission rate was nine cases/year. There were 57 males and 32 females with a male-to-female ratio of 1.8:1. Sixty-eight children (76%) were younger than 10 years of age.
Clinical features
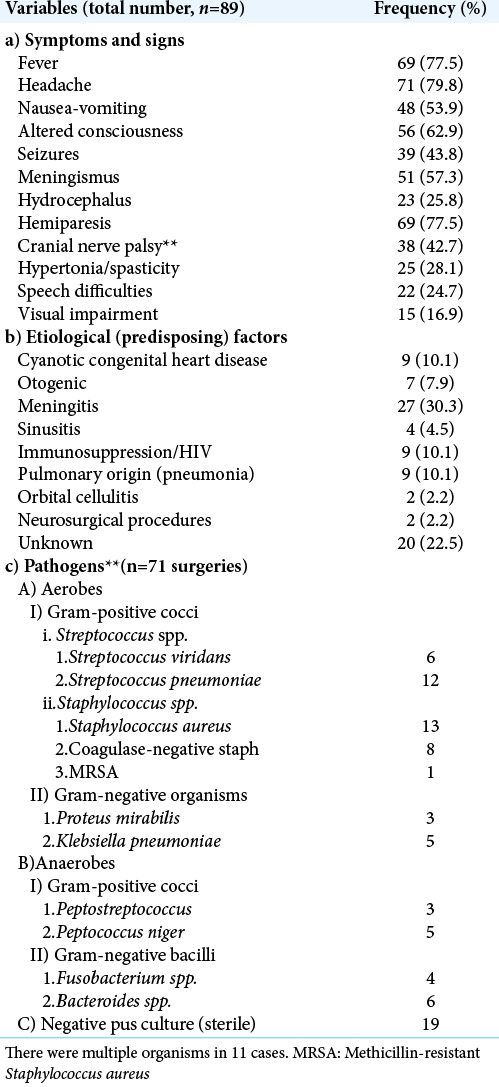
The duration of symptoms ranged from 19 to 92 days with a mean of 37.6 days. [Table 1a] shows the presenting features and their frequency in this series. The most frequent features at presentation were headache (80%), fever (78%), hemiparesis (78%), altered level of consciousness (63%), and meningismus (57%). Headache and fever being unspecific symptoms led to the children receiving empirical treatment for malaria or meningitis before diagnosis of abscess was made. Thirty-nine of 89 children (44%) had a history of seizure. Fifty-six children (63%) presented with altered sensorium. This included the 22 children (25%) that had speech impairment of varying degrees. The Glasgow Coma Score (GCS) was 13–15 in 22 of 89 children (24%), 10–12 in 25 children (28%), and <10/15 in 9 (10%). Visual impairment was seen in 15 children (17%). These were mostly older children. The classical triad of fever, headache, and focal neurologic deficit was seen in 31 children (35%).
Table 1:
Clinical profile and bacteriology of children with brain abscess.
Etiological/predisposing factors
The underlying diseases or predisposing factors are listed on [Table 1b]. The etiological factor was not known in 20 children (23%). Sixty-nine children (78%) had identifiable risk factors, the most common being meningitis (30%), cyanotic congenital heart disease (CHD) (9), pulmonary infections (9), and immunosuppression/HIV, each contributing 9 patients (10.1%) of the etiological factors. Others were otogenic infections (8%), sinusitis (5%), orbital cellulitis (2%), and neurosurgical interventions (2%). Of the nine children with CHD, two had tetralogy of Fallot, two had pulmonary atresia, one had ventricular septal defect, and one had tricuspid atresia, while the type of CHD was not specified in three others.
Diagnostic imaging and abscess characteristics
Radiologic diagnosis was made using CT scan in 56 (63%) children, (MRI) in 9 (10%), and TFUSS in 24 (27%) children. There were solitary abscesses in 63 (71%) and multiple abscesses in 26 children (29%). The abscesses were located in the supratentorial region in 69 children (78%), infratentorial in 7 children (8%), and in both regions in 9 children (10%). Of the total of 78 abscesses observed in the supratentorial region, 39 (50%) were on the left, 27 (35%) on the right, and 12 were bilateral. Some multiple abscesses affected only one hemisphere. Of the 63 cases of solitary abscesses, 62 were supratentorial, only one was infratentorial in location. Information on the specific lobe involved in each hemisphere was lacking. The multiple abscesses in the infratentorial region (15) affected the left side (6) more than the right (4) and were bilateral in five cases. The number of multiple abscesses ranged from 2 to 11.
