Abstract
TP53 mutations was reported to be correlated to the efficacy of program death-1 (PD-1) and program death ligand-1 (PD-L1). The role of co-mutations of TP53 with other recurrently mutated genes in outcome of anti-PD-(L)1 treatment for non-small cell lung cancer (NSCLC) is unknown. Here we mined a previously generated dataset to address the effect of co-mutations on the progression free survival (PFS) of NSCLC patients. Non-synonymous mutations and clinical data of 240 NSCLC patients with anti-PD-(L)1 based therapy was downloaded from cBioPortal. Totally 206 patients received monotherapy and 34 patients received combination therapy. In 240 NSCLC patients, TP53 mutation rate was 59.2%. For the monotherapy cohort, TP53 mutated NSCLC patients have a significantly longer PFS (4.3 vs. 2.5 months, P = 0.0019) compared with TP53 wild type NSCLC patients. The same tendency was also observed in the combination therapy cohort, but the difference in PFS (6.3 vs. 5.4 months, P = 0.12) was not significant. Ever-smoker had a longer PFS compared to never-smokers (4.0 vs. 2.7 months). For further co-mutation analysis with TP53 including KEAP1 mutation (53/240, 22.1%), KMT3C mutation (26/240, 10.8%), STK11 mutation (56/240, 23.3%), EGFR mutation (28/240, 11.7%) and KRAS mutation (86/240, 35.8%). Patients with both TP53 plus KEAP1 mutations in all 240 patients had a longer PFS compared with co-wild population (PFS 9.2 vs. 4.2 months, P = 0.012) when treated with PD-1/PD-L1 inhibitors. TP53 might be the dominating mutation correlating with longer PFS in PD-1/PD-L1 monotherapy. Different genes displayed distinct effect when co-mutated with TP53 in NSCLC patients.
Keywords: NSCLC, PD-1, PD-L1, PFS, TP53 co-mutation
Introduction
Checkpoint as PD-1 and PD-L1 inhibitors have emerged as the most promising therapeutics for non-small-cell lung cancer (NSCLC) which could prolong the 5-year survival in those responders.1 However, the efficacy of checkpoint inhibitors in NSCLC was limited with an objective response rate of around 20%.2 Identification of biomarkers with predictive power for the outcome of checkpoint inhibition could guide the clinical decision to employ checkpoint inhibitors. Currently, expression of PD-L1 and tumor mutation burden (TMB) are most wildly investigated as biomarkers to predict the effect of checkpoint inhibitors.3,4 However, some PD-L1 negative and TMB low NSCLC populations could still respond to PD-1/PD-L1 antibodies. The complexity of checkpoint inhibition has yet to be investigated.
TP53 is one of the well-studied genes in human. With another tumor suppressor gene CHEK2, p53 checks whether DNA mutations in a damaged cell can be repaired or the cell has to be destroyed.5 It was reported that TP53 mutation is independently correlated with longer OS in advanced NSCLC patients.6 This effect can be partially explained by the connection between TP53 and TMB. If the functionality of p53 is intact, the magnitude of mutations in cancer will be kept at minimal level. TP53 mutation was reported to significantly increase the expression of immune checkpoints and activated T-effector and interferon-signature and TP53/KRAS co-mutation NSCLC showed remarkable clinical benefit to PD-1 inhibitors in a small sampled study with 34 patients,7 which needs to be further confirmed.
The mutational landscape of cancer is rather complexed.8 For NSCLC, there are many other recurrently mutated genes with mutation frequency above 10%.9 Examples include KRAS, KEAP1, STK11 and EGFR. KRAS mutations leads to hyperactivated downstream signaling controlling cell proliferation.10 KEAP1 is an important regulator of antioxidant response, determining the cellular outcome after exposure to oxidative stress.11 STK11 is a major modulator of lung cancer differentiation and metastasis.12 EGFR is an important receptor regulating RAS/MAPK, PI3K/AKT signaling pathways, and the target of EGFR-TKI (Tyrosine Kinase Inhibitor). EGFR mutations critically impacts the clinical outcomes of NSCLC patients.13
It is not clear that how co-mutation of TP53 with oncogenes or other tumor suppressor genes influence the response of NSCLC patients to checkpoint inhibitors. Our study took advantage of a recently published cohort of NSCLC patients with mutation data and survival data after receiving either monotherapy using anti-PD-1/PD-L1 therapy or combination therapy of anti-PD-1/PD-L1 and anti-CTLA-4.14
Specifically, this study aims to investigate the impact of co-mutation pattern on progression free survival of NSCLC patients.
