Abstract
Introduction
The incidence of thromboembolism during COVID-19 and the use of thromboprophylaxis vary greatly between studies. Only a few studies have investigated the rate of thromboembolism post-discharge. This study determined the 90-day incidence of venous and arterial thromboembolic complications, risk factors for venous thromboembolic events and characterized the use of thromboprophylaxis during and after hospitalization.
Materials and methods
We retrospectively reviewed medical records for adult patients hospitalized for >24 h for COVID-19 before May 15, 2020, in ten Norwegian hospitals. We extracted data on demographics, thromboembolic complications, thromboembolic risk factors, and the use of thromboprophylaxis. Cox proportional hazards regression was used to determine risk factors for VTE.
Results
550 patients were included. The 90-day incidence of arterial and venous thromboembolism in hospitalized patients was 6.9% (95% CI: 5.1–9.3) overall and 13.8% in the ICU. Male sex (hazard ratio (HR) 7.44, 95% CI 1.73–32.02, p = 0.007) and previous VTE (HR 6.11, 95% CI: 1.74–21.39, p = 0.005) were associated with risk of VTE in multivariable analysis. Thromboprophylaxis was started in 334 patients (61%) with a median duration of 7 days (25th–75th percentile 3–13); in the VTE population 10/23 (43%) started thromboprophylaxis prior to diagnosis. After discharge 20/223 patients received extended thromboprophylaxis and 2/223 (0.7%, 95% CI: 0.3–1.9) had a thromboembolism.
Conclusions
The 90-day incidence of thromboembolism in COVID-19 patients was 7%, but <1% after discharge. Risk factors were male sex and previous VTE. Most patients received thromboprophylaxis during hospitalization, but only <10% after discharge.
Keywords: COVID-19, Thromboembolism, Thromboprophylaxis, Risk factors
1. Introduction
Coronavirus disease 2019 (COVID-19) predisposes to both arterial and venous thromboembolic complications, including venous thromboembolism (VTE), ischemic stroke and myocardial infarction (MI) [[1], [2], [3], [4]]. The rates of thrombotic complications are higher in acute COVID-19 than in non-COVID-19 acute respiratory distress syndrome (ARDS) [5].
Autopsy studies have demonstrated microvascular thrombi, excessive activation of neutrophils and platelets, as well as neutrophil-platelet aggregates in blood [6]. Further on, endothelial injury is evident from the direct invasion of endothelial cells by SARS-CoV-2, as well as from cytokines and various acute-phase reactants [7]. Elevated circulating prothrombotic factors contribute to a hypercoagulable state [7,8] and immobilization promotes stasis.
Few studies have systematically assessed risk factors for VTE in COVID-19 patients. General VTE risk assessment models might aid in identifying high risk patients, and the IMPROVE-DD score has been externally validated for COVID-19 patients [9,10]. Identified risk factors include active cancer, immobilization, previous VTE, ICU admission, advanced age and elevated D-dimer, amongst others.
The increased risk of VTE has prompted routine institution of thromboprophylaxis in hospitalized patients with COVID-19. However, despite the use of thromboprophylaxis, the incidence of VTE during acute COVID-19 ranges from 3% to 85% in published reports [11]. Variable incidence estimates are likely explained by variations in study design, populations, and assessment methods. Only a few studies have investigated the rate of thromboembolic complications after discharge and in ambulatory patients [12,13]. There is still a debate regarding the need for higher prophylactic dosage in this patient group [14], and whether thromboprophylaxis after hospital discharge is warranted [15].
The main aim of our study was to determine the 90-day incidence of arterial and venous thromboembolism. Secondary aims were to assess risk factors for VTE and describe the use of thromboprophylaxis.
2. Material and methods
2.1. Study design and population
This was a national multicenter retrospective cohort study in ten Norwegian hospitals comprising 550 patients hospitalized with COVID-19. We included subjects ≥18 years of age admitted for >24 h before May 15, 2020. Patients were identified through the hospital diagnosis registries at each hospital using the ICD-10 code for polymerase chain reaction (PCR) confirmed COVID-19 (U07.1).
The regional ethics committee approved the study (Helse Sør-Øst, approval no. 138629, 2020). An opt-out consent process was granted based on the distribution of study information to all participants, and consent exemption was approved for deceased patients.