Microbiology
Seventy-one (80%) children had surgical evacuation of the abscesses, of which 15 (21%) had negative cultures [Table 1]. Aerobes were isolated in 53 (75%) cases, 20 (28%) of them were Streptococcus species, 23 (32%) of Staphylococcus species, and 10 (14%) cases of Gram-negative aerobes. Anaerobes were isolated in 12 (17%) cases. The most common organisms isolated were Staphylococcus aureus (15; 21%) and Streptococcus pneumoniae (14; 20%). The other organisms are as listed on [Table 1c]. There were multiple cultures in 11 children and one case of methicillin-resistant S. aureus (MRSA).
Treatment
Drug therapy
All patients had parenteral antibiotics for a minimum duration of 4 weeks. Total duration of antibiotic therapy was 6–8 weeks. Unless otherwise dictated by sensitivity pattern, the most common antibiotics used were cefotaxime (or ceftriaxone), amikacin (or gentamycin), and metronidazole based on presumed etiologic agent. Vancomycin was used in the patients with MRSA or as indicated by sensitivity pattern. Other antibiotics used were meropenem, cefuroxime, cefpodoxime, and cotrimoxazole. Treatment was tailored in a multidisciplinary mode, to treat both the abscess and the source in each case. Additional procedures if required were performed by the comanaging team.
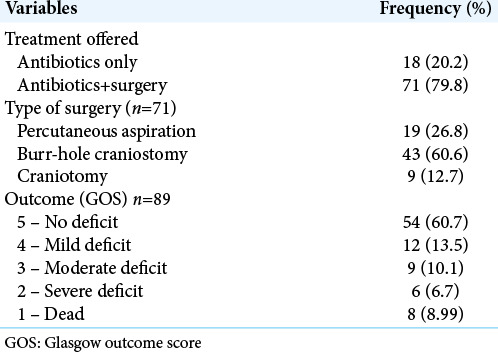
Eighteen patients (20%) received antibiotics alone while 71 patients (60%) had antibiotics and surgical drainage [Table 2]. Corticosteroid (dexamethasone) was administered to patients who had significant perilesional edema and considerable mass effect (midline shift on cranial imaging) at presentation or in the perioperative period for 3–5 days but not more than 7 days. Additional treatments given included antiepileptic drugs, physiotherapy, and speech therapy.
Table 2:
Treatment pattern and GOS.
Procedures
Of the 71 patients who had surgery, percutaneous aspiration was used in 19 patients (27%), burr-hole craniostomy in 43 (61%), and craniotomy in 9 children (13%) [Table 2].
Capsule excision is not routinely done at craniotomy, but three of the nine cases treated by craniotomy had partial capsule excision because they were located close to the surface.
In all cases, abscess cavity was irrigated with saline containing antibiotics. Gentamycin is our antibiotic of choice for irrigation but some cases were irrigated with vancomycin and additionally metronidazole. We did not record any case with ventricular rupture, so ventriculostomy was not needed in any of the patients in this series.
Outcome
A total of 75 patients (84%) had a favorable outcome: 54 (61%) had full recovery, 12 (14%) had mild deficits, and 9 (10.1%) had moderate deficits. Of the 14 (16%) who had unfavorable outcome, 8 (9%) died during admission while 6 (7%) had severe deficits [Table 2]. These fatalities and severe deficits were documented in patients who had delayed presentation, with poor GCS at presentation due to financial constraints. Consequently, diagnosis and intervention were delayed leading to poor outcome.
