Abstract
Genetically encoded fluorescent biosensors are versatile tools for studying brain metabolism and function in live tissue. The genetic information for these biosensors can be delivered into the brain by stereotaxic injection of engineered adeno-associated viruses (AAVs), which can selectively target different cell types depending on the capsid serotype and/or the viral promoter. Here, we describe a protocol for intracranial injections of two viral vectors encoding the metabolic biosensor Peredox and the calcium biosensor RCaMP1h. When combined with 2-photon microscopy and fluorescence lifetime imaging, this protocol allows the simultaneous quantitative assessment of changes in the cytosolic NADH/NAD+ ratio and the intracellular Ca2+ levels in individual dentate granule cells from acute hippocampal slices.
Graphic abstract:
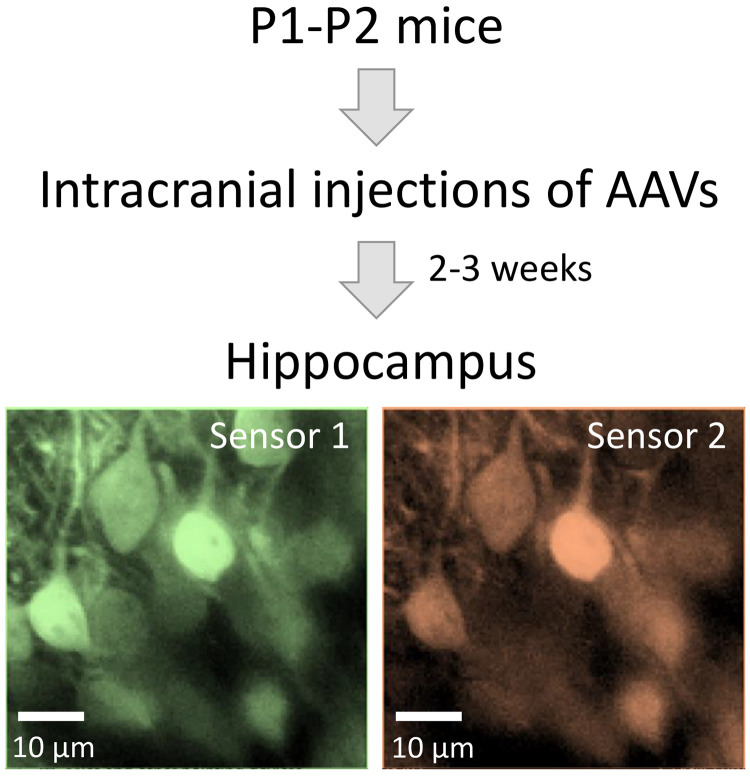
Workflow diagram for biosensor expression in the mouse hippocampus using intracranial injections of adeno-associated viruses.
Keywords: Adeno-associated viruses, Acute brain slices, Genetically-encoded fluorescent biosensors, Hippocampus, Intracranial injection, Dentate granule cells
Background
Energy supply is critical for proper brain health and function. Several methods like positron emission tomography, functional magnetic resonance imaging, and nuclear magnetic resonance, have been used for decades to study how energy metabolism responds to simulation and the mechanisms that regulate these responses. However, even with these powerful tools, it is challenging to obtain real-time dynamics of metabolites with cellular or subcellular resolution. On the other hand, the use of genetically encoded fluorescent biosensors can overcome these challenges ( Barros et al., 2018 ). These biosensors report on changes in metabolite levels by changing the intensity of the emitted light, and, in some cases, with changes in the fluorescence lifetime (Yellen and Mongeon, 2015).
Deploying these biosensors requires a vector for delivering recombinant DNA into cells. For gene delivery in vivo, adeno-associated viruses (AAVs) have been particularly useful: their genome size of ~4.7 kb is adequate for many fluorescent proteins, the tropism of their capsids can be harnessed to preferentially target different cell types in the brain, and they can be produced in typical laboratory conditions ( Bedbrook et al., 2018 ; Challis et al., 2019 ; Kimura et al., 2019 ).
