Abstract
The prevalence of kidney failure continues to rise globally. Dialysis is a treatment option for individuals with kidney failure; after the decision to initiate dialysis has been made, it is critical to involve individuals in the decision on which dialysis modality to choose. This review, based on evidence arising from the literature, examines the role of shared decision-making (SDM) in helping those with kidney failure to select a dialysis modality. SDM was found to lead to more people with kidney failure feeling satisfied with their choice of dialysis modality. Individuals with kidney failure must be cognizant that SDM is an active and iterative process, and their participation is essential for success in empowering them to make decisions on dialysis modality. The educational components of SDM must be easy to understand, high quality, unbiased, up to date, and targeted to the linguistic, educational, and cultural needs of the individual. All individuals with kidney failure should be encouraged to participate in SDM and should be involved in the design and implementation of SDM approaches.
Keywords: continuous renal replacement therapy, education, hemodialysis, peritoneal dialysis
Chronic kidney disease (CKD) is defined as kidney damage or an estimated glomerular filtration rate <60 ml/min per 1.73 m2 for ≥3 months, regardless of cause.1 Individuals with CKD can develop end-stage kidney disease, also known as kidney failure, which is defined as an estimated glomerular filtration rate <15 ml/min per 1.73 m2.1 In 2017, the estimated global prevalence of CKD was 9.1%, with 0.04% of the population receiving dialysis.2 The prevalence of kidney failure continues to rise worldwide and up to 5 million people are expected to require treatment by 2030, with the greatest increase in Asia.3,4 Equity of access to kidney replacement therapy varies between countries based on finance and rationing.5 Where available, kidney transplantation is the preferred option in eligible individuals.6 Dialysis options for individuals with kidney failure include hemodialysis (HD), which can either be undertaken in-center (ICHD) or at home (HHD), or peritoneal dialysis (PD), which can be continuous ambulatory PD or automated PD. Evidence suggests that individuals with kidney failure receiving PD treatment have a similar risk of death to those receiving ICHD.4,7, 8, 9, 10, 11, 12, 13, 14, 15, 16
Helping the Individual Make a Treatment Choice
There are different treatment decision-making models to aid individuals seeking health care.17
-
1.
In the parental or paternalistic model, the health care professional (HCP) decides on the treatment strategy.
-
2.
The HCP as best agent model also focuses on treatment options being decided by the HCP but considers the values and preferences of the individual seeking health care.
-
3.
The shared decision-making (SDM) model allows for open discussion between the individual seeking health care and the HCP and uses a collaborative approach to choose a treatment.
-
4.
Informed decision-making allows the individual seeking health care to make a decision on their treatment based on information supplied to them by the HCP without collaboration on the treatment decision (an “informed choice”).
Of course, people have the intrinsic right to refuse treatment.18 Although individual rights may vary by country or jurisdiction, the clinician has a duty of care to respect the rights of the individual seeking health care.18 Furthermore, patient involvement is increasingly recognized as essential in research, quality improvement, policy development, service reviews, and payer reviews.19,20 As such, individuals seeking health care should be more involved in decisions on their own treatment and care. Indeed, the phrase “no decision about me, without me” has become synonymous with SDM.21
SDM provides individuals seeking health care with the opportunity for engagement and has been defined as an “approach where clinicians and patients make decisions together using the best available evidence … Shared decision making respects patient autonomy and promotes patient engagement.”22 It has also been found that SDM can lead to increased satisfaction, reduce anxiety, improve treatment compliance, and lower demand for health care resources.23, 24, 25, 26, 27, 28
A randomized trial has found the importance of choice to individuals with kidney failure on to start dialysis. This trial, which compared starting therapy with ICHD versus PD, was stopped early because of difficulties with recruitment when only 38 of more than 700 eligible participants agreed to be randomized. The authors suggested that one possible explanation for the vast majority of eligible individuals with kidney failure refusing consent to participate in the trial was that they had already developed a treatment preference after receiving extensive education on the available options and were unwilling to be randomized to their nonpreferred choice.29 Following predialysis education, it has been suggested that up to 50% of informed individuals would choose home dialysis options,30 yet the reality remains that individuals with kidney failure receiving PD and HHD represent a small proportion of the global dialysis population.4,31,32
Systematic reviews of qualitative studies have revealed that HHD and PD improve an individual with kidney failure’s sense of independence, self-efficacy, and well-being, but they may also experience anxiety owing to isolation from medical and social support and need strategies to help improve resilience and self-confidence.33,34
This review, based on expert opinions and evidence arising from published literature, describes the processes that enable effective SDM, illustrates how SDM is used for predialysis education, identifies the barriers to the uptake of SDM, and provides suggestions on how to overcome these barriers. For literature regarding SDM and dialysis modality choice to be included in this narrative review, we performed a PubMed search using the terms “chronic kidney disease,” “shared decision-making,” and “dialysis.” Literature in English published after 2000 was evaluated, and all authors contributed to inclusion of articles and proposed additional literature based on expert opinion and experience in the area. Articles were screened by title, followed by abstract, and then by full text. Literature detailing treatment options for kidney failure other than dialysis was excluded.
The SDM Model
A number of resources from Healthwise (USA),35 the United Kingdom National Health Service,36 and the SHARE approach from the US Agency for Healthcare Research and Quality37 have outlined the core steps associated with SDM, including the following:
-
1.
Inviting the individual seeking health care to participate in the SDM process.
-
2.
Allowing information exchange between the person seeking health care and the HCP, supplemented with evidence-based resources, such as patient decision aids (PDAs).
-
3.
An assessment of the individual’s values and preferences to reach a common understanding on risks and benefits of the treatment options.
-
4.
Enabling the individual seeking health care to arrive at an initial/preferred treatment decision, which is then discussed with the HCP.
-
5.
Attaining and reviewing a final decision.
An ideal consultation leading to the choice of a dialysis modality would involve building a trusting relationship between the HCP and individual with kidney failure and attending to any care-associated emotional needs of the individual. Clinical findings should be discussed at a suitable health literacy level. This could result in an agreement on a unique dialysis management plan that respects the preferences of the individual with kidney failure and incorporates both quality- and quantity-of-life considerations.
