Abstract
Introduction
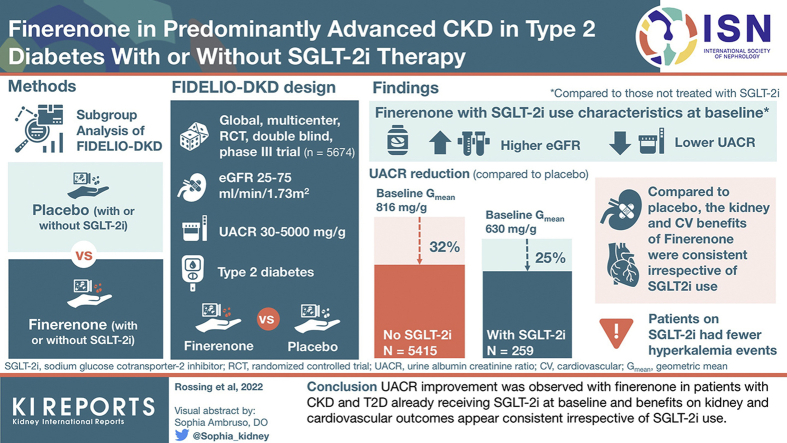
FIDELIO-DKD (FInerenone in reducing kiDnEy faiLure and dIsease prOgression in Diabetic Kidney Disease) investigated the nonsteroidal, selective mineralocorticoid receptor (MR) antagonist finerenone in patients with CKD and type 2 diabetes (T2D). This analysis explores the impact of use of sodium-glucose cotransporter-2 inhibitor (SGLT-2i) on the treatment effect of finerenone.
Methods
Patients (N = 5674) with T2D, urine albumin-to-creatinine ratio (UACR) of 30 to 5000 mg/g and estimated glomerular filtration rate (eGFR) of 25 to <75 ml/min per 1.73 m2 receiving optimized renin-angiotensin system (RAS) blockade were randomized to finerenone or placebo. Endpoints were change in UACR and a composite kidney outcome (time to kidney failure, sustained decrease in eGFR ≥40% from baseline, or renal death) and key secondary cardiovascular outcomes (time to cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) (ClinicalTrials.gov, NCT02540993).
Results
Of 5674 patients, 259 (4.6%) received an SGLT-2i at baseline. Reduction in UACR with finerenone was found with or without use of SGLT-2i at baseline, with ratio of least-squares means of 0.69 (95% CI = 0.66–0.71) and 0.75 (95% CI -= 0.62–0.90), respectively (Pinteraction = 0.31). Finerenone also significantly reduced the kidney and key secondary cardiovascular outcomes versus placebo; there was no clear difference in the results by SGLT-2i use at baseline (Pinteraction = 0.21 and 0.46, respectively) or at any time during the trial. Safety was balanced with or without SGLT-2i use at baseline, with fewer hyperkalemia events with finerenone in the SGLT-2i group (8.1% vs. 18.7% without).
Conclusion
UACR improvement was observed with finerenone in patients with CKD and T2D already receiving SGLT-2is at baseline, and benefits on kidney and cardiovascular outcomes appear consistent irrespective of use of SGLT-2i.
Keywords: albuminuria, chronic kidney disease, finerenone, sodium-glucose cotransporter-2 inhibitors, type 2 diabetes
Graphical abstract
Diabetes is the ptleading cause of kidney failure in many countries,1 with approximately 30% to 50% of adults with T2D having CKD.2,3 In patients with T2D, recommendations include a comprehensive treatment strategy including optimization of glycemic, blood lipid, and blood pressure control to reduce the risk or slow the progression of CKD.4, 5, 6 Guidelines have also long recommended the use of RAS inhibitor therapy (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) for patients with hypertension and albuminuria, and more recently the use of SGLT-2is based on findings from cardiovascular outcomes trials.4,6, 7, 8, 9 From 2018, use of an SGLT-2i has been recommended in patients with diabetes who have an eGFR ≥30 ml/min per 1.73 m2 and at least moderately increased albuminuria (microalbuminuria; UACR >30 mg/g) to reduce the risk of progression of CKD, cardiovascular events, or both.4, 5, 6
Results from the CREDENCE and DAPA-CKD trials, which were designed to determine outcomes in kidney disease in patients with a UACR of 200 mg/g or higher, revealed that SGLT-2is offer kidney protection in patients with CKD with or without T2D.10,11 Despite the use of guideline-recommended therapies including RAS inhibitors and SGLT-2is in CREDENCE and DAPA-CKD, CKD progression or kidney failure still occurred in approximately 10% of patients after a median follow-up of approximately 2.5 years.10,11 Further treatment strategies to combat progression of kidney disease are therefore needed.
Finerenone is a novel, selective, nonsteroidal MR antagonist developed to treat kidney and cardiovascular disease in patients with CKD and T2D.12,13 In the FIDELIO-DKD trial, finerenone significantly reduced the primary composite kidney outcome and the key secondary cardiovascular composite outcome compared with placebo in patients receiving optimized RAS inhibitor therapy with no effect on glycated hemoglobin.12
Although recommendations for the use of an SGLT-2i in patients with CKD and T2D were introduced after the initiation of FIDELIO-DKD in 2015, a limited number of patients received concomitant SGLT-2i treatment during the trial.12,14 The objective of this analysis was to explore the treatment effect of finerenone in patients with concomitant use of SGLT-2i, either at baseline or during the trial.
