Abstract
Introduction
Self-management is an integral component of CKD treatment. Nevertheless, many patients with CKD do not adequately engage in self-management behaviors, and little is known on the underlying reasons. We aimed to identify and describe the factors that influence self-management behaviors from the perspective of adults with CKD.
Methods
We conducted 30 semistructured interviews with adults with CKD stage 3 or 4 from an academic nephrology clinic in the United States. Interviews were analyzed thematically.
Results
The following are the 3 key phases of CKD self-management behavior engagement identified: (i) prioritization, (ii) performance, and (iii) maintenance. Prioritization was favorably influenced by optimism, stress management, and patient-provider communication and hampered by fatalism and competing priorities. Behavior performance was facilitated by motivating factors, self-efficacy, and support resources and impeded by comorbid conditions that caused treatment burden and adverse symptoms. Behavior maintenance relied on effective routines, influenced by similar factors as behavior performance, and reinforced by memory aids, goal setting, self-monitoring, and proactive preparation.
Conclusion
We identified modifiable facilitators and barriers that influence the incorporation of CKD self-management into daily life. Our findings have important implications for the care of patients with CKD by providing a framework for providers to develop effective, tailored approaches to promote self-management engagement.
Keywords: chronic kidney disease, health behavior, qualitative, self-care, self-efficacy, self-management
CKD affects close to 10% of the world’s population and is associated with high risks of cardiovascular disease and death.1 Although there are specific treatments for some causes of CKD, for many patients, CKD management consists principally of control of blood pressure (BP), blood glucose, volume status, and metabolic complications. This requires engagement by patients in self-management activities, such as monitoring BP and blood glucose, maintaining physical activity, and adhering to medications and dietary restrictions. Current CKD management guidelines consider promotion of self-management behaviors as standards of care in the effort to slow CKD progression and prevent its complications.2, 3, 4, 5, 6, 7
Inadequate engagement in self-management behaviors has been well-documented for many patients with CKD,8,9 but the underlying reasons are not well understood.10, 11, 12 Improving CKD self-management and developing effective support strategies require greater awareness of the patient’s daily lived experience. Qualitative research provides an opportunity to learn on how adults with CKD manage their health at home and allow for exploration of a full range of responses without the limitations set by questionnaires or survey instruments. Although qualitative studies evaluating self-management in kidney disease have been conducted, they have been restricted to patients with advanced CKD, including those with end-stage kidney disease and older age, or focusing only on certain behaviors (dietary restrictions, medication taking, and exercise/physical activity) mainly in isolation.12, 13, 14, 15, 16, 17, 18, 19, 20, 21
With the ultimate goal of developing interventions to improve CKD outcomes, we sought to identify facilitators and barriers for multiple self-management behaviors among middle- to older-aged adults with stage 3 or 4 CKD through a qualitative study using semistructured interviews. Patients were asked to describe their experiences living with CKD, including their efforts to adhere to treatment recommendations and other health-related and well-being activities.
Methods
Study Design
We conducted in-depth semistructured interviews and adhered to the Consolidated Criteria for Reporting Qualitative Research that was developed to promote explicit and comprehensive reporting of interviews and focus groups (see Supplementary Material for the COREQ checklist).22
Participant Selection
This study enrolled patients with CKD stage 3 or 4 from 2 outpatient nephrology clinics with 7 and 20 nephrologists, respectively, at a single, urban academic medical center in the United States. Patients had to be English speaking, aged ≥18 years, have a follow-up visit with a nephrologist, and be cognitively able to participate in an independent interview. We reviewed charts of patients scheduled for upcoming visits and purposively sampled to provide a mix of age (<65 and ≥65 years), sex, and racial backgrounds. With the permission of the treating nephrologist, an invitation letter was mailed to eligible patients 1 to 2 weeks before they were scheduled for an appointment, along with a follow-up telephone call. Patients who agreed to participate were scheduled for an interview on the same day as their clinic appointment between May 2019 and August 2019. The study protocol was approved by the institutional review board at the University of Pennsylvania. Verbal informed consent was obtained. Written consent was waived. A $25 gift card was provided to all participants.
