Abstract
The goal this review is to clarify the effects of the fat mass and obesity-associated protein (FTO) in lipid metabolism regulation and related underlying mechanisms through the FTO-mediated demethylation of m6A modification. FTO catalyzes the demethylation of m6A to alter the processing, maturation and translation of the mRNAs of lipid-related genes. FTO overexpression in the liver promotes lipogenesis and lipid droplet (LD) enlargement and suppresses CPT-1–mediated fatty acid oxidation via the SREBP1c pathway, promoting excessive lipid storage and nonalcoholic fatty liver diseases (NAFLD). FTO enhances preadipocyte differentiation through the C/EBPβ pathway, and facilitates adipogenesis and fat deposition by altering the alternative splicing of RUNX1T1, the expression of PPARγ and ANGPTL4, and the phosphorylation of PLIN1, whereas it inhibits lipolysis by inhibiting IRX3 expression and the leptin pathway, causing the occurrence and development of obesity. Suppression of the PPARβ/δ and AMPK pathways by FTO-mediated m6A demethylation damages lipid utilization in skeletal muscles, leading to the occurrence of diabetic hyperlipidemia. m6A demethylation by FTO inhibits macrophage lipid influx by downregulating PPARγ protein expression and accelerates cholesterol efflux by phosphorylating AMPK, thereby impeding foam cell formation and atherosclerosis development. In summary, FTO-mediated m6A demethylation modulates the expression of lipid-related genes to regulate lipid metabolism and lipid disorder diseases.
Keywords: Adipose tissue, FTO, Lipid disorder diseases, Lipid metabolism, Liver, Skeletal muscle
Abbreviations
- FTO
the fat amount and obesity-related protein
- SREBP1C
sterol regulatory element binding protein-1 c
- FAS
fatty acid synthase
- SCD
stearoyl-CoA desaturase
- ACC1
acetyl-CoA carboxylase 1
- DGAT
diacylglycerol acyltransferase 2
- CPT1
carnitine palmitoyltransferase 1
- ROS
reactive oxygen species
- CIDEC
cell death-inducing
- DFFA
(DNA fragmentation factor-α)-like effector C
- C/EBPβ
CCAAT/enhancer-binding protein β
- UCP-1
uncoupling protein
- RUNX1T1
Runt-related transcription factor 1
- SRSF2
serine/arginine-rich splicing factors 2
- PPARγ
peroxisome proliferators-activated receptors γ
- CTSB
cathepsin B
- ANGPTL4
angiopoietin–like protein 4
- LPL
lipoprotein lipase
- IRX3
Iroquois–class homeobox protein
- CUX1
cut–like homeobox 1
- RPGRIP1L
regulatory factor interaction protein-1-like.
Introduction
The prevalence of lipid disorders in the Western world has become increasingly alarming,1 and up to 58% of the adult population worldwide is predicted to be overweight or obese by 2030.2 Accumulating evidence indicates that obesity is closely associated with an increased risk of lipid disorder diseases such as NAFLD, type 2 diabetes mellitus (T2DM), dyslipidemia and atherosclerosis.1,3 The results of recent studies have shown that the N6 methyladenosine (m6A) modification is implicated in lipid metabolism and the progression of lipid disorders in the human body.4 As the most abundant epigenetic modification of eukaryotic messenger RNA (mRNA), m6A plays key roles in modulating of substrate mRNA metabolism, including that of some lipid-related genes.5
The fat mass and obesity-associated gene (FTO) was first discovered in a genome-wide association study (GWAS) of T2DM patients in Europe.6 The discovery of FTO as the first m6A mRNA demethylase established the concept of reversible RNA modifications.7 FTO is the primary m6A eraser that belongs to the ALKB family of Fe(II)/α-ketoglutarate–dependent dioxygenases, which preferentially bind to pre-mRNA strands in intronic regions, in the proximity of alternatively spliced exons and poly(A) sites.8 FTO has powerful demethylase activity toward multiple methylated RNA substrates, which affects mRNA splicing, stability, decay and translation. FTO is primarily localized in the nucleus and cytoplasm9 and controls the expression of lipid-related genes to regulate multiple processes associated with lipid metabolism.10, 11, 12
The tissue expression profile of FTO showed that it is distributed in adult and fetal livers, adipose tissue, the hypothalamus, islets, skeletal muscle and macrophages.13 The expression of hepatic FTO is regulated by metabolic signals, such as nutrients and hormones, which affect the metabolism of glucose and lipids in the liver. FTO overexpression was shown to lead a dose-dependent increase in body weight and fat mass, regardless of whether mice were fed a standard or high-fat diet.14 FTO was observed to alter changed the expression of genes related to glucose metabolism, resulting in the development of hyperlipidemia in T2DM patients.15 However, the regulatory roles and underlying mechanism of FTO in lipid metabolism are not well understood, which hinders the discovery of effective tactics to control FTO. Thus, additional investigations are also required to evaluate the role FTO in lipid disorders to prevent or treat certain lipid disorder diseases.
