Abstract
Background
Circulating tumor DNA (ctDNA) has potential as a specific, noninvasive, and cost‐effective new biomarker for patients with lung cancer. This study aimed to determine whether plasma ctDNA can be used to predict treatment outcomes in patients with lung cancer.
Methods
Pre‐ and in‐treatment blood samples were collected from 14 patients with lung cancer receiving chemotherapy. Based on next‐generation sequencing technology, we constructed a unique molecular identifier (UMI) library and performed targeted deep sequencing of 72 genes (15 000×). We used dVAF to evaluate the change level and trend of variant allele frequency (VAF).
Results
We identified MUC16, KMT2D, AMER1, and NTRK1 as the most‐frequently mutated genes in ctDNA associated with lung cancer. Furthermore, we showed that the change trend of dVAF in patients with lung cancer undergoing chemotherapy was closely related to the changes in both tumor volume and tumor biomarkers, including CEA, CA125, NSE, and CK (Cytokeratin). Moreover, the ctDNA analysis revealed disease progression of SCLC patients earlier than did computed tomography.
Conclusions
The dynamic detection of plasma ctDNA VAF has the potential value as a biomarker for evaluating the efficacy of chemotherapy in patients with SCLC and advanced NSCLC, and may predict the progression of lung cancer patients earlier than radiography.
Keywords: chemotherapy, circulating tumor DNA, lung cancer, unique molecular identifiers
The dVAF level in chemotherapy patients was closely associated with tumor size. High levels of tumor biomarkers were detected in patients with dVAF > 0. The dynamic detection of plasma ctDNA VAF has the potential value as a biomarker for evaluating the efficacy of chemotherapy in patients with SCLC and advanced NSCLC. Furthermore, the dynamic detection of plasma ctDNA VAF may predict the progression of lung cancer patients earlier than radiography.
INTRODUCTION
Tumors are the main cause of the increase in deaths worldwide. In 2020, although the incidence of lung cancer ranked second, its mortality rate was the highest worldwide. 1 According to the histopathological classification, lung cancer is divided into small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC). NSCLC accounts for more than 80% of all lung cancers, including squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. 2 In recent years, significant progress has been made in the diagnosis and treatment of lung cancer. In addition to traditional surgery, radiotherapy, and chemotherapy, targeted therapy and immunotherapy have increasingly benefited patients with advanced lung cancer. However, the latest statistics (2019) show that the 5‐year overall survival (OS) rate for lung cancer remains at only 18%–19%. 3 , 4 The relatively low OS of patients with lung cancer may be attributed to the lack of highly sensitive early‐screening diagnostics and precise targeted therapies; therefore, the optimal treatment window is missed in these patients.
At present, tumor biopsy remains the gold standard for the diagnosis of lung cancer. 5 However, the sensitivity of tissue biopsy is affected by multiple factors, such as the tumor growth site, size of specimens, and degree of tumor heterogeneity. 6 , 7 Moreover, the acquisition of biopsy tissue mainly relies on invasive procedures, including interventional puncture, bronchoscopy biopsy, and surgical resection, which may cause some complications, such as pneumothorax. 8 In addition, tissue biopsy cannot be used to monitor the condition of the disease in real time because it is difficult to perform a biopsy in a timely manner. 9 , 10 Therefore, it is of great interest to develop a highly specific, sensitive, and noninvasive approach for the monitoring of lung cancer.
Liquid biopsy refers to the analysis of tumor‐derived components in body fluids, among which circulating tumor DNA (ctDNA) has been used for dynamic monitoring of tumor changes, therapeutic effects, and patient prognosis. 11 ctDNA is a DNA fragment that is released from tumor tissues to the peripheral blood and carries all the genetic characteristics of the tumor tissue. 12 Compared with traditional tissue biopsy, ctDNA has the following advantages: first, ctDNA sampling is noninvasive and can be easily performed by blood drawing. Second, ctDNA harbors genomic DNA information from different regions of all tumors. The ctDNA analysis can reveal almost all changes in the tumor genome to a large extent, and solve the problem of tumor heterogeneity. Third, the detection of ctDNA allows the real‐time monitoring of tumor progression at the molecular level and may guide clinical treatments in a timely manner. Therefore, plasma ctDNA analyses may provide a noninvasive new method for the diagnosis of lung cancer, monitoring of tumor progression, and assessment of clinical treatment in real‐time. 13 , 14 , 15 , 16
Currently, few studies on ctDNA VAF are available in the field of lung cancer chemotherapy, and most of this research has focused on the quantification of plasma cfDNA levels at a single time point, and no further detailed dynamic analysis of ctDNA has been reported. 17 , 18 , 19 Moreover, in terms of research technology, most of the relevant experiments were performed using dPCR or next‐generation sequencing technology. Although next‐generation sequencing technology has been widely used in the field of tumor research, the variations in library preparation, target sequence capture, and sequencing process limit the further development of next‐generation sequencing technology. The molecular barcode (unique molecular identifiers, UMIs) method consists in connecting DNA fragments with a unique sequence barcode that can identify repeated fragments and false‐positive results. Using this method, mutation detection can be performed when the VAF is extremely low, which significantly reduces false‐positive results and greatly improves the accuracy of the findings. This project was based on next‐generation sequencing technology using targeted deep sequencing (15 000×) combined with UMIs to select 72 genes for sequencing in 43 samples dynamically collected from patients with advanced NSCLC or SCLC. We divided the samples into the NSCLC and SCLC groups according to pathological types, and analyzed the relationship between plasma ctDNA VAF and serum tumor markers and tumor size, while revealing the potential clinical application value of ctDNA VAF dynamic detection as a biomarker for evaluating the efficacy of chemotherapy for lung cancer.
