Abstract
Age-related macular degeneration (AMD) is a complex eye disorder and is the leading cause of incurable blindness worldwide in the elderly. Clinically, AMD initially affects the central area of retina known as the macula and it is classified as early stage to late stage (advanced AMD). The advanced AMD is classified into the nonexudative or atrophic form (dry AMD) and the exudative or neovascular form (wet AMD). More severe vision loss is typically associated with the wet form. Multiple genetic factors, lipid metabolism, oxidative stress and aging, play a role in the etiology of AMD. Dysregulation in genetic to AMD is established to 46%–71% of disease contribution, with CFH and ARMS2/HTRA1 to be the two most notable risk loci among the 103 identified AMD associated loci so far. Chronic cigarette smoking is the most proven consistently risk living habits for AMD. Deep learning algorithm has been developed based on image recognition to distinguish wet AMD and normal macula with high accuracy. Currently, anti-vascular endothelial growth factor (VEGF) therapy is highly effective at treating wet AMD. Several new generation AMD drugs and iPSC-derived RPE cell therapy are in the clinical trial stage and are promising to improve AMD treatment in the near future.
Keywords: Age-related macular degeneration, Diagnosis, Genetics, Mechanism, Target treatment
Introduction
The macula is a circular area of diameter 5.5 mm with a center located 17°, or 4.0–5.0 mm, temporal, and 0.53–0.8 mm inferior to the center of the optic disc. The fovea centralis is a small, central pit composed of closely packed cones in the human eye. The fovea is responsible for sharp central vision. The fovea is surrounded by the parafovea belt and the perifovea outer region (Fig. 1A, B). Macular degeneration is the leading cause of severe, irreversible vision loss in people over age 55. It occurs when the macula deteriorates. Because the disease develops as a person ages, it is often referred to as age-related macular degeneration (AMD).
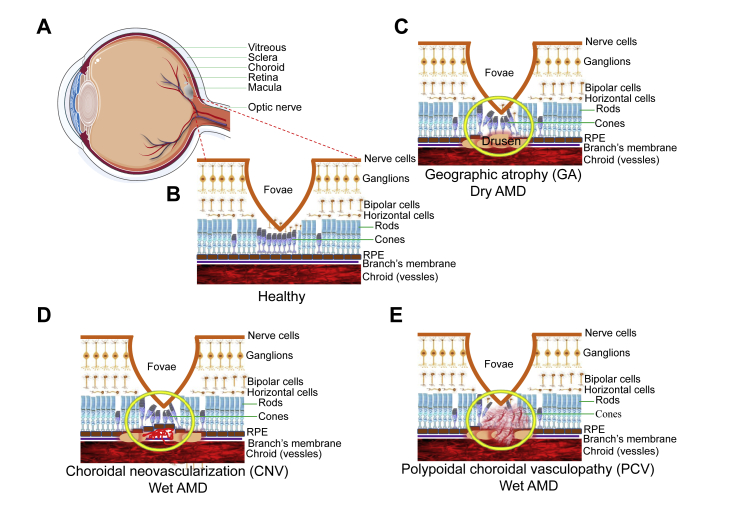
Figure 1.
Neovascularization and drusen formation. (A) A cross section of a normal human eye showing the location of the macula. An oval area with a diameter of 1.5 mm near the fovea is known as the macula. (B) It shows the important structure of the retina in the macular area. RPE cells have the function of removing metabolites produced by photoreceptors. The blood vessels in the choroid can transport nutrients and nourish the outer retina. (C) The formation and location of drusen is shown. Drusen formation will lead to retinal tissue atrophy and Bruch membrane calcification rupture, further leading to AMD. (D) This picture describes a choroid polypoid lesion. (E) This picture shows the proliferation of new blood vessels.
AMD can be classified into four stages.1, 2, 3 The first stage is normal ageing changes with only small - drusen and no pigment abnormalities. At this stage, the diameter of the drusen is < 63 μm. In the second stage (early AMD), there are some intermediate drusen whose diameter is ≥ 63 μm and ≤124 μm but with no retinal pigment epithelium (RPE) cell abnormalities. Stage 3rd is extensive, moderate, at least one large drusen (diameter ≥125 μm; intermediate AMD) and RPE abnormalities. Stage 4th, also known as advanced AMD, is concerned with the geographic atrophy (GA) of the fovea or any age-related characteristics of neovascular macular degeneration, as well as resulting in visual loss. Liew confirmed the robustness of the age-related eye disease study (AREDS) simplified severity scale.4 Early AMD has the smallest visual impairment, usually accompanied by decreased reading ability, visual distortion, and a black or gray spot in the central vision. Mid-term AMD is characterized by large or moderate vitreous wart membranes or imperfect pigmentation. The central vision of patients with advanced AMD is significantly affected.
Clinically, besides disciform scar which is at the end stage of advanced AMD, advanced AMD is also divided into two categories: dry AMD (Fig. 1C) and wet AMD. Dry AMD is also known as GA, which does not involve blood or serum leakage.5 It is characterized by symmetrical eyes, distorted vision, disordered macular pigmentation in both eyes, the disappearance of foveal reflex, varying posterior poles with yellowish-white drusen between Bruch's membrane (BrM) and RPE. In some advanced patients, map-like atrophy of the posterior retina can be observed. Wet AMD can be divided into choroidal neovascularization (CNV, Fig. 1D) and polypoidal choroidal vasculopathy (PCV, Fig. 1E), which involve blood or serum leakage. (At present, it is still controversial whether PCV belongs to wet AMD. On the one hand, PCV and CNV have different characteristics in clinical characteristics and epidemiology. On the other hand, PCV and CNV have similarities in environmental and genetic risk factors.6 In this review, PCV, and CNV are regarded as subtypes of wet AMD). Compared with dry AMD, the visual acuity of patients with wet AMD decreased more rapidly. Most patients with wet AMD suffer from one eye first, and the other eye may not develop for a long time. In CNV, choroidal capillaries grow to the RPE and BrM lesions, resulting in choroidal neovascularization. Because the structure of neovascularization is not perfect, it will lead to a series of pathological changes such as exudation, bleeding and scar, which will further lead to the loss of central vision. PCV is more prevalent in Asians.7 The clinical course of PCV is more stable and visual outcomes are more favorable from those of CNV and therefore been described as a separate clinical entity differing from CNV.6 In PCV condition, the polypoid neovascularization from the choroid layer can pass through the BrM but would not penetrate the RPE layer. PCV can be divided into type I and type II according to the morphology of the lesions. Type I had abnormal branching vascular network (BVN) and polypoid lesions at the end of the vascular network. Type II had isolated or clustered polypoid lesions, but no obvious branching vascular network or fine reticular vessels.8 In this condition, secondary subretinal hemorrhage or submacular hemorrhage may occur.
Epidemiology
Prevalence
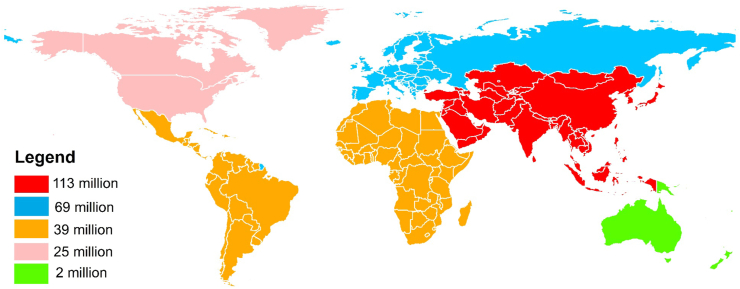
The population of AMD is increasing with ethnic and regional differences. From 1990 to 2010, the incidence of blindness and visual impairment caused by AMD was increased.9 With population aging worldwide, it is estimated that the number of AMD patients will be increased to 196 million in 2020, 288 million in 2040.10 Since Asia accounts for more than half of the world's population, the number of cases is expected to reach 113 million by 2040.10 Because of the highest prevalence of AMD in Europe, the number of future cases in this region is predicted to be second after Asia (Fig. 2).10,11 AMD is more common in whites than in blacks.12, 13, 14 The prevalence rate of AMD in European was 12.33%, Asian was 7.38%, and African was 7.53%10 Michele Reibaldi reported that latitude or longitude was negatively correlated with the original or age-standardized prevalence of AMD in the early and late stages (P < 0.001).15 Chinese population accounts for about one-fifth of the world's population. Song Peige reported that the prevalence of AMD varies in different geographical backgrounds in China16; the prevalence of AMD is concentrated in the most populous areas of south-central China, at 6.64% (95% CI = 5.12–8.52) and 6.74% (95% CI = 5.20–8.65) in 2000 and 2010, respectively, and increases with the decrease of latitude.
Figure 2.
The predicted global prevalence of AMD in 2040. TThis figure shows the estimated number of people worldwide with AMD in 2040. Data from REF.10.
General risk factors
There are several risk factors for AMD, such as ageing, race, blood pressure, and lifestyle. Ageing is a strong risk factor for the development of AMD. Blue iris is more likely to develop AMD than the brown iris.17 Systemic factors such as cardiovascular disease are also risk factors for AMD18; elevated blood pressure17,19 and atherosclerosis20 can increase the risk of AMD. The presence of diabetic retinopathy (DR), high-density lipoprotein (HDL),21 obesity and high systolic blood pressure increase the risk of AMD in diabetic patients.22 Also, Ronald et al reported that the baseline level of serum cystatin C was associated with the incidence of early AMD and wet AMD.23 Studies show that smoking and heavy drinking increase the risk of AMD.24, 25, 26 Lutein and zeaxanthin can reduce the risk of AMD, vitamin C, vitamin E, vitamin D, zinc oxide can prevent the occurrence of AMD.27,28 Increase fish intake can reduce the risk of AMD,29, 30, 31 DHA and EPA may slow the occurrence of AMD.32,33 In addition, lunch break is associated with a lower incidence of advanced AMD.34 People who divorced or separated were three times more likely to have advanced AMD than married people.34
Genetics
Associated loci
Genetic factors had a strong impact on the pathogenesis of AMD,35 totally 103 AMD-related genes or loci have been reported. CFH and HTRA1 loci are the two major associated loci for AMD. In 2005, researchers first linked the 402H allele of CFH to an increased risk of AMD.36, 37, 38 CFH is a key regulator of the complement pathway, located in chromosome 1q32. CFH 402H disable to transport oxidative lipids out of the RPE layer, destroys the regulatory function of CFH in inhibiting the activation of C3 to C3b and degrading C3b, leading to the over-activation of complement, thus increasing the risk of AMD.35, 36, 37, 38, 39, 40 CFB and C2 are activators of alternative and classical pathways, respectively. CFB R32Q and R32Q/IVS10 haplotype has a protective effect for AMD.41 The mutation R102G (rs2230199) of C3 is involved in the process of AMD from early to late. Lysate C3a of C3 exists in the vitreous verruca membrane.42 C3a can induce the expression of VEGF and promote the formation of CNV.43 CFI is regulated by CFH and is a cofactor of inactivation of C3b. Study showed that rs10033900, rs11728699, rs6854876, rs7439493 and rs13117504 had protective effects,44 while rs2285714 was associated with increased risk for AMD. HTRA1 is another major effect locus for AMD.45, 46, 47, 48, 49, 50, 51, 52, 53, 54 Rs10490924 in HTRA1 promoter region can increase the risk of AMD 15 times.49,50,52 HTRA1 transgenic mice showed BrM and choroid damage, with some pathological features of CNV and PCV.