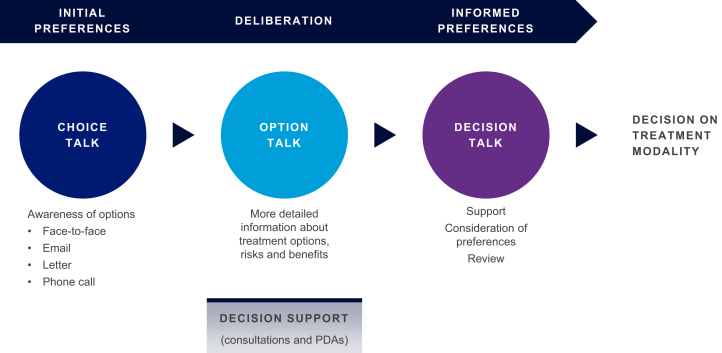
To help HCPs integrate SDM into their practice, a 3-step model, developed from a sample of the general population, has been proposed to guide consultations with individuals seeking health care (Figure 1). This model uses “Choice,” “Option,” and “Decision” talks, during which participants are provided with decision support materials to assist them in making their treatment decisions using a deliberative process.38
Figure 1.
The 3-talk shared decision-making model. Adapted from Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367.38 © The Author(s) 2012. This article is published as open access at https://link.springer.com/article/10.1007%2Fs11606-012-2077-6. PDA, patient decision aid.
The Role of PDAs in SDM and Predialysis Education
PDAs help individuals seeking health care arrive at informed choices on screening, treatment, or other interventions39; they are not designed to replace consultations with the HCP. PDAs can be delivered in a variety of formats, including booklets, interactive media, and video or audio sessions.39 It is important that the content of PDAs be evidence based, devoid of bias, and verifiable because of the influence the tool may have on decision-making.39 To support confidence in the quality of the PDA for both HCPs and individuals seeking health care, the International Patient Decision Aids Standards Collaboration developed a certification tool40 and a set of criteria for quality appraisal of PDAs. The key criteria were “content” (key information, probabilities, clarification of values, and disease-specific guidance), “development process” (generic design and developmental criteria for PDAs), and “effectiveness” (generic evidence of high-quality decision processes and a high-quality choice).39
A Cochrane review of PDAs for individuals seeking health care facing screening decisions reported that those who used a PDA, versus those who did not, felt they were better informed and had a more active role when choosing their treatments. Indeed, there was an increase in knowledge and understanding of risk perception and a decrease in decisional conflict and the number of individuals who were undecided on their treatment choices. Importantly, PDAs were also found to improve communication between individuals seeking health care and HCPs and improve satisfaction in their treatment decisions.41 Table 1 provides examples of PDAs that have been developed for individuals with kidney failure receiving or on to start dialysis treatment.
Table 1.
Examples of PDAs used for individuals with kidney failure receiving or on to start dialysis treatment
| Name | Country | Formata | Target | Link/reference |
|---|---|---|---|---|
| My Kidneys My Choice | Australia, NZ | Online, PDF, paper | Individuals who will experience kidney failure in 6–12 mo | https://kidney.org.au/your-kidneys/treatment/my-kidneys-my-choice |
| The Dialysis Decision Aid Booklet: Making the Right Choices For You | UK | PDF, paper | Individuals with CKD or their carers | https://kidneyresearchuk.org/wp-content/uploads/2019/05/KR-decision-Aid-DOWNLOAD.pdf |
| Ontario Renal Network SHERPA PDA | Canada | Individuals with kidney failure who would like to plan which dialysis treatment option is best for them and/or want to share their views with others | https://www.ontariorenalnetwork.ca/en/kidney-care-resources/clinical-tools/person-centred-care | |
Healthwise PDAs:
|
USA | Online | Individuals with the following:
|
https://www.healthwise.org/services.aspx |
| My Life, My Dialysis Choice | USA | Online | Individuals who have the following:
|
https://mydialysischoice.org/ |
| Preparing For Kidney Treatment: You Have a Choice | USA | Paper and video | Individuals with kidney failure receiving dialysis or yet to start treatment | Ameling et al.42 |
| iChoose Kidney | USA | Online | Individuals with kidney failure deciding on dialysis options vs. transplant | https://ichoosekidney.emory.edu/ |
CKD, chronic kidney disease; NZ, New Zealand; PDA, patient decision aid; UK, United Kingdom; USA, United States of America.
Online: interactive online platform; PDF: online digital material; paper: physical print (e.g., brochure); video: online video format.
A total of 17 PDAs designed to support individuals with kidney failure choose between dialysis and conservative management were identified in a systematic review by Winterbottom et al.43 Of these 17 PDAs, 8 focused on the choice of dialysis modality.