METHODS
Patients
A total of 14 patients diagnosed with lung cancer in the second hospital of the Dalian Medical University from December 2018 to August 2019 were included in this study. The inclusion criteria were as follows: (1) patients with primary lung cancer diagnosed by cytology or pathology, (2) those receiving first‐line treatment with platinum‐containing dual‐drug chemotherapy, and (3) those with complete clinical data. The following exclusion criteria were applied: (1) patients who had previously received chemotherapy, radiotherapy, targeted therapy, immunotherapy, or other treatments; and (2) those with a second primary malignant tumor. The first‐line platinum‐containing dual‐drug chemotherapy regimens used in the study were as follows: gemcitabine and platinum for patients with squamous cell carcinoma, pemetrexed and platinum for those with adenocarcinoma, and etoposide plus cisplatin for patients with SCLC. The outcome of chemotherapy was evaluated based on RECIST v1.1 criteria. Pre‐ and in‐treatment blood samples (8 ml for each) were collected in a K2‐EDTA tube. This study was approved by the Ethics Committee of the second hospital of the Dalian Medical University, and all participants signed a written informed consent.
Cell‐free DNA isolation and next‐generation sequencing
Blood samples were subjected to centrifugation at 3000 rpm for 10 min. Subsequently, the plasma was separated, centrifuged at 12 000 rpm for 15 min, immediately aliquoted, and stored at −80°C. The gDNA of leukocytes was extracted and quantified as a control. Cell‐free DNA (cfDNA) was extracted from 3 ml aliquots of plasma using the QIAamp Circulating Nucleic Acid Kit (55 114, Qiagen), concentrated using Agencourt Ampure XP beads, and quantified on a Qubit fluorometer. The unique molecular identifier (UMI) library construction and target enrichment were performed using QIAseq Targeted DNA Panels (12)‐Human Lung Cancer Panel (333502‐DHS‐005Z‐12, Qiagen). We used Illumina high‐throughput sequencing platform to perform targeted deep sequencing of 72 genes (15 000×). The sequencing strategy was based on PE 150 (double‐ended 150 bp). After NGS, data analysis was carried out using the QIAseq targeted DNA panel analysis software pipeline or Biomedical Genomics Workbench. Finally, interpretation of the detected variants was conducted using Qiagen clinical insight.
Data processing
All reads were aligned via BWA to GRCh37 using the default argument, and the smCounter2 pipeline was used to call single‐nucleotide variants (SNVs). We filtered any variant that had a VAF ≥ 0.05 and retained the VAF sites that were present in both white blood cells (WBCs) and tumors. Subsequently, the mean value of VAF for each sample was calculated, and each patient had a mean value at each time point. dVAF was the difference in VAF standard deviation between the tumor and WBCs, and was used to draw a graph for visualizing the data.
RESULTS
Patient characteristics
The clinical characteristics of all patients are summarized in Table 1. Among the 14 patients with lung cancer, the average age was 58 years and half had a history of smoking. Among the patients with NSCLC, one patient with adenocarcinoma achieved partial response (PR) after chemotherapy, and four patients had stable disease, whereas three had progressive disease (PD), and one had not undergone evaluation. Out of the five patients with SCLC patients, two patients achieved PR after chemotherapy, and one had PD, whereas the remaining two patients were not evaluated.
TABLE 1.
Clinical characteristics of patients
| Characteristics | N = 14 |
|---|---|
| Age, years | 58 (47–72) |
| Gender, male/female | 8/6 |
| Smoking, n (%) | 7 (50.00) |
| Stage at entry, n (%) | |
| Limited disease | 1 (7.14) |
| Extensive disease | 4 (28.57) |
| IIIB | 2 (14.29) |
| IV | 7 (50.00) |
| Histology, n (%) | |
| Squamous carcinoma | 2 (14.29) |
| Adenocarcinoma | 7 (50.00) |
| Small cell carcinoma | 5 (35.71) |
| ECOG/WHO PS at baseline, n (%) | |
| 0 | 9 (64.29) |
| 1 | 5 (35.71) |
| RECIST, n (%) | |
| CR | 0 |
| PR | 3 (21.43) |
| SD | 4 (28.57) |
| PD | 4 (28.57) |
| No evaluation | 3 (21.43) |
Characteristics of gene mutations in pretreatment ctDNA
We first used the highly sensitive single‐end specific UMI technology to perform targeted deep sequencing (15 000×) of 72 genes on baseline samples. The right side of Figure 1 shows the gene mutations detected in patients with NSCLC. The most commonly mutated genes in these patients were MUC16 (67%), AMER1 (67%), and KMT2D (56%). Three patients with NSCLC (50%) carried simultaneous mutations in the MUC16 and KMT2D genes. The left side of Figure 1 shows the gene mutations detected in patients with SCLC. The most commonly mutated genes in these patients were MUC16 (100%), KMT2D (80%), NTRK1 (80%), and NF1 (80%). Two cases of SCLC carried common mutations in the MUC16, KMT2D, AMER1, and NTRK1 genes.
FIGURE 1.
