Summary
Background
COVID-19 has worse mortality than influenza in American and European studies, but evidence from the Western Pacific region is scarce.
Methods
Using a large-scale multicenter inpatient claims data in Japan, we identified individuals hospitalised with COVID-19 in 2020 or influenza in 2017–2020. We compared patient characteristics, supportive care, and in-hospital mortality, with multivariable logistic regression analyses for in-hospital mortality overall, by age group, and among patients with mechanical ventilation.
Findings
We identified 16,790 COVID-19 patients and 27,870 influenza patients, with the different age distribution (peak at 70–89 years in COVID-19 vs. bimodal peaks at 0–9 and 80–89 years in influenza). On admission, the use of mechanical ventilation was similar in both groups (1·4% vs. 1·4%) but higher in the COVID-19 group (3·3% vs. 2·5%; p<0·0001) during the entire hospitalisation. The crude in-hospital mortality was 5·1% (856/16,790) for COVID-19 and 2·8% (791/27,870) for influenza. Adjusted for potential confounders, the in-hospital mortality was higher for COVID-19 than for influenza (adjusted odds ratio [aOR] 1·83, 95% confidence interval [CI] 1·64–2·04). In age-stratified analyses, the aOR (95%CI) were 0·78 (0·56–1·08) and 2·05 (1·83–2·30) in patients aged 20–69 years and ≥70 years, respectively (p-for-interaction<0·0001). Among patients with mechanical ventilation, the aOR was 0·79 (0·59–1·05).
Interpretation
Patients hospitalised with COVID-19 in Japan were more likely to die than those with influenza. However, this was mainly driven by findings in older people, and there was no difference once mechanical ventilation was started.
Funding
Ministry of Health, Labour and Welfare of Japan (21AA2007).
Research in context.
Evidence before this study
Similarities and differences in the clinical characteristics of COVID-19 and influenza have previously been described. We searched PubMed on July 26, 2021, with the terms (“influenza” OR “seasonal influenza”) AND (“COVID-19”), in any language without restrictions of the start and end dates of the search. We also conducted manual searches of the reference lists of identified previous studies. After excluding studies on COVID-19 and influenza co-infection, we identified 17 observational studies that compared patient characteristics and in-hospital mortality between COVID-19 and influenza. Previous studies were more likely to report adverse outcomes, including higher in-hospital mortality, among COVID-19 patients than influenza patients. However, most previous studies were conducted in North America or Europe, and evidence from the Western Pacific region was scarce.
Added value of this study
To the best of our knowledge, this is the first large-scale study from the Western Pacific region, which investigated the clinical differences between COVID-19 and influenza. Our results showed that in-hospital mortality was higher in patients admitted for COVID-19 in 2020 than in patients admitted for influenza in 2017-2020 in Japan, which was consistent with the direction of the effect, albeit less prominent, in American and EU countries. The higher risk of in-hospital death for COVID-19 than influenza was mainly driven by findings in older people, whereas there was no statistically significant difference in in-hospital mortality between COVID-19 and influenza among younger patients and among those with mechanical ventilation.
Implications of all the available evidence
This study supports the hypothesis that COVID-19 is more lethal than influenza in the Western Pacific region, consistent with evidence from American and European countries. As our stratified analysis by age group showed higher in-hospital mortality of COVID-19 compared to influenza only among older people, it is reasonable to prioritize the vaccination of COVID-19 for older citizens. In addition, since in-hospital mortality did not differ between COVID-19 and influenza once mechanical ventilation was started, improvement of the management and development of treatment options for COVID-19 before the introduction of mechanical ventilation may be important in lowering the in-hospital mortality of COVID-19.
Alt-text: Unlabelled box
Introduction
Coronavirus disease 2019 (COVID-19) has affected people's lives worldwide, with a total casualty of approximately five million by October 2021.1 To identify its clinical features and effective treatments, much effort is being made to illustrate the similarities and differences in characteristics between COVID-19 and other existing respiratory viral infections, such as influenza. COVID-19 and influenza are both caused by enveloped RNA viruses affecting respiratory organs, and both can cause similar symptoms, such as fever and cough.2 Nevertheless, previous studies largely reported worse outcomes among patients with COVID-19 than those with influenza, including higher mortality,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 higher morbidity of complications,3, 4, 5, 6, 7, 8,14, [15], 16, 17 and more excess use of healthcare resources such as mechanical ventilation,3,4,6,8,16 intensive care unit,3, 4, 5, 6, 7, 8, 9,11,13,14,16 and increased length of hospital stay.3, 4, 5, 6,9,11,14,17 These disparities may be partly explained by differences in pathogenicity or transmissibility,2,18 as well as the lack of well-established treatment, vaccines (which started in February 2021 in Japan), and herd immunity for COVID-19.
However, most of these comparative studies of COVID-19 and influenza focused on populations in North America3,7, 8, 9, 10, 11,16 or Europe,4, 5, 6,12,14 except for a few studies from China with smaller study populations.13,15,17 Thus, evidence from the Western Pacific region remains scarce. Between the Western Pacific region and American or European countries, some different clinical manifestations of COVID-19 patients have been reported, such as relatively low mortality and fewer morbidities in Japan.19 Therefore, it is necessary to confirm whether the comparative evaluation results from Europe or the Americas on COVID-19 and influenza are also applicable to the Western Pacific region.
Thus, in this study, we aimed to compare patient characteristics, supportive care, and in-hospital mortality between patients admitted for COVID-19 and influenza in Japan using large-scale multicenter administrative claims data.