Here we provide a detailed protocol for intracranial injections of AAVs into the mouse hippocampus, which is arguably one of the most studied regions of the mammalian brain. In our experience, the AAV serotypes 8 and 9 have been equally effective in transducing hippocampal dentate granule cells, using the neuron-specific hSyn promoter, or the pan-mammalian CAG promoter. Using this protocol, we have co-expressed the green metabolic biosensor Peredox ( Hung et al., 2011 ) and the red calcium-sensitive sensor RCaMP1h ( Akerboom et al., 2013 ), to simultaneously monitor the glycolytic production of NADH and the neuronal Ca2+ dynamics, respectively (Díaz- García et al., 2017 ). This technology has allowed us to study how neuronal activity instructs an increase in neuronal glycolysis, triggered by the energy demand resulting from ion pumping (Díaz- García et al., 2021 ). This same protocol has been used to express biosensors in both neurons and astrocytes throughout the juvenile mouse hippocampus ( Mongeon et al., 2016 ).
Materials and Reagents
Wiretrol® II disposable micropipets and plunger (Drummond, catalog number: 5-000-2005; VWR, catalog number: 53507-426)
MicroFil 28 Gauge, length: 67 mm, non-metallic syringe needle (World Precision Instruments, catalog number: MF28G67-5)
Electrophysiology pipette holder (Warner Instruments, catalog number: 64-0837)
Surgical tape (3M Micropore Medical Tape, catalog number: 1530-0)
70% isopropyl alcohol wipes (Covidien Curity Alcohol Prep, catalog number: 5750, stored at room temperature)
30 Gauge needle (BD, PrecisionGlide Needles, catalog number: 305106)
Parafilm (Parafilm, PM999)
Q-tips (Puritan, catalog number: 803-WC)
3 ml syringe (BD, catalog number: 309657)
Laboratory marker (Sharpie, catalog number: 13801)
Mineral oil (Sigma-Aldrich, catalog number: M8410-100ML, stored at room temperature)
-
Experimental animals
C57BL/6NCrl mice (species: Mus musculus; Charles River Laboratories, International Mouse Resource Center IMSR catalog number: CRL_27, RRID:IMSR_CRL:27).
Animals were housed in a barrier facility in individually ventilated cages with ad libitum access to standard chow diet (PicoLab 5053). Animals were provided with wood shavings as bedding, and shepherd shacks and nestlets for environmental enrichment. The offspring was used for injections at postnatal day 1 or 2 (P1-P2).
-
Viral vectors (stored at -80°C)
For the Peredox sensor, we used the AAV8 serotype and the CAG promoter (obtained from the Penn Vector Core, University of Pennsylvania, PA).
For the RCaMP1h sensor, we used the AAV9 serotype and the hSyn promoter [produced in the laboratory following the protocol of Kimura et al. (2019) , or obtained from different suppliers: the Penn Vector Core, University of Pennsylvania, PA, the Viral Core Facility from Children Hospital in Boston, MA, and the Center for Genomics and System Biology, New York University, Abu Dhabi, UAE, kindly provided by Dr. G. Fishell and Dr. J Dimidschstein].
