Abstract
Background:
Limited data exist about clinical outcomes and levels of inflammatory and immune markers among people hospitalized with COVID-19 by HIV serostatus and by HIV viral suppression.
Setting:
Large tertiary care health system in the Bronx, NY, USA.
Methods:
We conducted a retrospective cohort study of 4613 SARS-CoV-2 PCR-positive patients admitted between March 10, 2020, and May 11, 2020. We examined in-hospital intubation, acute kidney injury (AKI), hospitalization length, and in-hospital mortality by HIV serostatus, and by HIV-viral suppression and CD4 counts among people living with HIV (PLWH) using adjusted competing risks regression. We also compared immune and inflammatory marker levels by HIV serostatus and viral suppression.
Results:
Most patients were either non-Hispanic Black (36%) or Hispanic (37%); 100/4613 (2.2%) were PLWH, among whom 15 had detectable HIV viral load. PLWH compared to patients without HIV had increased intubation rates (adjusted hazard ratio 1.73 [95% CI: 1.12 to 2.67], P = 0.01). Both groups had similar rates of AKI, length of hospitalization, and death. No (0%) virally unsuppressed PLWH were intubated or died, versus 21/81 (26%, P = 0.04) and 22/81 (27%, P = 0.02) of virally suppressed PLWH, respectively. Among PLWH, higher CD4 T-cell counts were associated with increased intubation rates. C-reactive protein, IL-6, neutrophil counts, and ferritin levels were similar between virally suppressed PLWH and patients without HIV, but significantly lower for unsuppressed PLWH (all P < 0.05).
Conclusions:
PLWH had increased risk of intubation but similarly frequent rates of AKI and in-hospital death as those without HIV. Findings of no intubations or deaths among PLWH with unsuppressed HIV viral load warrant further investigation.
Keywords: COVID-19, Sars-CoV-2, HIV, AIDS, inflammatory markers
BACKGROUND
The COVID-19 pandemic, caused by the recently identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues its global spread with total infections over 15 million and deaths exceeding 600,000.1 Whether COVID-19 disproportionately affects people living with HIV (PLWH) is still unknown. Several factors, such as immunosuppression and a disproportionate burden of comorbidities associated with a poor prognosis, suggest that PLWH may be at increased risk for adverse outcomes after infection with SARS-CoV-2.2 However, other factors, such as the use of antiretroviral drugs shown to have in vitro activity against SARS-CoV-2, may attenuate manifestations of COVID-19 among PLWH.3,4 Despite several case series describing characteristics and outcomes of COVID-19 among people living with HIV,5-10 due to small sample sizes and/or lack of comparison groups of patients without HIV, the impact of COVID-19 on PLWH remains incompletely understood. In addition, most studies of PLWH and COVID-19 have predominantly reported on patients on antiretroviral therapy with suppressed viral load,5-12 thus limiting our understanding of how viral suppression may influence clinical outcomes.
New York City, a longstanding epicenter of the HIV epidemic in the United States, also became the epicenter of COVID-19 in the Spring of 2020. Within New York City, the Bronx borough (population 1.4 million) is disproportionately affected by both diseases with an existing 2.1% HIV prevalence (compared to 0.4% nationally in the United States),13 and at the COVID-19 epidemic’s peak to date, 859 COVID-19 hospitalizations per 100,000 people (compared to 659 COVID-19 hospitalizations per 100,000 people citywide).14 We sought to compare clinical outcomes of PLWH hospitalized with COVID-19 in a large health care system in the Bronx to those in hospitalized patients without HIV, and to examine whether, among PLWH, outcomes differed according to CD4 count and HIV viral suppression.
METHODS
Study Design, Population, and Data Source
We conducted a retrospective cohort study of all patients admitted to the Montefiore Health System (MHS) in the Bronx, New York, between March 10, 2020, and May 11, 2020 with a laboratory-confirmed diagnosis of COVID-19. Diagnosis of COVID-19 was confirmed by SARS-CoV-2 RNA polymerase chain reaction. Patients were categorized as HIV-positive or HIV-negative and followed until the earliest of in-hospital death, discharge, or censoring on May 11, 2020, if still hospitalized. If a patient was admitted with COVID-19 more than once, we considered only the first hospitalization. We excluded children (< 18 years of age), given the low prevalence of HIV in this group. The study was approved by the Albert Einstein College of Medicine Institutional Review Board.