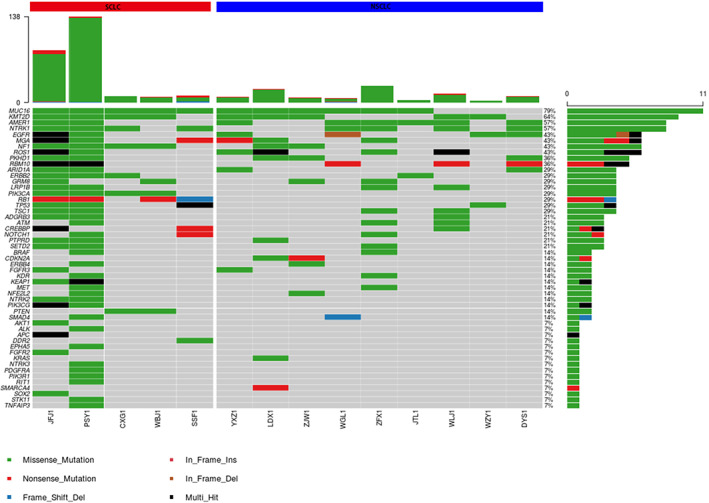
NGS‐based mutational analysis in the pretreatment ctDNA from 14 patients with lung cancer
Correlation between serum tumor markers and dVAF during chemotherapy in lung cancer patients
To determine whether the ctDNA VAF change was correlated with the level of tumor biomarkers, we compared the contents of tumor biomarkers, including CEA, CA125, NSE, SCC, and CK, between the increased dVAF group and decreased dVAF group. All p‐values were above 0.05 in the increased dVAF group and the decreased dVAF group among advanced NSCLC patients. There were no significant differences with regard to tumor markers (Figure 2).
FIGURE 2.
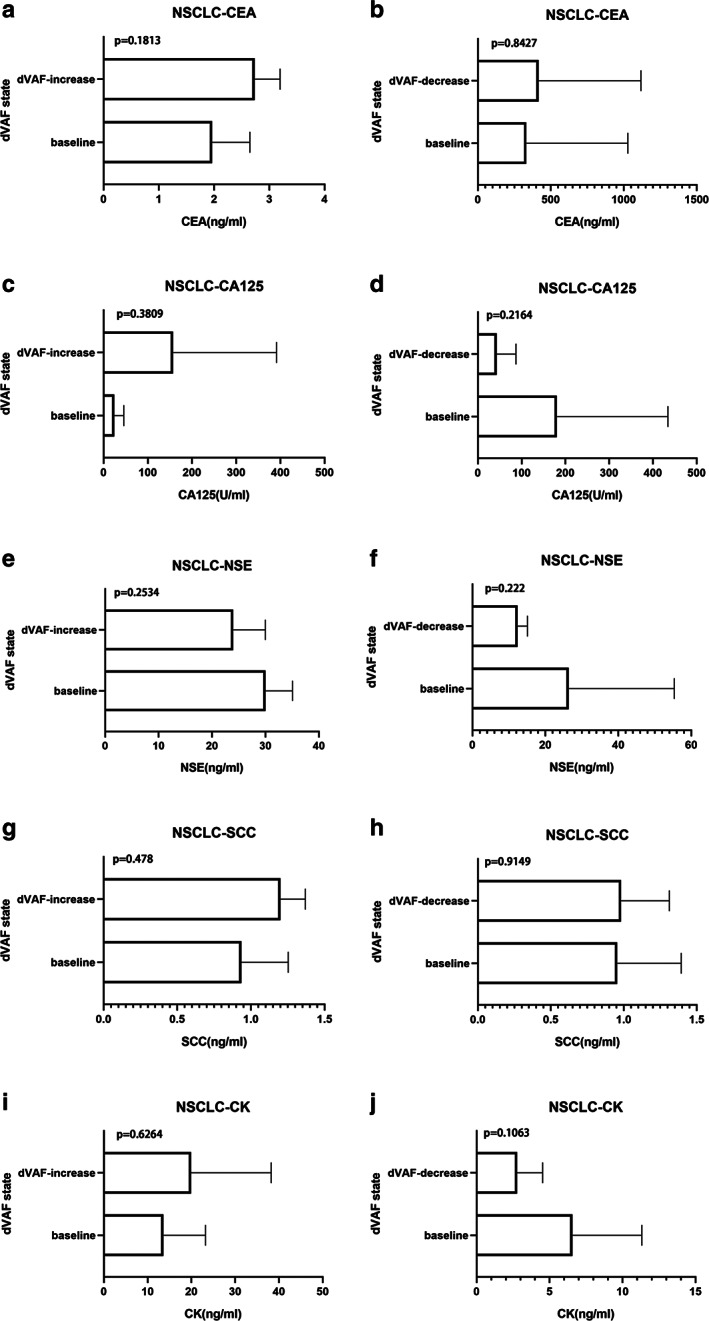
Correlation between serum tumor markers and dVAF during chemotherapy in patients with NSCLC
In patients with SCLC, p‐values were above 0.05 in the decreased dVAF group and there were no significant differences with regard to tumor markers. However, the level of the serum tumor marker CK and the trend of dVAF were consistent, regardless of whether the patients had advanced NSCLC or SCLC (Figure 3i,j,e). This suggests that the changes in dVAF in lung cancer patients during chemotherapy may be related to serum tumor marker levels.
FIGURE 3.