DISCUSSION
With the introduction of modern imaging, antibiotics, and stereotactic surgical techniques, the outcome of brain abscess has dramatically improved.[5,22,25,28,36,40,44] Despite the decline in mortality, brain abscess remains a serious illness that can result in severe morbidity or even mortality, especially if misdiagnosed, diagnosed late, or managed inappropriately.[6,14,23,28,33,40,43]
This study of 89 children with an average of 8.1 cases/year has higher incidence than the series published from Turkey, Korea, and the USA with a range of 1.67–2.7 cases/year.[5,21,37] A median age of 6.4 years is closer to the series in Turkey.[5,28] Delayed presentation is evident in this study with a mean duration of 37.6 days and symptoms lasting up to 92 days before presentation. This is comparable only to the study by Atiq et al. in India (sharing similar technological challenges with many parts of Africa) with delayed presentation of up to 120 days.[2] There are no data on incidence rate of brain abscess in Nigeria at the moment. In a Tunisian multicenter study involving 41 children over a 19-year period, the mean age was 4.9 years.[26]
Clinical findings in children are mostly nonspecific, especially in the younger age group.[12,14,18,28,36,45] The clinical features in our series of frequency were headache, fever, hemiparesis, altered consciousness, meningism, nausea, vomiting, seizures, cranial nerve palsy, and hydrocephalus. Headache and fever being nonspecific symptoms were seen in 80% and 78%, respectively, in our series. The triad of fever, headache, and vomiting in this study was more that seen in the Tunisian study.[26] Fever is a common condition in many childhood illnesses.[9] Most of the patients first received empirical therapy for malaria, an endemic condition in SubSaharan Africa, often presenting with pyrexia and headache, and might have coexisted with the brain abscess. This may account for the higher frequency in this series compared to most published series in children.[8,12,13,18,27,28,36] Seizure (partial or generalized) was present in 39 (44%) of the children in this series. Lee et al.[21] observed seizures in 48% of the patients in their series in Korea. Atiq et al.[2] observed seizures in 45% in their series from India. Seydoux and Francioli[35] observed that 25% of their patients presented with seizures as the first clinical manifestation of brain abscess.
There was no established predisposing factor in 20 (23%) of 89 children in this series. In published studies, this ranges from 8.3% to 40%.[1,2,5,21,27,30,36,43] In 69 children (78%) with identifiable risk factors, the most frequent was meningitis (30.3%), followed by CHD, pulmonary infections, and immunosuppression (from HIV/AIDS and childhood malignancies with chemotherapy), each occurring in 9 (10%) children. Many studies in literature found CHD to be the most common etiological factor accounting for 25–46%.[1-3,5,18,28,29,31,39,44] In other studies, suppurative otitis media was a major contributing etiologic factor.[8,10,21,36] In our study, CHD was responsible for 10% of abscess in children. The failure to diagnosis and treat CHD in developing world could lead to majority of them dying before intervention. Ignorance and lack of facilities accessible to the parents, especially in the rural area, may account for failure to diagnose and treat CHD. The evolution in antibiotic therapy has also led to effective treatment of otitis and sinogenic infections, and prophylactic antimicrobial therapy for CHD patients resulting in fewer complications.[10,13] Immunosuppressed children are often on treatment for HIV/AIDS and hematological malignancies, or organ transplant.[10] None of the children in this series had organ transplantation.
Imaging diagnosis was done with CT scan, TFUSS, and MRI in the ratio of 6.2:1:2.7. The use of TFUSS has proven very useful in our region and has been used to diagnose various intracranial conditions.[17] Ultrasonography was used in diagnosis of three of 25 cases reviewed in the study from Korea.[21] They, however, did not state the reason for the use of TFUSS in some of their cases. The difference in percentage of cases diagnosed with ultrasonography is related to cost or affordability of the superior imaging techniques, especially in developing countries.[17]
TFUSS is unable to accurately characterize the abscess or the count in multiple lesions and this contributed to paucity of the information regarding the number of abscesses in children who underwent this procedure. Because it is operator dependent, some of the information might have been missed out. However, TFUSS will characterize large solitary lesions (>2.5 cm in diameter) and lesions closer to the surface facilitating early diagnosis and therapy. TFUSS can be repeated severally without risk of exposure to irradiation.[16,17] Ultrasonography was deployed in the management of majority of the patients in the cohort reported by Yang.[44]
Our high rate of sterile culture is not uncommon. In several series, the culture-negative rate ranges from 10% to 56%.[3,12,17,18,28,33,36,41,45] Negative cultures may be due to recent use of antibiotics as the case for many children in this series; this is accounted for by previous use of antibiotics, inadequate sampling techniques, and difficulty in isolating some fastidious microorganisms.