The Einstein-Rockefeller-CUNY Center for AIDS Research (ERC-CFAR) Clinical Cohort Database contains data on patients living with HIV and receiving care within MHS.15 MHS is an integrated health care delivery system in the Bronx, NY, with 4 hospitals and >50 ambulatory care sites, including a network of substance abuse treatment programs. The database combines data from the electronic health record, including inpatient and outpatient visits; laboratory test results; prescription data; and intake data from New York State’s AIDS Institute Reporting System (AIRS),16 which records key risk factor information at facilities receiving Ryan White funding. Records are captured through existing electronic systems and combined in a centralized location, where they undergo standardized data checks. For this study, we supplemented information from the database with chart review for quality assurance purposes as well as to confirm values of selected variables.
Outcomes of Interest
We assessed the following outcomes for each hospitalization: intubation, acute kidney injury (AKI), length of hospitalization stay (LOS), and in-hospital death. These outcomes were analyzed primarily as time-to-event outcomes, including LOS, which was analyzed as time to discharge. The index date was the date of admission. Depending on the outcome, patients were censored at death, discharge, or were administratively censored on May 11, 2020, if still hospitalized.
AKI was defined as either hospital-acquired AKI using KDIGO criteria,17 or community-acquired AKI. Community-acquired AKI was defined as a >50% increase in serum creatinine between a “baseline” value (ie, mean of creatinine values recorded in the medical record, 7–365 days preceding admission) and the value recorded at admission. In those with no baseline creatinine, community-acquired AKI was defined as a >50% increase in serum creatinine between the maximum creatinine level and the minimum creatinine level during the hospitalization.18,19
Exposures of Interest
The primary exposure of interest was HIV-positive status. HIV-positivity was identified using a previously validated algorithm that requires either a positive HIV Western blot or Multispot; a detectable HIV-1 viral load; or at least 3 undetectable HIV-1 viral loads ordered concurrently with a CD4 T-cell count (N = 77).20 This last criterion captures PLWH who are missing documentation of HIV antibody testing but are well controlled on ART. Because the algorithm is optimized to identify PLWH receiving routine outpatient care in our system, we identified additional PLWH with COVID-19 who were initially categorized as HIV-negative (N = 23) through the assignment of ICD-10-CM code B20 (HIV disease) as a discharge diagnosis, prescription of at least one ART medication in the year before admission, or dispensing of ART during the hospitalization. All PLWH were further confirmed through chart review to exclude HIV-negative patients who were using certain ART medications for HIV pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP), hepatitis B treatment, or experimental treatment for COVID-19 (eg, lopinavir/ritonavir). All remaining patients were classified as HIV-negative, regardless of whether a negative HIV test was recorded in the chart. The likelihood of misclassifying an individual with known or undiagnosed HIV as being HIV-negative was likely low; a prior HIV seroprevalence study found only 0.2% of 4990 patients visiting our emergency department to have undiagnosed HIV.21
We further classified HIV-positive patients on the basis of CD4 T-cell count and HIV-1 RNA viral suppression (<40 copies/mL), using the most recent value available in the one year before admission. For patients without available CD4 T-cell counts or HIV RNA levels in the year before admission, we used the first value recorded during the hospitalization.
Confounders and Other Variables
Potential confounders extracted from the database for all patients included age at diagnosis; sex; race/ethnicity (black non-Hispanic, Hispanic, white non-Hispanic, and other); body mass index (BMI); smoking history; history of specific comorbidities including chronic lower respiratory disease, hypertension, diabetes mellitus, ischemic heart disease, and heart failure, based on diagnosis codes recorded up to 10 years before the admission; and calendar time (ie, date of admission). In addition, we extracted data on steroid use among PLWH by chart review.
We also extracted descriptive data on vital signs during the hospitalization including temperature (degrees Fahrenheit), heart rate (beats per minute), mean arterial pressure (mm Hg), O2 saturation (%), and respiratory rate (breaths per minute), as well as blood markers of immune function and inflammation including white blood cell count, lymphocyte count, neutrophil count, C-reactive protein (CRP), interleukin-6 (IL-6), erythrocyte sedimentation rate, procalcitonin, ferritin, and D-dimer.