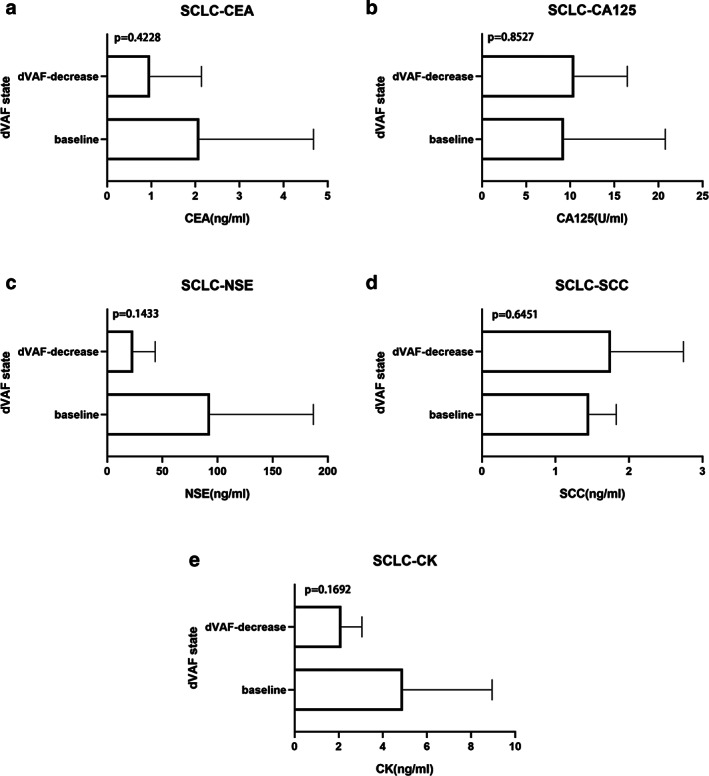
Correlation between serum tumor markers and dVAF during chemotherapy in patients with SCLC
dVAF level in patients receiving chemotherapy was closely associated with tumor size
Next, we investigated the relationship between the dVAF and tumor size in relation to chemotherapy. Among the patients with NSCLC, five patients had a decrease in dVAF after two cycles of first‐line chemotherapy and a corresponding reduction in tumor volume. In another patient, dVAF increased after two cycles of first‐line chemotherapy, and the tumor volume also increased correspondingly (Figure 4). Moreover, in 67% of patients with NSCLC, dVAF changes were consistent with the evolution of tumor size during chemotherapy.
FIGURE 4.
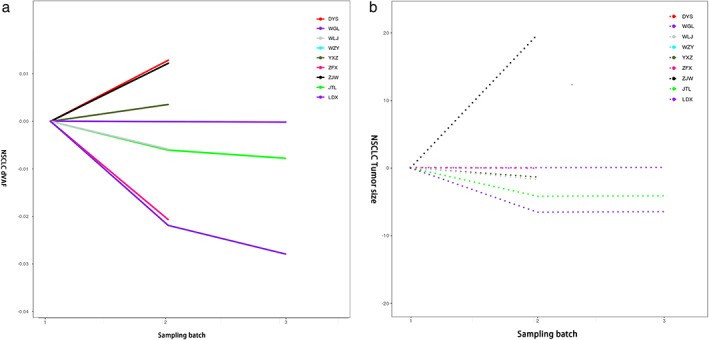
Changes in tumor size and dVAF during chemotherapy in patients with NSCLC
Among the patients with SCLC, three patients (60%) had a decrease in dVAF after two cycles of first‐line chemotherapy, and the tumor volume also decreased accordingly (Figure 5). It is suggested that the evolution of tumor size is consistent with the change trend of ctDNA VAF during chemotherapy, regardless of whether the patients had NSCLC or SCLC.
FIGURE 5.
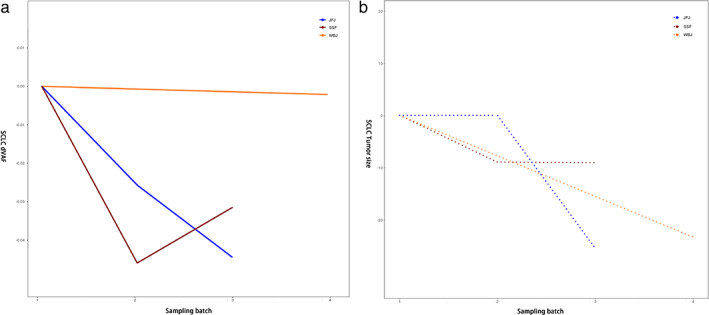
Changes in tumor size and dVAF during chemotherapy in patients with SCLC
Longitudinal ctDNA analysis to monitor lung cancer patient response to chemotherapy
To examine whether ctDNA sequencing can be used to monitor the response of the patients to chemotherapy, we analyzed serial plasma specimens from patients with lung cancer that were collected during the course of therapy. As described below, we reported typical cases in which ctDNA analysis was performed to monitor the response of patients with NSCLC and SCLC response to chemotherapy.
Dynamic collection of peripheral blood samples was performed in a patient with stage IV NSCLC at the baseline and after two cycles of chemotherapy (Figure 6). The frequency of tumor mutations was increased, and the VAF was deviated from the normal control compared with baseline after two cycles of gemcitabine combined with cisplatin chemotherapy, which indicated that the patient's condition was progressing. At this time, the patient's clinical efficacy evaluation was also PD. In other patients with stage IIIB–IV NSCLC, the dynamic changes of ctDNA VAF were also consistent with the clinical efficacy evaluation. Collectively, these findings show that ctDNA VAF analysis may be used to assess the response of patients with NSCLC to chemotherapy.
FIGURE 6.