Methods
Data source
We conducted a retrospective cohort study using both inpatient and outpatient administrative data and discharge summaries of inpatients at Japanese acute care hospitals under the Diagnosis Procedure Combination (DPC) payment system. In brief, the DPC payment system is a Japanese classification method for inpatients in the acute phase and includes demographic and clinical information such as diagnoses, pre-existing comorbidities, treatment, admission date, and discharge date.20 The number of hospitals covered by the DPC payment system increased since its introduction in 2003 and were almost constant at about 1,700 hospitals in 2017–2020.20 The current database, built by Medical Data Vision Co., Ltd. (Tokyo Japan) (MDV), consists of over 350 acute care hospitals (around 20% of the DPC hospitals).21 The MDV database consists of hospitals that used the business support system by the MDV Co., Ltd., and provided consent for secondary data uses for research purposes. The MDV database has been widely used in previous studies.22, 23, 24, 25 The age distribution of inpatients in the MDV database is roughly similar to that of all DPC hospitals, whereas the hospital volume (i.e., the number of beds) tends to be larger (Supplementary Table 1). Laboratory test values were available for approximately 10% of participating hospitals that agreed to provide the data. The study was approved by the Ethics Committee of the University of Tsukuba (approval no. 1624). Because the claims data were anonymized before the researchers received the data, individual participants’ consent was waived, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects.26
Study population
The study population included patients admitted and discharged for COVID-19 in 2020 or for influenza in 2017–2020 from hospitals which continuously contributed to the MDV database during the study period. For influenza, we included patients admitted not only in 2020 but also between 2017–2019 because the number of influenza cases in 2020 was reported to be very low in most countries including Japan,27,28 and also because the 3-year data from 2017 to 2019 was more likely to reflect the average characteristics of patients with influenza, given the well-known variation in clinical characteristics of influenza in different years. We identified individuals whose primary disease that triggered admission was COVID-19 (10th revision of the International Statistical Classification of Diseases [ICD-10] codes: U071) or influenza (ICD-10: J09, J10, J11). We categorized a few patients admitted for both COVID-19 and influenza (46 individuals) as patients with COVID-19.3 For those admitted for COVID-19 or influenza several times in the same year, we used only the first hospitalisation record for each year.
Variables
We obtained information on patient characteristics, including sex, age, body mass index (BMI), smoking history, and comorbidities, based on the inpatient or outpatient ICD-10 codes given on the admission day or before (since 2017): cancer (C00–C43, C45–C97), chronic lung disease (J40–J47), ischemic heart disease (I20–I25), heart failure (I50, I110), arrhythmia (I44, I45, I47–I49), hypertension (I10, I11, I12, I13, I15, I674), diabetes mellitus (E10–E14), cerebrovascular disease (I60–I69), chronic kidney disease (N18), end-stage renal disease (N185, N19), dementia (F00–F03, F051, G30), dyslipidaemia (E78), cirrhosis (K703, K717, K743, K744, K745, K746), deficiency anaemia (D50), and peripheral artery disease (I702, I739).
Furthermore, we extracted information on the supportive care (intensive care unit [ICU] admission, oxygen therapy, non-invasive positive pressure ventilation [NPPV], mechanical ventilation, extracorporeal membrane oxygenation [ECMO], renal replacement therapy, blood transfusion, and vasopressor use), and dexamethasone use, based on procedure codes (detailed definitions are shown in Supplementary Table 2), both on the day of admission and at any time during the entire hospitalisation. In addition, in the MDV database, laboratory test values were available from approximately 10% of the participating hospitals and were therefore extracted. The outcome of interest was in-hospital mortality, which is recorded and constantly monitored for accuracy in the DPC system.
Statistical analysis
First, we crudely compared the participants’ demographics and health-related characteristics between patients admitted for COVID-19 in 2020 and influenza in 2017–2020. Second, we compared the supportive care between COVID-19 and influenza patients, on the day of admission and during the entire hospitalisation. For patients discharged alive by the end of 2020, we also compared the length of stay (in hospital and in ICU). We used Student's t-test or the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. We also compared laboratory test values between the groups of patients admitted to hospitals with available laboratory results, by calculating the standardized mean difference. We then compared in-hospital mortality between COVID-19 and influenza groups and plotted these by ten-year age groups.
We conducted multivariable logistic regression analyses for in-hospital mortality in the whole study population, in line with a previous foreign study.11 We first adjusted for age group and sex, and then additionally adjusted for smoking history, BMI, and comorbidities. We then further adjusted for treatment on admission day (blood transfusion, mechanical ventilation, vasopressor use, renal replacement therapy, and dexamethasone use; hereafter referred to as the “fully adjusted model”). For missing data on smoking history and BMI, we conducted multiple imputation by chained equations (MICE) using all variables including outcomes.29 In this study, we generated five complete, filled-in datasets and replaced each missing value with a set of plausible substitutes. For sensitivity analysis, we also conducted complete case analyses, assuming that missing values for BMI and smoking history were missing not at random.
In addition, we conducted multivariable logistic regression analyses stratified by age group because the relative risk of in-hospital death between COVID-19 and influenza has been suggested to differ by age.3,4 We stratified the population into two age groups (20–69 years and 70 years or older) after we had plotted the in-hospital death mortality of COVID-19 and influenza by age, finding no fatal COVID-19 case under 20 years old. We also tested the interaction by age, with the multivariable logistic regression model including the interaction term between age and diseases (i.e., COVID-19 or influenza). Furthermore, we conducted a multivariable logistic regression analysis in patients with mechanical ventilation (any time during the entire hospitalisation period) to examine whether the association between COVID-19/influenza and mortality is different when patients are affected by more severe conditions requiring mechanical ventilation.
