Abstract
Ubiquitin-specific protease (USP7), also known as Herpesvirus-associated ubiquitin-specific protease (HAUSP), is a deubiquitinase. There has been significant recent attention on USP7 following the discovery that USP7 is a key regulator of the p53-MDM2 pathway. The USP7 protein is 130 kDa in size and has multiple domains which bind to a diverse set of proteins. These interactions mediate key developmental and homeostatic processes including the cell cycle, immune response, and modulation of transcription factor and epigenetic regulator activity and localization. USP7 also promotes carcinogenesis through aberrant activation of the Wnt signalling pathway and stabilization of HIF-1α. These findings have shown that USP7 may induce tumour progression and be a therapeutic target. Together with interest in developing USP7 as a target, several studies have defined new protein interactions and the regulatory networks within which USP7 functions. In this review, we focus on the protein interactions of USP7 that are most important for its cancer-associated roles.
Keywords: Cancer, Deubiquitinase, Protein network, Protein–protein interaction, Proteomics, USP7
The ubiquitin proteasome system (UPS)
Ubiquitination is a key post-translational modification governing protein turnover.1 This process is essential for the cellular viability and homeostasis which exist at multiple levels starting with the control of gene transcription and translation and finishing with the degradation of damaged, unnecessary or short-term proteins.1 The ubiquitin-proteasome system (UPS) has been identified as a key regulator for the targeting of proteins especially those involved in processes such as the cell cycle, gene transcription, apoptosis and DNA repair. Through these processes, the UPS plays a role in diverse diseases such as cancer, cardiovascular diseases and Alzheimer's disease. Colorectal adenocarcinoma is a well-studied example of how important protein turnover and the UPS is in tumorigenesis.1,2 As an example, the UPS regulates Wnt/β-catenin/APC/TCF4 signalling which is implicated in the proliferation of epithelial cells in the base of colonic crypts. The frequent mutations that alter Wnt signalling in colorectal and other solid tumours do so via stabilization of β-catenin leading to cell proliferation.3
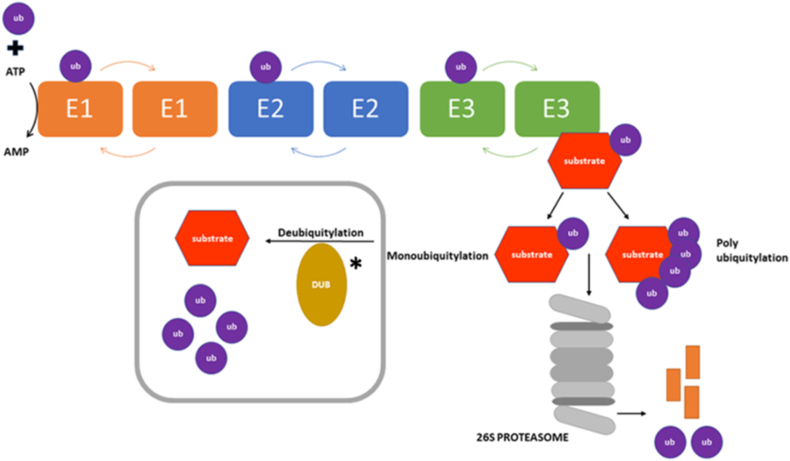
The UPS tags proteins for destruction by the covalent attachment of small protein ubiquitin (Ub) molecules and proteasomal degradation of the tagged protein (Fig. 1). The ubiquitination process requires three consecutive steps including the ATP-dependent activation performed by ubiquitin-activating (E1), ubiquitin conjugation (E2) and ubiquitin ligase (E3) enzymes of which the E3 is essentially in charge of substrate choice4 (Fig. 1). E3 stimulates the formation of an isopeptide bond between the lysine of the target protein and the carboxyl-terminus of ubiquitin. Polyubiquitination results from multiple ubiquitin links to a protein substrate and mono-Ub results from a single ubiquitin molecule linked to a protein substrate.1 In humans, there are around 600 such ubiquitin E3 ligases which are assembled into three noteworthy classes characterised by the sequence homology known as the HECT, RING or RING-between-RING.1,2 Notably, aberrant E3 ligases expression can function as tumour suppressors or oncogenes based on ubiquitin target substrates in colorectal cancer.5
Figure 1.
Key elements of the ubiquitin-proteasome system (UPS). Ubiquitin conjugation itself is mediated by the E1-E2-E3 enzymes respectively with the ubiquitin ligases (E3) targeting the ubiquitin to specific substrates. Deubiquitinase proteins (DUBs), (indicated by ∗) are proteases that act to cleave ubiquitin from substrate proteins and one of the most important effects of this is to antagonize the targeting of proteins for proteasomal degradation.
Deubiquitinating enzymes (DUBS)
Deubiquitinating enzymes (DUBs) oppose the E3 functionality by cleaving ubiquitin from from 1) ubiquitin precursor; 2) protein substrate; 3) another ubiquitin within a polyubiquitin chain.6 There are approximately 80 DUBs which are classified according to the sequence homology of the catalytic domain into six groups: ubiquitin-specific proteases (USP), ubiquitin carboxy-terminal hydrolases (UCH), ovarian-tumour proteases (OUT), Machado-Joseph disease protein domain proteases (MJD), JAMM/MPN domain-associated metallopeptidases (JAMM) and monocyte chemotactic protein-induced protein (MCPIP).6 Additionally, two groups of DUBs have been recently detected; the Monocyte Chemotactic Protein-Induced Proteins (MCPIPs) and the MINDY family (MIU-containing novel DUB family).6
Many DUBs can modify protein localization, trafficking and promote stability by deubiquitination of mono-Ub or poly-Ub chains.6 DUBs inhibitors represent a new strategy of anti-cancer therapeutic and several are now in preclinical stages of development7; in particular the USPs which are implicated in diverse human diseases and are the largest group of DUBS, comprising more than 60 human members.6
Ubiquitin-specific-peptidase 7 (USP7)
The focus of this review is USP7, also known as Herpesvirus-Associated Ubiquitin-Specific Protease (HAUSP). We focus here on the protein–protein interactions and functions of USP7. Significant progress is being made on the development of therapeutics targeting USP7 and we direct the reader to recent studies and reviews of this area which we will not cover here.8,9(p7) An early study of USP7 showed that it interacts with infected cell polypeptide 0 (ICP0) to activate Herpes simplex virus type 1 (HSV-1),10 hence the name HAUSP (in this review we refer to the protein as USP7). USP7 regulates many target proteins and interactors through its deubiquitinating activity11 and has been shown to have an essential role in the regulation of stability for proteins that are implicated in crucial cellular procedures such as mitosis, apoptosis, cell cycle, DNA replication, neuronal development, and epigenetic modulators.9 Aberrant USP7 expression was observed in several non-solid and solid tumours.9 The knockout strategy for the USP7 was lethal in mice due to the p53 activation which leads to a dramatic reduction in proliferation and termination during development.12 USP7 destabilizes the tumour-suppressor p53 creating interest in it as a target in oncology9 and recent work suggests that USP7 may also be a tumour-specific drug target for colorectal tumours with APC mutations.13
USP7 structure
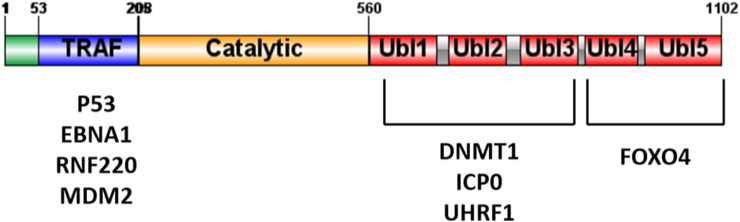
The multi-domain architecture of USP7 consists of 1102 amino acids, and facilitates its diverse roles (Fig. 2).11,14 USP7 contains a catalytic domain (208–560), N-terminal contains 50 amino acids followed by a TNF receptor-associated factor (TRAF) domain which is linked to a substrate peptide binding and five C-terminal ubiquitin-like (UBL) domains.11,14 The UBL domains 1, 2 and 3 are implicated in binding interactions with ICP0, DNA methyltransferase-1(DNMT1), MDM2, and Ubiquitin-like with PHD and Ring Finger Domains 1 (UHRF1) and UBL domains 4 and 5 are fundamental for the full deubiquitinated activity.14 The C-terminal residues are essential to activate the catalytic activity of USP7, while the N terminus of USP7 plays a role in nuclear localization. Of particular note, the TRAF domain is necessary for identification of target proteins but is not implicated in the deubiquitinating activity on Ub substrate.15 USP7 requires an accurate regulation of activity to function and in order to maintain full activity, its Ub-like domain is folded back onto the catalytic domain.11
Figure 2.