Data Collection
We developed an interview guide to elicit participants’ perceptions of CKD, management of CKD at home, and supports related to self-management. The interview questions were primarily guided by the recommendations in CKD management guidelines,23 including prespecified topics related to experiences with medication adherence, BP monitoring, dietary restrictions, physical activity participation, and appointment attendance, and with the goal of identifying factors that facilitate or impede participation in these behaviors (see Supplementary Material for the Interview Question Guide). All questions were open-ended. Standardized prompts and probes were used to elicit more details or seek clarification. The interviews were conducted in a private space within the clinics and lasted 30 to 60 minutes. To reduce bias, patients receiving care from the interviewer (SJS), a physician trained in qualitative research, were not enrolled. Participants self-reported sociodemographic information (age, sex, race/ethnicity, marital status, education level, employment status, annual income) and duration of CKD diagnosis using a survey. All interviews were audio recorded and transcribed verbatim. Deidentified transcripts were entered into QSR NVivo 12 (QSR International Pty Ltd., Victoria, Australia).
Data Analysis
We used thematic analysis to identify facilitators and barriers to engagement in CKD self-management behaviors derived from text within the transcripts.24 We performed inductive coding to identify themes without an a priori theoretical perspective. A codebook was developed by 2 trained individuals (SJS, ER) who evaluated the transcripts line-by-line to identify each coherent idea. The codebook was applied to all transcripts by SJS and ER, using the interrater reliability function in NVivo to ascertain interrater reliability. Through analysis and comparisons, the codes were grouped into conceptual categories based on descriptive themes from the data, and saturation was determined based on discussions among researchers (SJS, ER, CB). After identifying the final set of descriptive themes, we considered the fit of themes and codes with frequently used and widely accepted health behavior frameworks of behavior engagement, including self-regulation theory,25 integrated behavioral model,26 theory of reasoned action,27 and theory of planned behavior.28 These results were then triangulated with investigators from different disciplines (SA, FB). Member checking was conducted with 2 patient participants. Descriptive statistics (mean, median, and frequency) were used to report demographic characteristics of the study population.
We compared the number of individuals who expressed each facilitator and barrier across the categories of age (<65 and ≥65 years), sex (male, female), race (white or minority), education (<high school or ≥high school), marital status (married vs. other), CKD stage 3 or 4, and duration of CKD diagnosis awareness (1–5 or >5 years) to explore for differences in the responses of the participants. Descriptive statistics (mean, median and frequency) were used to report demographic characteristics using STATA software version 16.1 (StataCorp, College Station, TX).
Results
Invitation letters were sent to 179 patients, and on follow-up recruitment phone calls, 2 declined and 34 patients agreed to participate, with 30 ultimately interviewed between May 2019 and August 2019. Of those who volunteered but were not interviewed, 1 did not attend the scheduled appointment and 3 could not be scheduled owing to conflicts. Participant characteristics are found in Table 1.
Table 1.
Characteristics of interview participants (N = 30)
| Characteristics | Median (range) or n (%) |
|---|---|
| Age, yr | 64 (33–91) |
| Female | 15 (50.0) |
| Race | |
| White | 14 (46.7) |
| Black | 13 (43.3) |
| Hispanic | 2 (6.7) |
| Other | 1 (3.3) |
| Marital status | |
| Single | 10 (33.3) |
| Married | 13 (43.3) |
| Divorced | 3 (10.0) |
| Widowed | 4 (13.3) |
| Educationa | |
| Less than high school | 2 (7.1) |
| High school or GED | 9 (32.1) |
| Some college | 8 (28.6) |
| 4-yr college degree or more | 9 (32.1) |
| Employment statusa | |
| Employed | 6 (20.7) |
| Unemployed | 1 (3.5) |
| Retired | 13 (44.8) |
| Disabled/not able to work | 9 (31.0) |
| Yearly household income, $ | |
| <20,000 | 5 (16.7) |
| 20,000 to <40,000 | 9 (30.0) |
| 40,000 to <60,000 | 3 (10.0) |
| ≥60,000 | 5 (16.7) |
| Did not report | 8 (26.7) |
| CKD stage | |
| Stage 3 | 15 (50.0) |
| Stage 4 | 15 (50.0) |
| Known CKD diagnosis, yr | 5 (1–51) |
| 1–5 | 16 (53.3) |
| >5 to 10 | 8 (26.7) |
| >10 | 6 (20.0) |
CKD, chronic kidney disease; GED, general educational development.
Missing data for education (n = 2) and employment (n = 1).