Statistical Analysis
We compared demographic and clinical characteristics of COVID-19 patients by HIV serostatus and HIV viral suppression among PLWH. We examined differences in outcomes by HIV serostatus in unadjusted analyses using relevant statistical tests (eg, χ2 test for categorical outcomes and log-rank test for time-to-event outcomes). To appropriately account for competing risks for each outcome that could occur during the hospitalization, we used an extension of Cox proportional hazards regression that models the cause-specific hazard function. All models were adjusted for age, ex, race/ethnicity, BMI, history of chronic lower respiratory diseases, and calendar time. Among PLWH, we also examined differences in outcomes by CD4 T-cell count and viral suppression using the same methods. In sensitivity analyses, we further adjusted for smoking history and the remaining comorbidities (hypertension, diabetes mellitus, ischemic heart disease, and heart failure). Because PLWH-specific analyses had a more limited sample size, we created a single summary score for these comorbidities (range 0–4).
SAS 9.4 (SAS Institute, Cary, NC) was used for all analyses. We used multiple imputation (5 datasets) based on multivariate sequential regression to account for missing data on BMI (15%), smoking history (29%), and history of comorbidities (12%).22
RESULTS
Between March 10, 2020, and May 11, 2020, 4671 patients were hospitalized and tested PCR-positive for SARS-CoV-2. Of these, we excluded 58 patients younger than 18 years of age, resulting in a final analytic sample of N = 4613. Among this sample, 100 patients (2.2%) were HIV-positive.
Table 1 shows demographic and clinical characteristics of PLWH by viral suppression and those without HIV hospitalized with COVID-19. Females represented 44% of PLWH in the sample and 47% of the overall study population. Most patients in either group were a member of a racial or ethnic minority group (either non-Hispanic Black or Hispanic) and had public health insurance (Medicaid or Medicare). Among PLWH compared to patients without HIV, the median age was slightly younger (61 years versus 65 years), and a greater proportion were Black (43% versus 36%), and had a history of chronic lower respiratory disease (46% versus 32%). Clinical characteristics (temperature, heart rate, mean arterial pressure, and oxygen saturation) at the time of admission were similar between the groups.
TABLE 1.
Demographic and Clinical Characteristics of Laboratory-Confirmed COVID-19 Patients Admitted to MHS, October 3, 2020–November 5, 2020, by HIV Serostatus and Viral Suppression∥
| Without HIV, N = 4513 N (%) |
PLWH, Suppressed (<40 Copies/mL), N = 81 N (%) |
PLWH, Unsuppressed, N = 15 N (%) |
P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Median age at admission (IQR) | 65 (54–76) | 63 (52–69)¶ | 60 (53–62)** | 0.01 |
| Female gender* | 2111 (47) | 39 (48) | 4 (27) | 0.30 |
| Race/ethnicity | ||||
| Black, non-Hispanic | 1618 (36) | 36 (44) | 6 (40) | 0.66 |
| Hispanic (any race) | 1685 (37) | 29 (36) | 6 (40) | |
| White, non-Hispanic | 375 (8) | 3 (4) | 1 (7) | |
| Asian/Pacific Islander | 117 (3) | 0 (0) | 0 (0) | |
| Other/unknown | 718 (16) | 13 (16) | 2 (13) | |
| Primary language | ||||
| English | 3348 (74) | 65 (80) | 12 (80) | 0.21 |
| Spanish | 971 (22) | 16 (20) | 2 (13) | |
| Other | 194 (4) | 0 (0) | 1 (7) | |
| Insurance status | ||||
| Medicaid | 1239 (27) | 33 (41)f | 8 (53) | 0.01 |
| Medicare | 2345 (52) | 40 (49) | 6 (40) | |
| Commercial | 698 (15) | 8 (10) | 1 (7) | |
| Self-pay or other | 229 (5) | 0 (0) | 0 (0) | |
| Clinical characteristics | ||||
| Chronic lower respiratory diseases† | 997 (22) | 27 (33)¶ | 4 (27) | 0.06 |
| History of smoking reported | 1129 (25) | 35 (43)¶ | 4 (27) | 0.001 |
| Mean body mass index, kg/m2 (SD) | 29.6 (7.4) | 28.6 (7) | 27.5 (7) | 0.29 |
