Abstract
Infections caused by viruses are one of the foremost causes of morbidity and mortality in the world. Although a number of antiviral drugs are currently used for treatment of various kinds of viral infection diseases, there is still no available therapeutic agent for most of the viruses in clinical practice. Coumarin is a chemical compound which is found naturally in a variety of plants, it can also be synthetically produced possessing diverse biological effects. More recently, reports have highlighted the potential role of coumarin derivatives as antiviral agents. This review outlines the advances in coumarin-based compounds against various viruses including human immunodeficiency virus, hepatitis virus, herpes simplex virus, Chikungunya virus and Enterovirus 71, as well as the structure activity relationship and the possible mechanism of action of the most potent coumarin derivatives.
Keywords: Coumarin, Hepatitis virus, Human immunodeficiency virus, Infection, Molecular mechanism
Introduction
Viruses containing RNA or DNA has long been considered a serious threat to human being, causing various disease that affect global health and the economy.1 A wide range of viral diseases such as AIDS, measles, influenza, infectious hepatitis, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are highly contagious with high mortality rates. Currently, outbreak of pandemic Coronavirus disease (COVID-19) has caused several hundred thousand deaths, posing unprecedented threat to the public health.2 (Table 1, Table 2, Table 3, Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 9).
Table 1.
The coumarin derivatives with anti-CHIKV activity.
| Compound | Chemical structure | CC50a (μM) | EC50b (μM) | SI |
|---|---|---|---|---|
| 29 | 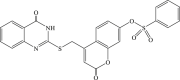 |
178 | 19.1 | 9.3 |
| 30 | 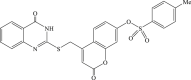 |
117 | 10.2 | 11.5 |
| 31 | 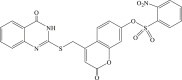 |
144 | 17.2 | 8.8 |
| 32 | 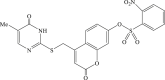 |
107 | 19.0 | 5.6 |
| 33 | 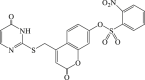 |
75.2 | 13.0 | 5.8 |
| 34 | 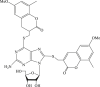 |
>212 | 9.9 | >21.7 |
| 35 | 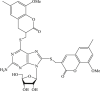 |
96.5 | 10.3 | 9.37 |
| 36 | 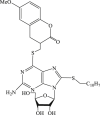 |
>227 | 13.9 | >16.3 |
| 37 | 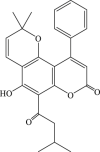 |
3150.0 | 10.7 | 295.2 |
| 38 | 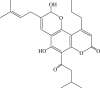 |
549.8 | 0.5 | 1021.0 |
CC50: the half cytotoxic concentration.
EC50: the median effective concentration.
Table 2.
The coumarin derivatives with anti-EV71 activity.
| Compound | Chemical structure | CC50 (μM) | EC50 (μM) | SI |
|---|---|---|---|---|
| 39 | 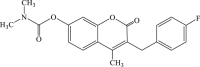 |
/ | 0.3 | / |
| 40 | >100 | 1.02 | >98 | |
| 41 | >100 | 3.92 | >25 | |
| 42 | 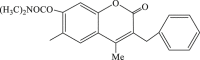 |
152 | 2.5 | 60.8 |
| 43 |  |
72.92 | 10 | 7.29 |
| 44 | 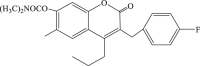 |
756.06 | 3.98 | 190 |
| 45 | 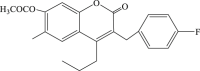 |
41.46 | 18.5 | 2.24 |
Table 3.
The coumarin derivatives with anti-HCV activity.
| Compound | Chemical structure | CC50 (μM) | EC50 (μM) | SI |
|---|---|---|---|---|
| 59 | 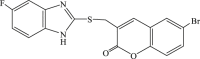 |
27 | 3.4 | 8 |
| 60 | 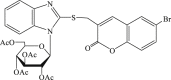 |
43 | 4.1 | 10 |
| 61 | 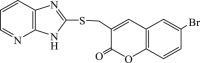 |
128 | 6.8 | 19 |
| 62 | 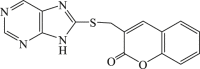 |
109 | 2.0 | 54 |
| 63 | 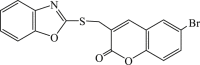 |
131 | 12 | 11 |
| 64 | 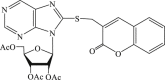 |
39 | 6.6 | 5.9 |
| 65 |  |
50 | 5.5 | 9.1 |
| 66 | 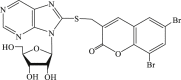 |
36 | 5.9 | 6.1 |
| 67 | 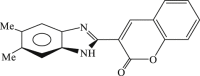 |
23 | 3.0 | 7.9 |
| 68 | 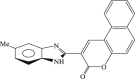 |
77 | 5.5 | 14 |
| 69 | 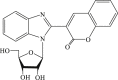 |
127 | 20 | 6.4 |
| 70 | 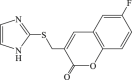 |
83 | 7.2 | 12 |
| 71 | 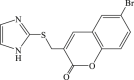 |
75 | 5.1 | 15 |
| 72 | 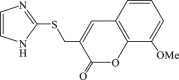 |
173 | 8.4 | 21 |
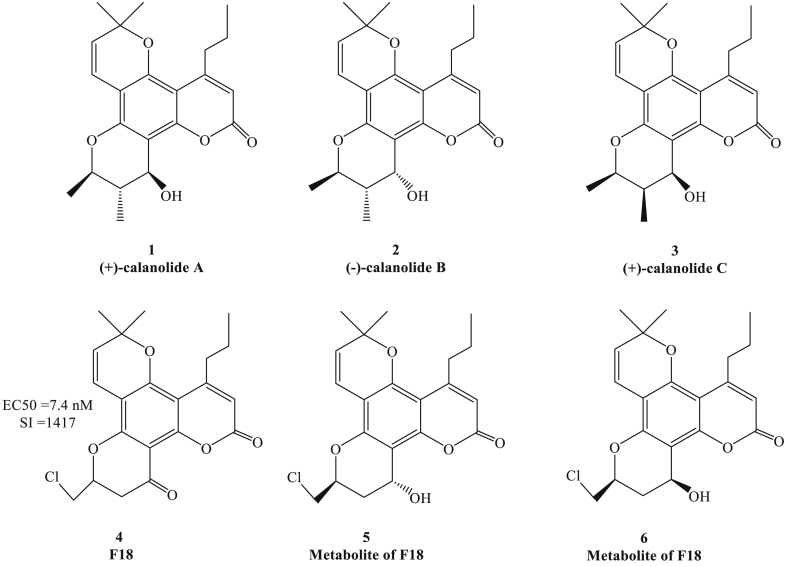
Figure 1.
Chemical structure of calanolides and its derivative.
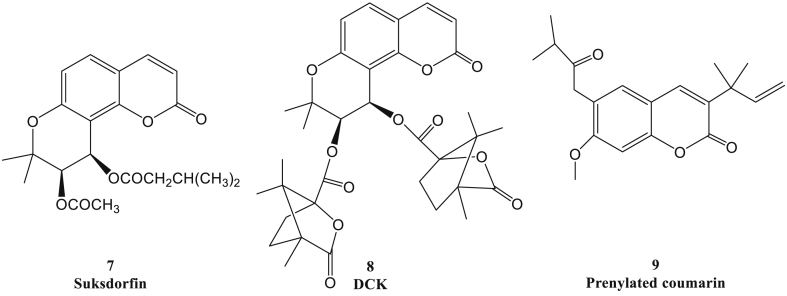
Figure 2.
Chemical structures of pyrano and prenylated coumarin derivatives.
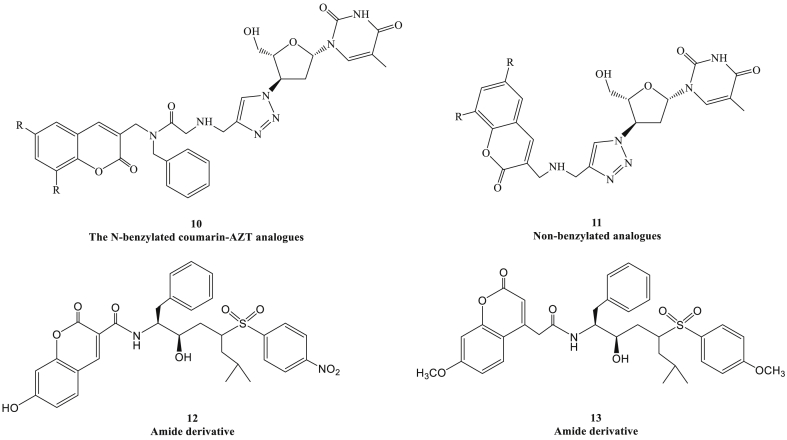
Figure 3.
