Abstract
Prestin (SLC26a5) is an integral membrane motor protein in outer hair cells (OHC) that underlies cochlear amplification. As a voltage-dependent protein, it relies on intrinsic sensor charge to respond to transmembrane voltage (receptor potentials), thereby effecting conformational changes. The protein’s electromechanical actively is experimentally monitored as a bell-shaped nonlinear capacitance (NLC), whose magnitude peaks at a characteristic voltage, Vh. This voltage denotes the midpoint of prestin’s charge-voltage (Q-V) Boltzmann distribution and region of maximum gain of OHC electromotility. It is an important factor in hearing capabilities for mammals. A variety of biophysical forces can influence the distribution of charge, gauged by shifts in Vh, including prior holding voltage or membrane potential. Here we report that the effectiveness of prior voltage augments during the delivery of prestin to the membranes in an inducible HEK cell line. The augmentation coincides with an increase in prestin density, maturing at a characteristic membrane areal density of 870 functional prestin units per square micrometer, and is likely indicative of prestin-prestin cooperative interactions.
Keywords: nonlinear capacitance, prestin cell line, voltage clamp, molecular memory, prestin density
Introduction
Prestin (SLC26a5) is a protein (Zheng et al., 2000) that is housed in abundance within the lateral membrane of outer hair cells (OHC) in the organ of Corti. The protein imparts robust electromechanical activity to the cell that is unlike any other form of cellular motility, notably associated with a voltage-dependent, bell-shaped nonlinear capacitance (NLC) that reports on conformational changes in the protein (Ashmore, 1990; Santos-Sacchi, 1991). This mechanical activity, termed electromotility, driven by receptor potentials (Evans and Dallos, 1993), is believed to feed back into the acoustically-driven vibration of the cochlear partition, thereby enhancing the mechanical stimulus that the inner hair cells (IHC) sense and transmit to the CNS (Ashmore et al., 2010). This process is called cochlear amplification, amounting to 40–60 dB of gain.
One of the interesting features of prestin is the protein’s ability to alter its state differentially depending upon initial voltage conditions, i.e., it show hysteresis. Simply put, the protein’s operating voltage range (conveniently characterized by Vh, the voltage at peak NLC, where half of its sensor charge is moved across the membrane field, and where electromotility gain is maximal) shifts depending upon prior holding potentials. This phenomenon has been measured in OHCs and in prestin-transfected cells (Santos-Sacchi et al., 1998; Santos-Sacchi et al., 2001). In OHCs, the voltage at peak capacitance (namely, Vh) differs by about 20 mV between pre-pulse holding potentials of +/− 100 mV. Besides magnitude of holding voltage, the shift depends on the polarity direction of voltage prior to the measurement of NLC. Finally, the shift evolves over time in a stretched exponential fashion, ranging from sub-milliseconds to seconds (Santos-Sacchi et al., 2009); thus, the duration of prior holding potential influences the amplitude of the Vh shift. This phenomenon likely reflects interactions among adjacent prestin molecules within the local membrane environment, just as ion channels show cooperative effects when expressed at high areal densities within the membrane (Molina et al., 2006). Here we denote this phenomenon observed in prestin Boltzmann characteristics as “molecular memory”, realizing that it may not be an intrinsic property of the protein prestin itself, but instead an interaction of prestin with itself and its local microenvironment (lipid, cytoskeletal) within the membrane. In mice, having an appropriate Vh value is critically important for normal hearing. In prestin knock-ins that have altered Vh, or with cochlear perilymphatic alterations of chloride that alter Vh, profound deafness ensues, the latter in a reversible manner (Santos-Sacchi et al., 2006; Dallos et al., 2008).
The development of prestin function has been studied in immature hair cells and in prestin-transfected cells (Oliver and Fakler, 1999; Abe et al., 2007; Bian et al., 2010; Bian et al., 2013; Seymour et al., 2016; Bai et al., 2019). We recently established a HEK cell line where prestin expression is tetracycline-inducible (Bian et al., 2010). We have already reported on some component Boltzmann characteristics of NLC maturation over hours following induction, and during the first few minutes following release of membrane trafficking block from low temperature (Bian et al., 2013). Prestin insertion into the membrane followed a sigmoidal function of time following induction. Here we utilize our model system to follow the maturation of prestin’s molecular memory as prestin density increases post-induction. We find that mature function arises after about 12 hours following induction, that is, when prestin charge density is greater than 11.1 fC/pF, or equivalently about 870 functional prestin units/μm2. We suggest that protein-protein interactions are optimized at and above this molecular density, such that negative cooperative effects among prestin units are maximized.