The most common micro-organisms in published literature, responsible for brain abscess are aerobic and anaerobic streptococci and staphylococci.[3,10,14,28,33,36,41,45] The treatment modality for brain abscess is still a subject of controversy in literature and indications are constantly being modified by new methods of minimally invasive therapy. Contemporary treatment is a centered around antimicrobial therapy and surgical intervention. The choice of therapeutic approach depends on the stage of the abscess, the location, origin, the number of abscesses, the microorganism isolated, patient’s immune status, the degree of cerebral edema, and available technological advances like image-guided minimally invasive surgery.[4,12,18,19] Antibiotic therapy alone is the preferred choice if the abscess is deep seated and <2 cm in diameter, if there are multiple abscesses and if the patient’s clinical status precludes surgery.[12] Surgical drainage (aspiration or excision) is preferred if the abscess diameter is >2.5 cm or if there is a mass effect followed by 6–8 weeks of intravenous antimicrobial therapy.[12,14,18,27,28,34,36,45] Stereotactic aspiration in combination with antimicrobial therapy or hyperbaric oxygen has been used to treat abscesses in difficult-to-reach areas such as the brain stem or eloquent areas and multiple abscesses, even in patients who are high surgical risk.[4,14,19,20]
In this series, 20% of patients received antimicrobial therapy alone. This falls within the range of 10–24% in published literature.[3,13,14,28] All the children who received only antimicrobial therapy had parenteral antibiotics for a minimum duration of 4 weeks.
Corticosteroid use in the management of brain abscesses has attracted controversies. Some authors have opined that steroid use is associated with increased morbidity and mortality.[14,46] Corticosteroid use has been linked with decreased antibiotic penetrance, inhibition of leukocyte migration, delayed capsule formation with increased risk of intraventricular rupture, and reduction of host defense.[24,46] However, a recent meta-analysis suggested that administration of dexamethasone does not increase mortality in brain abscesses treated with antibiotics.[38]
Sixty-two (87%) of the 71 patients who had surgical intervention received minimally invasive procedures, while only nine patients had craniotomy. Percutaneous aspiration was performed in 19 patients while burr-hole craniostomy was used in 43 patients. Stereotactic techniques are not yet available in our center. The use of craniotomy for the treatment of abscess has significantly decreased while minimally invasive or image-guided aspiration techniques are now preferred as first surgical choice for abscesses.[32,42]
Outcome in this study was unfavorable in 16% with a mortality rate of 9% [Table 2]. The deaths were due to delayed presentation, diagnosis, and intervention often attributable to poverty and ignorance.[16,17] This is common in resource-poor countries and radical changes in the health system and public education are necessary to reverse this trend. The health insurance coverage is still very low in Nigeria. A mortality rate of 9% falls within the range published in literature.[14,22,23,28,36,40]
CONCLUSION
Brain abscess is common in children with high incidence of morbidity and mortality often related to late presentation and therefore still poses a public health challenge in resource-limited regions. Successful management of childhood brain abscess with acceptable outcome is dependent on early diagnosis and intervention, the rational use of antimicrobial therapy, and adaptation of available technology. Despite the fact that Lagos has a multiethnic and multinational population, authors acknowledge that medical and demographic variations exist between countries.
Acknowledgment
The authors are grateful to Late James T. Goodrich MD, PhD (RIP) for his mentoring and contributions toward this study.
Footnotes
How to cite this article: Kanu OO, Ojo O, Esezobor C, Bankole O, Olatosi J, Ogunleye E, et al. Pediatric brain abscess – etiology, management challenges and outcome in Lagos Nigeria. Surg Neurol Int 2021;12:592.
Contributor Information
Okezie Obasi Kanu, Email: drkanu@gmail.com.
Omotayo Ojo, Email: tayoojo@unilag.edu.ng.
Christopher Esezobor, Email: cesezobor@unilag.edu.ng.