2.1.1. Medical record review
Investigators in the respective hospitals reviewed medical records, extracting the following data [1]: demographics including age, sex, weight and height, comorbidity, smoking status, previous arterial or venous thrombosis [2], clinical biochemical data [3], type, dosage and duration of anticoagulation during and after hospitalization [4], time and duration of admission to the intensive care unit (ICU) [5], use and duration of invasive mechanical ventilation and [6] study outcomes within 90 days of hospital admission. Thrombotic complications were ischemic stroke, myocardial infarction (MI), pulmonary embolism (PE), or deep vein thrombosis (DVT). Ischemic stroke and VTEs were radiologically confirmed, while MIs were diagnosed based on a rise and fall in cardiac biomarkers together with clinical symptoms, typical changes in an electrocardiogram or during percutaneous coronary intervention. Data on the following comorbidities were recorded to calculate Charlson Comorbidity Index score [16]: previous myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident or transient ischemic attack, connective tissue disease, dementia, chronic obstructive pulmonary disease, peptic ulcer disease, liver disease, diabetes, hemiplegia, kidney disease, solid tumor, leukemia, lymphoma, acquired immunodeficiency syndrome and immune thrombocytopenia.
2.2. Statistical analysis
Descriptive statistics and thromboembolic complications are presented with the mean and standard deviation (SD), median (25th–75th percentile) or absolute number (%). Groups were compared using Mann-Whitney U test or t-test for continuous data and Fisher's exact or chi-square test for categorical variables.
We calculated the incidence rates for arterial and venous thromboembolism and thromboprophylaxis practice and estimated 95% confidence intervals using the Wilson method. The cumulative incidence of VTE for males and females was presented using a Kaplan Meier curve.
We used Cox proportional hazards regression to determine risk factors for VTE during 90 days after admission to hospital for COVID-19 infection. We used time to VTE as the dependent variable and estimated hazard ratios (HR) with 95% confidence intervals. Observations were censored at 90 days. We checked the proportional hazards assumption using log-minus-log plots and a test of non-zero slope of Schoenfeld residuals and found the assumption to be acceptable.
We performed univariate analysis using age, sex (male or female), a history of previous VTE (yes or no), body mass index (BMI) ≥ 30 kg/m2 (yes or no), D-dimer ≥ 75 percentile (1.4 mg/L) fibrinogen equivalent units (FEU) at admission to hospital (yes or no), C-reactive protein (CRP) > 75th percentile (129 mg/L) at admission to hospital (yes or no) and Charlson comorbidity index (0–2 or >2) as independent variables. In the analysis that included D-dimer as a predictor, we excluded VTE events during the first 2 days after admission, as an elevated D-dimer could be considered the first sign of a VTE.
Because of a limited number of VTE events prior to the analysis, we initially chose four independent variables (age, sex, previous VTE and BMI) for inclusion in a multivariable analysis. As a rule of thumb, a minimum of five to ten events per independent variable is recommended [17]. There were too few arterial events to conduct meaningful analysis on potential risk factors.
We used Stata software version 17.0 (StataCorp, College Station, TX, USA) for all analyses, choosing a significance level of p < 0.05 in two-sided tests.
3. Results
We included and reviewed medical records of 550 patients who were admitted for COVID-19 during the study period. Of these, 61 patients (11%) had died during hospitalization. Mean age was 61.5 years (SD 16.4), and 345 (52%) were males. The majority (64.7%) of patients had a Charlson Comorbidity Index ≥3 (range: 0–11). The median (25th to 75th percentile) length of stay in the hospital was 7 days [[4], [5], [6], [7], [8], [9], [10], [11], [12]] overall. In total, 130 patients (23%) were admitted to the ICU, with a median length of stay in the ICU of 13 days [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]] (Table 1 ). There was a difference in sex, BMI, inflammatory biomarkers, such as CRP and D-dimer levels, length of stay, ICU admission rate and need of mechanical ventilation between the VTE and non-VTE group (all p<0.05) (Table 1).
Table 1.
Descriptive statistics, presented as number (%) or median (25th-75th percentile unless otherwise stated.
