Abstract
Background COVID-19 vaccines approved in the UK are highly effective in general population cohorts, however, data on effectiveness amongst individuals with clinical conditions that place them at increased risk of severe disease are limited.
Methods We used GP electronic health record data, sentinel virology swabbing and antibody testing within a cohort of 712 general practices across England to estimate vaccine antibody response and vaccine effectiveness against medically attended COVID-19 amongst individuals in clinical risk groups using cohort and test-negative case control designs.
Findings There was no reduction in S-antibody positivity in most clinical risk groups, however reduced S-antibody positivity and response was significant in the immunosuppressed group. Reduced vaccine effectiveness against clinical disease was also noted in the immunosuppressed group; after a second dose, effectiveness was moderate (Pfizer: 59.6%, 95%CI 18.0–80.1%; AstraZeneca 60.0%, 95%CI -63.6–90.2%).
Interpretation In most clinical risk groups, immune response to primary vaccination was maintained and high levels of vaccine effectiveness were seen. Reduced antibody response and vaccine effectiveness were seen after 1 dose of vaccine amongst a broad immunosuppressed group, and second dose vaccine effectiveness was moderate. These findings support maximising coverage in immunosuppressed individuals and the policy of prioritisation of this group for third doses.
Introduction
A range of clinical comorbidities have been associated with more severe COVID-19 disease and poor outcomes.1, 2, 3 COVID-19 vaccines have shown high levels of efficacy in older adults, healthcare workers and the general population both in clinical trials and real world effectiveness studies.4, 5, 6, 7, 8, 9 However, data on the effectiveness of these vaccines amongst individuals in clinical risk groups are limited.
The UK's Medicines and Healthcare Products Regulatory Agency (MHRA) gave emergency use authorisation to three vaccines against COVID-19 between December 2020 and January 2021, namely the Pfizer/BioNTech BNT162b2 mRNA, Oxford/AstraZeneca ChAdOx1 nCoV-19 adenoviral AZD1222; and Moderna mRNA-1273 vaccines. BNT162b2 and AZD1222 have been delivered through the national vaccination programme since 8 December 2020 and 4 January 2021, respectively. Rollout of the Moderna vaccine in England began 13th April 2021, though use of this vaccine has been more limited. Vaccination was initially prioritised for older people, health and social care workers and predefined clinical risk groups.10 As of 24 November 2021, over 110 million doses of vaccine have been delivered in the UK.11
The COVID-19 vaccine trials demonstrated high levels of efficacy.4, 5, 6 This has been further supported by real world vaccine effectiveness studies which indicate 50–70% protection against infection or mild disease after a single dose of either BNT162b2 or AZD1222, and 75–85% protection against hospitalisation or death. After two doses effectiveness reaches 65–90% against infection or mild disease, and 90–100% against severe disease.7 , 9 , 12, 13, 14, 15, 16, 17 These high levels of effectiveness are maintained in older adults, nevertheless, vaccine effectiveness estimates have not yet been reported for individuals in clinical risk groups.
Although age has been found to be the greatest risk factor for adverse outcomes following COVID-19 infection, clinical comorbidities may also increase the risk of severe disease. Diabetes, severe asthma, chronic heart disease, chronic kidney disease (CKD), chronic liver disease, neurological disease, and disease or therapy associated with immunosuppression have all been linked to an increased risk of hospitalisation or death with COVID-19.1, 2, 3 Individuals with these conditions have been prioritised for vaccination in many national programmes. In the UK, those at highest risk of severe disease have been advised to ‘shield’ by remaining isolated at home for long periods of the pandemic.18 This group was offered vaccination from January 2021 along with older adults. Individuals aged under 65 in other clinical risk groups were offered vaccination from February 2021.19
A number of studies have monitored antibody responses to vaccination in individuals with clinical comorbidities. Reduced seroconversion rates have been seen in transplant recipients, haematological malignancy, solid organ cancer patients and patients on some immunosuppressive therapies after one dose of vaccine. 20, 21, 22, 23, 24, 25 Reduced antibody responses have also been seen after two doses amongst patients with haematological malignancy and transplant recipients.23 , 26 , 27 Conversely other studies have found similar seroconversion rates amongst patients on immunosuppressive therapy, patients with end stage renal disease and solid organ cancer patients, in particular after 2 doses.22 , 23 , 25 , 28, 29, 30, 31 However, it is not yet clear how differences in antibody responses translate into changes in vaccine effectiveness, although there is some suggestion that higher antibody levels are associated with better protection with respect to more severe outcomes.
In this study we use computerised medical record (CMR) data from a cohort of general practice patients and sentinel antibody testing within the same cohort to estimate antibody responses and vaccine effectiveness against symptomatic medically attended COVID-19 amongst patients in different clinical risk groups.
Methods
Summary
We conducted cohort and nested test-negative case-control (TNCC) VE analyses. Our population of interest were individuals in risk groups and those advised to shield. Our outcome was medically attended COVID-19, with the diagnosis confirmed by PCR test.
Data sources
We used pseudonymised CMR data collected by the Oxford-Royal College of General Practitioners Research and Surveillance Centre (RSC),32 one of Europe's oldest primary care sentinel systems.33 A cohort was created to support the UKHSA COVID-19 VE studies comprising the registered patients from 718 English general practices (N = 7480,272), 11% of general practices and 10% of the population. These practices used the Systematised NOmenclature of MEDicine Clinical Terms (SNOMED CT) to record key data. Data were held in Oxford-RCGP Clinical Informatics Digital Hub (ORCHID), a trusted research environment (TRE).34
National COVID-19 testing results through community testing, hospital laboratories and public health laboratories are posted electronically into the general practice CMR. UK general practice has had a system of electronic laboratory links since 2004, allowing pathology results including COVID-19 test results to be sent through direct to that individual's record.
For a subset of sentinel surveillance practices, swabs were collected from individuals presenting with flu- or COVID-like symptoms and sent to the Virus Reference Laboratory at UKHSA for PCR testing for SARS-CoV-2, and other respiratory viruses including influenza and RSV.
A further subset of practices collected additional sera from patients presenting at their GP for a routine blood test as part of SARS-CoV-2 serological surveillance. Patients in older age groups and in clinical risk groups were oversampled to match the rollout of the vaccination programme. Samples were tested at UKHSA Porton using two assays from Roche diagnostics (Basel, Switzerland): the Elecsys Anti-SARS-CoV-2 spike (S) and nucleocapsid (N) assays. The N assay detects only antibodies acquired following natural infection, while the S assay detects both post-infection and vaccine-induced antibodies.