Due to the increasing importance of the demethylase FTO in lipid metabolism, we first summarized general information on FTO, including the gene locus, protein structure and catalytic activity before discussing the role of FTO in lipid synthesis and decomposition in the liver. Subsequently, we discuss the effect of FTO on the differentiation, transformation and lipid metabolism of adipose tissue. Finally, we summarize the role of FTO regulation in the occurrence of diabetic hyperlipidemia and macrophage lipid flux. The goal of this review is to clarify the exact roles and underlying mechanisms of the demethylase FTO in lipid metabolism to better understand and evaluate the FTO-driven pathogenesis of lipid disorder diseases.
FTO biology
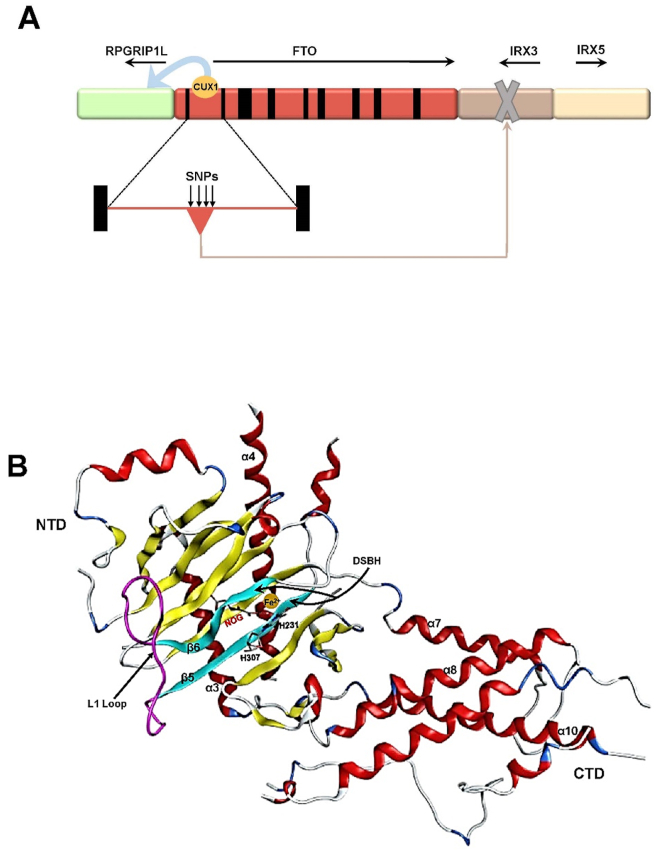
The FTO gene was initially confirmed as an obesity-susceptibility gene that predispose individuals to obesity by independent GWASs in obese cohorts of Belgian Caucasian and European American individuals.16,17 The mouse FTO gene is located on chromosome 8, while the human FTO gene is located on 16q12.2. Human FTO is approximately 400 kb in length, comprises 8 introns and 9 exons and encodes multiple protein products. The FTO gene is highly homologous among mammalian species such as mice, pigs and other mammals.18 To date, only a few SNPs have been identified in the first intron of the FTO gene.19 The approximately 3.4-kb region upstream of the human FTO gene contains a transcriptional initiation site for the retinitis pigmentosa GTPase regulator interacting protein 1-like (RPGRIP1L) gene. The first intron of FTO harbors a binding site for the transcription factor cut-like homeobox 1 (CUX1), which promotes the expression of RPGRIP1L after binding to this site.20 The region downstream of the FTO gene is adjacent to IRX3, IRX5 and IRX6 which belong to the Iroquois gene family (Fig. 1A). The first intron of the FTO gene possesses an enhancer sequence for the IRX3 gene, which binds to the promoter of IRX3 to boost its expression.19,20
Figure 1.
