Abstract
Drug shortages continue to pose a significant threat to public health and safety. Drug shortages not only worsen patient outcomes, but also significantly burden healthcare systems with additional costs. This study aimed to assess community pharmacy staff knowledge, opinion, and practice toward drug shortages in Saudi Arabia from November 2019 to March 2020. This was a cross-sectional study carried out among 1,008 community pharmacists from Saudi Arabia using a validated, self-administered and Internet-based survey. Analysis was done using chi square and fisher exact tests. Most participants were males (97.2%), less than 30 years old (48.1%), and non-Saudi citizens (94.4%), and 72.0% had good knowledge of drug shortages (score = 4–5). Around 36.0% reported that it takes 1–3 days to receive a response to the shortage report. There was a statistically significant association between the professional level of the community pharmacists and their opinion about reporting drug shortages (P < .05). Most community pharmacies (56.2%) did not receive any notification about drug shortages. In conclusion, most community pharmacists are knowledgeable and have good practice regarding drug shortages, but their opinions about drug shortages differ according to each pharmacy reporting policy.
Keyword: Pharmacist, Drug shortage, Perception, Knowledge, Saudi Arabia
1. Introduction
Drug shortages continue to pose a significant threat to public health and safety. According to the World Health Organization (WHO), drug shortages have been a serious global challenge to clinicians, pharmacists, and all healthcare providers in providing high-quality and cost-effective healthcare services to patients (WHO, 2016). Drug shortages in the United States (USA) are traced and monitored by the American Society of Health-System Pharmacists (ASHP) and the United States Food and Drug Administration (FDA), although they use different definitions of the term (US Department of Health and Human Services, 2011, Fox et al., 2014). The ASHP defines drug shortages as “a supply problem that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent” (Fox et al., 2009). The FDA defines drug shortage as “a situation in which the total supply of all clinically interchangeable versions of an FDA-regulated drug is inadequate to meet the current or projected demand at the patient level” (US Department of Health and Human Services, 2011).
Drug shortages not only worsen patient outcomes by delaying medical procedures, reducing patient adherence and increasing the risk of medication errors, but also significantly burden healthcare systems with additional costs. There appear to be no signs of improvements to the issue of drug shortages in the near future (Fox et al., 2014, World Health Organization, 2016, Tucker et al., 2020). The number of reported drug shortages has increased worldwide over the last decade (Ventola, 2011, Dal Moro, 2013, Fox et al., 2014, Butterfield et al., 2015). Drug shortages have been reported in many countries irrespective of the economic status (Lynas, 2010, Becker et al., 2013, Gupta and Huang, 2013, Jagsi et al., 2014, Caulder et al., 2015, De Weerdt et al., 2015a, De Weerdt et al., 2015b, Rosa et al., 2016, De Weerdt et al., 2017, Schwartzberg et al., 2017, Vanegas, 2019). Drug shortages have negatively affected most therapeutic classes and dosage forms of medications, mainly sterile injectable products and cancer medications (Link et al., 2012, Balkhi et al., 2013, Pauwels et al., 2015, Alsheikh et al., 2016, Awad et al., 2016, Walker et al., 2017, Alazmi and Alrashidi, 2019). It was reported that drug shortages affect mostly vulnerable populations, such as cancer and neonate patients (Butterfield et al., 2015, Alazmi and Alrashidi, 2019).
The root causes of drug shortages have been frequently studied in many countries and healthcare settings, including hospitals in Saudi Arabia (Balkhi et al., 2013, Pauwels et al., 2014, Pauwels et al., 2015, Alsheikh et al., 2016, Setayesh and Mackey, 2016, AlRuthia et al., 2017a, Alruthia et al., 2018, Tucker et al., 2020). Drug shortages are a multifaceted and complex issue that encompass every phase of a drug’s life cycle, such as manufacturing, quality, marketing, procurement, supply chain management and regulation (Pauwels et al., 2014, Alsheikh et al., 2016, Setayesh and Mackey, 2016, Alruthia et al., 2018, Tucker et al., 2020). In Saudi Arabia, the main cause of the drug shortages is the lack of an advanced warning system that can provide alerts regarding expected drug shortages (Alruthia et al., 2018). Unfortunately, there are no current regulations that require pharmaceutical companies and importers to notify the Saudi Food and Drug Authority (SFDA) of any potential shortages at least six months in advance, so there is no penalty for failure to notify. Additionally, there are no effective penalties for licensed pharmaceutical importers and pharmaceutical companies that do not abide by Saudi government regulations (Alruthia et al., 2018). Other factors that contribute to drug shortages in Saudi Arabia include defective supply chain management systems, low-profit margins of some vital necessary medications, strict regulatory requirements for biological medicinal products, insufficient local pharmaceutical manufacturing, and over-dependence on medication imports (Alruthia et al., 2018).
Most of the studies that have investigated drug shortages in Saudi Arabia were conducted to explore the prevalence and causes of drug shortages and how hospitals cope with them (Alaqeel et al., 2010, Alshehri and Alshammari, 2016, Alsheikh et al., 2016, AlRuthia et al., 2017a, Alruthia et al., 2018, Alazmi and Alrashidi, 2019). However, there are a lack of studies that assess drug shortages in community pharmacies in Saudi Arabia. There is only one published study that assessed the shortages of psychotropic medications in community pharmacies in Saudi Arabia. It was found that around 15 essential psychotropic medications were unavailable in more than half of the 248 community pharmacies surveyed in 2017 (Alruthia et al., 2017b). According to Pharmaceutical Group of European Union (PGEU) medicine shortages survey 2020 results, 23.0% of responding European countries revealed that their countries still lack shortage reporting systems that community pharmacists may utilize, and their community pharmacists received their information on drug shortages from medicine agencies (65.0%), manufacturers (57.0%), and wholesalers (50.0%). It was found that the percentage of drug shortages of the most common class of medications were cardiovascular agents (92.3%) and vaccines (88.4%) (PGEU, 2020). Other studies that have been conducted on Finnish and Australian community pharmacies found that drug shortages cause customer/patient dissatisfaction and raise workload of the pharmacies (Heiskanen et al., 2015, Tan et al., 2016).
Community pharmacists are the most accessible healthcare professionals to the general public and patients. Understanding their perception will play a significant role not only in improving patient outcomes but also in responding to drug shortages and planning for challenges regarding availability of drugs (Jovanović Lješković et al., 2021). The government of Saudi Arabia has put emphasis on the opportunity for community pharmacies to be part of Saudi Arabia's national Vision 2030 (Aljedai et al., 2016, The Government of Saudi Arabia, 2019). To the authors’ knowledge, this is the first study assessing community pharmacy staff knowledge, opinion, and practice toward drug shortages in Saudi Arabia.