MATERIALS AND METHODS
Experiments were performed on OHCs and HEK cells. Full details on OHC measurements have been reported previously (Santos-Sacchi et al., 2009). Full details on cell culture and induction of our tetracycline-inducible, highly-expressing monoclonal prestin HEK 293 cell lines have been reported previously (Bian et al., 2010; Bian et al., 2013). Briefly, cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 50 U/ml each of penicillin and streptomycin, 10% fetal bovine serum at 37°C in a 5% CO2 incubator. 4 μg/ml of blasticidin and 130 μg/ml of zeocin were supplemented in the growth media. Induction was begun by treating with 1.0 μg/ml tetracycline. At time point 2, 4, 6, 10 and 24 hr, coverslips of grown cells were transferred to recording media (see below).
Whole cell patch-clamp measurements were made with an Axopatch 200B patch clamp amplifier (Axon Instruments) and a Digidata 1322A digitizer, with sampling rates of 10 μs. Experiments were performed at room temperature. Blocking solutions were used to remove ionic currents, limiting confounding effects on NLC determination and voltage delivery under voltage clamp (Santos-Sacchi, 1991; Santos-Sacchi and Song, 2016). Extracellular solution was (in mM): NaCl 100, TEA-Cl 20, CsCl 20, CoCl2 2, MgCl2 1, CaCl2 1, Hepes 10. Intracellular solution was (in mM): CsCl 140, MgCl2 2, Hepes 10 and EGTA 10. All chemicals were purchased from Sigma-Aldrich.
Pipettes had initial resistances of about 2.5 MΩ. Stray capacitance was compensated with amplifier controls prior to whole-cell establishment. Corrections for series resistance were made post-hoc. For our HEK cell studies, following establishment of whole-cell recording conditions, cells were held at 0 mV. Thirty second pre-pulse holding potential steps to −100 or +50 mV preceded NLC collections. Whole cell recordings were made on single cells growing on a coverslip at 2h, 4h, 6h, 10h and 24h after tetracycline induction. The number of cells recorded for each time point was 9, 9, 10, 10 and 9, respectively.
Membrane capacitance was measured with jClamp software (Scisoft, CT; www.SciSoftCo.com) using a continuous high resolution (2.56 ms sampling) two-sine stimulus protocol (10 mV peak at both 390.6 and 781.2 Hz) superimposed onto the voltage ramp (Santos-Sacchi et al., 1998; Santos-Sacchi, 2004). Briefly, real and imaginary components of membrane current at harmonic frequencies were determined by FFT in jClamp, corrected for the roll-off of recording system admittance (Gillis, 1995). Rs, Rm and Cm were extracted using the dual-sine, 3-parameter solution of the standard patch clamp model (Santos-Sacchi et al., 1998; Santos-Sacchi, 2004), based on the original single sine solution (Pusch and Neher, 1988). In order to extract Boltzmann parameters, capacitance-voltage data were fit to the first derivative of a two-state Boltzmann function.
| (eq. 1) |
Qmax is the maximum nonlinear charge moved, Vh is voltage at peak capacitance or equivalently, at half-maximum charge transfer, Vm is Rs-corrected membrane potential, z is valence, Clin is linear membrane capacitance, e is electron charge, kB is Boltzmann’s constant, and T is absolute temperature. Csa is a component of capacitance that characterizes sigmoidal changes in specific membrane capacitance (Santos-Sacchi and Navarrete, 2002; Santos-Sacchi and Song, 2014). ΔCsa is the total sum of unitary changes per prestin motor protein. Qsp denotes charge density, namely Qmax/Clin.
The time-dependent change in Qsp in Figure 3B is fit by a sigmoidal function, f=a/(1+exp(−(x-x0)/b)), as we have done previously (Bian et al., 2010), where x is time, x0 is the midpoint, b is the slope indicator, and a is the asymptotic value. Two parameter exponential fits were made using the formula f=a*(1-exp(−b*x)), where x is time for Fig. 3A, and x is Qsp for Fig. 4. The parameter a denotes the asymptotic value of either Qsp or ΔVh (see plots). The parameter 1/b is the characteristic “time constant” to achieve the final value a. For Fig. 4, to estimate charge density at “steady state”, we use the value reached at 5 times the “time constant”.