Evaluation of the identified PDAs found variations in the quality of information provided on dialysis modality choice. The Dialysis Decision Aid Booklet: Making the Right Choices For You highlighted in Table 1 scored highly in quality assessments; however, none of the PDAs identified included information on treatment failure or switching. They also found variations in how dialysis modality options were described.43
The Yorkshire Dialysis Decision Aid (YoDDA) is distributed in The Dialysis Decision Aid Booklet. This evidence-based booklet was developed for individuals with kidney failure and their families to facilitate making an informed choice between either HD (ICHD or HHD) or PD (automated PD or continuous ambulatory PD). The decision aid was developed using rigorous decision analysis and behavioral decision support guidance methodology. Clinical guidelines, surveys of dialysis choice, and existing patient information were reviewed as part of the development process.44 Content included information on CKD and progressive kidney disease, kidney failure, dialysis options, decision aids focusing on lifestyle activities, and treatment preferences. A study comparing usual care with or without the Yorkshire Dialysis Decision Aid booklet found that individuals with kidney failure who received the Yorkshire Dialysis Decision Aid booklet had higher scores for understanding kidney disease, understanding advantages and disadvantages regarding treatment options, feeling in control over dialysis choice, and sharing their decision with their family compared with those who did not receive the booklet. Individuals deciding on a treatment option considered the views of their family and HCPs as important in decision-making, but the views of others with kidney failure were not rated as important.44
In a Spanish registry study, individuals with kidney failure who participated in an education process (4 phases: identifying values, provision of balanced information, deliberation/question and answer, decision-making) with PDAs chose PD significantly more often than those who did not participate (47.8% vs. 6.5%, respectively; P < 0.001). Moreover, the use of PDAs, even in the scenario of an unplanned start for dialysis, led to high levels of agreement between the modality of choice and the actual modality that was started in those who had participated in the education process. This suggests that individuals with kidney failure who undertook the education process were more confident in their modality choice.45
Challenges With PDAs
There are numerous challenges regarding the use of PDAs. Clear guidance is needed on who is responsible for updating these tools and how frequently updates are made. It is critical that the information contained within PDAs is evidence based and current as poor-quality, time-consuming, inaccurate, imbalanced, or misleading tools can be harmful to individuals seeking health care.46 Clear pathways for funding and certification of PDAs are also lacking.40,46
Another challenge with PDAs is the changing values individuals with kidney failure may have owing to the worsening of their disease. A PDA that is suitable at the beginning of their disease may not be suitable a few years further on, with treatment plans needing to be updated in line with the person’s current situation and values.43
Individuals with kidney failure may have cognitive changes that can impair mental skills such as executive function and memory. It is vital to consider the timing of PDAs so the individual with kidney failure can participate in SDM before their decision-making ability has declined.47
The challenges with PDAs have been highlighted by The Dialysis Guide, an application for mobile devices developed for a Danish cross-sectional study to help individuals with kidney failure make decisions on dialysis modality. The application included information on kidney failure, dialysis choice, and dialysis modalities, and, although it seemed to help predialysis individuals with kidney failure decide on a dialysis modality, it did not reduce decisional conflict.48 The lack of effect on decisional conflict may be because of the fact that 91% of individuals with kidney failure had already attended education sessions or a consultation with an HCP to discuss dialysis options. The usability of the application was rated as low by the participants, whose average age was 65 years. This highlights the need to consider the appropriate timing of PDA deployment when individuals are choosing a dialysis modality, with the educational needs of the individual factored into the material.
SDM for Predialysis Individuals With Kidney Failure
Individuals with kidney failure who are yet to start dialysis are often not given enough information to make an informed choice or have their choices made for them. Indeed, almost one-third of participants (32.2%) interviewed in the Empowering Patients On Choices for Renal Replacement Therapy (EPOCH-RRT) study, of whom 46.8% started ICHD and only 2.6% started PD, did not think the decision to start dialysis was primarily their choice.49 These data suggest that individuals who received limited information and opportunity to make an informed choice were unlikely to choose PD as a dialysis modality.
Shared Decision Making and Dialysis Choice (SDM-DC) program is an initiative received by 348 individuals with kidney failure at 4 hospitals in Denmark who were making decisions on starting HD or PD. The intervention was based on the “three-talk model”38 and included a PDA, videos, and 3 meetings between individuals with kidney failure and a dialysis coordinator. As a result of partaking in the Shared Decision Making and Dialysis Choice program, participants felt that they were in control of their dialysis choice. They reported that the meetings were critical to their decision-making and provided them with time and opportunities to ask questions. The PDA was also found to be essential, with individuals in the study reporting that they could not have made a decision without the tool.50,51 A mixed-methods (questionnaire and semistructured interviews) descriptive study of the Shared Decision Making and Dialysis Choice program found that more than 80% of individuals in the trial experienced SDM and made a “high-quality” decision (based on chosen dialysis modality and knowledge scores). The decision quality was the same for those who chose HD or PD.52
Of interest is an SDM program conducted in Taiwan that included physician training and a PDA, interviews with individuals with kidney failure, and clinical consultations. Physicians attended a short course that introduced SDM and gave guidance on how to inform and encourage their patients to participate in SDM. Individuals with kidney failure who participated in the program were more likely to choose PD, had higher confidence in their choices for treatment, and experienced less decisional conflict.53
The International Society for Peritoneal Dialysis has released key practice recommendations that include SDM: “PD should be prescribed using SDM between the person doing PD and the care team. The aim is to establish realistic care goals that (1) maintain quality of life for the person doing PD as much as possible by enabling them to meet their life goals, (2) minimize symptoms and treatment burden while (3) ensuring high-quality care is provided” (key recommendation 1) and “The PD prescription should take into account the local country resources, the wishes and lifestyle considerations of people needing treatment, including those of their families/caregivers’, especially if providing assistance in their care” (key recommendation 2).54
Barriers to the Implementation of SDM and How to Overcome Them
Emotional Burden Experienced by Individuals With Kidney Failure
Individuals with kidney failure may be fearful or overwhelmed when making a decision on initiating dialysis and which modality to choose, and this emotional overload may make it impossible to learn.55 Improving feelings of hope can help combat these negative emotions and has been found to significantly reduce fear, anxiety, and depression.56, 57, 58 An 8-week stress management intervention provided to individuals having ICHD by HD nurses was found to significantly increase hope compared with those who did not receive the intervention (P < 0.001).57 Hope was also found to be an independent predictor for psychological adjustment of individuals with kidney failure on dialysis and was inversely associated with anxiety and depression and positively associated with mental health quality of life.58 The integration of interventions to promote hope within the SDM process could lead to better engagement and outcomes for individuals with kidney failure, who in turn may act as patient ambassadors involved in the SDM process, alleviating feelings of fear and anxiety in other individuals with kidney failure.
Psychosocial Factors and Willingness to Engage in SDM
Despite having a strong desire for more information, individuals with kidney failure are not always willing to participate in SDM, especially those who have low ratings for autonomy, are older, male, or of non-White race.59 Studies have found that males were more likely to delay making a decision on a dialysis modality than females, with females more willing to seek information regarding dialysis modalities.60 Ethnic minorities face a number of barriers to access SDM, one of which may be a mistrust in medical professionals.61 Involving the individual’s spouse, family, and community can help with informal education of the SDM participant and identification of factors that influence preferences for engagement may help the provision of individually tailored care.