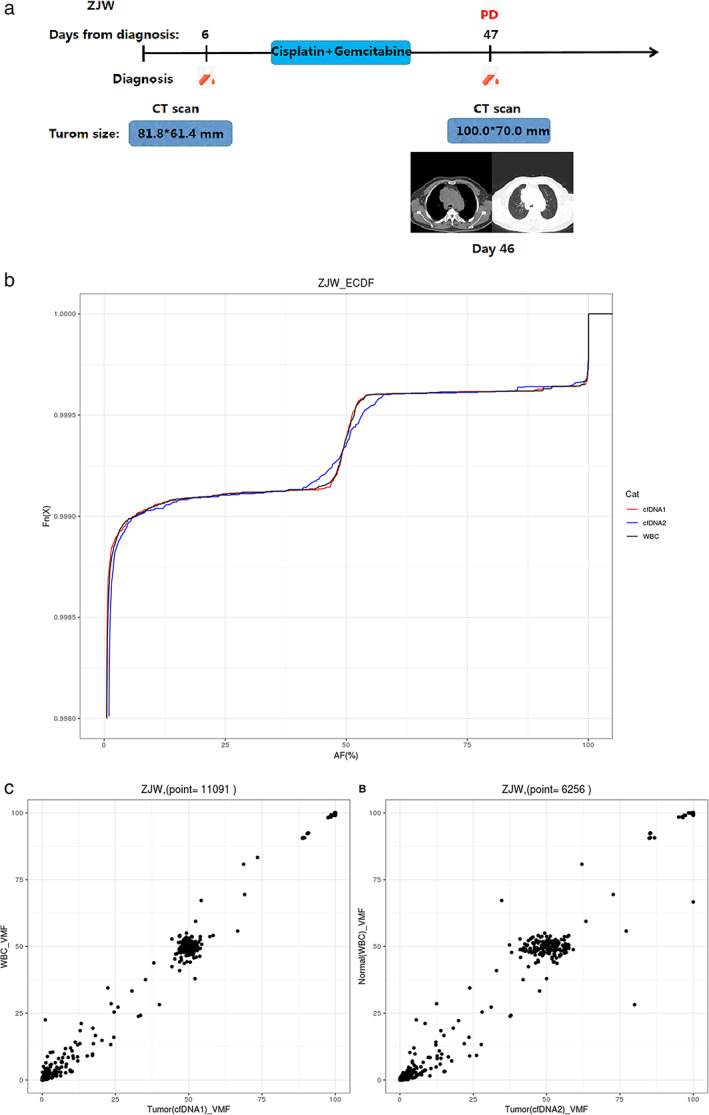
Longitudinal ctDNA analysis to monitor the response of a patient with lung cancer (ZJW) to chemotherapy. (a) Flow chart of the clinical course of patient ZJW, starting from the time of diagnosis and throughout the treatment. The light‐blue bars represent the treatment time frame, whereas the red dots indicate the time points of blood collection. Radiographic images were acquired at day 46. PD in red described the clinical outcome of chemotherapy in the patient. (b) Mutation allele frequency of patient ZJW during chemotherapy. The different colored curves correspond to different time points of blood collection. The abscissa is the variant allele frequency. The ordinate Fn (x) represents the cumulative percentage. (c) Frequency of tumor mutations compared with the normal control at different time points. VMF is variant mutation frequency
In one patient with SCLC (Figure 7), we found that the baseline VAF was significantly different from that of the normal control. After receiving two cycles of chemotherapy (day 45), the clinical efficacy was evaluated as PR in this patient. At this time, the frequency of tumor mutations was decreased, and the VAF was close to normal compared with the baseline, which also indicated that the patient's condition was relieved and the tumor was under control. The patient's third sample (day 84) was collected after receiving four cycles of chemotherapy. At this time, the frequency of tumor mutations was significantly reduced, and VAF was closer to normal than it was on day 45, indicating that the patient's condition continued to be relieved. In addition, the clinical efficacy was evaluated as PR in this patient. In addition to the patients with SCLC detailed above, the dynamic changes of ctDNA VAF were also consistent with the clinical efficacy evaluation in other patients with SCLC.
FIGURE 7.
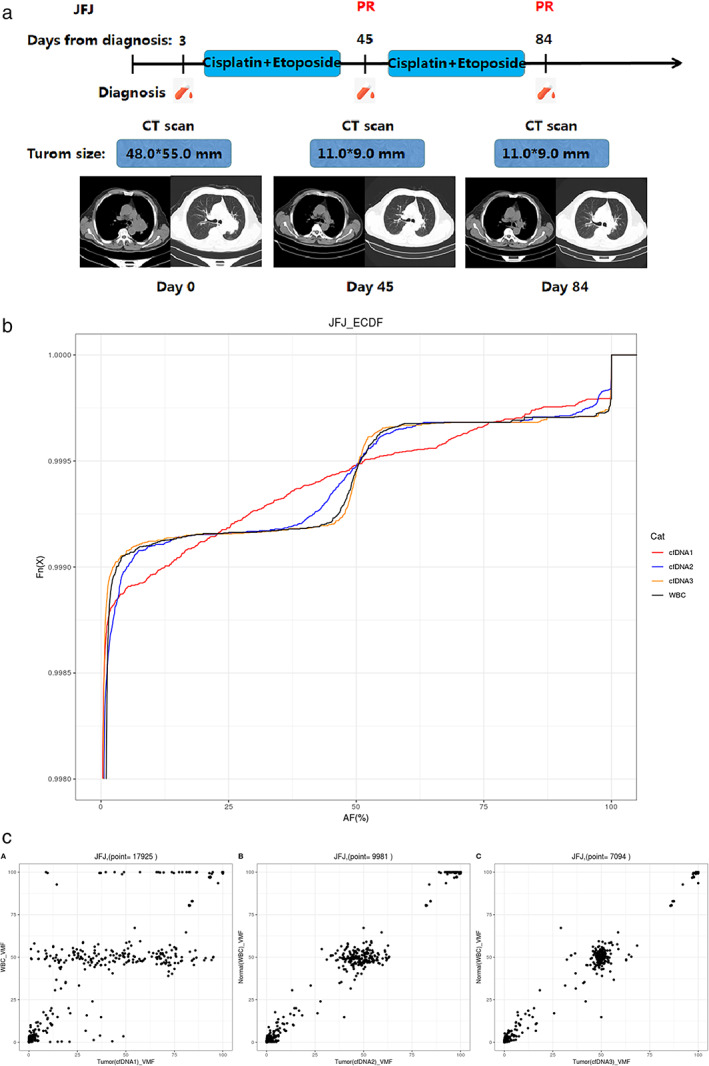
Longitudinal ctDNA analysis to monitor the response of a patient with lung cancer (JFJ) to chemotherapy. (a) Flow chart of the clinical course of patient JFJ, starting from the time of diagnosis and throughout the treatment. The light‐blue bars represent the treatment time frame, whereas the red dots indicate the time points of blood collection. Radiographic images were acquired at days 0, 45, and 84. PR in red indicates the clinical outcome of chemotherapy in the patient at different time points. (b) Mutation allele frequency of patient JFJ during chemotherapy. The different color curves correspond to different time points of blood collection. The abscissa is the variant allele frequency. The ordinate Fn (x) represents the cumulative percentage. (c) Frequency of tumor mutations compared with the normal control at different time points. VMF is variant mutation frequency
Longitudinal ctDNA analysis reveals resurgence of disease earlier than radiography
We collected peripheral blood samples after the baseline, two cycles of chemotherapy, and four cycles of chemotherapy (Figure 8). After two cycles of chemotherapy (day 48), the VAF was very close to normal. Moreover, the imaging revealed a PR status that was in accordance with the change trend of VAF. Strikingly, we found that the patient's mutation frequency was increased, and VAF deviated from the normal control compared with day 48. At this time, radiography revealed a remarkable response in pulmonary parenchymal disease. The patient received an additional two cycles of chemotherapy. However, the patient's condition had progressed, as assessed by imaging‐based clinical evaluation, after he/she received six cycles of chemotherapy. In this case, the ctDNA test revealed the patient's disease progression in advance, which was not found in imaging studies. Therefore, longitudinal ctDNA analysis could identify disease progression before radiographic evidence.