Statistical significance was set at p<0·05. Stata 15 (StataCorp, TX, USA) and Microsoft Excel for Mac 16.56 (Microsoft, WA, USA) were used for all analyses.
Role of the funding source
This work was supported by a grant-in-aid from the Ministry of Health, Labour and Welfare Policy Research Grants, Japan (grant number: 21AA2007). The funder did not play any role in the conception, design, conduct, or reporting of this study.
Results
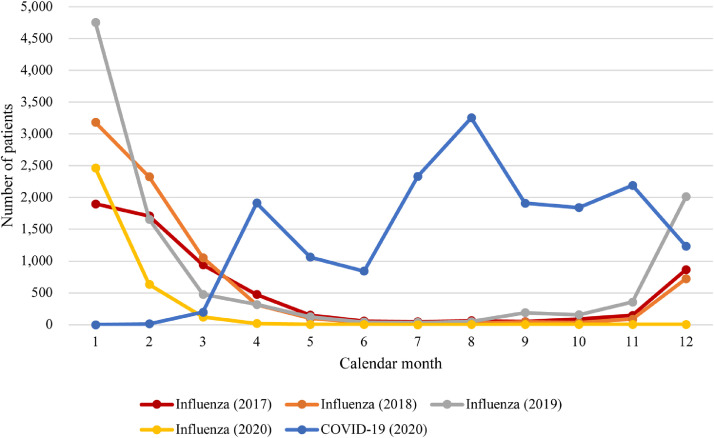
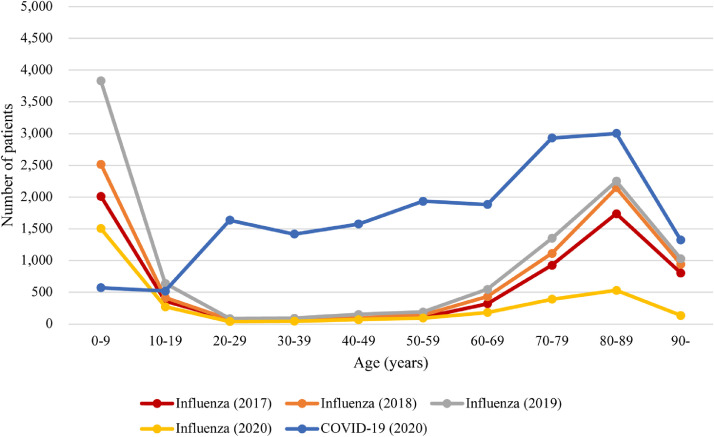
We identified 16,790 patients admitted for COVID-19 in 2020 and 27,870 patients admitted for influenza in 2017–2020. The number of patients with influenza in 2020 was one-half to one-third of that between 2017–2019 (Table 1). By calendar month, COVID-19 showed two waves in 2020, in line with Japanese national statistics,1 whereas influenza showed a single peak every year around January (Figure 1). The age distribution was largely different (Figure 2): the number of COVID-19 patients was almost constant among people aged 20–69 years and peaked at 70–89 years, whereas influenza showed a bimodal distribution with peaks at ages 0–9 years and 80–89 years. Patients with COVID-19 were more likely to be men, younger, overweight or obese, have a smoking history, and have comorbidities including cancer, hypertension, diabetes mellitus, chronic kidney disease, end-stage renal disease, dyslipidaemia, cirrhosis, and deficiency anaemia than patients with influenza (Table 1). Among patients with available laboratory test values, whereas most parameters were different with statistical significance (i.e., p<0·0001), the standardized mean difference for each parameter, which measures the magnitude of effect,30 was generally small (Supplementary Table 3).
Table 1.
Baseline characteristics of participants at admission to a hospital in Japan for COVID-19 or influenza.
| COVID-19 (n=16,790) | Influenza (n=27,870) | p | |
|---|---|---|---|
| Age, median (IQR), year | 64 (40–80) | 67 (5–84) | <0·0001 |
| Sex (women), n (%) | 7,242 (43·1) | 13,039 (46·8) | <0·0001 |
| Calendar year, n (%) | |||
| 2017 | − | 6,491 (23·3) | |
| 2018 | − | 7,943 (28·5) | |
| 2019 | − | 10,170 (36·5) | |
| 2020 | 16,790 (100) | 3,266 (11·7) | |
| BMI, n (%), kg/m2 | <0·0001 | ||
| <25 | 10,563 (62·9) | 19,492 (69·9) | |
| 25 to <30 | 2,601 (15·5) | 2,645 (9·5) | |
| 30≤ | 910 (5·4) | 740 (2·7) | |
| Missing | 2,716 (16·2) | 4,993 (17·9) | |
| Smoking history, n (%) | <0·0001 | ||
| Yes | 4,473 (26·6) | 3,665 (13·2) | |
| No | 9,487 (56·5) | 21,966 (78·8) | |
| Missing | 2,830 (16·9) | 2,239 (8·0) | |
| Comorbidities, n (%) | |||
| Cancer | 2,149 (12·8) | 2,385 (8·6) | <0·0001 |
| Chronic lung disease | 3,008 (17·9) | 8,067 (29·0) | <0·0001 |
| Ischemic heart disease | 1,436 (8·6) | 6,712 (24·1) | <0·0001 |
| Heart failure | 2,517 (15·0) | 4,246 (15·2) | 0·49 |
| Arrhythmia | 1,608 (9·6) | 2,831 (10·2) | 0·047 |
| Hypertension | 5,192 (30·9) | 7,965 (28·6) | <0·0001 |
| Diabetes mellitus | 3,602 (21·5) | 4,804 (17·2) | <0·0001 |
| Cerebrovascular disease | 1,739 (10·4) | 3,659 (13·1) | <0·0001 |
| Chronic kidney disease | 1,048 (6·2) | 1,507 (5·4) | <0·0001 |
| End-stage renal disease | 690 (4·1) | 803 (2·9) | <0·0001 |
| Dementia | 1,522 (9·1) | 3,064 (11·0) | <0·0001 |
| Dyslipidaemia | 2,759 (16·4) | 4,052 (14·5) | <0·0001 |
| Cirrhosis | 230 (1·4) | 252 (0·9) | <0·0001 |
| Deficiency anaemia | 1,652 (9·8) | 2,165 (7·8) | <0·0001 |
| Peripheral artery disease | 233 (1·4) | 435 (1·6) | 0·14 |
Abbreviations: BMI, body mass index; IQR, interquartile range. Data are reported as number (%) for categorical variables and median (IQR) for continuous variables. We conducted Student's t-test or the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables.