Chemical structures of benzylaminomethyl and amide coumarin derivatives.
Figure 4.
Chemical structures of coumarin derivatives targeting NF-κB pathway.
Figure 5.
Chemical structures of coumarin derivatives targeting HIV-1 integrase.
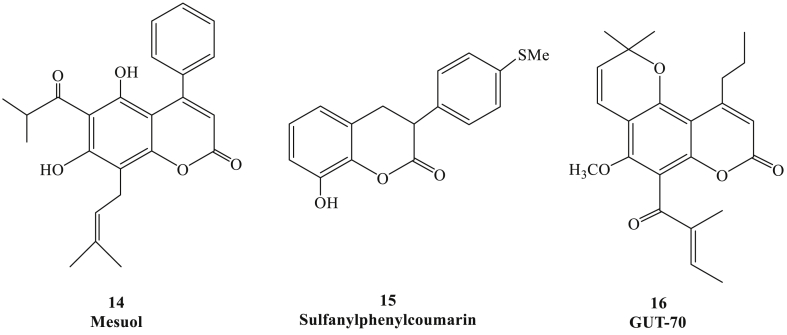
Figure 6.
Chemical structures of coumarin derivatives targeting other proteins.
Figure 9.
The chemical structures of coumarin derivatives with anti-HSV activity.
Since the first nucleoside analog antiviral drug idoxuridine was approved by FDA in 1963, more than 90 antiviral drugs have been approved for the treatment of human infectious diseases induced by viruses, and most of them are targeting the key enzyme such as DNA polymerase, revertase and neuraminidase.3 However, because of the mutation of viral genes during antiviral drug exposure, the success of therapy is being threatened by the increasing prevalence of drug resistant strains clinically.4 Therefore, developing new antiviral drugs has become the spotlight in medicinal chemistry field nowadays.
Natural products have been proved to be a particularly crucial source of compounds with antiviral activities, which can be processed into semisynthetic and synthetic drugs, applying to antiviral chemotherapy.5 Coumarin is a chemical compound found in a variety of different plants, which is composed of fused benzene and α-pyrone rings. Currently, at least 1300 different coumarins have been identified, and they are presenting a wide variety of biological activities such as antioxidant, analgesic, anti-inflammatory, and anti-mutagenic properties. In recent years, coumarins and their derivatives isolated from various plants or synthesized also exhibit bioactivities against many viruses.6 Meanwhile, coumarins are used as precursors to screen out many derivatives that remarkably restrain viruses, which can further get chemically modified thereon.7, 8, 9 Besides, the coumarin-containing conjugated compounds, combination of coumarin nucleus through different linkers with other antiviral compounds, are also the field to overcome the drug resistance.10 This review focuses on the status of coumarin derivatives purposing approaches for therapeutic potential against the various viral diseases, and provided the recent advances in the possible structure-activity relationship and molecular mechanisms of coumarin derivatives as antiviral agents.
Therapeutic potential and molecular target of coumarins as antiviral agents
Coumarin derivatives against RNA virus
Anti-human immunodeficiency virus
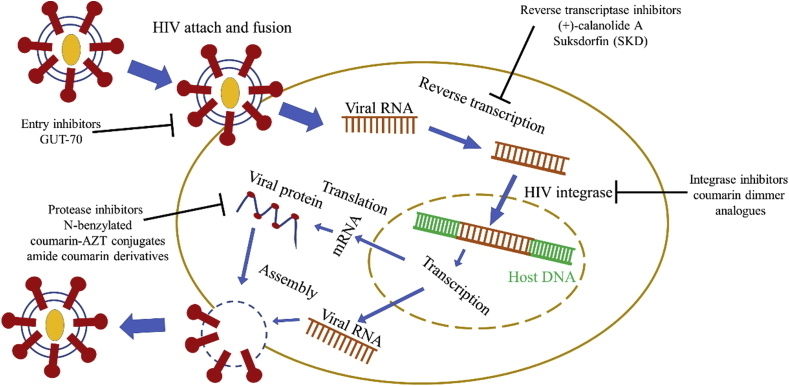
Human immunodeficiency virus (HIV) is a retrovirus that attacks the immune system by destroying CD4+ T cells.11 At present, antiretroviral treatment (ART) is playing a pivotal role in the reduction of HIV-related morbidity and mortality, which applies non-nucleoside reverse transcriptase inhibitor (NNRTI), nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), protease inhibitor (PI), as well as integrase inhibitors (INIs).12, 13, 14
Natural coumarin compounds with anti-HIV activity have been studied for decades.15 In 1992, researchers discovered (+)-calanolide A (Fig. 1, compound 1), which was extracted from tropical plants of Calophyllurn langierum, had the potent inhibitory activity against HIV-1 virus. This was the first coumarin reported to inhibit HIV-1 reverse transcriptase (HIV-1 RT) with high safety and inhibitory activity against a variety of drug-resistant strains.16, 17, 18 Furthermore, the dipyranocoumarins (−)-calanolide B (compound 2) and (+)-calanolide C (compound 3), the leaf hexane extracts of C. brasiliense, attributed to the properties of anti-HIV-1 RT with slight toxicity in mice.16 Then a new derivative of Calanolide A, which was termed 10-chloromethyl-11-desmethyl-12-oxy-calanolide-A (F18) (compound 4), showed the inhibitory effect to the HIV-1 nonnucleoside reverse transcriptase against both wild-type and Y181C mutation HIV-1 with higher synergy interaction (SI).19 Recently, researchers identified that two metabolites of compound 4 (compound 5, 6) had the more potent activity against HIV-1, and the 12-carbonyl group contributes to the bioactivity significantly.20
The sulphonic coumarin Suksdorfin (Fig. 2, SKD, compound 7), was isolated from Lomatium suksdorfii and Angelica morii in 1994, which had strong anti-HIV activity with EC50 1.3 μmol/L.21 As a leading compound, the chemical structure of SKD was modified to a series of analogues, and one of the derivatives named DCK (compound 8) exhibited extremely potent inhibitory activity to HIV-1 replication. Furthermore, the structure analysis showed that the angular pyrano-coumarin skeleton and di-O-(−)-camphanoyl moieties of R-configuration at the 3′- and 4′-positions were crucial for anti-HIV activity.22, 23, 24, 25, 26, 27, 28, 29 The underlying mechanism of these compounds was through uniquely inhibiting HIV-1 RT.30 In contrast to the currently used nucleoside anti-HIV counterparts such as zidovudine and zalcitabine, DCK and most of its analogues had the potent anti-HIV activities with extremely high selective index (SI) value, which indicated that these coumarins had the more selective activity against the HIV-infected cells.31, 32, 33, 34, 35 Besides, the prenylated coumarins isolated from the fruits of Manilkara zapota also displayed anti-HIV activities with EC50 values in range of 0.12–8.69 μM, and compound 9 showed the most efficient activity against HIV RT with an EC50 value at 0.12 μM.36
Temitope et al synthesized a series of N-benzylated coumarin-azidothymidine (AZT) conjugates (Fig. 3, compound 10) and non-benzylated analogues (compound 11), and the AZT compounds had the dual-action as the HIV-1 protease (HIV-1 PR) and RT inhibitors. Molecular docking indicated that the benzyl group of these compounds could occupy the hydrophobic pocket.10 In addition, a series of amide coumarin derivatives were synthesized and characterized by various linkers, among which compound 12 and compound 13 manifested potency against both PR and RT.37 It suggested that some coumarin derivatives had the potential to become dual HIV-1 PR/RT inhibitors, and this possible mechanism promotes the coumarins to be new candidates for the treatment of drug-resistant HIV.