Sterile 0.9% NaCl saline solution (Growcells, catalog number: MSDW-1000, stored at room temperature, shelf life 3 years)
Ice block (Nordic Ice Brick, catalog number: NB15, stored at -20°C)
Equipment
Dumont # 5 forceps (Fine Science Tools, catalog number: 11251-30)
P10 pipette (Gilson, catalog number: F144802)
Flaming/Brown micropipette puller (Sutter Instrument Co., model: P-97)
Digital stereotaxic instrument (Parkland Scientific, catalog number: 51730D)
Microsyringe pump (World Precision Instruments, model UMP3) and controller (World Precision Instruments, model: SYS-MICRO4)
DC temperature controller (FHC, catalog number: 40-90-8D-01)
Heating pad (FHC, catalog number: 40-90-2-02) and thermistor probe (FHC, catalog number: 40-90-5D-02)
Surgical lamp, dual arm gooseneck illuminator (Dolan-Jenner Industries, model: Fiber-Lite Mi-150)
Precision stereo zoom binocular microscope (World Precision Instruments, model: PZMIV-BS)
Procedure
-
Make micropipettes for injections
-
Pull a micropipette from a 10 µl glass capillary tube using a micropipette puller. The micropipettes (from both halves of the capillary) should have a total length of 4.0-4.5 cm, and a taper length (i.e., neck-to-tip) of ~0.5 cm,
Note: The following settings are an example for a P-97 Flaming/Brown Micropipette Puller: Heat = 550, Pull = 37, Vel = 40, Time = [leave field empty]. These settings are intended as a starting point, some adjustments may be required due to instrumental variability.
-
Break the tip of the micropipette using Dumont #5 forceps.
Note: The final outer diameter should be 10-25 µm, such that it is sharp enough to pierce through the skull, and sufficiently wide to not break during the process. The dimensions of the tip can be confirmed using a light microscope equipped with a reticle.
-
-
Prepare the viral mix
-
Thaw aliquots of the two desired AAVs on ice.
Note: Typically, a different combination of serotype/promoter was used for each biosensor: AAV8.CAG.Peredox (Mongeon et al., 2016; Addgene #73807), and AAV9.hSyn.RCaMP1h (Díaz-García et al., 2017).
-
Mix the viral samples at a 1:1 ratio and let the viral mix equilibrate for 10 min. The viral mix is prepared and stored on ice (4°C) until use.
Note: When mixing different preparations with similar viral titers, a ratio of 1:1 should result in a sufficient number of cells co-expressing both biosensors. Nevertheless, this ratio may require some empirical optimization given the variability in titers (range: 1010-1014 gc/ml) and efficiency of infection among different AAV preparations (see note at the end). If desired, a viral preparation can be diluted using sterile physiological saline (0.9% NaCl).
-
-
Load viral mix into micropipette
Fully load one micropipette with mineral oil using a 3 ml syringe with the MicroFil 28 Gauge needle.
-
Place the plunger and the pipette holder onto the microinjector system.
Place the plunger first and then place the pipette holder with the side steel tube inlet resting on the plastic mount attached to the stereotaxic instrument.
-
Mount the micropipette in the micropipette holder on the stereotaxic instrument, which must be connected to the microsyringe pump controller.
Ensure that the plunger enters the open end of the loaded micropipette. Ideally, this should result in some of the mineral oil coming out of the sharp end of the micropipette.
-
Using the microsyringe pump controller, carefully eject 500 nl of the mineral oil to further confirm that the tapered end of the micropipette is sufficiently open for injections, and that the micropipette does not move with the injector. Eject more mineral oil until 300-500 nl remain in the pipette.
Note: For better visualization, drop the oil onto an extended piece of parafilm covering a hollow case.
Pipette viral mix (typically 4-6 µl to inject 6 pups) onto the parafilm away from the oil drop.
-
Under visual control with the stereo zoom microscope, use the stereotaxic instrument to place the sharp end of the micropipette into the viral suspension.
Note: Having no hard surface underneath the parafilm should avoid any accidental break of the micropipette tip if it touches the parafilm when approaching the viral suspension.
If the viral suspension is repelled during this process, immerse the micropipette tip into a drop of saline first.
-
Use the microsyringe pump controller to withdraw the viral mix into the micropipette; avoid taking up air bubbles.
Note: The microsyringe pump is set to withdraw 300 nl per withdrawing event, at a rate of 150 nl/s. Perform this operation as many times as necessary to load the complete volume of viral mix into the micropipette. Avoid taking up mineral oil into the micropipette.
Move the injection holder away.
-
Induce cryoanesthesia
Place the mouse pups at postnatal day 1 or 2 (P1-P2) in a clean cage and keep them wrapped in a paper towel.