Methods
Study Design and Participants
The design of the phase 3, randomized, double-blind, placebo-controlled, multicenter FIDELIO-DKD study and the results of the primary analysis and full trial protocol have been published previously.12,14 Recruitment for the trial was completed in June 201814 before the results of the first dedicated SGLT-2i kidney outcome trials, CREDENCE and DAPA-CKD, were published.10,11 Briefly, adults (≥18 years of age) with CKD and T2D who were receiving optimized RAS inhibitor therapy were eligible to participate if they had a serum potassium level up to 4.8 mmol/l at screening. CKD was defined as persistent, moderately elevated albuminuria (UACR 30 to <300 mg/g), an eGFR of 25 to <60 ml/min per 1.73 m2 (calculated using the CKD Epidemiology Collaboration formula), and a history of diabetic retinopathy or persistent, severely elevated albuminuria (UACR = 300–5000 mg/g) with an eGFR of 25 to <75 ml/min per 1.73 m2. The trial protocol was approved by the institutional review board at each study site, and all participants provided written informed consent.
Patients were randomized 1:1 to receive oral finerenone (10 mg or 20 mg) or matching placebo once daily. Randomization was based on computer-generated randomization schedule stratified by region (North America, Europe, Asia, Latin America, other), albuminuria at screening (moderately increased, severely increased), and eGFR at screening (25–<45, 45–<60, ≥60 ml/min per 1.73 m2). All participants and study personnel (except for the independent data monitoring committee) were masked to treatment allocation.
Procedures and Outcomes
The present analysis reports outcomes and safety data in subgroups of patients by SGLT-2i use at baseline. Separate analyses were also performed for patients who were treated with an SGLT-2i at any time during the trial, with SGLT-2i use as a time-dependent covariate.
Urinalysis was performed centrally during run-in and screening visits and at baseline, month 4, month 12, and then every 12 months thereafter. Central laboratory values, including serum potassium and serum creatinine, were obtained at all study visits.
The primary kidney outcome of the study was evaluated in a time-to-event analysis using the composite outcome of kidney failure, a sustained decrease of 40% or more in eGFR from baseline (for ≥4 weeks), or death owing to renal causes (renal death). Kidney failure was defined as end-stage kidney disease or an eGFR <15 ml/min per 1.73 m2, confirmed by a second measurement at the earliest 4 weeks after the initial measurement. The key secondary outcome was a composite cardiovascular outcome of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. Other secondary outcomes included death from any cause, hospitalization from any cause, change in UACR from baseline to month 4, and a secondary composite kidney outcome of kidney failure, a sustained decrease of 57% or more in eGFR from baseline (for ≥4 weeks), or renal death, all evaluated in a time-to-event analysis.
Statistical Analysis
Change in UACR from baseline to month 4 was tested using an analysis of covariance model adjusted for treatment group, stratification factors, and baseline value as described previously.12 Change in UACR and systolic blood pressure throughout the entire study was analyzed using a mixed-model approach that incorporated additional variables to account for differences in baseline characteristics between SGLT-2i baseline subgroups. The mixed-model included treatment group, stratification factors (region, albuminuria category at screening, and eGFR category at screening), time during the study, treatment over time, log-transformed baseline value nested within type of albuminuria at screening, and log-transformed baseline value over time as covariates. Covariance patterns were estimated within patients to adjust for the within-patient variation. For each treatment group, a separate covariance pattern was estimated based on unstructured covariance.
Statistical analysis methods for the primary and secondary outcomes in FIDELIO-DKD have also been published previously.12,14 Further details are provided in the Supplementary Appendix. Exploratory analysis for the primary and secondary efficacy outcomes was prespecified for subgroups by the use of an SGLT-2i at baseline. Treatment effect for time-to-event outcomes in patients stratified by SGLT-2i use at baseline (expressed as a hazard ratio [HR] with corresponding 95% CIs and P values for SGLT-2i use at baseline-by-treatment interaction) was derived from a stratified Cox proportional hazards model including treatment (finerenone vs. placebo), subgroup of SGLT-2i use at baseline, and subgroup of SGLT-2i use at baseline-by-treatment interaction term as fixed effects.
A time-to-event analysis was also performed to estimate the treatment effect of finerenone on the primary and key secondary outcomes and investigator-reported hyperkalemia accounting for the use of an SGLT-2i at any point during the trial. Stepwise selection methods were used to account for confounding factors that may have influenced initiation of SGLT-2is and the outcome of interest. Further details are provided in the Supplementary Appendix.
Safety analyses were performed in the safety analysis set, consisting of all randomized patients without critical Good Clinical Practices violations who took 1 or more doses of the study drug.
Results
Patients
Of 13,911 patients screened for eligibility to participate in the FIDELIO-DKD study across 48 countries between September 2015 and June 2018, a total of 5674 randomized patients were included in the full analysis set and were followed up for a median of 2.6 years.12 Of these, 259 of 5674 patients (4.6%) were receiving an SGLT-2i at baseline, comprising 124 of 2833 (4.4%) in the finerenone group and 135 of 2841 (4.8%) in the placebo group (Table 1). SGLT-2i treatment was initiated during the trial in a further 328 patients (5.8%) who did not receive an SGLT-2i at baseline (153 patients in the finerenone group and 175 in the placebo group; Supplementary Table S1). The mean time on an SGLT-2i throughout the study was 735 and 788 days in the finerenone and placebo groups, respectively. Mean time to treatment initiation with an SGLT-2i post baseline was 629 days with finerenone and 565 days with placebo. Approximately half of the patients in the trial received empagliflozin either at baseline or at any time during the trial (Supplementary Table S2).