Antibody response to vaccination
BNT162b2 and AZD1222 post vaccination S antibody responses were assessed in N-negative individuals i.e. in those who had no evidence of antibodies from prior infection, and who had received dose 1 vaccination at least 28 days prior or dose 2 14 days prior. Percentage (%) positive and median (IQR) antibody levels were calculated. Approximate adjusted prevalence ratios on proportion seropositive following dose 1 were calculated using multivariable Poisson regression with robust error variance and including terms for each specific risk group, age group, sex and time since dose. Multivariable regression was also used to calculate geometric mean ratios of levels following dose 1 and dose 2, with the same adjustments as above and additionally dosing schedule for dose 2. Some levels were capped at 2500 and interval regression was used to account for this censoring. All analyses were repeated without specific risk groups, but including a term for non-risk / risk (non-shielding) / shielding.
N-assay based seropositivity by risk group is also described in supplementary material S4.
VE outcomes and exposures
Box 1: symptomatic COVID-19 outcome definition
|
| • Diagnosis of COVID-19 infection confirmed by positive virology test |
| AND |
| • Symptoms of COVID 19 in the 10 days before/after the virology test |
| ▪ Cough |
| ▪ Fatigue |
| ▪ Fever |
| ▪ Diarrhoea |
| ▪ Headache |
| ▪ Anosmia |
| ▪ Loss of taste |
| ▪ Sore Throat |
| ▪ Shortness of Breath |
| ▪ Nausea |
| ▪ Myalgia |
| OR |
|
| ▪ Influenza-like-illness |
| ▪ Acute bronchitis |
| ▪ Pneumonia or pneumonitis |
| ▪ Lower respiratory infection |
| ▪ Upper respiratory infection |
Our primary outcome was a case of acute symptomatic COVID-19, defined as symptoms or clinical illness consistent with COVID-19 within 10 days before or after a positive PCR test for COVID-19 (Box 1) recorded in the practice CMR entry. Secondary outcomes were hospitalisation and death following a PCR positive test: methods and results are described in supplementary material S6. The CMR entry was usually an encounter (phone or face-to-face) with the GP, though may have been an entry from a hospital, emergency or out of hours encounter. The PCR test was conducted ether as part of sentinel surveillance or through the national testing process.
Symptom onset dates were not available, so we used whichever came first of test or consultation date.
Test-negative study controls met the same case-definition and symptoms within 10 days of a negative test. We excluded negative tests with symptoms if within 21 days before or 90 days after any positive test, and we allowed a maximum of one negative test within a 21 day period because these could represent a single illness episode.
The exposure of interest was COVID-19 vaccination. Our dataset included available information in GP records on the date and dose of vaccine given, manufacturer and batch number. Where manufacturer was unavailable, we inferred vaccine brand from the batch number or vaccination date (Pfizer if before 4th Jan 2021). Dose 1 vaccine effectiveness was considered as 28–90 days after the first dose and dose 2 as 14+ days after the second dose.
VE statistical analyses
The study start date was 7th December 2021 and the study end date was 16th May 2021 to correspond with the Alpha-variant dominant period in England; individuals were censored at death, deregistration or at the last recorded vaccination date within a patient's registered GP practice.
Cohort analyses were conducted using acute symptomatic COVID-19 as outcomes (Box 1). We used Poisson regression on outcomes, including vaccination status as a time-varying covariate and further adjusting for time and region by fitting cubic splines over weeks for each NHS region, and demographic and clinical variables. Time after first event was retained in analyses and probable re-infections were included.
TNCC analyses also included people with acute symptomatic COVID-19. Logistic regression was used for analysis, including vaccination status at the event time and further adjusting for time-region interaction, demographic and clinical variables as for the cohort study.
Adjusted analyses for demographic and clinical variables included only those with complete data. Adjustments were made for: age group (in 5-year bands, then 90+), sex, ethnicity, index of multiple deprivation (IMD) quintile, GP record indicating prior COVID-19, large household (< 10, divided into those with a median age < 70 and ≥ 70 years old), GP consultation rate quartile, comorbidity, shielding recommendation, and latest smoking status.
The first set of analyses presented are for the population aged 16–64, and aged 65 and above. These comprise: all individuals, those not in risk groups, those in predefined risk groups,10 and people who had a shielding recommendation. We checked two-way interactions with vaccination status (any manufacturer, for simplicity) for all covariates. Since health and social care workers are not flagged in GP records, the analysis for 16–64 year olds was initiated from 1st February 2021 and excluded those who were vaccinated before or experienced an event between 7th December and this date. Results are presented 28–90 days post first dose and 14+ days post second dose both separately for AstraZeneca and Pfizer and combined for all manufacturers. The second set of analyses focused on people in predefined risk groups, and results are presented for VE within each risk group. Results are presented 28–90 days post first dose and 14+ days post second dose combined for all manufacturers.
All statistical analyses were carried out using STATA version 14.2.
Ethical considerations
Surveillance and COVID-19 VE studies were approved by the PHE/UKHSA Caldicott Guardian as Health Protection and permitted under Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002.
Role of the funding source
Funding was provided through PHE/UKHSA. The funder of the study had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
The full vaccine effectiveness cohort included 7480,272 individuals. After exclusions, listed in Supplementary material S1, the cohort included 5591,142 individuals, of which 1274,718 were aged 65 years and above and 1533,879 belonged to a risk group.
Descriptive characteristics and case fatality rates are shown in Supplementary material S2 and vaccine coverage in Supplementary material S3. Chronic heart disease and vascular disease (CHD), CKD, chronic respiratory and neurological risk groups saw a higher proportion of individuals vaccinated during December-January, the earliest phase of vaccine rollout. People aged 65+ were largely vaccinated during January and February, with a fairly even split between AZD1222 and BNT162b2 vaccines. Vaccination rollout is ongoing in the healthy 16–64 cohort; more have received AstraZeneca.
Since the cohort start date up to 16th August 2021, 16,180 serology samples were collected, of which we were able to link 11,911 to GP records; all were members of the vaccine effectiveness cohort. 7080 linked serology samples were taken post vaccination, of which 6473 were N antibody negative (without clear evidence of past infection): 1933 fell within in the period 28–90 days after dose 1 and 2548 were taken 14+ days after dose 2.
Nucleocapsid seropositivity
Modelled and adjusted N-assay based seropositivity is given in Supplementary Fig. S4.1. For individuals not belonging to a risk group seropositivity was 9.7% (95% CI 8.4–11.1%). Seropositivity was lower for individuals in risk groups and shielding, though not significantly. For the specific risk groups seropositivity was a little lower for the CHD, chronic respiratory, immunosuppressed, and chronic liver groups, with seropositivity of around 7%.