| Obese (body mass index 30 or more) | 1568 (41) | 28 (37) | 6 (40) | 0.80 |
| Hypertension | 3069 (69) | 60 (74) | 10 (67) | 0.60 |
| Diabetes mellitus | 2044 (46) | 34 (42) | 7 (47) | 0.76 |
| Ischemic heart disease | 868 (20) | 13 (16) | 3 (20) | 0.77 |
| Heart failure | 622 (14) | 16 (20) | 1 (7) | 0.26 |
| Temperature at admission, °F (median, IQR) | 98.9 (98.2–100.1) | 99.0 (98.2–99.8) | 98.5 (97.8–99.3) | 0.40 |
| Highest recorded temperature, °F (median, IQR) | 101.0 (99.7–102.6) | 100.6 (99.2–102.7) | 100.1 (99.6–101.1) | 0.20 |
| Heart rate at admission, beats per min (median, IQR) | 99 (85–113) | 97 (83–110) | 106 (87–117) | 0.66 |
| Mean arterial pressure at admission, mm Hg (median, IQR) | 87 (77–96) | 87 (75–96) | 89 (86–97) | 0.36 |
| Oxygen saturation at admission, % (median, IQR) | 95 (91–98) | 94 (90–98) | 95 (93–98) | 0.54 |
| Lowest oxygen saturation, % (median, IQR) | 90 (82–93) | 89 (84–92) | 95 (93–98)#** | 0.02 |
| Respiratory rate, breaths per min (median, IQR) | 20 (18–22) | 19 (18–22) | 19 (18–20) | 0.21 |
| HIV Transmission category | ||||
| Men who have sex with men risk | — | 4 (5) | 4 (27)# | 0.04 |
| Injection drug use history | — | 12 (15) | 3 (20) | |
| Heterosexual transmission | — | 31 (38) | 3 (20) | |
| Other/unknown or missing | — | 34 (42) | 5 (33) | |
| Median CD4+ count‡, cells/uL (IQR)‡ | — | 412 (237–636) | 250 (107–535)# | 0.03 |
| Any ART use in past yr§ | — | 78 (96) | 12 (80)# | 0.047 |
| Any tenofovir use§ | — | 56 (69) | 8 (53) | 0.25 |
P-value denotes difference across all groups, by the Kruskal-Wallis or Fisher exact test as appropriate.
Includes one transgender female in the PLWH suppressed group and one transgender female in the unsuppressed.
Based on at least one of the following diagnosis codes: ICD-9-CM 490–496 and ICD-10-CM J40-J47.
Most recent value recorded in the yr before admission.
Includes ART use during the hospitalization.
4 PLWH did not have HIV RNA measurements available.
P<0.05 for comparison between patients without HIV and suppressed PLWH.
P<0.05 for comparison between suppressed PLWH and unsuppressed PLWH.
P<0.05 for comparison between patients without HIV and unsuppressed PLWH.
COVID-19, coronavirus disease 2019; IQR, interquartile range.
Among PLWH hospitalized with COVID-19, 96 patients had HIV viral load data available, of whom most (n = 90, 96%) had evidence of any ART use in the past year, and 81 (84%) patients had suppressed HIV viral load. Median CD4 count was higher among virally suppressed PLWH compared to unsuppressed PLWH (412 cells/uL [IQR, 237–636] versus 250 cells u/L [IQR, 107–535], P = 0.03). A greater proportion of virally suppressed than unsuppressed PLWH received corticosteroids (24/81 [30%] versus 2/15 [13%]), although this difference was not statistically significant (P = 0.34).
Association of HIV Serostatus and Characteristics With Clinical Outcomes
Table 2 shows the unadjusted and adjusted associations of HIV serostatus with clinical outcomes. Overall, 21 (21%) PLWH and 636 (14%) patients without HIV were intubated (P = 0.05). In analyses adjusting for age, sex, race/ethnicity, BMI, history of chronic lower respiratory diseases, and calendar time, there was an increased hazard of intubation among PLWH compared to patients without HIV (adjusted HR 1.73 [95% CI: 1.12 to 2.67], P = 0.01). The incidence of AKI was 55% in PLWH and 50% in patients without HIV (P = 0.32). Median (IQR) length of hospital stay was 5 days (3–9) for both PLWH and patients without HIV (P = 0.92), and the proportion who died in the hospital was also similar for both groups: 22 (22%) of PLWH and 1104 (24%) of patients without HIV (P = 0.73). Adjusted associations were similar. All effect estimates were virtually unchanged in sensitivity analyses that further adjusted for smoking history and other comorbidities (data not shown).