Figure 1.
Distribution of patients admitted for COVID-19 in 2020 or influenza in 2017–2020 by calendar month of admission.
Figure 2.
Distribution of patients admitted for COVID-19 in 2020 or influenza in 2017–2020 by age.
On admission day, patients with COVID-19 were more likely to enter the ICU and receive a blood transfusion and dexamethasone, whereas patients with influenza received oxygen therapy, NPPV, and vasopressors more often (Table 2). We did not observe statistically significant differences in the proportions of mechanical ventilation, ECMO, and renal replacement therapy on admission between COVID-19 and influenza patients. However, during the entire hospitalisation period, patients with COVID-19 were more likely to receive any of the treatments above, except for oxygen therapy and NPPV, compared to patients with influenza, indicating that patients with COVID-19 were more likely to become severely ill during hospitalisation. For patients discharged alive (15,934 for COVID-19 and 27,079 for influenza), the median length of stay was longer for COVID-19 (10 [IQR, 7–16] days) than for influenza (5 [4–10] days), whereas the median length of stay in ICU for patients with COVID-19 (3 [1–8] days) and patients with influenza (4 [2–8] days) were not statistically different.
Table 2.
Treatment of patients admitted to a hospital in Japan for COVID-19 or influenza, as well as their length of stay.
| COVID-19 (n=16,790) | Influenza (n=27,870) | p | |
|---|---|---|---|
| Treatment on admission day, n (%) | |||
| ICU admission | 749 (4·5) | 207 (0·7) | <0·0001 |
| Oxygen therapy | 3,707 (22·1) | 7,938 (28·5) | <0·0001 |
| NPPV | 34 (0·2) | 119 (0·4) | <0·0001 |
| Mechanical ventilation | 241 (1·4) | 376 (1·4) | 0·45 |
| ECMO | 6 (0·04) | 9 (0·03) | 0·85 |
| Renal replacement therapy | 108 (0·6) | 168 (0·6) | 0·60 |
| Blood transfusion | 167 (1·0) | 67 (0·2) | <0·0001 |
| Vasopressor use | 194 (1·2) | 436 (1·6) | <0·0001 |
| Dexamethasone use | 506 (3·0) | 272 (1·0) | <0·0001 |
| Treatment during hospitalisation, n (%) | |||
| ICU admission | 937 (5·6) | 293 (1·1) | <0·0001 |
| Oxygen therapy | 5,573 (33·2) | 9,860 (35·4) | <0·0001 |
| NPPV | 188 (1·1) | 306 (1·1) | 0·83 |
| Mechanical ventilation | 546 (3·3) | 691 (2·5) | <0·0001 |
| ECMO | 36 (0·2) | 24 (0·1) | <0·0001 |
| Renal replacement therapy | 407 (2·4) | 395 (1·4) | <0·0001 |
| Blood transfusion | 621 (3·7) | 400 (1·4) | <0·0001 |
| Vasopressor use | 609 (3·6) | 891 (3·2) | 0·015 |
| Dexamethasone use | 1,465 (8·7) | 459 (1·7) | <0·0001 |
| Length of stay, median (IQR), days* | 10 (7–16) | 5 (4–10) | <0·0001 |
| Length of stay in ICU, median (IQR), days‡ | 3 (1–8) | 4 (2–8) | 0·089 |
Abbreviations: NPPV, Non-invasive Positive Pressure Ventilation; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range. Data are reported as number (%) for categorical variables and median (IQR) for continuous variables. We conducted the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables.
Length of stay was calculated for patients discharged alive (15,934 for COVID-19 and 27,079 for influenza).