Thematic Framework
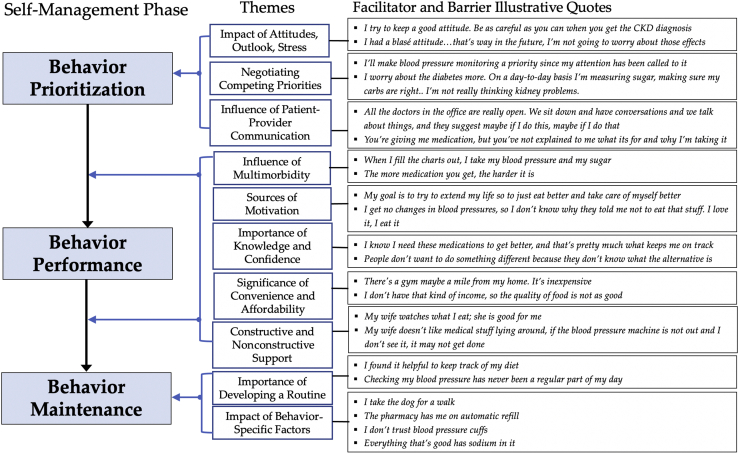
From the final set of descriptive themes, we identified 3 main elements that were consistently described by patients on their behavior engagement—prioritization, performance, and maintenance—which mirrored self-regulation theory, a frequently used theoretical perspective of understanding behavior change.29 The process of self-regulation focuses on the attainment and maintenance of personal goals and is distinguished by the following: (i) goal selection, (ii) active goal pursuit, and (iii) goal attainment and maintenance.25 We describe the facilitators and barriers in-depth according to the 3 phases corresponding to self-regulation—behavior prioritization, performance, and maintenance—and provide illustrative quotes for each theme (Figure 1).
Figure 1.
Phases of self-management behavior engagement in CKD along with supporting themes and illustrative quotes of facilitators and barriers corresponding to each theme. CKD, chronic kidney disease.
Behavior Prioritization
Impact of Attitudes, Outlook, and Stress
Keeping a “good attitude” and optimism seemed to affect patients’ prioritization of desirable health behaviors. Some participants reported using meditation to manage stress and BP by “putting myself in the zone” and for supporting general well-being. Others felt a sense of impotence on being able to affect CKD and reported worry, dissatisfaction, stress, and having a “really bad attitude,” which impeded prioritization of desirable behaviors.
Negotiating Competing Priorities
Participants described the ways in which different priorities in their life, including “schoolwork” and “going to the gym,” and day-to-day choices influenced and competed with their decisions to engage in health behaviors. Many participants explicitly noted that a given behavior was a priority and important for management of CKD in saying “that’s important for kidney disease, watch your diet,” but others noted that competing priorities did not leave them time to perform the recommended behavior (e.g., serving as primary caretaker of an ill family member). Others shared that “I’m not really thinking kidney problems” because it was felt to have less of an effect on their daily life, as some worried more on cancer treatment or “more about the diabetes.” Other participants felt that a particular behavior was just not important in their lives and did not “care who tells me whatever.”
Influence of Patient-Provider Communication
Many participants cited examples of how both effective and ineffective patient-provider communication affected their decisions to engage in health-promoting behaviors for their CKD. Effective patient-provider communication was described “really open.” Ineffective patient-provider communication centered on participants feeling that they had not received adequate education on behaviors “they are not explaining to me what its for” nor encouragement to engage in the behaviors in sharing that “I’ve asked, ‘is there anything else I should do’ but my nephrologist says not really. So, I don’t know there’s nothing that I have to do.”
The following 5 themes tracked with both Behavior Performance and Behavior Maintenance (Figure 1).
Influence of Multimorbidity
Multimorbidity, which caused treatment burden, including “more medication” and adverse symptoms, was one of the most widely reported impediments to behavior performance. Some participants discussed on how symptoms such as shortness of breath, pain, fatigue, and having “half my front right foot removed” negatively affected their ability to adhere to certain treatment recommendations, particularly attending doctors’ appointments and participating in physical activity. Burdensome, complex treatment plans for comorbid conditions “puzzled” participants and led to feelings that the recommendations had “opposite” goals, particularly related to diet and medications, and navigating the prioritization of treatments for different diseases also created barriers. Concomitant “depressed mood” and anxiety typically reduced desire to engage in behaviors. Compatible treatment plans for coexisting conditions facilitated both self-management behavior performance and maintenance, such as self-monitoring and “writing down” BP and blood sugar measurements at the same time.