2. Material and Methods
2.1. Study design and participants
This was a cross-sectional survey-based study carried out from November 2019 to March 2020 among a convenient sample of community pharmacy staffs from Saudi Arabia to assess their knowledge, opinion and practice toward drug shortages.
2.1.1. Inclusion criteria and Exclusion criteria
Eligibility for inclusion in this study was all pharmacists and technicians who work at community pharmacies in Saudi Arabia of both genders and all nationalities. Exclusion criteria were hospital pharmacists, those who are not registered as community pharmacists or technicians (unlicensed) with the Saudi Commission for Health Specialties, and people over 60 years old.
2.1.2. Sample size determination
In Saudi Arabia, there are 8,419 community pharmacists out of 24,395 licensed pharmacists (34.5%) working in different pharmacy sectors (Alruthia et al., 2018). For this study, it was determined that surveying at least 842 pharmacists from different regions across Saudi Arabia would be representative (i.e., 10%).
2.2. The survey
The survey consisted of four parts, including demographic characteristics, knowledge, opinion, and practice. Participants were asked to rate statements on descriptions of drug shortages, factors contributing to drug shortages, impacts of drug shortages, and sources of information of drug shortages on 5-point Likert scale, starting from “not at all” =1, “not well” = 2, “average” = 3, “well” = 4, and “very well” = 5. In this analysis, a score of 4–5 represents good knowledge, a score of 2–3 represents fair knowledge, and a score of 0–1 represents poor knowledge. Prescription medications were classified using USP therapeutic categories model guidelines (U.S. Food and Drug Administration, 2018). All survey questions were obtained from relevant literature and drafted by two expert academic pharmacists. Validation of the survey for face and content was performed via feedback from five pharmacy staff from the Clinical Pharmacy department at the College of Pharmacy at Taif University. In addition, the survey was piloted the same way as the main study among 10 community pharmacists with pharmacy practice and research background. The survey questions were written in both English and Arabic to prevent misunderstanding. The survey was simple and straightforward so it could be filled-out by participants in as little time as possible, estimated to be 10–15 min.
2.3. Data collection
The data were collected randomly across Saudi Arabia from November 2019 to March 2020 using a self-administered, Internet-based survey.
2.4. Study procedure
The sampling was a convenient sampling technique. The survey was created to be completed online using Google Forms. The questionnaire link was circulated to community pharmacist professional groups in Saudi Arabia via the WhatsApp application.
2.5. Ethical considerations
Ethical approval was granted by the research committee at Taif University in September 2019. Consents were obtained from all participants who were informed that their provided information was completely confidential and that the results would be reported anonymously. The data was downloaded anonymously from Google Forms in an Excel sheet. Nothing can be used to identify the identity of the participants.
2.6. Statistical analyses
Statistical analyses were performed using Microsoft Excel 2016 and IBM® SPSS Statistics Version 25.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics (means [M), standard deviations [SD), frequencies [N), and percentages [%)) were employed to describe the knowledge, awareness, and opinion of community pharmacists toward drug shortages. Fisher's exact test and the chi-square test were used to assess differences in proportions. The statistically significant level was set a priori at p < 0.05.
3. Results
3.1. Demographic Characteristics
From November 2019 to March 2020, 1,008 participants responded to the online survey. Most participants were males (97.2%), <30 years old (48.1%), and non-Saudi citizens (94.3%). Six hundred forty four (63.8%) of the participants were classified by the Saudi Commission for Health Specialties as pharmacists while 322 (31.9%) were senior pharmacists. Most respondents had a B-Pharm degree (85.8%) with>6 years of work experience in community pharmacies (56.4%). The highest percentages of the respondents came from chain community pharmacies (97.7%), community pharmacies in cities (90.7%), and the west region (47.6%) (Table 1).
Table 1.
Demographic Characteristics.
| Variables | N = 1008 | % | |
|---|---|---|---|
| Age | |||
| <30 | 485 | 48.1% | |
| 31–40 | 459 | 45.5% | |
| 41–50 | 63 | 6.3% | |
| 51–60 | 1 | 0.1% | |
| Gender | |||
| Male | 980 | 97.2% | |
| Female | 28 | 2.8% | |
| Nationality | |||
| Non-Saudi | 951 | 94.4% | |
| Saudi | 57 | 5.7% | |
| Educational Level | |||
| B-Pharm | 865 | 85.8% | |
| Pharm-D | 125 | 12.4% | |
| Others (PhD, Master and Diploma) | 18 | 1.8% | |
| Professional Level | |||
| Pharmacist | 644 | 63.9% | |
| Senior pharmacist | 322 | 31.9% | |
| others (Consultant pharmacist, Technician) | 42 | 4.2% | |
| Years of Experience | |||
| ˃ 6 | 568 | 56.4% | |
| ≤ 6 | 440 | 43.7% | |
| Pharmacy Type | |||
| Chain pharmacy | 985 | 97.7% | |
| Independent pharmacy | 23 | 2.3% | |
| Pharmacy Region | |||
| West | 480 | 47.6% | |
| Central | 229 | 22.7% | |
| South | 174 | 17.3% | |
| East | 102 | 10.1% | |
| North | 23 | 2.3% | |
| Pharmacy Location | |||
| In a city | 914 | 90.7% | |
| In a village | 94 | 9.3% | |
3.2. Perceived knowledge about drug shortages
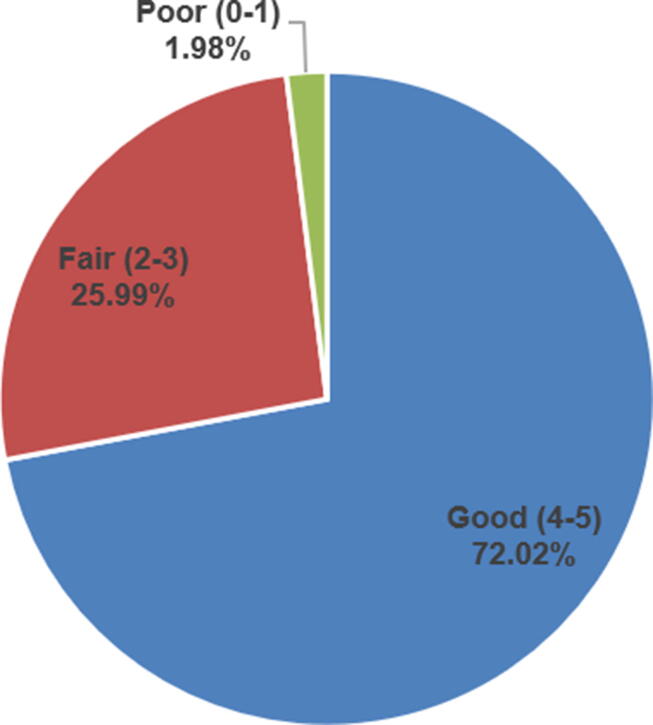
Participants were asked to report their perceived knowledge about drug shortages. Fig. 1 shows that 72.0 %, 26.0% and 2.0% of the participants perceived their knowledge to be good (score = 4–5), fair (score = 2–3) and poor (score = 0–1), respectively. Overall, the FDA definition, “Inadequate drug supply to meet the current or projected demand at the patient level” (M score = 2.9 out of 5, SD = 1.1, 57.1%), and the ASHP definition, “Lack of supply that affects how the pharmacy prepares or dispenses a drug depending on the patient's condition” (M score = 2.8 out of 5, SD = 1.1, 56.7%) were the statements rated higher by the participants. Recalls or withdraw from the markets (M score = 3.3 out of 5, SD = 1.2, 66.3%), discontinuations (M score = 3.1 out of 5, SD = 1.2, 62.5%), and stop production (M score = 3.1 out of 5, SD = 1.2, 62.4%) were the most important factors contributing to drug shortages (Table 2).