Length of stay in ICU was calculated for patients who stayed in ICU and discharged alive (658 for COVID-19 and 180 for influenza)
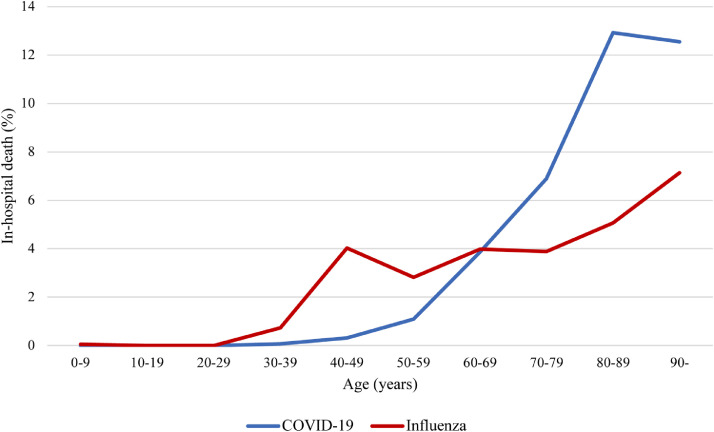
Overall, the in-hospital mortality was higher in patients admitted for COVID-19 (856/16,790 [5·1%]) than in patients with influenza (791/27,870 [2·8%] between 2017–2020, consisting of 2·2% [142/6,491] in 2017, 3·4% [272/7,943] in 2018, 3·0% [303/10,170], and 2·3% [74/3,266] in 2020). However, the association between COVID-19 or influenza and in-hospital mortality varied with age (Figure 3). Among people younger than 20 years, the in-hospital mortality was not significantly different between COVID-19 (0/1,092 [0%]) and influenza (6/11,558 [0·05%]). Among individuals aged between 20 and 69 years, it was higher in influenza patients (92/2,957 [3·1%]) than in COVID-19 patients (100/8,443 [1·2%]; p<0·0001), whereas in patients 70 years of age or older, it was higher among COVID-19 patients (756/7,255 [10·4%]) than among influenza patients (693/13,355 [5·2%]; p<0·0001; Figure 3).
Figure 3.
In-hospital death of patients admitted for COVID-19 in 2020 or influenza in 2017–2020 by age.
In the multiple logistic regression analyses after multiple imputation, COVID-19 patients had a higher risk of in-hospital death than influenza patients in the age-sex adjusted model (odds ratio [OR] 1·95, 95% confidence interval [CI] 1·76−2·16), in the model further adjusting for smoking history, BMI, and comorbidities (OR 1·86, 95%CI 1·68–2·07), and in the fully adjusted model (OR 1·83, 95%CI 1·64–2·04) (Table 3).
Table 3.
Risk of in-hospital death of patients hospitalized for COVID-19 or influenza
| COVID-19 | Influenza | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|
| In-hospital death, n (%) | Adjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | ||
| Full cohort, n (%) | 856/16,790 (5·1) | 791/27,870 (2·8) | 1·95 (1·76-2·16) | 1·86 (1·68-2·07) | 1·83 (1·64–2·04) |
| Patients aged 20–69 years old, n (%) | 100/8,443 (1·2) | 92/2,957 (3·1) | 0·58 (0·43–0·79) | 0·72 (0·53–0·99) | 0·78 (0·56–1·08) |
| Patients aged 70 or older, n (%) | 756/7,255 (10·4) | 693/13,355 (5·2) | 2·24 (2·01–2·49) | 2·10 (1·88–2·35) | 2·05 (1·83–2·30) |
| Mechanical ventilation cohort, n (%) | 178/546 (32·6) | 229/691 (33·1) | 0·81 (0·63–1·05) | 0·80 (0·61–1·06) | 0·79 (0·59–1·05) |
Abbreviations: CI, confidence interval. Influenza was treated as reference. Model 1 adjusted for sex and age. Model 2 additionally adjusted for smoking history, body mass index, and comorbidities (cancer, chronic lung disease, ischemic heart disease, heart failure, arrhythmia, hypertension, diabetes mellitus, cerebrovascular disease, chronic kidney disease, dementia, dyslipidaemia, cirrhosis, deficiency anaemia, and peripheral artery disease). Model 3 additionally adjusted for treatment on admission day (blood transfusion, mechanical ventilation, vasopressor use, renal replacement therapy, and dexamethasone use).
When stratified by age group, in patients aged 70 years or older, the risk of in-hospital death was higher among COVID-19 patients than influenza patients (fully adjusted OR 2·05, 95%CI 1·83−2·30), but there was no statistically significant difference among patients aged 20−69 (fully adjusted OR 0·78, 95%CI 0·56−1·08) (p-for-interaction<0·0001) (Table 3).
Among patients with mechanical ventilation (any time during the entire hospitalisation period), we did not observe a statistically significant difference in the risk of in-hospital death (crude in-hospital mortality 32·6% [178/546] in COVID-19 patients and 33·1% [229/691] in influenza patients; fully adjusted OR 0·79, 95%CI 0·59–1·05) (Table 3).
The complete case analyses excluding the participants with missing values of BMI and smoking history showed similar results (Supplementary Table 4).
Discussion
In this large Japanese cohort study using administrative claims data in Japan, we compared the characteristics, treatment, and in-hospital mortality between patients with COVID-19 and those with influenza in Japan. We found that overall, patients with COVID-19 were more likely to die in the hospital than patients admitted for influenza. Nevertheless, we did not observe higher in-hospital mortality for COVID-19 than influenza among patients younger than 70 years of age and among patients who received mechanical ventilation during hospitalisation.
To the best of our knowledge, this is the first large-scale study from the Western Pacific region to investigate the clinical differences between COVID-19 and influenza. A few reports from China13,15,17 compared the characteristics of COVID-19 and influenza, but their results were limited due to the small sample size13,15,17 or the indirect comparison of separate cohorts at different locations.13,15 The strength of the current study is that we directly compared data for COVID-19 and influenza patients using a large database of acute care hospitals across Japan.
Our results were largely compatible with those of previous studies from Europe or North America regarding the higher mortality3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and the longer hospital stay3, 4, 5, 6,9,11,14 among patients with COVID-19 than those with influenza. Differences in the risks of death between COVID-19 and influenza might be partly explained by the fact that effective vaccination and appropriate treatments are available for influenza, but not for COVID-19, at least by the end of 2020 in Japan. It should also be noted that, in line with previous studies,27,28 the number of patients with influenza in 2020 was one-half to one-third of that between 2017–2019, probably because the prevention measures for COVID-19, such as mask and physical distancing, were effective in preventing influenza infection. The influenza vaccination coverage in Japan was almost constant at 48.2% in 2017, 47.9% in 2018, and 50.4% in 2019, among people ≥ 65 and people aged 40–64 with specific conditions (heart, kidney, or respiratory disease, and HIV).31 Thus, there is seemingly no obvious correlation between in-hospital mortality of patients with influenza (i.e., 2·2% in 2017, 3·4% in 2018, and 3·0% in 2019) and vaccination coverage for influenza between 2017-2019, although the influenza vaccination rate in 2020 is not yet publicly available.