Sources of Motivation
The expectation of a positive outcome motivated many participants (“benefits-based motivation”), such as improved kidney function and “to extend my life a little longer.” Others were motivated by the avoidance of an undesired outcome (“consequences-based motivation”), most often to “keep from going on dialysis.” Lastly, worrying symptoms motivated some to think on behaviors (“symptoms as cue”), such as “my ankles will swell” if they have eaten too much salt or “I can tell right away” if they missed taking BP medications.
A lack of motivation served as a barrier to engaging in self-management behaviors. Participants expressed feeling “lazy” to adhere to behavior recommendations despite knowing that they would be health promoting. The absence of negative consequences to inadequate self-management engagement, such as “I don’t get no changes in my body and blood pressure levels,” reinforced behavior nonparticipation.
Importance of Knowledge and Confidence
Confidence (or self-efficacy) facilitated engagement in behaviors. Participants who felt self-confident and “thinking I can be in control of this” or reported receiving “more information, the better you feel” seemed to have greater capacity to carry out the behaviors. Another facilitator was the act of seeking out health information through patient-provider interactions or on their own by “Googling it” or “reading all the pamphlets.” Frequently, this motivation came on because participants perceived they received inadequate information from their health care providers by sharing that “no one’s actually told me ‘you need to eat this, you need to eat that’ and “if the blood pressure reading is way high, what am I gonna do?” Some participants shared that insufficient understanding on health behaviors for CKD management made them feel uncomfortable engaging in the behaviors and they often expressed frustration or helplessness and a desire for more education and greater understanding of behaviors to help with CKD management.
Significance of Convenience and Affordability
Participants cited the lack of safe or affordable places to exercise and lacking “that kind of income” to purchase healthy food as barriers to adhere to recommended physical activity and diet plans, respectively. Several participants noted cost-related inconveniences, including “co-pays,” “fixed income,” and “running out of coverage” as barriers to behavior engagement.
Constructive and Nonconstructive Support
Participants reported that friends or family “watch what I eat” and provided them with motivation, reassurance, and accountability to perform behaviors and tell them to “take the medicine.” Many participants also reported their “church is very supportive.” Material support included assistance with reminders to perform a behavior, food preparation within dietary guidelines, and transportation to doctors’ appointments. The absence of social support served as a major impediment, including difficulty attending numerous doctors’ appointments when “my wife is not always there” and lack of emotional support as shared “I can’t talk to my husband, he doesn’t like to talk about sickness.” Several participants shared that friends and family members hindered participation in behaviors by actively not supporting behavior performance (“non-constructive support”), such as “my wife doesn’t like to have the blood pressure stuff lying around.”
Behavior Maintenance
Behavior maintenance was closely linked to the aforementioned factors described for behavior performance and was reinforced by routines and habits, including goal setting, monitoring, memory aids, and proactive preparation (Figure 1).
Importance of Developing a Routine
Participants reported the days they felt “best are the days that I’m in a routine.” They found that setting goals and tracking progress using a “FitBit” and “food log” reinforced self-monitoring of their weight, daily steps, and/or sodium intake. The use of memory aids or reminders, such as using “pillboxes” or using “colored baskets” for medications, served as cues to maintain routines within the context of day-to-day life. Proactive preparation, such as grocery shopping with dietary restrictions in mind, filling a pillbox “every Saturday night,” or refilling medication in a timely manner, also reinforced behavior maintenance.
Conversely, lacking a routine or the behavior “never being a regular part of my day” eroded the ability to maintain the behavior. Routine disruptions, in the form of an increase in severity of a comorbid condition, a medication dose adjustment, or stressors from family obligations, negatively affected behavior maintenance by “losing touch with what I used to do”.