Overview of motif structure of the USP7 protein showing the TNF-receptor associated factor (TRAF) domain, papain-like catalytic domain (Catalytic), and multiple Ubiquitin-like (Ubl) domains. The TRAF and Ubl domains of USP7 mediate distinct protein–protein interactions.
USP7 regulation of Wnt signalling
CID-deleted APC mutants lead to accumulation of β-catenin, activating Wnt signalling,16,17 and a role for USP7 in this has been discovered. Specifically, USP7 binds the N-terminus of β-catenin when the APC losses CID confirming the essential role of USP7 in the activation of Wnt signalling and cell survival in colorectal cancers.13 Ma et al showed that the USP7/RNF220 complex activates the Wnt pathway while knockdown of USP7 using a small interfering RNA (siRNA) strategy, reduces the expression of Wnt.18 Conversely, a recent study proposed that USP7 deubiquitinates β-catenin and RNF220 independently and works as a tumour-specific condition when APC is mutated. Additionally, USP7 inhibition does not affect the Wnt activation physiologically.13
Adenomatous Polyposis Coli (APC) is one of the components of the destruction complex that regulates β-catenin through phosphorylation and ubiquitination leading to its degradation in the cytoplasm.19 β-catenin is the key modulator of the Wnt signalling pathway with a mutation in APC causing an aberrant Wnt activation which is observed in most colorectal cancers.13 Previous studies revealed that the CID domain in the APC, which is located between the second and third twenty amino acid repeats, is responsible for inhibiting β-catenin.16 The CID is located in the right of the mutation cluster region (MCR) which is mutated in most colorectal cancer patients. Despite the significance of the CID role, the DUBs role in regulating β-catenin is not fully understood in colorectal cancer.13 The data of Novellasdemunt et al highlighted the relationship between USP7 and APC in Wnt signalling, particularly in colorectal cancer. The CID domain in the APC protects β-catenin from USP7-mediated deubiquitination and promotes E3 ligase b-TrCP ubiquitination.13
The study of Ma et al suggested that the USP7 binds the β-catenin in conjunction with the E3 ligase RNF220 whereas the Novellasdemunt et al data showed that the interaction between them is RNF220 and p53 independent.13 RNF220 is a RING-type E3 ubiquitin ligase and has been identified as a regulator of β-catenin.13,18,20 RNF220 stabilizes β-catenin instead of promoting its ubiquitination and proteasomal degradation and therefore promotes canonical Wnt signalling in colon cancer cells. USP7 interacts with RNF220 forming a complex that plays a role in deubiquitinating the β-catenin. The ring domain which is located in the C-terminus of RNF220 is responsible for interacting with β-catenin18 while RNF220 interacts with the MATH domain of USP7, which is the same domain shown to interact with MDM2 and p53.21
Interestingly, recent work has also showed that USP7 can negatively regulate canonical Wnt signalling.22 Ji et al showed that Axin, a key scaffolding protein and component of the β-catenin destruction complex, is stabilized by USP7-mediated deubiquitination through an interaction with the Axin TRAF domain. Knocking down USP7 promoted Axin degradation and Wnt/β-catenin activity. In addition, this function of USP7 was showed to be important in diverse cellular systems including adipocyte and osteoblast differentiation.22
USP7 and epigenetic regulators
DNA methyltransferase 1 (DNMT1) is an enzyme with a key role in DNA methylation through methylation of CpG islands located close to the regulatory regions of the genes throughout replication. These regions have an important role in the cellular process and develop cancer. DNA methylation is a key controller of many epigenetic processes; for instance, it regulates differentiation, transcription and repairs the DNA. During replication, DNMT1 plays a role in maintaining the methylation on the newly synthesised daughter strand.23 DNMT1 is responsible also for various epigenetic pathways and the E3 ubiquitin ligase UHRF1 ubiquitinates DNMT1 and USP7 deubiquitinates it.24,25 Notably, DNMT1 stability is linked to the expression levels of these proteins and inactivation of DNMT1 activity results in several diseases, including cancer. The over expression of DNMT1 has been found in different tumour types including bladder renal and prostate cancers.26 Ubiquitin-like, containing PHD and RING finger domains, 1 (UHRF1) is E3 ubiquitin ligase and plays an essential role in regulating the cellular process such as DNMT1 ubiquitination.24 UHRF1 is a multi-domain protein with an N-terminus composed of a ubiquitin-like (UBL) domain, tandem Tudor domain (TTD), a plant homeodomain (PHD), a SET-and-RING-associated (SRA) domain and a RING domain. The first two UBL domains of USP7 and polybasic region (PBR) of UHRF1 mediate the interaction between the USP7 and UHRF1.24 In vivo, USP7 regulates UHRF1 stability by targeting it for deubiquitination, whereas, the enzymatic activity of DNMT1 was catalysed by USP7 in vivo and in vitro.27 Autoubiquitylation activity has been shown to decrease due to the interaction between USP7, C223S and UHRF1, while autoubiquitylation was eliminated by an active USP7.27 Functionally, USP7, in addition to its role in deubiquitination, is also required for deactivating the intra-molecular TTD-PBR interaction during the S phase,24 whereas, USP7 dissociates from the UHRF1 resulting in its ubiquitination during cell mitosis.28 Cellular growth in colon cancer cell lines HCT116 and SW620 was suppressed by knockdown of UHRF1 expression. UHRF1 expression was increased in about two-thirds of samples in a colorectal cancer study, suggesting that UHRF1 may play a role in the cellular proliferation of colorectal cancer.29
Overexpressed SUMO-1 was found in human colon cancer and caused accumulation of p53 protein, which may be involved in tumour aggressiveness.30 Interestingly, the high levels of SUMO and low levels of ubiquitin environment is necessary for the DNA replication to induct a nascent chromatin. USP7 helps to reach this level of SUMO/Ub by deubiquitinating SUMO during the firing and replication forks progression.31 These findings identified SUMO2/3 as a substrate of USP7 and defined the role of USP7 in stabilization of other SUMOylated proteins during DNA replication.31 SUMO2 is deubiquitinated by USP7 which cleaves mono and polyubiquitin from the SUMO2 Chains.32
USP7 is not the only factor that controls the Ub/SUMO levels at the replication forks; segregase p97 collaborate with the USP7 in this process.33 Of note, the USP7 inhibitor P22077 decreases 5-ethynyl-2′-deoxyuridine (EdU) and raises the phosphorylated histone H2AX (γH2AX) leading to the conclusion that USP7 inhibits replication stress.32 Moreover, the study of Lecona et al using HCT116 cells treated with USP7 inhibitors P22077, P5091 and HBX19818 showed a marked reduction fork speed rate and the firing of new origins.