We also identified differences from previous studies in the Americas and Europe. The overall relative risk of in-hospital death for COVID-19 compared to influenza (adjusted OR of 1·8) was lower in the current study than in previous studies; four large cohort studies from the United States,3,11 France,4 and Canada9 reported the relative risk of in-hospital death ranging from 2·9 to 19·8. While the in-hospital mortality of influenza in our study (2·8%) was comparable with reported rates overseas (1·4–6·1%),3,4,9,11 the in-hospital mortality of COVID-19 in our study (5·1%) was lower than that reported in previous foreign studies (13·7–19·9%),3,4,9,11 which can be the main reason for the lower relative risk in our study. Second, the difference in in-hospital COVID-19 deaths between Japan and American or EU countries may be due to the difference in the capacity of medical care services. The number of COVID-19 cases in Japan was much lower than those in the United States and France (with total numbers per million people by the end of 2020: 1,800 in Japan, 59,000 in the United States, and 38,000 in France).1 Thus, Japan could maintain access to health care (as suggested by the bed occupancy rate <100% in each prefecture) for patients with COVID-19 during 2020, during the first wave (February–May, 2020), the second wave (June–September, 2020) and the third wave (October 2020–February 2021).32 Japan may have been able to preserve the quality of medical care services, leading to lower mortality of COVID-19. In the early phase of the COVID-19 pandemic, the Ministry of Health, Labour and Welfare of Japan initially requested all patients with COVID-19 to be hospitalised, regardless of severity, as of February 2020. However, since March 2020, in response to the rapid increase in the number of patients, the government suggested only patients with moderate to severe symptoms and patients at higher risk (such as pregnant women and individuals aged 65 or older) be eligible for admission, whereas the others (i.e., asymptomatic, or mild cases) should stay at designated facilities (such as hotels) and their home.33,34 Therefore, we do not believe that the change in hospitalisation indication at the early stage had much effect on our results because the number of cases before March 2020 was very small (at less than 1%) in our data set.
As for the supportive care, the necessity of mechanical ventilation, ECMO, and renal replacement therapy did not differ between COVID-19 and influenza patients on the admission day, but COVID-19 patients were more likely to require these treatments during the entire hospitalisation period. Patients with COVID-19 tended to experience a deterioration of their condition after hospitalisation compared to those with influenza, which seemingly led to a higher risk of in-hospital death. Notably, when mechanical ventilation was started, the in-hospital mortality (both crude and adjusted) did not differ between the study groups. To reduce the overall in-hospital mortality of COVID-19 and bring it closer to that of influenza infection, improvement in the management or development of treatment options for patients with COVID-19 before initiating mechanical ventilation may be important.,
The stratified analysis by age group showed that people aged 70 years or older with COVID-19 had a higher risk of in-hospital death than those with influenza. Our results were largely compatible with those of previous studies reporting that in-hospital mortality was higher in the COVID-19 group than in the influenza group, especially among older individuals.3,4,11 Among people aged 20–69, we did not find a statistically significant difference in in-hospital mortality, which was in line with a report from France.4 Our results suggest that it is reasonable to prioritize the vaccination of COVID-19 for older people. A French study reported that COVID-19 patients younger than 18 years had a higher risk of in-hospital death,4 which we could not confirm because the death of COVID-19 patients younger than 20 years was not observed in our study population.
This study has several limitations. First, although our database covered a large number of cases admitted to more than 350 acute care hospitals across Japan, the results may not represent the entire population in Japan. The hospital volume of the MDV database tends to be larger than that of all DPC hospitals. We could not evaluate the representativeness of hospitals in the MDV database in terms of hospital ownership (i.e., public or private) and socio-economic status of hospitalised patients due to lack of information. However, in the Japanese healthcare system, all acute care hospitals are eligible for reimbursement by the national health insurance, and people can be hospitalised for any acute care hospitals regardless of socio-economic status. In addition, we found that the age distribution of inpatients in the MDV database was roughly similar to that of all DPC hospitals, although information on the sex distribution was unavailable. More importantly, the crude in-hospital mortality for COVID-19 (5·1%) and influenza (2·8%) in our cohort were similar to those reported in previous Japanese studies.35, 36, 37 Second, when we defined comorbidities based on inpatient or outpatient ICD-10 codes recorded in the database, we may have missed some comorbidities if patients received routine health care in medical institutions other than the hospitals to which the patients were admitted. In addition, miscoding of diagnoses and procedures may be possible in claims data, although a previous study reported that the diagnoses and procedure records of DPC data had a high validity in general.38 For example, although the proportion of patients with oxygen therapy in our data is roughly similar to other Japanese statistics of COVID-19 and influenza,19,37,39 we might have failed to detect some use of oxygen therapy in case a temporal use of a small amount of oxygen was not recorded or reimbursed by the hospitals. If such misclassification bias existed, it is likely to be non-differential and, therefore, diluting the relative risk between COVID-19 and influenza (meaning that the true relative risk would be even larger than our estimate). These issues may be also true to blood transfusion, although the proportion of blood transfusion in the current study (3.7%) was compatible with the reported value (4.8%) in a previous large-scale Japanese study on COVID-19.19 Third, as we used data from administrative claims data, we could not acquire information including vital signs, the severity of comorbidities, or socioeconomic status. Thus, we could neither compare these characteristics between COVID-19 and influenza nor consider these factors as potential confounders or mediators in our analysis. Fourth, laboratory test values were available for only 10% of the hospitals that agreed to provide the data in the MDV database; therefore, laboratory test results were unadjusted in our multivariable analysis. Fifth, we conducted the MICE for missing values of BMI and smoking history based on the assumption that these variables were missing at random; however, we could not deny the possibility that they were missing not at random. Thus, we also conducted the complete case analyses to confirm similar results. Sixth, our study did not include patients staying in hospital at the end of 2020. Thus, the length of stay