Place one mouse at a time between two paper towels on top of an ice block (3-4°C) for 2-5 min.
-
Lean a plastic bag filled with crushed ice over the paper towel covering the mouse.
Note: Make sure the pup is not in direct contact with the ice block or the crushed ice to avoid freeze damage to the skin.
Confirm anesthesia by gently squeezing a paw and monitor the lack of withdrawal reflex after 5 min, then every two minutes.
-
Perform intracranial injections
-
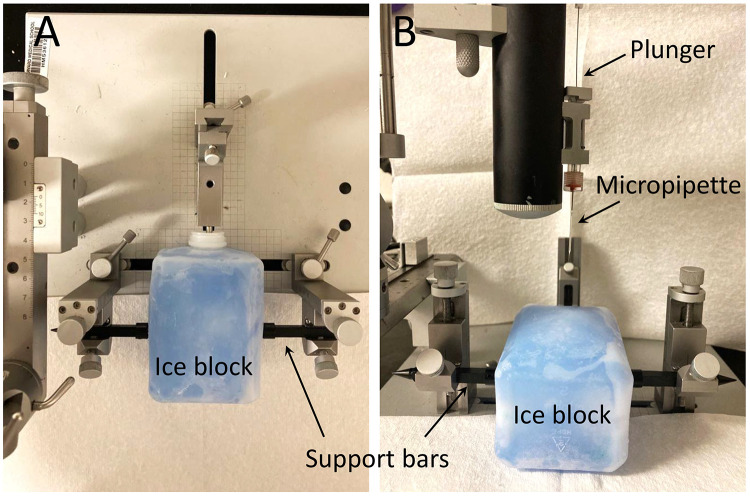
Place another ice block touching the metallic end of the inhalator attached to the stereotaxic instrument, and secure it laterally with the blunt ends of small support bars (Figure 1).
Note: Place a thin paper towel on top to ensure that the pup is not in direct contact with the ice block to avoid freeze damage to the skin.
-
Transfer the pup from the ice block used for cryoanesthesia to the ice block secured in the stereotaxic instrument, and position the mouse flat in prone position.
Note: The sagittal suture should appear parallel to the longitudinal axis of the stage, while the imaginary line between the ears should be parallel to the transverse axis of the stage.
-
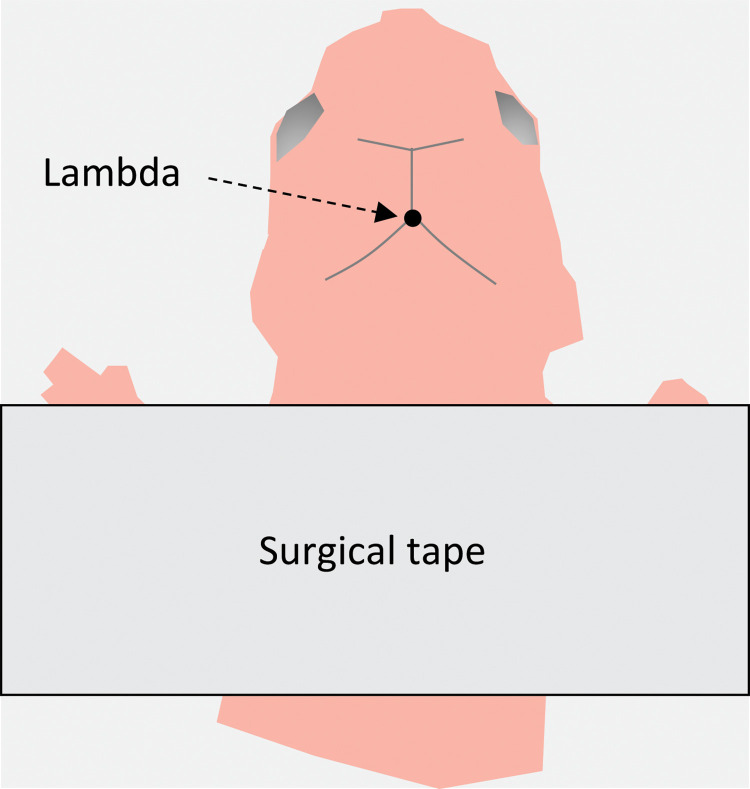
Gently secure the pup to the ice block using surgical tape (Figure 2).