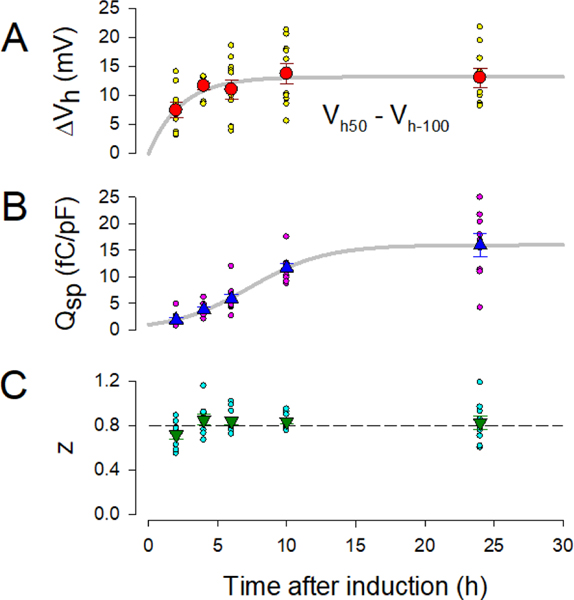
Figure 3.
Prestin behavior alters over post-induction time. A) The magnitude of Vh change (ΔVh) increases in an exponential manner over time. Fitted exponential parameters: a= 12.6 mV, 1/b = 1.9 h (see Methods). R2:(correlation coefficient of variation): 0.89. B) Prestin charge density also increases in an exponential manner. Fitted exponential parameters: a= 20.1 fC/pF, 1/b = 14.9 h (see Methods). R2: 0.99. C) On the other hand, the Boltzmann parameter z, which increases very early following induction (Bian et al., 2010), is stable after 4 hr. Error bars are SEM.
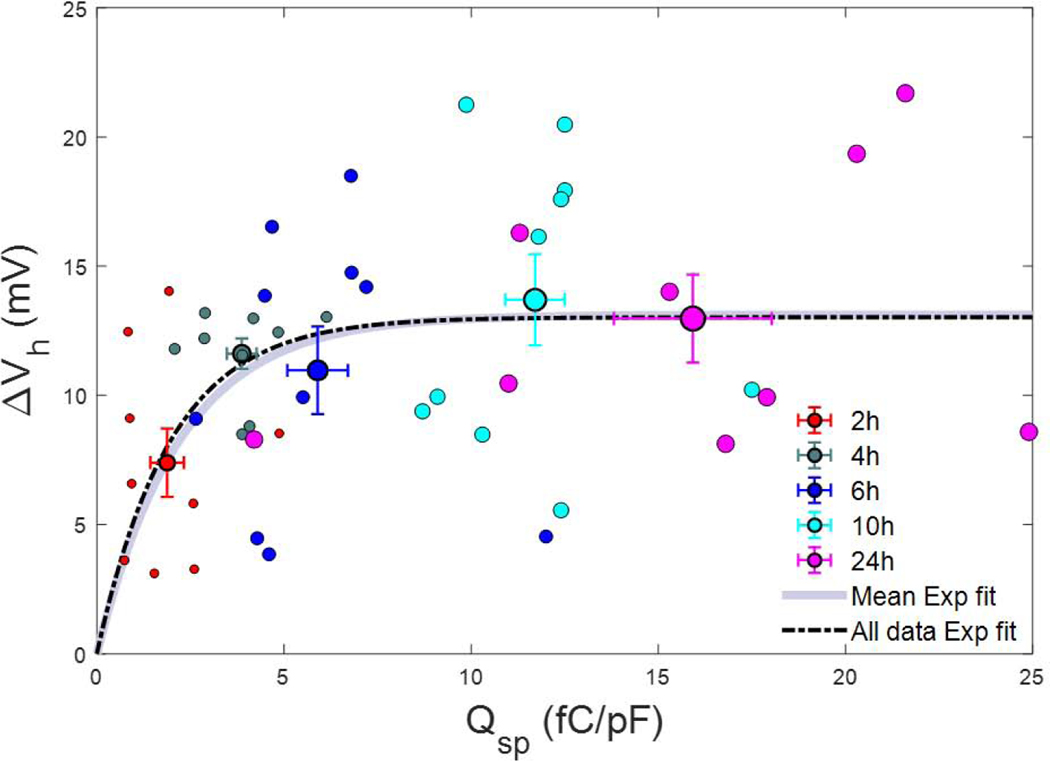
Figure 4.