Fig. 1.
Perceived Knowledge about Drug Shortages.
Table 2.
Perceived Knowledge of Community Pharmacy Staff toward Drug Shortages.
| Variables | M Scores out of 5 (SD) | % |
|---|---|---|
| Statements Best Describing Drug Shortages | ||
| Inadequate drug supply to meet the current or projected demand at the patient level | 2.9 (1.1) | 57.1% |
| Lack of supply affects how the pharmacy prepares or dispenses a drug depending on the patient's condition | 2.8 (1.1) | 56.7% |
| Increase demand for medicines of medication supplies | 2.8 (1.1) | 56.3% |
| Drug is not available for purchase | 2.8 (1.2) | 55.9% |
| Drug is on back order from manufacturers | 2.8 (1.2) | 55.4% |
| Factors Contribute to Drug Shortages | ||
| Recalls or withdraw from the markets | 3.3 (1.2) | 66.3% |
| Discontinuations | 3.1 (1.2) | 62.5% |
| Stop production | 3.1 (1.2) | 62.4% |
| Logistical and regulatory challenges | 3.0 (1.1) | 61.0% |
| Manufacturing problems | 3.0 (1.2) | 60.0% |
| Poor medication supply chain management | 3.0 (1.2) | 59.3% |
| Quality problems | 3.0 (1.1) | 59.2% |
| Lack of raw materials | 3.0 (1.2) | 59.1% |
| Business and Economic Issues | 2.9 (1.1) | 58.6% |
| Lack of incentives to produce less profitable drugs | 2.9 (1.2) | 57.5% |
| Increased global demand (Natural disaster, winter seasons, Hajj season etc.) | 2.8 (1.1) | 56.2% |
| Misuse/abuse medications | 2.8 (1.1) | 56.0% |
| Increase off label use | 2.8 (1.1) | 55.1% |
| Low prices of generic medications | 2.7 (1.1) | 54.5% |
| Impacts of Drug Shortages | ||
| Decrease patient quality of life | 3.5 (1.2) | 69.6% |
| Affect pharmacy's reputation | 3.4 (1.2) | 67.3% |
| Reduce patient's adherence | 3.3 (1.2) | 66.0% |
| Increase health care cost | 3.3 (1.1) | 65.2% |
| Delaying medical or surgical procedure | 3.1 (1.2) | 63.0% |
| Increase prescribing inaccuracies | 2.9 (1.2) | 57.2% |
| Increase dispensing and administration errors | 2.6 (1.1) | 52.3% |
| Sources of Information of Drug Shortages | ||
| Saudi Food and Drug Authority (SFDA) | 3.4 (1.3) | 68.9% |
| Pharmaceutical Companies | 3.2 (1.3) | 64.5% |
| World Health Organization (WHO) | 3.1 (1.3) | 62.3% |
| United States Food and Drug Administration (FDA) | 3.1 (1.4) | 61.0% |
| Professional News/Social Media | 2.8 (1.3) | 55.0% |
| American Society of Health-Care Pharmacists (ASHP) | 2.7 (1.2) | 54.6% |
| Conferences/Seminars | 2.7 (1.2) | 53.5% |
Another question was about the impacts of drug shortages. Decreasing patient quality of life (M score = 3.5 out of 5, SD = 1.2, 69.6%), affecting pharmacy's reputation (M score = 3.4 out of 5, SD = 1.2, 67.3%), reducing patient's adherence (M score = 3.3 out of 5, SD = 1.2, 66.0%), and increasing health care cost (M score = 3.3 out of 5, SD = 1.1, 65.2%) were the most frequent possible outcomes of drug shortages reported by respondents. Participants were also asked about their sources of information to learn about drug shortages. SFDA (M score = 3.4 out of 5, SD = 1.3, 68.9%), pharmaceutical companies (M score = 3.2 out of 5, SD = 1.3, 64.5%), WHO (M score = 3.1 out of 5, SD = 1.3, 62.3%), and the FDA (M score = 3.0 out of 5, SD = 1.4, 61.0%) were the most important sources of information reported by participants. Other sources were used less for finding information about drug shortages (Table 2).