4-phenyl coumarin derivative Mesuol (Fig. 4, compound 14) was isolated from the leaf of Marila pluricostata in 2005, which could restrain HIV-1 replication through targeting the pathway of nuclear factor-κB (NF-κB).38 Antiviral RV assay showed that sulfanylphenyl coumarin (compound 15) was one of the most valid HIV-1 replication dual-target inhibitors, it also could target the NF-κB pathway and block HIV-1 replication.39 As a tricyclic coumarin, GUT-70(compound 16), which was extracted from the stem bark of Chlophyllum brasiliense, was able to suppress HIV-1 replication by inhibiting NF-κB.40 Interestingly, it was also confirmed that GUT-70 could block HIV-1 to enter the mammalian cells by stabilizing cell membrane fluidity, and down-regulate the expression of the HIV-1 receptor CD4.41 These evidences suggested that NF-κB was one of the possible targets of coumarin against HIV infection.
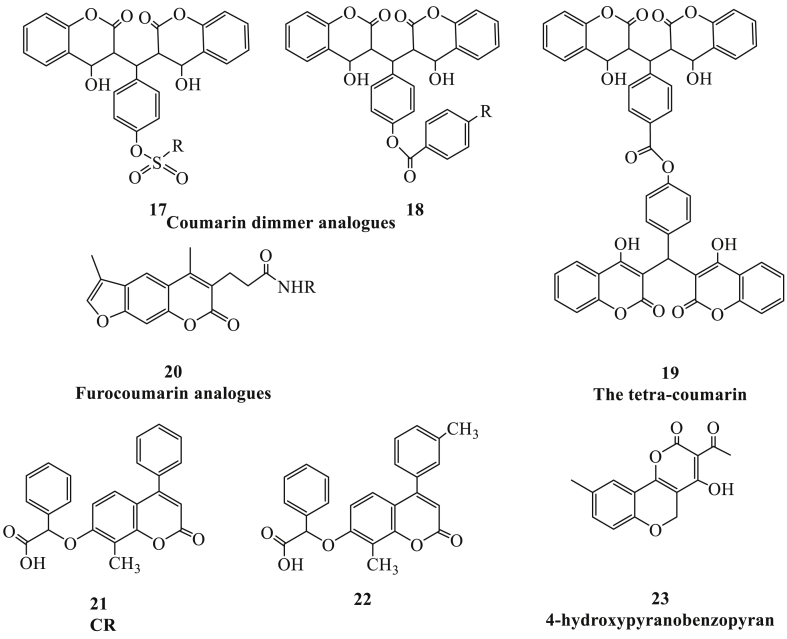
A series of 4-hydroxycoumarin dimmers analogues such as compound 17 and compound 18 (Fig. 5) also exhibited inhibitory activities to the HIV-1 integrase, thereby blocking viral replicative cycle. All of these chemical molecules consist of hydrophobic moiety on the linker, and aryl-substituted structure was attached by the benzoyloxy or sulfonyloxy group, which might contribute to the inhibitory activity.42 However, it seemed unlikely for these compounds to form covalent bond to the binding site of HIV-1 integrase. Consequently, a novel series of bis-and tetra-coumarin derivatives were synthesized, and the latter (compound 19) with the longest extended linker showed the increased inhibitory activity to HIV-1 integrase, the spatial distance between each coumarin moiety and linker being crucial to the activity.43 Furthermore, furocoumarin (compound 20) was also confirmed as potentialHIV-1 integrase inhibitor.44 In recent years, lens epithelium-derived growth factor/p75 (LEDGF/p75) was confirmed as a cellular binding partner of HIV-1 integrase and a crucial cofactor for HIV-1 replication.45 The coumarin termed CR (compound 21) could block the interaction of HIV-1 integrase and LEDGF/p75. On the basis of the chemical structure of CR, addition of 8-methyl group (compound 22) was also proved as an HIV-1 integrase-LEDGF/p75 interaction inhibitor after computational study and biological screening.9 Meanwhile, one of the 4-hydroxypyranobenzopyrancoumarin derivatives (compound 23) was also able to inhibit the activity of both theHIV-1 integrase and RT-associated Ribonuclease H (RNase H).46
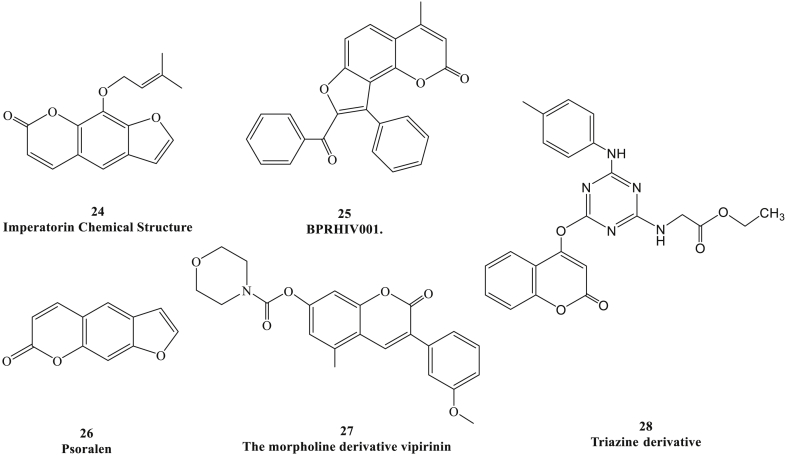
Furanocoumarin imperatorin (Fig. 6, compound 24) is distributed in citrus fruits and culinary herbs, which can inhibit gp160 protein enveloped recombinant HIV-1 infection in several T cell lines and in HeLa cells, and arrest the cells at the G (1) phase of the cell cycle through Sp1 transcription factor dependent pathway.47,48 Similarly, BPRHIV001 (compound 25) also had strong inhibitory activity to HIV-1 replication, but it might act on Tat-mediated HIV-1transactivation by suppressing the PI3K/Akt pathway,49 while Psoralen (compound 26) extracted from the dried aerial parts of Prangos tschimganica also showed the potent anti-HIV activity by inhibiting HIV-1 replication.50 Because HIV-1 viral protein R (Vpr) plays important roles at multiple stages of the HIV-1 viral life cycle, it can promote virus replication and disease progression.51,52 3-phenyl coumarin derivative vipirinin (compound 27) could arrest the cell cycle by binding to a hydrophobic region of HIV-1Vpr, and 5-methoxy moiety of the phenyl ring is essential to its antiviral activity.53 2-(coumarin-4-yloxy)-4,6-(substituted)-s-triazine (compound 28) was reported as a novel non-nucleoside reverse transcriptase inhibitor (NNRTI) with anti-HIV effect, and modification at position 4 and 6 by introducing amino acid or various aryl ureas and aryl amines could enhance the anti-HIV activity of this kind of coumarin derivatives.54
Hence, the above evidences indicated that different coumarin derivatives could target different stages of HIV. For example, GUT-70 could block the attachment and fusion stage of HIV to the cellular wall or plasma membrane. (+)-calanolide A could inhibit the reverse transcription, while coumarin dimmer analogues could inhibit the HIV integrase, N-benzylated coumarin-AZT conjugates and amide coumarin derivatives could affect the assembly of HIV (Figure 7, Figure 8).
Figure 7.
Anti-HIV mechanisms of coumarin derivatives.
Figure 8.
Chemical structures of coumarin derivatives against HBV.