Table 1.
Baseline characteristics by baseline SGLT-2i use
| Characteristic | SGLT-2i at baseline (n = 259) | No SGLT-2i at baseline (n = 5415) |
|---|---|---|
| Age, yr, mean (SD) | 63.1 (9.6) | 65.7 (9.0) |
| Male sex, n (%) | 188 (72.6) | 3795 (70.1) |
| Race, n (%) | ||
| White | 180 (69.5) | 3412 (63.0) |
| Asian | 58 (22.4) | 1382 (25.5) |
| Black/African American | 9 (3.5) | 255 (4.7) |
| Duration of diabetes, yr, mean (SD) | 16.9 (8.9) | 16.6 (8.8)a |
| HbA1c, %, mean (SD) | 8.00 (1.20) | 7.66 (1.35)a |
| Systolic blood pressure, mm Hg, mean (SD) | 134.7 (14.4) | 138.2 (14.4)b |
| eGFR, ml/min per 1.73 m2, mean (SD) | 51.1 (11.9) | 44.0 (12.5)c |
| Distribution, n (%) | ||
| <25 | 0 | 135 (2.5) |
| 25–<45 | 76 (29.3) | 2905 (53.6) |
| 45–<60 | 125 (48.3) | 1775 (32.8) |
| ≥60 | 58 (22.4) | 598 (11.0) |
| UACR, mg/g, median (IQR) | 619 (370–1258) | 866 (456–1653)d |
| Distribution, n (%) | ||
| <30 | 0 | 23 (0.4) |
| 30 to <300 | 39 (15.1) | 646 (11.9) |
| ≥300 | 220 (84.9) | 4743 (87.6) |
| Serum potassium, mmol/l, mean (SD) | 4.28 (0.42) | 4.38 (0.46)c |
| Medication use at baseline, n (%) | ||
| ACEi | 77 (29.7) | 1865 (34.4) |
| ARB | 182 (70.3) | 3543 (65.4) |
| β-blocker | 137 (52.9) | 2831 (52.3) |
| Diuretic | 145 (56.0) | 3069 (56.7) |
| Statin | 223 (86.1) | 3992 (73.7) |
| Potassium-lowering agent | 5 (1.9) | 131 (2.4) |
| Glucose-lowering therapies | 259 (100) | 5265 (97.2) |
| Insulin and analogs | 173 (66.8) | 3464 (64.0) |
| GLP-1RA | 48 (18.5) | 346 (6.4) |
| SGLT-2i | 259 (100) | 0 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; IQR, interquartile range; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; UACR, urine albumin-to-creatinine ratio.
Letters indicate data missing for the stated number of patients: an = 11; bn = 5; cn = 2; dn = 3.
Baseline demographics and disease characteristics were generally similar between those who received an SGLT-2i at baseline and those who did not, with some notable exceptions (Table 1 and Supplementary Table S3). Baseline mean eGFR was higher and median UACR was lower in patients treated with an SGLT-2i at baseline compared with those who were not (eGFR was 51.1 ml/min per 1.73 m2 with an SGLT-2i vs. 44.0 ml/min per 1.73 m2 without an SGLT-2i, and UACR was 619 mg/g vs. 866 mg/g, respectively). There was a higher proportion of White patients in the SGLT-2i group, although no regional variations were observed (Supplementary Table S4). Baseline serum potassium levels and mean systolic blood pressure were slightly lower in patients who received an SGLT-2i at baseline compared with those who did not. A higher proportion of patients treated with an SGLT-2i at baseline also received a glucagon-like peptide-1 receptor agonist than those who did not receive an SGLT-2i at baseline (18.5% vs. 6.4%).
Efficacy
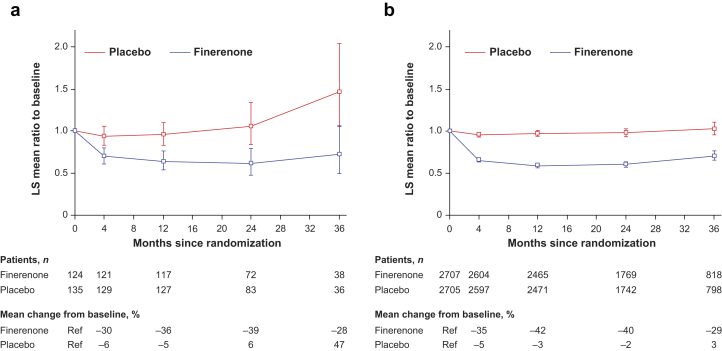
In the 5451 patients included in the UACR analysis, finerenone was associated with a 31% greater reduction in UACR from baseline to month 4 versus placebo (ratio of least-squares means = 0.69, 95% CI = 0.66–0.71).12 A similar reduction in UACR from baseline to month 4 was observed after treatment with finerenone in those who received an SGLT-2i at baseline and those who did not, with a 25% and a 31% reduction versus placebo, respectively (ratio of least-squares means = 0.75, 95% CI = 0.62–0.90 with an SGLT-2i and 0.69, 95% CI = 0.66–0.71 without an SGLT-2i, Pinteraction = 0.31). The lower mean UACR observed with finerenone compared with placebo at month 4 was maintained for the duration of the study with no apparent effect of SGLT-2i treatment at baseline (Figure 1).