Materials and methods
Data collection
Non-synonymous mutations and clinical data of 240 NSCLC patients with anti-PD-(L)1 based therapy was downloaded from cBioPortal.14,15 Patient samples were analyzed by MSK-IMPACT assay as previously described. Sequencing libraries were generated for a custom panel of 341 (56 patients, version 1), 410 (164 patients, version 2) and 468 (20 patients, version 3) genes. In total, 206 patients received monotherapy with PD-1/PD-L1 inhibitors and 34 patients received combination therapy with PD-1/PD-L1 inhibitor and anti-CTLA-4 therapy. All patients were enrolled in Memorial Sloan Kettering Cancer Center between April 2011 and January 2017.
Survival analysis
Survival analysis was performed with Kaplan–Meier method. Survminer was used to implement survival analysis. All plots were generated with R statistical programming environment. For each patient stratification method, survival curves were plotted for the monotherapy cohort, the combination therapy cohort and the complete patient cohort.
To determine single-cell mutation and double-gene mutation, only non-synonymous mutations were considered. Kaplan–Meier curves analysis of progression-free survival (PFS) were compared using the log-rank test.
Statistics
No statistical method was carried out to estimate the sample number. All reported P values are two-tailed, and for all analyses, P less than 0.05 is considered statistically significant, unless otherwise specified. Hazard ratios (HRs) were calculated by the Mantel–Haenszel test. Given that smoking acts as a possible treatment selection bias, we performed multivariable extended cox regression when accessing the effect of co-mutation.
Results
In total, there were 206 patients who received monotherapy with PD-1/PD-L1 inhibitor and 34 patients who received combination therapy with PD-1/PD-L1 inhibitor and anti-CTLA-4 therapy. First, we evaluated the effect of all the patients' characteristics on PFS (Table 1), and results showed that smoking status (4.0 vs. 2.7 months, P = 0.031), lines of treatment (7.5 vs. 2.7 months, P = 0.00046), TP53 mutation (4.3 vs. 2.5 months, P = 0.00019), EGFR mutation (3.1 vs. 3.3 months, P = 0.0038) and KMT2C mutation (7.3 vs. 3.2 months, P = 0.049) were significantly correlated with PFS in the entire cohort of patients. For monotherapy of PD-1 and PD-L1 inhibitors, only smoking status (3.3 vs. 2.1 months, P = 0.0025) and TP53 mutation (4.0 vs. 2.5 months, P = 0.0078) had significant effect on PFS.
Table 1.
Patients characteristics.