| Total | VTE | Non-VTE | P** | |
|---|---|---|---|---|
| Patients, n | 550 | 23 | 527 | |
| Age (years), mean (SD) | 61.5 (16.4) | 60.2 (14) | 61.5 (17) | 0.65 |
| Sex, males | 345 (52) | 21 (91) | 324 (62) | 0.003 |
| Body mass index, kg/m2, mean (SD) | 28.0 (5.6) | 31.0 (5.2) | 27.8 (5.6) | 0.008 |
| Current smokers | 13 (4) | 0 | 13 (4) | 0.571 |
| Former smokers | 165 (35) | 8 (38) | 157 (35.3) | 0.818 |
| Diabetes mellitus type 2 | 101 (18) | 2 (18) | 99 (18) | 0.281 |
| Chronic obstructive lung disease | 28 (5) | 0 | 28 (5) | 0.622 |
| Chronic kidney disease moderate/severe* | 21 (4) | 0 | 21 (4) | 1 |
| Previous venous thromboembolism | 21 (4) | 3 (13) | 18 (3) | 0.052 |
| Charlson comorbidity index | 3 (2-5) | 3 (2-4) | 3 (2-5) | 0.604 |
| 0–2 | 194 (35) | 6 (26) | 188 (35) | |
| ≥3 | 356 (64) | 17 (73) | 339 (64) | |
| Laboratory values at admission | ||||
| D-dimer (mg/L FEU), n = 366 | 0.8 (0.4–1.4) | 2.6 (0.9–4.1) | 0.7 (0.4–1.3) | < 0.001 |
| C-reactive protein (mg/L) | 60 (25–129) | 115 (70–186) | 60 (24–128) | 0.002 |
| Creatinine (μmol/L) | 81.5 (67–100) | 83 (76–102) | 81 (66–100) | 0.098 |
| Laboratory values, highest | ||||
| D-dimer (mg/L FEU) | 1.3 (0.7–3.1) | 4.1 (4.1–19.2) | 1.2 (0.6–2.8) | < 0.001 |
| C-reactive protein (mg/L) | 130 (55–235) | 268 (146–340) | 128 (52–224) | < 0.001 |
| Creatinine (μmol/L) | 86 (68–111) | 88 (77–145) | 85 (68–110) | 0.445 |
| Days from positive PCR to admission | 0 (0–3) | 0 (0–5) | 0 (0–3) | 0.469 |
| Days hospitalized | 7 (4-12) | 11 (7-27) | 7 (4-12) | 0.001 |
| Intensive care unit | 130 (23) | 13 (56) | 117 (22) | < 0.001 |
| Days | 13 (6-20) | 18 (13-20) | 12 (6-20) | 0.189 |
| Thromboprophylaxis | 118 (91) | 13 (100) | 105 (90) | 0.227 |
| Invasive mechanical ventilation | 96 (17) | 12 (52) | 84 (16) | < 0.001 |
| Days | 13 (9-20) | 15 (12-21) | 13 (9-20) | 0.541 |
| Deceased | 61 (11) | 2 (9) | 59 (11) | 0.709 |
| Thromboprophylaxis initiated in hospital | 334 (61) | 10 (43)*** | 324 (61) | 0.009 |
| Duration of thromboprophylaxis, days | 7 (3-13) | 5 (2-20) | 7 (3-13) | 0.005 |
PCR = polymerase chain reaction; VTE = venous thromboembolism; * (creatinine >0.27 mmol/L).
**Bold font indicate significance *** Initiated minimum 2 days prior to VTE diagnosis.
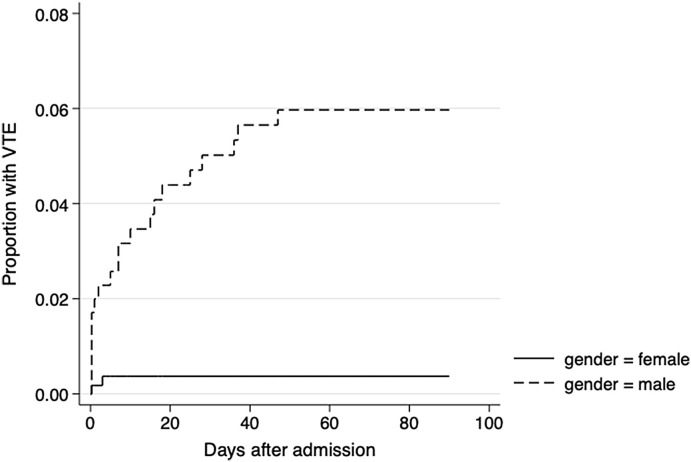
In total, 38 out of 550 patients (6.9%, 95% CI: 5.1–9.3) had an arterial thromboembolic event (ATE) or VTE within 90 days of admission (Table 2 ). In the ICU 18/130 patients (13.8%, 95% CI: 8.9–20.8) had a thromboembolic event. The cumulative incidence of VTE was higher in male and female patients with COVID-19 with an increased incidence of VTE among male than in female patients (Fig. 1 ).
Table 2.
Incidence of thromboembolic complications 30- and 90-days after admission to hospital, presented as number of events and incidence rates with 95% confidence intervals (CI).