Histone methyltransferase (SUV39H1) mediates histone H3 lysine9 trimethylation (H3K9) at pericentric heterochromatin. SUV39H1/2 plays a role in the H3 K9me3 modification at telomeric heterochromatin and the knockout of SUV39H1 decreased pericentric H3 K9me3 levels.34 SUV39H1 has a crucial role in heterochromatin maintenance and it regulates the efficacy of double-strand breaks repair.34 SUV39H1 is ubiquitinated and targeted for degradation by MDM2, specifically lysine 87. In the absence of p53, USP7 deubiquitinates SUV39H1 providing protection from MDM2 ubiquitination and USP7, MDM2 and SUV39H1 form a DNA independent trimeric complex.35
Finally, the role of US7 in regulation of epigenetic regulators is evidenced by the interaction of USP7 with LSD1. Lysine-specific demethylase 1 (LSD1) was the first characterized histone that demethylases mono-methyl and di-methyl from histone H3 lysine4 (H3K4) and H3 lysine 9 (H3K9) through a flavin adenine dinucleotide (FAD)-dependent monoamine oxidoreductase.36 LSD1 has been shown to act in diverse cancer signalling pathways and is considered a promising new epigenetic target in particular for leukaemias and lung cancers.37 LSD1 and USP7 were shown to be co-associated in vitro and in vivo and their expression levels were higher in glioma patients and were correlated with glioma progression,38 and LSD1 has been proposed as a target of USP7 in glioma.38
USP7 roles in the p53 pathway
USP7 plays a fundamental role in stabilizing both p53 and MDM2 by deubiquitinating p53. Mutations in the p53 gene, specifically the DNA binding domain, were discovered in 1979 and have been linked to half of the human cancer cases. p53, as a tumour-suppressor, exerts an essential role in signalling associated with the control of cell survival and aberrant p53 signalling results in unchecked cell growth and the initiation of cancer.
USP7 interacts with a range of protein targets in the p53 pathway (p53, MDMX, MDM2)39 and MDM2 plays a key role in p53 stabilization by increasing the ubiquitination process.39 USP7 deubiquitinates MDM2 and its functional regulator MdmX and stabilizes p53.39 USP7 apparently has 2 independent p53 regulatory functions, stabilizing p53 through deubiquitylation and regulating the sequence-specific DNA binding of p53.40 Within the USP7, MDM2 and p53 complex, p53 and the MDM2 have been shown to bind USP7 through the TRAF domain.39 Under normal conditions, USP7 prefers MDM2 as a substrate rather than p53 and MDM2 has a higher binding affinity for USP7 compared to p53.41 Reduction of USP7 expression levels leads to p53 stabilisation, and destabilization of MDM2. In vivo, USP7 ablation in mouse embryos showed p53 activation, without an increase in apoptosis.12 Previous studies have shown that MDM2 represents the second direct target of USP7 and MDMX as a third target link to the p53 pathway.39 In addition, MDM2 promotes the ubiquitination of MDMX and its proteasomal degradation.42 It has also been shown that both up or down-regulation of USP7 protein expression reduces cell proliferation in colon cancer and tumour development in vivo, as a consequence of increasing p53 levels.43
Interleukin-6 (IL-6) stimulates STAT3 activation in normal physiological conditions and is controlled via negative feedback mechanisms. In pathological conditions, aberrant IL-6 affects STAT3 signalling in cancer cells which play a crucial role in cancer initiation and development and thus tumour progression. USP7 mRNA and protein levels are decreased in colon cancer cells and IL-6 stimulates STAT3 activation suppressing USP7 expression, resulting in the degradation of p53.44,45
Recent studies have shown new interacting proteins for USP7. FAM188B is a novel gene and its mRNA is found overexpressed in many solid tumours including colorectal tumours. Knockdown of FAM188B stimulated cell growth inhibition, through an increase of apoptosis in colon cancer cell lines.46 Proteomic analysis of FAM188B immunocomplexes identified p53 and USP7 as interaction partners of FAM188B. Knockdown of FAM188B led to an overall decrease in ubiquitination of p53 immunocomplexes and p53, further implicating USP7 in p53 deubiquitination.46
USP7 and the cell cycle
E3 ubiquitin-protein ligase CHFR is a checkpoint protein with FHA and RING domains and a member of the RING family and it is known as a mitotic checkpoint. The phospho–protein interaction occurs in the N-terminal FHA domain in Chfr whereas the RING finger domain is responsible for protein ubiquitination.47 For the ligase activity and for autoubiquitination, the Chfr requires the RING finger domain. Chfr has a key role in tumour suppression, cell cycle progression and in controlling the key mitotic proteins expression levels to ensure chromosomal stability.47 Chfr is a tumour suppressor gene which promoter CpG island methylation silence it in many human cancers and the CHFR promoter hypermethylation was detected in 40% in colorectal cancer. Chfr hypermethylation is a valuable prognostic marker for colorectal cancer.48 USP7 interacts with Chfr, avoiding degradation through deubiquitination and so increasing the stability of Chfr, both, in vivo and vitro.47
Proliferating Cell Nuclear Antigen (PCNA) was found as an independent prognostic factor for colorectal cancer49 PCNA post-translational mono-ubiquitination is a significant phenomenon in regulating pathways of the translesion DNA synthesis (TLS) pathway. In order to stalled replication forks, the recruitment of human DNA polymerase h, which is held responsible for the Xeroderma pigmentosum variant (cancer-prone syndrome), as well as other DNA polymerases (Y-family) —i (Poli), REV1 and polymerase k (Polk) have PCNA and ubiquitin-interacting domains.50 The error-prone DNA polymerases are activated by the Mono-ubiquitinated PCNA; thus, the regulation of PCNA is fundamental to avoiding mutagenesis. USP7 can deubiquitinate PCNA alongside a related DUB, USP1.50 As a response to the DNA damage, the E3 ubiquitin ligase RAD18 ubiquitinates PCNA stimulated by UV irradiation or interactive oxygen species.50 USP7 engages in DNA damage responses, for instance, it interacts with UV-stimulated scaffold protein A (UVSSA) to repair transcription-coupled nucleotide excisions.51 USP7 also regulates the chromatin structure to repair oxidative DNA lesions. Moreover, the USP7 enhances UV and H2O2 stimulates the PCNA mono-ubiquitination by regulating the stability of Polη; therefore, the USP7 inhibits the H2O2 stimulated mutagenesis including DNA repair by the down-regulation of RAD18.50 RAD18 promotes translesion synthesis (TLS) to simplify the replication of damaged genomes, thus a loss of Rad18 causes an increase in stalled forks resulting in a deficient genome replication. USP7 deubiquitinates RAD18 which leads to stabilisation and up-regulation of PCNA ubiquitination. USP7 can releases Rad18-dependent poly-ubiquitin chains both in vivo and vitro.52