Anti-chikungunya virus
Chikungunya virus (CHIKV), a cytoplasmic positive single-stranded RNA virus, which is mainly transmitted by mosquito, has caused major epidemic outbreaks in Africa, Asia and Americas.55 Although compounds targeting key inflammatory pathways, as well as attenuated virus vaccines, have shown some success in animal models, there is still no available anti-virus therapeutic against CHIKV infection, and the treatment mainly focuses on relieving symptoms, such as taking acetaminophen or paracetamol to reduce fever and pain.56
Recently, the progress of coumarin derivatives with anti-CHIKV activity was obtained. Five uracil-coumarin-aromatics compounds (Table 1, compound 29–33) showed significant inhibitory activity to CHIKV in Vero cells, and the structure–activity relationship indicated that the coumarin nucleus played an important role in anti-CHIKV activity. Moreover, the structure of benzouracil-SCH2-coumarin-OSO2-arene also presented the efficient anti-CHIKV activity, and the triply conjugated compounds combined by the linking unit in coumarin-arene through –OSO2- joint were of vital importance to anti-CHIKV bioactivity.57 In 2018, based on the above structures, a series of new coumarin-thioguanosine conjugates were synthesized, three of which (compound 34–36) exhibited inhibitory effect against CHIKV replication in Vero cells with an EC50 value ranging from 9.9 μM to 13.9 μM, and the coumarin moiety of these compounds was indispensable with regard to their activity, which was further enhanced by the coumarin-guanosine conjugates containing the eOMe group.58 Meanwhile, some coumarins extracted from natural products also showed the activity against CHIKV. For instance, two coumarins (compound 36, compound 38) isolated from the seeds of M. americana could remarkably inhibit both entry into the cell and replication of CHIKV in VERO cells.59
Anti-enterovirus 71
Enterovirus 71 (EV71) is a non-enveloped, positive-sense, single-stranded RNA virus, it is an important neurotropic enterovirus and one of the major pathogens causing both neurological and mucocutaneous diseases, and the latter most commonly presents as hand, foot and mouth disease (HFMD) or herpangina in infants and young children.60 In recent years, serious outbreaks of HFMD were reported frequently in the Asia-Pacific region, including China and Korea.61 Since HFMD caused by EV71 tends to be associated with fatal complications, there is an urgent need to develop the vaccine or therapeutic to treat EV71 infection.62
Increasing evidence show that Ras/Raf/MEK/ERK, one of the mitogen-activated protein kinase (MAPK) pathways, is involved in EV71 RNA replication after entering the host cell, which suggests that blocking of this path way might contribute to the impairment of the replication of EV71.63 In 2005, carbamate-substituted coumarin G8935 (Table 2, compound 39) was confirmed as the inhibitor of MEK activation.64 Then a series of derivatives with coumarin scaffold as ATP-noncompetitive MEK1/2 inhibitor were designed and synthesized, and these compounds could suppress the EV71 replication effectively, such as 3-benzyl-1,3-benzoxazine-2,4-diones(compound 40, compound 41) could restrain the unphosphorylation of MEK1, and then inhibit the replication of EV71 without obvious cytotoxicity,65 while 3-benzyl-coumarins (compound 42–45) had potent MEK1 binding affinity as well as significant inhibitory effect of ERK pathway, thereby inhibiting the replication of EV71. Moreover substituent group of N,N-dimethylcarbamoyloxyl or acetoxyl substitution at C7 position contributed to antiviral activity.66
Anti-hepatitisC virus
Viral hepatitis is a major threat to human health associated with significant morbidity and mortality. Five major biologically hepatotropic viruses including hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV) and hepatitis E virus (HEV), except the HBV belongs to the DNA virus, other hepatitis virus belong to RNA virus. The hepatitis virus cause most of the global burden of viral hepatitis,67 and most deaths from viral hepatitis are due to HBV and HCV.68,69
HCV is the cause of hepatitis C and some cancers such as liver cancer and lymphomas in humans.70 Since 2011, many anti-HCV drugs such as interferons alpha, DNA and RNA polymerase inhibitors, NS3/4A RNA protease inhibitors, NS5 RNA serine protease inhibitors, NS5B RNA polymerase inhibitors have been approved for clinical use, while the effectiveness of anti-HCV drugs depends on the presence of resistance associated variants (RAV), and it was demonstrated that 5–20% RAV occurred at the second day of monotherapy.71,72 Therefore, the new chemical compounds inhibiting directly the function of HCV proteins or reduced viral access to human cells, thus inhibiting viral replication, being hopeful for RAV treatment.
In 2008, Neyts et al synthesized and found that two benzimidazole–SCH2–coumarin (Table 3, compound 59, compound 60) conjugates could inhibit HCV subgenomic replicon replication. Structure-activity relationship (SAR) revealed that the bromo or methoxyl group on the coumarin nucleus enhanced the inhibitory activity of these compounds to the HCV.73 Then they designed and synthesized another series of analogues by incorporating heteroatoms into the benzimidazole moiety, and connected the heterocyclic including imidazopyridine, purine, benzoxazole with coumarin nucleus, forming three conjugated coumarin derivatives(compound 61–63), which exhibited excellent antiviral potency.74 In 2011, a novel class of conjugated compounds with 3-(chloromethyl) coumarins and 9-(β-D-ribofuranosyl) purine-8-thiones bearing different substituents were synthesized, three of which exhibited inhibitory effects on HCV subgenomic replicon replication (compound 64–66), and introducing coumarin moiety in the conjugated compounds was essential to the anti-HCV activity of these compounds.75 In 2013, the same research team synthesized a series of hinged benzimidazole-coumarin hybrids and β-d-ribofuranosides, and three of them could inhibit HCV replication remarkably (compound 67–69), and the attachment of a methyl group to the benzimidazole nucleus attributed to the anti-HCV activity. On this basis, the triple conjugated compound also exhibited anti-HCV activity with a significant SI value.76 In 2016, they also synthesized a sequence of imidazole–coumarin conjugates compounds with a –SCH2-linking group, some of which showed significant anti-HCV activity (compound 70–72). In accordance with the SAR, hybrid compounds bearing a simple imidazole nucleus showed greater anti-HCV activity. Hence, introducing a substituent into the coumarin nucleus could improve both potency and selectivity of the conjugates.77 Due to the new chemical structure and novel anti-viral mechanism, coumarins will be the possible candidates against the resistance-associated variant.
Coumarin derivatives against DNA virus
Anti-hepatitisB virus
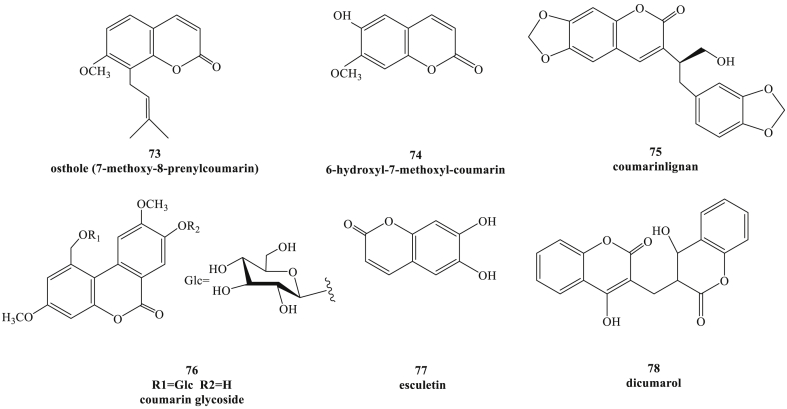
HBV is a small double-stranded DNA virus with unusual features similar to retroviruses.78,79 It is estimated that more than 250 million individuals worldwide are infected with chronic HBV infection. Although vaccine can prevent hepatitis B, so far there is no specific therapeutic agents.80 Several classes of coumarins from natural products or coumarin derivatives have been evaluated for inhibitory effects against HBV. 7-Methoxy-8-prenylcoumarin Osthol (Fig. 8, compound 73) mainly extracted from the root of Angelica pubescens, could reduce hepatitis B surface antigen (HBsAg) secretion up to 70% by glycosylation, and exhibited the anti-HBV activity.81 6-Hydroxyl-7-methoxyl-coumarin (compound 74), one of the extract compound from the root and bark of streblus asper, showed the anti-HBV activity, and the C-6 hydroxy groups contributed to the potency of this compound.82 A coumarin compound (compound 75) from the stem of Kadsura heteroclite also exhibited inhibitory activity up to 57% and 48% towards HBsAg and hepatitis B e-antigen (HBeAg) in HpeG 2.2.15 cells, respectively,83 and a new coumarin glycoside (compound 76) from the seeds of H. caudigerum was proved as a promising anti-HBV agent by suppressing the secretion of HBsAg.84 Recently, a coumarin compound esculetin (compound 77) was isolated from Microsorium fortunei (Moore) Ching, and it could obviously inhibit the expression of not only HBsAg and HBeAg, but HBV DNA and the hepatitis B virus X (HBx) in vitro. Furthermore, esculetin also could suppress the DHBV replication in vivo, alleviating liver injury caused by HBV, as well as reducing the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).85 HBV covalently closed circular DNA (cccDNA) serves as the transcription template for HBV RNAs, and is responsible for the establishment of viral infection and persistence.86,87 According to the reported literature, the host molecules that modulated the cccDNA minichromosome were potential targets of anti-HBV therapy.88 At present, only one research showed that dicumarol (compound 78), one of the coumarin derivatives, could inhibit HBV replication and reduce the HBV-ccDNA level in HBV infected cells.79
Anti-herpes simplex virus
Herpes simplex virus (HSV) is a double-stranded DNA virus which was categorized into two distinct herpesvirus species, herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2) due to the differences in antigenicity.89 WHO estimated that about 3.7 billion people under the age of 50 had HSV-1 infection, and 491 million people aged 15–49 years worldwide were living with the genital herpes infection caused by HSV-2.90 However, there are only a few therapies targeting the worldwide prevalent virus with no preventive vaccine.