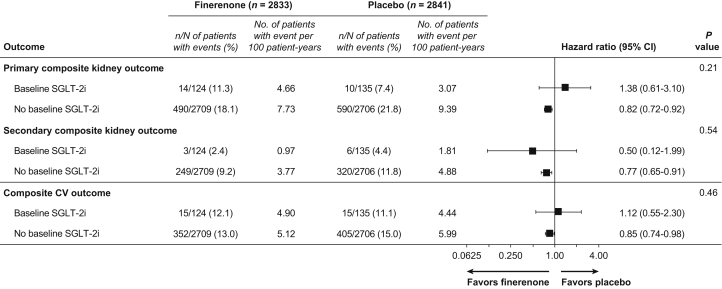
Figure 1.
Analysis of primary and secondary composite outcomes by baseline SGLT-2i use. CV, cardiovascular; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
Finerenone significantly reduced the primary composite kidney outcome and the key secondary cardiovascular composite outcome compared with placebo (HR = 0.82, 95% CI = 0.73–0.93 and HR = 0.86, 95% CI = 0.75–0.99, respectively) in the primary analysis.12 A total of 24 of 259 events (9.3%) were observed in patients who were treated with an SGLT-2i at baseline versus 1080 of 5415 events (19.9%) in patients who were not (Figure 1). The efficacy of finerenone compared with placebo seemed to be consistent for the primary composite kidney outcome (HR = 1.38, 95% CI = 0.61–3.10 with an SGLT-2i vs. HR = 0.82, 95% CI = 0.72–0.92 without an SGLT-2i, Pinteraction = 0.21; Figure 2). Similarly, the efficacy of finerenone compared with placebo seemed to be independent of SGLT-2i treatment at baseline for the key secondary composite cardiovascular outcome (HR = 1.12, 95% CI = 0.55–2.30 with an SGLT-2i vs. HR = 0.85, 95% CI = 0.74–0.98 without an SGLT-2i, Pinteraction = 0.46) and secondary composite kidney outcome (HR = 0.50, 95% CI = 0.12–1.99 with an SGLT-2i vs. HR = 0.77, 95% CI = 0.65–0.91 without an SGLT-2i, Pinteraction = 0.54; Figure 1). The event rates for these 3 outcomes were lower in patients who were treated with an SGLT-2i at baseline compared with those who were not, regardless of whether patients received finerenone or placebo (Figure 2).
Figure 2.
Effect on albuminuria over time by baseline SGLT-2i use. Mixed-model analysis of UACR levels in patients who were (a) with or (b) without an SGLT-2i at baseline. Analysis included the following covariates: treatment group, stratification factors (region, albuminuria category at screening, eGFR category at screening), time, treatment over time, log-transformed baseline value nested within type of albuminuria at screening, and log-transformed baseline value over time. The change in UACR at month 4 was consistent irrespective of treatment with an SGLT-2i at baseline (Pinteraction = 0.31). eGFR, estimated glomerular filtration rate; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; LS, least-squares; UACR, urine albumin-to-creatinine ratio.
The effect of SGLT-2i use at any time during the trial was also investigated. Finerenone benefit for either the primary composite kidney or key secondary composite cardiovascular outcome seemed to be unaffected by SGLT-2i use at any time (Pinteraction = 0.83 and 0.26, respectively; Supplementary Figures S1 and S2).
Safety Outcomes and Vital Signs
Incidence of any treatment-emergent adverse event (AE) or treatment-emergent serious AE seemed similar with finerenone and placebo in patients who were treated with an SGLT-2i at baseline and in those who were not (Table 2). The incidence of hypoglycemia tended to be lower in patients treated with finerenone (5.3% for finerenone and 6.9% for placebo) and in patients who were treated with an SGLT-2i at baseline (4.2% with SGLT-2is and 6.2% without SGLT-2is; Supplementary Table S5). The numbers of acute kidney injury and hypovolemia events were low (<5% of patients who were treated with finerenone or placebo, with or without an SGLT-2i).
Table 2.
Overall safety, treatment-emergent hyperkalemia-related events, and central laboratory assessments by baseline SGLT-2i use
| Event | SGLT-2i at baseline |
No SGLT-2i at baseline |
||
|---|---|---|---|---|
| Finerenone (n = 124) |
Placebo (n = 135) |
Finerenone (n = 2703) |
Placebo (n = 2696) |
|
| n (%) | n (%) | n (%) | n (%) | |
| Any AE | 113 (91.1) | 117 (86.7) | 2355 (87.1) | 2361 (87.6) |
| Related to study drug | 25 (20.2) | 15 (11.1) | 621 (23.0) | 434 (16.1) |
| Leading to discontinuation | 5 (4.0) | 7 (5.2) | 202 (7.5) | 161 (6.0) |
| Any serious AE | 39 (31.5) | 40 (29.6) | 863 (31.9) | 931 (34.5) |
| Related to study drug | 1 (0.8) | 0 | 47 (1.7) | 34 (1.3) |
| Leading to discontinuation | 4 (3.2) | 1 (0.7) | 71 (2.6) | 77 (2.9) |
| AE with outcome death | 1 (0.8) | 2 (1.5) | 30 (1.1) | 49 (1.8) |
| Hyperkalemia-related events | ||||
| Any AE | 10 (8.1) | 4 (3.0) | 506 (18.7) | 251 (9.3) |
| Related to study drug | 5 (4.0) | 3 (2.2) | 328 (12.1) | 132 (4.9) |
| Leading to discontinuation | 1 (0.8) | 1 (0.7) | 63 (2.3) | 24 (0.9) |
| Any serious AE | 1 (0.8) | 0 | 43 (1.6) | 12 (0.4) |
| Related to study drug | 1 (0.8) | 0 | 25 (0.9) | 5 (0.2) |
| Leading to discontinuation | 1 (0.8) | 0 | 4 (0.1) | 1 (<0.1) |
| Reported as life threatening | 0 | 0 | 3 (0.1) | 3 (0.1) |
| Leading to hospitalization | 1 (0.8) | 0 | 39 (1.4) | 8 (0.3) |
| Central laboratory assessments | ||||
| Treatment-emergent serum potassium > 6.0 mmol/l | 0 | 0 | 126 (4.7) | 38 (1.4) |
| Treatment-emergent serum potassium > 5.5 mmol/l | 8 (6.5) | 4 (3.0) | 589 (21.8) | 252 (9.3) |
AE, adverse event; SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
The numerical incidence of treatment-emergent hyperkalemia-related AEs was higher in patients receiving finerenone compared with placebo, with an approximate 2-fold increase with finerenone. The overall rates of treatment-emergent hyperkalemia-related AEs were numerically lower in patients receiving an SGLT-2i at baseline (8.1% for finerenone and 3.0% for placebo) than in patients not receiving an SGLT-2i at baseline (18.7% for finerenone and 9.3% for placebo; Table 2). Most treatment-emergent hyperkalemia-related events were mild to moderate in severity, and few serious events led to treatment discontinuation (n = 6; 1 with an SGLT-2i [0.4%] and 5 without an SGLT-2i [<0.1%]; Table 2). When considering SGLT-2i use at any time during the trial, there seemed to be no significant difference in the risk of hyperkalemia that was observed owing to the use of an SGLT-2i (Pinteraction = 0.61).