Some AMD genes related to lipid metabolism. APOE is a key regulator of lipid and cholesterol transport in the central nervous system. Allele haplotypes of APOE were ε2, ε3 and ε4. The risk of AMD in carriers of ε4 is lower than that in people with ε3 genotype. The good mobility of ε4 enables lipid, cholesterol and RPE degradation products to better cross BrM from RPE, otherwise, these substances accumulate in BrM to cause drusen and AMD.55, 56, 57 APOE ε2 seems to enhance the expression of vascular growth factor and fibroblast growth factor RPE cells.58 Therefore, APOE ε4 can reduce the incidence and progression of AMD compared with APOE ε2. In addition, TIMP3 is involved in RPE aging and sorbite dystrophy.59,60 The rs9621532 allele and nearby TIMP3 variants were associated with increased AMD risk. LIPC is also associated with AMD, among which rs10468017 is most related.61 CETP, LPL, and ABA1 have been demonstrated to express in the retina,61,62 which is associated with AMD may through affecting the formation of dursen. Rs2511989 encodes C1INH in SEPRING1 gene. C1INH is a suppressor of complement classic and lectin pathway activation. It is highly genotypically related to AMD.63
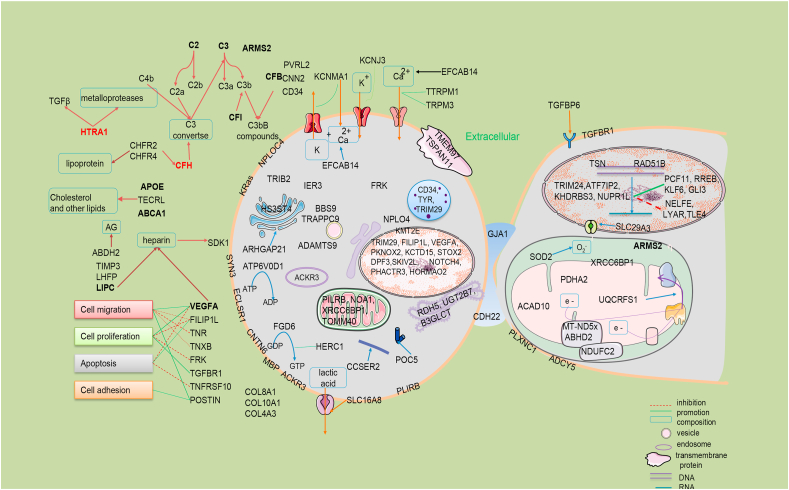
So far, at least 103 AMD associated loci have been identified according to GWAS Catalog (https://www.ebi.ac.uk/gwas/) and other publications (Table 1). They are involved in complement factors, lipid metabolism, angiogenesis, immune, cell motion, cytoskeleton, apoptosis and proliferation, cell junction, collagen and etc. Most of the reported genes are associated with all types of AMD and involved in multiple functions (Fig. 3 and Supplementary materials 1). TLR3 is only associated with dry AMD, which can participate in innate immunity and plays a role as pathogen recognition receptors in RPE, leading to apoptosis when induced by cytokines. A rare SNP rs77466370 in FGD6 is only associated with PCV.64
Table 1.
Classification of AMD related genes.
| Type | Genes (region, SNP) |
|---|---|
| Complement factors | C9 (5p13.1, rs62358361), C3 (19p13.3, rs2230199), C2 (6p21.33, rs116503776), CFHR4 (1q31.3, rs2171106), CFH(1q31.3, rs10922109), MBL2 (10q21.1, rs6480975), CFI(4q25, rs10033900),CFB(6p21.33, rs116503776),CFHR2 (1q31.3, rs2171106) |
| Lipid metabolism | CETP (16q13, rs5817082), APOE (19q13.32, rs429358), LIPC(15q21.3, rs2043085), ABCA1(9q31.1, rs2740488), LHFP (13q14.11, rs8002574), ABHD2 (15q26.1, rs4932480), TIMP3(22q12.3, rs9621532) |
| Angiogenesis | FILIP1L (3q12.1, rs13081855), VEGFA (6p21.1, rs943080) |
| Immune | PVRL2 (19q13.32, rs6857), CD34 (1q32.2, rs1967689), PILRB (7q22.1, rs7803454), SDK1 (7p22.2, rs55869773), ACKR3(2q37.3, rs56072732) |
| Cell motion | POSTN (13q13.3, rs9646096), TNXB (6p21.32, rs12153855), CNN2(19p13.3, rs67538026) |
| Cytoskeleton | CCSER2(10q23.1, rs113544501), POC5(5q13.3, rs79069165), HTRA1 (10q26.13, rs3750846), ARMS2(10q26.13, rs3750846) |
| Apoptosis and proliferation | TNFRSF10A (8p21.3, rs13278062), CTRB2 (16q23.1, rs72802342), NOTCH4 (6p21.32, rs2071277), FRK (6q22.1, rs1999930), PHACTR3 (20q13.32, rs843818), PKNOX2 (11q24.2, rs1077952), KRAS (12p12.1, rs73296436), IER3(6p21.33, rs3130783), TRIM29 (11q23.3, rs61900473), TGFBR1(9q22.33, rs334353) |
| Cell junction | GJA1 (6q22.31, rs9482193), CDH22 (20q13.12, rs6032755) |
| Mitochondrial | NDUFC2(11q14.1, rs113553030), XRCC6BP1(12q14.1, rs12368533), UQCRFS1 (19q12, rs741449),TOMM40 (19q13.32, rs6857), SOD2 (6q25.3, rs2842992), ACAD10 (12q24.12, rs61941274), MT–ND5 x ABHD2 (15q26.1, rs267606894 x rs4932480) |
| Ionic channel | KCNMA1 (10q22.3, rs76150532), KCNJ3 (2q24.1, rs1445653), TRPM1 (15q13.3, rs7182946), EFCAB14 (1p33, rs59182762), KCTD15 (19q13.11, rs10404384), TRPM3 (9q21.12, rs71507014) |
| Ubiquitination | NPLOC4(17q25.3, rs6565597) |
| Transcription | PCF11(11q14.1, rs4293143),TRIM24 (chr7:138166004,hg19), NELFE (6p21.33, rs116503776), RREB1 (6p24.3, rs11755724), GLI3 (7p14.1, rs2049622), KLF6 (10p15.1, rs12411753), ATF7IP2(16p13.13, rs28368872), BAZ1A (14q13.2, rs6571690), EFCAB14 (1p33, rs59182762), KHDRBS3(8q24.23, rs200534628), LYAR(4p16.3, rs150938341), NUPR1L (7p11.2, rs148800247), TLE4 (9q21.31, rs11545434) |
| Transport | SLC16A8 (22q13.1, rs8135665), SLC29A3 (10q22.1, rs7091537), SLC44A4 (6p21.33, rs12661281), TRAPPC9(8q24.3, rs117659209), ADAMTS9 (3p14.1, rs6795735) |
| Nerve | SYN3(22q12.3, rs5754227), CELSR1 (22q13.31, rs2337055), CNTN6 (3p26.3, rs77360121), DPF3(14q24.2, rs12887388), MBP(18q23, rs1789110), TNR (1q25.1, rs58978565), HERC1 (15q22.31, 15q22.31) |
| Cluster protein family | PLXNC1(12q22, rs17296444) |
| Helicases | SKIV2L (6p21.33, rs406936) |
| Adenylate cyclase | ADCY5(3q21.1, rs6762009) |
| GTPase or ATPase | ARHGAP21 (10p12.1, rs12357257), ATP6V0D1 (16q22.1, rs1471142), FGD(12q22, rs10507047) |
| Tyrosinase | TYR (11q14.3, rs621313) |
| BBSsome | BBS9(7p14.3, rs202162020) |
| Transmembrane protein | TMEM97 (17q11.2, rs11080055), TSPAN11 (12p11.21, rs55916253) |
| DNA binding protein | RAD51B (14q24.1, rs61985136), TSN(2q14.3, rs72837798) |
| Transferase | KMT2E (7q22.3, rs1142), B3GALTL (13q12.3, rs9564692), UGT2B7 (4q13.2, rs112243525), HS3ST4 (16p12.1, rs79590629) |
| Collagen | COL8A1 (3q12.1, rs140647181), COL10A1 (6q22.1, rs1999930), COL4A3 (2q36.3, rs11884770) |
| Noncoding RNA | ADAMTS9-AS2 (3p14.1, rs62247658), LINC00470(18p11.32, rs2186849), LINC00588(8q12.1, rs72657107), LINC00900(11q23.3, rs431911), PCDH9-AS3 (13q21.32, rs1359191), LOC101060498(4p14, rs12498917), LOC101927280 (5q23.1, rs61287758), LOC101927797(21q21.1, rs2205502), LOC101929681 (5p13.3, rs139161960), LOC153910(6q24.2, rs63337561), LOC729987(1p21.3, rs12727789) |
| Others | INHBB(2q14.2,rs6721654), ZPLD1 (3q12.3, rs17822656), HORMAD2 (22q12.2, rs713875), CST5 (20p11.21, rs4815244), CTRB2 (16q23.1, rs72802342), EXOC5(14q22.3, rs75165563), IGFBP6(12q13.13, rs11170417), PDHA2 (4q22.3, rs62315917), PRLR (5p13.2, rs114092250), RDH5 (12q13.2, rs3138141), STOX2 (4q35.1, rs11132213), TRIB2(2p24.3, rs10191751), C20orf85 (20q13.32, rs201459901), C4orf14 (4q12, rs1713985), C6orf223 (6p21.1, rs2295334), C80rf42 (8p23.3, rs722782), C9orf91 (9q32, rs41278671) |
Figure 3.
Schematic diagram of AMD genetics related genes/proteins. Some of these genes are distributed in the nucleus to participate in the regulation of transcription process, the methylation of histone and so on, some are distributed in the mitochondria to participate in the electron transmission of respiratory chain, and some are distributed in the cytoplasmic matrix and serve as the cytoskeleton used in the cytoskeleton to participate in the regulation of cell life activities. AG: arachidonic acid glyceride.