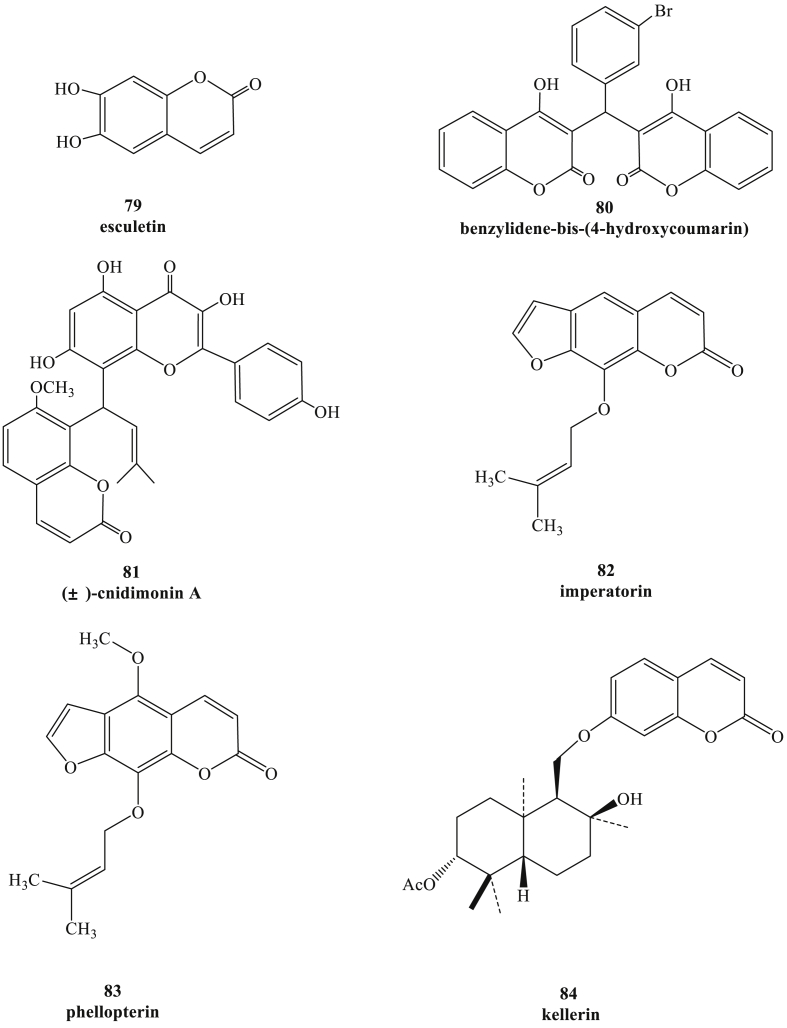
In 2009, esculetin (Fig. 9, compound 79) was the first reported coumarin compound with anti-HSV-1 activity at a concentration of 0.14 mM, which was evaluated in VERO cells by cytopathic effect (CPE) assay.91 Then the 4-bromobenzylidene derivative of bis-(4-hydroxycoumarin) (compound 80) exerted potential inhibitory activity against HSV-1and HSV-2 in human embryonic lung (HEL) cell cultures, and 50% inhibitory concentration (EC50) values of both were 9 μM, while minimum cytotoxic concentration (MCC) was more than 20 μM.92 As coumarin−flavonol compound (±)-cnidimonin A (compound 81) was found in the fruits of Cnidium monnieri, and the bioassay data showed that this racemic mixture (±)-1 possessed strong activity against HSV-1 with EC50 values of 1.23 μM in VERO cells.93 In additional, these compounds extracted from fruits of Angelica archangelica L also had antiviral activity against HSV-1, which was proved using cytopathic effect (CPE) inhibitory assay and virus titer reduction assay in the Vero cell line. Barbara Rajtar and colleagues found that imperatorin (compound 82) and phellopterin (compound 83) had the potent anti-HSV-1 activity. These coumarin derivatives could reduce the virus replication markedly with the concentration ranging from 3.90 μg/mL to 31.25 μg/mL, and the SAR manifested that the isopentenyloxy moiety at C-8 position contributed significantly to the antiviral activity.94 As a sesquiterpene coumarin separated from the gum-resin of Ferula assa-foetida, kellerin (compound 84) was a promising antiviral agent by suppressing the replication of HSV-1with EC50 at 38 μg/mL in Vero cells, and it also could significantly inhibit cytopathic effects (CPE), reducing the viral titer of HSV-1 DNA viral strain KOS.95
Conclusion
As a natural product, coumarin has received more and more attention in the field of drug development since its discovery in 1812. In recent years, with the development of drug screening technologies and methods, coumarin and its semi-synthetic derivatives have showed antiviral activity, and many coumarin-based anti-viral agents with high efficiency and low side effects were obtained. This review provides the main insights on current development in the area of antiviral agents of coumarin and its derivatives, and also discusses the structure activity relationship of potent coumarin derivatives.
Although there is no coumarin candidate used in clinical as antiviral drug, some coumarin candidates were developed as the anticoagulants (dicoumarol, warfarin, acenocoumarol), antibiotics (novobiocin, coumermycin) and antidermatosis drugs (methoxsalen). These evidence indicated that coumarin derivatives had the potential druggability.96, 97, 98 Furthermore, numerous attempts have been made for modifying the different position of coumarin nucleus to improve their anti-viral activities, and make the coumarin such as DCK and the analogues as the promising candidates against HIV. Meanwhile, some coumarin derivatives have obvious toxic effects in rodents and the toxicity of coumarins in different animals and organs are significantly different, and toxic reactions occurred mainly in the coumarin metabolism after high dose oral administration.99,100 Other possible reasons include the high liposolubility and photosensitization of some coumarins, certainly, and the toxicity and efficiency varies based on the chemical structure.
Since modifications on the nucleus of chemical structure can increase the inhibitory activity of coumarin against viruses including many RNA and DNA viruses, it results in a large number of compounds having diverse mechanism of actions, and the selective targeting proteins of these compounds include not only the specific stages of certain virus, but the common process of viral life cycle, such as viral attachment to the cells or replication in the cells.
In this article we mainly focus on the current developments of coumarin-based antiviral agents and the hybrid compounds bearing coumarin nucleus with antiviral activity. At present, these coumarins are undergoing pre-clinical research, and in-depth study of the antiviral activity mechanism of such compounds is still to be explored. We believe this review is helpful for medicinal chemists or pharmacologist to rational design and development of potential coumarin-related antiviral drugs with more activity and less toxicity.