Vaccine-induced spike antibodies
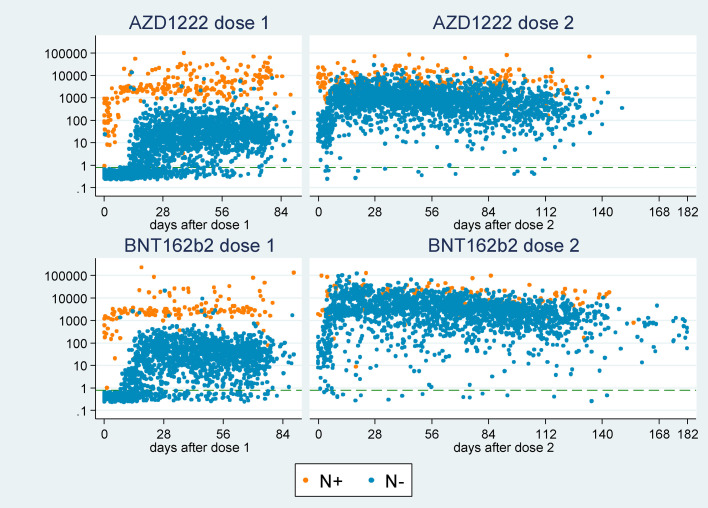
Spike (S) serology outcomes were available for 1933 adults with no evidence of naturally acquired N antibodies who had received dose 1 vaccination 28–90 days prior; we assume S positive outcomes in N negative individuals indicate vaccine-induced antibodies. Fig. 1 shows the S antibody level plotted against time since vaccination, with points coloured by vaccine manufacturer and N outcome. Given the high specificity of the N assay (99.8%), we assume that individuals with a positive N outcome have experienced a past infection and developed antibodies to the S protein. These antibodies appear to be boosted by vaccination, and most N positive individuals reach very high S antibody levels within 7–10 days of first vaccination, with 100% S positive 7+ days after dose 1. We do not consider these individuals further in analyses and instead focus on N negative individuals only.
Fig. 1.
Spike (S) antibody level following 1 and 2 vaccination doses, coloured by nucleocapsid (N) antibody status.
Table 1 gives estimates from multivariable regression models of S seropositivity and S levels including all specific risk groups, and Table 1 also includes estimates for no risk group / risk (non-shielding) / shielding (fitted separately). Results for model covariates (age, sex, days since dose, schedule) are presented in Supplementary material S4. Within the specific risk groups, dose 1 seropositivity is significantly lower for the diabetes and immunosuppressed groups for both vaccines and CHD for BNT162b2. The immunosuppressed group especially stands out as having a less vaccine-induced antibodies: 75% and 77% for AZD1222 and BNT162b2, respectively, as compared with 96% in non-immunosuppressed individuals. Individuals who were shielding or belonging to a risk group had significantly lower seropositivity than those not in a risk group. Reduced S-antibody levels after dose 1 were seen in the immunosuppressed and diabetes risk groups for both vaccines, and also in the CKD and severe asthma groups for AZD1222, and the CHD and morbid obesity groups for BNT162b2. After 2 doses, there were only 13 individuals in the whole cohort who were not S-antibody positive (Table 2 ). Reduced levels were seen in the immunosuppressed (AZD1222 30% reduction [95%CI 8–46%]; BNT162b2 77% reduction [95% CI 71–82%]) and chronic respiratory disease groups (AZD1222 41% reduction [95%CI 23–55%]; BNT162b2 36% reduction [95% CI 17–51%]), and also CKD for AZD1222 only (25% reduction [95% CI 5–40%]) and diabetes for BNT162b2 only (22% reduction [95% CI 6–35%]).
Table 1.
Presence and levels of spike (S) antibodies 28–90 days after dose 1 COVID-19 vaccination in N-negative individuals by risk group status: N samples, % positive and approximate adjusted prevalence ratios, median response (IQR) with adjusted geometric mean (GM) ratio of responses.
| absence / presence in risk group | N | n pos (%) | prevalence ratio (95% CI)1 | median (IQR) | adjusted GM ratio (95% CI)1 |
|---|---|---|---|---|---|
| Astra Zeneca AZD1222: risk groups | |||||
| not CHD | 777 | 740 (95%) | 1 (ref) | 30.3 (11 - 64.4) | 1 (ref) |
| CHD | 244 | 218 (89%) | 0.96 (0.92 - 1.01) | 24.1 (7.4 - 52.7) | 0.85 (0.66 - 1.09) |
| not diabetes | 853 | 811 (95%) | 1 (ref) | 30.6 (11.1 - 63.5) | 1 (ref) |
| diabetes | 168 | 147 (88%) | 0.94 (0.89 - 1)* | 17.3 (5.8 - 51.7) | 0.71 (0.54 - 0.93)* |
| not neurological | 905 | 850 (94%) | 1 (ref) | 29.6 (10.8 - 64.1) | 1 (ref) |
| neurological | 116 | 108 (93%) | 1.01 (0.96 - 1.07) | 21.4 (6.1 - 48.6) | 0.73 (0.53 - 1.01) |
| not chronic kidney | 905 | 856 (95%) | 1 (ref) | 28.9 (10.8 - 63.5) | 1 (ref) |