Interaction between genes and environmental factors
Some AMD-related genes and environmental factors (such as smoking and so on) interact together to influence the occurrence and development of AMD. Smoking changed the binding of CFH to C3, lowered the level of CFH in plasma,65,66 and the rs1061170 variant changed the binding ability of CFH to C3b. Therefore, rs1061170 carriers have a stronger impact on AMD risk among smokers.39,50,67, 68, 69, 70 Smoking also increases the risk of all people with the HTRA1 genotype.71 AREDS reported a significant interaction between CFH 402H and BMI.70 Blue Mountains Eye Study (BMES) found that increasing fish intake in CFH 402Y carriers had a stronger protective effect on late AMD.72 Intake of antioxidant nutrients also reduced the risk of early AMD in people with high genetic risk of CFH and HTRA1 loci.73,74 Similar to CFH variants, when both HTRA1 variants and higher C-reactive protein (CRP) levels are present, the risk of AMD increases.75 In addition, the potential interaction between chromosome 6q16.2 and 18q22.1 and smoking significantly increased the risk of AMD in long-term smokers.76 Schmidt et al found that APOE genotype had an impact on the smoking-related risk of AMD, and had a greater impact on CNV, and smoking was the most harmful to carriers of apolipoprotein ε2.77,78
Mechanism/pathophysiology
RPE cell senescence
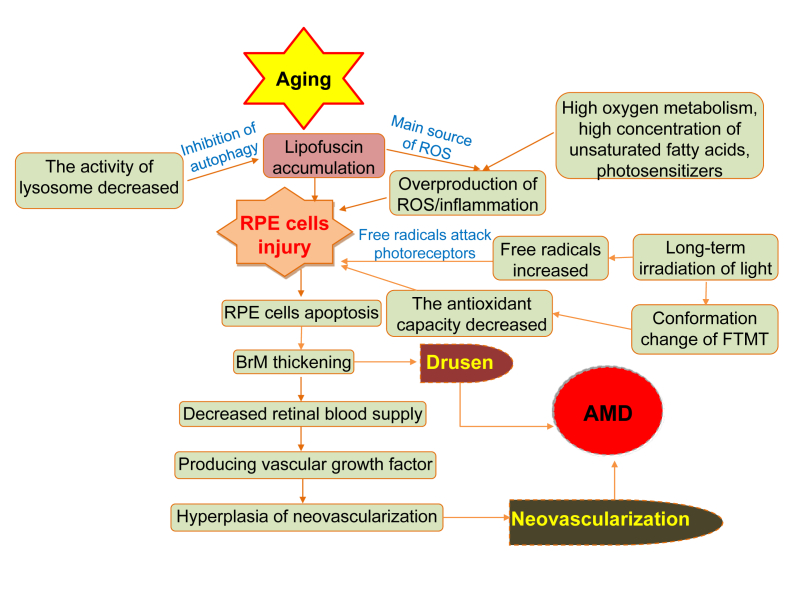
A series of changes caused by aging of RPE cells can lead to AMD. The ageing RPE cells break the balance of enzymes in the extracellular matrix in the macular area gathers on the BrM.79,80 Metabolites accumulate on the BrM, forming vitreous warts, damaging adjacent retinal tissues, reducing the blood supply of retina. RPE cell senescence leads the immune cells to produce VEGF. Calcification, rupture, and phagocytosis of the Bruch membrane produce blood vessels which eventually leads to AMD (Fig. 4).
Figure 4.
The formation of AMD during aging. This diagram shows the non-genetic mechanisms of AMD induced by RPE cell senescence, oxidative stress, hemodynamics and so on during aging.
Oxidative stress
The retina is one of the tissues with the highest oxygen consumption in the human body. Accumulation of oxidative stress plays an important role in the pathophysiological process of AMD.81 The cumulative oxidative damage of RPE is mainly caused by the imbalance between the production and elimination of reactive oxygen species (ROS). The presence of high oxygen metabolism, high concentration of polyunsaturated fatty acids, and photosensitizers can lead to the excessive production of ROS in the retina.82,83 Lipofuscin is the main source of reactive oxygen species, which accumulates in the RPE with age, further enhancing oxidative stress in the retina.84 Excessive lipofuscin is associated with AMD.85 In addition, the incidence of AMD in people exposed to sunlight for a longer period was significantly higher than that in the local general population.86,87 Antioxidative damage may be the goal of the prevention and treatment of related diseases. However, it was also reported that the mixture of antioxidants had little effect on reducing the rate of progression to CNV.88 Hyttinen and others suggested that modeling of AMPK-mTOR axis might prevent the development of AMD by helping cells adapt to oxidative stress.89,90
Lipid metabolism
High-density lipoprotein cholesterol (HDL-C) levels were associated with increased risk of AMD, while high total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) were associated with reduced risk of AMD.91 Researchers have found advanced lipid peroxidation end products (ALEs) in lipofuscin,92,93 vitreous warts, and BrM of AMD patients. Accumulation of advanced lipid ALEs interferes with protein stability and leads to apoptosis of photoreceptors and RPE cells.81,92,93
Inflammation and immunity
AMD drusen contain a variety of pro-inflammatory factors,81 which indicates that local inflammation is a marker of early AMD. The complement system is a component of the innate immune system, which plays an important role in monitoring and maintaining homeostasis of the intraocular microenvironment. It was found that the level of CRP was higher in AMD patients.94 CRP can recruit CFH to destroy necrotic tissue and prevent the release of pro-inflammatory cytokines. When the risk protein 402H is combined with CRP, the binding ability between 402H and CRP is much lower than that between 402Y in CFH and CRP, which leads to complement activation and inflammation.95 Most complement proteins do not diffuse through BrM, but C5a released after the activation of complements of AMD pass through BrM, leading to inflammation and angiogenesis.96 There is growing evidence that the targeted regulation of complement-specific proteins is expected to become a therapeutic method for AMD. Local inhibition of complement activation is considered a promising treatment for advanced AMD. At present, some studies have found potential complement cascade inhibitors, which may slow down the progress of AMD.97 Also, Calippe et al found that the combination of CFH and mononuclear phagocytes (MP) inhibited CD47-mediated MPs clearance, while MPs maintained the steady state of subretinal space.98 Therefore, inflammation and immunity are also closely related to the occurrence of AMD.
Neovascularization
VEGF is one of the main risks of wet AMD, which plays an important role in the occurrence of CNV and PCV.64,69 Overexpression of vascular-promoting cytokines (such as VEGF, PDGF) and reduction of expression of inhibitory cytokines (ES, TIMP and so on) can lead to excessive proliferation of neovascularization. Studies have shown that the Pulsatile ocular blood flow (POBF) and pulse amplitude (PA) in exudative AMD patients are lower than those in non-exudative AMD patients. The thickening of BrM may lead to increased choroidal vascular resistance, and the decrease of choroidal blood flow may be related to the formation of CNV.99, 100, 101, 102 In addition, choroidal vitreous warts and abnormal pigmentation also increase the risk of AMD. After one eye progresses to advanced AMD, the five-year risk of occurrence of AMD of the second eye is estimated to be between 30% and 40%, which is much higher than that in the normal population.103
Other mechanisms
In addition to the above factors that are closely related to the occurrence and development of AMD, other factors, such as autophagy, hemodynamics and circadian rhythm, are also believed to be related to AMD. Autophagy is a lysosomal pathway for the degradation of cytoplasmic proteins and damaged organelles. Protein aggregates are transported to lysosomes via autophagy. With the increase of age and RPE cells aging, lysosomal enzyme activity decreased. Autophagy was reduced to remove mitochondria and protein aggregates, outside cells, thus accelerating the accumulation of lipofuscin, further leading to the development of AMD.104,105 Golestaneh et al found that the autophagic flux of RPE in AMD patients was lower than that in normal RPE controls.106
There is a classical theory about the occurrence and development of AMD, namely hemodynamics, which holds that choroidal vascular dysfunction is the basis of AMD. Atherosclerotic changes in the blood supply to the eye are now known as neovascular AMD. The modern hemodynamic theory proposed by Friedman in 1997 that AMD occurs because of the change of choroidal blood flow caused by the increase of choroidal and scleral sclerosis.107 Blood lipid deposition and atherosclerosis result in thickening of the vascular wall, stenosis of vascular lumen, and decreased compliance of vascular wall, resulting in decreased choroidal blood flow and abnormal choroidal perfusion, resulting in insufficient perfusion of RPE cells or Bruch membrane injury, which ultimately leads to AMD. The view that choroidal hemodynamics contributes to the progress of AMD has been supported by recent clinical and experimental data.108
Some studies have found that cyclic genes may play a role in regulating retinal vascularization signals in retinopathy.109 In proliferative neovascularization, the expression of retinal rhythm genes is destroyed.110 Whether circadian rhythm disorder will affect the occurrence and development of AMD remains to be further studied. In addition, the imbalance of protein homeostasis may be one of the potential mechanisms leading to AMD.
Diagnosis
The diagnostic methods of AMD include fundus imaging, fluorescein angiogenesis (FA), indocyanine green angiography (ICGA), optical coherence tomography (OCT), Optical coherence tomography angiography (OCTA) and artificial intelligence (AI). These techniques play an important role in the diagnosis of AMD and have their unique advantages.
Fundus imaging
Fundus imaging methods include color photography, monochromatic photography, automatic fluorescence imaging, and fundus angiography, which are useful for AMD diagnosis. Microperimetry is a non-invasive visual function test for the analysis of fixation and central visual field defects, especially for AMD. Compared with standard automated perimetry (SAP), microperimetry has the advantages of real-time imaging of fundus and eye tracking.111 GA can be observed in the eyes of patients with dry AMD drusen, subretinal hemorrhage and pigment epithelial detachment (PED) are common in the eyes of patients with wet AMD. These phenotypes can be easily observed in fundus imaging.
FA and indocyanine green angiography (ICGA)
Dyes used for fundus angiography include sodium fluorescein and indocyanine green. FA is a useful method for detecting wet AMD. Indocyanine green angiography (ICGA) is the gold standard diagnostic tool for PCV. Both FA and ICGA are safe, but occasionally serious reactions may occur. Therefore, the potential risk factors of patients must be carefully screened and the risk of angiography should be informed. Under FA, compared with CNV, serous PED or atypical serous PED is more common in PCV. Under ICGA, CNV showed a neovascularization network without polypoid structure, while abnormal vessels in PCV were more robust with terminal polypoid or aneurysmal cluster with high fluorescence.