Note: Place the surgical tape near the ears and slightly pull the skin of the skull towards the back for better visualization of the skull landmarks. Sterilize the pup’s head one time with a 70% isopropyl alcohol wipe.
-
Using the stereo microscope, visually locate the zero coordinate point (lambda) and mark it with a laboratory marker.
Note: Use a fiber optic surgical lamp to illuminate the surgical field, as an incandescent bulb may inadvertently cause surface warming.
Under the stereo microscope, lower the micropipette on this zero coordinate point—tip touching the skull.
Set coordinates to zero on the digital manipulator attached to the stereotaxic instrument.
Move the micropipette up to avoid breaking the pipette tip, and then move it to the desired anterior-posterior (AP, Y-axis) and medial-lateral (ML, X-axis) coordinates, using the digital manipulator.
Gently touch the skull with the tip of the micropipette, and set the dorso-ventral coordinate (DV, Z-axis) on the manipulator to zero.
Move up the pipette and mark the location in the skin with a laboratory marker.
-
Make a hole in this spot for micropipette injection by pricking the skin and piercing through the skull with a 30 G needle.
Note: If some blood comes out, absorb it with a piece of sterile paper towel or a Q-tip.
For hippocampal injections, two injections per hemisphere are done in the following sites (Table 1):
*The plus/minus symbols in the X-axis indicate different hemispheres (right/left).
-
Lower the micropipette to the desired depth (Z-axis).
Note: Go an extra 0.2 mm in depth and then come back to the correct Z coordinate (for instance, if Z= -2.0 mm, then go down to -2.2 mm first, and from there to -2.0 mm).
-
Use the microinjector system to inject 150 nl of virus at a rate of 50 nl/min.
Note: With slightly different settings, our group has been also able to express biosensors in the hippocampus with a single 200-300 nl injection per hemisphere, as described elsewhere (Díaz-García et al., 2017; Martínez-François et al., 2018).
Wait 1-2 min before withdrawing the micropipette; then withdraw slowly to avoid backflow of the virus to the surface.
-
Before the next injection and monitoring under the stereoscope, confirm that the pipette is not clogged by ejecting 10 nl of viral mix from the pipette.
Note: If the micropipette tip is clogged, either use a new one or carefully break the end of the tip with a pair of forceps, making sure that the micropipette tip remains narrow and sharp.
Perform the second injection and repeat step 10.
Repeat the same process to inject the other hemisphere (Steps E10-E15).
After finishing all injections, dispose the micropipette into a BSL-2 waste container for sharps, and remove the pipette holder and Wiretrol II plunger.
-
-
Post-injection recovery
After injections, place the pup on a heating pad until the mouse begins to move again and the skin color returns to normal.
Place the pups in a clean cage until they recover their normal movement.
-
Return the injected pups to their original cage.
Note: Put the pups away from the nest and gently mix them with the bedding. The mother should search for the pups and take them inside the nest.
Fill a surgery card and a surgery log to notify the veterinarians about the procedure.
Perform a subcutaneous injection of Ketoprofen (10 mg/kg), once a day for 3 days.