Regulation of transcription factors
Fork head box O (FOXO) are transcription factors which regulate various biological processes such as cellular metabolism, apoptosis and cell-cycle regulation. Multiple post-translational modifications such as phosphorylation, acetylation and polyubiquitination regulate the FOXO activity. Increases in cellular oxidative stress results in the monoubiquitination of FOXO.53 Monoubiquitination of FOXO4 stimulates nuclear localization and raises transcriptional activity. USP7 links to FOXO4 to deubiquitinate it in response to oxidative stress, and USP7 do not affect the half-life of FOXO protein. The results of the interaction between the USP7 and FOXO4 in H1299 human lung carcinoma cells that lack p53 suggested that the interaction is p53 independent.45,53 However, p53 and FOXOs have noticeable similarities such as the ability to stimulate cell-cycle arrest and cell death and they are both regulated by USP7.53
N-Myc protein expression and hence its function is destabilized in a knockdown of USP7 resulting in decreased tumorigenesis both in vivo and vitro. Interestingly, USP7 inhibitors can regulate N-Myc protein levels to prevent tumorigenesis in a p53-independent manner.54 c-MYC is one of the oncogenic transcription factors which has recently been found to be a USP7 substrate. USP7 regulates c-MYC expression and USP7 over-expression leads to up-regulation of c-MYC at both the protein and mRNA level, implicating USP7 in cancer cell signalling.55
USP7 affects the immune response
Foxp3+T-regulatory (Treg) cells play a role in suppressing the immune responses to diverse solid tumours and the maintenance of self-tolerance. The histone/protein acetyltransferase KAT5 which is commonly known as Tip60 promotes acetylation and functioning in Treg cells.56 The development of the regulatory T (Treg) cells function requires the stability of the Foxp3 expression.45
Indeed, USP7 controls over the Treg function primarily by ensuring the stability of Tip60 and Foxp3 expression and stimulating their multimerization. This leads to target USP7 genetically or pharmacologically to reduce the suppressive functions of Foxp3+ Treg whereas the normal T cell responses remain intact.56 In other words, USP7 deubiquitinates Foxp3, increasing Foxp3 protein levels and subsequently up-regulating Treg cells.
A knockdown of USP7 expression in the Treg cells leads to a decrease in the Foxp3 protein levels reducing the suppression of the Treg-cell in vitro and vivo.45 USP7 inhibitors can affect the tumour growth limitation in immunocompetent mice and may have a key role in future cancer immunotherapies.56
USP7 has also been implicated in regulation of the inflammasome. The inflammasome is a major immune signalling pathway that is regulated by post-translational modifications, including ubiquitination and counteracting deubiquitination. When macrophages recognize danger signals, they assemble a molecular complex called the NLRP3 inflammasome. Inflammasome activation is regulated by USP7 and USP47 in macrophages, and the inhibition of USP7 and USP47 leads to alterations in the ubiquitination status of NLRP3. In response to inflammasome activators, the activity of USP7 and USP47 is increased in macrophages; vice versa, knocking down both USP7 and USP47 decreases inflammasome activation. These results suggest that targeting USP7 and USP47 using inhibitors could be a potential therapeutic approach for inflammatory disease.57
USP7 regulates viral proteins
USP7 has been shown to interact with proteins from several different viruses,58 and one of the best understood interactions is with EBNA1, the Epstein–Barr virus nuclear antigen 1. EBNA1 is one of the USP7 interacting partners which can affect cellular processes such as p53 function. EBNA1 is also known as a DNA-binding protein that is necessary for the replication, segregation and maintenance of the EBV genome.59 EBV infection caused considerable differences in the expression of EBNA-1 associated with colorectal carcinoma in both high and low grade.60 EBNA1 interacts with USP7 via the same N-terminal domain (TRAF) which also binds p53 and many other USP7 interaction partners. Interestingly, EBNA1 may have higher affinity for USP7 than other host cellular proteins thus preventing the deubiquitination of other substrates such as p53, which may enhance the survival of EBV-infected cells.61 USP7 binds EBNA1 at amino acids 395–450 and a mutation in EBNA1 which disrupts USP7 binding causes a 4-fold increase in EBNA1 replication activity. However, no impact was shown on either cell-surface presentation or EBNA1 turnover. According to the findings, USP7 is capable of regulating of EBNA1, whereas EBNA1 might have an impact on the cellular process through the sequestering of important regulatory proteins.58,62
USP7 and hypoxia
Hypoxia Inducible Factor 1 (HIF-1α) is a master regulator of the cellular response to hypoxia and is implicated in diverse tissues and diseases where hypoxic conditions are found. In colorectal cancer, for example, HIF-1α was overexpressed in 142 tumours across 731 colorectal cancers and was associated with higher mortality. HIF-1α plays an oncogenic role in colorectal cancer by up-regulating numerous proangiogenic factors.63,64 One important role in many solid tumours is in regulation of Epithelial-mesenchymal transition (EMT). This process is promoted by the Hypoxia/HIF-1α and regulated by transcriptional regulators such as the ZEB1, Snail, and Twist1.65 HIF-1α is stabilized at the post-translational modification during ubiquitination by oncogenic MDM2 and p53 interacts with HIF-1a but indirectly via MDM2.66 USP7 deubiquitinates and stabilizes HIF-1α which can promote EMT and metastasis.65
Sirtuin 7 (SIRT7), one of the NAD+-dependent class of histone 3 deacetylases (HDACs)has regulatory roles in diverse cellular processes such as ageing, cellular homeostasis, DNA repair and cancer.67 SIRT7 is a marker of colorectal cancer prognosis due to its essential role in the development and progression of colorectal cancer.68 It also plays a role in resisting several stresses such as low glucose levels, DNA damage and hypoxia.69 The control of glycolysis is linked to SIRT7, attributed to its role in glucose metabolism. A number of transcriptional and post-transcriptional mechanisms underpin the regulation of SIRT7 activity. USP7 has been shown to interact with SIRT7, both, in vivo and in vitro.70 SIRT polyubiquitinates at Lys 63 which is deubiquitinated by the USP7.70 Interestingly, USP7 was not shown to have an effect on SIRT7 stability although it does repress its enzyme activity. An important function of this interaction seems to be the regulation of gluconeogenesis as USP7 and SIRT7 were shown to regulate glucose-6-phosphatase catalytic sub-unit (G6PC) expression.70