Conflict of interests
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Abe T., Marutani Y., Shoji I. Cytosolic DNA-sensing immune response and viral infection. Microbiol Immunol. 2019;63(2):51–64. doi: 10.1111/1348-0421.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev. 2016;29(3):695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumlova M., Ruml T. In vitro methods for testing antiviral drugs. Biotechnol Adv. 2018;36(3):557–576. doi: 10.1016/j.biotechadv.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 6.Ren Q.C., Gao C., Xu Z., et al. Bis-coumarin derivatives and their biological activities. Curr Top Med Chem. 2018;18(2):101–113. doi: 10.2174/1568026618666180221114515. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y., Chen W., Shen Y., Zhu B., Wang G.X. Synthesis and antiviral activity of coumarin derivatives against infectious hematopoietic necrosis virus. Bioorg Med Chem Lett. 2019;29(14):1749–1755. doi: 10.1016/j.bmcl.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Khomenko T.M., Zarubaev V.V., Orshanskaya I.R., et al. Anti-influenza activity of monoterpene-containing substituted coumarins. Bioorg Med Chem Lett. 2017;27(13):2920–2925. doi: 10.1016/j.bmcl.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 9.De Luca L., Agharbaoui F.E., Gitto R., et al. Rational design, synthesis and evaluation of coumarin derivatives as protein-protein interaction inhibitors. Mol Inform. 2016;35(8–9):460–473. doi: 10.1002/minf.201501034. [DOI] [PubMed] [Google Scholar]
- 10.Olomola T.O., Klein R., Mautsa N., Sayed Y., Kaye P.T. Synthesis and evaluation of coumarin derivatives as potential dual-action HIV-1 protease and reverse transcriptase inhibitors. Bioorg Med Chem. 2013;21(7):1964–1971. doi: 10.1016/j.bmc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Guarner J. Human immunodeficiency virus: diagnostic approach. Semin Diagn Pathol. 2017;34(4):318–324. doi: 10.1053/j.semdp.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Parikh U.M., McCormick K., van Zyl G., Mellors J.W. Future technologies for monitoring HIV drug resistance and cure. Curr Opin HIV AIDS. 2017;12(2):182–189. doi: 10.1097/COH.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chimukangara B., Varyani B., Shamu T., et al. HIV drug resistance testing among patients failing second line antiretroviral therapy. Comparison of in-house and commercial sequencing. J Virol Methods. 2017;243:151–157. doi: 10.1016/j.jviromet.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waheed A.A., Tachedjian G. Why do we need new drug classes for HIV treatment and prevention? Curr Top Med Chem. 2016;16(12):1343–1349. doi: 10.2174/1568026616999151013124606. [DOI] [PubMed] [Google Scholar]
- 15.Laila U., Akram M., Shariati M.A., et al. Role of medicinal plants in HIV/AIDS therapy. Clin Exp Pharmacol Physiol. 2019;46(12):1063–1073. doi: 10.1111/1440-1681.13151. [DOI] [PubMed] [Google Scholar]
- 16.Cesar G.Z., Alfonso M.G., Marius M.M., et al. Inhibition of HIV-1 reverse transcriptase, toxicological and chemical profile of Calophyllum brasiliense extracts from Chiapas, Mexico. Fitoterapia. 2011;82(7):1027–1034. doi: 10.1016/j.fitote.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Eiznhamer D.A., Creagh T., Ruckle J.L., et al. Safety and pharmacokinetic profile of multiple escalating doses of (+)-calanolide A, a naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy HIV-negative volunteers. HIV Clin Trials. 2002;3(6):435–450. doi: 10.1310/9gde-f2r1-w2rl-e9fj. [DOI] [PubMed] [Google Scholar]
- 18.Butler M.S. Natural products to drugs: natural product derived compounds in clinical trials. Nat Prod Rep. 2005;22(2):162–195. doi: 10.1039/b402985m. [DOI] [PubMed] [Google Scholar]
- 19.Xue H., Lu X., Zheng P., et al. Highly suppressing wild-type HIV-1 and Y181C mutant HIV-1 strains by 10-chloromethyl-11-demethyl-12-oxo-calanolide A with druggable profile. J Med Chem. 2010;53(3):1397–1401. doi: 10.1021/jm901653e. [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Zhang Q., Guo J., et al. Metabolism of F18, a derivative of calanolide A, in human liver microsomes and cytosol. Front Pharmacol. 2017;8:479. doi: 10.3389/fphar.2017.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee T.T., Kashiwada Y., Huang L., Snider J., Cosentino M., Lee K.H. Suksdorfin: an anti-HIV principle from Lomatium suksdorfii, its structure-activity correlation with related coumarins, and synergistic effects with anti-AIDS nucleosides. Bioorg Med Chem. 1994;2(10):1051–1056. doi: 10.1016/s0968-0896(00)82054-4. [DOI] [PubMed] [Google Scholar]
- 22.Yu D., Brossi A., Kilgore N., Wild C., Allaway G., Lee K.H. Anti-HIV agents. Part 55: 3'R,4'R-Di-(O)-(-)-camphanoyl-2',2'-dimethyldihydropyrano[2,3-f]chromone (DCP), a novel anti-HIV agent. Bioorg Med Chem Lett. 2003;13(9):1575–1576. doi: 10.1016/s0960-894x(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z.Y., Xia Y., Xia P., Cosentino L.M., Lee K.H. Anti-AIDS agents. 31. Synthesis and anti-HIV activity of 4-substituted 3',4'-di-O-(-)-camphanoyl-(+)-cis-khellactone (DCK) thiolactone analogs. Bioorg Med Chem Lett. 1998;8(12):1483–1486. doi: 10.1016/s0960-894x(98)00254-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z.Y., Xia Y., Xia P., Brossi A., Cosentino L.M., Lee K.H. Anti-AIDS agents part 41: synthesis and anti-HIV activity of 3',4'-di-o-(-)-camphanoyl-(+)-cis-khellactone (DCK) lactam analogues. Bioorg Med Chem Lett. 2000;10(10):1003–1005. doi: 10.1016/s0960-894x(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 25.Xie L., Takeuchi Y., Cosentino L.M., McPhail A.T., Lee K.H. Anti-AIDS agents. 42. Synthesis and anti-HIV activity of disubstituted (3'R,4'R)-3',4'-di-O-(S)-camphanoyl-(+)-cis-khellactone analogues. J Med Chem. 2001;44(5):664–671. doi: 10.1021/jm000070g. [DOI] [PubMed] [Google Scholar]
- 26.Xie L., Takeuchi Y., Cosentino L.M., Lee K.H. Anti-AIDS agents. 37. Synthesis and structure-activity relationships of (3'R,4'R)-(+)-cis-khellactone derivatives as novel potent anti-HIV agents. J Med Chem. 1999;42(14):2662–2672. doi: 10.1021/jm9900624. [DOI] [PubMed] [Google Scholar]
- 27.Xie L., Takeuchi Y., Cosentino L.M., Lee K.H. Anti-AIDS agents. 33. Synthesis and anti-HIV activity of mono-methyl substituted 3',4'-di-O-(-)-camphanoyl-(+)-cis-khellactone (DCK) analogues. Bioorg Med Chem Lett. 1998;8(16):2151–2156. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 28.Xie L., Allaway G., Wild C., Kilgore N., Lee K.H. Anti-AIDS agents. Part 47: synthesis and anti-HIV activity of 3-substituted 3',4'-Di-O-(S)-camphanoyl-(3'R,4'R)-(+)-cis-khellactone derivatives. Bioorg Med Chem Lett. 2001;11(17):2291–2293. doi: 10.1016/s0960-894x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang L., Kashiwada Y., Cosentino L.M., et al. Anti-AIDS agents. 15. Synthesis and anti-HIV activity of dihydroseselins and related analogs. J Med Chem. 1994;37(23):3947–3955. doi: 10.1021/jm00049a014. [DOI] [PubMed] [Google Scholar]
- 30.Huang L., Yuan X., Yu D., Lee K.H., Chen C.H. Mechanism of action and resistant profile of anti-HIV-1 coumarin derivatives. Virology. 2005;332(2):623–628. doi: 10.1016/j.virol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 31.Xia P., Yin Z.J., Chen Y., et al. Anti-AIDS agents. Part 58: synthesis and anti-HIV activity of 1-thia-di-O-(-)-camphanoyl-(+)-cis-khellactone (1-thia-DCK) analogues. Bioorg Med Chem Lett. 2004;14(12):3341–3343. doi: 10.1016/j.bmcl.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 32.Yu D., Chen C.H., Brossi A., Lee K.H. Anti-AIDS agents. 60. Substituted 3'R,4'R-di-O-(-)-camphanoyl-2',2'-dimethyldihydropyrano[2,3-f]chromone (DCP) analogues as potent anti-HIV agents. J Med Chem. 2004;47(16):4072–4082. doi: 10.1021/jm0400505. [DOI] [PubMed] [Google Scholar]