| (n = 550) | 30 days |
90 days |
||
|---|---|---|---|---|
| No. of events | Incidence % (95% CI) | No. of events | Incidence % (95% CI) | |
| ATE or VTE | 344 | 6.2 (1.0–8.5) | 38 | 6.9 (5.1–9.3) |
| In-hospital | 32 | 5.8 (4.2–8.1) | 34 | 6.2 (4.5–8.5) |
| Intensive care unit, n = 130 | 13 | 10.0 (5.9–16.4) | 18 | 13.8 (8.9–20.8) |
| Post discharge* | 2 | 0.4 (0.0–1.3) | 4 | 0.7 (0.3–2.1) |
| Venous thrombosis | 20 | 3.6 (2.4–5.5) | 23 | 4.2 (2.8–6.2) |
| Only deep vein thrombosis | 2 | 0.4 (0.0–1.3) | 2 | 0.4 (0.0–1.3) |
| Pulmonary embolism** | 17 | 3.1 (1.8–4.9) | 20 | 3.6 (2.2–5.6) |
| Catheter-related VTE | 1 | 0.2 (0.0–1.0) | 1 | 0.2 (0.0–1.0) |
| Arterial thrombosis | 14 | 2.5 (2.0–4.0) | 15 | 2.7 (1.7–4.5) |
| Myocardial infarction | 10 | 1.8 (1.0–3.3) | 11 | 2.0 (1.0–3.6) |
| Ischemic stroke | 4 | 0.7 (0.2–1.9) | 4 | 0.7 (0.2–1.9) |
ATE = arterial thromboembolic event; VTE = venous thromboembolic event; * 2 pulmonary embolisms, 1 myocardial infarction and 1 ischemic stroke. **10 central and 13 peripheral pulmonary embolisms*** 1 ST-elevation myocardial infarction, the rest non-ST-elevation myocardial infarction.
Fig. 1.
Kaplan Meier plot of the cumulative incidence of venous thromboembolic events (VTE) during and after hospitalization for males and females.
A VTE occurred in 23 patients (4.2%, 95% CI: 2.8–6.2), 20/23 (87%) were pulmonary embolisms, of which 10 were central (pulmonary trunk or main pulmonary arteries) and 13 were peripheral (segmental or subsegmental pulmonary arteries). Among those diagnosed with a VTE 13/23 (56%) had been treated in the ICU compared to 22% in the non-VTE group. Four thromboembolic events were diagnosed after discharge, giving a post-discharge 90-day incidence rate of 0.7% (95% CI: 0.3–1.9) with a median time from discharge to thromboembolic event of 17 days (25th–75th percentile 3.5–34 days).
ATE occurred in 15 patients (2.7%, 95% CI: 1.7–4.5) within 90 days of admission to hospital, of which 11 were MI and 4 ischemic strokes. Among the patients with MIs, 10/11 (91%) were non-ST-elevation myocardial infarctions, 4/11 (36%) were admitted to the ICU and 1 occurred after discharge from hospital. Only 1/4 (25%) patients with ischemic stroke were admitted to the ICU and 1 occurred after discharge from hospital.
Thromboprophylaxis was initiated in 334/550 patients (61%, 95% CI: 56.6–64.7); dosages are shown in Table 3 . The median duration of the thromboprophylaxis was 7 days (25th–75th percentile 3–13 days). Dosages were frequently changed depending on fluctuations in clinical and biochemical parameters. Of the patients admitted in the hospital wards 214/420 (51%) received thromboprophylaxis and 6 (2.7%) of those developed VTE, whereas of those admitted in the ICU 118/130 (91%) received thromboprophylaxis and 13 (11%) of these developed a VTE. In a subgroup of 223 patients with data on discharge medications, 20/223 (9%) were discharged with thromboprophylaxis initiated during the hospital stay. Further on, 15/223 (6.7%) were discharge with newly initiated anticoagulation due to other causes such as treatment of established VTE, atrial fibrillation or unknown/unspecified in the remaining. Low-molecular-weight heparin (LMWH) was most often prescribed (71.4%).
Table 3.
Anticoagulation pattern during and after hospitalization, presented as number (%) with 95% confidence intervals (CI), unless otherwise specified.
| Variables | n = 550 | %, 95% CI |
|---|---|---|
| Anticoagulation initiated during hospitalization | 341 | 62 (57.9–66.0) |
| Low-molecular-weight heparin | 334 | 61 (56.6–64.7) |
| Intermediate-therapeutic intensity* | 71 | 22 (17.2–26.0) |
| Prophylactic intensity | 249 | 77 (72.0–81.1) |
| Direct oral anticoagulation | 22 | 7 (4.3–9.) |
| Duration, days, median (25th-75th percentile) | 7 (3-13) | |
| Discharged with newly initiated anticoagulation** | 35 | 16 (11.5–21.1) |
| Indication*** | ||
| Venous thromboembolism | 9 | 26 (14.2–42.1) |
| Atrial fibrillation | 2 | 6 (1.6–18.6) |
| Thromboprophylaxis | 20 | 57 (40.9–72.0) |
| Unknown/other | 4 | 11 (4.5–26.0) |
| Duration*** | ||
| Unknown | 10 | 29 |
| ≤10 days | 10 | 29 |
| ≥2 weeks <3 months | 5 | 14 |
| 3 months | 5 | 14 |
| Indefinite | 5 | 14 |
| Type*** | ||
| Low-molecular-weight heparin | 25 | 71 |
| Direct oral anticoagulation | 10 | 28 |
*Intermediate intensity refers to standard prophylactic dose twice daily (enoxaparin 40 mg bd or dalteparin 5000 mg bd),**n = 223, ***n = 35.