| Item | number | mPFS (all) | logrank_P | cox_P | HR | 0.95LCI | 0.95UCI | mPFS (mono) | logrank_P | cox_P | HR | 0.95LCI | 0.95UCI | mPFS (combination) | logrank_P | cox_P | HR | 0.95LCI | 0.95UCI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis Age | 18–60 | 76 | 3.13 | 0.39 | 3.05 | 0.91 | 5.73 | 0.26 | ||||||||||||
| ≥60 | 164 | 3.50 | 0.393 | 1.1 | 0.84 | 1.5 | 3.07 | 0.915 | 1 | 0.73 | 1.4 | 7.9 | 0.267 | 1.6 | 0.7 | 3.6 | ||||
| Sex | Female | 122 | 3.07 | 0.54 | 2.77 | 0.74 | 6.33 | 0.43 | ||||||||||||
| Male | 118 | 3.50 | 0.542 | 1.1 | 0.83 | 1.4 | 3.23 | 0.738 | 1.1 | 0.78 | 1.4 | 7.90 | 0.427 | 1.4 | 0.63 | 3 | ||||
| smoking | Ever | 193 | 4.00 | 0.031 | 3.3 | 0.0025 | 6.33 | 0.93 | ||||||||||||
| never | 47 | 2.67 | 0.032 | 1.4 | 1 | 2 | 2.1 | 0.003 | 1.8 | 1.2 | 2.6 | 11.83 | 0.933 | 1 | 0.43 | 2.5 | ||||
| Pathology | squamous cell carcinioma | 34 | 2.92 | LUAD-LUSC: 0.9782 | 0.981 | 1.01 | 0.67 | 1.5 | 3.23 | LUAD-LUSC: 0.8644 | 0.426 | 0.84 | 0.54 | 1.3 | 1.83 | LUAD-LUSC: 0.0709 | 0.043 | 3.96 | 1.04 | 15 |
| adenocarcinoma | 186 | 3.50 | LUAD-Others: 0.9639 | 3.07 | LUAD-Others: 0.8644 | 8.63 | LUAD-Others: 0.6416 | |||||||||||||
| Others | 20 | 3.68 | LUSC-Others: 0.9639 | 0.535 | 0.84 | 0.47 | 1.5 | 2.52 | LUSC-Others: 0.8644 | 0.753 | 0.91 | 0.49 | 1.7 | 6.33 | LUSC-Others: 0.2571 | 0.668 | 0.73 | 0.17 | 3.1 | |
| Lines.of.treatment of PD-1/PD-L1 | 1 st | 51 | 7.50 | 0.00046 | 5.47 | 0.2 | 10.46 | 0.087 | ||||||||||||
| ≥2 | 189 | 2.73 | 0.005 | 1.7 | 1.2 | 2.4 | 2.67 | 0.201 | 1.3 | 0.86 | 2 | 4.33 | 0.093 | 2 | 0.89 | 4.5 | ||||
| Detection panel | IMPACT341 | 56 | 2.92 | IMPACT341-IMPACT410: 0.4389 | 2.1 | IMPACT341-IMPACT410: 0.4389 | 7.9 | IMPACT341-IMPACT410: 0.4057 | ||||||||||||
| IMPACT410 | 164 | 3.50 | IMPACT341-IMPACT468: 0.4389 | 0.401 | 0.87 | 0.63 | 1.2 | 3.17 | IMPACT341-IMPACT468: 0.4389 | 0.035 | 0.69 | 0.48 | 0.97 | 6.33 | IMPACT341-IMPACT468:0.4057 | 0.405 | 1.4 | 0.61 | 3.3 | |
| IMPACT468 | 20 | 4.17 | IMPACT410-IMPACT468: 0.4389 | 0.238 | 0.68 | 0.36 | 1.3 | 6.03 | IMPACT410-IMPACT468: 0.4389 | 0.038 | 0.5 | 0.26 | 0.96 | 3.43 | IMPACT410-IMPACT468:0.4057 | 0.209 | 4 | 0.46 | 34.2 | |
| KEAP1 | yes | 53 | 2.80 | 0.53 | 0.538 | 0.9 | 0.64 | 1.3 | 2.5 | 0.77 | 22.63 | 0.1 | 0.12 | 0.31 | 0.072 | 1.4 | ||||
| no | 187 | 3.50 | 3.07 | 0.777 | 0.95 | 0.67 | 1.4 | 5.43 | ||||||||||||
| KMT2C | yes | 26 | 7.33 | 0.049 | 0.052 | 0.62 | 0.39 | 1 | 4.17 | 0.11 | 22.43 | 0.23 | 0.247 | 0.42 | 0.099 | 1.8 | ||||
| no | 214 | 3.17 | 2.9 | 0.118 | 0.67 | 0.41 | 1.1 | 5.43 | ||||||||||||