Optical coherence tomography (OCT)
OCT is a non-invasive method that can display anatomical cross-sectional information about retina, RPE, and choroid. In early AMD, abnormalities in drusen and RPE can be observed by OCT, which helps doctors to determine the annual signs of disease activity and the risk of progression. In advanced AMD, the activity of disease was monitored by qualitative and quantitative analysis of fluid pools, such as intraluminal cystic fluid, subretinal fluid, and PED, by using OCT.112 Spectral OCT (SD-OCT) has the advantage of sampling information of multiple locations in the optical path at the same time, which improves the speed of diagnosis. It can easily detect and measure the changes in the photoreceptor layer (PRL) on verruca vitreosa.113 Under OCT, the choroid thickness of wet AMD is greater than that of normal human, while that of dry AMD is less than that of normal human.114
Optical coherence tomography angiography (OCTA)
Optical Coherence Tomography Angiography (OCTA) is a new non-invasive imaging technology, which provides non-invasive characterization and quantification of microvascular system under different retinal conditions.115 OCTA can be used to observe chorionic changes in patients with dry AMD, to detect the presence of CNV and PCV. OCTA has a good resolution of CNV structure in patients with wet AMD, and can observe and classify the orientation of CNV from cross-section.116,117 Overall sensitivity of active CNV detection by OCTA was 80.7%.118 Compared with FA, OCTA is less occluded by subretinal hemorrhage.119,120 For PCV, a combination of structural OCT and OCTA can be used to screen for PCV with 82.6% sensitivity and 100% specificity.121 Therefore, OCTA can provide clearer vascular details and better detect and quantify CNV lesions in AMD patients. The superficial and deep vascular density of dry AMD patients was significantly lower than that of healthy people. In patients with wet AMD, significant loss of deep choroid vessels and villi muscle was observed. Among them, CNV patients have reduced chorionic capillary perfusion and deeper choroid.122
Artificial intelligence diagnosis technology
With the development of artificial intelligence (AI) technology, AI has great potential in the diagnosis of ophthalmic diseases. Kermany et al have proved that the classification effect of the model based on the neural network resume on AMD and diabetic macular edema is equivalent to that of human experts.123 Similarly, Treder et al used deep learning to train and verify 1112 SD-OCT images of AMD to distinguish exudative macula and normal macula. The classifier model established has achieved good results (the training accuracy and validation accuracy are 100%, the average score of AMD experimental group and control group are more than 0.9).124 With the development of network technology and the advent of 5G era, AI technology will be constantly optimized. The advantages of AI technology in assisting ophthalmologists to judge the patient's condition and to assist doctors to formulate the optimal treatment plan will also become increasingly prominent. However, the application of AI depends on database, so the establishment of high-quality database is still a challenge for the application of AI in disease diagnosis.
Treatment
Laser and radiotherapy
In 1999, Reichel et al began to treat CNV by transpupillary thermotherapy (TTT).125 Because its therapeutic effect is only to block the existing CNV, it will also produce complications, such as choroidal atrophy. It is easy to recur, so it is seldom used at present. In addition, studies126,127 have shown that TTT combined with other angiogenic drugs, such as triamcinolone acetonide or ranibizumab, can be used as a therapeutic regimen for wet AMD, but its efficacy needs further evaluation. Besides TTT, laser therapy includes photodynamic therapy (PDT). PDT can be combined with ranibizumab. PDT combined with anti-vascular therapy for PCV is an effective method.128 Although laser therapy can inhibit the growth of new blood vessels, it can not ensure that no new blood vessels grow. The laser may also destroy normal tissue around the retina. Therefore, it is necessary and important to improve the existing treatment methods and develop new treatment methods for AMD.
Anti-VEGF treatment
The key to the treatment of exudative AMD is to inhibit the formation of neovascularization,129 and intraocular injection of drugs to inhibit VEGF has become the main treatment for wet AMD.130, 131, 132 Blindness and the density of choroidal capillaries decreases in patients treated with anti-vascular therapy.133,134 The main drugs include pegaptanib, ranibizumab, bevacizumab, afiibercept and conbercept (Table 2). Pegaptanib135,136 and ranibizumab are anti-VEGF monoclonal antibody fragment. Afiibercept is a fusion protein of VEGF binding. It is formed by the second binding of human VEGF receptor (VEGFR) −1 to the third binding domain of human VEGF receptor-2 (VEGFR2), and then binding to the Fc region of human immunoglobulin G-1. Afiibercept can bind to all subtypes of VEGFA and VEGFB. It is a new generation of anti-VEGF after pegaptanib and ranibizumab were approved by the FDA in November 2011. Intravitreal injection of afiibercept two months or monthly after the initial dose of three months was similar safety and tolerance to that of ranibizumab monthly.137,138 Conbercept is composed of the binding domain of human VEGFR-1 and VEGFR-2 and the Fc part of human immunoglobulin G-1. It had a strong anti-angiogenesis effect.139, 140, 141 Intravitreal injection of conbercept was safe and effective and could prevent the growth of lesions and CNV. Brolucizumab is A humanized, single-stranded antibody that targets VEGF and binds to the VEGF-A subtype. It showed good results in phase 3 clinical trials.142 It was approved by the FDA for the treatment of wet AMD. Faricimab is a bispecific antibody drug that can bind and inactivate angiopoietin-2 (Ang-2) and VEGF-A. The effect of faricimab is similar to that of ranibizumab.143 Abicipar pegol is an anti VEGF molecule that uses the designed ankyrin repeat protein (darpin) technology to reduce the level of VEGF. Its half-life in the eye is longer and the effect is more lasting.144 At keast 13 drugs for VEGF related targets are still in developing. Abicipar pegol, Faricimab, TypeOPT-302 and Dorzolamide-timolol are in their phase III clinical trials; RGX-314, GB-102 (Sunitinib), X-82 (Vorolanib), HMR59 (AAVCAGsCD59), CM082 tablets and RG7716 are in their phase II clinical trials; IBI302, ADVM-022 and TAB014 are in their phase I clinical trials. For dry AMD, there is no effective treatment method currently. However, Heavy dietary intake of specific antioxidants such as carotene, vitamin C, D, E, and zinc may have been shown to slow the progression of a few dry AMD patients.145
Table 2.
Current therapeutic drugs for wet AMD.
| Drug | Attribute | Mechanism of action | Date of approval | Note |
|---|---|---|---|---|
| Pegaptanib (Macugen) | Anti-VEGF aptamer | Binding to vascular endothelial growth factor 165 prevents its binding to vascular endothelial growth factor receptor | FDA approved it for wAMD treatment in 2004 | Visual acuity can not be stabilized well after treatment. |
| Ranibizumab | Anti-VEGF monoclonal antibody | Binding with VEGF 165, 121 and 110 | It was approved by FDA in 2006 for DR and in Europe in 2009 for visual impairment caused by wAMD, RVO, DR and CNV. | Therapeutic effect is better than photodynamic therapy. |
| Bvacizumab | Humanized anti-VEGF monoclonal antibody | Inhibition of angiogenesis | FDA approved it as a cancer inhibitor in 2004, but it has not yet been approved for ophthalmic treatment | Target molecule and effect are similar to those of ranibizumab |
| Afiibercept (Eylea) | Humanized anti-VEGF fusion protein | Binding to VEGF-A, VEGF-Band PGF | FDA approval for diabetic macular edema and DR in 2011 | It has the same effect as ranibizumab |
| Conbercept | Anti-VEGF Fusion Protein | Binding to VEGF-A, VEGF-Band PGF | In 2013, it was approved by CFDA for the treatment of wAMD, and was awarded “the most innovative drug with clinical value” in phase III clinic of USA. | Adverse reactions are weak and can be recovered without treatment, which is more economical than ranibizumab |
| Triamcinolone acetonide | Glucocorticoid | Inhibition of angiogenesis, the specific mechanism of action is not yet clear. | On February 4, 2019, the FDA accept the application for new drug triamcinolone acetonide suspension (xipere) | The effect of single use is not significant. |
| Brolucizumab (Beovu, RTH258) | anti-VEGF antibody | binds to the VEGF-A subtype | approved in the United States in 2019 | After one year of treatment, patients treated with BEOVU showed similar visual improvement as patients treated with aflibercept. |
| Abicipar pegol | anti-VEGF molecule | Using DARP in technique to mimic antibody, target VEGF | Not yet approved, phase III completed | |
| Faricimab | Bi-specific antiboy | anti-Ang-2 antibody and anti-VEGF-A antibody | Not yet approved, phase III completed | |
| OPT-302 | A fusion protein composed of the 1–3 of VEGFR-3 and Fc | By binding to VEGF-C or VEGF-D, they block their binding to VEGFR-2 or VEGFR-3 on the surface of endothelial cells in blood vessels or lymphatic vessels. | Phase III clinical trials are planned for 2021 | The effect of combination with ranibizumab is better than that of using alone |
| Dorzolamide-timolol | Carbonic anhydrase inhibitor | Acting by decreasing the production of aqueous humour | phase III clinical trials are underway | Combined with anti-VEGF Drugs to reduce retinal effusion |
| RGX-314 | A gene vector containing anti-VEGF gene | Adenovirus AAV8 carries the monoclonal antibody gene of VEGF and is injected under the retina | Phase II clinical trials are underway. Good results have been achieved in phase I/IIa clinical trials. | A gene therapy method |
| GB-102 (Sunitinib) | multi-target receptor tyrosine kinase inhibitors | VEGF is blocked by inhibiting the VEGF receptor ((VEGFR1, VEGFR2, VEGFR3) | Phase II clinical trials begin in September 2019. | |
| X-82 (Vorolanib) | VEGFR and PDGFR inhibitors | Antiangiogenesis is achieved by inhibiting VEGFR and PCGFR | Phase I clinical trials were completed in December 2019, phase II clinical trials are underway | |
| HMR59 (AAVCAGsCD59) | Adenovirus vector containing e soluble protein CD59 (sCD59) | Making the retinal cells continue to produc (sCD59) soluble proteins to block the final step of the complement cascade | phase II clinical trials are plan for 2020 | |
| CM082 tablets | VEGFR/PDGFR dual inhibitors | Antiangiogenesis is achieved by inhibiting VEGFR and PCGFR | Phase II clinical trials began in 2019 | Oral drug |
| RG7716 | Bi-specific monoclonal antibody | Binds and inactivates VEGF-A and ang-2 | Phase II clinical trials were completed in 2018 and phase III clinical trials are planned | Vitreous injection, it works better in combination with ranibizumab |
| IBI302 | Dual target antibody | Both VEGF and complement are targeted | Phase I clinical trials began in 2019 | |
| ADVM-022 | A gene vector containing anti-VEGF gene | AAV-anti-VEGF therapy method | Phase I clinical trials. are underway, start to collect participants in the second half of 2020 | It's injected into the vitreous instead of under the retina |
| TAB014 | anti-VEGF antibody | Blocking the signal transmission mediated by VEGF can inhibit the growth of new blood vessels | Phase I clinical trials have been completed in January 2020 | Registered as a class 1 new drug in China |
PDGF: platelet-derived growth factor; PGF: placental growth factor; wAMD: wet age-related macular degeneration; DR: diabetic retinopathy; RVO: retinal vein occlusion; CNV: choroidal neovascularization; Ang-2: angiopoietin-2; DARP: designed ankyrin repeat protein; PDGFR: platelet-derived growth factor receptor, VEGFR: vascular endothelial growth factor receptor.