TABLE 2.
Hospitalization Outcomes of Laboratory-Confirmed COVID-19 Patients Admitted to MHS, October 3, 2020–November 5, 2020, by HIV Serostatus
| Outcomes by HIV Serostatus, Unadjusted |
Adjusted Association of Positive HIV Serostatus with Outcome (Reference: HIV-Negative) |
|||||
|---|---|---|---|---|---|---|
| Outcome | PLWH Events/Total Eligible (%) |
Without HIV Events/Total Eligible (%) |
P (Unadjusted) |
Outcome | Adjusted Hazard Ratio (95% CI) |
P (Adjusted) |
| Intubation | 21/100 (21%) | 636/4513 (14%) | 0.05 | Time to intubation | 1.73 (1.12 to 2.67) | 0.01 |
| Acute kidney injury* | 46/83 (55%) | 2072/4149 (50%) | 0.32 | Time to acute kidney injury | 1.21 (0.96 to 1.52) | 0.11 |
| Length of stay†, d (median, IQR) | 5 (3–9) | 5 (3–9) | 0.92 | Time to discharge | 0.99 (0.79 to 1.25) | 0.94 |
| Death in hospital | 22/100 (22%) | 1104/4513 (24%) | 0.73 | Time to death | 1.20 (0.78 to 1.83) | 0.41 |
Associations of positive HIV serostatus with each outcome are adjusted for age, sex, race/ethnicity, history of chronic lower respiratory disease, BMI, calendar time, and account for competing risks of other outcomes as appropriate.
Excludes patients with end-stage renal disease (N = 369).
Includes patients discharged during study period (N = 3487).
Clinical outcomes by HIV viral suppression status are shown in Table 3. There were no (0%) intubations among the 15 patients with unsuppressed HIV viral load, compared to 21 (26%) among 81 patients who were virally suppressed (P = 0.04). Similarly, there were no (0%) deaths among PLWH with unsuppressed viral load compared to 22 (27%) among patients who were suppressed (P = 0.02). Because there were no intubations or deaths in the unsuppressed group, we could not adjust for potential confounders. We did not observe statistically significant differences in AKI or length of stay by HIV suppression status.
TABLE 3.
Hospitalization Outcomes of PLWH With Laboratory-Confirmed COVID-19 Admitted to MHS, October 3, 2020–November 5, 2020, by HIV Viral Suppression Status
| Outcomes by HIV Viral Suppression Status, Unadjusted |
Adjusted Association of Unsuppressed Viral Load with Outcome (Reference: Suppressed Viral Load, <40 Copies/mL) |
|||||
|---|---|---|---|---|---|---|
| Outcome | Unsuppressed Events/Total Eligible (%) |
Suppressed (<40 Copies/mL) Events/Total Eligible (%) |
P (Unadjusted) |
Outcome | Adjusted Hazard Ratio (95% CI) |
P (Adjusted) |
| Intubation | 0/15 (0%) | 21/81 (26%) | 0.04 | Time to intubation | N/A | N/A |
| Acute kidney injury* | 7/13 (54%) | 38/67 (57%) | 0.85 | Time to acute kidney injury | 0.89 (0.45 to 1.74) | 0.74 |
| Length of stay†, d (median, IQR) | 7 (4–12) | 5 (3–8) | 0.40 | Time to discharge | 1.58 (0.97 to 2.55) | 0.06 |
| Death in hospital | 0/15 (0%) | 22/81 (27%) | 0.02 | Time to death | N/A | N/A |
Associations of unsuppressed viral load with each outcome are adjusted for age, sex, race/ethnicity, history of chronic lower respiratory disease, BMI, calendar time, and account for competing risks of other outcomes as appropriate. Four HIV-positive patients did not have HIV RNA measurements available. Adjusted associations of unsuppressed viral load with time to intubation and time to death could not be estimated due to no events among unsuppressed.