| STK11 | yes | 56 | 2.54 | 0.23 | 0.229 | 1.2 | 0.88 | 1.7 | 2.47 | 0.21 | 9.10 | 0.65 | 0.651 | 0.78 | 0.27 | 2.3 | ||||
| no | 184 | 3.80 | 3.23 | 0.207 | 1.2 | 0.89 | 1.8 | 6.33 | ||||||||||||
| EGFR | yes | 28 | 3.07 | 0.038 | 0.04 | 1.6 | 1 | 2.4 | 3.07 | 0.12 | 2.92 | 0.044 | 0.052 | 2.8 | 0.99 | 8 | ||||
| no | 212 | 3.50 | 3.03 | 0.123 | 1.4 | 0.9 | 2.3 | 7.9 | ||||||||||||
| KRAS | yes | 86 | 3.43 | 0.83 | 0.824 | 0.97 | 0.72 | 1.3 | 3.07 | 0.56 | 4.88 | 0.79 | 0.783 | 1.1 | 0.5 | 2.5 | ||||
| no | 154 | 3.30 | 2.8 | 0.559 | 0.91 | 0.67 | 1.2 | 8.63 | ||||||||||||
| TP53 | yes | 142 | 4.27 | 0.0019 | 0.002 | 0.64 | 0.49 | 0.85 | 4.00 | 0.0078 | 6.33 | 0.12 | 0.127 | 0.55 | 0.25 | 1.2 | ||||
| no | 98 | 2.47 | 2.47 | 0.008 | 0.67 | 0.49 | 0.9 | 5.43 | ||||||||||||
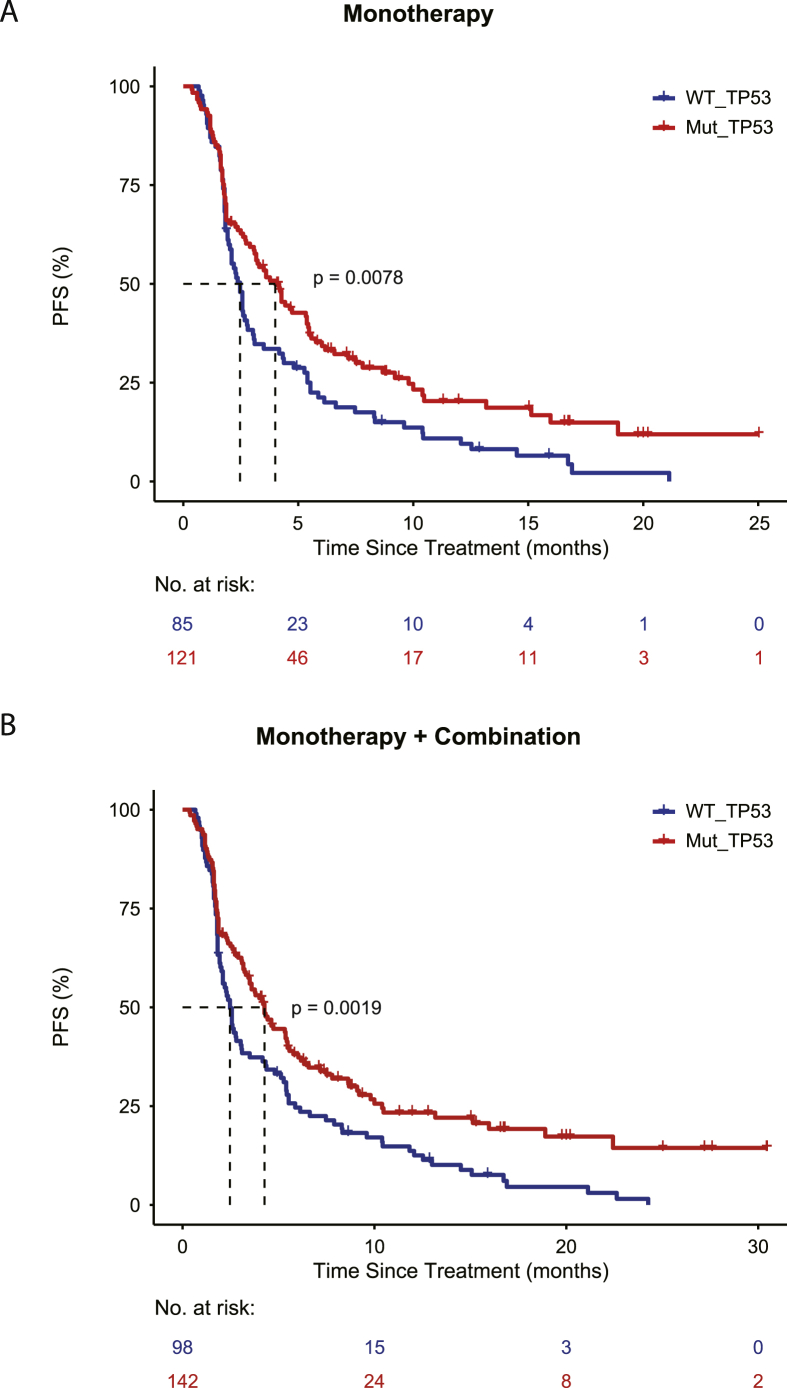
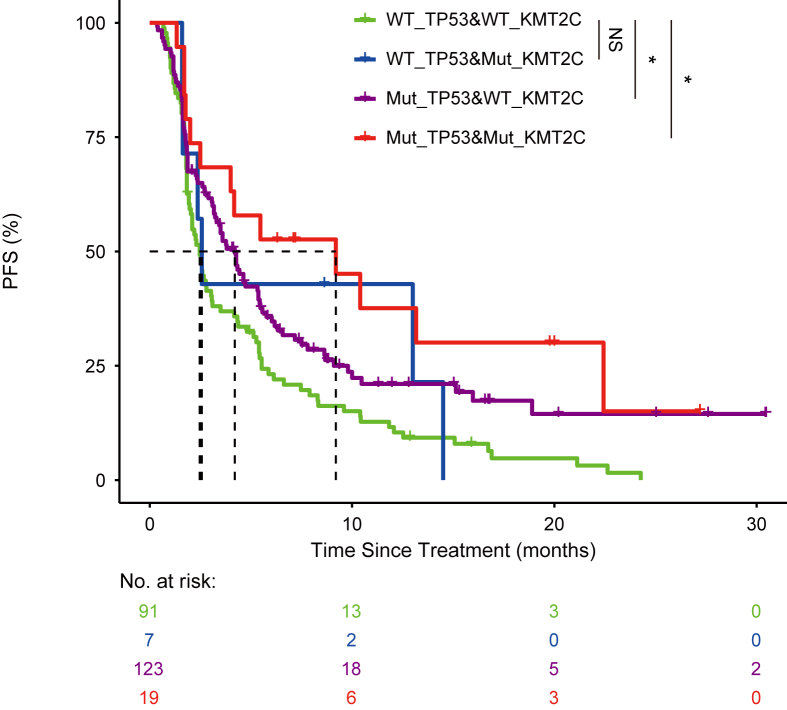
Then we plotted the survival curve for both the monotherapy cohort and the combination cohort (Fig. 1). Patients were stratified based on the status of TP53 mutation. In 240 NSCLC patients, TP53 mutation rate was 59.2%. Consistently, it was found that NSCLC patients with TP53 mutations had significantly longer PFS either using the monotherapy cohort or the entire cohort. For the monotherapy (PD-1, PD-L1) cohort, TP53 mutated NSCLC patients have a significantly longer PFS (4.3 months vs. 2.5 months, P = 0.0019) compared with TP53 wild type NSCLC patients. The same tendency was also observed in the combination therapy cohort, but the difference in PFS (6.3 months vs. 5.4 months, P = 0.12) was not significant due to limited number of patients. We focused on three recurrently mutated tumor suppressor genes: KMT2C, STK11 and KEAP1 (Table 2). Patients with co-mutation in TP53 and KMT2C have longer PFS (9.2 months vs. 2.5 months, P = 0.005) compared with patients without TP53 and KMT2C mutations (Fig. 2). Co-mutations seemed to confer a favorable survival compared with the patients with only mutation in one gene. For co-mutation analysis, patients with TP53 and STK11 co-mutations have better PFS (3.3 months vs. 2.6 months), but this was not statistically significant. Patients with mutant TP53 and wild type STK11 had significantly longer PFS (4.3 months vs. 2.6 months) as compared with patients with wild type TP53 and STK11. Similarly, in the case of TP53 and KEAP1 co-mutation, it seemed that TP53 mutation was dominating the outcome of checkpoint inhibition. KEAP1 mutation diminished the effect of TP53 mutation.