FIGURE 8.
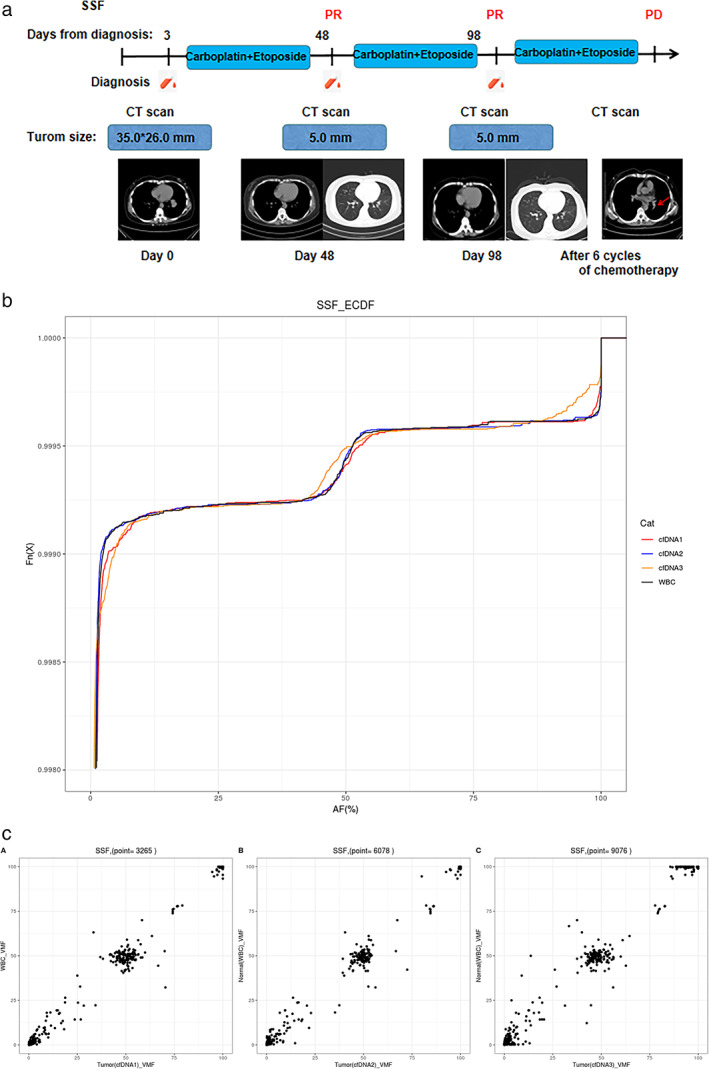
Longitudinal ctDNA analysis to monitor the response of a patient with lung cancer (SSF) to chemotherapy. (a) Flow chart of the clinical course of patient SSF, from the time of diagnosis and throughout the treatment. The light‐blue bar represents the treatment time frame, whereas the red dots indicate the time points of blood collection. Radiographic images were acquired at day 0, day 48, and day 98. PR or PD in red indicates the clinical outcome of chemotherapy in the patient at different time points. (b) Mutation allele frequency in patient SSF during chemotherapy. The different colored curves correspond to different time points of blood collection. The abscissa is the variant allele frequency. The ordinate Fn (x) represents the cumulative percentage. (c) Frequency of tumor mutations compared with the normal control at different time points. VMF is variant mutation frequency
DISCUSSION
Liquid biopsy has important prospects in the field of tumors. ctDNA is a safe, convenient, low‐invasive, highly specific, and reproducible biomarker. 20 , 21 Serum tumor markers are widely used in clinical practice. It is not only used for early screening and diagnosis of tumors, but also for monitoring therapeutic efficacy among patients with tumors. The commonly used clinical serum tumor markers for lung cancer include CEA, CA125, NSE, SCC, and CK. Some researchers have analyzed the correlation between cfDNA and tumor markers in patients with colorectal cancer, and found that the concentration of cfDNA is correlated with the levels of CEA, CA199, CA125, NSE, and LDH. 22 In another 30 patients with colorectal cancer, some researchers used ddPCR to quantify ctDNA and found that the increase in ctDNA occurred earlier than the increase in CEA, and that the changes in ctDNA were more sensitive than those in CEA. 23 To analyze whether there is a similar relationship between ctDNA VAF and serum tumor markers in patients with NSCLC and SCLC during chemotherapy, we adopted the UMI deep‐targeted sequencing technology to improve the accuracy and reliability of the results. We found that in patients with NSCLC, the levels of CEA, CA125, SCC, and CK in the elevated dVAF group were increased during chemotherapy. The levels of CA125, NSE, and CK in the dVAF decreased group were also decreased. In patients with SCLC, CEA, NSE, and CK levels were decreased in the dVAF‐reduced group during chemotherapy. Among them, CK maintained the same trend as dVAF in both the NSCLC and SCLC groups. There were no significant differences statistically, which may be due to the small number of samples and large individual differences in the cohort. These findings suggest that, although there is a certain relationship between serum tumor marker levels and ctDNA VAF changes during chemotherapy for NSCLC and SCLC, we need to expand the sample size in future research.