Importantly, the emotional, psychosocial, and cognitive states of the individual with kidney failure when considering starting dialysis have an impact on the choices made. Anxiety is highly prevalent among individuals with kidney failure undergoing dialysis and is associated with an increase in the 1-year hospitalization rate, length of hospital stay, and all-cause mortality.62 Individuals with kidney failure were found to be less likely to regret their choice to start dialysis when they had discussions with their HCP on life expectancy and were more likely to regret their decision if they chose to have dialysis to please family members or their doctor.63 As expected, treatment satisfaction was higher in those who had participated in SDM and had a “good” psychological state (less anxious and/or depressed) when starting treatment.64
Lack of HCP Engagement
Individuals with kidney failure are not the only ones who may not engage in SDM59; HCPs have reported that the challenges to implementing SDM include the lack of available tools and training, their patients not wanting to participate in SDM, and high workload.65, 66, 67, 68 Time constraints may be resolved by asking patients to work through a tool before the visit with the HCP or for another member of the multidisciplinary team to engage with the individual, in addition to the physician.
The medical community must ensure young and emerging nephrologists are trained and confident with SDM. Unless they are fully equipped with the information to make decisions, individuals with kidney failure will continue to miss out on opportunities to participate in their own care. It is also important to identify whether education tools need to be developed or if existing tools are of an adequate standard and the barrier to effective SDM is access to resources. Moreover, it is necessary that the nephrology community ensures they have adequate knowledge and understanding of new and emerging evidence—presenting outdated and biased evidence is no longer acceptable. The modern era has seen advances in outcome data, such as technique survival and patient survival, demonstrating, for example, that PD is no longer inferior to HD.4,7, 16,69,70 HCPs need to be trained in a variety of aspects of SDM, including support for the individual with kidney failure, identification of cultural barriers, communication strategies, and recognizing psychosocial factors, such as anxiety. However, a general review of SDM training programs for HCPs identified that many are not evaluated—a major barrier to the improvement of this important aspect of SDM.71
HCPs have been found to be poor at evaluating their patients’ preferred treatment and level of involvement in decision-making, even in institutions that have implemented SDM.72 It has been found that “paternalist” and “institutionalist” HCPs often perceive the initiation of dialysis for older individuals as a success, a view often at odds with the perception of the individual themselves. HCPs who engaged in SDM with their patients focused more on quality of life and aligning values with treatment and were the only ones who offered conservative care to those of an older age.73 It is important that the values and preferences of the HCP are not transferred to the individual seeking health care as the preferred option and that the choice of modality for dialysis is based on the unbiased information provided. Nevertheless, it must be acknowledged that HCPs are bound by local policy. In countries with a PD-first policy, the treatment options that HCPs can offer may be limited.
HCPs may be unwilling to participate in SDM if it is not initiated by the individual seeking health care. In a study of physician interactions with their patients (primary care patients, individuals with systemic lupus erythematosus, or individuals with lung cancer), it was reported that 84% of SDM-like behaviors in medical consultations were not initiated by the HCP.74 Individuals seeking health care who engaged in active participation were more likely to receive facilitative communication from the HCP, be educated, and be of White race.74 Another study in individuals with depression found that HCPs only initiated SDM after specific requests regarding treatment were made.75 It has been suggested that raising awareness of SDM through public campaigns may help prepare individuals seeking health care to ask for SDM during consultations and subsequently increase uptake.76 HCPs should actively encourage predialysis individuals with kidney failure to participate in SDM to ensure that treatment decisions are not made without the participation of the individual and/or their family/caregiver.
SDM in Emergency Situations
SDM may not be possible or may be limited in scenarios where urgent or unplanned kidney replacement therapy is required. Urgent kidney replacement therapy refers to dialysis initiation required within 48 hours of presentation, and unplanned dialysis is when the individual with kidney failure may require hospitalization to begin dialysis or when the modality is not their choice. HD and PD are options in most cases of urgent and unplanned dialysis; however, if the situation is critical, HCPs may not have the time or information to provide an SDM process. It is important that the individual with kidney failure is subsequently provided with the support and information required to transfer to the modality of their choice at a later date, if feasible.77
Environmental Barriers to Uptake of SDM and PDAs
Environmental barriers to SDM may include lack of access to mobile technology or e-health. Another consideration is that older individuals seeking health care may not readily engage with technology and may not have ready access to or understand the complex use of technology in health care.78
Language, educational level, health literacy, and cultural barriers can prevent predialysis individuals with kidney failure from engaging in the SDM process.61 Older individuals with kidney failure are likelier to have poorer e-Health literacy, which may preclude their access to certain resources. Those from culturally or linguistically diverse backgrounds may be disadvantaged during SDM because of a lack of sufficient or understandable information and HCP time pressures.79 Consequently, they may miss out on opportunities to be an informed participant during the process. PDAs should be available in local languages and provided at an appropriate reading level. Individuals seeking health care can be influenced by many information sources that are readily accessible, and this can limit the degree to which they accept evidence-based knowledge provided by their HCP. Family members or other treatment-experienced individuals may influence new individuals with kidney failure, regardless of their level of knowledge, especially older adults who may lack autonomy on decision-making for their health.61 Indeed, filial piety, the honor and respect shown to parents, grandparents, and elderly relatives by children, is very influential in some cultures, particularly in Asia.80 Within these cultures, individuals may not be responsible for making their own treatment decisions, relying instead on family members to make decisions regarding their health care.
Learning styles vary between adults, with some preferring visual, auditory, or kinesthetic tools.81 This means that the delivery of SDM and PDAs should be adapted to meet the needs of the individual, without compromising the integrity of the material.
The COVID-19 Pandemic
The COVID-19 pandemic poses barriers for continuing health care delivery, including SDM, with limitations on physical contact/proximity between HCPs and individuals with kidney failure. Challenges exist for predialysis individuals with kidney failure making decisions on starting dialysis and which options are most suitable.82 SDM meetings may have to be remote and the materials provided need to be available in a format suitable to be accessed from home-based settings. Of concern is that predialysis individuals may become more reliant on HCPs to advise them on treatment options.82 Predialysis individuals with kidney failure may need to be given more time to read/look at materials, as the social interactions and dynamics of virtual or telephone meetings differ from those during face-to-face consultations. This in turn may result in additional burden on the individual with kidney failure and the HCP in terms of time and resources. Use of COVID-19–specific decision aids may help initiate the dialogue needed for SDM in these circumstances.82 HCP training and support for SDM tailored to the COVID-19 era will be critical to ensure decision aids are used appropriately.82 Promoting home-based therapies may be beneficial in mitigating the challenges of in-center attendance (including possible transmission of coronavirus to individuals with kidney failure) posed by the COVID-19 pandemic, reducing the burden on dialysis care centers and prioritizing access to individuals who need it most. Similarly, those currently receiving ICHD may be encouraged to transition to HHD or PD where this is an option to reduce the impact on ICHD units and promote social distancing.82 It is important that risks and benefits to the individual be taken into consideration before suggesting these options.