Impact of Behavior-Specific Reinforcing or Undermining Factors
Pets were often identified as catalysts to physical activity, primarily through the participants’ commitment to their pets’ need for regular exercise and getting them “motivated up, and moving around more.” Physical therapy and rehabilitation services provided a structured routine to engage in physical activity and fostered confidence by “learning” and “feeling comfortable with how to move around.” Assistance from pharmacy staff by medication advice and “automatic refills” aided medication-taking behavior. Technical issues and discomfort related to home BP monitors caused participants to “doubt the blood pressure is accurate” and to distrust the machine or stop measuring their BP altogether. Participants also noted that dietary restrictions, particularly simultaneous limitations on sodium and glucose, were barriers to healthy eating. Some were upset that they were no longer allowed to eat foods they enjoyed, and others felt that finding a dietary substitution was difficult and frustrating because “everything you touch has sugar” and “everything that’s good has sodium in it.”
Discussion
Effective management of CKD depends on recognizing that the patient is the principal manager of their illness. Nevertheless, to date, little research has explored through qualitative means on how people with CKD perceive their health and what influences their ability to engage in multiple recommended self-management behaviors in their daily lives. Most studies to date have largely included those with advanced CKD and older adults, but younger adults with earlier stages of CKD may face different behavioral recommendations and unique barriers.13,14,30,31 Furthermore, few studies have used health behavior theory to understand behavior change in this population. On the basis of our in-depth interviews, we identified key themes that support the premise that self-regulation theory25 is a salient model to frame how adults with CKD stage 3 or 4 prioritize and incorporate CKD self-management behaviors into their daily lives and that many of these themes were consistent with those found in studies with advanced CKD, suggesting they may be unique to people living with kidney disease.
Self-regulation theory can help determine where a patient with CKD is at in terms of engagement in self-management behaviors by outlining the following 3 distinct phases: (i) goal selection (behavior prioritization), (ii) active goal pursuit (behavior performance), and (iii) goal attainment and maintenance (behavior maintenance). The facilitators and barriers related to each phase can help guide discussions between patients and providers to optimize patients’ ability to proceed to the next phase and/or maintain the behavior. For instance, if patients are struggling to view a behavior as a priority, health care providers can provide essential support for prioritization by emphasizing the importance of the behaviors and providing education and encouragement for behavior engagement. Studies in cardiology have revealed that adherence to dietary restriction is greater when patients are informed of the positive gains from their action.32 Learning on the benefits of self-management behaviors can lead to benefit-based motivation (e.g., better BP control or improved physical fitness) or consequence-based motivation (e.g., slowing CKD progression, avoiding dialysis), which influence behavioral performance and maintenance. Lopez-Vargas et al.33 concluded from a focus group study on information needs for CKD management among people with CKD stages 1 to 4 that the motivation to maintain a specific diet and monitor fluid intake was driven by the belief that these actions could prevent CKD progression. In our study, communication with doctors was often described by patients, especially those from minority groups, as lacking in detail on the importance of self-management behaviors, even though most expressed keen interest in learning more leading to behavior performance. These perceptions may be due to disparities in delivery of health information or medical mistrust. Future research should focus on how to assist providers in delivering health information to patients in ways that are culturally appropriate and tailored to each individual’s health status and life to potentially improve management of CKD, such as developing specific communication training. Furthermore, these findings provide data to support policy changes to address barriers that providers face, including limited time and resources, such as alternative reimbursement models to allow for and encourage this type of care delivery.
Self-efficacy empowered participants to engage in behaviors and cope with barriers and to seek out additional information or education. Information seeking has been reported to allay anxieties and help patients cope with living with kidney disease,34 thereby supporting behavioral performance. Finding ways to increase patient self-efficacy can improve behavior performance and enhance patients’ sense of control to overcome barriers to self-management, such as by specifically addressing patients’ information needs on CKD and its management during clinical encounters.35 Measuring patient activation, a construct of knowledge to understand treatment options, confidence to communicate effectively with providers, and skills to apply this information,36 in routine nephrology clinical practice could help identify those who might benefit from tailored educational programs and skill-building interventions to improve self-efficacy and self-management. Recent research has revealed that the 13-item Patient Activation Measure is reliable and valid for evaluating patient activation in CKD.37
The finding that social support is important for behavior performance and maintenance across multiple behaviors is consistent with previous studies that focused on single behaviors.19,29,38 Participants in our study described that community support, through churches and senior centers, encouraged disease management and enhanced general well-being, which is not well-described in this population and represents an opportunity for future work. Community-focused interventions can leverage the trust of community members to overcome sociocultural barriers and medical mistrust and by developing research partnerships with patients and their communities for intervention development.