Figure 1.
(A) Patients treated with monotherapy were stratified with TP53 mutation status. The survival curve was plotted with PFS for the distinct group. Wild type TP53 is shown with blue and mutated TP53 is displayed with red. (B) Patients treated either with monotherapy or combination therapy were stratified with TP53 mutation status. The survival curve was plotted with PFS for the distinct group. Wild type TP53 is shown with blue and mutated TP53 is displayed with red.
Table 2.
Co-mutation status with PFS of PD-1/PDL1 treatment.
| Number of case | mPFS (all) | log-rank_P | COX P | rmsmoker_cox_P | mPFS (mono) | log-rank_P | COX P | rmsmoker_cox_P | mPFS (combine) | log-rank_P | COX P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP53 mut | KEAP1 mut | 34 | 3.38 | 0.1348 | 0.037 (0.62, 0.39–0.97) | 0.093 | 3.17 | 0.3252 | 0.109 (0.68, 0.42–1.09) | 0.314 | NA | 0.3489 | 0.083 (0.16, 0.02–1.3) |
| TP53 mut | KEAP1 wild | 108 | 4.27 | 0.0086 | 0.002 (0.61, 0.44–0.84) | 0.009 | 4.27 | 0.0231 | 0.005 (0.61, 0.43–0.86) | 0.032 | 6.33 | 0.5283 | 0.191 (0.59, 0.26–1.3) |
| TP53 wild | KEAP1 mut | 19 | 2.27 | 0.4491 | 0.363 (0.78, 0.46–1.33) | 0.566 | 2.18 | 0.4712 | 0.351 (0.76, 0.43–1.34) | 0.684 | 22.63 | 0.5283 | 0.379 (0.4, 0.05–3.1) |
| TP53 wild | KEAP1 wild | 79 | 2.57 | 2.57 | 5.28 | ||||||||
| TP53 mut | KMT2C mut | 19 | 9.2 | 0.0204 | 0.005 (0.44, 0.24–0.78) | 0.012 | 5.47 | 0.061 | 0.02 (0.48, 0.26–0.89) | 0.056 | 22.43 | 0.3828 | 0.141 (0.21, 0.028–1.7) |
| TP53 mut | KMT2C wild | 123 | 4.2 | 0.0204 | 0.007 (0.67, 0.5–0.9) | 0.019 | 3.6 | 0.061 | 0.021 (0.69, 0.5–0.95) | 0.071 | 4.63 | 0.3828 | 0.205 (0.59, 0.266–1.3) |
| TP53 wild | KMT2C mut | 7 | 2.57 | 0.5119 | 0.42 (0.71, 0.31–1.63) | 0.523 | 2.47 | 0.671 | 0.571 (0.77, 0.31–1.91) | 0.769 | 13 | 0.8451 | 0.64 (0.61, 0.079–4.8) |
| TP53 wild | KMT2C wild | 91 | 2.47 | 2.47 | 5.28 | ||||||||
| TP53 mut | STK11 mut | 21 | 3.3 | 0.5177 | 0.285 (0.75, 0.44–1.28) | 0.58 | 2.83 | 0.7648 | 0.527 (0.83, 0.47–1.48) | 0.932 | 9.1 | 0.6947 | 0.267 (0.42, 0.094–1.9) |
| TP53 mut | STK11 wild | 121 | 4.27 | 0.0349 | 0.007 (0.64, 0.46–0.88) | 0.043 | 4.2 | 0.0732 | 0.013 (0.64, 0.45–0.91) | 0.119 | 6.33 | 0.6947 | 0.165 (0.55, 0.237–1.3) |