Lack of High-Quality Evidence
There is a lack of high-quality evidence regarding the effectiveness of interventions focusing on increasing the use of SDM among HCPs. A Cochrane systematic review recommended that future studies of SDM should be designed to minimize bias and methods and results should be fully reported. It highlighted that further research is needed to develop better patient-derived measures of SDM. Studies should be conducted across multiple clinical backgrounds and cost-effectiveness should be reported.83
There is also a lack of data regarding outcomes of SDM on dialysis modality choice, apart from the previously discussed positive impact on mental health. The impact of SDM on clinical outcomes, such as survival, is important for policy makers who may not see the utility of SDM without this information.
The barriers to SDM and proposed solutions are summarized in Table 2.
Table 2.
Barriers to SDM and proposed solutions
| Barrier to SDM | Solutions |
|---|---|
| Emotional burden experienced by individuals with kidney failure | |
|
|
| Psychosocial factors and willingness to engage in SDM | |
|
|
| Lack of HCP engagement | |
|
|
| SDM in emergency situations | |
|
|
| Environmental barriers to uptake of SDM and PDAs | |
|
|
| COVID-19 pandemic | |
|
|
| Lack of high-quality evidence | |
|
|
HCP, health care provider; KRT, kidney replacement therapy; PDA, patient decision aid; SDM, shared decision-making.
SDM Clinical Trials for Predialysis Individuals With Kidney Failure
There are several trials currently exploring SDM for predialysis individuals that will provide further guidance on the use of SDM.
PREPARE NOW
PREPARE NOW (NCT02722382) is a cluster-randomized controlled trial in the United States. The “Patient Centered Kidney Transition Care” program uses multicomponent interventions to improve patient preparedness for kidney replacement therapy through helping providers focus on patient values and treatment preferences. A primary outcome of the study is the change in the proportion of patients feeling in control of their decision-making at 36 months of follow-up. The study was completed in October 2020 and enrolled 1572 patients.84,85
CKD-EDU
The Feasibility of Enhanced Dialysis Education Intervention for Chronic Kidney Disease Patients (CKD-EDU) trial (NCT03465449) is a randomized, parallel controlled trial comparing palliative care-based dialysis decision-making with usual care. Patients will be ≥75 years of age and have CKD (stage 4/5). Primary outcomes are the number of patients receiving the intervention and the acceptability of the CKD-EDU. Estimated enrollment is 60 patients, and estimated completion is January 2022.86
DIAL-SDM
The Shared Decision Making in Dialysis (DIAL-SDM) trial is a randomized, single-blind pilot study (NCT04392440) planning to recruit patients aged ≥65 years with kidney failure/CKD who are facing challenges in decision-making on dialysis. The study will compare the DIAL-SDM intervention with usual care. Nephrologists in the intervention group will receive 3 communication training sessions, delivered by a standardized patient instructor. Patients (and caregivers, if available) will receive 2 coaching sessions provided by health coaches, who will explore each patient’s relevant contextual information (values, preferences, and goals) and help them identify and practice important questions for their nephrologist. Estimated enrollment is 60 participants, and estimated study completion is May 2023.87
Summary and Recommendations
Access to SDM is a right that can lead to improved outcomes and can be integrated into the choice of treatment modality for predialysis individuals with kidney failure. SDM can empower people faced with decisions on which dialysis modality to choose and can lead to increased treatment satisfaction. It is immensely important that the perspective of “no decision about me, without me” be respected by HCPs when discussing their patients’ health care. For SDM to be successful, individuals seeking health care must be aware that the process is both active and iterative and requires their participation. Individuals with kidney failure must be equal partners if their long-term care is to be successful, and this partnership should begin at the very start of their dialysis journey. Within this partnership, HCPs should be mindful of the influences that may affect their patients’ decisions when choosing a treatment option. These can include personal values and preferences, age, or emotional state.
PDAs are integral to the SDM process. Although PDAs have been successfully integrated into kidney care in some health care systems, it is imperative that these tools are objective, evidence based, and up to date.
We recommend that all individuals with kidney failure are encouraged to participate in SDM to discuss their treatment options and should be involved in the design and implementation of recommendations regarding the SDM approach for those with CKD/kidney failure. This can be achieved by the formation of groups who are actively involved in providing input on SDM processes when choosing dialysis modality. Of course, some individuals will not want to participate in SDM and would prefer their HCPs make all their treatment choices. In this case, the individuals’ wishes should be respected.
Table 3 reveals our recommendations for a predialysis modality SDM consultation process between those seeking health care and HCPs, based on the 3-talk SDM model found in Figure 1. Although Table 3 describes our recommendations for predialysis individuals, it could be applied at any point on the dialysis journey as some individuals receive dialysis education only after commencing treatment (e.g., those who start dialysis in an emergency). It should also be noted that this process is dynamic, with changes in an individual’s medical condition or personal circumstances meaning that the process may need to be repeated with possible changes to previously made decisions.
Table 3.
Recommendations for a predialysis modality SDM consultation process
| Recommendations |
|---|
Choice talk
|
Option talk
|
Decision talk
|
APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; HCP, health care professional; HD, hemodialysis; ICHD, in-center hemodialysis; IPDAS, International Patient Decision Aids Standards; PDA, patient decision aid; SDM, shared decision-making.
It is incumbent on us all to ensure nephrology teams are trained in both SDM and the tools used to support this process.
Disclosure
XY reports receiving grants from Baxter Healthcare Corporation, Kyowa-Kirin, China, Wanbang Company, AstraZeneca, and GlaxoSmithKline and providing consultancy for Baxter Healthcare Corporation. YLK reports receiving personal fees for speaker honorarium from Fibrogen. DS reports that Baxter is a sponsor of the nonprofit Medical Education Institute’s Home Dialysis Central website each year. RRQ reports receiving speaker fees from Baxter Corporation and having a patent “Dialysis Measurement Analysis and Reporting System” issued. MD is a full-time employee of Baxter Healthcare Pty Ltd. (Associate Medical Director, Medical Affairs—Baxter Healthcare ANZ). All the other authors declared no competing interests.