Proteomics analyses of the USP7 interaction network
The majority of proteins rarely perform only a single function, but function in a variety of cellular processes interacting with a range of different molecules. The complexity of these interactions is further increased by the temporal and spatial variation of proteins, and temporally or spatially-dependent interactions may be regulated by post-translational modifications. Defining protein–protein interactions through proteomics can lead to dramatic leaps in our understanding of protein function. Proteomic techniques are providing detailed maps of protein–protein interaction networks using techniques such as two-hybrid, mass-spectrometry, protein fragment complementation assays (PCAs) and protein microarrays and mass spectrometry.71,72
USP7 has been the subject of several proteomic studies, and here we highlight selected studies to illustrate the different approaches. A landmark study of DUB protein interactions aimed to identify the Human Deubiquitinating Enzyme Interaction Landscape by a global proteomic analysis of Dubs and their associated protein complexes.73 One of the significant challenges in the protein interactome studies is to distinguish the interactions from those that are specifically associated with the isolated protein of interest. Sowa and his lab group developed the Comparative Proteomic Analysis Software Suite (CompPASS), which utilizes an unbiased method for the identification of High Confidence Candidate Interacting Proteins (HCIPs). Out of the 2458 identified proteins in their Dub data set, 774 HCIPs are associated with 75 Dubs.73 In addition, interactome topology classification, Gene Ontology, sub-cellular localization and functional studies were used to link the Dubs to various processes such as DNA damage, protein turn-over, RNA processing, transcription and endoplasmic reticulum-associated degradation.73
In gastric carcinoma cells, affinity purification, in conjunction with mass spectrometry, was performed for pinpointing USP7 binding targets.74,75 The findings of this study are in concordance with those of previous reports as per which TRIP12 E3 ubiquitin ligase, PPM1G phosphatase, and USP11 were associated with USP7. Furthermore, in addition to demonstrating these bindings' specificity, new interactions have been identified for USP7, DDX24 and DHX40. DHX40 uses the Ubl2 domain to engage with USP7. On the other hand, USP7 is bound by DDX24, USP11, PPM1G, and TRIP12 via its TRAF domain.74 Another study used affinity purification in conjunction with mass spectrometry for profiling USP7 interaction on FLAG-tagged USP7 expressed in AGS gastric carcinoma cells. According to the data, USP7 rescues FBXO38 from proteasomal degradation and plays a role in FBXO38 stability. Subsequently, a BioID approach was used for profiling the protein interactions and FBXO38's putative functions. Notably, the BioID approach is a viable beneficial method of checking both neighbouring and interacting proteins in their natural cellular environment.75,76
Other key protein–protein interactions discussed in this review were initially identified through mass-spectrometry. The interaction between USP7, DNMT1 and UHRF1 has been characterized using mass-spectrometry.25,27,77 It was shown that UHRF1 plays role in ubiquitination and subsequent proteasomal degradation of DNMT1 and UHRF1 ubiquitinates DNMT1 tightly and temporally during the cell cycle. USP7 helps ubiquitin ligase activity of UHRF1 to be under control by first counteracting UHRF1 enzymatic activity via direct deubiquitylation of DNMT1. Next, the E3 ubiquitin ligase activity of UHRF1 is hypothesized to be directly decreased by USP7. In agreement with the hypothesis with the hypothesis, UHRF1 overexpression has been shown higher ubiquitination of a DNMT1 lacking the USP7 interaction domain in comparison with full-length DNMT1. For this reason, USP7 must be cleaved from DNMT1 complexes before DNMT1 degradation.25
A related approach using global quantitative proteomics rather than affinity-purification mass-spectrometry used knockdown of USP7 to determine USP7 interactions and identify its biological functions.78 Stably transfected cells were generated that carried inducible shRNA expression plasmids, the USP7 and USP7 mRNA levels' protein expression was strongly down-regulated 48–72 h after shRNA induction. The proteomics experiment entailed the comparison of a selected clone to whole cell proteomes before and after the knockdown of USP7. It was found that 36 proteins were altered after knockdown of USP7 and were analysed using mass spectrometry. Among these 36 proteins is Alix/HP95, a protein involved in endosomal organization that also plays a key role in virus budding, was reduced after downregulating USP7 levels.78
Finally, in myeloma cells, mass spectrometry was used for identifying proteins associated with MafB ubiquitination. According to the findings, the presence of USP7 was observed in the MafB interactome. Also, USP7 interacts with MafA, as well as c-Maf, preventing their degradation and polyubiquitination. Consistently, USP7 knockdown led to the degradation of Maf in addition to increased levels of polyubiquitination. At the same time, USP7 was observed to be upregulated in myeloma cells besides having a negative association with the survival of myeloma patients. USP7 stimulates myeloma cell survival, and inhibition of USP7 levels using P5091 Inhibitor caused apoptosis in myeloma cell lines.79
Summary
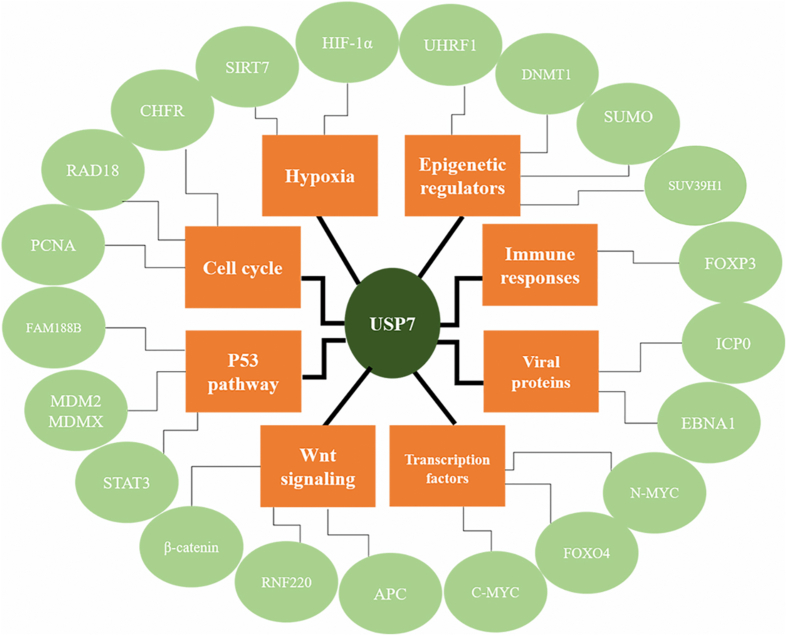
In summary, multiple proteins are known to interact with USP7 and it is possible that these proteins are also USP7 targets (Fig. 3). They may also comprise entirely novel complexes, and E3 ubiquitin ligases account for a large number of these, for example, the RNF220 complex with USP7 TRAF domain, stimulating deubiquitination of β-catenin in the canonical Wnt pathway.11 Fig. 3 summarizes the known USP7 protein network with respect to cancer and tumorigenesis. Although perhaps it best studied role is as a regulator of the p53–MDM2 pathway, emerging studies are defining the interactions and roles of USP7 in the Wnt pathway, cell cycle and hypoxia. In addition, more evidence has shown that USP7 is a protein stabiliser that stabilises tumor suppressors, transcription factors and proteins responsible for immune responses. Targeting USP7 may open up new horizons as a novel molecular target for the treatment of cancers including colorectal cancer.