| TP53 wild | STK11 mut | 35 | 2.27 | 0.9195 | 0.884 (1.03, 0.67–1.59) | 0.507 | 2.27 | 0.9021 | 0.941 (0.98, 0.62–1.55) | 0.481 | 12.13 | 0.6947 | 0.77 (0.8, 0.173–3.7) |
| TP53 wild | STK11 wild | 63 | 2.6 | 2.47 | 5.43 | ||||||||
| TP53 mut | EGFR mut | 20 | 3.4 | 0.633 | 0.707 (1.1, 0.66–1.84) | 0.747 | 3.3 | 0.7633 | 0.782 (1.08, 0.61–1.92) | 0.902 | 3.5 | 0.3627 | 0.449 (1.58, 0.48–5.2) |
| TP53 mut | EGFR wild | 122 | 4.33 | 0.0069 | 0.001 (0.61, 0.45–0.82) | 0.003 | 4.2 | 0.0315 | 0.005 (0.63, 0.46–0.87) | 0.018 | 9.1 | 0.0963 | 0.082 (0.46, 0.19–1.1) |
| TP53 wild | EGFR mut | 8 | 2.47 | 0.4153 | 0.336 (1.46, 0.67–3.18) | 0.428 | 3.07 | 0.7633 | 0.662 (1.2, 0.52–2.78) | 0.974 | 1.27 | 0.0574 | 0.022 (26.78, 1.61–446.3) |
| TP53 wild | EGFR wild | 90 | 2.52 | 2.33 | 6.66 | ||||||||
| TP53 mut | KRAS mut | 32 | 5.77 | 0.0113 | 0.005 (0.47, 0.27–0.8) | 0.024 | 5.47 | 0.0142 | 0.005 (0.42, 0.23–0.77) | 0.042 | 6.33 | 0.5253 | 0.354 (0.57, 0.17–1.9) |
| TP53 mut | KRAS wild | 110 | 3.6 | 0.1495 | 0.122 (0.75, 0.52–1.1) | 0.282 | 3.3 | 0.1031 | 0.048 (0.66, 0.44–1) | 0.247 | 8.63 | 0.6536 | 0.58 (0.76, 0.29–2) |
| TP53 wild | KRAS mut | 54 | 2.33 | 0.5746 | 0.718 (1.08, 0.72–1.6) | 0.478 | 2.37 | 0.4977 | 0.468 (0.85, 0.54–1.32) | 0.988 | 1.63 | 0.3114 | 0.179 (2.18, 0.7–6.8) |
| TP53 wild | KRAS wild | 44 | 2.57 | 2.47 | 9.87 | ||||||||
Figure 2.
Patients treated either with monotherapy or combination therapy were stratified with TP53 mutation status and KMT2C mutation status. The survival curves were plotted for four distinct groups, including wild type TP53 and wild type KMT2C (green), wild type TP53 and mutated KMT2C (blue), mutated TP53 and wild type KMT2C (purple), mutated TP53 and mutated KMT2C (red).
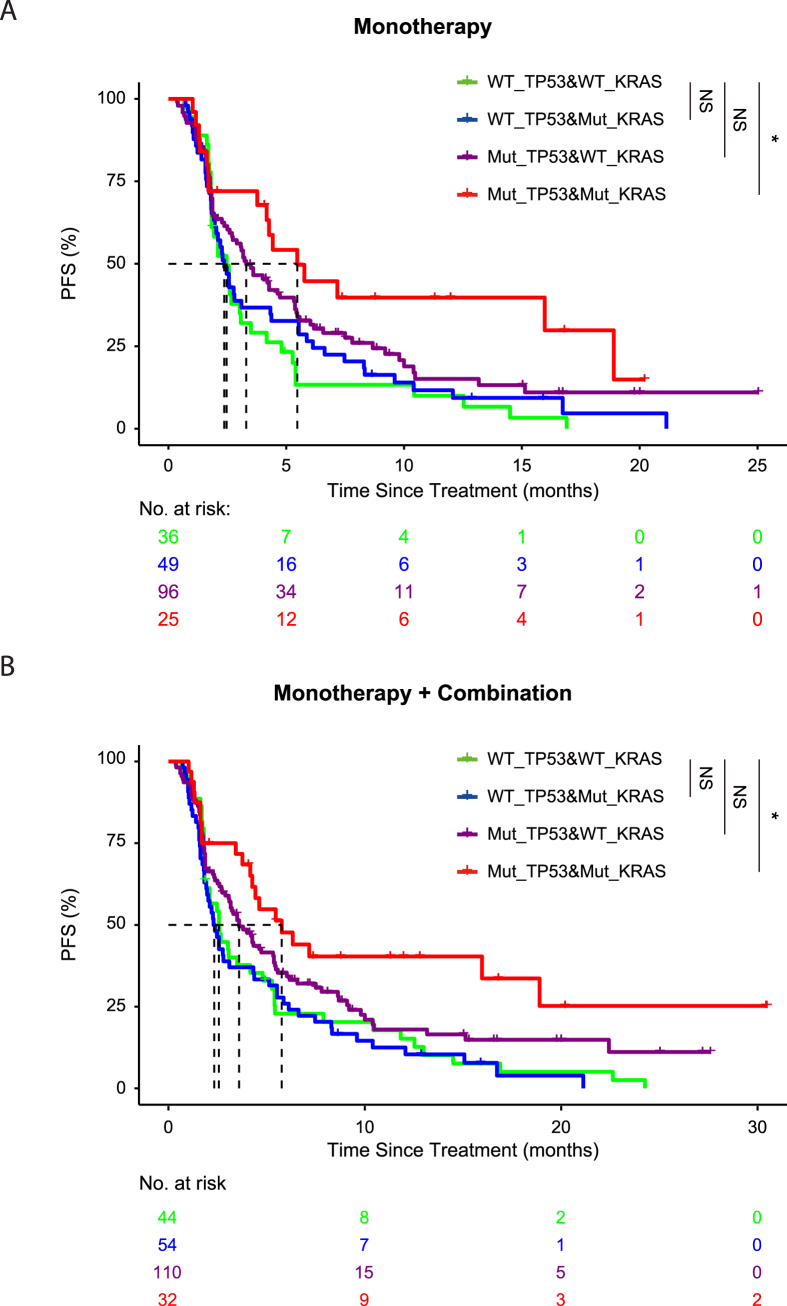
We next checked the effect of KRAS co-mutation on the outcome of patients in response to checkpoint inhibitors (Fig. 3). We found that patients with co-mutation of TP53 and KRAS had significantly longer PFS (5.8 months vs. 2.6 months, P = 0.005), as compared to patients harboring wild type TP53 and KRAS. Patient with TP53 mutation and wild type KRAS had a median PFS of 3.6 months. When smoking factor is included in multivariate analysis, only the TP53/KRAS