Finerenone had a modest effect on systolic blood pressure in patients without an SGLT-2i at baseline; the least-square mean change from baseline to month 12 was −0.94 and 1.12 mm Hg for finerenone and placebo, respectively, in patients with an SGLT-2i at baseline, and −2.12 and 0.84 mm Hg in patients without an SGLT-2i at baseline (Supplementary Figure S3).
Discussion
In the FIDELIO-DKD study, finerenone was associated with a 31% greater reduction in UACR from baseline to month 4 versus placebo. In this prespecified exploratory subanalysis, a similar reduction in UACR after treatment with finerenone from baseline to month 4 was observed in patients who received an SGLT-2i at baseline and in patients who did not, and the reduction also seemed to be independent of SGLT-2i use at baseline. The analyses performed for UACR in this study had greater statistical power than those for the kidney composite and cardiovascular composite outcomes, given that UACR was measured in all available patients at specific time points throughout the study and data analysis did not depend on the occurrence of a clinical end point. The assessment of UACR at month 4 was also close to the start of the trial, which was more reflective of the effect of concomitant use of finerenone and an SGLT-2i at baseline before changes in other medications occurred. The reduction in UACR from baseline to month 4 with finerenone seemed to be consistent irrespective of SGLT-2i use at baseline.
The data reveal that finerenone improved UACR reduction by 25% in patients who were already receiving an SGLT-2i, supporting the efficacy of finerenone in patients already being treated with a drug known to reduce UACR. In 2018, a workshop led by the National Kidney Foundation evaluated whether changes in albuminuria or eGFR could be surrogate end points for kidney disease progression, and it was concluded that a UACR reduction of 21% to 27% is predictive of a benefit in clinical outcome in patients with moderately or severely increased albuminuria.15 In addition, a recent post hoc analysis of the CREDENCE trial revealed that each 30% UACR reduction over the first 26 weeks of canagliflozin treatment was independently associated with a lower risk of cardiorenal events; and residual albuminuria after 26 weeks of canagliflozin therapy was associated with similarly poor outcomes to patients who received placebo at baseline.16 These findings underscore the likelihood that any therapies that confer further lowering of UACR on top of that from SGLT-2is, as is the case with finerenone, are likely to provide additional kidney and cardiovascular benefits beyond those of SGLT-2is alone.
A reduction in UACR of 31% from baseline has also been observed with canagliflozin compared with placebo in the CREDENCE trial over 6 to 42 months10; changes in UACR over time are yet to be reported for DAPA-CKD.11 Finerenone improves albuminuria on top of treatment with an SGLT-2i; therefore, it is reasonable to hypothesize that these agents have complementary effects because of their different mechanisms of action. Data regarding combination therapy with an SGLT-2i and a nonsteroidal MR antagonist in CKD in T2D have not been reported previously. Nevertheless, the benefits of SGLT-2is in patients with heart failure and reduced ejection fraction in the DAPA-HF study and the EMPEROR-Reduced trial were observed on top of concomitant treatment with a steroidal MR antagonist without any attenuation of response.17,18 In FIDELIO-DKD, patients with a clinical diagnosis of chronic heart failure with reduced ejection fraction and persistent symptoms at the run-in visit were excluded from the trial, which limits the ability to compare these results with findings from DAPA-HF and EMPEROR-Reduced.12
In the FIDELIO-DKD primary analysis, finerenone significantly reduced the primary composite kidney outcome and the key secondary cardiovascular composite outcome compared with placebo in patients with CKD and T2D receiving optimized RAS inhibitor therapy.12 This analysis revealed that these benefits of finerenone did not seem to be modified by use of a guideline-recommended SGLT-2i in addition to optimized RAS inhibitor therapy at baseline. Initiation of SGLT-2is during the trial was similar between treatment arms (153 of 2833 [5.4%] for finerenone and 175 of 2841 [6.2%] for placebo), suggesting an increased use of SGLT-2is in the placebo arm was unlikely to confound the results.