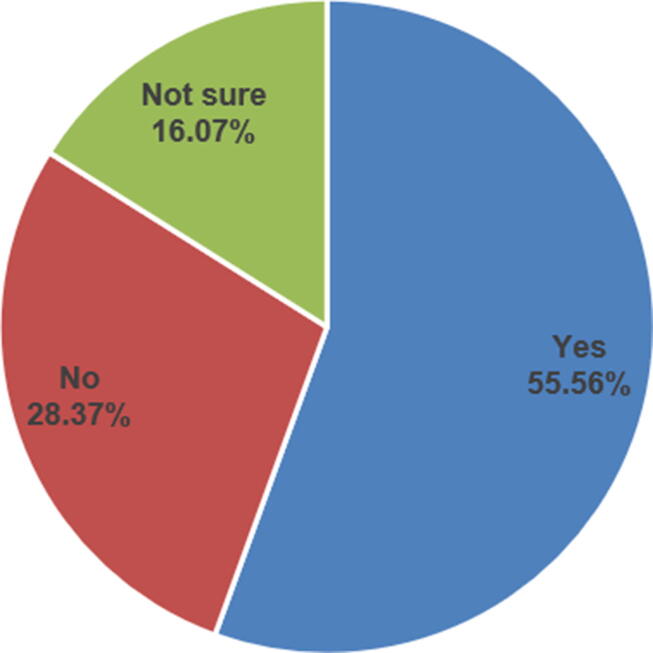
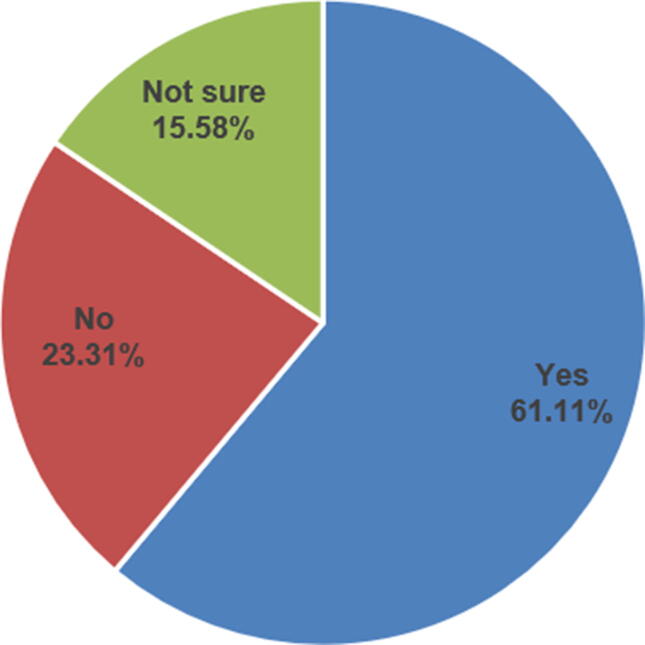
Fig. 2 shows the participant response to the question “Do you know how to report a drug shortage?”. Overall, most of the respondents (55.6%) know how to report a drug shortage. Fig. 3 shows the participants’ response to the question “In your opinion, do community pharmacists need training courses about drug shortage?” Most of the respondents (61.1%) agreed about the need for training courses on drug shortages, and there was a statistically significant difference in their responses to the question by their level of education (P < .05). Also, there were statistically significant differences in the community pharmacists’ perceived knowledge about drug shortages by their educational level (P < .05), professional level (P < .05), and years of experiences (P < .05) (Table 3). There was no other significant difference in pharmacists’ perceived knowledge by other demographic variables.
Fig. 2.
Knowing How to Report a Drug Shortage.
Fig. 3.
The Need for Training Courses about Drug Shortages.
Table 3.
Knowledge and Perception of Community Pharmacy Staffs toward Drug Shortages by Respondents Characteristics.
| Items | Comparisons | P-Value | ||
|---|---|---|---|---|
| Self-Reporting of Perceived Knowledge toward Drug Shortages | Educational Level | |||
| Pharm-D | B-Pharm | Others | ||
| Good (4–5) | 81 (64.8%) | 634 (73.3%) | 11 (61.1%) | |
| Fair (2–3) | 37 (29.6%) | 218 (25.2%) | 7 (38.9%) | |
| Poor (0–1) | 7 (5.6%) | 13 (1.5%) | 0 (0.0%) | <.05 |
| Total | 125 (100%) | 865 (100%) | 18 (100%) | |
| Level of Knowledge toward Drug Shortages | Professional Level | |||
| Pharmacist | Senior Pharmacist | Others | ||
| Good (4–5) | 440 (68.3%) | 252 (78.3%) | 34 (81.0%) | |
| Fair (2–3) | 188 (29.2%) | 66 (20.5%) | 8 (19.0%) | |
| Poor (0–1) | 16 (2.5%) | 4 (1.2%) | 0 (0.0%) | <.05 |
| Total | 644 (100.0%) | 322 (100.0%) | 42 (100.0%) | |
| Level of Knowledge toward Drug Shortages | Years of Experience | |||
| ≤6 | >6 | |||
| Good (4–5) | 296 (67.3%) | 430 (75.7%) | ||
| Fair (2–3) | 133 (30.2%) | 129 (22.7%) | <.05 | |
| Poor (0–1) | 11 (2.5%) | 9 (1.6%) | ||
| Total | 440 (100%) | 568 (100%) | ||
| The Need for Training Courses about Drug Shortages | Educational Level | |||
| Pharm-D | B-Pharm | Others | ||
| Yes | 89 (71.2%) | 515 (59.5%) | 12 (66.7%) | <.05 |
| No | 20 (16.0%) | 209 (24.2%) | 6 (33.3%) | |
| Not Sure | 16 (12.8%) | 141 (16.3%) | 0 (0.0%) | |
| Total | 125(100%) | 865 (100%) | 18 (100%) | |
3.3. Practice toward drug shortages
Most community pharmacists (56.2%) did not receive any notification about drug shortages from SFDA, and 44.3% reported that they had drug shortages of ≥ 8 drugs shorted: 36.1% had 4–7 drug shortages and 19.6% had ≤ 3 drug shortages. According to the respondents, solving drug shortage problems ranges from weeks (36.8%) to months (37.2%), and it takes a year or more in some rare cases (0.5%). According to respondents, prescription-only medications (Rx) most commonly affected by drug shortages were blood glucose regulators (41.8%), antidepressants (29.0%), cardiovascular agents (24.0%), and hormonal agents (23.5%). Over the counter (OTC) medications most commonly affected by drug shortages were heartburn (47.8%), motion sickness (47.1%), antidiarrheal (36.1%), and laxatives (34.3%). When a drug shortage concerning a prescription medication occurs, community pharmacists likely suggest an alternative medication (78.6%), refer patient to their physician to change the prescribed drug (13.6%), advise the patient to go to another pharmacy (3.9%), or advise the patient to come back to the pharmacy later (4.0%) (Table 4).
Table 4.