Multimorbidity, which leads to treatment burden, adverse symptoms, and contradictory treatment advice, was a common impediment to behavior performance. In a systematic review of qualitative studies (N = 80 with CKD stages 1–5), multimorbidity was identified as a barrier to diet and fluid restrictions when it led to feelings of being overwhelmed and facing conflicting advice.14 We found that compatible comorbid treatment plans aided in behavior performance. Collaborative multidisciplinary care and case management could help minimize conflicting treatments and help patients prioritize treatment plans to optimize CKD self-management and overall health and well-being.
Participants indicated that the main catalyst to behavior maintenance was establishing a routine that was tailored to fit their lives, and we observed that there were several key factors that reinforced these routines. These factors, such as self-monitoring, could provide well-defined practical targets for interventions to support CKD self-management. For example, reasons for not self-monitoring BP at home (e.g., validity concerns of machine, confusion of how to communicate the BP readings to their doctor) could be readily addressed by calibrating the cuff in the office, providing clear communication instructions, and using a mobile health application and a wireless device (e.g., Bluetooth-enabled BP monitor) to directly communicate BP readings to the health care team. Mobile health applications and wireless devices allow patients to self-monitor and directly communicate behavior performance, such as BP readings, daily step count totals, and dietary information to the health care team, which could increase patients’ self-efficacy13 and the ability of the health care team to identify those at risk for nonadherence. A recent study found that 70% of 932 patients with CKD had access to a smartphone and 80% were interested in using mobile health applications for disease management.39
The results of our study need to be interpreted in the context of its limitations. First, our sample was from a single academic institution and, therefore, the themes identified may not generalize to other CKD clinic settings. Second, interviews were conducted in a medical setting, and this may have led to some reluctance to speak freely on the topic of health-related behaviors. Despite these limitations, our study has important strengths including people with earlier stages of CKD and in younger adult years to provide new knowledge on self-management because many of the previous qualitative studies have focused mostly on older people with advanced CKD, those receiving dialysis, or kidney transplant recipients. Additional strengths include purposive sampling of the study population for race, age, and sex to increase the range of issues that were identified. Trustworthiness of the data was enhanced by evaluating intercoder reliability, peer debriefing, and maintaining an audit trail during data analysis.
We believe that the insights provided by this study have important implications for the care of patients with CKD and to stimulate future research to identify and intervene on specific pathways that mediate participation in deleterious behaviors and suboptimal engagement in health-promoting behaviors. By identifying perceived barriers to self-management behaviors, our study offers answers to the under-researched question of why most patients do not succeed in adhering to recommended self-management behaviors. Our findings suggest that, owing to the diverse influences on behavior engagement, a strategy to increase self-management would be to work with patients to prioritize behavior targets based on their preferences, health goals, and life context. We provide practical suggestions to implement in nephrology clinical practice to improve CKD self-management, primarily by the patient-provider interaction by leveraging facilitators and developing creative strategies to overcome barriers (Table 2). Interventions can be designed to target specific needs of patients across the entire process of behavior engagement by overcoming barriers and supporting facilitators, such as strengthening self-efficacy, placing greater attention on providing adequate health information, and harnessing psychosocial and practical support, to improve engagement in self-management behaviors.
Table 2.
Potential approaches for health care providers to leverage facilitators and overcome barriers to increase self-management in CKD
| Approach | Targeted facilitator/barrier | Additional considerations |
|---|---|---|
|
|
May require additional communication training for health care providers Important to evaluate patients’ ability to read |
|
|
May require multidisciplinary care coordination |
|
|
Requires asking patients what they are currently doing, what they have access to, and what gets in the way. |
|
|
CKD, chronic kidney disease; PEER, Partnerships for Enhanced Engagement in Research; YMCA, Young Men’s Christian Association.
Brief video: https://www.youtube.com/watch?v=ZhWHpJN3KaY; National Institute of Diabetes, Digestive and Kidney Diseases website: https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/managing.
National Institute of Aging website and video: https://www.nia.nih.gov/health/real-life-benefits-exercise-and-physical-activity; exercise example videos: https://www.nutrition.gov/topics/exercise-and-fitness/exercise-examples-and-videos.
National Institute of Diabetes, Digestive and Kidney Diseases website: https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/eating-nutrition.
Example of Bluetooth-enabled blood pressure machine and mobile application: https://omronhealthcare.com/service-and-support/connected-health/.