It should be noted that recruitment for FIDELIO-DKD was completed close to guideline updates that recommended the use of SGLT-2is to reduce the risk of cardiovascular and kidney events.4, 5, 6 This is likely to have contributed to the low level of SGLT-2i use in this patient population. Furthermore, use of SGLT-2is to reduce cardiorenal risk was not licensed in many countries at the time of study initiation and the approved indication was for treatment initiation in patients with a higher eGFR level than the recommended guidelines (≥60 ml/min per 1.73 m2 vs. ≥30 ml/min per 1.73 m2, respectively).4, 5, 6 In FIDELIO-DKD, only approximately 12% of patients met the eGFR criteria for initiation of an SGLT-2i, and 54% of patients did not have a history of cardiovascular disease at baseline.19 In the course of FIDELIO-DKD, there was an approximately 2.5% increase in the use of SGLT-2is in this patient population, potentially reflecting an increased uptake owing to changes made to treatment recommendations while the trial was ongoing.
Patients who were treated with an SGLT-2i at baseline had a higher mean eGFR and a lower median UACR, indicative of a lower level of kidney disease, which is likely reflected in the low kidney event rate observed in the trial. These characteristics were expected because SGLT-2is were not recommended for use in patients with an eGFR <45 ml/min per 1.73 m2 at the time of trial recruitment (with the exception of dapagliflozin, which was not recommended in patients with an eGFR <60 ml/min per 1.73 m2). More than 70% of patients who received an SGLT-2i at baseline had an eGFR >45 ml/min per 1.73 m2, compared with 44% in patients not receiving an SGLT-2i. The low kidney event rate observed in these patients might also be partly attributed to the kidney-protective effect of an SGLT-2i. The relatively small number of patients taking an SGLT-2i at baseline and during the trial (∼4.5% and ∼10.5% of the overall trial population, respectively) with few kidney and cardiovascular events observed in this group makes it difficult to interpret the data from the primary and secondary composite end points in this subgroup. Nevertheless, there was no indication of treatment heterogeneity between the patients treated with an SGLT-2i and without an SGLT-2i based on the P values for interaction. Furthermore, the efficacy of finerenone seemed to be consistent in patients treated with an SGLT-2i at any time during the study.
Hyperkalemia is a major complication of CKD, and its incidence is increased in patients who have diabetes, including in those who are treated with RAS inhibitor therapy.20 Finerenone was found to have a lower incidence of hyperkalemia compared with the steroidal MR antagonist spironolactone.21 In FIDELIO-DKD, incidence of hyperkalemia was higher with finerenone versus placebo (∼2-fold higher with finerenone in patients with or without SGLT-2i use at baseline), but most cases were mild or moderate and few patients discontinued treatment as a result. Although data are limited and should be interpreted with caution, the rates of treatment-emergent hyperkalemia-related AEs in FIDELIO-DKD were numerically lower in patients receiving an SGLT-2i at baseline than in patients not receiving an SGLT-2i at baseline. In DAPA-HF, the rate of hyperkalemia in patients with heart failure was also numerically lower with dapagliflozin in those who were also receiving an MR antagonist (although Pinteraction = 0.08 did not reach the threshold for significance).18 In the CREDENCE trial, investigator-reported hyperkalemia was numerically lower (HR = 0.80, 95% CI = 0.65–1.00) with canagliflozin versus placebo.10 Furthermore, in the CREDENCE trial, serious hyperkalemia (serum potassium ≥6.0 mmol/l) occurred less frequently with canagliflozin versus placebo, with fewer investigator-reported hyperkalemia events.22 Similar outcomes were also observed in the EMPA-REG OUTCOME study of empagliflozin versus placebo in patients with T2D and established cardiovascular disease, including those with concomitant CKD.23 We also observed that baseline mean serum potassium levels were lower in patients who received an SGLT-2i at baseline than those who did not. Therefore, SGLT-2is may protect against hyperkalemia through increased potassium excretion because of natriuresis, osmotic diuresis, and improved kidney function.24
Our findings of numerically lower rates of hyperkalemia-related AEs, together with an apparent reduction in UACR in patients receiving both finerenone and an SGLT-2i, suggest that this combination may provide additional benefits above and beyond those associated with each individual therapy, although further data are needed to verify these findings. A subgroup analysis of patients according to SGLT-2i treatment in the ongoing finerenone study FIGARO-DKD13 and dedicated combination studies may substantiate these findings in the future. It is important to note that the proportion of patients in FIGARO-DKD who received an SGLT-2i at baseline was higher than those in FIDELIO-DKD (∼8.3% vs. 4.6%).12,13 The possibility of independent (or even synergistic) effects with the combination of finerenone and an SGLT-2i is supported by their complementary mechanisms of action. The kidney-protective and cardioprotective effects of SGLT-2is are believed to be primarily owing to their glucosuric and hemodynamic effects.25,26 In addition, alternative mechanisms of action for renal benefit with SGLT-2is have been proposed, such as indirect effects on inflammation, the sympathetic nervous system, the vascular system, obesity, and the heart.27 In contrast, finerenone has no effect on blood glucose, and its kidney-protective effect is driven by its anti-inflammatory and antifibrotic mode of action, translated from preclinical models, in concert with natriuresis.28,29 Preclinical data support the hypothesis that complementary mechanisms of action between finerenone and SGLT-2is may be beneficial for kidney protection. Indeed, in a nondiabetic cardiorenal rat model, the combination of finerenone and an SGLT-2i was associated with an early, significant, and sustained reduction in albuminuria that was greater than the sum of the individual effects of each respective monotherapy (Kolkhof P, Pavkovic M, Hartmann E, et al. American Society of Nephrology. Virtual, 19–25 October 2020, Abstract P00642). Combination treatment was also associated with an improvement in survival in this preclinical model.