The correlation between ctDNA and tumor size is in the research and exploratory stage. Other researchers collected blood samples from 28 patients with NSCLC treated with EGFR‐TKI, and used ddPCR to detect ctDNA. Their results showed that the baseline detection of ctDNA is associated with a higher tumor burden. 24 In addition, several researchers have also obtained a similar result in immunotherapy for lung cancer. Among the patients with NSCLC who received immune checkpoint inhibitor monotherapy, the average tumor volume of patients with a dVAF < 0 at 6 weeks of immunotherapy was reduced by 39%, whereas the average tumor volume of patients with dVAF > 0 was increased by 36%. 19 Therefore, ctDNA changes may be a marker that benefits targeted therapy and immunotherapy in patients with NSCLC. To examine whether ctDNA VAF has the same effect on patients with NSCLC during chemotherapy, we dynamically collected blood samples and clinical examination results, for comprehensive comparison and analysis between the baseline and chemotherapy. In patients with advanced NSCLC, the dynamic changes in ctDNA VAF were consistent with the clinical efficacy evaluation. In addition, in patients with SCLC, the dynamic changes in ctDNA VAF were also consistent with the clinical efficacy evaluation results. Our findings suggest that the dynamic detection of ctDNA VAF may have the potential value of evaluating the efficacy of chemotherapy in patients with advanced NSCLC and SCLC.
At present, the commonly used clinical efficacy evaluation standard for patients with solid tumors is the measurement of the tumor lesion volume using imaging methods such as CT/MRI/PET‐CT, followed by the evaluation of the treatment effect. However, imaging examinations often fail to detect small lesions or small changes in tumor burden, and imaging measurements have inherent errors. In addition, the sensitivity and stability of the serum tumor markers that are often detected in clinical practice are relatively low. Some researchers found that ctDNA SNVs can detect disease recurrence in patients with early NSCLC prior to its detection via imaging examinations, and can identify different clones or subclones of the primary tumor and molecular changes in metastatic tumors. 25 Therefore, ctDNA has the potential to be used to assess the progression and prognosis of lung cancer. We used ctDNA VAF as a new biomarker to explore its role in evaluating the efficacy of chemotherapy for NSCLC and SCLC. Although no positive results were obtained in patients with NSCLC, the dynamic changes in ctDNA VAF detected in patients with SCLC can predict disease progression in advance, before disease progression can be recognized by imaging. This suggests that ctDNA VAF detects changes in the condition of patients with SCLC during chemotherapy earlier than imaging, which can help predict changes in the condition of patients, change treatments in a timely manner, and improve the survival of patients with lung cancer.
We found that ctDNA VAF dynamic detection was related to the evolution of serum tumor markers and tumor size during chemotherapy in patients with both NSCLC and SCLC. Moreover, it had the ability to evaluate the efficacy of chemotherapy. Although we found that ctDNA VAF predicted disease progression in advance during chemotherapy in patients with SCLC, a similar phenomenon was not observed in patients with NSCLC. It is worth noting that the VAF changes detected in some patients in this study were inconsistent with clinical indicators. This represents a particularly interesting area for future research, to understand better whether ctDNA VAF dynamic detection can help improve the evaluation of chemotherapy efficacy in patients with lung cancer. This study had clinical significance for patients with advanced NSCLC and SCLC undergoing chemotherapy; however, it also had limitations. First, although samples were collected from each patient at multiple time points, the number of patients enrolled in the cohort was relatively small. At present, open‐source databases include mostly tissue samples, and no blood samples collected at multiple consecutive time points are available. Moreover, there no WBCs are available as a control, and there no additional data sets exist for further verification of our results, which should be the focus of future research. Second, for practical reasons, the follow‐up period of the patients was shorter; a longer follow‐up period will be needed to evaluate whether ctDNA VAF can be used as a predictor of survival in patients with lung cancer. Although ctDNA VAF has a potential for clinical application during chemotherapy in patients with advanced NSCLC and SCLC, it is not possible to use a single index to evaluate treatment efficacy. In clinical applications, ctDNA VAF should be combined with imaging, serum tumor marker levels, and other clinical indicators to comprehensively evaluate patient status and the effects of treatment. We will develop this to its maximum clinical value and achieve individualized and precise treatment, to maximize the survival of patients.