Acknowledgments
We thank Dr. Mark Marshall, former director of Medical Affairs, Baxter Healthcare (Asia Pacific), for providing the initial concept for this review and for conducting advisory board meetings. We also thank the participants of the predialysis SDM advisory board meetings that took place in 2019. This review was funded by Baxter Australia who provided financial support for medical writing assistance. We thank Cameron Ward, AMICULUM Ltd., for medical editorial assistance with this manuscript. This review was funded by Baxter Australia who provided financial support for medical writing assistance.
References
- 1.Levey A.S., Eckardt K.-U., Tsukamoto Y., et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.xv. [DOI] [PubMed] [Google Scholar]
- 2.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liyanage T., Ninomiya T., Jha V., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 4.2020 USRDS annual data report United States Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2020 Accessed August 2021.
- 5.White S.L., Chadban S.J., Jan S., et al. How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ. 2008;86:161–240. doi: 10.2471/blt.07.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodger R.S. Approach to the management of endstage renal disease. Clin Med (Lond) 2012;12:472–475. doi: 10.7861/clinmedicine.12-5-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.W., Lee N.R., Son S.K., et al. Comparative study of peritoneal dialysis versus hemodialysis on the clinical outcomes in Korea: a population-based approach. Sci Rep. 2019;9:5905. doi: 10.1038/s41598-019-42508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y.K., Hsu C.C., Hwang S.J., et al. A comparative assessment of survival between propensity score-matched patients with peritoneal dialysis and hemodialysis in Taiwan. Medicine (Baltimore) 2012;91:144–151. doi: 10.1097/MD.0b013e318256538e. [DOI] [PubMed] [Google Scholar]
- 9.Heaf J.G., Wehberg S. Relative survival of peritoneal dialysis and haemodialysis patients: effect of cohort and mode of dialysis initiation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer A., Pippias M., Noordzij M., et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2016: a summary. Clin Kidney J. 2019;12:702–720. doi: 10.1093/ckj/sfz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steenkamp R., Pyart R., Fraser S. Survival and cause of death in UK adult patients on renal replacement therapy in 2016. Nephron. 2018;139(suppl 1):117–150. doi: 10.1159/000490963. [DOI] [PubMed] [Google Scholar]
- 12.Sukul N., Mukhopadhyay P., Schaubel D.E., et al. Peritoneal dialysis and mortality, kidney transplant, and transition to hemodialysis: trends from 1996-2015 in the United States. Kidney Med. 2020;2:610–619.e1. doi: 10.1016/j.xkme.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi J.Y., Jang H.M., Park J., et al. Survival advantage of peritoneal dialysis relative to hemodialysis in the early period of incident dialysis patients: a nationwide prospective propensity-matched study in Korea. PLoS One. 2013;8 doi: 10.1371/journal.pone.0084257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho Y.W., Chau K.F., Choy B.Y., et al. Hong Kong renal registry report 2012. HK J Nephrol. 2013;15:28–43. doi: 10.1016/j.hkjn.2013.03.005. [DOI] [Google Scholar]
- 15.Wong B., Ravani P., Oliver M.J., et al. Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis. 2018;71:344–351. doi: 10.1053/j.ajkd.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Quinn R.R., Hux J.E., Oliver M.J., Austin P.C., Tonelli M., Laupacis A. Selection bias explains apparent differential mortality between dialysis modalities. J Am Soc Nephrol. 2011;22:1534–1542. doi: 10.1681/ASN.2010121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaburg L., Hess E.P., Coylewright M., Ting H.H., McLeod C.J., Montori V.M. Shared decision making in atrial fibrillation: where we are and where we should be going. Circulation. 2014;129:704–710. doi: 10.1161/CIRCULATIONAHA.113.004498. [DOI] [PubMed] [Google Scholar]
- 18.A declaration on the promotion of patients’ rights in Europe. European consultation on the rights of patients. Amsterdam 28–30 March, 1994. World Health Organization; Published June 28, 1994. https://www.who.int/genomics/public/eu_declaration1994.pdf Accessed August 2021. [Google Scholar]
- 19.Bombard Y., Baker G.R., Orlando E., et al. Engaging patients to improve quality of care: a systematic review. Implement Sci. 2018;13:98. doi: 10.1186/s13012-018-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandy L.G., Tuckson R.V., Stevens S.L. UnitedHealthcare experience illustrates how payers can enable patient engagement. Health Aff (Millwood) 2013;32:1440–1445. doi: 10.1377/hlthaff.2012.1082. [DOI] [PubMed] [Google Scholar]
- 21.Coulter A., Collins A. The King’s Fund; London, United Kingdom: 2011. Making Shared Decision-Making a Reality—No Decision About Me, Without Me. [Google Scholar]
- 22.Elwyn G., Laitner S., Coulter A., Walker E., Watson P., Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341:c5146. doi: 10.1136/bmj.c5146. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn D., Wellman R., Westbrook E., et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff (Millwood) 2012;31:2094–2104. doi: 10.1377/hlthaff.2011.0686. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Zacharia A., Adamson M., Boyd A., et al. Impact of shared decision making on disease-modifying drug adherence in multiple sclerosis. Int J MS Care. 2018;20:287–297. doi: 10.7224/1537-2073.2017-070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hack T.F., Degner L.F., Watson P., Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psycho Oncol. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 26.Joosten E.A., DeFuentes-Merillas L., de Weert G.H., Sensky T., van der Staak C.P., de Jong C.A. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 27.Wennberg D.E., Marr A., Lang L., O’Malley S., Bennett G. A randomized trial of a telephone care-management strategy. N Engl J Med. 2010;363:1245–1255. doi: 10.1056/NEJMsa0902321. [DOI] [PubMed] [Google Scholar]
- 28.Wilson S.R., Strub P., Buist A.S., et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korevaar J.C., Feith G.W., Dekker F.W., et al. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int. 2003;64:2222–2228. doi: 10.1046/j.1523-1755.2003.00321.x. [DOI] [PubMed] [Google Scholar]
- 30.Schiller B., Munroe H., Neitzer A. Thinking outside the box—identifying patients for home dialysis. NDT Plus. 2011;4(suppl 3):iii11–iii13. doi: 10.1093/ndtplus/sfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P.K., Chow K.M., Van de Luijtgaarden M.W., et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90–103. doi: 10.1038/nrneph.2016.181. [DOI] [PubMed] [Google Scholar]
- 32.Walker R.C., Howard K., Morton R.L. Home hemodialysis: a comprehensive review of patient-centered and economic considerations. Clinicoecon Outcomes Res. 2017;9:149–161. doi: 10.2147/CEOR.S69340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong A., Lesmana B., Johnson D.W., Wong G., Campbell D., Craig J.C. The perspectives of adults living with peritoneal dialysis: thematic synthesis of qualitative studies. Am J Kidney Dis. 2013;61:873–888. doi: 10.1053/j.ajkd.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Walker R.C., Hanson C.S., Palmer S.C., et al. Patient and caregiver perspectives on home hemodialysis: a systematic review. Am J Kidney Dis. 2015;65:451–463. doi: 10.1053/j.ajkd.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 35.8 Steps to shared decision making success. Healthwise. https://www.healthwise.org/resources/shared-decision-making-success.aspx Accessed August 2021.