RPE transplantation
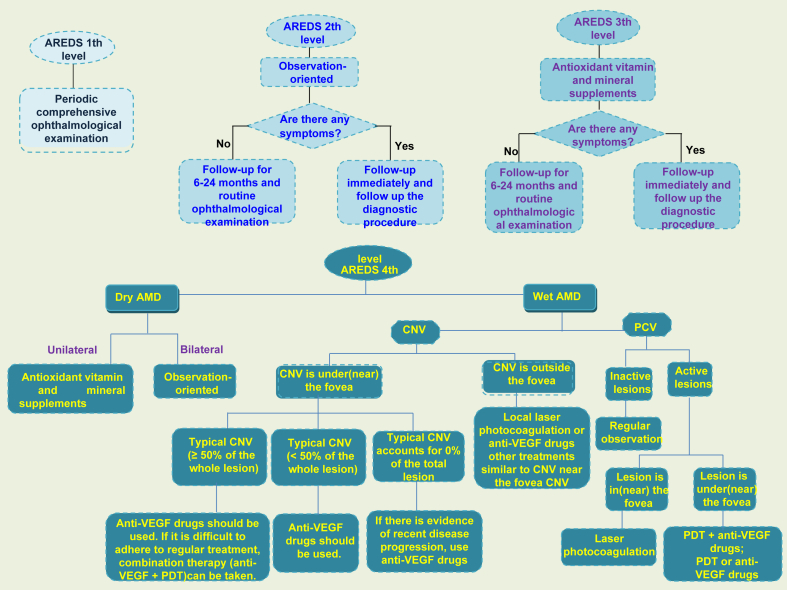
Because AMD is mainly related to damage RPE cells which are easy to cultivate and independent of synaptic connections,146, 147, 148 RPE cells can be transplanted to repair or replace RPE cells in patients. RPE transplantation mainly includes macular translocation, autologous RPE-choroidal transplantation, and RPE cell suspension injection. Macular translocation refers to the displacement of macula on RPE, which can be regarded as functional RPE transplantation. Although it has been proved that the operation can bring long-term visual stability,149 there will be pigment epithelial cell atrophy under the displaced macula,150 and it is prone to complications. The advantage of autologous RPE-choroidal transplantation is that the graft can attach to the choroidal vessels in a short time, and the pigment epithelium usually does not grow excessively. Transplantation remains a major challenge for eye surgeons. Researchers also tried traditional clamp, aspiration reflux tube into the subretinal space to release implants, micro-thermal gripper using heat-induced graft attachment and peeling, and even using customized devices to deliver implants.151,152 RPE cell suspension injection has much fewer complications than macular translocation,153 but the transport of cell suspension is still an issue. Researchers have tried to inject cell suspension154 through scleral choroidal pathway with blunt needle and transport cell suspension155,156 through glass cannula. Although RPE cell transplantation is a way for AMD treatment in the clinic, there are still some problems to be solved urgently, such as the transportation of cell suspension and implant delivery, source of cells, the residual RPE removal, the prevention and control of PVR, and the quality of transplanted cells.157 In addition to RPE transplantation, there are artificial cone transplantation, lower localized macular displacement, 360° macular transposition, and macular transposition combined with 360° retinotomy.158, 159, 160 Doctors often treat patients differently depending on the severity and type of AMD (Fig. 5).
Figure 5.
Suggestions on treatment and follow-up of AMD. This is the treatment strategy for AMD. Routine ophthalmic examination and follow-up were the main methods for early and middle stage AMD patients, while appropriate treatment was adopted for advanced AMD patients according to their characteristics.
Stem cell therapy
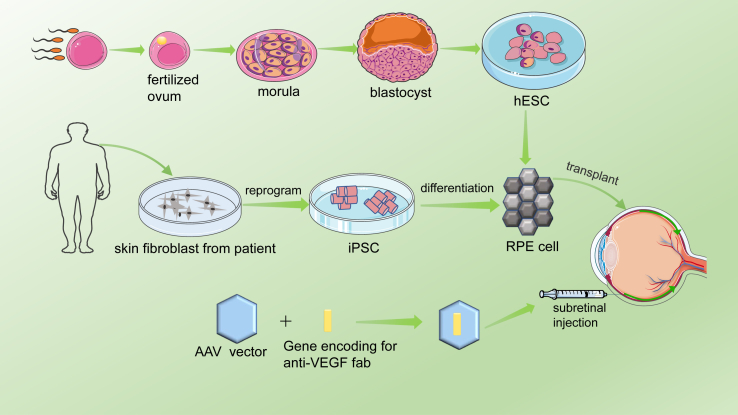
Stem cell therapy is a new potential therapeutic method for dry AMD treatment. In 2012, researchers first reported the transplantation of human embryonic stem cells (hESC) derived RPE cells into elderly patients with dry AMD.161 They successfully transplanted the cultured hESC-RPE cells into the patients, and no pathological conditions such as teratoma, abnormal proliferation of hESC-RPE cells were found. Generally speaking, the patients were well tolerated, and the patients' best-corrected visual acuity was also obtained. Researchers designed a biological patch consisting of RPE monolayer derived from hESC.162 In their clinical trials, the vision of two AMD patients with severe visual impairment was stabilized and improved. Their study demonstrates the feasibility and safety of hESC-RPE patch transplantation as a treatment for dry AMD. One disadvantage of this technique is that local immunosuppression is necessary for hESC-RPE. There is no evidence of adverse proliferation, tumorigenicity or other serious ocular or systemic safety problems associated with transplanted patients. Mandai et al studied the feasibility of transplantation of RPE cells derived from pluripotent stem cells (IPSC) for the treatment of AMD.163 IPSC was generated by skin fibroblasts of patients (Fig. 6). The patients' best corrected visual acuity did not improve or deteriorate, but their VFQ-25 score improved.
Figure 6.
Schematic diagram of cell therapy and gene therapy for AMD. The fertilized ovum was obtained by in vitro fertilization, and the fertilized ovum developed into a morula and then a blastocyst. The inner cell population of the blastula was proliferated in vitro to obtain embryonic stem cells, which were induced to differentiate into RPE cells and transplanted into the patients’ eyes. In addition, skin fibroblasts from the patient were induced to produce pluripotent stem cells, which differentiated into RPE cells and were transplanted into the patients’ eyes. Gene therapy is performed mainly by injecting AAV vector carrying vVEGF-binding protein into the rsubetinal space of patients.
Gene therapy
Gene therapy is also one of the potential therapies for AMD. Because the eye is a small enclosed microenvironment and has a blood-retinal barrier, it can maintain a high concentration for a long time by injecting a small amount of reagent without being affected by the immune system. Gene therapy achieves the goal of treating diseases by introducing genetic material into patients (Fig. 6). In AMD, AAV2 carriers with VEGF binding proteins are used to inhibit angiogenesis. AAV2-sFLT01 and AAV2-sFLT02 are fused into human IgG1 Fc region or its methyl domain and can be used to inhibit CNV.164,165 Studies have shown that AAV2 vector-mediated therapy is safe and effective, and can be used as a potential long-term treatment for wet AMD.166, 167, 168, 169, 170 In addition, viral vectors carrying endostatin and angiostatin also inhibited angiogenesis.171, 172, 173 Thumann et al successfully transfected PEDF gene using Sleeping Beauty (SB100X) transposon subsystem and microplasmid without antibiotic resistance marker (pFAR4),174 and isolated iris pigment epithelium (IPE) cells. Li W et al constructed a target inhibitor CR2-sFlt1, which can significantly reduce the expression of VEGF in RPE cells. The CR2 targeting fragment can locate CR2-sflt1 at the site of complement deposition in CNV region. Their research shows that the targeting inhibitor can inhibit CNV with high selectivity.175 In addition, cytokines are the basis of targeted combination therapy for wet AMD, among which TNF, PDGF or fibroblast growth factor are potential targets for effective combination therapy.176
Outlook
AMD is a complex disease. There are few effective treatments for dry AMD. Healthy lifestyles, such as proper exercise, maintaining a normal weight, control blood pressure, and cholesterol, are important to reduce the risk of AMD.177 No smoking, a high level of lutein/zeaxanthin diet such as vitamin C, vitamin E, zinc oxide, lutein/zeaxanthin can reduce the risk of AMD. Also, fish and green vegetables can also reduce the risk of AMD. Treatment strategies for AMD should incorporate a variety of risk factors to avoid and improve drug interventions, and also need to take into account personalized genetic information. The activation of complement components further promotes the development of AMD, so the local inhibition of complement is considered to be a choice for the prevention and treatment of early AMD. Besides, CFH, CFB, C2, C3 and CFI in complement pathway, HTRA1, lipids metabolism related genes such as CETP, APOE etc. are closely related to AMD and are expected to become therapeutic targets besides VEGF pathway. Emerging therapies are likely to open up new avenues for AMD treatment. With the development of technology and research progress, the emergence of new and more effective AMD treatment methods will improve patients’ visual acuity,178 and improve patients' quality of life.
Author contributions
L.H. designed the study. L.H., Y.D., L.Q., M.D., C.Q., J.L. and L.W. performed the literature review and writing. Y. D. and L.H. wrote the manuscript. All authors critically revised, and provided the final approval for this manuscript.
Conflict of interests
The authors declare no competing financial interests related to this paper.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 81670895 and 81970839 to L.H., 81700841 to J.L.) and the Department of Science and Technology of Sichuan Province, China (No. 21ZDYF0551 to L.H; 2016FZ0091 to Ling Wan).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2021.02.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary materials 1. Interaction between genes. The lake blue lines and the magenta lines represent known interactions, the lake bl ue represents what is obtained from the database, and the magenta represents what has been proved experimentally. The green, red and dark blue lines represent the predicted interaction. The green lines repreents gene neighborhood, the red line r-represents gene fusions, the dark blue lines represent gene co-occurrence. The yellow lines represents textmining, the black lin-es represents co-expression, the purple lines represents protein homology.