- 33.Xie L., Yu D., Wild C., et al. Anti-AIDS agents. 52. Synthesis and anti-HIV activity of hydroxymethyl (3'R,4'R)-3',4'-di-O-(S)-camphanoyl-(+)-cis-khellactone derivatives. J Med Chem. 2004;47(3):756–760. doi: 10.1021/jm030416y. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M., Li Y., Smith P.C., et al. Anti-AIDS agents 65: investigation of the in vitro oxidative metabolism of 3',4'-Di-O-(-)-camphanoyl-(+)-cis-khellactone derivatives as potent anti-hiv agents. Drug Metab Dispos. 2005;33(11):1588–1592. doi: 10.1124/dmd.105.004218. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Zhang Q., Zhang B., et al. Anti-AIDS agents. Part 56: synthesis and anti-HIV activity of 7-thia-di-O-(-)-camphanoyl-(+)-cis-khellactone (7-thia-DCK) analogs. Bioorg Med Chem. 2004;12(24):6383–6387. doi: 10.1016/j.bmc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y.P., Yan G., Guo J.M., et al. Prenylated coumarins from the fruits of Manilkara zapota with potential anti-inflammatory effects and anti-HIV activities. J Agric Food Chem. 2019;67(43):11942–11947. doi: 10.1021/acs.jafc.9b04326. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M., Ma L., Wen J., et al. Rational design and Structure-Activity relationship of coumarin derivatives effective on HIV-1 protease and partially on HIV-1 reverse transcriptase. Eur J Med Chem. 2020;186 doi: 10.1016/j.ejmech.2019.111900. [DOI] [PubMed] [Google Scholar]
- 38.Marquez N., Sancho R., Bedoya L.M., et al. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-kappaB pathway. Antivir Res. 2005;66(2–3):137–145. doi: 10.1016/j.antiviral.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Olmedo D., Sancho R., Bedoya L.M., et al. 3-Phenylcoumarins as inhibitors of HIV-1 replication. Molecules. 2012;17(8):9245–9257. doi: 10.3390/molecules17089245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo E., Taura M., Matsuda K., et al. Inhibition of HIV-1 replication by a tricyclic coumarin GUT-70 in acutely and chronically infected cells. Bioorg Med Chem Lett. 2013;23(3):606–609. doi: 10.1016/j.bmcl.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 41.Matsuda K., Hattori S., Kariya R., et al. Inhibition of HIV-1 entry by the tricyclic coumarin GUT-70 through the modification of membrane fluidity. Biochem Biophys Res Commun. 2015;457(3):288–294. doi: 10.1016/j.bbrc.2014.12.102. [DOI] [PubMed] [Google Scholar]
- 42.Mao P.C., Mouscadet J.F., Leh H., Auclair C., Hsu L.Y. Chemical modification of coumarin dimer and HIV-1 integrase inhibitory activity. Chem Pharm Bull (Tokyo) 2002;50(12):1634–1637. doi: 10.1248/cpb.50.1634. [DOI] [PubMed] [Google Scholar]
- 43.Chiang C.C., Mouscadet J.F., Tsai H.J., Liu C.T., Hsu L.Y. Synthesis and HIV-1 integrase inhibition of novel bis- or tetra-coumarin analogues. Chem Pharm Bull (Tokyo) 2007;55(12):1740–1743. doi: 10.1248/cpb.55.1740. [DOI] [PubMed] [Google Scholar]
- 44.Olomola T.O., Mosebi S., Klein R., et al. Novel furocoumarins as potential HIV-1 integrase inhibitors. Bioorg Chem. 2014;57:1–4. doi: 10.1016/j.bioorg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Sharma S., Cermakova K., De Rijck J., et al. Affinity switching of the LEDGF/p75 IBD interactome is governed by kinase-dependent phosphorylation. Proc Natl Acad Sci U S A. 2018;115(30):E7053–E7062. doi: 10.1073/pnas.1803909115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esposito F., Ambrosio F.A., Maleddu R., et al. Chromenone derivatives as a versatile scaffold with dual mode of inhibition of HIV-1 reverse transcriptase-associated Ribonuclease H function and integrase activity. Eur J Med Chem. 2019;182 doi: 10.1016/j.ejmech.2019.111617. [DOI] [PubMed] [Google Scholar]
- 47.Sancho R., Marquez N., Gomez-Gonzalo M., et al. Imperatorin inhibits HIV-1 replication through an Sp1-dependent pathway. J Biol Chem. 2004;279(36):37349–37359. doi: 10.1074/jbc.M401993200. [DOI] [PubMed] [Google Scholar]
- 48.Deng M., Xie L., Zhong L., Liao Y., Liu L., Li X. Imperatorin: a review of its pharmacology, toxicity and pharmacokinetics. Eur J Pharmacol. 2020;879:173124. doi: 10.1016/j.ejphar.2020.173124. [DOI] [PubMed] [Google Scholar]
- 49.Lin P.H., Ke Y.Y., Su C.T., et al. Inhibition of HIV-1 Tat-mediated transcription by a coumarin derivative, BPRHIV001, through the Akt pathway. J Virol. 2011;85(17):9114–9126. doi: 10.1128/JVI.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shikishima Y., Takaishi Y., Honda G., et al. Chemical constituents of Prangos tschiniganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem Pharm Bull (Tokyo) 2001;49(7):877–880. doi: 10.1248/cpb.49.877. [DOI] [PubMed] [Google Scholar]
- 51.Lv L., Wang Q., Xu Y., et al. Vpr targets TET2 for degradation by CRL4(VprBP) E3 ligase to sustain IL-6 expression and enhance HIV-1 replication. Mol Cell. 2018;70(5):961–970. doi: 10.1016/j.molcel.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Q., Su L. Vpr enhances HIV-1 env processing and virion infectivity in macrophages by modulating TET2-dependent IFITM3 expression. mBio. 2019;10(4) doi: 10.1128/mBio.01344-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ong E.B., Watanabe N., Saito A., et al. Vipirinin, a coumarin-based HIV-1 Vpr inhibitor, interacts with a hydrophobic region of VPR. J Biol Chem. 2011;286(16):14049–14056. doi: 10.1074/jbc.M110.185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahajan D.H., Pannecouque C., De Clercq E., Chikhalia K.H. Synthesis and studies of new 2-(coumarin-4-yloxy)-4,6-(substituted)-S-triazine derivatives as potential anti-HIV agents. Arch Pharm (Weinheim) 2009;342(5):281–290. doi: 10.1002/ardp.200800149. [DOI] [PubMed] [Google Scholar]
- 55.Fatma B., Kumar R., Singh V.A., et al. Alphavirus capsid protease inhibitors as potential antiviral agents for Chikungunya infection. Antivir Res. 2020;179 doi: 10.1016/j.antiviral.2020.104808. [DOI] [PubMed] [Google Scholar]
- 56.Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372(13):1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 57.Hwu J.R., Kapoor M., Tsay S.C., et al. Benzouracil-coumarin-arene conjugates as inhibiting agents for chikungunya virus. Antivir Res. 2015;118:103–109. doi: 10.1016/j.antiviral.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Hwu J.R., Huang W.C., Lin S.Y., et al. Chikungunya virus inhibition by synthetic coumarin-guanosine conjugates. Eur J Med Chem. 2019;166:136–143. doi: 10.1016/j.ejmech.2019.01.037. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Calderon C., Mesa-Castro C., Robledo S., et al. Antiviral effect of compounds derived from the seeds of Mammea americana and Tabernaemontana cymosa on Dengue and Chikungunya virus infections. BMC Compl Alternative Med. 2017;17(1) doi: 10.1186/s12906-017-1562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong J., Xia Y., Hua L., et al. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif Cells Nanomed Biotechnol. 2019;47(1):3485–3491. doi: 10.1080/21691401.2019.1640716. [DOI] [PubMed] [Google Scholar]
- 61.Zhou D., Zhao Y., Kotecha A., et al. Unexpected mode of engagement between enterovirus 71 and its receptor SCARB2. Nat Microbiol. 2019;4(3):414–419. doi: 10.1038/s41564-018-0319-z. [DOI] [PubMed] [Google Scholar]
- 62.Woodman A., Lee K.M., Janissen R., et al. Predicting intraserotypic recombination in enterovirus 71. J Virol. 2019;93(4) doi: 10.1128/JVI.02057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang B., Zhang H., Zhu M., Luo Z., Peng Y. MEK1-ERKs signal cascade is required for the replication of Enterovirus 71 (EV71) Antivir Res. 2012;93(1):110–117. doi: 10.1016/j.antiviral.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Han S., Zhou V., Pan S., et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg Med Chem Lett. 2005;15(24):5467–5473. doi: 10.1016/j.bmcl.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 65.Sun J., Niu Y., Wang C., et al. Discovery of 3-benzyl-1,3-benzoxazine-2,4-dione analogues as allosteric mitogen-activated kinase kinase (MEK) inhibitors and anti-enterovirus 71 (EV71) agents. Bioorg Med Chem. 2016;24(16):3472–3482. doi: 10.1016/j.bmc.2016.05.055. [DOI] [PubMed] [Google Scholar]