Olufemi Bankole, Email: bbankole@unilag.edu.ng.
John Olatosi, Email: jolatosi@unilag.edu.ng.
Ezekiel Ogunleye, Email: ezekielolla@yahoo.com.
Chinyere Asoegwu, Email: casoegwu@unilag.edu.ng.
Morgan Eghosa, Email: morganeghosa@gmail.com.
Bamidele Adebayo, Email: drdeleadebayo@gmail.com.
Rita Oladele, Email: oladelerita@gmail.com.
Clement Nwawolo, Email: clementnwawolo@yahoo.com, cnwawolo@unilag.edu.ng.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Aebi C, Kaufmann F, Schaad UB. Brain abscess in childhood-long-term experiences. Eur J Pediatr. 1991;150:282–6. doi: 10.1007/BF01955533. [DOI] [PubMed] [Google Scholar]
- 2.Atiq M, Ahmed US, Allana SS, Chishti KN. Brain abscess in children. Indian J Pediatr. 2006;73:401–4. doi: 10.1007/BF02758560. [DOI] [PubMed] [Google Scholar]
- 3.Auvichayapat N, Auvichayapat P, Aungwarawong S. Brain abscess in infants and children: A retrospective study of 107 patients in northeast Thailand. J Med Assoc Thai. 2007;90:1601–7. [PubMed] [Google Scholar]
- 4.Boviatsis EJ, Kouyialis AT, Stranjalis G, Korfias S, Sakas DE. CT-guided stereotactic aspiration of brain abscesses. Neurosurg Rev. 2003;26:206–9. doi: 10.1007/s10143-003-0257-x. [DOI] [PubMed] [Google Scholar]
- 5.Canpolat M, Ceylan O, Per H, Koc G, Tumturk A, Kumandas S, et al. Brain abscesses in children: Results of 24 children from a reference center in Central Anatolia, Turkey. J Child Neurol. 2015;30:458–67. doi: 10.1177/0883073814549247. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane DD. Consultation with the specialist. Brain abscess. Pediatr Rev. 1999;20:209–15. doi: 10.1542/pir.20-6-209. [DOI] [PubMed] [Google Scholar]
- 7.Cole TS, Clark ME, Jenkins AJ, Clark JE. Pediatric focal intracranial suppuration: A UK single-center experience. Childs Nerv Syst. 2012;28:2109–14. doi: 10.1007/s00381-012-1877-7. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein S, Williams B, Shingadia D, Coxon L, Riordan A, Demetriades AK, et al. Clinical and microbiologic features guiding treatment recommendations for brain abscesses in children. Pediatr Infect Dis J. 2013;32:129–35. doi: 10.1097/INF.0b013e3182748d6e. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JA, Christiansen CL, Platt R. Fever in pediatric primary care: Occurrence, management, and outcomes. Pediatrics. 2000;105(Suppl 2):260–6. [PubMed] [Google Scholar]
- 10.Frazier JL, Ahn ES, Jallo GI. Management of brain abscesses in children. Neurosurg Focus. 2008;24:E8. doi: 10.3171/FOC/2008/24/6/E8. [DOI] [PubMed] [Google Scholar]
- 11.Garvey G. Current concepts of bacterial infections of the central nervous system. Bacterial meningitis and bacterial brain abscess. J Neurosurg. 1983;59:735–44. doi: 10.3171/jns.1983.59.5.0735. [DOI] [PubMed] [Google Scholar]
- 12.Gelabert-Gonzalez M, Serramito-Garcia R, Garcia-Allut A, Cutrin-Prieto J. Management of brain abscess in children. J Paediatr Child Health. 2008;44:731–5. doi: 10.1111/j.1440-1754.2008.01415.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodkin HP, Harper MB, Pomeroy SL. Intracerebral abscess in children: Historical trends at Children’s Hospital Boston. Pediatrics. 2004;113:1765–70. doi: 10.1542/peds.113.6.1765. [DOI] [PubMed] [Google Scholar]
- 14.Hakan T, Ceran N, Erdem I, Berkman MZ, Goktas P. Bacterial brain abscesses: An evaluation of 96 cases. J Infect. 2006;52:359–66. doi: 10.1016/j.jinf.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Kagawa M, Takeshita M, Yato S, Kitamura K. Brain abscess in congenital cyanotic heart disease. J Neurosurg. 1983;58:913–7. doi: 10.3171/jns.1983.58.6.0913. [DOI] [PubMed] [Google Scholar]