The structure of human FTO. (A) Human FTO is approximately 400 kb in length, comprises 8 introns and 9 exons and encodes multiple protein products. The approximately 3.4-kb region upstream of the human FTO gene contains a transcriptional initiation site for the RPGRIP1L gene. The first intron of FTO harbors a binding site for the transcription factor CUX1 which promotes the expression of RPGRIP1L after binding to this site. The region downstream of the FTO gene is adjacent to IRX3 and IRX5 which belong to the Iroquois gene family. The first intron of the FTO gene possesses an enhancer sequence for the IRX3 gene, which binds to the promoter of IRX3 to promote its expression. (B) The conformation of FTO protein. The FTO protein contains an NTD and a CTD with a novel fold. The catalytic core of NTD consists of a DSBH with the highly conserved residues: His231, Asp233 and His307, which are coordinated with Fe(II), and Arg316 and Arg322, which form a salt bridge with NOG. One side of the DSBH motif is buttressed by two α-helices α3 and α4, whereas the other side is covered by a long loop linking β5 and β6. The CTD contains approximately 170 residues and primarily forms α-helixes, of which α7, α8 and α10 form a triple helical bundle.
The FTO protein is a member of the non-heme dioxygenase [Fe(II)- and 2-oxoglutarate-dependent dioxygenases] superfamily.21,22 The FTO protein contains an amino-terminal AlkB-like domain (also referred to as amino–terminal domain; NTD) and a carboxy–terminal domain (CTD) with novel fold (Fig. 1B). The conserved sequence of the AlkB-like domain is located at amino acid positions 57–324 of the N-terminus of the FTO protein. The catalytic core of NTD consists of a double–stranded β-helix motif (DSBH; also referred to as the jelly roll motif) with the highly conserved residues His231, Asp233 and His307 coordinated with Fe(II), and Arg316 and Arg322 forming a salt bond with N-oxalylglycine (NOG). One side of the DSBH motif is buttressed by two α-helices (α3 and α4), while the other side is covered by a long loop (called an L1 ring) linking β5 and β6. The interaction of the L1 loop with the jelly-roll motif primarily occurs through Trp230-mediated hydrophobic contacts. Moreover, the L1 loop completely covers this region and makes it less positively charged. Hydrophobic interactions also stem from the packing of Leu109 and Val228 against the sugar ring of 3-methylthymidine (3-meT) in the single–stranded DNA, indicating that FTO has a strong preference for 3-meT or 3-methyluracil (3-meU) in single–stranded DNA. The 31-residue truncated N-terminal region of FTO (FTOΔ31) was shown to bind to the single nucleotide 3-meT to participate in substrate sorting, and NOG was used for the formation of a catalytically inert FTO-substrate complex. The CTD of the FTO protein does not possess any known structure that is significantly homologous to the other members of the non-heme dioxygenase superfamily and represents a new fold. The CTD contains approximately 170 residues and primarily forms α-helixes, of which α7, α8 and α10 form a triple helical bundle. One end of the helical bundle has extensive interactions with the NTD, suggesting that the CTD primarily plays an important role in stabilizing the conformation of the NTD.23 Therefore, the catalytic activity of FTO is achieved by the interaction between the NTD and CTD.22