Relationship between susceptibility to voltage pre-pulse (ΔVh) versus prestin charge density (Qsp). ΔVh increases exponentially with charge density, reaching at 11.1 fC/pF at 5 times 1/b, the characteristic “time constant”. Fitted exponential parameters of the mean data points: a= 13.11 mV, 1/b = 2.2 fC/pF (see Methods). R2: 0.89. Error bars are SEM. All data points were used for an exponential fit, as well. Fitted exponential parameters are similar: a= 13.02 mV, 1/b = 2.0 fC/pF.
RESULTS
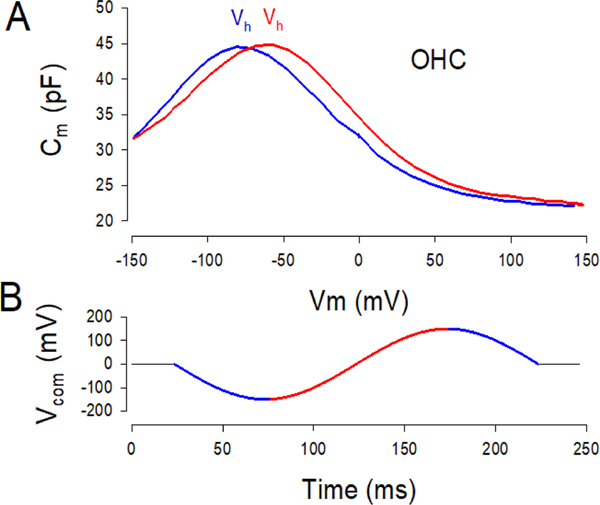
The voltage range over which prestin works is not fixed, but shifts depending on previous state of the protein/membrane environment set by holding voltage. This is most apparent in native isolated OHCs. Figure 1 illustrates the effect of prior holding voltage on prestin’s state/charge distribution as revealed through capacitance measures in the guinea pig OHC. In this case, dual sine stimuli were superimposed on a slow sinusoidal holding voltage (Fig. 1b), which spanned from −150 to +150 mV. The direction of holding potential change over time influences the position of Vh along the voltage axis (Fig. 1a); in the hyperpolarizing direction (blue lines), Vh is shifted towards depolarized voltages and vice versa (red lines). Steady state pre-pulse voltages can likewise cause shifts in Vh.
Figure 1.
Prestin state memory. A) NLC functions in a guinea pig OHC measured during hyperpolarizing direction (blue) and depolarizing direction (red) of sinusoidal change in holding potential. Prior hyperpolarizations cause movements of Vh in the opposite direction. The shift in voltage at peak capacitance (Vh) indicates redistribution of charge as a function of prior voltage. B) Holding potential sinusoid. Two sine stimuli have been removed.
Our HEK cell line allows us to monitor NLC characteristics over the course of prestin insertion into the membrane. Thirty seconds holding potential pre-pulses to either −100 or +50 mV were used to gauge the molecular memory of prestin in our HEK cell line. Following these pre-pulses, NLC was measured. Fig. 2 illustrates the dependence of Vh on post-induction time. Here we average our voltage protocol-generated currents prior to Cm determination. Clear differences are found between 2 and 24 hr post-induction. Analysis of cells on an individual basis allowed statistical comparisons. At 2 hr post induction, the average difference in Vh between 2 and 24 hr pre-pulse protocols (ΔVh) is smaller than that measured after 24 hr post induction (unpaired t-test, p=0.02). The average difference (mean +/− SEM) at 2 hr was 7.39 +/− 1.32 mV (n=9) and that at 24 hr was 12.97 +/− 1.70 mV (n=9). Figure 3 A plots results for 2, 4, 6, 12, and 24 hours post induction (mean +/− SEM). ΔVh rises exponentially over induction time, with a time constant of 2.2 h. Along with the growth of molecular memory, the density of prestin, gauged as Qsp, increases more slowly in a sigmoidal fashion (Fig. 3 B), with a time at half magnitude of 7.3 h and reaching an asymptotic value of 16.0 fC/μm2 based on the fit. This is in line with our previous observations over this time course (Bian et al., 2010). Clearly, while the density of prestin continues to rise, ΔVh asymptotes during the early phase of prestin delivery to the membrane. The Boltzmann parameter, z, on the other hand is stable after 4 hr post induction near 0.8 (Fig. 3 C).