Excludes patients with end-stage renal disease (N = 17).
Includes patients discharged alive during study period (N = 78).
Among PLWH, increased CD4 T-cell counts were associated with an increased hazard of intubation (adjusted HR 1.13 [95% CI: 1.06 to 1.20] per every 100 cells/uL increase in CD4). CD4 T-cell count was not associated with AKI, length of stay, or death (data not shown).
Selected Markers of Immune and Inflammatory Function by HIV Serostatus and Viral Suppression
Table 1, Supplemental Digital Content, http://links.lww.com/QAI/B576 shows the peak levels of selected markers of immune and inflammatory function during hospitalization by HIV serostatus and viral suppression, when they were measured. For most markers, levels were similar between suppressed PLWH and patients without HIV, whereas PLWH who were unsuppressed had markedly lower levels. Differences among these groups were statistically significant for CRP, IL-6, neutrophil count, and ferritin levels (all P < 0.05) (Fig. 1).
FIGURE 1.
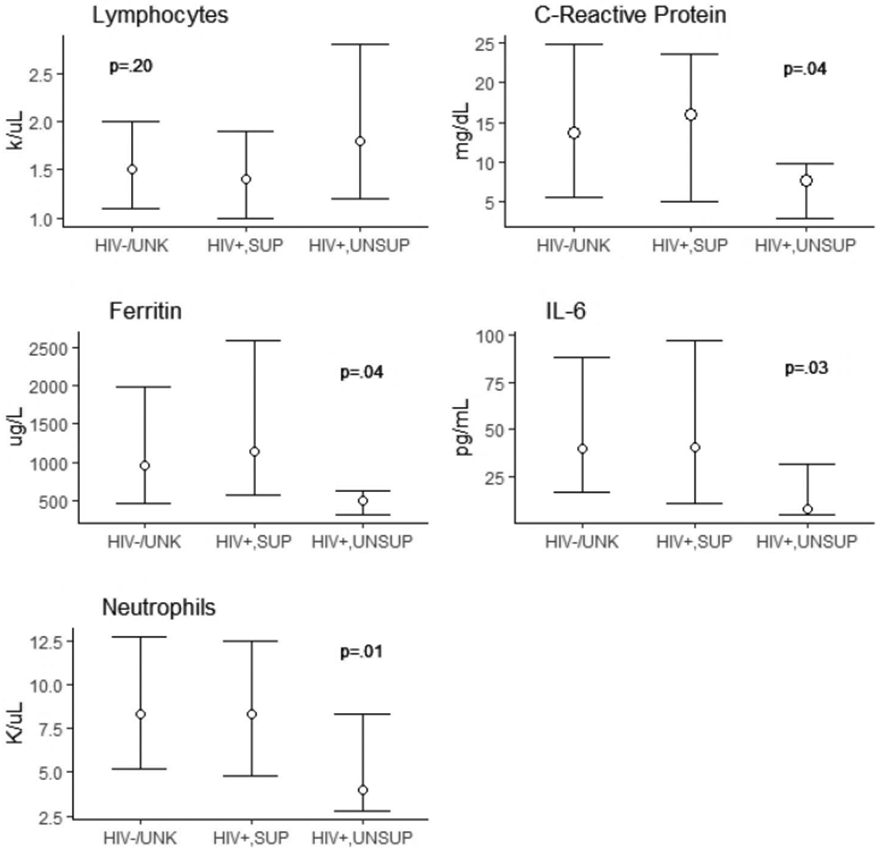
Selected markers of immune and inflammatory function. HIV−/UNK, patients without HIV; HIV+, SUP, PLWH with suppressed HIV viral load;HIV+.UNSUP, PLWH with unsuppressed viral load. P-value represents Kruskal–Wallis test for distributional differences across all 3 groups.
DISCUSSION
We report on the largest cohort to date comparing PLWH hospitalized with COVID-19 to patients hospitalized for COVID-19 without HIV. In this large and diverse sample, we found that AKI, length of hospitalization, and mortality were similar between PLWH and patients without HIV. However, we did find that hospitalized PLWH with COVID-19 were more likely to be intubated compared to those without HIV. Notably, this finding was driven exclusively by patients with suppressed viral load. In addition, this is the first study to the best of our knowledge to report on differences in clinical outcomes by viral suppression status among PLWH infected with SARS-CoV-2.