The cumulative incidence of VTE was higher in male than in female patients (Fig. 1). In univariate proportional hazard regression analysis, male sex (HR 6.13, 95% CI: 1.44–26.20, p = 0.014) and previous venous thromboembolism (HR 4.58, 95% CI: 1.35–15.55, p = 0.015) were associated with risk of VTE (Table 4 ). In further multivariable analysis, male sex (HR 7.44, 95% CI: 1.74–32.02, p = 0.007) and history of previous VTE (HR 6.11, 95% CI: 1.74–21.39, p = 0.005) were associated with risk of VTE.
Table 4.
Univariate and multivariable analysis of risk factors of venous thromboembolism, presented as hazard ratios with 95% confidence intervals (CI) and p-values.
| Variable | Univariate**** |
Multivariable***** |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Age, per years | 0.99 | 0.97–1.0 | 0.70 | 0.99 | 0.97–1.03 | 0.998 |
| Sex, males | 6.13 | 1.44–26.20 | 0.014 | 7.44 | 1.73–32.02 | 0.007 |
| Previous venous thromboembolism | 4.58 | 1.35–15.55 | 0.015 | 6.11 | 1.74–21.39 | 0.005 |
| Body mass index ≥30 kg/m2 * | 1.87 | 0.79–4.43 | 0.155 | 2.33 | 0.95–5.71 | 0.065 |
| Charlson comorbidity index >2 | 1.30 | 0.53–3.14 | 0.57 | |||
| D-dimer ≥ 75th percentile (1.4 mg/L FEU) ** | 2.89 | 0.77–10.69 | 0.116 | |||
| C-reactive protein >75th percentile (129mg/L)*** | 2.04 | 0.86–4.82 | 0.106 | |||
*n = 407; ** At admission to hospital; n = 359, events the first two days of hospitalization are excluded.
***n = 542, at admission to hospital; ****n=23; *****n = 407, number of events = 23.
4. Discussion
There are three main findings in this study; First, the 90-day incidence of thromboembolic events during hospitalization was about 7% in a population where 62% already had received thromboprophylaxis, mainly standard prophylactic intensity. Second, the rate of thrombotic events after discharge was very low (0.7%), despite subgroup analysis revealing that less than 10% had further prophylactic anticoagulation upon discharge from hospital. Third, male sex and a history of previous VTE were associated with an increased risk of VTE.
Early reports indicated very high rates of VTE up to 85% in patients admitted for COVID-19, even in patients receiving standard thromboprophylaxis, which led to suggestions of the possible need for higher prophylactic dosage [4,11,18]. Some recent studies indicate a lower incidence of thromboembolic complications than earlier studies, but reports still show a large variation [[19], [20], [21], [22], [23], [24], [25]]. In a recent meta-analysis of more than 30.000 patients, the overall incidence of VTE during hospitalization was 12.8%; 24.1% in patients admitted to the ICU, and 7.7% in non-ICU patients [20], not considering distal deep vein thrombosis (DVT). A study of 399 patients admitted for COVID-19 reported ATE (MI and stroke) in 15/399 patients (3.8%) and VTE in 51/399 patients (12.8%) [26].
Our findings are in the lower end of previously reported rates of VTE, but similar to reported rates of ATE [19,26]. Previously reported VTE rates for hospitalized patients with COVID-19 may be overestimated due to selected high-risk populations, reports from ICU populations, routine use of chest CT, or intensive case findings by screening asymptomatic patients for VTE. This may have influenced the rates both in solitary studies and in meta-analyses. Several factors may have affected our VTE rate estimate and contributed to the low incidence of VTE. For instance, our study included patients hospitalized in the early days of the pandemic, when awareness of thromboembolic complications was limited.
In this early period, restrictions based on institutional infection control measures may have led to conservative use of radiological imaging. Because the incidence of COVID-19 infection in Norway has been lower than in other areas of Europe, and hospital capacity has not been overwhelmed, the threshold for admission may have been lower than in some other countries [27]. Lastly, only a small subset of fatal COVID-19 cases were autopsied, and the presence of unrecognized VTE and ATE in these patients cannot be excluded [28].
A large proportion of the patients that developed VTE had thromboprophylaxis at the time of diagnosis, however, the majority only received standard prophylactic intensity. It is possible that selected patients may benefit from higher intensity anticoagulation, although this could not be assessed in the present study. As our data did not include assessment of bleeding complications, we cannot conclude on the net benefit on mortality and morbidity. Whether the incidence of VTE in COVID-19 differs from that of other viral and bacterial pneumonia remains uncertain.