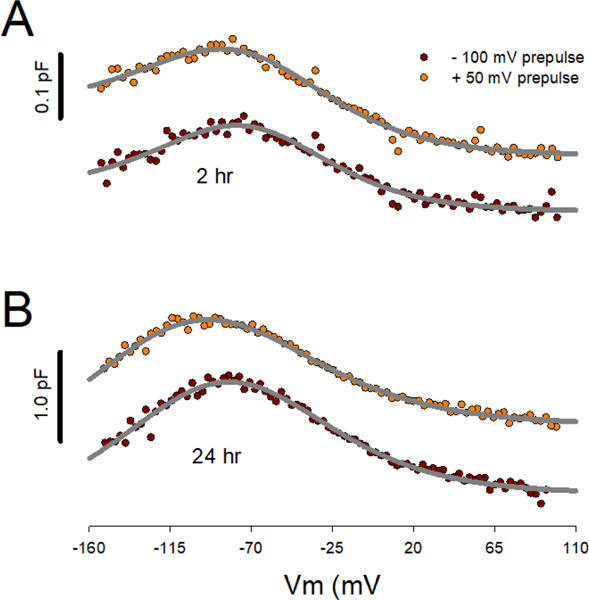
Figure 2.
Illustration of post-induction time dependence of Vh on steady state holding potential. Individual cell currents were averaged prior to Cm determination from averaged currents. A) At 2 hr post tetracycline induction, 0.5 minute prepulse to −100 or +50 mV has smaller effects on Vh than at 24 hr post induction (B). Vh for fits at 2 hr, −100 mV prepulse: −73.3 mV; +50 mV prepulse: −76.4 mV. Fits at 24 hr, −100 mV prepulse: −84.7 mV; +50 mV prepulse: −102.3 mV. Traces offset for visual clarity.
In order to relate prestin density and ΔVh, we plot the two against each other in Fig. 4. The relation shows an exponential growth in ΔVh as density increases, reaching at 5 times the characteristic “time constant”, 1/b (5 * 2.2 fC/pF = 11.1 fC/pF), the value of 12.6 mV. The number of prestin units per square micrometer is determined by Qsp/(z*e), where e is electron charge. Taking the classical value for linear membrane capacitance of 1μF/cm2 (Hille, 1992), 1pF = 100 μm2, and based on 11.1 fC/pF, we estimate that beyond a critical density of about 870 functional units of prestin per square micrometer, ΔVh has matured. The nonlinear relationship between charge density and ΔVh we take to mean that above this critical density, interactions among prestin molecules are likely.
DISCUSSION
All hair cells utilize apical membrane components (stereocilia) for forward transduction of sound into receptor potentials that evoke neurotransmitter release onto afferent nerve fibers (Flock, 1965). However, in addition, the OHC has evolved special lateral membrane components to reverse transduce those receptor potentials into mechanical energy, a process called electromotility (Evans and Dallos, 1993).
The responsible molecular motor was identified in 2000 (Zheng et al., 2000), and remarkably was found to be a solute carrier of the SLC26 family. Named as prestin, for its presumed fast kinetics (electromotility was measured beyond 80 kHz (Frank et al., 1999)), the protein has been studied extensively. Recently, this ultrafast capability of electromechanical activity has been challenged (Santos-Sacchi and Tan, 2018; Santos-Sacchi, 2019; Santos-Sacchi et al., 2019). Regardless, prestin is a voltage-dependent protein (Santos-Sacchi and Dilger, 1988) that has been successfully modelled with modifications of a two-state Boltzmann process, where motors are either in an expanded or compact state. Displacement currents or correspondingly a nonlinear capacitance (NLC) arises from these conformational changes (Ashmore, 1990; Santos-Sacchi, 1990, 1991). Based on these measures, membrane density of the motor in the OHC lateral membrane has been estimated to be up to 8000/μm2 (Huang and Santos-Sacchi, 1993; Gale and Ashmore, 1997). Specific membrane sensor charge correlates well with biochemical measures of membrane content (Bian et al., 2010; Seymour et al., 2016). Interestingly, as might be expected of an anion solute carrier, anion binding plays a pivotal role in its function (Oliver et al., 2001; Rybalchenko and Santos-Sacchi, 2003), though it likely does not work solely as an extrinsic voltage sensor (Song and Santos-Sacchi, 2010; Santos-Sacchi and Song, 2016). Concerning our present work, we have previously shown that chloride effects on prestin do not underlie its molecular memory (Santos-Sacchi et al., 2009).