Figure 3.
A summary of the roles and known interaction partners of USP7 in cancer. The principal known processes and pathways within which USP7 functions are shown in orange and known interaction partners and substrates corresponding to these functional categories are shown in green.
Conflicts of interests
The authors declare no conflicts of interest.
Funding
This work was supported by the Medical Research Council, UK (No. MR/S01411X/1) and the Saudi Arabia Cultural Bureau in London (No. DMU500).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Ahood Al-Eidan, Email: A.A.A.Aleidan@soton.ac.uk.
Yihua Wang, Email: yihua.wang@soton.ac.uk.
Paul Skipp, Email: pjss@soton.ac.uk.
Rob M. Ewing, Email: rob.ewing@soton.ac.uk.
References
- 1.Voutsadakis I.A. The ubiquitin-proteasome system in colorectal cancer. Biochimica et Biophysica Acta. 2008;1782(12):800–808. doi: 10.1016/j.bbadis.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Mofers A., Pellegrini P., Linder S., D'Arcy P. Proteasome-associated deubiquitinases and cancer. Cancer Metastasis Rev. 2017;36(4):635–653. doi: 10.1007/s10555-017-9697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauriello D.V.F., Maurice M.M. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle. 2010;9(18):3700–3709. doi: 10.4161/cc.9.18.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu C.W., Jacobson A.D. Functions of the 19S complex in proteasomal degradation. Trends Biochem Sci. 2013;38(2):103–110. doi: 10.1016/j.tibs.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L., Wong C.C., Gong B., Yu J. Functional significance and therapeutic implication of ring-type E3 ligases in colorectal cancer. Oncogene. 2018;37(2):148–159. doi: 10.1038/onc.2017.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes-Turcu F.E., Ventii K.H., Wilkinson K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78(1):363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Lello P., Pastor R., Murray J.M., et al. Discovery of small-molecule inhibitors of ubiquitin specific protease 7 (USP7) using integrated NMR and in-silico techniques. J Med Chem. 2017;60(24):10056–10070. doi: 10.1021/acs.jmedchem.7b01293. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull A.P., Ioannidis S., Krajewski W.W., et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature. 2017;550(7677):481–486. doi: 10.1038/nature24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Kang W., You Y., et al. USP7: novel drug target in cancer therapy. Front Pharmacol. 2019;10:427. doi: 10.3389/fphar.2019.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R.D., Meredith M., Orr A., Cross A., Kathoria M., Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16(3):566–577. doi: 10.1093/emboj/16.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim R.Q., Sixma T.K. Regulation of USP7: a high incidence of E3 complexes. J Mol Biol. 2017;429(22):3395–3408. doi: 10.1016/j.jmb.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Kon N., Kobayashi Y., Li M., Brooks C.L., Ludwig T., Gu W. Inactivation of HAUSP in vivo modulates p53 function. Oncogene. 2010;29(9):1270–1279. doi: 10.1038/onc.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novellasdemunt L., Foglizzo V., Cuadrado L., et al. USP7 is a tumor-specific WNT activator for APC -mutated colorectal cancer by mediating β-catenin deubiquitination. Cell Rep. 2017;21(3):612–627. doi: 10.1016/j.celrep.2017.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rougé L., Bainbridge T.W., Kwok M., et al. Molecular understanding of USP7 substrate recognition and C-terminal activation. Structure. 2016;24(8):1335–1345. doi: 10.1016/j.str.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Faesen A.C., Dirac A.M.G., Shanmugham A., Ovaa H., Perrakis A., Sixma T.K. Mechanism of USP7/HAUSP activation by its C-Terminal ubiquitin-like domain and allosteric regulation by GMP-synthetase. Mol Cell. 2011;44(1):147–159. doi: 10.1016/j.molcel.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Kohler E.M., Chandra S.H.V., Behrens J., Schneikert J. Beta-Catenin degradation mediated by the CID domain of APC provides a model for the selection of APC mutations in colorectal, desmoid and duodenal tumours. Hum Mol Genet. 2009;18(2):213–226. doi: 10.1093/hmg/ddn338. [DOI] [PubMed] [Google Scholar]
- 17.Roberts D.M., Pronobis M.I., Poulton J.S., et al. Deconstructing the sscatenin destruction complex: mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol Biol Cell. 2011;22(11):1845–1863. doi: 10.1091/mbc.E10-11-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma P., Yang X., Kong Q., et al. The ubiquitin ligase RNF220 enhances canonical Wnt signaling through USP7-mediated deubiquitination of -catenin. Mol Cell Biol. 2014;34(23):4355–4366. doi: 10.1128/MCB.00731-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong Q., Zeng W., Wu J., Hu W., Li C., Mao B. RNF220, an E3 ubiquitin ligase that targets Sin3B for ubiquitination. Biochem Biophys Res Commun. 2010;393(4):708–713. doi: 10.1016/j.bbrc.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 21.Hu M., Gu L., Li M., Jeffrey P.D., Gu W., Shi Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: implications for the regulation of the p53-MDM2 pathway. PLoS Biol. 2006;4(2):228–239. doi: 10.1371/journal.pbio.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji L., Lu B., Zamponi R., et al. USP7 inhibits Wnt/β-catenin signaling through promoting stabilization of Axin. Nat Commun. 2019;10(1):4184. doi: 10.1038/s41467-019-12143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitag M., Selker E.U. Controlling DNA methylation: many roads to one modification. Curr Opin Genet Dev. 2005;15(2):191–199. doi: 10.1016/j.gde.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z.M., Rothbart S.B., Allison D.F., et al. An allosteric interaction links USP7 to deubiquitination and chromatin targeting of UHRF1. Cell Rep. 2015;12(9):1400–1406. doi: 10.1016/j.celrep.2015.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Z., Song J., Wang Y., et al. DNMT1 stability is regulated by proteins coordinating deubiquitination and acetylation-driven ubiquitination. Sci Signal. 