Practice of Community Pharmacy Staff toward Drug Shortages.
| Variables | N = 1008 | % |
|---|---|---|
| Notification about drug shortages from SFDA | ||
| No | 566 | 56.2% |
| Yes | 442 | 43.9% |
| Having drug shortages in past 3 months | ||
| Yes | 935 | 92.8% |
| No | 73 | 7.2% |
| Time needed to solve drug shortages | ||
| Not applicable | 43 | 4.3% |
| Hours | 27 | 2.7% |
| Days | 187 | 18.6% |
| Weeks | 371 | 36.8% |
| Months | 375 | 37.2% |
| Years | 5 | 0.5% |
| Percentage of drug shortages for the top ten classes of prescription medications (Rx) | ||
| Blood glucose regulators | 421 | 41.8% |
| Antidepressants | 292 | 29.0% |
| Cardiovascular agents | 242 | 24.0% |
| Hormonal agents | 237 | 23.5% |
| Anticonvulsants | 233 | 23.1% |
| Anti-viral | 216 | 21.4% |
| Anti-emetics | 188 | 18.7% |
| Anti-bacterial | 174 | 17.3% |
| Anti-migraine agents | 159 | 15.8% |
| Anti-Parkinson agent | 154 | 15.3% |
| Percentage of drug shortages for the top ten classes of over-the counter medications (OTC) | ||
| Heartburn | 482 | 47.8% |
| Motion-sickness | 475 | 47.1% |
| Anti-diarrheal | 364 | 36.1% |
| Laxatives | 346 | 34.3% |
| Multi-Symptoms Gastrointestinal (GI) | 258 | 25.6% |
| Hemorrhoid treatment | 240 | 23.8% |
| Upper respiratory | 221 | 21.9% |
| Gas relief | 221 | 21.9% |
| Female contraceptive | 159 | 15.8% |
| Vitamins and others supplement | 145 | 14.4% |
| Pharmacist practice in case of drug shortages | ||
| Advise her/him to come back to the pharmacy later | 40 | 4.0% |
| Advise her/him to go to another pharmacy | 39 | 3.9% |
| Refer her/him to their physicians to change the drug | 137 | 13.6% |
| Suggest an alternative medication | 792 | 78.6% |
3.4. Participants experiences and opinions about reporting of drug shortages
Most respondents reported that it usually takes 1–3 days to get a response to the report (36.0%). Most respondents (48.0%) selected “all products in short supply” as a major criterion for reporting shortages, and substantial proportion of participants (39%) selected “medically necessary products only that have the greatest impact on public health” as a major criterion. Most respondents (91.3%) reported having an electronic system checking for low stock or drug shortages in their pharmacies. However, drug shortage probability appeared to be the same among those having such systems and those do not. Pharmacists were asked to rate several solutions based on importance in preventing drug shortages on a 5-point Likert scale (1 = least important, 5 = most important). Establishing an allocation program tracking drug shortage (M score = 3.6, SD = 1.1, 72.5%), finding other suppliers (M score = 3.6, SD = 1.1, 71.9%), and performing more research to discover the root causes of drug shortages in Saudi Arabia (M score = 3.6, SD = 1.1, 71.1%) were the solutions rated highest by participants (Table 5).
Table 5.
Community Pharmacy Staff Experience and Opinion about Drug Shortages.
| Variables | % | |
|---|---|---|
| Length Needed to Get Feedback after Reporting | N = 1008 | |
| Never responded back | 147 | 14.6% |
| <24 h | 280 | 27.8% |
| 1–3 days | 365 | 36.2% |
| 4–7 days | 113 | 11.2% |
| >7 days | 103 | 10.2% |
| Criteria for Reporting Drug Shortages | N = 1008 | |
| All products in short supply | 488 | 48.4% |
| Medically necessary products only that have the greatest impact on public health | 393 | 39.0% |
| Shortages which involve only a strength or package size, which have a substitute strength and package size | 97 | 9.6% |
| Nothing | 30 | 3.0% |
| Importance of the Following Solutions in Preventing Drug Shortages | M Scores out of 5 (SD) | |
| Establish an allocation program tracking drug shortage | 3.6 (1.1) | 72.5% |
| Find other suppliers | 3.6 (1.1) | 71.9% |
| Performing more research to discover the root causes of drug shortages in Saudi Arabia | 3.6 (1.1) | 71.1% |
| Implementing suggested programs from other countries | 3.5 (1.1) | 70.1% |
| Creating data sharing system | 3.5 (1.1) | 69.0% |
| Providing financial incentives to local manufactures to solve shortages | 3.3 (1.2) | 66.5% |
| Employing pharmacists monitoring drug shortage | 3.1 (1.3) | 61.3% |
| Lengthened expiration dates | 2.9 (1.2) | 58.1% |
3.5. Presence of drug Shortages, to whom shortages are Reported, and how reporting is done
There were statistically significant differences by professional level of the community pharmacists regarding their response to the question, “Which one of the following do you think about first when you need to report a drug shortage?” (P < .05). Other factors regarding whether the community pharmacists experienced drug shortages included access to an automated/electronic inventory system (P < .01) and the preferred method of reporting a drug shortage (P < .05) (Table 6).
Table 6.
Presence of Drug Shortages, to Whom Shortages are Reported, and How Reporting is Done.
| Items | Comparisons | P-Value | ||
|---|---|---|---|---|
| Having Drug Shortages | Having Automated/Electronic Inventory System | |||
| Yes | No | Not sure | ||
| Yes | 858 (93.4%) | 50 (84.7%) | 25 (83.3%) | |
| No | 61 (6.6%) | 9 (15.3%) | 5 (16.7) | <.01 |
| Total | 919 (100%) | 59 (100%) | 30 (100%) | |
| Which one of the following do you think about first when you need to report a drug shortage? | Professional Level | |||
| Pharmacist | Senior Pharmacist | Others | ||
| Pharmacy director | 384 (59.6%) | 159 (49.4%) | 23 (54.8%) | |
| Saudi Food and Drug Administration (SFDA) | 144 (22.4%) | 89 (27.6%) | 10 (23.8%) | <.05 |
| Pharmaceutical companies | 64 (9.9%) | 53 (16.5%) | 6 (14.3%) | |
| None | 52 (8.1%) | 21 (6.5%) | 3 (7.1%) | |
| Total | 644 (100%) | 322 (100%) | 42 (100%) | |
| Preferred Method of Reporting Drug Shortages |
Years of Experience |
|||
| ≤6 | >6 | |||
| Telephone | 52 (11.8%) | 65 (11.4%) | ||
| Electronic reporting form | 106 (24.1%) | 190 (33.5%) | ||
| 139 (31.6%) | 154 (27.1%) | <.05 | ||
| Direct Contact | 109 (24.8%) | 115 (20.2%) | ||
| Others | 34 (7.7%) | 43 (7.6%) | ||
| Total | 440 (100%) | 568 (100%) | ||
4. Discussion
A few studies have investigated drug shortages in hospital pharmacies in Saudi Arabia. Most of them were conducted to explore the prevalence and causes of drug shortages and how hospitals cope with shortages (Alaqeel et al., 2010, Alshehri and Alshammari, 2016, Alsheikh et al., 2016, AlRuthia et al., 2017a, Alruthia et al., 2018, Alazmi and Alrashidi, 2019). However, there are a lack of studies assessing drug shortages in community pharmacies in Saudi Arabia. The present study has mainly focused on assessing the perceived knowledge, opinion, and practices of Saudi community pharmacists.