- 16.Kanu OO, Esezobor CI, Ojo OA, Asoegwu CN, Nnoli C, Dawang Y, et al. Infantile supratentorial subdural empyema managed by percutaneous aspiration: An outcome study in a Nigerian city. Sudan J Paediatr. 2019;19:37–43. doi: 10.24911/SJP.106-1520470056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanu OO, Nnoli C, Olowoyeye O, Ojo O, Esezobor C, Adeyomoye A, et al. Infantile subdural empyema: The role of brain sonography and percutaneous subdural tapping in a resource-challenged region. J Neurosci Rural Pract. 2014;5:355–9. doi: 10.4103/0976-3147.139978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao KL, Wu KG, Chen CJ, Wu JJ, Tang RB, Chang KP, et al. Brain abscesses in children: Analysis of 20 cases presenting at a medical center. J Microbiol Immunol Infect. 2008;41:403–7. [PubMed] [Google Scholar]
- 19.Kutlay M, Colak A, Yildiz S, Demircan N, Akin ON. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery. 2005;57:1140–6. discussion 1140-6. [PubMed] [Google Scholar]
- 20.Kutlay M, Colak A, Yildiz S, Demircan N, Akin ON. Stereotactic aspiration and antibiotic treatment combined with hyperbaric oxygen therapy in the management of bacterial brain abscesses. Neurosurgery. 2008;62(Suppl 2):540–6. doi: 10.1227/01.neu.0000316257.21478.5b. [DOI] [PubMed] [Google Scholar]
- 21.Lee CG, Kang SH, Kim YJ, Shin HJ, Choi HS, Lee JH, et al. Brain abscess in Korean children: A 15-year single center study. Korean J Pediatr. 2010;53:648–52. doi: 10.3345/kjp.2010.53.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mamelak AN, Mampalam TJ, Obana WG, Rosenblum ML. Improved management of multiple brain abscesses: A combined surgical and medical approach. Neurosurgery. 1995;36:76–85. doi: 10.1227/00006123-199501000-00010. discussion 85-6. [DOI] [PubMed] [Google Scholar]
- 23.Mampalam TJ, Rosenblum ML. Trends in the management of bacterial brain abscesses: A review of 102 cases over 17 years. Neurosurgery. 1988;23:451–8. doi: 10.1227/00006123-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–79. doi: 10.1086/515541. quiz 780-1. [DOI] [PubMed] [Google Scholar]
- 25.Miller ES, Dias PS, Uttley D. CT scanning in the management of intracranial abscess: A review of 100 cases. Br J Neurosurg. 1988;2:439–46. doi: 10.3109/02688698809029597. [DOI] [PubMed] [Google Scholar]
- 26.Miniar T, Amel BA, Khalil S, Ben Helal BH, Gueddiche GM, Tilouche TS, et al. Pyogenic brain abscess in children: A Tunisian multi-center experience. Afr Health Sci. 2018;18:560–8. doi: 10.4314/ahs.v18i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain abscess: Management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg. 2011;75:716–26. doi: 10.1016/j.wneu.2010.11.043. discussion 612-7. [DOI] [PubMed] [Google Scholar]
- 28.Ozsurekci Y, Kara A, Cengiz AB, Celik M, Ozkaya-Parlakay A, Karadag-Oncel E, et al. Brain abscess in childhood: A 28-year experience. Turk J Pediatr. 2012;54:144–9. [PubMed] [Google Scholar]
- 29.Pandian JD, Moosa NV, Cherian PJ, Radhakrishnan K. Brainstem abscess complicating tetralogy of Fallot successfully treated with antibiotics alone. Neurol India. 2000;48:272–5. [PubMed] [Google Scholar]
- 30.Pit S, Jamal F, Cheah FK. Microbiology of cerebral abscess: A four-year study in Malaysia. J Trop Med Hyg. 1993;96:191–6. [PubMed] [Google Scholar]