| chronic kidney | 116 | 102 (88%) | 0.96 (0.9 - 1.03) | 21.7 (4.7 - 52.6) | 0.71 (0.52 - 0.98)* |
| not morbid obesity | 953 | 895 (94%) | 1 (ref) | 28.9 (9.9 - 61.7) | 1 (ref) |
| morbid obesity | 68 | 63 (93%) | 0.99 (0.93 - 1.06) | 22.8 (9.3 - 60.9) | 0.82 (0.55 - 1.23) |
| not chronic respiratory | 951 | 894 (94%) | 1 (ref) | 28.7 (10.3 - 61) | 1 (ref) |
| chronic respiratory | 70 | 64 (91%) | 1.01 (0.95 - 1.08) | 20.3 (5.8 - 74.3) | 1.03 (0.69 - 1.55) |
| not immunosuppressed | 934 | 893 (96%) | 1 (ref) | 30.1 (11.5 - 64.4) | 1 (ref) |
| immunosuppressed | 87 | 65 (75%) | 0.78 (0.7 - 0.88)⁎⁎ | 10.5 (0.7 - 33.9) | 0.25 (0.17 - 0.36)⁎⁎ |
| not chronic liver | 969 | 911 (94%) | 1 (ref) | 28.4 (9.7 - 61) | 1 (ref) |
| chronic liver | 52 | 47 (90%) | 0.98 (0.9 - 1.07) | 32.2 (11.6 - 76.3) | 1.22 (0.77 - 1.93) |
| not severe asthma | 1002 | 944 (94%) | 1 (ref) | 28.8 (10 - 62.6) | 1 (ref) |
| severe asthma | 19 | 14 (74%) | 0.79 (0.61 - 1.02) | 10.9 (0.7 - 40.9) | 0.34 (0.16 - 0.72)⁎⁎ |
| Astra Zeneca AZD1222: risk status | |||||
| not in any risk group | 468 | 458 (98%) | 1 (ref) | 38.4 (15.7 - 71.8) | 1 (ref) |
| any risk group (non-shielding) | 516 | 471 (91%) | 0.94 (0.92 - 0.97)⁎⁎ | 22.1 (7.2 - 53.6) | 0.57 (0.46 - 0.7)⁎⁎ |
| shielding | 37 | 29 (78%) | 0.82 (0.69 - 0.96)⁎⁎ | 17.3 (2.7 - 40.5) | 0.39 (0.22 - 0.68)⁎⁎ |
| Pfizer Bio N-Tech BNT162b2: risk groups | |||||
| not CHD | 570 | 543 (95%) | 1 (ref) | 37.9 (14.6 - 84.2) | 1 (ref) |
| CHD | 339 | 308 (91%) | 0.96 (0.92 - 1)* | 20.3 (6.9 - 53.9) | 0.76 (0.59 - 0.99)* |
| not diabetes | 721 | 683 (95%) | 1 (ref) | 33.5 (11.9 - 80.7) | 1 (ref) |
| diabetes | 188 | 168 (89%) | 0.95 (0.9 - 1)* | 20.7 (5.2 - 55.9) | 0.67 (0.5 - 0.89)⁎⁎ |
| not neurological | 781 | 735 (94%) | 1 (ref) | 33.4 (11.5 - 78.3) | 1 (ref) |
| neurological | 128 | 116 (91%) | 0.98 (0.93 - 1.04) | 19.7 (7.2 - 55.5) | 0.89 (0.64 - 1.25) |
| not chronic kidney | 717 | 672 (94%) | 1 (ref) | 33.8 (11.5 - 78.9) | 1 (ref) |
| chronic kidney | 192 | 179 (93%) | 1.04 (0.99 - 1.08) | 19.9 (8.3 - 58.8) | 1.27 (0.95 - 1.72) |
| not morbid obesity | 872 | 817 (94%) | 1 (ref) | 30.7 (10.6 - 77.1) | 1 (ref) |
| morbid obesity | 37 | 34 (92%) | 0.95 (0.86 - 1.05) | 35.9 (16.3 - 52.7) | 0.52 (0.29 - 0.93)* |
| not chronic respiratory | 797 | 746 (94%) | 1 (ref) | 32.5 (11.3 - 78.1) | 1 (ref) |
| chronic respiratory | 112 | 105 (94%) | 1.01 (0.95 - 1.07) | 20.8 (5.8 - 49.6) | 0.85 (0.59 - 1.22) |
| not immunosuppressed | 811 | 776 (96%) | 1 (ref) | 33.4 (12.6 - 79.2) | 1 (ref) |
| immunosuppressed | 98 | 75 (77%) | 0.79 (0.71 - 0.89)⁎⁎ | 8 (1.3 - 37.4) | 0.2 (0.14 - 0.3)⁎⁎ |
| not chronic liver | 855 | 800 (94%) | 1 (ref) | 30.8 (10.9 - 76.8) | 1 (ref) |
| chronic liver | 54 | 51 (94%) | 1.05 (0.98 - 1.12) | 27.8 (5.9 - 72.2) | 1.12 (0.69 - 1.81) |
| not severe asthma | 879 | 822 (94%) | 1 (ref) | 31.1 (10.7 - 75.8) | 1 (ref) |
| severe asthma | 30 | 29 (97%) | 1.06 (0.97 - 1.16) | 24.8 (6.6 - 68.8) | 1.35 (0.7 - 2.62) |
| Pfizer Bio N-Tech BNT162b2: risk status | |||||
| not in any risk group | 289 | 282 (98%) | 1 (ref) | 55.1 (23.4 - 97.7) | 1 (ref) |
| any risk group (non-shielding) | 578 | 534 (92%) | 0.96 (0.93 - 0.99)⁎⁎ | 23.8 (7.5 - 65.6) | 0.69 (0.52 - 0.91)⁎⁎ |
| shielding | 42 | 35 (83%) | 0.86 (0.75 - 0.98)* | 21.9 (3.1 - 65) | 0.46 (0.25 - 0.84)⁎⁎ |
statistically significant at 5% level,
statistically significant at 1% level,
adjusted for age, sex, days since dose.
Table 2.
Levels of spike (S) antibodies 14+ days after dose 2 COVID-19 vaccination in N-negative individuals by risk group status: N samples,% positive, median response and geometric mean (GM) ratio of responses.