- 36.Shared decision making. National Health Service. https://www.england.nhs.uk/shared-decision-making/ Accessed August 2020.
- 37.The SHARE approach. Agency for Healthcare Research and Quality (AHRQ) https://www.ahrq.gov/health-literacy/curriculum-tools/shareddecisionmaking/index.html Accessed August 2021.
- 38.Elwyn G., Frosch D., Thomson R., et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elwyn G., O’Connor A., Stacey D., et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333:417. doi: 10.1136/bmj.38926.629329. AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph-Williams N., Newcombe R., Politi M., et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Mak. 2014;34:699–710. doi: 10.1177/0272989X13501721. [DOI] [PubMed] [Google Scholar]
- 41.Stacey D., Légaré F., Lewis K., et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ameling J.M., Auguste P., Ephraim P.L., et al. Development of a decision aid to inform patients’ and families’ renal replacement therapy selection decisions. BMC Med Inform Decis Mak. 2012;12:140. doi: 10.1186/1472-6947-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winterbottom A.E., Mooney A., Russon L., et al. Kidney disease pathways, options and decisions: an environmental scan of international patient decision aids [published correction appears in Nephrol Dial Transplant. 2021;36:1140-1143] Nephrol Dial Transplant. 2020;35:2072–2082. doi: 10.1093/ndt/gfaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterbottom A.E., Gavaruzzi T., Mooney A., et al. Patient acceptability of the Yorkshire Dialysis Decision Aid (YoDDA) Booklet: a prospective non-randomized comparison study across 6 predialysis services. Perit Dial Int. 2016;36:374–381. doi: 10.3747/pdi.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prieto-Velasco M., Quiros P., Remon C. Spanish Group for the Implementation of a Shared Decision Making Process for RRT Choice with Patient Decision Aid Tools. The concordance between patients’ renal replacement therapy choice and definitive modality: is it a utopia? PLoS One. 2015;10 doi: 10.1371/journal.pone.0138811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elwyn G., Burstin H., Barry M.J., et al. A proposal for the development of national certification standards for patient decision aids in the US. Health Policy. 2018;122:703–706. doi: 10.1016/j.healthpol.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Berger I., Wu S., Masson P., et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016;14:206. doi: 10.1186/s12916-016-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Therkildsen S.B., Hansen L.H., Jensen L.E.D., Finderup J. A patient decision aid app for patients with chronic kidney disease: questionnaire study. JMIR Form Res. 2019;3 doi: 10.2196/13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dahlerus C., Quinn M., Messersmith E., et al. Patient perspectives on the choice of dialysis modality: results from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) study. Am J Kidney Dis. 2016;68:901–910. doi: 10.1053/j.ajkd.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Finderup J., Dam Jensen J., Lomborg K. Evaluation of a shared decision-making intervention for dialysis choice at four Danish hospitals: a qualitative study of patient perspective. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finderup J., Jensen J.K.D., Lomborg K. Developing and pilot testing a shared decision-making intervention for dialysis choice. J Ren Care. 2018;44:152–161. doi: 10.1136/bmjopen-2019-029090. [DOI] [PubMed] [Google Scholar]
- 52.Finderup J., Lomborg K., Jensen J.D., Stacey D. Choice of dialysis modality: patients’ experiences and quality of decision after shared decision-making. BMC Nephrol. 2020;21:330. doi: 10.1186/s12882-020-01956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho Y.F., Lin C.C. Applying shared decision making with end-stage renal disease patients undergoing renal replacement therapy to reduce decision making conflicts. Article in Chinese. Hu Li Za Zhi. 2020;66:95–102. doi: 10.6224/JN.201908_66(4).12. [DOI] [PubMed] [Google Scholar]
- 54.Brown E.A., Blake P.G., Boudville N., et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40:244–253. doi: 10.1177/0896860819895364. [DOI] [PubMed] [Google Scholar]
- 55.Lindström B.R., Bohlin G. Threat-relevance impairs executive functions: negative impact on working memory and response inhibition. Emotion. 2012;12:384–393. doi: 10.1037/a0027305. [DOI] [PubMed] [Google Scholar]
- 56.Hsu S.Y., Huang H.S. Improving depression, hope, and quality of life in dialysis patients using health promotion education groups. Article in Chinese. Hu Li Za Zhi. 2019;66:29–39. doi: 10.6224/JN.201908_66(4).05. [DOI] [PubMed] [Google Scholar]
- 57.Poorgholami F., Abdollahifard S., Zamani M., et al. The effect of stress management training on hope in hemodialysis patients. Glob J Health Sci. 2015;8:165–171. doi: 10.5539/gjhs.v8n7p165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Billington E., Simpson J., Unwin J., Bray D., Giles D. Does hope predict adjustment to end-stage renal failure and consequent dialysis? Br J Health Psychol. 2008;13:683–699. doi: 10.1348/135910707X248959. [DOI] [PubMed] [Google Scholar]
- 59.Jayanti A., Neuvonen M., Wearden A., et al. Healthcare decision-making in end stage renal disease-patient preferences and clinical correlates. BMC Nephrol. 2015;16:189. doi: 10.1186/s12882-015-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harwood L., Clark A.M. Dialysis modality decision-making for older adults with chronic kidney disease. J Clin Nurs. 2014;23:3378–3390. doi: 10.1111/jocn.12582. [DOI] [PubMed] [Google Scholar]