- 31.Puthucheary SD, Parasakthi N. The bacteriology of brain abscess: A local experience in Malaysia. Trans R Soc Trop Med Hyg. 1990;84:589–92. doi: 10.1016/0035-9203(90)90052-g. [DOI] [PubMed] [Google Scholar]
- 32.Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment-78 years: Aspiration versus excision. World Neurosurg. 2011;76:431–6. doi: 10.1016/j.wneu.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Saez-Llorens X. Brain abscess in children. Semin Pediatr Infect Dis. 2003;14:108–14. doi: 10.1053/spid.2003.127227. [DOI] [PubMed] [Google Scholar]
- 34.Sarmast AH, Showkat HI, Bhat AR, Kirmani AR, Kachroo MY, Mir SF, et al. Analysis and management of brain abscess; a ten year hospital based study. Turk Neurosurg. 2012;22:682–9. doi: 10.5137/1019-5149.JTN.5458-11.3. [DOI] [PubMed] [Google Scholar]
- 35.Seydoux C, Francioli P. Bacterial brain abscesses: Factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394–401. doi: 10.1093/clind/15.3.394. [DOI] [PubMed] [Google Scholar]
- 36.Shachor-Meyouhas Y, Bar-Joseph G, Guilburd JN, Lorber A, Hadash A, Kassis I. Brain abscess in children-epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr. 2010;99:1163–7. doi: 10.1111/j.1651-2227.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 37.Sheehan JP, Jane JA, Ray DK, Goodkin HP. Brain abscess in children. Neurosurg Focus. 2008;24:E6. doi: 10.3171/FOC/2008/24/6/E6. [DOI] [PubMed] [Google Scholar]
- 38.Simjian T, Muskens IS, Lamba N, Yunusa I, Wong K, Veronneau R, et al. Dexamethasone administration and mortality in patients with brain abscess: A systematic review and meta-analysis. World Neurosurg. 2018;115:257–63. doi: 10.1016/j.wneu.2018.04.130. [DOI] [PubMed] [Google Scholar]
- 39.Takeshita M, Kagawa M, Yonetani H, Izawa M, Yato S, Nakanishi T, et al. Risk factors for brain abscess in patients with congenital cyanotic heart disease. Neurol Med Chir (Tokyo) 1992;32:667–70. doi: 10.2176/nmc.32.667. [DOI] [PubMed] [Google Scholar]
- 40.Tseng JH, Tseng MY. Brain abscess in 142 patients: Factors influencing outcome and mortality. Surg Neurol. 2006;65:557–62. doi: 10.1016/j.surneu.2005.09.029. discussion 562. [DOI] [PubMed] [Google Scholar]
- 41.Tsou TP, Lee PI, Lu CY, Chang LY, Huang LM, Chen JM, et al. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: A 10-year experience. J Microbiol Immunol Infect. 2009;42:405–12. [PubMed] [Google Scholar]
- 42.Wu S, Wei Y, Yu X, Peng Y, He P, Xu H, et al. Retrospective analysis of brain abscess in 183 patients: A 10-year survey. Medicine (Baltimore) 2019;98:e17670. doi: 10.1097/MD.0000000000017670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC. Brain abscess: Clinical experience and analysis of prognostic factors. Surg Neurol. 2005;63:442–9. doi: 10.1016/j.surneu.2004.08.093. discussion 449-50. [DOI] [PubMed] [Google Scholar]
- 44.Yang SY. Brain abscess: A review of 400 cases. J Neurosurg. 1981;55:794–9. doi: 10.3171/jns.1981.55.5.0794. [DOI] [PubMed] [Google Scholar]
- 45.Yogev R, Bar-Meir M. Management of brain abscesses in children. Pediatr Infect Dis J. 2004;23:157–9. doi: 10.1097/01.inf.0000110272.67271.a2. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Hu L, Wu X, Hu G, Ding X, Lu Y. A retrospective study on the aetiology, management, and outcome of brain abscess in an 11-year, single-centre study from China. BMC Infect Dis. 2014;14:311. doi: 10.1186/1471-2334-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]