In our current experiments we have employed our tetracycline-inducible prestin cell line which provides specific nonlinear charge (sensor charge/linear capacitance) values of up to 20 fC/pF after 24 hrs, far greater than transient transfection can provide (Bian et al., 2010). This efficiency allows us to monitor characteristics of NLC resulting during prestin delivery and insertion into the plasma membrane. Specifically, we used this approach to study the development of prestin’s ability to respond to its prior state, established by pre-pulse holding voltage. We find that ΔVh, a metric for prestin’s molecular memory, increases as post-induction time and the number of membrane-bound prestin molecules increase. The correspondence between areal density and ΔVh indicates that at a critical density of prestin within the membrane, namely, about 870 functional prestin units per square micrometer, molecular memory matures. If maturation were simply a consequence of time spent within the membrane, we would not have expected asymptotic behavior, because insertion of new proteins into the membrane continues well beyond the observed maturation. The exponential fit of ΔVh as charge density increases is simply used to provide an estimate of the asymptotic behavior; earlier time point measures of this phenomenon could reveal non-exponential behavior, just as charge density itself matures in a sigmoidal fashion (Fig. 2B) (Bian et al., 2010). Nevertheless, the identification of asymptotic behavior within our evaluated time points would not be changed by including earlier time points of ΔVh, which would be exceedingly difficult to measure. Interestingly, we recently have found that prestin kinetics augments, that is, the frequency response of NLC increases, during early development of mouse OHCs in organ explants (Bai et al., 2019). However, it is unlikely that development of prestin density may be a controlling factor in prestin’s ability to alter conformation at kilohertz rates. In that study, the estimated frequency cut-off (Fc) was found to asymptote beyond postnatal day p17–18, where charge density has also matured -- a density well beyond maturation of molecular memory. Of course, for OHCs, other factors beyond prestin kinetics could be at play. For example, not only intrinsic (e.g., membrane components or cytoskeleton) but also extrinsic (e.g., viscoelastic interactions with supporting cells within the organ) mechanical loads could influence our frequency response measures, as we have recently observed (Santos-Sacchi et al., 2019). These mechanical impediments to charge movement have as their basis prestin’s piezoelectric-like behavior, where load can influence prestin’s state (Iwasa, 1993; Gale and Ashmore, 1994; Kakehata and Santos-Sacchi, 1995).
The piezoelectric-like mechanical sensitivity of prestin may also underlie its molecular memory, and result from interactions among functional prestin units within the membrane. Indeed, we previously modelled molecular memory as resulting from such behavior (Santos-Sacchi et al., 1998), where the voltage-induced conformational changes in one unit could directly affect those of adjacent units through the viscoelastic plasma membrane. We reasoned that when prestin moves from an extended state to a compact state, forces generated within the plane of the membrane will influence the state in adjacent molecules, as lipid redistributes around compact prestin and acts on neighbors. Recapitulation of experimental data with the model was successful (Santos-Sacchi et al., 1998). Of course, the effectiveness of such negative cooperativity will depend upon intermolecular distances. At a critical density of about 870 functional units per square micrometer, intermolecular distances would be on the order of 34 nm, assuming a uniform distribution. Physiological evidence for dimerization has been obtained for prestin (Navaratnam et al., 2005; Detro-Dassen et al., 2008), and recent cryo-EM observations on SLC26a9, a family member of prestin, also suggests dimerization. Given that the widest dimension of a prestin dimer within the membrane may be 10 nm based on SLC26a9 cryo-EM structure, the closest proximity of adjacent molecular edges would be 17 nm. Of course, this is founded on a uniform distribution, but local, restricted diffusion cannot be discounted (Santos-Sacchi and Zhao, 2003; Organ and Raphael, 2007; Yamashita et al., 2015), nor can subsequent clustering be ignored. To be sure, in our cell line prestin-fused YFP often presents as visible fluorescent patches within the membrane. Modelling of ion channels indicates that at areal densities greater than 103/μm2, close to our observed critical prestin density, cooperative viscoelastic interactions are possible (Ursell et al., 2007). Indeed, clustering in a variety of ion channels can impact on channel activity (see (Molina et al., 2006)). Thus, for the OHC, where densities of prestin are up to 10000/μm2 (Huang and Santos-Sacchi, 1993; Gale and Ashmore, 1997; Mahendrasingam et al., 2010), the ensemble might be considered one huge cluster permitting negative cooperativity among molecules spanning across several molecular distances. These interactions are expected to impact OHC influence on cochlear amplification and hearing, since those resulting Vh shifts may be viewed as amplificatory in nature (Santos-Sacchi et al., 2009).