In our study, thromboprophylaxis was initiated in about 2 of 3 patients during hospitalization. At the start of the pandemic, there was no national or local guidelines of thromboprophylaxis for patients with COVID-19. However, currently a standard prophylactic dosing is recommended in hospital wards and mainly intermediate dosing (standard dose x 2) for patients in the ICU in Norway. Many hospitals have used the Padua score for thromboprophylaxis on COVID-19 patients. Current international guidelines are based on expert opinions and very low certainty of evidence, which may explain the diversity in recommendations [15,29,30]. A minimum of prophylactic intensity anticoagulation (40 mg enoxaparin or 5000IE dalteparin) is recommended for most patients after careful assessment of bleeding risk. Published evidence suggests no benefit of intermediate intensity (standard intensity bd) over standard intensity thromboprophylaxis [31], however, therapeutic intensity (1 mg/kg enoxaparin qd or 200IU/kg dalteparin qd) seems to be beneficial in non-critically ill patients [[32], [33], [34]].
We found that only 0.7% of patients had an ATE or VTE after hospital discharge, which is in line with previous reports of 0.2–2.5% [13,35,36]. Less than 10% of patients in the present study were discharged with continued prophylaxis. This practice is still heterogenous in Norway, as only some of the participating hospitals have guidelines recommending post-discharge thromboprophylaxis with low dose direct oral anticoagulation (DOAC) for 1–4 weeks to patients treated in the ICU with a complicated course of illness. Large randomized controlled trials (RCTs) investigating the impact of thromboprophylaxis after discharge and in an ambulatory setting are currently ongoing; one of these is the ACTIV-4C trial (NCT04650087), part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines initiative coordinating research on COVID-19, which aims to determine whether extended thromboprophylaxis after hospitalization reduces risk of thromboembolism and mortality following discharge from the hospital [37]. Meanwhile, The American College of Chest Physicians (CHEST) guidelines recommend against thromboprophylaxis after hospital discharge with exceptions like a history of previous VTE or active cancer [15]. International Society of Thrombosis and Haemostasis (ISTH) guidelines recommend considering post-discharge thromboprophylaxis for all high-risk patients, including advanced age, ICU stay, D-dimer > 2 times ULN or IMPROVE VTE score of more than 4 [15,30]. Bleeding risk must also be taken into consideration.
In our study, male sex and a history of previous thrombosis were associated with time to VTE in multivariable analysis, consistent with results from previous studies [38,39]. In univariate analysis, D-dimer ≥ 75 percentile (1.4 mg/L FEU) was also associated with risk of VTE. However, because all patients did not have D-dimer measured at the time of admission, the possibility of an existing thrombosis at the time of admission, and the possible impact of inflammation on D-dimer values during COVID-19, we chose not to include D-dimer in the multivariable analysis. Further on, we chose to dichotomize D-dimer because different laboratories report different upper and lower limits, which makes a continuous variable problematic. Some studies have also indicated that biomarkers like D-dimer and CRP have a high predictive value for VTE [40], as well as clinical factors like severity of COVID-19, ICU admission, active malignancy and male sex [38,41]. The literature on age and association with VTE during COVID-19 is inconsistent, as both advanced age and young age may be associated with a higher incidence of VTE [9,39,42,43].
Risk assessment models (RAM), e.g., IMPROVE-DD, Padua or Caprini, can assist in the identification of COVID-19 patients at high risk for VTE [9,10,44]. Despite male sex being a prominent risk factor for VTE during COVID-19 in several studies, including the current study, sex is not included in any of these risk models [41,43,45]. The male predominance described among critically ill and ICU-admitted COVID-19 patients probably explains the increased risk of VTE among males during acute COVID-19 infection [38,39]. Further studies and external validation of prediction models are required to determine the optimal risk stratification scheme for this patient population. Male sex could be considered as a risk factor in future risk models for VTE during COVID-19.
The present study has some limitations. The study period was from the early days of the pandemic; thus, it does not take into account the increased awareness around thromboembolic complications and thromboprophylaxis, mutations of the virus and the effect of vaccines. Norwegian hospitals have not been overwhelmed during the pandemic; consequently, the population admitted to hospitals in Norway might differ from that of other countries. The hospitals included in this study received more than 50% of admitted COVID-19 patients in Norway, which supports generalization to a setting without severely constrained hospital capacity. In our opinion, the retrospective study design is not a major limitation, as the same patients are likely to have been included if the study was prospective. However, it is possible that some patients are missed due to insufficient ICD-10 coding or misclassification at the time of discharge. Further on, the number of thromboembolic events were few, which limits the multivariable analysis. We did not include variables describing the severity of the infection, which could have been interesting to assess potential associations with risk of VTE.