References
- 1.Davis M.D., Gangnon R.E., Lee L.Y., et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris F.L., Davis M.D., Clemons T.E., et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim L.S., Mitchell P., Seddon J.M., Holz F.G., Wong T.Y. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 4.Liew G., Joachim N., Mitchell P., Burlutsky G., Wang J.J. Validating the AREDS simplified severity scale of age-related macular degeneration with 5- and 10-year incident data in a population-based sample. Ophthalmology. 2016;123(9):1874–1878. doi: 10.1016/j.ophtha.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Fine S.L., Berger J.W., Maguire M.G., Ho A.C. Age-related macular degeneration. N Engl J Med. 2000;342(7):483–492. doi: 10.1056/NEJM200002173420707. [DOI] [PubMed] [Google Scholar]
- 6.Laude A., Cackett P.D., Vithana E.N., et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29(1):19–29. doi: 10.1016/j.preteyeres.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Varma R., Choudhury F., Chen S., et al. Prevalence of age-related macular degeneration in Chinese American adults: the Chinese American eye study. JAMA Ophthalmol. 2016;134(5):571–577. doi: 10.1001/jamaophthalmol.2016.0588. [DOI] [PubMed] [Google Scholar]
- 8.Yeung L., Kuo C.N., Chao A.N., et al. Angiographic subtypes of polypoidal choroidal vasculopathy in taniwan: a prospective multicenter study. Retina. 2018;38(2):263–271. doi: 10.1097/IAE.0000000000001556. [DOI] [PubMed] [Google Scholar]
- 9.Jonas J.B. Global prevalence of age-related macular degeneration. Lancet Glob Health. 2014;2(2):65–66. doi: 10.1016/S2214-109X(13)70163-3. [DOI] [PubMed] [Google Scholar]
- 10.Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki R., Yasuda M., Song S.J., et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Friedman D.S., O'Colmain B.J., Muñoz B., et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 13.Klein R., Klein B.E., Knudtson M.D., et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Klein R., Chou C.F., Klein B.E., Zhang X., Meuer S.M., Saaddine J.B. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129(1):75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 15.Reibaldi M., Longo A., Pulvirenti A., et al. Geo-Epidemiology of age-related macular degeneration: new clues into the pathogenesis. Am J Ophthalmol. 2016;161:78–93. doi: 10.1016/j.ajo.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Song P., Du Y., Chan K.Y., Theodoratou E., Rudan I. The national and subnational prevalence and burden of age-related macular degeneration in China. J Glob Health. 2017;7(2) doi: 10.7189/jogh.07.020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy U., Elisabeth P., Astrid F., et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10(1) doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastogi N., Smith R.T. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv Ophthalmol. 2016;61(4):422–433. doi: 10.1016/j.survophthal.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Choudhury F., Varma R., McKean-Cowdin R., Klein R., Azen S.P. Risk factors for four-year incidence and progression of age-related macular degeneration: the los angeles latino eye study. Am J Ophthalmol. 2011;152(3):385–395. doi: 10.1016/j.ajo.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer T. The age-related macular degeneration as a vascular disease/part of systemic vasculopathy: contributions to its pathogenesis. Orv Hetil. 2015;156(9):358–365. doi: 10.1556/OH.2015.30017. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan S., Swaminathan G., Kulothungan V., Ganesan S., Sharma T., Raman R. Age-related macular degeneration in a South Indian population, with and without diabetes. Eye (Lond). 2017;31(8):1176–1183. doi: 10.1038/eye.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J.K., Lym Y.L., Moon J.W., Shin H.J., Cho B. Diabetes mellitus and early age-related macular degeneration. Arch Ophthalmol. 2011;129(2):196–199. doi: 10.1001/archophthalmol.2010.355. [DOI] [PubMed] [Google Scholar]
- 23.Klein R., Knudtson M.D., Lee K.E., Klein B.E. Serum cystatin C level, kidney disease markers, and incidence of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2009;127(2):193–199. doi: 10.1001/archophthalmol.2008.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houston T. Smoking and age-related macular degeneration. Am Fam Phys. 2001;63(2):826. [PubMed] [Google Scholar]
- 25.Bhatiwada N., Bangera Sheshappa M., Prabhu P., Baskaran V. Dietary and lifestyle risk factors associated with age-related macular degeneration: a hospital based study. Indian J Ophthalmol. 2013;61(12):722–727. doi: 10.4103/0301-4738.120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chong E.W., Kreis A.J., Wong T.Y., Simpson J.A., Guymer R.H. Alcohol consumption and the risk of age-related macular degeneration: a systematic review and meta-analysis. Am J Ophthalmol. 2008;145(4):707–715. doi: 10.1016/j.ajo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Humphries J.M., Khachik F. Distribution of lutein, zeaxanthin, and related geometrical isomers in fruit, vegetables, wheat, and pasta products. J Agric Food Chem. 2003;51(5):1322–1327. doi: 10.1021/jf026073e. [DOI] [PubMed] [Google Scholar]
- 28.Merle B.M.J., Silver R.E., Rosner B., Seddon J.M. Associations between vitamin D intake and progression to incident advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4569–4578. doi: 10.1167/iovs.17-21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W., Wu Y., Meng Y.F., Xing Q., Tao J.J., Lu J. Fish consumption and age-related macular degeneration incidence: a meta-analysis and systematic review of prospective cohort studies. Nutrients. 2016;8(11):743–756. doi: 10.3390/nu8110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weikel K.A., Chiu C.J., Taylor A. Nutritional modulation of age-related macular degeneration. Mol Aspects Med. 2012;33(4):318–375. doi: 10.1016/j.mam.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swenor B.K., Bressler S., Caulfield L., West S.K. The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmology. 2010;117(12):2395–2401. doi: 10.1016/j.ophtha.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu C.J., Klein R., Milton R.C., Gensler G., Taylor A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009;93(9):1241–1246. doi: 10.1136/bjo.2008.143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merle B.M., Benlian P., Puche N., et al. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol. 2014;55(3):2010–2019. doi: 10.1167/iovs.14-13916. [DOI] [PubMed] [Google Scholar]
- 34.Anastasopoulos E., Haidich A.B., Coleman A.L., et al. Risk factors for age-related macular degeneration in a Greek population: the thessaloniki eye study. Ophthalmic Epidemiol. 2018;25(5–6):457–469. doi: 10.1080/09286586.2018.1512634. [DOI] [PubMed] [Google Scholar]
- 35.Fritsche L.G., Fariss R.N., Stambolian D., Abecasis G.R., Curcio C.A., Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genom Hum Genet. 2014;15:151–171. doi: 10.1146/annurev-genom-090413-025610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein R.J., Zeiss C., Chew E.Y., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haines J.L., MHauser M.A., Schmidt S., et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 38.Edwards A.O., Ritter R., Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 39.Sepp T., Khan J.C., Thurlby D.A., et al. Complement factor H variant Y402H is a major risk determinant for geographic atrophy and choroidal neovascularization in smokers and nonsmokers. Invest Ophthalmol Vis Sci. 2006;47(2):536–540. doi: 10.1167/iovs.05-1143. [DOI] [PubMed] [Google Scholar]
- 40.Despriet D.D., Klaver C.C.W., Witteman J.C.M., et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296(3):301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 41.Kaur L., Katta S., Reddy R.K., et al. The involvement of complement factor B and complement component C2 in an Indian cohort with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51(1):59–63. doi: 10.1167/iovs.09-4135. [DOI] [PubMed] [Google Scholar]
- 42.Johnson L.V., Leitner W.P., Staples M.K., Anderson D.H. Complement activation and inflammatory processes in Drusen formation and age-related macular degeneration. Exp Eye Res. 2001;73(6):887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 43.Nozaki M., Raisler B.J., Sakurai E., et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103(7):2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ennis S., Gibson J., Cree A.J., Collins A., Lotery A.J. Support for the involvement of complement factor I in age-related macular degeneration. Eur J Hum Genet. 2010;18(1):15–16. doi: 10.1038/ejhg.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deangelis M.M., Ji F., Adams S., et al. Alleles in the HtrA serine peptidase 1 gene alter the risk of neovascular age-related macular degeneration. Ophthalmology. 2008;115(7):1209–1215. doi: 10.1016/j.ophtha.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dewan A., Liu M., Hartman S., et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314(5801):989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 47.Fritsche L.G., Loenhardt T., Janssen A., et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat Genet. 2008;40(7):892–896. doi: 10.1038/ng.170. [DOI] [PubMed] [Google Scholar]
- 48.Hughes A.E., Nick O., Chris P., et al. Neovascular age-related macular degeneration risk based on CFH, LOC387715/HTRA1, and smoking. PLoS Med. 2007;4(12) doi: 10.1371/journal.pmed.0040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera A., Fisher S.A., Fritsche L.G., et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt S., Hauser M.A., Scott W.K., et al. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet. 2006;78(5):852–864. doi: 10.1086/503822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seddon J.M., Francis P.J., George S., Schultz D.W., Rosner B., Klein M.L. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297(16):1793–1800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 52.Wang G., Spencer K.L., Scott W.K., et al. Analysis of the indel at the ARMS2 3'UTR in age-related macular degeneration. Hum Genet. 2010;127(5):595–602. doi: 10.1007/s00439-010-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Z., Camp N.J., Sun H., et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 54.Yang Z., Tong Z., Chen Y., et al. Genetic and functional dissection of HTRA1 and LOC387715 in age-related macular degeneration. PLoS Genet. 2010;6(2) doi: 10.1371/journal.pgen.1000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishida B.Y., Bailey K.R., Duncan K.G., et al. Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J Lipid Res. 2004;45(2):263–271. doi: 10.1194/jlr.M300306-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Anderson D.H., Ozaki S., Nealon M., et al. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol. 2001;131(6):767–781. doi: 10.1016/s0002-9394(00)00961-2. [DOI] [PubMed] [Google Scholar]
- 57.Li C.M., Clark M.E., Chimento M.F., Curcio C.A. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Invest Ophthalmol Vis Sci. 2006;47(7):3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- 58.Eiriksdottir G., Aspelund T., Bjarnadottir K., et al. Apolipoprotein E genotype and statins affect CRP levels through independent and different mechanisms: AGES-Reykjavik Study. Atherosclerosis. 2006;186(1):222–224. doi: 10.1016/j.atherosclerosis.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Strunnikova N.V., Maminishkis A., Barb J.J., et al. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet. 2010;19(12):2468–2486. doi: 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gliem M., Müller P.L., Mangolde E., et al. Sorsby fundus dystrophy: novel mutations, novel phenotypic characteristics, and treatment outcomes. Invest Ophthalmol Vis Sci. 2015;56(4):2664–2676. doi: 10.1167/iovs.14-15733. [DOI] [PubMed] [Google Scholar]