co-mutation stood out as a significant factor (P = 0.024). Finally, we evaluated the effect of co-occurring TP53 and EGFR mutations. Patients with mutated TP53 and wild type EGFR had significantly longer PFS (4.3 months vs. 2.5 months, P = 0.001) as compared with patients with wild type TP53 and EGFR, while patients with co-occurring TP53 and EGFR mutations exerted no significant improvement of PFS (3.4 months vs. 2.5 months, P = 0.707).
Figure 3.
(A) Patients treated with monotherapy were stratified with TP53 mutation status and KRAS mutation status. The survival curves were plotted for four distinct groups, including wild type TP53 and wild type KRAS (green), wild type TP53 and mutated KRAS (blue), mutated TP53 and wild type KRAS (purple), mutated TP53 and mutated KRAS (red). (B) Patients treated either with monotherapy or combination therapy were stratified with TP53 mutation status and KRAS mutation status. The survival curves were plotted for four distinct groups, including wild type TP53 and wild type KRAS (green), wild type TP53 and mutated KRAS (blue), mutated TP53 and wild type KRAS (purple), mutated TP53 and mutated KRAS (red).
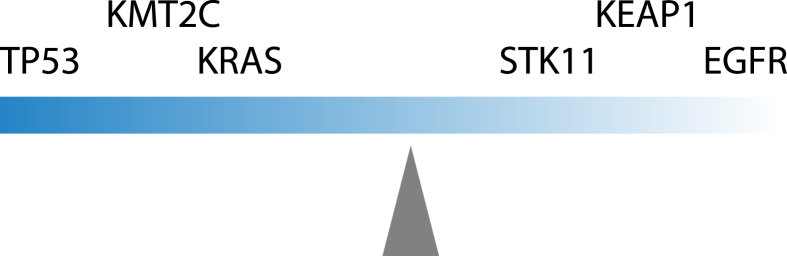
To sum up, we proposed a model to explain the effect of mutations in key driver genes on the sensitivity of ICI treatment (Fig. 4). The effect of individual gene could be additive or subtractive to TP53 mutations.
Figure 4.
Proposed model for the influence of distinct TP53 co-mutation patterns on the outcome of checkpoint inhibition.
Discussion
Our study approached the problem of patient stratification in immune checkpoint inhibition by extensive data mining and re-analysis of a publicly available dataset, uncovering a complex interplay between recurrently occurring mutations. We confirmed the beneficial effect of TP53 mutations in immune checkpoint inhibition treated patients. This is in agreement with previous reports.6,9,14,16 However, the outcome of immune checkpoint inhibition depends on multiple factors, rendering a multivariate analysis necessary. Here we comprehensively surveyed the effect of TP53 with co-occurring mutations in common oncogenes and other tumor suppressor genes on the response to immune checkpoint inhibition.