This analysis has certain limitations. Only a small number of patients in FIDELIO-DKD received an SGLT-2i at baseline, and the imbalance in baseline eGFR and UACR between patients who received an SGLT-2i at baseline versus those who did not increased the potential for bias. Furthermore, analyses by SGLT-2i subgroups during the trial were not defined until after randomization had taken place and, therefore, are nonrandomized. An imbalance in glucagon-like peptide-1 receptor agonist treatment between patients with and without an SGLT-2i at baseline (∼19% vs. ∼6%, respectively) may also have confounded the results observed owing to the known UACR-lowering effects of these agents.30, 31, 32
In conclusion, the addition of finerenone to an SGLT-2i may offer clinically relevant improvement in albuminuria compared with SGLT-2i alone in patients with predominantly advanced CKD and T2D receiving optimized RAS inhibitor therapy. Furthermore, in the FIDELIO-DKD study, finerenone reduced the risk of kidney disease progression and cardiovascular events compared with placebo, and this effect seemed to be independent of whether patients received an SGLT-2i or not. The combination of finerenone on top of an SGLT-2i may also lower the risk for hyperkalemia with finerenone. Additional studies are needed to further substantiate these findings.
Disclosure
PR reports receiving personal fees from Bayer during the conduct of the study; research support and personal fees from AstraZeneca and Novo Nordisk; and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi, and Vifor. All fees are given to Steno Diabetes Center Copenhagen. GF reports receiving lecture fees and/or serving as a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor; being a Senior Consulting Editor for JACC Heart Failure; and receiving research support from the European Union. RA reports receiving personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals Inc. during the conduct of the study; receiving personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Fresenius, Janssen, Relypsa, Sanofi, and Vifor Pharma; receiving personal fees from Ironwood Pharmaceuticals, Lexicon, Merck & Co., and Reata; receiving nonfinancial support from E. R. Squibb & Sons, OPKO Pharmaceuticals, and Otsuka America Pharmaceutical; serving as a member of data safety monitoring committees for Amgen, AstraZeneca, and Celgene; serving as a member of steering committees of randomized trials for Akebia Therapeutics, Bayer, Janssen, and Relypsa; serving as a member of adjudication committees for AbbVie, Bayer, Boehringer Ingelheim, and Janssen; serving as associate editor for the American Journal of Nephrology and Nephrology Dialysis and Transplantation and an author for UpToDate; and receiving research grants from the US Veterans Administration and the National Institutes of Health. SDA reports receiving research support from Abbott Vascular and Vifor International and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier, and Vifor International. BP reports receiving consultant fees from Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; having stock options for Ardelyx, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, and Vifor/Relypsa; and having a patent for site-specific delivery of eplerenone to the myocardium (US patent #9931412) and a provisional patent for histone acetylation-modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045,784). LMR reports receiving consultancy fees from Bayer. JCNC reports receiving grants and/or honoraria for consultancy or giving lectures from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Hua Medicine, Lee Powder, Merck Serono, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier. AK reports receiving research funding paid to the Bethesda Diabetes Research Center Netherlands, from AstraZeneca, Merck Sharpe & Dohme, Novo Nordisk, and Sanofi Aventis. KM reports receiving research funding from AstraZeneca and consultancy/speaker honoraria from AstraZeneca, Bayer, Napp, Oncacare, Pharmacosmos, and Vifor Fresenius. GS reports receiving honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Mundipharma, and Takeda. CW reports receiving advisory board and lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, MSD, and Mundipharma. AJ and MFS report being full-time employees of Bayer AG, Germany. MFS is also a shareholder in AstraZeneca, Bayer, Eli Lilly, and Novo Nordisk. CS reports being a full-time employee of Bayer PLC, United Kingdom. GLB reports receiving research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study; receiving research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; acting as a consultant and received personal fees from for Alnylam, Merck, and Relypsa; serving as an editor for the American Journal of Nephrology, Nephrology, and Hypertension, and Section Editor for UpToDate; and serving as an associate editor for Diabetes Care and Hypertension Research.
Acknowledgments
The authors are indebted to the patients who have participated in this trial, the FIDELIO-DKD trial investigators, and the study centers that supported the trial. The FIDELIO-DKD study and subanalyses were funded by Bayer AG. Medical writing assistance was provided by Kate Weatherall, PhD, on behalf of Chameleon Communications International, and was funded by Bayer AG.
Author Contributions
The Executive Committee designed the study in conjunction with the sponsor. PR wrote the first draft of the report. All authors were involved in data analysis and interpretation and in drafting and critically revising the report. All authors had access to study results and the first and corresponding authors assume responsibility for the integrity and accuracy of the data reported. All authors reviewed and approved the final submitted version of the report.
DATA AVAILABILITY
The data supporting the findings of this study are not currently available; they will be openly available in an electronic repository at a date to be confirmed by Bayer AG.
Footnotes
Table S1. Change in SGLT-2i use during the FIDELIO-DKD study.
Table S2. Type of SGLT-2i administered throughout FIDELIO-DKD.
Table S3. Baseline characteristics according to treatment and by baseline SGLT-2i use.
Table S4. SGLT-2i use at baseline according to region in the FIDELIO-DKD study.
Table S5. Treatment-emergent adverse events affecting >5% of patients in any treatment group.