2010;3(146):ra80. doi: 10.1126/scisignal.2001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varol N., Konac E., Bilen C.Y. Does Wnt/β-catenin pathway contribute to the stability of DNMT1 expression in urological cancer cell lines? Exp Biol Med. 2015;240(5):624–630. doi: 10.1177/1535370214556951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felle M., Joppien S., Németh A., et al. The USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1 and regulates the stability of UHRF1. Nucleic Acids Res. 2011;39(19):8355–8365. doi: 10.1093/nar/gkr528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H., Chen H., Guo X., et al. M phase phosphorylation of the epigenetic regulator UHRF1 regulates its physical association with the deubiquitylase USP7 and stability. Proc Natl Acad Sci Unit States Am. 2012;109(13):4828–4833. doi: 10.1073/pnas.1116349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kofunato Y., Kumamoto K., Saitou K., et al. UHRF1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol Rep. 2012;28(6):1997–2002. doi: 10.3892/or.2012.2064. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Kuai X., Ji Z., Li Z., Shi R. Over-expression of small ubiquitin-related Modifier-1 and sumoylated p53 in colon cancer. Cell Biochem Biophys. 2013;67(3):1081–1087. doi: 10.1007/s12013-013-9612-x. [DOI] [PubMed] [Google Scholar]
- 31.Lecona E., Fernandez-Capetillo O. A SUMO and ubiquitin code coordinates protein traffic at replication factories. Bioessays. 2016;38(12):1209–1217. doi: 10.1002/bies.201600129. [DOI] [PubMed] [Google Scholar]
- 32.Lecona E., Rodriguez-Acebes S., Specks J., et al. USP7 is a SUMO deubiquitinase essential for DNA replication. Nat Struct Mol Biol. 2016;23(4):270–277. doi: 10.1038/nsmb.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits V.A.J., Freire R. USP7/HAUSP: a SUMO deubiquitinase at the heart of DNA replication. Bioessays. 2016;38(9):863–868. doi: 10.1002/bies.201600096. [DOI] [PubMed] [Google Scholar]
- 34.Zheng H., Chen L., Pledger W.J., Fang J., Chen J. P53 promotes repair of heterochromatin DNA by regulating JMJD2b and SUV39H1 expression. Oncogene. 2014;33(6):734–744. doi: 10.1038/onc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mungamuri S.K., Qiao R.F., Yao S., Manfredi J.J., Gu W., Aaronson S.A. USP7 enforces heterochromatinization of p53 target promoters by protecting SUV39H1 from MDM2-mediated degradation. Cell Rep. 2016;14(11):2528–2537. doi: 10.1016/j.celrep.2016.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Jie W., Yan W., Zhou K., Xiao Y. Lysine-specific histone demethylase 1 (LSD1): a potential molecular target for tumor therapy. Crit Rev Eukaryot Gene Expr. 2012;22(1):53–59. doi: 10.1615/critreveukargeneexpr.v22.i1.40. [DOI] [PubMed] [Google Scholar]
- 37.Fu X., Zhang P., Yu B. Advances toward LSD1 inhibitors for cancer therapy. Future Med Chem. 2017;9(11):1227–1242. doi: 10.4155/fmc-2017-0068. [DOI] [PubMed] [Google Scholar]
- 38.Yi L., Cui Y., Xu Q., Jiang Y. Stabilization of LSD1 by deubiquitinating enzyme USP7 promotes glioblastoma cell tumorigenesis and metastasis through suppression of the p53 signaling pathway. Oncol Rep. 2016;36(5):2935–2945. doi: 10.3892/or.2016.5099. [DOI] [PubMed] [Google Scholar]
- 39.Sheng Y., Saridakis V., Sarkari F., et al. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nat Struct Mol Biol. 2006;13(3):285–291. doi: 10.1038/nsmb1067. [DOI] [PubMed] [Google Scholar]
- 40.Sarkari F., La Delfa A., Arrowsmith C.H., Frappier L., Sheng Y., Saridakis V. Further insight into substrate recognition by USP7: structural and biochemical analysis of the HdmX and Hdm2 interactions with USP7. J Mol Biol. 2010;402(5):825–837. doi: 10.1016/j.jmb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Colland F., Formstecher E., Jacq X., et al. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol Cancer Therapeut. 2009;8(8):2286–2295. doi: 10.1158/1535-7163.MCT-09-0097. [DOI] [PubMed] [Google Scholar]
- 42.Pan Y., Chen J. MDM2 promotes ubiquitination and degradation of MDMX. Mol Cell Biol. 2003;23(15):5113–5121. doi: 10.1128/MCB.23.15.5113-5121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becker K., Marchenko N.D., Palacios G., Moll U.M. A role of HAUSP in tumor suppression in a human colon carcinoma xenograft model. Cell Cycle. 2008;7(9):1205–1213. doi: 10.4161/cc.7.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhi Y., Shoujun H., Yuanzhou S., et al. STAT3 repressed USP7 expression is crucial for colon cancer development. FEBS Lett. 2012;586(19):3013–3017. doi: 10.1016/j.febslet.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 45.VanLoosdregt J., Fleskens V., Fu J., et al. Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases treg-cell-suppressive capacity. Immunity. 2013;39(2):259–271. doi: 10.1016/j.immuni.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi E.S., Lee H., Sung J.Y., et al. FAM188B enhances cell survival via interaction with USP7. Cell Death Dis. 2018;9(6):633. doi: 10.1038/s41419-018-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh Y.M., Yoo S.J., Seol J.H. Deubiquitination of Chfr, a checkpoint protein, by USP7/HAUSP regulates its stability and activity. Biochem Biophys Res Commun. 2007;357(3):615–619. doi: 10.1016/j.bbrc.2007.03.193. [DOI] [PubMed] [Google Scholar]
- 48.Sun Z., Liu J., Jing H., Dong S.X., Wu J. The diagnostic and prognostic value of CHFR hypermethylation in colorectal cancer, a meta-analysis and literature review. Oncotarget. 2017;8(51):89142–89148. doi: 10.18632/oncotarget.19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovac D., Rubinic M., Krasevic M., et al. Proliferating cell nuclear antigen (PCNA) as a prognostic factor for colorectal cancer. Anticancer Res. 1995;15(5 B):2301–2302. [PubMed] [Google Scholar]
- 50.Kashiwaba S.I., Kanao R., Masuda Y., Kusumoto-Matsuo R., Hanaoka F., Masutani C. USP7 is a suppressor of PCNA ubiquitination and oxidative-stress-induced mutagenesis in human cells. Cell Rep. 2015;13(10):2072–2080. doi: 10.1016/j.celrep.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Schwertman P., Lagarou A., Dekkers D.H.W., et al. UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat Genet. 2012;44(5):598–602. doi: 10.1038/ng.2230. [DOI] [PubMed] [Google Scholar]