KMT2C is a gene frequently mutated in non-small cell lung cancer. Our analysis suggested that co-mutation of TP53 with KMT2C seemed to confer a favorable response of NSCLC patients to immune checkpoint inhibition. Co-occurring KMT2C mutations significantly enhanced the response of NSCLC patients to ICIs, serving as proof of principle that finer patient stratification is more informative to guide clinical decision. The other two tumor suppressor genes STK11 and KEAP1 analyzed in this study did not significantly alter the response profile of NSCLC patients to immune checkpoint inhibitors. There is still limited evidence to completely rule out roles played by those tumor suppressor genes, as functionality is always context dependent.
Recently, there was a case report documenting a durable response to combination therapy with PD-1 antibody and chemotherapy in a NSCLC patient with co-occurring TP53 and KRAS mutations.17 One potential explanation for this is that TP53 and KRAS double mutated patients had significantly higher expression of PD-L1 in their cancer samples.7 PD-L1 is a well-accepted biomarker to predict the sensitivity to immune checkpoint inhibition.18
EGFR mutations were shown to correlate with a worse response of patients to immune checkpoint inhibition.19,20 Despite this, the effect of EGFR mutations might be context dependent.21 The negative effect of EGFR mutations and the positive effect of TP53 mutations seemed to neutralize each other, as double mutants were similar to double wild type. This suggested that the first line therapy for TP53/EGFR double mutated NSCLC patients should be TKIs. To sum up previously discussed points, it is clear that the mechanisms for a cancer gene mutation to alter the ICIs response are decoupled from its roles played in tumorigenesis as an oncogene or tumor suppressor gene.
Conclusions
Immune checkpoint inhibition has emerged as a promising cancer therapeutic that can induce durable clinical benefit in a subset of patients. However, many patients are insensitive to checkpoint inhibitors, while the mechanistic insights remain lacking. It's urgent to develop a finer patient stratification method to guide clinical decision. As next generation sequencing had become routine in clinic to inform clinical decision regarding the use of targeted drugs,22 the mutation status of recurrently mutated genes analyzed in this study is generally available for cancer patients. Thus, future studies using a larger population of patients are merited to further confirm the effect of distinct co-mutation patterns on the response of NSCLC patients to immune checkpoint inhibition.
Author contributions
Ning Li and Xiaoyun Huang contributed to concept and design. Shuhang Wang and Miaomiao Jiang contributed to the literature search, data acquisition, data analysis, statistical analysis, manuscript preparation. Zuozhen Yang contributed to manuscript editing and manuscript review.
Conflict of interests
All authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Xiaoyun Huang, Email: x.huang@intelliphecy.com.
Ning Li, Email: lining@cicams.ac.cn.
Funding
This work was supported by Chinese Academy of Medical Sciences (No. 2019XK320068).
References
- 1.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J., Reckamp K.L., Baas P., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cyriac G., Gandhi L. Emerging biomarkers for immune checkpoint inhibition in lung cancer. Semin Cancer Biol. 2018;52(Pt 2):269–277. doi: 10.1016/j.semcancer.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Goodman A.M., Kato S., Bazhenova L., et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams A.B., Schumacher B. p53 in the DNA-Damage-Repair Process. Cold Spring Harb Perspect Med. 2016;6(5):a026070. doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assoun S., Theou-Anton N., Nguenang M., et al. Association of TP53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer. Lung Cancer. 2019;132:65–71. doi: 10.1016/j.lungcan.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z.Y., Zhong W.Z., Zhang X.C., et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. doi: 10.1158/1078-0432.CCR-16-2554. [DOI] [PubMed] [Google Scholar]
- 8.Alexandrov L.B., Nik-Zainal S., Wedge D.C., et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 11.Cuadrado A., Rojo A.I., Wells G., et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18(4):295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 12.Ji H., Ramsey M.R., Hayes D.N., et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 13.Gazdar A.F. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–S31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizvi H., Sanchez-Vega F., La K., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao J., Aksoy B.A., Dogrusoz U., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biton J., Mansuet-Lupo A., Pecuchet N., et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin Cancer Res. 2018;24(22):5710–5723. doi: 10.1158/1078-0432.CCR-18-0163. [DOI] [PubMed] [Google Scholar]
- 17.Fang C., Zhang C., Zhao W.Q., Hu W.W., Wu J., Ji M. Co-mutations of TP53 and KRAS serve as potential biomarkers for immune checkpoint blockade in squamous-cell non-small cell lung cancer: a case report. BMC Med Genomics. 2019;12(1):136. doi: 10.1186/s12920-019-0592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 19.Lisberg A., Cummings A., Goldman J.W., et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C.K., Man J., Lord S., et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastings K., Yu H.A., Wei W., et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol. 2019;30(8):1311–1320. doi: 10.1093/annonc/mdz141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagan J., Van Allen E.M. Next-generation sequencing to guide cancer therapy. Genome Med. 2015;7(1):80. doi: 10.1186/s13073-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]