Figure S1. Analysis of the primary composite outcome considering the effect of SGLT-2i use at any time during FIDELIO-DKD.
Figure S2. Analysis of the key secondary CV composite outcome. considering the effect of SGLT-2i use at any time during FIDELIO-DKD.
Figure S3. Change in SBP over time by baseline SGLT-2i use.
STROBE Statement.
Supplementary Material
Table S1. Change in SGLT-2i use during the FIDELIO-DKD study.
Table S2. Type of SGLT-2i administered throughout FIDELIO-DKD.
Table S3. Baseline characteristics according to treatment and by baseline SGLT-2i use.
Table S4. SGLT-2i use at baseline according to region in the FIDELIO-DKD study.
Table S5. Treatment-emergent adverse events affecting >5% of patients in any treatment group.
Figure S1. Analysis of the primary composite outcome considering the effect of SGLT-2i use at any time during FIDELIO-DKD.
Figure S2. Analysis of the key secondary CV composite outcome. considering the effect of SGLT-2i use at any time during FIDELIO-DKD.
Figure S3. Change in SBP over time by baseline SGLT-2i use.
STROBE Statement (PDF)
Supplementary information is available at KI Report's website.
References
- 1.Incidence, prevalence, patient characteristics, and treatment modalities United States Renal Data System. Published 2020. Accessed 13 April 2021. https://adr.usrds.org/2020/end-stage-renal-disease/1-incidence-prevalence-patient-characteristics-and-treatment-modalities
- 2.Wu B., Bell K., Stanford A., et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007–2012. BMJ Open Diabetes Res Care. 2016;4 doi: 10.1136/bmjdrc-2015-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piperidou A., Loutradis C., Sarafidis P. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens. 2021;35:12–25. doi: 10.1038/s41371-020-00393-4. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(suppl 1):S1–S244. doi: 10.2337/dc21-Sdis. [DOI] [PubMed] [Google Scholar]
- 5.Buse J.B., Wexler D.J., Tsapas A., et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018 A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [published correction appears in Diabetologia. 2020;63:1667] Diabetologia. 2020;63:221–228. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) diabetes work group KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 8.American Diabetes Association Standards of medical care in diabetes-2015. Diabetes Care. 2015;38(suppl):S1–S94. [Google Scholar]
- 9.Cosentino F., Grant P.J., Aboyans V., et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD [published correction appears in Eur Heart J. 2020;41:4317] Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 11.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 12.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 13.Ruilope L.M., Agarwal R., Anker S.D., et al. Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am J Nephrol. 2019;50:345–356. doi: 10.1159/000503712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakris G.L., Agarwal R., Anker S.D., et al. Design and baseline characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease trial. Am J Nephrol. 2019;50:333–344. doi: 10.1159/000503713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Gansevoort R.T., Coresh J., et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75:84–104. doi: 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Oshima M., Neuen B.L., Li J., et al. Early change in albuminuria with canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE trial. J Am Soc Nephrol. 2020;31:2925–2936. doi: 10.1681/ASN.2020050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira J.P., Zannad F., Pocock S.J., et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-Reduced. J Am Coll Cardiol. 2021;77:1397–1407. doi: 10.1016/j.jacc.2021.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Shen L., Kristensen S.L., Bengtsson O., et al. Dapagliflozin in HFrEF patients treated with mineralocorticoid receptor antagonists: an analysis of DAPA-HF. JACC Heart Fail. 2021;9:254–264. doi: 10.1016/j.jchf.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Filippatos G., Anker S.D., Agarwal R., et al. Finerenone and cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes. Circulation. 2021;143:540–552. doi: 10.1161/CIRCULATIONAHA.120.051898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Nicola L., Di Lullo L., Paoletti E., et al. Chronic hyperkalemia in non-dialysis CKD: controversial issues in nephrology practice. J Nephrol. 2018;31:653–664. doi: 10.1007/s40620-018-0502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitt B., Kober L., Ponikowski P., et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuen BL, Oshima M, Perkovic V, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. Published online August 23, 2021. https://doi.org/10.1093/eurheartj/ehab497 [DOI] [PubMed]
- 23.Herrington W.G., Preiss D., Haynes R., et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study [published correction appears in Clin Kidney J. 2019;13:722] Clin Kidney J. 2018;11:749–761. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippatos T.D., Tsimihodimos V., Liamis G., Elisaf M.S. SGLT2 inhibitors-induced electrolyte abnormalities: an analysis of the associated mechanisms. Diabetes Metab Syndr. 2018;12:59–63. doi: 10.1016/j.dsx.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Heerspink H.J., Perkins B.A., Fitchett DH Husain M., Cherney D.Z. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 26.Cherney D.Z.I., Zinman B., Inzucchi S.E., et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 27.Margonato D., Galati G., Mazzetti S., et al. Renal protection: a leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail Rev. 2020;26:337–345. doi: 10.1007/s10741-020-10024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolkhof P., Delbeck M., Kretschmer A., et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64:69–78. doi: 10.1097/FJC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 29.Kolkhof P., Jaisser F., Kim S.Y., Filippatos G., Nowack C., Pitt B. Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol. 2017;243:271–305. doi: 10.1007/164_2016_76. [DOI] [PubMed] [Google Scholar]
- 30.Mann J.F.E., Orsted D.D., Brown-Frandsen K., et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 31.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle K.R., Lakshmanan M.C., Rayner B., et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605–617. doi: 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are not currently available; they will be openly available in an electronic repository at a date to be confirmed by Bayer AG.