| absence / presence in risk group | N | n pos (%) | median (IQR)1 | adjusted GM ratio (95% CI)2 |
|---|---|---|---|---|
| Astra Zeneca AZD1222: risk groups | ||||
| not CHD | 955 | 951 (100%) | 782 (401 - 1536) | 1 (ref) |
| CHD | 303 | 301 (99%) | 719 (273 - 1552) | 0.88 (0.74 - 1.04) |
| not diabetes | 1034 | 1028 (99%) | 774.5 (364 - 1533) | 1 (ref) |
| diabetes | 224 | 224 (100%) | 714 (333 - 1580.5) | 1.02 (0.85 - 1.23) |
| not neurological | 1149 | 1144 (100%) | 767 (360 - 1547) | 1 (ref) |
| neurological | 109 | 108 (99%) | 708 (349 - 1494) | 1.05 (0.82 - 1.34) |
| not chronic kidney | 1121 | 1117 (100%) | 781 (377 - 1552) | 1 (ref) |
| chronic kidney | 137 | 135 (99%) | 628 (237 - 1297) | 0.75 (0.6 - 0.95)* |
| not morbid obesity | 1197 | 1192 (100%) | 759 (360 - 1530) | 1 (ref) |
| morbid obesity | 61 | 60 (98%) | 862 (337 - 2217) | 1.09 (0.79 - 1.52) |
| not chronic respiratory | 1164 | 1159 (100%) | 779 (375 - 1543.5) | 1 (ref) |
| chronic respiratory | 94 | 93 (99%) | 517 (190 - 1496) | 0.59 (0.45 - 0.77)⁎⁎ |
| not immunosuppressed | 1169 | 1164 (100%) | 777 (366 - 1540) | 1 (ref) |
| immunosuppressed | 89 | 88 (99%) | 631 (198 - 1479) | 0.7 (0.54 - 0.92)* |
| not chronic liver | 1205 | 1199 (100%) | 767 (362 - 1551) | 1 (ref) |
| chronic liver | 53 | 53 (100%) | 544 (320 - 1431) | 0.8 (0.57 - 1.14) |
| not severe asthma | 1216 | 1210 (100%) | 763 (360 - 1543.5) | 1 (ref) |
| severe asthma | 42 | 42 (100%) | 810.5 (339 - 1404) | 1.11 (0.75 - 1.64) |
| Astra Zeneca AZD1222: risk status | ||||
| not in any risk group | 569 | 568 (100%) | 845 (449 - 1678) | 1 (ref) |
| any risk group (non-shielding) | 640 | 635 (99%) | 683 (300 - 1464.5) | 0.74 (0.64 - 0.86)⁎⁎ |
| shielding | 49 | 49 (100%) | 801 (359 - 1722) | 0.87 (0.6 - 1.26) |
| Pfizer Bio N-Tech BNT162b2: risk groups | ||||
| not CHD | 779 | 774 (99%) | 2934 (1378 - 5861) | 1 (ref) |
| CHD | 496 | 494 (100%) | >2500 (804 - 4895.5) | 1.07 (0.91 - 1.26) |
| not diabetes | 1002 | 996 (99%) | 2763.5 (1234 - 5669) | 1 (ref) |
| diabetes | 273 | 272 (100%) | >2500 (752 - 4798) | 0.78 (0.65 - 0.94)⁎⁎ |
| not neurological | 1084 | 1079 (100%) | 2638 (1192 - 5629) | 1 (ref) |
| neurological | 191 | 189 (99%) | >2500 (838 - 4792) | 0.91 (0.74 - 1.12) |
| not chronic kidney | 1014 | 1010 (100%) | 2845 (1273 - 5807) | 1 (ref) |
| chronic kidney | 261 | 258 (99%) | 2402 (739 - 4204) | 0.92 (0.76 - 1.11) |
| not morbid obesity | 1215 | 1208 (99%) | 2503 (1070 - 5417) | 1 (ref) |
| morbid obesity | 60 | 60 (100%) | 3812.5 (1789.5 - 12,600) | 0.92 (0.64 - 1.3) |
| not chronic respiratory | 1161 | 1157 (100%) | 2657 (1149 - 5694) | 1 (ref) |
| chronic respiratory | 114 | 111 (97%) | 2312.5 (552 - 4032) | 0.64 (0.49 - 0.83)⁎⁎ |
| not immunosuppressed | 1161 | 1159 (100%) | 2788 (1370 - 5709) | 1 (ref) |
| immunosuppressed | 114 | 109 (96%) | 767 (202 - 2645) | 0.23 (0.18 - 0.29)⁎⁎ |
| not chronic liver | 1212 | 1205 (99%) | 2524 (1081 - 5487.5) | 1 (ref) |
| chronic liver | 63 | 63 (100%) | 2815 (1591 - 6193) | 1.31 (0.93 - 1.84) |
| not severe asthma | 1237 | 1230 (99%) | 2569 (1097 - 5521) | 1 (ref) |
| severe asthma | 38 | 38 (100%) | >2500 (1149 - 4922) | 1.02 (0.65 - 1.59) |
| Pfizer Bio N-Tech BNT162b2: risk status | ||||
| not in any risk group | 373 | 373 (100%) | 3576 (1893 - 6684) | 1 (ref) |
| any risk group (non-shielding) | 836 | 829 (99%) | >2500 (880 - 5072) | 0.74 (0.62 - 0.88)⁎⁎ |
| shielding | 66 | 66 (100%) | 2064 (547 - 3996) | 0.71 (0.49 - 1.04) |
statistically significant at 5% level,.
statistically significant at 1% level.
some S levels were capped at 2500, hence some results can only be given as >2500.
adjusted for age, sex, days since dose and dosing schedule.
Vaccine effectiveness
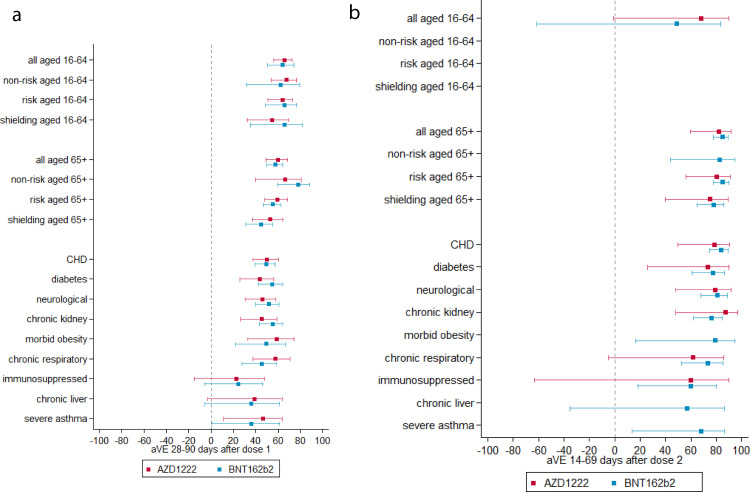
Table 3 and Fig. 2 show vaccine effectiveness estimates by age group or risk group using the cohort analysis. VE after 1 dose was approximately 60% for both vaccines, with little variation by age group. After 2 doses, in the 16–64 years cohort VE was low, but these results should be interpreted with caution since most individuals in this age group would not have been eligible for a second dose for the majority of the study period. Only small numbers of health and social care workers and clinical risk groups are likely to have received a second vaccine dose. In the 65 years and older cohort VE with BNT162b2 was 84.7% (95% CI 77.7% - 89.5%) and with AZD1222 was 81.7% (95% CI 59.6% - 91.7%). The TNCC, generally gave slightly higher dose 2 estimates (Supplementary Table S5.1 and Supplementary Fig. S5.1a, b).
Table 3.