for all COVID-19 patients hospitalised in 2020 could have been even longer than that observed in this study (which was longer than that for influenza patients). Seventh, similar to previous studies in the United States,3,11 France,4 and Canada,9 this study compared patients hospitalised for COVID-19 and influenza, and therefore may suffer from selection or collider bias due to different threshold of admission for each disease. However, our analysis demonstrated that patients admitted for COVID-19 had a higher risk of in-hospital death than those admitted for influenza, even after adjusting for comorbidities and treatment on admission day. Finally, it should be noted that the latest characteristics of COVID-19 in 2021 may be different from our results on COVID-19 cases in 2020. The rapid development of treatments and vaccines may have lessened the outcome differences between COVID-19 and influenza in 2021 compared to 2020. At the same time, it is also possible that the emergence of more transmissible variants, such as the Delta variant,40,41 first detected in late 2020 in India, has recently worsened health outcomes.
In conclusion, we showed that in-hospital mortality was higher in patients admitted for COVID-19 in 2020 than in patients admitted for influenza in 2017–2020 in Japan, which was consistent with the direction of the effect, albeit less prominent, in American and EU countries. However, this was mainly driven by findings in older people, whereas we did not observe higher mortality for COVID-19 than influenza among younger patients and among those with mechanical ventilation.
Authors’ contributions
YT designed the study, conducted data processing, analysed the data, and wrote the initial draft. MAI contributed to conception, acquisition of data, study design, interpretation, and revision of the manuscript. TK contributed to conception, study design, interpretation, and revision of the manuscript. JK, MA and TS accessed and verified the data, and were involved in data processing, interpretation, and revision of the manuscript. TA, MII, AM, MS, HO, YM, SI, TK and NT substantially contributed to study design, interpretation of the data, and revision of the manuscript. All authors approved the final version and had final responsibility for the decision to submit for publication.
Declaration of Competing Interest
Atsushi Miyawaki has a joint research project with Medical Data Vision Co., Ltd. outside of this study and received labour contributions in the financial year 2020.
Acknowledgments
Acknowledgements
We would like to express our gratitude to the personnel of Medical Data Vision Co., Ltd., especially Masaki Nakamura and Shogo Atsuzawa, for their contribution to the preparation of the administrative claims data. We also thank Editage (www.editage.com) for English language editing.
Data sharing
We obtained data from the Medical Data Vision Co., Ltd. (MDV), and we are not allowed to share these data with other parties. Researchers who meet the criteria for access can acquire de-identified participant data from the MDV (https://en.mdv.co.jp).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2021.100365.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) dashboard.; https://covid19.who.int. Accessed Novermber 20, 2021.
- 2.Jiang C, Yao X, Zhao Y, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020;22(6–7):236–244. doi: 10.1016/j.micinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y, Bowe B, Maddukuri G, Al-Aly Z. Comparative evaluation of clinical manifestations and risk of death in patients admitted to hospital with COVID-19 and seasonal influenza: cohort study. BMJ. 2020;371:m4677. doi: 10.1136/bmj.m4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brehm TT, van der Meirschen M, Hennigs A, et al. Comparison of clinical characteristics and disease outcome of COVID-19 and seasonal influenza. Sci Rep. 2021;11(1):5803. doi: 10.1038/s41598-021-85081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig M, Jacob J, Basedow F, Andersohn F, Walker J. Clinical outcomes and characteristics of patients hospitalized for influenza or COVID-19 in Germany. Int J Infect Dis. 2021;103:316–322. doi: 10.1016/j.ijid.2020.11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cates J, Lucero-Obusan C, Dahl RM, et al. Risk for in-hospital complications associated with COVID-19 and influenza - veterans health administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1528–1534. doi: 10.15585/mmwr.mm6942e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnino MW, Moskowitz A, Thompson GS, et al. Comparison between patients hospitalized with influenza and COVID-19 at a tertiary care center. J Gen Intern Med. 2021;36(6):1689–1695. doi: 10.1007/s11606-021-06647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma AA, Hora T, Jung HY, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ. 2021;193(12):E410–E418. doi: 10.1503/cmaj.202795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb NL, Sathe NA, Duan KI, et al. Vol. 18. 2021. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza; pp. 632–640. (Ann Am Thorac Soc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot HK, Martin ET, Gaglani M, et al. Coronavirus disease 2019 (COVID-19) versus influenza in hospitalized adult patients in the United States: Differences in demographic and severity indicators. Clin Infect Dis. 2021:ciab123. doi: 10.1093/cid/ciab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieber P, Flury D, Güsewell S, et al. Characteristics of patients with coronavirus disease 2019 (COVID-19) and seasonal influenza at time of hospital admission: a single center comparative study. BMC Infect Dis. 2021;21(1):271–274. doi: 10.1186/s12879-021-05957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Ding D, Huang X, et al. Differentiation of COVID-19 from seasonal influenza: A multicenter comparative study. J Med Virol. 2021;93(3):1512–1519. doi: 10.1002/jmv.26469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auvinen R, Nohynek H, Syrjänen R, et al. Comparison of the clinical characteristics and outcomes of hospitalized adult COVID-19 and influenza patients - a prospective observational study. Infect Dis (Lond) 2021;53(2):111–121. doi: 10.1080/23744235.2020.1840623. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Du RH, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1–7. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng LS, Yuan J, Ding L, et al. Comparison of patients hospitalized with COVID-19, H7N9 and H1N1. Infect Dis Poverty. 2020;9(1):163–165. doi: 10.1186/s40249-020-00781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose R, Ikegaya H, Naito Y, et al. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19. Clin Infect Dis. 2020:ciaa1517. doi: 10.1093/cid/ciaa1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsunaga N, Hayakawa K, Terada M, et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 REGISTRY JAPAN. Clin Infect Dis. 2020:ciaa1470. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashida K, Murakami G, Matsuda S, Fushimi K. History and profile of diagnosis procedure combination (DPC): Development of a real data collection system for acute inpatient care in Japan. Journal of Epidemiology. 2021;31(1):1–11. doi: 10.2188/jea.JE20200288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medical Data Vision Co., Ltd. MDV Database.; Available at: https://en.mdv.co.jp. Accessed June 28, 2021.