- 66.Wang C., Zhang H., Xu F., et al. Substituted 3-benzylcoumarins as allosteric MEK1 inhibitors: design, synthesis and biological evaluation as antiviral agents. Molecules. 2013;18(5):6057–6091. doi: 10.3390/molecules18056057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanini S., Ustianowski A., Pisapia R., Zumla A., Ippolito G. Viral hepatitis: etiology, epidemiology, transmission, diagnostics, treatment, and prevention. Infect Dis Clin. 2019;33(4):1045–1062. doi: 10.1016/j.idc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Foster M.A., Hofmeister M.G., Kupronis B.A., et al. Increase in hepatitis A virus infections - United States, 2013-2018. MMWR Morb Mortal Wkly Rep. 2019;68(18):413–415. doi: 10.15585/mmwr.mm6818a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beer A., Holzmann H., Pischke S., et al. Chronic Hepatitis E is associated with cholangitis. Liver Int. 2019;39(10):1876–1883. doi: 10.1111/liv.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medvedev R., Ploen D., Hildt E. HCV and oxidative stress: implications for HCV life cycle and HCV-associated pathogenesis. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/9012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed A., Felmlee D.J. Mechanisms of hepatitis C viral resistance to direct acting antivirals. Viruses. 2015;7(12):6716–6729. doi: 10.3390/v7122968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zajac M., Muszalska I., Sobczak A., Dadej A., Tomczak S., Jelinska A. Hepatitis C - new drugs and treatment prospects. Eur J Med Chem. 2019;165:225–249. doi: 10.1016/j.ejmech.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 73.Hwu J.R., Singha R., Hong S.C., et al. Synthesis of new benzimidazole-coumarin conjugates as anti-hepatitis C virus agents. Antivir Res. 2008;77(2):157–162. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Neyts J., De Clercq E., Singha R., et al. Structure-activity relationship of new anti-hepatitis C virus agents: heterobicycle-coumarin conjugates. J Med Chem. 2009;52(5):1486–1490. doi: 10.1021/jm801240d. [DOI] [PubMed] [Google Scholar]
- 75.Hwu J.R., Lin S.Y., Tsay S.C., De Clercq E., Leyssen P., Neyts J. Coumarin-purine ribofuranoside conjugates as new agent against hepatitis C virus. J Med Chem. 2011;54(7):2114–2126. doi: 10.1021/jm101337v. [DOI] [PubMed] [Google Scholar]
- 76.Tsay S.C., Hwu J.R., Singha R., et al. Coumarins hinged directly on benzimidazoles and their ribofuranosides to inhibit hepatitis C virus. Eur J Med Chem. 2013;63:290–298. doi: 10.1016/j.ejmech.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Tsay S.C., Lin S.Y., Huang W.C., et al. Synthesis and structure-activity relationships of imidazole-coumarin conjugates against hepatitis C virus. Molecules. 2016;21(2) doi: 10.3390/molecules21020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang T.J. Hepatitis B: the virus and disease. Hepatology. 2009;49(5 Suppl):S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takeuchi F., Ikeda S., Tsukamoto Y., et al. Screening for inhibitor of episomal DNA identified dicumarol as a hepatitis B virus inhibitor. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0212233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang B.Y., Chai D.P., Wu Y.H., et al. Potential drug targets against hepatitis B virus based on both virus and host factors. Curr Drug Targets. 2019;20(16):1636–1651. doi: 10.2174/1389450120666190729115646. [DOI] [PubMed] [Google Scholar]
- 81.Huang R.L., Chen C.C., Huang Y.L., et al. Osthole increases glycosylation of hepatitis B surface antigen and suppresses the secretion of hepatitis B virus in vitro. Hepatology. 1996;24(3):508–515. doi: 10.1002/hep.510240307. [DOI] [PubMed] [Google Scholar]
- 82.Li L.Q., Li J., Huang Y., et al. Lignans from the heartwood of Streblus asper and their inhibiting activities to hepatitis B virus. Fitoterapia. 2012;83(2):303–309. doi: 10.1016/j.fitote.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Su W., Zhao J., Yang M., et al. A coumarin lignanoid from the stems of Kadsura heteroclita. Bioorg Med Chem Lett. 2015;25(7):1506–1508. doi: 10.1016/j.bmcl.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 84.Xu B., Liu S., Fan X.D., Deng L.Q., Ma W.H., Chen M. Two new coumarin glycosides from Herpetospermum caudigerum. J Asian Nat Prod Res. 2015;17(7):738–743. doi: 10.1080/10286020.2014.996137. [DOI] [PubMed] [Google Scholar]
- 85.Huang S.X., Mou J.F., Luo Q., et al. Anti-hepatitis B virus activity of esculetin from microsorium fortunei in vitro and in vivo. Molecules. 2019;24(19) doi: 10.3390/molecules24193475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu S., Zhou B., Valdes J.D., Sun J., Guo H. Serum hepatitis B virus RNA: a new potential biomarker for chronic hepatitis B virus infection. Hepatology. 2019;69(4):1816–1827. doi: 10.1002/hep.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohd-Ismail N.K., Lim Z., Gunaratne J., Tan Y.J. Mapping the interactions of HBV cccDNA with host factors. Int J Mol Sci. 2019;20(17) doi: 10.3390/ijms20174276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levrero M., Pollicino T., Petersen J., Belloni L., Raimondo G., Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51(3):581–592. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 89.Lloyd J., Copaciu R., Yahyabeik A., et al. Characterization of polyclonal antibodies to herpes simplex virus types 1 and 2. J Histotechnol. 2019;42(4):202–214. doi: 10.1080/01478885.2019.1683132. [DOI] [PubMed] [Google Scholar]
- 90.James C., Harfouche M., Welton N.J., et al. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–329. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian L.W., Pei Y., Zhang Y.J., Wang Y.F., Yang C.R. 7-O-methylkaempferol and -quercetin glycosides from the whole plant of Nervilia fordii. J Nat Prod. 2009;72(6):1057–1060. doi: 10.1021/np800760p. [DOI] [PubMed] [Google Scholar]
- 92.Zavrsnik D., Muratovic S., Makuc D., et al. Benzylidene-bis-(4-hydroxycoumarin) and benzopyrano-coumarin derivatives: synthesis, (1)H/(1)(3)C-NMR conformational and X-ray crystal structure studies and in vitro antiviral activity evaluations. Molecules. 2011;16(7):6023–6040. doi: 10.3390/molecules16076023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su F., Zhao Z., Ma S., et al. Cnidimonins A-C, three types of hybrid dimer from Cnidium monnieri: structural elucidation and semisynthesis. Org Lett. 2017;19(18):4920–4923. doi: 10.1021/acs.orglett.7b02290. [DOI] [PubMed] [Google Scholar]
- 94.Rajtar B., Skalicka-Wozniak K., Swiatek L., Stec A., Boguszewska A., Polz-Dacewicz M. Antiviral effect of compounds derived from Angelica archangelica L. on Herpes simplex virus-1 and Coxsackievirus B3 infections. Food Chem Toxicol. 2017;109(Pt 2):1026–1031. doi: 10.1016/j.fct.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 95.Ghannadi A., Fattahian K., Shokoohinia Y., Behbahani M., Shahnoush A. Anti-viral evaluation of sesquiterpene coumarins from Ferula assa-foetida against HSV-1. Iran J Pharm Res (IJPR) 2014;13(2):523–530. [PMC free article] [PubMed] [Google Scholar]
- 96.Wittkowsky A.K. Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. Semin Vasc Med. 2003;3(3):221–230. doi: 10.1055/s-2003-44457. [DOI] [PubMed] [Google Scholar]
- 97.Feng D., Zhang A., Yang Y., Yang P. Coumarin-containing hybrids and their antibacterial activities. Arch Pharm (Weinheim) 2020;353(6) doi: 10.1002/ardp.201900380. [DOI] [PubMed] [Google Scholar]
- 98.Guillon C.D., Jan Y.H., Foster N., et al. Synthetically modified methoxsalen for enhanced cytotoxicity in light and dark reactions. Bioorg Med Chem Lett. 2019;29(4):619–622. doi: 10.1016/j.bmcl.2018.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka Y., Fujii W., Hori H., Kitagawa Y., Ozaki K. Relationship between coumarin-induced hepatocellular toxicity and mitochondrial function in rats. Food Chem Toxicol. 2016;90:1–9. doi: 10.1016/j.fct.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Guo P.J., Lin Z.J., Zhang X.M., Zou L.N., Guo F.F., Zhang B. [Toxicological research and safety consideration of coumarins] Zhongguo Zhongyao Zazhi. 2020;45(3):518–522. doi: 10.19540/j.cnki.cjcmm.20190827.401. [DOI] [PubMed] [Google Scholar]