In this study, perceived knowledge of the community pharmacists about drug shortages was satisfactory. There were statistically significant differences in the community pharmacists’ perceived knowledge about drug shortages by their educational level, professional status, and years of experiences. As the level of education and professional status increased, their perceived knowledge directly increased as well. In addition to the level of education and profession, the years of experience played a vital role in the previous two factors within perceived knowledge and opinion of pharmacists in conditions like a drug shortage, to whom a drug shortage should be reported, and through which method they would report shortages. Years of experience seemed to affect the method used for reporting shortages, as years of experience increased preference for electronic reporting. The presence or absence of an automated electronic inventory system did not affect conditions of drug shortage positively or negatively. The frequent outcomes of drug shortages in Saudi Arabia include negative reputation of pharmacy, increase in healthcare costs, decrease in patient quality of life, and reduction in patient adherence. The majority of the medical experts agreed about the need for training in case of drug shortages.
In the current study, “antidepressants” were the second highest class of medications subjected to shortages in community pharmacies, and this is consistent with Alruthia et al. findings (Alruthia et al., 2017b). “Cardiovascular agents” were one of the most common medications classes affected by shortages. Community pharmacists received their information about drug shortages from medicine agencies and pharmaceutical companies. These findings agreed with published studies that have been conducted across the European countries (Heiskanen et al., 2015, Pharmaceutical Group of European Union, 2020).
One of the previous studies stated that continuity of mentally challenged patients is challenged by lax regulations governing licensing and work of different retail community pharmacies (Alruthia et al., 2018). As the result of lax regulations, there is shortage of indispensable medicines in the retail community pharmacies. The SFDA argues that the reasons for the unavailability of some psychiatric drugs at retail community pharmacies may be due to the lack of any notifications of shortages of the examined psychiatric drugs to the pharmacies themselves.
According to Alruthia et al., a critical factor that has contributed toward the issue of drug shortage is low-profit medication. The continuous supply of any medicinal product is dependent on market economic factors. In situations where the profit margin of a product becomes too narrow, especially in situations where a patent has expired, manufacturers cease production because they are simply unable to sustain low profits margins (Alruthia et al., 2018). This starts a vicious cycle of drug shortage. For example, in China, the government imposed strict price control, and this led to shortages because manufacturers were not able to sustain slow profit margins (Alruthia et al., 2017a). Keeping this phenomenon in mind, it would be suitable for SFDA to maintain a balance. They should not make the polices too stringent nor let them become too lax. The regulatory policies should allow manufacturers to earn a decent profit while not placing a burden on consumers due to increased manufacturing costs.
According to Adamski et al., outdated procurement policy is also one of the key reasons drug shortages are being experienced in Saudi Arabia. The current policy of the Saudi government with regards to procurement is based on competitive bidding process, which is also known as open tenders (Adamski et al., 2010). According to World Health Organization, tender is defined as “any formal and competitive procurement procedure through which offers are requested, received and evaluated for the procurement of goods, works or services, and as a consequence of which an award is made to the tenderer whose tender/offer is the most advantageous. The ultimate goal of the tendering process should be to obtain the most effective and high-quality pharmaceutical products in the most favorable pricing” (WHO, 2016). However, the issue is that the current policies of the government focus primarily on price and do not take into consideration other issues such as safety, quality, and effectiveness. A policy focusing on the quantitative aspects and not the qualitative aspect is bound to lead to issues such as drug shortages because the only variable taken into account by the policy is price (Adamski et al., 2010). Therefore, one of the implications of the findings of this study is that there is a need to review current government procurement policy of pharmaceuticals for compromising pioneering payment procedures like outcome-based contracts.
According to Rasooldeen, overdependence on prescription drug imports contributes to drug shortage issue in Saudi Arabia (Rasooldeen, 2017). Further, it showed that local pharmaceutical manufacture has grown significantly in the last ten years. Despite the fact that there are more than 32 pharmaceutical production units listed, roughly 27 are operational. The domestic producers only cover 20–25% of the Saudi Arabia’s prescription medicine consumption. Another study by Morris explained development of a strategy to provide better management of shortages (Morris, 2018). One of the most important decisions was to include a process for centralized systematic assessment and organization of shortages, along with the creation and maintenance of a medicine watch list, so that supply issues could be escalated to a National Medicines Action Group for advice on possible alternative treatments, if necessary. The American Society of Health-System Pharmacists developed guidelines for managing drug product shortages and mentioned two key factors: the role of pharmacists in this problem and the need for pharmacy leaders to educate all members of the healthcare team. There is need to address the challenging and complex nature of the drug shortage problem (Fox and McLaughlin, 2018).
Furthermore, the pharmacist must verify that the organization has the appropriate infrastructure and a well-established management plan in place to respond to a drug shortage. A drug shortage team used to establish mechanisms for approving alternative medicines and resolving ethical issues and a resource allocation committee are all essential parts of infrastructure that must be in place before medication shortages develop (Fox and McLaughlin, 2018).
Numerous solutions to manage drug shortages have been reported in the previous published studies (Traynor, 2011, Link et al., 2012, Fox and Tyler, 2013). However, only one study so far has examined the shortage of drugs in community pharmacies in Saudi Arabia (Alruthia et al., 2017b). So, a few solutions are recommended that are thought to help minimize this problem in the context of community pharmacies. The SFDA initiated a new program or service called pharmacovigilance for the detection of adverse effects and management (Alshammari, 2017). This will be a great solution to have a service or a plan for managing and detecting shortages of drugs. There is a need to provide training courses on managing drug shortages not only for community pharmacists but also for all health professions. The majority of participants in this study thought that training courses would play a vital role when community pharmacists face drug shortages. The ASHP’s new guidelines focused on pharmacist’s role (Fox and McLaughlin, 2018), requiring effective training and excellent qualifications.
The shortages are a global health problem in every country and region whether the countries are rich or poor. A lack of information about this problem in Saudi Arabia has been noticed. The present study provided good insights about community pharmacists’ knowledge, opinion, and practice toward drug shortages in Saudi Arabia and highlighted possible causes that led to drug shortages.
4.1. Study strength
The most important strength is that this study is the first to explore the community pharmacists’ knowledge, opinion and practice toward drug shortages in Saudi Arabia, and it is considered primary data in this area. Crucial drug classes and classes susceptible to a high rate of deficiencies were identified. The study detected the most common and impactful reasons for drug shortages and suggested practical solutions and recommendations.