Cohort study adjusted vaccine effectiveness (aVE) 28–90 days post dose one and 14–69 days post dose 2, for Pfizer Bio N-Tech BNT162b2 and AstraZeneca AZD1222 vaccines.
| Unvaccinated | Dose 1: 28–90 days | Dose 2: 14–69 days | ||||||
|---|---|---|---|---|---|---|---|---|
| group | cases | person years | cases | person years | aVE | cases | person years | aVE |
| Pfizer Bio N-Tech BNT162b2 | ||||||||
| all, ages 16–64 | 3802 | 796,910.6 | 44 | 33,667.5 | 64.1% (50.1% - 74.1%) | 3 | 2645.2 | 48.6% (−61.5% - 83.7%) |
| non-risk, ages 16–64 | 2884 | 724,485.2 | 12 | 14,103.5 | 62.3% (31.5% - 79.3%) | 3 | 1447.8 | −57.2% (−393.5% - 49.9%) |
| risk group, ages 16–64 | 860 | 68,083.6 | 31 | 18,746.5 | 65.3% (48.6% - 76.6%) | 0 | 1109 | |
| shielding, ages 16–64 | 260 | 15,287.4 | 11 | 4712.3 | 65.7% (35.1% - 81.9%) | 0 | 495.6 | |
| all, ages 65+ | 5509 | 204,073.2 | 238 | 66,564.1 | 57.7% (49.7% - 64.3%) | 33 | 37,124.6 | 84.7% (77.7% - 89.5%) |
| non-risk, ages 65+ | 958 | 89,426.2 | 11 | 23,509.2 | 78.0% (59.7% - 88.0%) | 3 | 10,578.6 | 82.2% (44.0% - 94.3%) |
| risk group, ages 65+ | 4470 | 112,251.6 | 225 | 42,294.1 | 55.3% (46.7% - 62.5%) | 30 | 26,074.2 | 84.9% (77.7% - 89.8%) |
| shielding, ages 65+ | 1869 | 29,713.5 | 130 | 11,835.9 | 44.5% (31.3% - 55.2%) | 21 | 8252.2 | 77.7% (65.0% - 85.7%) |
| CHD | 4593 | 110,956.1 | 175 | 31,740 | 49.0% (39.3% - 57.2%) | 21 | 17,246.5 | 83.7% (74.6% - 89.6%) |
| diabetes | 3534 | 78,448.8 | 83 | 19,827.8 | 54.4% (41.8% - 64.2%) | 15 | 8539 | 77.1% (60.9% - 86.6%) |
| neurological | 2796 | 63,891.1 | 113 | 15,593.3 | 51.6% (39.9% - 61.0%) | 15 | 8338.9 | 81.0% (67.6% - 88.9%) |
| chronic kidney | 1826 | 37,385.4 | 87 | 14,879.4 | 55.1% (43.1% - 64.6%) | 19 | 9860.1 | 76.0% (62.0% - 84.9%) |
| morbid obesity | 1675 | 40,601.7 | 28 | 6277.4 | 49.0% (21.7% - 66.8%) | 2 | 1858.3 | 79.2% (16.2% - 94.8%) |
| chronic respiratory | 1547 | 33,239 | 59 | 9813.9 | 45.0% (27.4% - 58.3%) | 12 | 5172.4 | 73.3% (52.5% - 85.0%) |
| immunosuppressed | 987 | 24,995.1 | 42 | 6394.5 | 24.3% (−5.9% - 46.0%) | 8 | 3150.2 | 59.6% (18.0% - 80.1%) |
| chronic liver | 939 | 25,853.2 | 17 | 4413.4 | 36.1% (−5.8% - 61.4%) | 3 | 1503 | 56.9% (−35.5% - 86.3%) |
| severe asthma | 880 | 15,538.8 | 26 | 3006.8 | 35.7% (0.8% - 58.3%) | 4 | 1331.8 | 68.0% (13.7% - 88.2%) |
| Astra Zeneca AZD1222 | ||||||||
| all, ages 16–64 | 3802 | 796,910.6 | 98 | 116,739.7 | 65.3% (56.2% - 72.5%) | 3 | 3324.6 | 67.9% (−1.1% - 89.8%) |
| non-risk, ages 16–64 | 2884 | 724,485.2 | 42 | 80,304.7 | 67.1% (53.9% - 76.5%) | 0 | 1359 | |
| risk group, ages 16–64 | 860 | 68,083.6 | 53 | 34,252.8 | 63.7% (50.8% - 73.2%) | 3 | 1783.4 | 56.6% (−36.8% - 86.2%) |
| shielding, ages 16–64 | 260 | 15,287.4 | 32 | 10,577.9 | 54.4% (32.1% - 69.4%) | 0 | 1078 | |
| all, ages 65+ | 5509 | 204,073.2 | 137 | 79,647.8 | 59.8% (49.2% - 68.2%) | 8 | 19,781.3 | 81.7% (59.6% - 91.7%) |
| non-risk, ages 65+ | 958 | 89,426.2 | 13 | 33,409.1 | 66.0% (39.6% - 80.8%) | 0 | 6749.3 | |
| risk group, ages 65+ | 4470 | 112,251.6 | 123 | 45,413.4 | 59.4% (48.2% - 68.1%) | 8 | 12,766.1 | 80.1% (56.1% - 91.0%) |
| shielding, ages 65+ | 1869 | 29,713.5 | 74 | 12,303.2 | 52.5% (36.7% - 64.4%) | 6 | 4309.7 | 74.9% (39.7% - 89.6%) |
| CHD | 4593 | 110,956.1 | 105 | 35,729 | 50.1% (37.4% - 60.2%) | 6 | 7955.1 | 78.2% (49.9% - 90.5%) |
| diabetes | 3534 | 78,448.8 | 75 | 23,776.3 | 43.2% (26.0% - 56.3%) | 4 | 4459 | 72.9% (25.8% - 90.1%) |
| neurological | 2796 | 63,891.1 | 89 | 19,686.1 | 45.9% (30.6% - 57.8%) | 5 | 4727.5 | 78.9% (47.8% - 91.4%) |
| chronic kidney | 1826 | 37,385.4 | 56 | 13,743.7 | 45.1% (26.2% - 59.1%) | 2 | 3963.2 | 87.2% (47.9% - 96.9%) |
| morbid obesity | 1675 | 40,601.7 | 21 | 9861 | 58.4% (32.8% - 74.3%) | 0 | 1103.2 | |
| chronic respiratory | 1547 | 33,239 | 29 | 11,726.2 | 57.3% (37.6% - 70.8%) | 4 | 2836.9 | 61.7% (−4.9% - 86.0%) |
| immunosuppressed | 987 | 24,995.1 | 31 | 8163.5 | 22.5% (−15.2% - 47.9%) | 2 | 1795.8 | 60.0% (−63.6% - 90.2%) |
| chronic liver | 939 | 25,853.2 | 16 | 6614.2 | 38.8% (−3.3% - 63.7%) | 1 | 938.8 | |
| severe asthma | 880 | 15,538.8 | 18 | 4382.3 | 46.6% (11.4% - 67.8%) | 1 | 788.9 | |
* adjusted for week-NHS region interaction, 5-yr age group, sex, ethnicity, IMD quintile, GP record of prior COVID-19, large household, GP consultation quartile, chapter count, shielding recommendation, overall PRIMIS risk group status (overall only) and latest smoking status.