Prestin (SLC26a5) underlies enhanced mammalian hearing
The function of prestin depends on prior membrane potential
Here we study the development of this functional dependence in HEK cells
Maturation of the response corresponds to prestin density in the membrane
We suggest at a critical density cooperative effects among prestin molecules are maximal
Acknowledgments
This research was supported by NIH-NIDCD R01 DC000273, R01 DC016318 and R01 DC008130 to JSS and DN, and a National Natural Science Foundation of China grant to FZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Kakehata S, Kitani R, Maruya S, Navaratnam D, Santos-Sacchi J, Shinkawa H (2007) Developmental expression of the outer hair cell motor prestin in the mouse. J Membr Biol 215:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J, Avan P, Brownell WE, Dallos P, Dierkes K, Fettiplace R, Grosh K, Hackney CM, Hudspeth AJ, Juelicher F, Lindner B, Martin P, Meaud J, Petit C, Sacchi JRS, Canlon B (2010) The remarkable cochlear amplifier. Hear Res 266:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF (1990) Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl 12:S39–S50. [DOI] [PubMed] [Google Scholar]
- Bai J-P, Navaratnam D, Santos-Sacchi J (2019) Prestin kinetics and corresponding frequency dependence augment during early development of the outer hair cell within the mouse organ of Corti. Sci Rep in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Navaratnam D, Santos-Sacchi J (2013) Real time measures of prestin charge and fluorescence during plasma membrane trafficking reveal sub-tetrameric activity. PLoS One 8:e66078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S, Koo BW, Kelleher S, Santos-Sacchi J, Navaratnam DS (2010) A highly expressing Tet-inducible cell line recapitulates in situ developmental changes in prestin’s Boltzmann characteristics and reveals early maturational events. AmJPhysiol Cell Physiol 299:C828–C835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J (2008) Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron 58:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C (2008) Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. JBiolChem 283:4177–4188. [DOI] [PubMed] [Google Scholar]
- Evans BN, Dallos P (1993) Stereocilia displacement induced somatic motility of cochlear outer hair cells. Proc Natl Acad Sci U S A 90:8347–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A (1965) Transducing mechanisms in the lateral line canal organ receptors. Cold Spring HarbSympQuantBiol 30:133–145. [DOI] [PubMed] [Google Scholar]
- Frank G, Hemmert W, Gummer AW (1999) Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci U S A 96:4420–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF (1994) Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc R Soc Lond B Biol Sci 255:243–249. [DOI] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF (1997) The outer hair cell motor in membrane patches. Pflugers Arch 434:267–271. [DOI] [PubMed] [Google Scholar]
- Gillis KD (1995) Techniques for Membrane Capacitance Measurements. In: Single Channel Recording (Sakmann B, Neher E, eds), pp 155–198. New York: Plenum Press. [Google Scholar]
- Hille B (1992) Ionic channels of excitable membranes, 2nd Edition. Sunderland, Mass.: Sinauer Associates. [Google Scholar]
- Huang G, Santos-Sacchi J (1993) Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. Biophys J 65:2228–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa KH (1993) Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J 65:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J (1995) Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys J 68:2190–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendrasingam S, Beurg M, Fettiplace R, Hackney CM (2010) The ultrastructural distribution of prestin in outer hair cells: a post-embedding immunogold investigation of low-frequency and high-frequency regions of the rat cochlea. Eur J Neurosci 31:1595–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina ML, Barrera FN, Fernandez AM, Poveda JA, Renart ML, Encinar JA, Riquelme G, Gonzalez-Ros JM (2006) Clustering and coupled gating modulate the activity in KcsA, a potassium channel model. J Biol Chem 281:18837–18848. [DOI] [PubMed] [Google Scholar]
- Navaratnam D, Bai JP, Samaranayake H, Santos-Sacchi J (2005) N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein. Biophys J 89:3345–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Fakler B (1999) Expression density and functional characteristics of the outer hair cell motor protein are regulated during postnatal development in rat [In Process Citation]. JPhysiol (Lond) 519 Pt 3:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B (2001) Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292:2340–2343. [DOI] [PubMed] [Google Scholar]