- 61.Neale B.M., Fagerness J., Reynoldset R., et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC) Proc Natl Acad Sci USA. 2010;107(16):7395–7400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tserentsoodol N., Gordiyenko N.V., Pascual I., Lee J.W., Fliesler S.J., Rodriguez I.R. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 63.Ennis S., Jomary C., Mullins R., et al. Association between the SERPING1 gene and age-related macular degeneration: a two-stage case-control study. Lancet. 2008;372(9652):1828–1834. doi: 10.1016/S0140-6736(08)61348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang L., Zhang H., Chen C.Y., et al. A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat Genet. 2016;48(6):640–647. doi: 10.1038/ng.3546. [DOI] [PubMed] [Google Scholar]
- 65.Kew R.R., Ghebrehiwet B., Janoff A. Cigarette smoke can activate the alternative pathway of complement in vitro by modifying the third component of complement. J Clin Invest. 1985;75(3):1000–1007. doi: 10.1172/JCI111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esparza-Gordillo J., Soria J.M., Buil A., et al. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56(2):77–82. doi: 10.1007/s00251-004-0660-7. [DOI] [PubMed] [Google Scholar]
- 67.DeAngelis M.M., Ji F., Kim I.K., et al. Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125(1):49–54. doi: 10.1001/archopht.125.1.49. [DOI] [PubMed] [Google Scholar]
- 68.Schaumberg D.A., Hankinson S.E., Guo Q., Rimm E., Hunter D.J. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch Ophthalmol. 2007;125(1):55–62. doi: 10.1001/archopht.125.1.55. [DOI] [PubMed] [Google Scholar]
- 69.Scott W.K., Schmidt S., Hauser M.A., et al. Independent effects of complement factor H Y402H polymorphism and cigarette smoking on risk of age-related macular degeneration. Ophthalmology. 2007;114(6):1151–1156. doi: 10.1016/j.ophtha.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 70.Seddon J.M., George S., Rosner B., Klein M.L. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2006;61(3):157–165. doi: 10.1159/000094141. [DOI] [PubMed] [Google Scholar]
- 71.Sobrin L., Seddon J.M. Nature and nurture- genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. doi: 10.1016/j.preteyeres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J.J., Rochtchina E., Smith W., et al. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol. 2009;169(5):633–641. doi: 10.1093/aje/kwn358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ho L., van Leeuwen R., Witteman J.C., et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol. 2011;129(6):758–766. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 74.Wang J.J., Buitendijk G.H.S., Rochtchina E., et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology. 2014;121(3):667–675. doi: 10.1016/j.ophtha.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 75.Seddon J.M., Gensler G., Rosner B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology. 2010;117(8):1560–1566. doi: 10.1016/j.ophtha.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naj A.C., Scott W.K., Courtenay M.D., et al. Genetic factors in nonsmokers with age-related macular degeneration revealed through genome-wide gene-environment interaction analysis. Ann Hum Genet. 2013;77(3):215–231. doi: 10.1111/ahg.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt S., Haines J.L., Postel E.A., et al. Joint effects of smoking history and APOE genotypes in age-related macular degeneration. Mol Vis. 2005;11:941–949. [PubMed] [Google Scholar]
- 78.Schmidt S., Saunders A.M., De La Paz M.A., et al. Association of the apolipoprotein E gene with age-related macular degeneration: possible effect modification by family history, age, and gender. Mol Vis. 2000;6:287–293. [PubMed] [Google Scholar]
- 79.Del Priore L.V., Kuo Y.H., Tezel T.H. Age-related changes in human RPE cell density and apoptosis proportion in situ. 2002;43(10):3312–3318. [PubMed] [Google Scholar]
- 80.Ciulla T.A. Evolving pathophysiological paradigms for age related macular degeneration. Br J Ophthalmol. 2001;85(5):510–512. doi: 10.1136/bjo.85.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Lookeren Campagne M., LeCouter J., Yaspan B.L., Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232(2):151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 82.Beatty S., Koh H., Phil M., Henson D., Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 83.Khandhadia S., Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expet Rev Mol Med. 2010;12 doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 84.Feeney-Burns L., Hilderbrand E.S., Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25(2):195–200. [PubMed] [Google Scholar]
- 85.Schmitz-Valckenberg S., Fleckenstein M., Scholl H.P., Holz F.G. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol. 2009;54(1):96–117. doi: 10.1016/j.survophthal.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Sui G.Y., Liu G.C., Liu G.Y., et al. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br J Ophthalmol. 2013;97(4):389–394. doi: 10.1136/bjophthalmol-2012-302281. [DOI] [PubMed] [Google Scholar]
- 87.Clemons T.E., Milton R.C., Klein R., Seddon J.M., Ferris F.L. Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology. 2004;112(4):533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hanus J., Anderson C., Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24(PtB):286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyttinen J.M., Petrovski G., Salminen A., Kaarniranta K. 5'-Adenosine monophosphate-activated protein kinase--mammalian target of rapamycin axis as therapeutic target for age-related macular degeneration. Rejuvenation Res. 2011;14(6):651–660. doi: 10.1089/rej.2011.1220. [DOI] [PubMed] [Google Scholar]
- 90.Hyttinen J.M.T., Błasiak J., Niittykoski M., et al. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells-implications for age-related macular degeneration (AMD) Ageing Res Rev. 2017;36:64–77. doi: 10.1016/j.arr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y., Wang M., Zhang X., et al. The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients. 2016;8(10):663–677. doi: 10.3390/nu8100663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sparrow J.R., Fishkin N., Zhou, et al. A2E, a byproduct of the visual cycle. Vis Res. 2003;43(28):2983–2990. doi: 10.1016/s0042-6989(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 93.Zhou J., Jang Y.P., Kim S.R., Sparrow J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2006;103(44):16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seddon J.M., Gensler G., Milton R.C., Klein M.L., Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291(6):704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 95.Lauer N., Mihlan M., Hartmann A., et al. Complement regulation at necrotic cell lesions is impaired by the age-related macular degeneration-associated factor-H His402 risk variant. J Immunol. 2011;187(8):4374–4383. doi: 10.4049/jimmunol.1002488. [DOI] [PubMed] [Google Scholar]
- 96.Clark S.J., McHarg S., Tilakaratna V., Brace N., Bishop P.N. Bruch’s membrane compartmentalizes complement regulation in the eye with implications for therapeutic design in age-related macular degeneration. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.01778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park D.H., Connor K.M., Lambris J.D. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calippe B., Augustin S., Beguier F., et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity. 2017;46(2):261–272. doi: 10.1016/j.immuni.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Campochiaro P.A., Soloway P., Ryan S.J., Miller J.W. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis. 1999;5:34. [PubMed] [Google Scholar]
- 100.Mori F., Konno S., Hikichi T., Yamaguchi Y., Ishiko S., Yoshida A. Pulsatile ocular blood flow study: decreases in exudative age-related macular degeneration. Br J Ophthalmol. 2001;85(5):531–533. doi: 10.1136/bjo.85.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman E., Krupsky S., Lane A.M., et al. Ocular blood flow velocity in age-related macular degeneration. Ophthalmology. 1995;102(4):640–646. doi: 10.1016/s0161-6420(95)30974-8. [DOI] [PubMed] [Google Scholar]
- 102.Friedman E., Ivry M., Ebert E., Glynn R., Gragoudas E., Seddon J. Increased scleral rigidity and age-related macular degeneration. Ophthalmology. 1989;96(1):104–108. doi: 10.1016/s0161-6420(89)32936-8. [DOI] [PubMed] [Google Scholar]
- 103.Tufail A.F., Holz D., Pauleikhoff R.F., Spaide A.C., Bird Age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245(9):1409–1410. doi: 10.1007/s00417-007-0649-7. [DOI] [PubMed] [Google Scholar]
- 104.Bowes Rickman C., Farsiu S., Toth C.A., Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54(14):68–80. doi: 10.1167/iovs.13-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferrington D.A., Sinha D., Kaarniranta K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res. 2016;51:69–89. doi: 10.1016/j.preteyeres.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Golestaneh N., Chu Y., Xiao Y.Y., Stoleru G.L., Theos A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8(1) doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997;124(5):677–682. doi: 10.1016/s0002-9394(14)70906-7. [DOI] [PubMed] [Google Scholar]
- 108.Gelfand B.D., Ambati J.A. Revised hemodynamic theory of age-related macular degeneration. Trends Mol Med. 2016;22(8):656–670. doi: 10.1016/j.molmed.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhatwadekar A.D., Yan Y., Qi X., et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62(1):273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Busik J.V., Tikhonenko M., Bhatwadekar A., et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206(13):2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cassels N.K., Wild J.M., Margrain T.H., Chong V., Acton J.H. The use of microperimetry in assessing visual function in age-related macular degeneration. Surv Ophthalmol. 2018;63(1):40–55. doi: 10.1016/j.survophthal.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Schmidt-Erfurth U., Klimscha S., Waldstein S.M., Bogunovic H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye. 2017;31(1):26–44. doi: 10.1038/eye.2016.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schuman S.G., Koreishi A.F., Farsiu S., Jung S.H., Izatt J.A., Toth C.A. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009;116(3):488–496. doi: 10.1016/j.ophtha.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manjunath V., Goren J., Fujimoto J.G., Duker J.S. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(4):663–668. doi: 10.1016/j.ajo.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cicinelli M.V., Rabiolo A., Sacconi R., et al. Optical coherence tomography angiography in dry age-related macular degeneration. Surv Ophthalmol. 2018;63(2):236–244. doi: 10.1016/j.survophthal.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Boltz A., Luksch A., Wimpissingeret B., et al. Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51(8):4220–4225. doi: 10.1167/iovs.09-4968. [DOI] [PubMed] [Google Scholar]
- 117.Bhutto I.A., Uno K., Merges C., Zhang L., McLeod D.S., Lutty G.A. Reduction of endogenous angiogenesis inhibitors in Bruch's membrane of the submacular region in eyes with age-related macular degeneration. Arch Ophthalmol. 2008;126(5):670–678. doi: 10.1001/archopht.126.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cicinelli M.V., Cavalleri M., Consorte A.C., et al. Swept-source and spectral domain optical coherence tomography angiography versus dye angiography in the measurement of type 1 neovascularization. Retina. 2019;40(3):499–506. doi: 10.1097/IAE.0000000000002452. [DOI] [PubMed] [Google Scholar]
- 119.Moult E., Choi W.J., Waheed N.K., et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imag Ret. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jia Y., Bailey S.T., Wilson D.J., et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheung C.M.G., Yanagi Y., Akiba M., et al. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina. 2019;39(9):1655–1663. doi: 10.1097/IAE.0000000000002228. [DOI] [PubMed] [Google Scholar]