Our findings are consistent with 2 other studies evaluating clinical outcomes between individuals with and without HIV from different health care systems in New York City. Although those studies had smaller numbers of PLWH and used different comparison approaches (primarily matching), findings were similar in that they showed no differences in mortality. One of these studies compared 21 PLWH matched to 42 non-PLWH, finding no significant difference in mortality or length of hospitalization, but did observe a trend toward increased intubation among PLWH.23 Another study of 88 PLWH in New York City also found no differences in mortality by HIV status.24 The need for increased intubation among PLWH warrants further research, particularly given that it did not seem to lead to greater mortality.
Of note, findings from this study and others conducted in higher income countries differ from a large population-based cohort study from Western Cape, South Africa.25 The study reported a doubling of mortality risk due to COVID-19 among PLWH compared to patients without HIV, both among hospitalized patients and overall, irrespective of viral suppression. These disparate findings support the need for studies from other contexts to understand whether PLWH are differentially at risk for poor clinical outcomes due to HIV itself or other contextual and environmental factors. For example, there may be a differential distribution of comorbidities (eg, tuberculosis, food insecurity, or other syndemic conditions), or there may be stigma toward PLWH leading to differential treatment for COVID-19 and resulting worsened outcomes, or likely a combination of factors.
We observed no deaths or intubations among PLWH who had unsuppressed viremia, whereas those with suppressed viral load experienced outcomes at rates similar to patients without HIV. In addition, peak levels of markers of immune and inflammatory function, including CRP, IL-6, and neutrophil count, were significantly lower among PLWH with unsuppressed viremia than among PLWH who were suppressed or patients without HIV. These findings support the notion that the intense cytokine storm observed in COVID-19 is likely a key mediator of poor clinical outcomes, and that attenuation of the immune response in HIV virally unsuppressed patients may be protective from these adverse outcomes. Indeed, recent studies in persons with and without HIV have reported associations of CRP and IL-6 levels with adverse COVID-19 outcomes including respiratory failure and death.26,27 Most case reports of PLWH and COVID-19 to date have primarily included patients with high rates of viral suppression, which may explain why this finding has not yet been reported. Further work is needed to elucidate the pathways linking lower levels of immune activation and inflammation with clinical outcomes. These insights may eventually provide better avenues for treatment among both PLWH and patients without HIV infected with SARS-CoV-2.
Although this study did not find significant differences in most clinical outcomes, longitudinal follow-up studies are needed to understand potential long-term sequelae or consequences of SARS-CoV-2 infection among PLWH (eg, do PLWH have increased long-term sequelae or morbidity compared to other patients?). There is an increasing recognition that COVID-19 may have a myriad of postinfectious syndromes that are not yet well understood.28 Monitoring for such longer-term complications among PLWH (with and without detectable HIV viremia) will be important to understand and determine whether any observed differences may be due to HIV itself or related to syndemic conditions surrounding HIV.
As with any study, ours has limitations. Our study population, including a large number of PLWH with COVID-19, comprised people living primarily in the Bronx, NY, a highly urbanized area with some of the poorest urban neighborhoods in the United States, and it is unclear how generalizable our findings are to other places. However, given the disproportionate prevalence of both HIV and COVID-19 in disadvantaged areas with largely Black and Latinx populations,29,30 our observations may be relevant to similar settings. We were not able to able to account for experimental treatments that may have been administered to COVID-19 patients (eg, remdesivir and IL-6 receptor antagonists), although we have no reason to believe that this differed by HIV serostatus because HIV was not an exclusion criteria for the experimental treatments. Although we controlled for many confounders believed to increase severity of complications and that may differ by HIV status, including age, BMI, smoking history, and several comorbidities including hypertension, chronic lower respiratory disease, and heart disease, the potential for residual confounding remains. Finally, we were unable to look at the impact of specific antiretroviral medications, in particular tenofovir, on clinical outcomes due to sample size constraints.