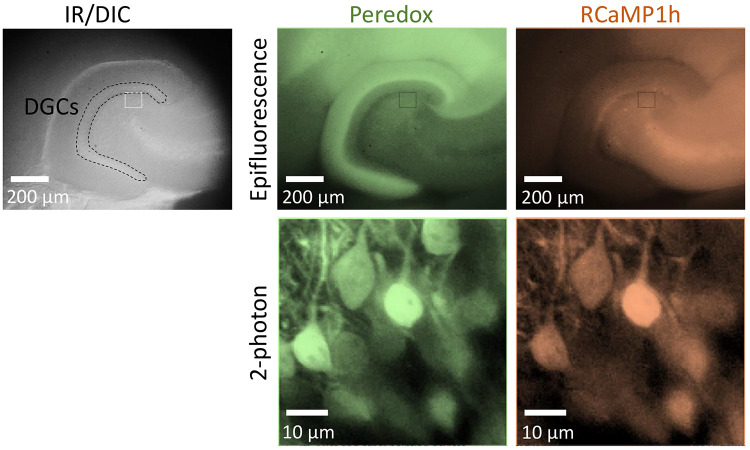
Fluorescence can be verified in acute hippocampal slices after two weeks (Figure 3).
Figure 1. Typical setup for cryoanesthesia and intracranial injections.
A. Top view of the stereotaxic instrument with the ice block secured by support bars. B. Front view of the setup showing the micropipette and plunger connected to the stereotaxic instrument.
Figure 2. Schematic representation of a mouse pup under a stereo microscope.
Top view of the mouse placed onto the paper-covered ice block in the stereotaxic instrument. The zero coordinate point (lambda) is located in the conjunction of the cranial sutures in the posterior part of the skull.
Table 1. Coordinates for intracranial injections (mm).
|
Anterior-Posterior (Y-axis) |
Medial- Lateral (X-axis) |
Dorsal-Ventral (Z-axis) |
|
| Injection 1 | 0 | ±1.9 | -2.0 |
| Injection 2 | 0 | ±2.0 | -2.3 |
Figure 3. Intracranial injection of AAVs induces biosensor expression in the mouse hippocampus.
Top left: Low magnification picture of a horizontal slice from the hippocampus, using infra-red differential interference contrast imaging (DIC). The dotted contour encloses the layer of granule cells (DGCs) in the dentate gyrus of the hippocampus. Top right: Biosensor expression in the dentate gyrus, revealed by epifluorescence microscopy. Bottom: Co-expression of the biosensors Peredox and RCaMP1h in individual DGCs. The high magnification images were obtained using 2-photon microscopy and an excitation wavelength of 790 nm, as described elsewhere (Díaz- García et al., 2021 ). The images have been pseudo-colored to illustrate the co-expression of different sensors in DGCs. This pattern of expression is observed after 2-3 weeks post-injections.
Notes
AAV titers may be misleading: even in the same viral preparation, different titers may result when amplifying different regions of the viral genome ( Kimura et al., 2019 ). Therefore, make sure to know what primers were used for the titration by qPCR.
Some viral preparations with high titers (determined by qPCR) and purity (determined by SDS-PAGE and silver staining) can paradoxically produce little to no expression of the biosensor of interest, as revealed by a low fluorescence signal in brain tissue. A likely explanation could be particle aggregation, which could be prevented by avoiding excessive concentration during the purification of AAVs ( Mueller et al., 2012 ), or using a high ionic strength solution during this process ( Wright et al., 2005 ).
If the experimenter has no previous experience with stereotaxic injections, peer-reviewed video-protocols are a great resource as a first approximation to the procedure (Lowery and Majewska, 2010; Kim et al., 2014 ; Chen et al., 2018 ; Berg et al., 2020 ).
Acknowledgments
This work was supported by NIH grants R01 NS102586 from the National Institute of Neurological Disorders and Stroke, R01 GM124038 from the National Institute of General Medical Sciences, and DP1 EB016986 from the NIH Office of the Director (to G.Y.); NIH fellowship F32 NS100331 from the National Institute of Neurological Disorders and Stroke (to C.M.D-G).; and a Fix Fund Postdoctoral Fellowship from the Department of Neurobiology, Harvard Medical School (to C.M.D-G.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
This protocol was derived from the study of Díaz-García et al. (2021), published in eLife (DOI: 10.7554/eLife.64821).
Competing interests
The authors do not have any competing interest to declare.