4.2. Study limitations
A limitation of this study was the relatively low number of participants from some regions in Saudi Arabia. In addition, reporting bias cannot be totally ruled out because of self-reporting.
4.3. Study implications and recommendations
The study findings can be used to develop targeted interventions by concerned authorities, such as SFDA, aimed to decrease drug shortage and improve the continuous availability of medication. A clear policy and guidelines about drug shortages and standardized format for reporting should be established urgently. Future studies should act to reveal the impact of newly established drug policy, guidelines, and/or reporting programs targeting drug shortages.
5. Conclusion
Most community pharmacists are aware of and reported suitable practices regarding drug shortages, but their personal experiences and opinion toward the problem differ according to each pharmacy’s policy because there is no standardized program available for reporting shortages.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The author is very thankful to all the associated personnel in any reference that contributed to this research.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mona Y. Alsheikh, Email: a.mona@tu.edu.sa.
Ali M. Alshahrani, Email: a.shahrani@tu.edu.sa.
Ahmed I. Fathelrahman, Email: aihassan@tu.edu.sa.
Moudi M. Alasmari, Email: Asmarim@ksau-hs.edu.sa, Alasmarimo1@ngha.med.sa.
Amal F. Alotaibi, Email: afotaibi@uqu.edu.sa.
References
- Adamski J., Godman B., Ofierska-Sujkowska G., Osińska B., Herholz H., Wendykowska K., Laius O., Jan S., Sermet C., Zara C., Kalaba M., Gustafsson R., Garuolienè K., Haycox A., Garattini S., Gustafsson L.L. Risk sharing arrangements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. 2010;10:153. doi: 10.1186/1472-6963-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaqeel S.A., Al-Salloum H.F., Abanmy N.O., Al-Shamrani A.A. Undispensed prescriptions due to drug unavailability at a teaching hospital in Saudi Arabia. Int. J. Health Res. 2010;3:213–216. [Google Scholar]
- Alazmi A., Alrashidi F. Medication Exchange and Sharing Network Program (MESNP) initiative to cope with drug shortages in the Kingdom of Saudi Arabia (KSA) Risk. Manag. Healthc. Policy. 2019;12:115. doi: 10.2147/RMHP.S198375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljedai A., Qaisi S., Al-meman A. Pharmacy practice and the health care system in Saudi Arabia. Can. J. Hosp. Pharm. 2016;69:231. doi: 10.4212/cjhp.v69i3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlRuthia Y.S., AlKofide H., AlAjmi R., Balkhi B., Alghamdi A., AlNasser A., Alayed A., Alshammari M., Alsuhaibani D., Alathbah A. Drug shortages in large hospitals in Riyadh: a cross-sectional study. Ann. Saudi. Med. 2017;37(5):375–385. doi: 10.5144/0256-4947.2017.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ruthia Y.S., Mansy W., Barasin M., Ghawaa Y.M., AlSultan M., Alsenaidy M.A., Alhawas S., AlGhadeer S. Shortage of psychotropic medications in community pharmacies in Saudi Arabia: Causes and solutions. Saudi. Pharm. J. 2017;25(5):744–749. doi: 10.1016/j.jsps.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alruthia Y.S., Alwhaibi M., Alotaibi M.F., Asiri S.A., Alghamdi B.M., Almuaythir G.S., Alsharif W.R., Alrasheed H.H., Alswayeh Y.A., Alotaibi A.J., Almeshal M., Aldekhail S.N., Alhusaini A., Alrashed S.A., Alrumaih A.M., Dahhas M.A., Alghamdi M.A., Aleheidib M.S., Alhaidari M.H., Alharbi J.A., Alshamsan A. Drug shortages in Saudi Arabia: Root causes and recommendations. Saudi. Pharm. J. 2018;26(7):947–951. doi: 10.1016/j.jsps.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari T.M., Alshakka M., Aljadhey H. Pharmacovigilance system in Saudi Arabia. Saudi. Pharm. J. 2017;25(3):299–305. doi: 10.1016/j.jsps.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri S., Alshammari A. Drug supply shortages in pharmacies: causes and solutions; a case study in King Khaled Eye Special Hospital. Int. Bus. Manag. 2016;10:2453–2459. [Google Scholar]
- Alsheikh M., Seoane-Vazquez E., Rittenhouse B., Fox E.R., Fanikos J. A comparison of drug shortages in the hospital setting in the United States and Saudi Arabia: an exploratory analysis. Hosp. Pharm. 2016;51(5):370–375. doi: 10.1310/hpj5105-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H., Al-Zu'bi Z.M.F., Abdallah A.B. A quantitative analysis of the causes of drug shortages in Jordan: a supply chain perspective. Int. Bus. Res. 2016;9(6):53. doi: 10.5539/ibr.v9n6p53. [DOI] [Google Scholar]
- Balkhi B., Araujo-Lama L., Seoane-Vazquez E., Rodriguez-Monguio R., Szeinbach S.L., Fox E.R. Shortages of systemic antibiotics in the USA: how long can we wait? J. Pharma. Health. Serv. Res. 2013;4:13–17. [Google Scholar]
- Becker D.J., Talwar S., Levy B.P., Thorn M., Roitman J., Blum R.H., et al. Impact of oncology drug shortages on patient therapy: unplanned treatment changes. J. Oncol. Pract. 2013;9:e122–e128. doi: 10.1200/JOP.2012.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield, L., Cash, J., Pham, K. and Advocacy Committee for the Pediatric Pharmacy Advocacy Group, 2015. Drug shortages and implications for pediatric patients. J. Pediatr. Pharmacol. Ther. 20, 149-152. [DOI] [PMC free article] [PubMed]
- Caulder C., Mehta B., Bookstaver P., Sims L., Stevenson B. South Carolina Society of Health-Sy. Impact of drug shortages on health system pharmacies in the southeastern United States. Hosp. Pharm. 2015;50:279–286. doi: 10.1310/hpj5004-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Moro F. BCG shortage in Europe. Prevent. Med. 2013;57(2):146. doi: 10.1016/j.ypmed.2013.04.019. [DOI] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Casteels M., Huys I. Toward a European definition for a drug shortage: a qualitative study. Front. Pharmacol. 2015;6:1–9. doi: 10.3389/fphar.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Hombroeckx L., Casteels M., Huys I. Causes of drug shortages in the legal pharmaceutical framework. Regul. Toxicol. Pharmacol. 2015;71(2):251–258. doi: 10.1016/j.yrtph.2015.01.005. [DOI] [PubMed] [Google Scholar]