We found male sex to be a distinct risk factor of VTE during COVID-19, which could be considered a risk factor in future risk assessment models. Higher intensity thromboprophylaxis might be warranted in certain high-risk patients. Still, results from large RCTs, including both benefits of thromboprophylaxis and risk of bleeding, are needed to recommend specific dosage for different subpopulations.
5. Conclusion
The incidence of thrombotic complications in patients hospitalized for COVID-19 was lower in this study compared to many other early studies. However, many patients were diagnosed with VTEs despite receiving standard thromboprophylaxis. Male sex and previous thrombosis were associated with increased risk of VTE, emphasizing the need for thorough consideration of thromboprophylaxis in these patients.
Authors contributions
All authors have contributed to drafting, revision and approval of the manuscript. BT and KS analyzed the data.
Ethics approval
The study has approval from the Norwegian Regional Ethics committee (REK).
Funding
Funding was provided by a grant from Helse Sør-Øst (grant nr 2021008)
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Waleed Ghanima reports fees for participation in Advisory board from Amgen, Novartis, Pfizer, Principia Biopharma Inc- a Sanofi Company, Sanofi, SOBI, Griffols, UCB, Argenx. Lecture honoraria from Amgen, Novartis, Pfizer, Bristol Myers Squibb, SOBI, Griffols, Sanofi. Research grants from Bayer, and BMS/Pfizer.
Galina Tsykunova reports lecture honoraria from Amgen, Janssen, Ablynx, Sanofi and Sobi. Research grants from Janssen.
Acknowledgments
None.
References
- 1.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemostasis. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhania N., Bansal S., Nimmatoori D.P., Ejaz A.A., McCullough P.A., Singhania G. Current overview on hypercoagulability in COVID-19. Am. J. Cardiovasc. Drugs. 2020;20(5):393–403. doi: 10.1007/s40256-020-00431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S., Zimba O., Gasparyan A.Y. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow's triad. Clin. Rheumatol. 2020;39(9):2529–2543. doi: 10.1007/s10067-020-05275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldin M., Lin S.K., Kohn N., Qiu M., Cohen S.L., Barish M.A., et al. External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19. J. Thromb. Thrombolysis. 2021:1–4. doi: 10.1007/s11239-021-02504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Zheng T., Wang S., Yu Y., Wang P., Song Y., et al. Association between risk of venous thromboembolism and mortality in patients with COVID-19. Int. J. Infect. Dis. 2021;108:543–549. doi: 10.1016/j.ijid.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porfidia A., Valeriani E., Pola R., Porreca E., Rutjes A.W.S., Di Nisio M. Venous thromboembolism in patients with COVID-19: systematic review and meta-analysis. Thromb. Res. 2020;196:67–74. doi: 10.1016/j.thromres.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tholin B., Ghanima W., Einvik G., Aarli B., Brønstad E., Skjønsberg O.H., et al. Incidence of thrombotic complications in hospitalised and non-hospitalised patients after COVID-19 diagnosis. Br. J. Haematol. 2021;194(3):542–546. doi: 10.1111/bjh.17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashidi F., Barco S., Kamangar F., Heresi G.A., Emadi A., Kaymaz C., et al. Incidence of symptomatic venous thromboembolism following hospitalization for coronavirus disease 2019: prospective results from a multi-center study. Thromb. Res. 2021;198:135–138. doi: 10.1016/j.thromres.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Samkari H. Finding the optimal thromboprophylaxis dose in patients with COVID-19. JAMA. 2021;325(16):1613–1615. doi: 10.1001/jama.2021.4295. [DOI] [PubMed] [Google Scholar]
- 15.Moores L.K., Tritschler T., Brosnahan S., Carrier M., Collen J.F., Doerschug K., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chron. Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Vittinghoff E., McCulloch C.E. Relaxing the rule of ten events per variable in logistic and Cox regression. Am. J. Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 18.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansory E.M., Srigunapalan S., Lazo-Langner A. Venous thromboembolism in hospitalized critical and noncritical COVID-19 patients: a systematic review and meta-analysis. TH Open. 2021;5(3):e286–e294. doi: 10.1055/s-0041-1730967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha M.J.S., Pinto C.A.V., Guerra J.C.C., Tachibana A., Portugal M.F.C., Ferraz L.J.R., et al. Incidence, diagnosis, treatment methods, and outcomes of clinically suspected venous thromboembolic disease in patients with COVID-19 in a quaternary hospital in Brazil. J Vasc Bras. 2021;20 doi: 10.1590/1677-5449.200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avruscio G., Camporese G., Campello E., Bernardi E., Persona P., Passarella C., et al. COVID-19 and venous thromboembolism in intensive care or medical ward. Clin Transl Sci. 2020;13(6):1108–1114. doi: 10.1111/cts.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemostasis. 2020;18(7):1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquez F.G., Riviello-Goya S., Vargas Ruiz A.G., Ortíz Brizuela E., Gil López F., Gutierrez Marín A., et al. Low rate of thrombosis in Mexican patients with COVID-19 infection. A benefit of higher doses anticoagulants or a sub diagnosis? Blood. 2020;136(Supplement 1):29–30. [Google Scholar]