Ethics
All experiments were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act. The Harvard Medical Area Standing Committee on Animals approved all procedures involving animals. (Animal Welfare Assurance Number A3431-01, Protocol IS00001113-3).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Akerboom J., Carreras Calderon N., Tian L., Wabnig S., Prigge M., Tolo J., Gordus A., Orger M. B., Severi K. E., Macklin J. J., et al.(2013). Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barros L. F., Bolanos J. P., Bonvento G., Bouzier-Sore A. K., Brown A., Hirrlinger J., Kasparov S., Kirchhoff F., Murphy A. N., Pellerin L., et al.(2018). Current technical approaches to brain energy metabolism. Glia 66(6): 1138-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedbrook C. N., Deverman B. E. and Gradinaru V.(2018). Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Annu Rev Neurosci 41: 323-348. [DOI] [PubMed] [Google Scholar]
- 4. Berg L., Gerdey J., and Masseck O. A.(2020). Optogenetic Manipulation of Neuronal Activity to Modulate Behavior in Freely Moving Mice. J Vis Exp 164. doi: 10.3791/61023. [DOI] [PubMed] [Google Scholar]
- 5. Chen S.-Y., Kuo H.-Y., and Liu F.-C.(2018). Stereotaxic Surgery for Genetic Manipulation in Striatal Cells of Neonatal Mouse Brains. J Vis Exp 137: 57270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Challis R. C., Ravindra Kumar S., Chan K. Y., Challis C., Beadle K., Jang M. J., Kim H. M., Rajendran P. S., Tompkins J. D., Shivkumar K., et al.(2019). Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat Protoc 14(2): 379-414. [DOI] [PubMed] [Google Scholar]
- 7. Díaz-García C. M., Meyer D. J., Nathwani N., Rahman M., Martinez-Francois J. R. and Yellen G.(2021). The distinct roles of calcium in rapid control of neuronal glycolysis and the tricarboxylic acid cycle. Elife 10: e64821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Díaz-García C. M., Mongeon R., Lahmann C., Koveal D., Zucker H. and Yellen G.(2017). Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab 26(2): 361-374 e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung Y. P., Albeck J. G., Tantama M. and Yellen G.(2011). Imaging cytosolic NADH-NAD+ redox state with a genetically encoded fluorescent biosensor . Cell Metab 14(4): 545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim J.-Y., Grunke S. D., Levites Y., Golde T. E., and Jankowsky J. L.(2014). Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J Vis Exp 91: 51863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimura T., Ferran B., Tsukahara Y., Shang Q., Desai S., Fedoce A., Pimentel D. R., Luptak I., Adachi T., Ido Y., Matsui R. and Bachschmid M. M.(2019). Production of adeno-associated virus vectors for in vitro and in vivo applications . Sci Rep 9(1): 13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowery R. L. and Majewska A. K.(2010). Intracranial injection of adeno-associated viral vectors. J Vis Exp 45: 2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martínez-François J. R., Fernandez-Aguera M. C., Nathwani N., Lahmann C., Burnham V. L., Danial N. N. and Yellen G.(2018). BAD and KATP channels regulate neuron excitability and epileptiform activity . Elife 7: e32721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mongeon R., Venkatachalam V. and Yellen G.(2016). Cytosolic NADH-NAD+ Redox Visualized in Brain Slices by Two-Photon Fluorescence Lifetime Biosensor Imaging . Antioxid Redox Signal 25(10): 553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mueller C., Ratner D., Zhong L., Esteves-Sena M. and Gao G.(2012). Production and discovery of novel recombinant adeno-associated viral vectors. Curr Protoc Microbiol Chapter 14: Unit14D 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright J. F., Le T., Prado J., Bahr-Davidson J., Smith P. H., Zhen Z., Sommer J. M., Pierce G. F., and Qu G.(2005). Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation . Mol Ther J Am Soc Gene Ther 12(1): 171-178. [DOI] [PubMed] [Google Scholar]
- 17. Yellen G. and Mongeon R.(2015). Quantitative two-photon imaging of fluorescent biosensors. Curr Opin Chem Biol 27: 24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]