- De Weerdt E., Simoens S., Casteels M., Huys I. Clinical, economic and policy implications of drug shortages in the European Union. Appl. Health Econ. Health Policy. 2017;15(4):441–445. doi: 10.1007/s40258-016-0264-z. [DOI] [PubMed] [Google Scholar]
- Fox E.R., Birt A., James K.B., Kokko H., Salverson S., Soflin D.L. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am. J. Health Syst. Pharm. 2009;66:1399–1406. doi: 10.2146/ajhp090026. [DOI] [PubMed] [Google Scholar]
- Fox E.R., Tyler L.S. Call to action: finding solutions for the drug shortage crisis in the United States. Clin Pharmacol Ther. 2013;93:145–147. doi: 10.1038/clpt.2012.225. [DOI] [PubMed] [Google Scholar]
- Fox E.R., Sweet B.V., Jensen V. Drug shortages: a complex health care crisis. Mayo. Clin. Proc. 2014;89(3):361–373. doi: 10.1016/j.mayocp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Fox E.R., McLaughlin M.M. ASHP guidelines on managing drug product shortages. Am. J. Health-Syst. Pharm. 2018;75:1742–1750. doi: 10.2146/ajhp180441. [DOI] [PubMed] [Google Scholar]
- Gupta D.K., Huang S.M. Drug shortages in the United States: a critical evaluation of root causes and the need for action. Clin. Pharmacol. Ther. 2013;93:133–135. doi: 10.1038/clpt.2012.229. [DOI] [PubMed] [Google Scholar]
- Heiskanen K., Ahonen R., Karttunen P., Kanerva R., Timonen J. Medicine shortages–a study of community pharmacies in Finland. Health Policy. 2015;119(2):232–238. doi: 10.1016/j.healthpol.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Jagsi R., Spence R., Rathmell W.K., Bradbury A., Peppercorn J., Grubbs S., Moy B. Ethical considerations for the clinical oncologist in an era of oncology drug shortages. Oncol. 2014;19(2):186–192. doi: 10.1634/theoncologist.2013-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanović Lješković N., Jovanović Galović A., Stojkov S., Jojić N., Gigov S. Medicine Shortages in Serbia: Pharmacists’ Standpoint and Potential Solutions for a Non-EU Country. Pharmaceutics. 2021;13(4):448. doi: 10.3390/pharmaceutics13040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link M.P., Hagerty K., Kantarjian H.M. Chemotherapy drug shortages in the United States: genesis and potential solutions. J Clin. Oncol. 2012;30(7):692–694. doi: 10.1200/JCO.2011.41.0936. [DOI] [PubMed] [Google Scholar]
- Lynas K. Pharmacists in Canada challenged to deal with drug shortages. Can. Pharm. J. 2010;143:164. [Google Scholar]
- Morris S. Medicine shortages in Australia–what are we doing about them? Aust. Prescr. 2018;41:136. doi: 10.18773/austprescr.2018.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels K., Huys I., Casteels M., Simoens S. Drug shortages in European countries: a trade-off between market attractiveness and cost containment? BMC Health Services Res. 2014;14:438. doi: 10.1186/1472-6963-14-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels K., Simoens S., Casteels M., Huys I. Insights into European drug shortages: a survey of hospital pharmacists. PLoS. One. 2015;10 doi: 10.1371/journal.pone.0119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceutical Group of European Union, 2020. PGEU medicine shortages survey results. Available online: https://www.pgeu.eu/wp-content/uploads/2019/03/2020-PGEU-Medicine-Shortages-Survey-Results-v2.pdf (accessed 30 Jun 2021).
- Rasooldeen M. Saudi Arabia Aims to Boost Pharmaceutical Industry. Arab News (Jeddah, Saudi Arabia) 2017 [Google Scholar]
- Rosa M.B., Reis A.M.M., Perini E., Rosa M.B., Reis A.M.M., Perini E. Drug shortage: a public health problem. Cad. Saude. Publica. 2016;32:1–3. doi: 10.1590/0102-311X00086916. [DOI] [PubMed] [Google Scholar]
- Schwartzberg E., Ainbinder D., Vishkauzan A., Gamzu R. Drug shortages in Israel: regulatory perspectives, challenges and solutions. Isr. J. Health. Policy. Res. 2017;6:17. doi: 10.1186/s13584-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setayesh S., Mackey T.K. Addressing the impact of economic sanctions on Iranian drug shortages in the joint comprehensive plan of action: promoting access to medicines and health diplomacy. Glob. Health. 2016;12:31. doi: 10.1186/s12992-016-0168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y.X., Moles R.J., Chaar B.B. Medicine shortages in Australia: causes, impact and management strategies in the community setting. Int. J. Clin. Pharm. 2016;38(5):1133–1141. doi: 10.1007/s11096-016-0342-1. [DOI] [PubMed] [Google Scholar]
- The Government of Saudi Arabia, 2019. The Kingdom of Saudi Arabia vision 2030. Available from: http://www.vision2030.gov.sa/download/file/fid/417.
- Traynor K. Drug shortage solutions elude stakeholders. Am. J. Heal Pharmacy. 2011;68:2106–2108. doi: 10.2146/news110074. [DOI] [PubMed] [Google Scholar]
- Tucker E.L., Cao Y., Fox E.R., Sweet B.V. The Drug Shortage Era: A Scoping Review of the Literature 2001–2019. Clin. Pharmacol. Ther. 2020;108:1150–1155. doi: 10.1002/cpt.1934. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2018. USP therapeutic categories model guidelines. Available online: https://www.fda.gov/regulatory-information/fdaaa-implementation-chart/usp-therapeutic-categories-model-guidelines (accessed 30 Jul 2021).
- US Department of Health and Human Services, 2011. Drug shortages. ASPE Issue Brief. https://aspe.hhs.gov/sites/default/files/ pdf/108986/ib.pdf. (accessed 1 Jan 2021)
- Vanegas E.P., Acosta A., Rovira J., Godman B., Bochenek T. Medicines shortages: Gaps between countries and global perspectives. Front. Pharmacol. 2019;10:763. doi: 10.3389/fphar.2019.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola C.L. The drug shortage crisis in the United States: causes, impact, and management strategies. P. T. 2011;36:740–742. [PMC free article] [PubMed] [Google Scholar]
- Walker J., Chaar B.B., Vera N., Pillai A.S., Lim J.S., Bero L., et al. Medicine shortages in Fiji: A qualitative exploration of stakeholders’ views. PLoS One. 2017;5 doi: 10.1371/journal.pone.0178429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2016. Medicines shortages: global approaches to addressing shortages of essential medicines in health systems. WHO Drug Information. 30, 180-185. https://apps.who.int/iris/handle/10665/331028 (accessed 1 Jan 2021).