- 61.Cassidy B.P., Getchell L.E., Harwood L., et al. Barriers to education and shared decision making in the chronic kidney disease population: a narrative review. Can J Kidney Health Dis. 2018;5 doi: 10.1177/2054358118803322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schouten R.W., Haverkamp G.L., Loosman W.L., et al. Anxiety symptoms, mortality, and hospitalization in patients receiving maintenance dialysis: a cohort study. Am J Kidney Dis. 2019;74:158–166. doi: 10.1053/j.ajkd.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Saeed F., Ladwig S.A., Epstein R.M., Monk R.D., Duberstein P.R. Dialysis regret: prevalence and correlates. Clin J Am Soc Nephrol. 2020;15:957–963. doi: 10.2215/CJN.13781119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinski M., Mau W., Wienke A., Girndt M. The Choice of Renal Replacement Therapy (CORETH) project: dialysis patients’ psychosocial characteristics and treatment satisfaction. Nephrol Dial Transplant. 2017;32:315–324. doi: 10.1093/ndt/gfv464. [DOI] [PubMed] [Google Scholar]
- 65.Joseph-Williams N., Lloyd A., Edwards A., et al. Implementing shared decision making in the NHS: lessons from the MAGIC programme [published correction appears in BMJ. 2017;357:j2005] BMJ. 2017;357:j1744. doi: 10.1136/bmj.j1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bae J.M. Shared decision making: relevant concepts and facilitating strategies. Epidemiol Health. 2017;39 doi: 10.4178/epih.e2017048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coulter A. Shared decision making: everyone wants it, so why isn’t it happening? World Psychiatry. 2017;16:117–118. doi: 10.1002/wps.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeuner R., Frosch D.L., Kuzemchak M.D., Politi M.C. Physicians’ perceptions of shared decision-making behaviours: a qualitative study demonstrating the continued chasm between aspirations and clinical practice. Health Expect. 2015;18:2465–2476. doi: 10.1111/hex.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehrotra R., Devuyst O., Davies S.J., Johnson D.W. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27:3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaza G., Rugiu C., Trubian A., et al. How has peritoneal dialysis changed over the last 30 years: experience of the Verona dialysis center. BMC Nephrol. 2015;16:53. doi: 10.1186/s12882-015-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diouf N.T., Menear M., Robitaille H., et al. Training health professionals in shared decision making: update of an international environmental scan. Patient Educ Couns. 2016;99:1753–1758. doi: 10.1016/j.pec.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 72.Couet N., Desroches S., Robitaille H., et al. Assessments of the extent to which health-care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18:542–561. doi: 10.1111/hex.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ladin K., Pandya R., Perrone R.D., et al. Characterizing approaches to dialysis decision making with older adults: a qualitative study of nephrologists. Clin J Am Soc Nephrol. 2018;13:1188–1196. doi: 10.2215/CJN.01740218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Street R.L., Jr., Gordon H.S., Ward M.M., Krupat E., Kravitz R.L. Patient participation in medical consultations: why some patients are more involved than others. Med Care. 2005;43:960–969. doi: 10.1097/01.mlr.0000178172.40344.70. [DOI] [PubMed] [Google Scholar]
- 75.Young H.N., Bell R.A., Epstein R.M., et al. Physicians’ shared decision-making behaviors in depression care. Arch Intern Med. 2008;168:1404–1408. doi: 10.1001/archinte.168.13.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adisso E.L., Borde V., Saint-Hilaire M.E., et al. Can patients be trained to expect shared decision making in clinical consultations? Feasibility study of a public library program to raise patient awareness. PLoS One. 2018;13z doi: 10.1371/journal.pone.0208449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan C.T., Blankestijn P.J., Dember L.M., et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96:37–47. doi: 10.1016/j.kint.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 78.Bonner A., Gillespie K., Campbell K.L., et al. Evaluating the prevalence and opportunity for technology use in chronic kidney disease patients: a cross-sectional study. BMC Nephrol. 2018;19:28. doi: 10.1186/s12882-018-0830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muscat D.M., Kanagaratnam R., Shepherd H.L., Sud K., McCaffery K., Webster A. Beyond dialysis decisions: a qualitative exploration of decision-making among culturally and linguistically diverse adults with chronic kidney disease on haemodialysis. BMC Nephrol. 2018;19:339. doi: 10.1186/s12882-018-1131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chow N. The practice of filial piety and its impact on long-term care policies for elderly people in Asian Chinese communities. Asian J Gerontol Geriatr. 2006;1:31–35. [Google Scholar]
- 81.Russell S.S. An overview of adult-learning processes. Urol Nurs. 2006;26:349–352. 370. [PubMed] [Google Scholar]
- 82.Martin D.E., Parsons J.A., Caskey F., et al. Ethics of kidney care in the era of COVID-19. Kidney Int. 2020;98:1424–1433. doi: 10.1016/j.kint.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Legare F., Adekpedjou R., Stacey D., et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7:CD006732. doi: 10.1002/14651858.CD006732.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green J.A., Ephraim P.L., Hill-Briggs F.F., et al. Putting patients at the center of kidney care transitions: PREPARE NOW, a cluster randomized controlled trial. Contemp Clin Trials. 2018;73:98–110. doi: 10.1016/j.cct.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. NCT02722382. Putting patients at the center of kidney care transitions (PREPARE NOW). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02722382 Accessed August 2021. [DOI] [PMC free article] [PubMed]
- 86. NCT03465449. Feasibility of enhanced dialysis education (EDU) intervention for chronic kidney disease (CKD) patients (CKD-EDU). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03465449 Accessed August 2021.
- 87. NCT04392440. Shared decision making in dialysis (DIAL-SDM). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04392440 Accessed August 2021.