Fig. 2.
(a) cohort vaccine effectiveness 28–90 days after dose one of vaccination. (b) cohort vaccine effectiveness 14–69 days after dose 2 of vaccination.
When considering all risk groups together, there was generally no reduction in VE compared to those not in risk groups. Slightly reduced VE was seen for those shielding, but not significantly so. When stratifying into groups of specific conditions the immunosuppressed group had most notably diminished VE. In the cohort analysis, VE after 1 dose was 24.3% (95% CI −5.9% - 46.0%) for BNT162b2 and 22.5% (95% CI −15.2% - 47.9%) for AZD1222. However, after 2 doses this increased to 59.6% (18.0% - 80.1%) for BNT162b2 and around 60.0% (−63.6% - 90.2%) for AZD1222. In the TNCC, VE was higher but still notably lower than most other groups. Lower VE estimates were also seen for the chronic liver and severe asthma groups, but these groups were smaller with greater uncertainty in estimates. amongst other risk groups, VE estimates do not differ significantly from those in non-risk groups.
Discussion
This study provides evidence of a strong S-antibody response and high levels of effectiveness of COVID-19 vaccines against symptomatic medically attended disease in most clinical risk groups. We see reduced S-antibody response and reduced VE amongst the immunosuppressed group, though VE in this group is higher after the second dose and the wide confidence intervals overlap with those in non-risk groups.
The overall immunogenicity and VE findings are similar to those reported previously. Like other studies we found lower antibody levels with advancing age and in males, and after dose 2 with shorter intervals between doses.24 The levels of VE in our study after 1 and 2 doses are similar to those previously reported in other real world studies.7 , 9 , 12, 13, 14, 15, 16 However, dose 2 VE estimates for the 16–64 age group appeared low, but only those in clinical risk groups and health and social care workers are likely to have received dose 2 by the end of the study. Health and social care workers are at higher risk of exposure to infection, which is likely to have impacted results.
Our finding of reduced S-antibody positivity and antibody levels in immunosuppressed individuals is in line with that seen in other immunogenicity studies of specific immunosuppressed groups.20, 21, 22, 23, 24, 25 One other study found reduced S antibody levels amongst individuals with cardiovascular disease, in particular amongst those on statin therapy.24 Mechanisms for any reduction in vaccine response in this group are unclear though the association between statins and lipid nanoparticle vaccines merits further investigation. VE against clinical outcomes has not previously been reported. Our findings suggest that the reduced S-antibody response after 1 dose translate into reduced VE in immunosuppressed individuals, but after a second dose VE is higher. After 2 doses of mRNA vaccine previous studies have suggested that individuals on immunosuppressive therapy maintain an immune response,30 , 31 however other studies have reported reduced immune response amongst individuals with haematological malignancy.23 , 26 Considering a broad immunosuppressed group we found only a modest and non-significant reduction in VE after 2 doses of either vaccine. There were 10 cases after 2 doses amongst immunosuppressed individuals, the majority of whom were over 70 years of age. Cases under 70 years had autoimmune conditions (Crohn's disease; type 1 diabetes and multiple sclerosis; psoriatic arthritis) and were on immune modulating therapy.
These results support maximising coverage with two doses of vaccine amongst immunosuppressed groups. In the context of high rates of COVID-19 in the population, there may be a case for reducing the interval between doses in order to maximise coverage. However, other studies have suggested that longer dosing intervals result in improved immune response, therefore such a move may be counterproductive, in particular in the context of low COVID-19 activity, a finding that we also see in our serology data (Table 2).35 The findings also support prioritising immunosuppressed individuals for third doses, and to maximise antibody levels prior to a new variant and wave of infections. The main findings are based on medically attended symptomatic disease, protection against severe disease after one dose, including hospitalisation and death, appears to be greater (supplementary material S4).
This study has a number of strengths: we rely on cases attending general practice and having relevant symptoms recorded by a medical practitioner, which is likely to be more reliable than self-reporting. We also have a large amount of data on previous medical history and demographic characteristics from the full clinical record which allows us to adjust for a large number of possible confounders. Furthermore, we have both immunogenicity data and vaccine effectiveness data (with two distinct methods to estimate VE) and in general the findings from these different analyses are concordant.
As with any observational study, there are also limitations. Disease epidemiology and testing policies have changed over the period of study. For example, with increased use of lateral flow devices in the community, PCR testing may have shifted toward more confirmatory testing of lateral flow outcomes, which could introduce temporal bias, especially in the TNCC design. We adjust for week which should help to control for such temporal changes. Risk of COVID-19 is likely be greater in health and social care workers (HSCW) who are at high risk of transmission and were amongst the first to be vaccinated. Care home residents were almost all offered vaccination before the end of January 2021, and since severity increases with age, GP consultation with symptoms may be more likely amongst the oldest age group. While it was possible to control for age effects, HSCW status is not known and our large household variable is a limited proxy for care home resident status. The lack of control for HSCW status is likely to explain the lower 2 dose VE in the 16–64 year old age group in the cohort analysis as HSCW are one of the few groups in this age cohort that will have received second doses during the study period but are also likely to have higher exposure risk. Imperfect control of these important variables will introduce bias, including temporal biases given the timing of vaccination in these groups, especially affecting the cohort study.
Unvaccinated individuals are likely to differ from vaccinated individuals in an important way. Vaccination coverage shows that individuals aged 90+, aged 65–69 and belonging to black, Asian and minority ethnic (BAME) groups, especially black ethnicities, are less likely to be vaccinated. Disparities in transmission rates are understood to exist by these sociodemographic characteristics. Those close to the end of life may be less likely to vaccinate. Those who have had recent infection are expected to wait 28 days from resolution of symptoms before vaccinating. We control for many of these factors, but some residual confounding is likely. While the cohort under study is large, once stratifying by clinical risk groups, numbers in some groups remain small and we were unable to further stratify, for example by specific cause of immunosuppression. It is likely that there are differences in immune response and VE according to the severity of immunosuppression.
In most clinical risk groups, immune response to vaccination is maintained and high levels of VE are seen with both the Pfizer and AstraZeneca vaccines. The immunosuppressed group stands out as having reduced response to vaccination after one and two doses. However, after second doses moderate vaccine effectiveness against clinical disease was seen in this group. In this study we are unable to further breakdown the impact of differing levels of immunosuppression on vaccine effectiveness. Our findings support maximising coverage of immunosuppressed individuals with two doses as well as prioritising this group for third doses. Further research is needed to understand vaccine effectiveness against severe disease amongst immunosuppressed groups, including the added value of 3rd and 4th doses.