- 25.Hanif A., Khan S., Mantri N., Hanif S., Saleh M., Alla Y., et al. Thrombotic complications and anticoagulation in COVID-19 pneumonia: a New York City hospital experience. Ann. Hematol. 2020;99(10):2323–2328. doi: 10.1007/s00277-020-04216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piazza G., Campia U., Hurwitz S., Snyder J.E., Rizzo S.M., Pfeferman M.B., et al. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(18):2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkehelseinstituttet. COVID-19 dagsrapport fredag 15. https://www.fhi.no/sv/smittsomme-sykdommer/corona/dags--og-ukerapporter/dags--og-ukerapporter-om-koronavirus/ mai 2020 [Available from:
- 28.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuker A., Tseng E.K., Nieuwlaat R., Angchaisuksiri P., Blair C., Dane K., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Advances. 2021;5(3):872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spyropoulos A.C., Levy J.H., Ageno W., Connors J.M., Hunt B.J., Iba T., et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemostasis. 2020;18(8):1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadeghipour P., Talasaz A.H., Rashidi F., Sharif-Kashani B., Beigmohammadi M.T., Farrokhpour M., et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goligher E.C., Bradbury C.A., McVerry B.J., Lawler P.R., Berger J.S., Gong M.N., et al. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N. Engl. J. Med. 2021;385(9):777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawler P.R., Goligher E.C., Berger J.S., Neal M.D., McVerry B.J., Nicolau J.C., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N. Engl. J. Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spyropoulos A.C., Goldin M., Giannis D., Diab W., Wang J., Khanijo S., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern. Med. 2021 doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts L.N., Whyte M.B., Georgiou L., Giron G., Czuprynska J., Rea C., et al. Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood. 2020;136(11):1347–1350. doi: 10.1182/blood.2020008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136(11):1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Talasaz A.H., Sadeghipour P., Kakavand H., Aghakouchakzadeh M., Kordzadeh-Kermani E., Van Tassell B.W., et al. Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021;77(15):1903–1921. doi: 10.1016/j.jacc.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahuja N., Bhinder J., Nguyen J., Langan T., Jr., O'Brien-Irr M., Montross B., et al. Venous thromboembolism in patients with COVID-19 infection: risk factors, prevention, and management. Semin. Vasc. Surg. 2021;34(3):101–116. doi: 10.1053/j.semvascsurg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J.Y., Wang H.F., Yin P., Li D., Wang D.L., Peng P., et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J. Thromb. Haemostasis. 2021;19(4):1038–1048. doi: 10.1111/jth.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dujardin R.W.G., Hilderink B.N., Haksteen W.E., Middeldorp S., Vlaar A.P.J., Thachil J., et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID-19 patients. Thromb. Res. 2020;196:308–312. doi: 10.1016/j.thromres.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thondapu V., Montes D., Rosovsky R., Dua A., McDermott S., Lu M.T., et al. Venous thrombosis, thromboembolism, biomarkers of inflammation, and coagulation in coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. 2021;9(4):835–844. doi: 10.1016/j.jvsv.2020.11.006. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirshblum S.C., DeLauter G., Eren F., Pomeranz B., DeLuca R., Hammerman S., et al. Screening for deep vein thrombosis in persons with COVID-19 upon admission to an inpatient rehabilitation hospital. Am. J. Phys. Med. Rehabil. 2021;100(5):419–423. doi: 10.1097/PHM.0000000000001729. [DOI] [PubMed] [Google Scholar]
- 43.Xiong X., Chi J., Gao Q. Prevalence and risk factors of thrombotic events on patients with COVID-19: a systematic review and meta-analysis. Thromb. J. 2021;19(1):32. doi: 10.1186/s12959-021-00284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caprini J.A., Arcelus J.I., Hasty J.H., Tamhane A.C., Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin. Thromb. Hemost. 1991;17(Suppl 3):304–312. [PubMed] [Google Scholar]
- 45.Motaganahalli R.L., Kapoor R., Timsina L.R., Gutwein A.R., Ingram M.D., Raman S., et al. Clinical and laboratory characteristics of patients with novel coronavirus disease-2019 infection and deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2021;9(3):605–614.e2. doi: 10.1016/j.jvsv.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]