Conclusions
People living with HIV who are hospitalized due to COVID-19 seem to have increased risk for intubation but are not at increased risk for in-hospital death. Peak markers of the immune and inflammatory response were similar between PLWH who were virally suppressed and patients without HIV, but notably lower among PLWH with unsuppressed HIV. The observed differences in outcomes between suppressed and unsuppressed PLWH may be in part due to this difference in immune response and warrant further investigation. Finally, longer-term follow-up studies are needed of PLWH and individuals without HIV infected with COVID-19 to understand whether differences in clinical outcomes emerge over time.
Supplementary Material
Acknowledgments
Supported in part by the Einstein-Rockefeller-CUNY Center for AIDS Research P30AI124414 (PI: Harris Goldstein), K23MH1063686 (PI: U.R.F.), and R00-DA043011 (PI: M.J.A.), and K01-HL-137557 (PI: D.B.H.).
Footnotes
The authors have no conflicts of interest to disclose.
These results were partially presented at AIDS2020 Virtual conference as a late-breaker, International AIDS Society; July 9, 2020; San Francisco, CA.
REFERENCES
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiau S, Krause KD, Valera P, et al. The burden of COVID-19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020;24:2244–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173:536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo W, Ming F, Dong Y, et al. A survey for COVID-19 among HIV/AIDS patients in two Districts of Wuhan, China. In: AIDS Patients in Two Districts of Wuhan, China (3/April/2020). Available at: https://ssrn.com/abstract=3550029 or 10.2139/ssrn.3550029. [DOI] [Google Scholar]
- 7.Ridgway JP, Farley B, Benoit JL, et al. A case series of five people living with HIV hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDS. 2020;34:331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toombs JM, Van den Abbeele K, Democratis J, et al. COVID-19 in 3 people living with HIV in the United Kingdom. J Med Virol. 2020. Available at: 10.1002/jmv.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shalev N, Scherer M, LaSota ED, et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID-19. Clin Infect Dis. 2020;71:2294–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haerter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;48:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Childs K, Post FA, Norcross C, et al. Hospitalized patients with COVID- 19 and human immunodeficiency virus: a case series. Clin Infect Dis. 2020;71:2021–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Center for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveill Supplemental Rep. 2019;24:1–89. [Google Scholar]
- 14.New York City Department of Health and Mental Hygiene. COVID-19: Data. Available at: https://www1.nyc.gov/site/doh/covid/covid-19-data.page. Accessed July 13, 2020.
- 15.Hanna DB, Felsen UR, Ginsberg MS, et al. Increased antiretroviral therapy use and virologic suppression in the Bronx in the context of multiple HIV prevention strategies. AIDS Res Hum Retroviruses. 2016;32:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AIDS Institute Reporting System, New York State Department of Health, AIDS Institute. Available at: http://www.airsny.org. Accessed July 24, 2020.
- 17.Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl 2012;2:1–138. [Google Scholar]
- 18.Siew ED, Matheny ME. Choice of reference serum creatinine in defining acute kidney injury. Nephron. 2015;131:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsen UR, Bellin EY, Cunningham CO, et al. Development of an electronic medical record-based algorithm to identify patients with unknown HIV status. AIDS Care. 2014;26:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torian LV, Felsen UR, Xia Q, et al. Undiagnosed HIV and HCV infection in a New York city emergency department, 2015. Am J Public Health. 2018;108:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol. 2001;27:85–96. [Google Scholar]
- 23.Karmen-Tuohy S, Carlucci PM, Zervou FN, et al. Outcomes among HIV-positive patients hospitalized with COVID-19. J Acquir Immune Defic Syndr. 2020;85:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigel K, Swartz T, Golden E, et al. Covid-19 and people with HIV infection: outcomes for hospitalized patients in New York city. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies MA. HIV and risk of COVID-19 death: a population cohort study from the Western Cape Province, South Africa. medRxiv. 2020. doi: 10.1101/2020.07.02.20145185. [DOI] [Google Scholar]
- 26.Ho HE, Peluso MJ, Margus C, et al. Clinical outcomes and immunologic characteristics of Covid-19 in people with HIV. J Infect Dis. 2020. doi: 10.1093/infdis/jiaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35:3036–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID-19 on black Communities. Ann Epidemiol. 2020;47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Diaz CE, Guilamo-Ramos V, Mena L, et al. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann Epidemiol. 2020;52:46–53.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