Author contributions
HW, RT, JLB, SdeL, GA drafted the manuscript, with direction from MR. HW, NA, MS, SdeL, JLB designed the vaccine effectiveness study. HW carried out all statistical analyses. NA, JLB, SdeL FDRH, SA provided oversight of vaccine effectiveness. MZ, JE were responsible for overseeing testing of sentinel surveillance swabs. PSP, RB, JS for data management and linkage of swab data. JW, HC, EL, JH, JS for data management and linkage of serology data. EB, WV, GH for data quality and sampling from contributing GPs. AO oversaw testing of serology samples. GA, KB provided oversight of serology.
Declaration of Competing Interest
Simon de Lusignan is the Director of the Oxford-RCGP RSC and has received funding through his University for studies from Astra-Zeneca, Eli Lilly, Sanofi, GSK, MSD. Seqirus and Takeda; and been member of advisory boards for Astra-Zeneca, Seqirus and Sanofi.
Ezra Linley reports that the UKHSA Vaccine Evaluation Unit performs contract research on behalf of GSK, Sanofi and Pfizer which is outside the submitted work.
Acknowledgments
Patients at Oxford-RCGP Research and Surveillance Centre (RSC) practices who do not opt out of data sharing and who consented to additional blood samples being taken for serology, and consent to virology sampling. Our RSC member practices who share data and for sampling. Collaboration of EMIS, TPP, In-Practice systems and Wellbeing to facilitate pseudonymised data extract. Take-a-Test who provide an online self-swabbing kit supply service. Members of the Practice Liaison team for supporting RSC practices with data quality and sampling: Carole Aspen, Sharon Howe, Jack Macartney, Jessica Smylie and Alice Williams. University of Oxford Medical Sciences Division has supports the Oxford-Royal College of GPs Clinical Informatics Digital Hub (ORCHID) trusted research environment (TRE).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.12.044.
Appendix. Supplementary materials
References
- 1.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clift A.K., Coupland C.A.C., Keogh R.H., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020 doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrotri M., Krutikov M., Palmer T., et al. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of Long-Term Care Facilities (VIVALDI study) MedRxiv. 2021 doi: 10.1016/S1473-3099(21)00289-9. 2021.03.26.21254391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health England COVID-19: the green book, chapter 14a. Immunisation against infectious diseases. Public Health Engl. 2020 [Google Scholar]
- 11.Public Health England. Coronavirus (COVID-19) in the UK - Vaccinations in United Kingdom. 2021. coronavirus.data.gov.uk/details/vaccinations (accessed 24 November 2021).
- 12.Ismail S.A., Vilaplana T.G., Elgohari S., et al. Effectiveness of BNT162b2 mRNA and ChAdOx1 adenovirus vector COVID-19 vaccines on risk of hospitalisation among older adults in England: an observational study using surveillance data. PHE Preprints 2021. https://khub.net/documents/135939561/430986542/Effectiveness+of+BNT162b2+mRNA+and+ChAdOx1+adenovirus+vector+COVID-19+vaccines+on+risk+of+hospitalisation+among+older+adults+in+England.pdf/9e18c525-dde6-5ee4-1537-91427798686b (accessed 4th April 2022).
- 13.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on mortality following COVID-19. MedRxiv. 2021 2021.05.14.21257218. [Google Scholar]
- 14.Lopez Bernal J., Andrews N., Gower C., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. MedRxiv. 2021 doi: 10.1056/NEJMc2113090. 2021.05.22.21257658. [DOI] [PubMed] [Google Scholar]
- 15.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. The New England journal of medicine. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson M.G., Burgess J.L., Naleway A.L., et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—Eight US locations, December 2020–March 2021. Morbid Mortal Weekly Rep. 2021;70(13):495. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasileiou E., Simpson C.R., Shi T., et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarif A., Joy M., Sherlock J., et al. The impact of primary care supported shielding on the risk of mortality in people vulnerable to COVID-19: english sentinel network matched cohort study. J Infect. 2021 doi: 10.1016/j.jinf.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020#contents (accessed 18 January 2021).
- 20.Benotmane I., Gautier-Vargas G., Cognard N., et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021 doi: 10.1016/j.kint.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billany R.E., Selvaskandan H., Adenwalla S.F., et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: a call to arms. Kidney Int. 2021;99(6):1492–1494. doi: 10.1016/j.kint.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyarsky B.J., Ruddy J.A., Connolly C.M., et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220289. annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrotri M., Fragaszy E., Geismar C., et al. Spike-antibody responses to ChAdOx1 and BNT162b2 vaccines by demographic and clinical factors (Virus Watch study) MedRxiv. 2021 2021.05.12.21257102. [Google Scholar]
- 25.Yi S.G., Knight R.J., Graviss E.A., et al. Kidney transplant recipients rarely show an early antibody response following the first COVID-19 vaccine administration. Transplantation. 2021 doi: 10.1097/TP.0000000000003764. [DOI] [PubMed] [Google Scholar]
- 26.Agha M., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. MedRxiv. 2021 doi: 10.1093/ofid/ofab353. 2021.04.06.21254949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peled Y., Ram E., Lavee J., et al. BNT162b2 vaccination in heart transplant recipients: clinical experience and antibody response. J Heart Lung Transpl. 2021 doi: 10.1016/j.healun.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attias P., Sakhi H., Rieu P., et al. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021 doi: 10.1016/j.kint.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220272. annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong S.Y., Dixon R., Martinez Pazos V., et al. Serologic response to messenger rna coronavirus disease 2019 vaccines in inflammatory bowel disease patients receiving biologic therapies. Gastroenterology. 2021 doi: 10.1053/j.gastro.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lusignan S., Lopez Bernal J., Byford R., et al. Influenza and respiratory virus surveillance, vaccine uptake, and effectiveness at a time of cocirculating COVID-19: protocol for the english primary care sentinel system for 2020-2021. JMIR Public Health Surveill. 2021;7(2):e24341. doi: 10.2196/24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lusignan S., Correa A., Smith G.E., et al. RCGP research and surveillance centre: 50 years’ surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. 2017;67(663):440. doi: 10.3399/bjgp17X692645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Lusignan S., Jones N., Dorward J., et al. The oxford royal college of general practitioners clinical informatics digital hub: protocol to develop extended COVID-19 surveillance and trial platforms. JMIR Public Health Surveill. 2020;6(3):e19773. doi: 10.2196/19773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voysey M., Costa Clemens S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.