National Kidney Foundation PEERs program video: https://www.youtube.com/watch?v=HC0_CJa2cRY&feature=emb_logo; learn more about the PEERs program: https://www.kidney.org/patients/peers.
Disclosure
LMD and HIF report receiving compensation from the National Kidney Foundation for their roles on the Editorial Board of the American Journal of Kidney Diseases. LMD reports receiving consulting fees from Merck, AstraZeneca, and Cara Therapeutics. HIF reports receiving fees from Kyowa Kirin Inc. and InMed Inc. SJS reports receiving support by K23 DK118198-01A1 and L30 DK110819. SA reports receiving support by DK110749, DK209886, and HD209886. ER reports receiving support by T32NR009356-12. All the other authors declared no competing interests.
Footnotes
Supplementary Material
COREQ Checklist.
Interview Question Guide.
STROBE Statement (PDF)
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of DIsease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittier W., Lewis E. In: National Kidney Foundation’s Primer on Kidney Disease. 5th ed. Gilbert S.J.W.D., editor. Elsevier Saunders; 2014. Pathophysiology of chronic kidney disease; pp. 448–457. [Google Scholar]
- 3.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(suppl 1):S1–S266. [PubMed] [Google Scholar]
- 5.MacGregor M.S., Taal M.W. Renal Association Clinical Practice Guidelines on detection, monitoring and care of patients with CKD. Nephron Clin Pract. 2011;118(suppl 1):c71–c100. doi: 10.1159/000328062. [DOI] [PubMed] [Google Scholar]
- 6.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M., et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortlaity in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98:S1–S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Ricardo A.C., Anderson C.A., Yang W., et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2015;65:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muntner P., Judd S.E., Gao L., et al. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J Am Soc Nephrol. 2013;24:1159–1165. doi: 10.1681/ASN.2012070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havas K., Bonner A., Douglas C. Self-management support for people with chronic kidney disease: patient perspectives. J Ren Care. 2016;42:7–14. doi: 10.1111/jorc.12140. [DOI] [PubMed] [Google Scholar]
- 11.Molzahn A.E., Bruce A., Sheilds L. Learning from stories of people with chronic kidney disease. Nephrol Nurs J. 2008;35:13–20. [PubMed] [Google Scholar]
- 12.Tong A., Sainsbury P., Chadban S., et al. Patients’ experiences and perspectives of living with CKD. Am J Kidney Dis. 2009;53:689–700. doi: 10.1053/j.ajkd.2008.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Walker R., James H., Burns A. Adhering to behaviour change in older pre-dialysis populations -- what do patients think? A qualitative study. J Ren Care. 2012;38:34–42. doi: 10.1111/j.1755-6686.2012.00262.x. [DOI] [PubMed] [Google Scholar]
- 14.Hollingdale R., Sutton D., Hart K. Facilitating dietary change in renal disease: investigating patients’ perspectives. J Ren Care. 2008;34:136–142. doi: 10.1111/j.1755-6686.2008.00034.x. [DOI] [PubMed] [Google Scholar]
- 15.Constantini L., Beanlands H., McCay E., Cattran D., Hladunewich M., Francis D. The self-management experience of people with mild to moderate chronic kidney disease. Nephrol Nurs J. 2008;35:147–155. [PubMed] [Google Scholar]
- 16.King N., Carroll C., Newton P., Dornan T. “You can’t cure it so you have to endure it”: the experience of adaptation to diabetic renal disease. Qual Health Res. 2002;12:329–346. doi: 10.1177/104973202129119928. [DOI] [PubMed] [Google Scholar]
- 17.Rifkin D.E., Laws M.B., Rao M., Balakrishnan V.S., Sarnak M.J., Wilson I.B. Medication adherence behavior and priorities among older adults with CKD: a semistructured interview study. Am J Kidney Dis. 2010;56:439–446. doi: 10.1053/j.ajkd.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Brito-Ashurst I., Perry L., Sanders T.A., Thomas J.E., Yaqoob M.M., Dobbie H. Barriers and facilitators of dietary sodium restriction amongst Bangladeshi chronic kidney disease patients. J Hum Nutr Diet. 2010;24:86–95. doi: 10.1111/j.1365-277X.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams A., Manias E. Exploring motivation and confidence in taking prescribed medicines in coexisting diseases: a qualitative study. J Clin Nurs. 2014;23:471–481. doi: 10.1111/jocn.12171. [DOI] [PubMed] [Google Scholar]