In conclusion, the dynamic detection of plasma ctDNA VAF has potential value as a biomarker for evaluating the efficacy of chemotherapy in patients with SCLC and advanced NSCLC, and may predict the progression of lung cancer patients earlier than radiography..
CONFLICT OF INTEREST
The authors have nothing to declare.
ACKNOWLEDGMENTS
This work was supported by the Dalian Institute of Physics‐The Second Hospital of Dalian Medical University “Individualized Diagnosis and Treatment Collaborative Innovation Center” Joint Trust (dy2yhws201801).
Zhang M, Huang C, Zhou H, Liu D, Chen R, Li X, et al. Circulating tumor DNA predicts the outcome of chemotherapy in patients with lung cancer. Thorac Cancer. 2022;13:95–106. 10.1111/1759-7714.14230
Funding information The Dalian Institute of Physics‐The Second Hospital of Dalian Medical University "Individualized Diagnosis and Treatment Collaborative Innovation Center" Joint Trust, Grant/Award Number: dy2yhws201801
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Ahmedin Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;0:1–41. [DOI] [PubMed] [Google Scholar]
- 2. Zappa C, Mousa SA. Non‐small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 5. Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel‐node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53. [DOI] [PubMed] [Google Scholar]
- 7. Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT‐guided cutting‐needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326–30. [DOI] [PubMed] [Google Scholar]
- 8. Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol. 2013;31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fenizia F, De Luca A, Pasquale R, Sacco A, Forgione L, Lambiase M, et al. EGFR mutations in lung cancer: from tissue testing to liquid biopsy. Future Oncol. 2015;11:1611–23. [DOI] [PubMed] [Google Scholar]
- 10. Chouaid C, Dujon C, Do P, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re‐biopsy in advanced non small‐cell lung cancer: a prospective multicenter study in a real‐world setting (GFPC study 12‐01). Lung Cancer. 2014;86:170–3. [DOI] [PubMed] [Google Scholar]
- 11. He J, Tan W. Circulating tumor cells and DNA for real‐time EGFR detection and monitoring of non‐small‐cell lung cancer. Future Oncol. 2017;13:787–97. [DOI] [PubMed] [Google Scholar]
- 12. Shu Y, Wu X, Tong X, Wang X, Chang Z, Mao Y, et al. Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple cancer types. Sci Rep. 2017;7:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamal‐Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the evolution of non‐small‐cell lung cancer. N Engl J Med. 2017;376:2109–21. [DOI] [PubMed] [Google Scholar]
- 14. Isbell JM, Jones DR, Li BT. Circulating tumor DNA: a promising biomarker to guide postoperative treatment and surveillance of non‐small cell lung cancer. J Thorac Cardiovasc Surg. 2018;155:2628–31. [DOI] [PubMed] [Google Scholar]
- 15. Schwarzenbach H, Hoon DS, Pantel K. Cell‐free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. [DOI] [PubMed] [Google Scholar]
- 16. Donaldson J, Park BH. Circulating tumor DNA: measurement and clinical utility. Annu Rev Med. 2018;69:223–34. [DOI] [PubMed] [Google Scholar]
- 17. Normando SRC, Delgado PO, Rodrigues A, Filho WJD, Fonseca FLA, Cruz FJSM, et al. Circulating free plasma tumor DNA in patients with advanced gastric cancer receiving systemic chemotherapy. BMC Clin Pathol. 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patsch K, Matasci N, Soundararajan A, Diaz P, Agus DB, Ruderman D, et al. Monitoring dynamic cytotoxic chemotherapy response in castration‐resistant prostate cancer using plasma cell‐free DNA (cfDNA). BMC Res Notes. 2019;12(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raja R, Kuziora M, Brohawn PZ, Higgs BW, Gupta A, Dennis PA, et al. Early reduction in ctDNA predicts survival in patients with lung and bladder cancer treated with Durvalumab. Clin Cancer Res. 2018;24(24):6212–22. [DOI] [PubMed] [Google Scholar]
- 20. Hahn AW, Stenehjem D, Nussenzveig R, Carroll C, Bailey E, Batten J, et al. Evolution of the genomic landscape of circulating tumor DNA (ctDNA) in metastatic prostate cancer over treatment and time. Cancer Treat Res Commun. 2019;19:100120. [DOI] [PubMed] [Google Scholar]
- 21. Alvarez JGB, Dittmar K, Janse SA, Kiourtsis S, Owen DH, Bertino EM, et al. Initiation of targeted therapy based on ctDNA only in metastatic NSCLC. J Clin Oncol. 2019;37(15 Suppl):e20687‐e. [Google Scholar]
- 22. Xu X, Yu Y, Shen M, Liu M, Wu S, Liang L, et al. Role of circulating free DNA in evaluating clinical tumor burden and predicting survival in Chinese metastatic colorectal cancer patients. BMC Cancer. 2020;20(1):1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zou D, Day R, Cocadiz JA, Parackal S, Mitchell W, Black MA, et al. Circulating tumor DNA is a sensitive marker for routine monitoring of treatment response in advanced colorectal cancer. Carcinogenesis. 2020;41(11):1507–17. [DOI] [PubMed] [Google Scholar]
- 24. Ding PN, Becker TM, Bray VJ, Chua W, Ma YF, Lynch D, et al. The predictive and prognostic significance of liquid biopsy in advanced epidermal growth factor receptor‐mutated non‐small cell lung cancer: a prospective study. Lung Cancer. 2019;134:187–93. [DOI] [PubMed] [Google Scholar]
- 25. Abbosh C, Birkbak NJ, Wilson GA, Jamal‐Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early‐stage lung cancer evolution. Nature. 2017;545:446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