- 52.Zlatanou A., Sabbioneda S., Miller E.S., et al. USP7 is essential for maintaining Rad18 stability and DNA damage tolerance. Oncogene. 2016;35(8):965–976. doi: 10.1038/onc.2015.149. [DOI] [PubMed] [Google Scholar]
- 53.van der Horst A., de Vries-Smits A.M.M., Brenkman A.B., et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8(10):1064–1073. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 54.Tavana O., Sun H., Gu W. Targeting HAUSP in both p53 wildtype and p53-mutant tumors. Cell Cycle. 2018;4101:1–18. doi: 10.1080/15384101.2018.1456293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhattacharya S., Ghosh M.K. HAUSP regulates c-MYC expression via de-ubiquitination of TRRAP. Cell Oncol (Dordr) 2015;38(4):265–277. doi: 10.1007/s13402-015-0228-6. [DOI] [PubMed] [Google Scholar]
- 56.Wang L., Kumar S., Dahiya S., et al. Ubiquitin-specific protease-7 inhibition impairs Tip60-dependent Foxp3+T-regulatory cell function and promotes antitumor immunity. EBioMedicine. 2016;13:99–112. doi: 10.1016/j.ebiom.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palazón-Riquelme P., Worboys J.D., Green J., et al. USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 2018;19(10):e44766. doi: 10.15252/embr.201744766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bojagora A., Saridakis V. USP7 manipulation by viral proteins. Virus Res. 2020;286:198076. doi: 10.1016/j.virusres.2020.198076. [DOI] [PubMed] [Google Scholar]
- 59.Frappier L. In: Münz C., editor. vol. 391. Current Topics in Microbiology and Immunology. Springer International Publishing; 2015. EBNA1; pp. 3–34. (Epstein Barr Virus Volume 2). [DOI] [PubMed] [Google Scholar]
- 60.Simatupang E.P., Simadibrata M. The difference expressions of EBNA- - 1 in Epstein- - Barr virus infection in low and high grade colorectal carcinoma. Indones J Gastroenterol Hepatol Dig Endosc. 2012;13(1):2–7. [Google Scholar]
- 61.Saridakis V., Sheng Y., Sarkari F., et al. Structure of the p53 binding domain of HAUSP/USP7 bound to Epstein-Barr nuclear antigen 1. Mol Cell. 2005;18(1):25–36. doi: 10.1016/j.molcel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 62.Holowaty M.N., Zeghouf M., Wu H., et al. Protein profiling with Epstein-Barr nuclear antigen-1 reveals an interaction with the herpesvirus-associated ubiquitin-specific protease HAUSP/USP7. J Biol Chem. 2003;278(32):29987–29994. doi: 10.1074/jbc.M303977200. [DOI] [PubMed] [Google Scholar]
- 63.Baba Y., Nosho K., Shima K., et al. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176(5):2292–2301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ioannou M., Paraskeva E., Baxevanidou K., et al. HIF-1α in colorectal carcinoma: review of the literature. J Buon. 2015;20(3):680–689. [PubMed] [Google Scholar]
- 65.Wu H.T., Kuo Y.C., Hung J.J., et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1α and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun. 2016;7:13644. doi: 10.1038/ncomms13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brahimi-Horn C., Mazure N., Pouysségur J. Signalling via the hypoxia-inducible factor-1α requires multiple posttranslational modifications. Cell Signal. 2005;17(1):1–9. doi: 10.1016/j.cellsig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Vakhrusheva O., Braeuer D., Liu Z., Braun T., Bober E. Sirt7-dependent inhibition of cell growth and proliferation might be instrumental to mediate tissue integrity during aging. J Physiol Pharmacol. 2008;59:201–212. [PubMed] [Google Scholar]
- 68.Yu H., Ye W., Wu J., et al. Overexpression of Sirt7 exhibits oncogenic property and serves as a prognostic factor in colorectal cancer. Clin Canc Res. 2014;20(13):3434–3445. doi: 10.1158/1078-0432.CCR-13-2952. [DOI] [PubMed] [Google Scholar]
- 69.Kiran S., Anwar T., Kiran M., Ramakrishna G. Sirtuin 7 in cell proliferation, stress and disease: rise of the Seventh Sirtuin! Cell Signal. 2015;27(3):673–682. doi: 10.1016/j.cellsig.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Jiang L., Xiong J., Zhan J., et al. Ubiquitin-specific peptidase 7 (USP7)-mediated deubiquitination of the histone deacetylase SIRT7 regulates gluconeogenesis. J Biol Chem. 2017;292(32):13296–13311. doi: 10.1074/jbc.M117.780130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun P., Gingras A.C. History of protein-protein interactions: from egg-white to complex networks. Proteomics. 2012;12(10):1478–1498. doi: 10.1002/pmic.201100563. [DOI] [PubMed] [Google Scholar]
- 72.Vidal M., Fields S. The yeast two-hybrid assay: still finding connections after 25 years. Nat Methods. 2014;11(12):1203–1206. doi: 10.1038/nmeth.3182. [DOI] [PubMed] [Google Scholar]
- 73.Sowa M.E., Bennett E.J., Gygi S.P., Harper J.W. Defining the human deubiquitinating enzyme interaction Landscape. Cell. 2009;138(2):389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Georges A., Marcon E., Greenblatt J., Frappier L. Identification and characterization of USP7 targets in cancer cells. Sci Rep. 2018;8(1):15833. doi: 10.1038/s41598-018-34197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Georges A., Coyaud E., Marcon E., Greenblatt J., Raught B., Frappier L. USP7 regulates cytokinesis through FBXO38 and KIF20B. Sci Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-39368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roux K.J., Kim D.I., Raida M., Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng J., Yang H., Fang J., et al. Molecular mechanism for USP7-mediated DNMT1 stabilization by acetylation. Nat Commun. 2015;6:7023. doi: 10.1038/ncomms8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kessler B.M., Fortunati E., Melis M., Pals C.E., Clevers H., Maurice M.M. Proteome changes induced by knock-down of the deubiquitylating enzyme HAUSP/USP7. J Proteome Res. 2007;6(11):4163–4172. doi: 10.1021/pr0702161. [DOI] [PubMed] [Google Scholar]
- 79.He Y., Wang S., Tong J., et al. The deubiquitinase USP7 stabilizes Maf proteins to promote myeloma cell survival. J Biol Chem. 2020;295(7):2084–2096. doi: 10.1074/jbc.RA119.010724. [DOI] [PMC free article] [PubMed] [Google Scholar]