- 20.Baay S., Hemmelgarn B., Tam-Tham H., et al. Understanding adults with chronic kidney disease and their caregivers’ self-management experiences: a qualitative study using the theoretical domains framework. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119848126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Washington T., Zimmerman S., Browne T. Factors associated with chronic kidney disease self-management. Soc Work Public Health. 2016;31:58–69. doi: 10.1080/19371918.2015.1087908. [DOI] [PubMed] [Google Scholar]
- 22.Tong A., Sainsbury P., Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 24.Bryant A., Charmaz K. Sage; 2007. The SAGE Handbook of Grounded Theory. [Google Scholar]
- 25.Maes S., Karoly P. Self-regulation assessment and intervention in physical health and illness: a review. Appl Psychol. 2005;54:267–299. doi: 10.1111/j.1464-0597.2005.00210.x. [DOI] [Google Scholar]
- 26.Montano D.E., Kaspryzk D. In: Health Behavior and Health Education: Theory, Research, and Practice. 4th ed. Glanz K., Rimer B., Viswanath K., editors. Jossey-Bass; 2008. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model; pp. 67–96. [Google Scholar]
- 27.Fishbein M., editor. Readings in Attitude Theory and Measurement. Wiley; New York, NY: 1967. [Google Scholar]
- 28.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Proc. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 29.Meuleman Y., ten Brinke L., Kwakernaak A.J., et al. Perceived barriers and support strategies for reducing sodium intake in patients with chronic kidney disease: a qualitative study. Int J Behav Med. 2015;22:530–539. doi: 10.1007/s12529-014-9447-x. [DOI] [PubMed] [Google Scholar]
- 30.Bowling C.B., Vandenberg A.E., Phillips L.S., McClellan W.M., Johnson T.M., 2nd, Echt K.V. Older patients’ perspectives on managing complexity in CKD self-management. Clin J Am Soc Nephrol. 2017;12:635–643. doi: 10.2215/CJN.06850616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griva K., Ng H.J., Loei J., Mooppil N., McBain H., Newman S.P. Managing treatment for end-stage renal disease--a qualitative study exploring cultural perspectives on facilitators and barriers to treatment adherence. Psychol Health. 2013;28:13–29. doi: 10.1080/08870446.2012.703670. [DOI] [PubMed] [Google Scholar]
- 32.Beswick A.D., Rees K., West R.R., et al. Improving uptake and adherence in cardiac rehabilitation: literature review. J Adv Nurs. 2005;49:538–555. doi: 10.1111/j.1365-2648.2004.03327.x. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Vargas P.A., Tong A., Phoon R.K., Chadban S.J., Shen Y., Craig J.C. Knowledge deficit of patients with stage 1-4 CKD: a focus group study. Nephrology (Carlton) 2014;19:234–243. doi: 10.1111/nep.12206. [DOI] [PubMed] [Google Scholar]
- 34.Ormandy P. Information topics important to chronic kidney disease patients: a systematic review. J Ren Care. 2008;34:19–27. doi: 10.1111/j.1755-6686.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 35.Lederer S., Fischer M.J., Gordon H.S., Wadhwa A., Popli S., Gordon E.J. A question prompt sheet for adult patients with chronic kidney disease. BMC Nephrol. 2016;17:155. doi: 10.1186/s12882-016-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hibbard J.H., Stockard J., Mahoney E.R., Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lightfoot C.J., Wilkson T.J., Memory K.E., Palmer J., Smith A.C. Reliability and validity of the Patient Activation Measure in kidney disease: results of Rasch analysis. Clin J Am Soc Nephrol. 2021;16:880–888. doi: 10.2215/CJN.19611220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke A.L., Young H.M., Hull K.L., Hudson N., Burton J.O., Smith A.C. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant. 2015;30:1885–1892. doi: 10.1093/ndt/gfv208. [DOI] [PubMed] [Google Scholar]
- 39.Schrauben S.J., Appel L., Rivera E., et al. Mobile Health (mHealth) technology: assessment of availability, acceptability, and use in CKD. Am J Kidney Dis. 2021;77:941–950.e1. doi: 10.1053/j.ajkd.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.