- 122.Ma J., Desai R., Nesper P., Gill M., Fawzi A., Skondra D. Optical coherence tomographic angiography imaging in age-related macular degeneration. Ophthalmol Eye Dis. 2017;9:1–7. doi: 10.1177/1179172116686075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kermany D.S., Goldbaum M., Cai W., et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. 2018;172(5):1122–1131. doi: 10.1016/j.cell.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 124.Treder M., Lauermann J.L., Eter N. Automated detection ofexudative age-related macular degeneration in spectral domain op-tical coherence tomography using deep learning. Graefe ArchClin Exp. 2018;256(2):259–265. doi: 10.1007/s00417-017-3850-3. [DOI] [PubMed] [Google Scholar]
- 125.Reichel E., Berroca A., Michael I., et al. Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age-related macular degeneration. Ophthalmology. 1999;106(10):1908–1914. doi: 10.1016/S0161-6420(99)90400-1. [DOI] [PubMed] [Google Scholar]
- 126.Agnieszka K.T. Transpupillary thermotherapy (TTT) with injections of triamcinolone acetonide under posterior Tenon's capsule in the treatment of exudative age-related macular degeneration (AMD) Klin Oczna. 2010;112(10–12):289–292. [PubMed] [Google Scholar]
- 127.Söderberg A.C., Algvere P.V., Hengstler J.C., Söderberg P., Seregard S., Kvanta A. Combination therapy with low-dose transpupillary thermotherapy and intravitreal ranibizumab for neovascular age-related macular degeneration: a 24-month prospective randomised clinical study. Br J Ophthalmol. 2012;96(5):714–718. doi: 10.1136/bjophthalmol-2011-300721. [DOI] [PubMed] [Google Scholar]
- 128.Kumar Chhablani J. Photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2010;150(5):754–755. doi: 10.1016/j.ajo.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Chappelow A.V., Kaiser P.K. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68(8):1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 130.Hamilton W.S. New treatments for age-related macular degeneration. Lancet. 2007;370(9597):1480–1481. doi: 10.1016/S0140-6736(07)61627-4. [DOI] [PubMed] [Google Scholar]
- 131.Rosenfeld P.J., Brown D.M., Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 132.Brown D.M., Kaiser P.K., Michels M., et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 133.Cheung N., Wong T.Y. Changing trends of blindness: the initial harvest from translational public health and clinical research in ophthalmology. Am J Ophthalmol. 2012;153(2):193–195. doi: 10.1016/j.ajo.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 134.Hikichi T., Agarie M. Reduced vessel density of the choriocapillaris during anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2019;60(4):1088–1095. doi: 10.1167/iovs.18-24522. [DOI] [PubMed] [Google Scholar]
- 135.Ng E.W., Shima D.T., Calias P., Cunningham E.T., Jr., Guyer D.R., Adamis A.P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5(2):123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 136.Gragoudas E.S., Adamis A.P., Cunningham E.T., Jr., Feinsod M., Guyer D.R. VEGF inhibition study in ocular neovascularization clinical trial group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 137.Holash J., DavisS, Papadopoulos N., et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heier J.S., Brown D.M., Chonget V., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 139.Zhang M., Zhang J., Yan M., Li H., Yang C., Yu D. Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis. 2008;14:37–49. [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang M., Yu D., Yang C., et al. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharmaceut Res. 2009;26(1):204–210. doi: 10.1007/s11095-008-9718-9. [DOI] [PubMed] [Google Scholar]
- 141.Li X., Xu G., Wang Y., et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121(9):1740–1747. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 142.Dugel P.U., Adrian Koh F., Ogura Y., et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 143.Khanani A.M., Patel S.S., Ferrone P.J., et al. Efficacy of every four monthly and quarterly dosing of faricimab vs ranibizumab in neovascular age-related macular degeneration the STAIRWAY phase 2 randomized clinical tria. JAMA Ophthalmol. 2020;138(9):964–972. doi: 10.1001/jamaophthalmol.2020.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kunimoto D., Ohji M., Maturi R.K., et al. Evaluation of abicipar pegol (ananti-VEGF DARPIN therapeutic) in patients with neovascular age-relatedmacular degeneration: studies in Japan and the United States. Ophthal Surg Lasers Imag Ret. 2019;50:e10–e12. doi: 10.3928/23258160-20190129-13. [DOI] [PubMed] [Google Scholar]
- 145.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Flood M.T., Gouras P., Kjeldbye H. Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Investig Ophthalmol Vis Sci. 1980;19(11):1309–1320. [PubMed] [Google Scholar]
- 147.Boulton M.E., Marshall J., Mellerio J. Human retinal pigment epithelial cells in tissue culture: a means of studying inherited retinal diseases. Birth Defects Orig Artic Ser. 1982;18(6):101–118. [PubMed] [Google Scholar]
- 148.Hu D.N., Del Monte M.A., Liu S., Maumenee I.H. Morphology, phagocytosis, and vitamin A metabolism of cultured human retinal pigment epithelium. Birth Defects Orig Artic Ser. 1982;18(6):67–79. [PubMed] [Google Scholar]
- 149.Chen F.K., Patel P.J., Uppal G.S., Tufail A., Coffey P.J., Da Cruz L. Long-term outcomes following full macular translocation surgery in neovascular age-related macular degeneration. Br J Ophthalmol. 2010;94(10):1337–1343. doi: 10.1136/bjo.2009.172593. [DOI] [PubMed] [Google Scholar]
- 150.Cahill M.T., Freedman S.F., Toth C.A. Macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2003;121(1):132–133. doi: 10.1001/archopht.121.1.132. [DOI] [PubMed] [Google Scholar]
- 151.Thumann G., Viethen A., Gaebler A., et al. The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials. 2009;30(3):287–294. doi: 10.1016/j.biomaterials.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 152.Stanzel B.V., Liu Z., Brinken R., Braun N., Holz F.G., Eter N. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Invest Ophthalmol Vis Sci. 2012;53(1):490–500. doi: 10.1167/iovs.11-8260. [DOI] [PubMed] [Google Scholar]
- 153.Lappas A., Foerster A.M., Weinberger A.W., Coburger S., Schrage N.F., Kirchhof B. Translocation of iris pigment epithelium in patients with exudative age-related macular degeneration: long-term results. Graefe’s Arch Clin Exp Ophthalmol. 2004;242(8):638–647. doi: 10.1007/s00417-003-0764-z. [DOI] [PubMed] [Google Scholar]
- 154.Li L.X., Turner J.E. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47(6):911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 155.Lopez R., Gouras P., Brittis M., Kjeldbye H. Transplantation of cultured rabbit retinal epithelium to rabbit retina using a closed-eye method. Invest Ophthalmol Vis Sci. 1987;28(7):1131–1137. [PubMed] [Google Scholar]
- 156.Gouras P., Algvere P. Retinal cell transplantation in the macula: new techniques. Vis Res. 1996;36(24):4121–4125. doi: 10.1016/s0042-6989(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 157.Alexander P., Thomson H.A., Luff A.J., Lotery A.J. Retinal pigment epithelium transplantation: concepts, challenges, and future prospects. Eye. 2015;29(8):992–1002. doi: 10.1038/eye.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akyurt A. Cyclotropia and surgical treatment after macular translocation. Klin Monbl Augenheilkd. 2002;219(1–2):50–54. doi: 10.1055/s-2002-23501. [DOI] [PubMed] [Google Scholar]
- 159.Machemer R. Macular translocation. Am J Ophthalmol. 1998;125(5):698–700. doi: 10.1016/s0002-9394(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 160.Polito A., Cereda M., Romanelli F., Pertile G. Macular translocation with 360-degree retinotomy f or management of retinal pigment epithelial tear: long-term results. Br J Ophthalmol. 2011;95(1):74–78. doi: 10.1136/bjo.2009.170381. [DOI] [PubMed] [Google Scholar]
- 161.Schwartz S.D., Hubschman J.P., Heilwell G., et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379(9817):713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 162.Da Cruz L., Fynes K., Georgiadis O., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36(4):328–337. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 163.Mandai M., Watanabe A., Kurimoto Y., et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376(11):1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- 164.Ludwig P.E., Freeman S.C., Janot A.C. Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa. Int J Ret Vitr. 2019;5(1):1–7. doi: 10.1186/s40942-019-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pechan P., Rubin H., Lukason M., et al. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009;16(1):10–16. doi: 10.1038/gt.2008.115. [DOI] [PubMed] [Google Scholar]
- 166.Constable I.J., Pierce C.M., Lai C.M., et al. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heier J.S., Kherani S., Desai S., et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial. Lancet. 2017;390(10089):50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 168.Igarashi T., Miyake K., Masuda I., Takahashi H., Shimada T. Adeno-associated vector (type 8)-mediated expression of soluble Flt-1 efficiently inhibits neovascularization in a murine choroidal neovascularization model. Hum Gene Ther. 2010;21(5):631–637. doi: 10.1089/hum.2009.153. [DOI] [PubMed] [Google Scholar]
- 169.Maclachlan T.K., Lukason M., Collins M., et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rakoczy E.P., Lai C.M., Magno A.L., et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: one year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386(10011):2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 171.Mori Keisuke, Ando Akira, Gehlbach Peter, et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol. 2001;159(1):313–320. doi: 10.1016/S0002-9440(10)61697-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raisler B.J., Berns K.I., Grant M.B., Beliaev D., Hauswirth W.W. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1-3 of angiostatin reduce retinal neovascularization. Proc Natl Acad Sci USA. 2002;99(13):8909–8914. doi: 10.1073/pnas.122247299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai L.J., Xiao X., Wu J.H. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14(3):313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 174.Thumann G.P.-D.M., Izsvak Z. Transposon-based, targeted ex vivo gene therapy to treat age-related macular degeneration (target AMD). Human gene therapy. Clin Develop. 2015;26(2):97–100. doi: 10.1089/humc.2015.2519. [DOI] [PubMed] [Google Scholar]
- 175.Li W., Dong L., Ma M., et al. Preliminary in vitro and in vivo assessment of a new targeted inhibitor for choroidal neovascularization in age-related macular degeneration. Drug Des Dev Ther. 2016;10:3415–3423. doi: 10.2147/DDDT.S115801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.De Oliveira Dias J.R., Rodrigues E.B., Maia M., Magalhães O., Jr., Penha F.M., Farah M.E. Cytokines in neovascular age-related macular degeneration: fundamentals of targeted combination therapy. Br J Ophthalmol. 2011;95(12):1631–1637. doi: 10.1136/bjo.2010.186361. [DOI] [PubMed] [Google Scholar]
- 177.Goodman D.M., Parmet S., Lynm C., Livingston E.H. Age-related macular degeneration. JAMA. 2012;308(16) doi: 10.1001/jama.2012.4091. [DOI] [PubMed] [Google Scholar]
- 178.Kaiser P.K. Emerging therapies for neovascular age-related macular degeneration: drugs in the pipeline. Ophthalmology. 2013;120(5S):S11–S15. doi: 10.1016/j.ophtha.2013.01.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials 1. Interaction between genes. The lake blue lines and the magenta lines represent known interactions, the lake bl ue represents what is obtained from the database, and the magenta represents what has been proved experimentally. The green, red and dark blue lines represent the predicted interaction. The green lines repreents gene neighborhood, the red line r-represents gene fusions, the dark blue lines represent gene co-occurrence. The yellow lines represents textmining, the black lin-es represents co-expression, the purple lines represents protein homology.