- Organ LE, Raphael RM (2007) Application of fluorescence recovery after photobleaching to study prestin lateral mobility in the human embryonic kidney cell. JBiomedOpt 12:021003. [DOI] [PubMed] [Google Scholar]
- Pusch M, Neher E (1988) Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch 411:204–211. [DOI] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J (2003) Cl- flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol 547:873–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J (1990) Fast outer hair cell motility: how fast is fast? In: The Mechanics and Biophysics of Hearing (Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR, eds), pp 69–75. Berlin: Springer-Verlag. [Google Scholar]
- Santos-Sacchi J (1991) Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci 11:3096–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J (2004) Determination of cell capacitance using the exact empirical solution of dY/dCm and its phase angle. Biophys J 87:714–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J (2019) The speed limit of outer hair cell electromechanical activity. HNO 67:159–164. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dilger JP (1988) Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res 35:143–150. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Navarrete E (2002) Voltage-dependent changes in specific membrane capacitance caused by prestin, the outer hair cell lateral membrane motor. Pflugers Arch 444:99–106. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Zhao HB (2003) Excitation of fluorescent dyes inactivates the outer hair cell integral membrane motor protein prestin and betrays its lateral mobility. Pflugers Arch in press. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Song L (2014) Chloride and Salicylate Influence Prestin-Dependent Specific Membrane Capacitance: Support for the Area Motor Model. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Song L (2016) Chloride anions regulate kinetics but not voltage-sensor Qmax of the solute carrier SLC26a5. Biophys J 110:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Tan W (2018) The Frequency Response of Outer Hair Cell Voltage-Dependent Motility Is Limited by Kinetics of Prestin. J Neurosci 38:5495–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Kakehata S, Takahashi S (1998) Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J Physiol 510 ( Pt 1):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Navarrete E, Song L (2009) Fast electromechanical amplification in the lateral membrane of the outer hair cell. Biophys J 96:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Iwasa KH, Tan W (2019) Outer hair cell electromotility is low-pass filtered relative to the molecular conformational changes that produce nonlinear capacitance. J Gen Physiol in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Shen W, Zheng J, Dallos P (2001) Effects of membrane potential and tension on prestin, the outer hair cell lateral membrane motor protein. J Physiol 531:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Song L, Zheng J, Nuttall AL (2006) Control of mammalian cochlear amplification by chloride anions. J Neurosci 26:3992–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour ML, Rajagopalan L, Duret G, Volk MJ, Liu H, Brownell WE, Pereira FA (2016) Membrane prestin expression correlates with the magnitude of prestin-associated charge movement. Hear Res 339:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Santos-Sacchi J (2010) Conformational state-dependent anion binding in prestin: evidence for allosteric modulation. Biophys J 98:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T, Huang KC, Peterson E, Phillips R (2007) Cooperative gating and spatial organization of membrane proteins through elastic interactions. PLoS Comput Biol 3:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Hakizimana P, Wu S, Hassan A, Jacob S, Temirov J, Fang J, Mellado-Lagarde M, Gursky R, Horner L, Leibiger B, Leijon S, Centonze VE, Berggren PO, Frase S, Auer M, Brownell WE, Fridberger A, Zuo J (2015) Outer Hair Cell Lateral Wall Structure Constrains the Mobility of Plasma Membrane Proteins. PLoS Genet 11:e1005500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155. [DOI] [PubMed] [Google Scholar]