- 22.Miyawaki A, Tomio J, Nakamura M, Ninomiya H, Kobayashi Y. Changes in Surgeries and Therapeutic Procedures During the COVID-19 Outbreak: A Longitudinal Study of Acute Care Hospitals in Japan. Ann Surg. 2021;273(4):e132–e134. doi: 10.1097/SLA.0000000000004528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano K, Nakamura M, Ninomiya H, Kobayashi Y, Miyawaki A. Large decrease in paediatric hospitalisations during the COVID-19 outbreak in Japan. BMJ Paediatrics Open. 2021;5(1) doi: 10.1136/bmjpo-2020-001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abe K, Miyawaki A, Nakamura M, Ninomiya H, Kobayashi Y. Trends in hospitalizations for asthma during the COVID-19 outbreak in Japan. J Allergy Clin Immunol Pract. 2021;9(1):494–496. doi: 10.1016/j.jaip.2020.09.060. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohsaka S, Lam CSP, Kim DJ, et al. Risk of cardiovascular events and death associated with initiation of SGLT2 inhibitors compared with DPP-4 inhibitors: an analysis from the CVD-REAL 2 multinational cohort study. Lancet Diabetes Endocrinol. 2020;8(7):606–615. doi: 10.1016/S2213-8587(20)30130-3. [DOI] [PubMed] [Google Scholar]
- 26.Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare. Ethical guidelines for medical and health research involving human subjects. https://www.lifescience.mext.go.jp/files/pdf/n2181_01.pdf. Accessed November 13, 2021.
- 27.Sakamoto H, Ishikane M, Ueda P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. JAMA. 2020;323(19):1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawakami T, Karako K, Song P. Behavioral changes adopted to constrain COVID-19 in Japan: What are the implications for seasonal influenza prevention and control? Global Health & Medicine. 2021;3(3):125–128. doi: 10.35772/ghm.2021.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moons KG, Donders RA, Stijnen T, Harrell FE., Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33(12):700–711. [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health, Labour and Welfare. Number of people who received routine vaccinations (in Japanese). https://www.mhlw.go.jp/topics/bcg/other/5.html. Accessed December 3, 2021.
- 32.Karako K, Song P, Chen Y, Tang W, Kokudo N. Overview of the characteristics of and responses to the three waves of COVID-19 in Japan during 2020-2021. Biosci Trends. 2021;15(1):1–8. doi: 10.5582/bst.2021.01019. [DOI] [PubMed] [Google Scholar]
- 33.Ministry of Health, Labour and Welfare. Eligibility for recuperation at designated facilities or at home for patients with mild cases of COVID-19, and preparations for response by local governments (in Japanese). https://www.mhlw.go.jp/content/000618525.pdf. Accessed November 15, 2021.
- 34.Ministry of Health, Labour and Welfare. Transition of each countermeasure (surveillance, prevention of spread of infection, and medical care delivery system) in case the number of patients with COVID-19 increases in the community (in Japanese). https://www.mhlw.go.jp/content/000601816.pdf. Accessed November 15, 2021.
- 35.Saito S, Asai Y, Matsunaga N, et al. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J Infect. 2021;82(4):84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama T, Fujisawa T, Suga S, et al. Outcomes and prognostic features of patients with influenza requiring hospitalization and receiving early antiviral therapy: a prospective multicenter cohort study. Chest. 2016;149(2):526–534. doi: 10.1378/chest.14-2768. [DOI] [PubMed] [Google Scholar]
- 37.Ishii M, Terai H, Kabata H, et al. Clinical characteristics of 345 patients with coronavirus disease 2019 in Japan: A multicenter retrospective study. J Infect. 2020;81(5):e3–e5. doi: 10.1016/j.jinf.2020.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27(10):476–482. doi: 10.1016/j.je.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sruamsiri R, Ferchichi S, Jamotte A, Toumi M, Kubo H, Mahlich J. Impact of patient characteristics and treatment procedures on hospitalization cost and length of stay in Japanese patients with influenza: A structural equation modelling approach. Influenza Other Respir Viruses. 2017;11(6):543–555. doi: 10.1111/irv.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh A, McMenamin J, Taylor B, Robertson C. Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.