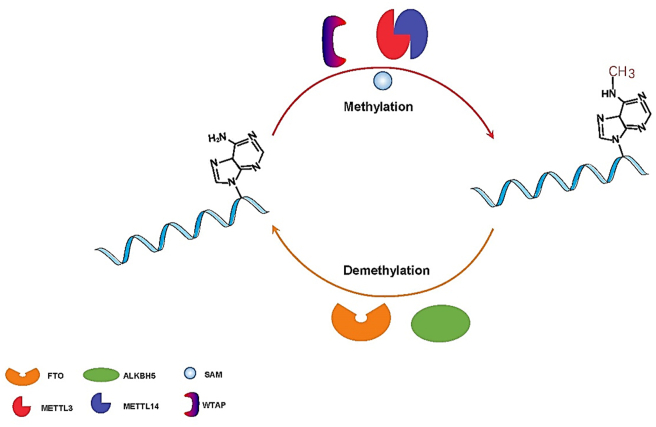
FTO, the first identified RNA m6A eraser, has been shown to have strong catalytic activities toward multiple RNA methylation substrates, including m6Am and m6A at the 5’ cap of mRNAs and snRNAs.24 The RNA m6A modification involves the catalytic transfer of methyl groups from reactive methyl compounds (such as S-adenosyl-l-methionine) onto the sixth position nitrogen atom (N) of adenine (A) on the RNA strand by the m6A writers METTL3, METTL14 and WTAP.25 RNA m6A modification can be reversibly removed by the m6A erasers FTO and ALKHB5 from the methylated mRNA strand (Fig. 2). As an m6A erasers, FTO reversibly catalyzes the demethylation of m6A. First, FTO oxidizes m6A to the intermediate N6-hydroxymethyl adenosine (hm6 A) by intermediate modification of ferrous ions and 2-oxoglutarate-dependent dioxygenase. Second, the FTO protein further oxidizes metastable hm6A in the same manner to form N6-formyladenosine (f6A). It is possible that additional protein factors are involved in the FTO-mediated demethylation of m6A by catalyzing the hydrolysis of hm6A and f6A. Finally, hm6A and f6A naturally decompose into adenine (A) in aqueous solution.26 After the m6A modification is removed by FTO, the original sites of m6A modification cannot be recognized by “reader” proteins (such as YTH or non-YTH proteins), and some RNA binding proteins cannot interact with these sites, which affects the splicing, maturation, translation or degradation of target RNAs.
Figure 2.
The reversible processes of methylation and demethylation of m6A modification. METTL3, METTL 4 and WTAP act as methylases to transfer the methyl-group onto the sixth carbon atom of substrate mRNA from the reactive methyl compound SAM. The demethylase FTO and ALKBH5 erase the m6A modification from the methylated mRNA strand.
In vivo and in vitro studies have shown that m6A is closely associated with lipid metabolism. For instance the m6A modification was shown to be increased in patients with fatty livers.9 In addition, an increase in m6A levels was confirmed to cause obesity in both children and adult populations.22 These findings suggest that the abnormal expression and function of FTO is implicated in the development of lipid disorder diseases. An analysis of FTO protein expression profile showed that it is primarily distributed in the liver, hypothalamus, pancreas and adipose tissue, but little is currently known regarding the regulation and underlying mechanism of FTO in lipid metabolism.27 Therefore, our review primarily focuses on the role and regulatory mechanism of FTO in lipid metabolism and related lipid disorder diseases in these tissues.
FTO and lipid metabolism in the liver
FTO and FTO-dependent m6A demethylation intimately participate in various aspects of hepatic lipid metabolism. The expression and function of FTO is modulated in response to alterations in hepatic lipid metabolism.28 Significant increases in FTO mRNA and protein levels has been observed in the livers of in NAFLD patients,29 where FTO expression was shown to be significantly increased and large amounts of fat were accumulated in the liver in NASH patients. In vitro FTO depletion reduces palmitic acid-induced lipotoxicity in HepG2 cells by alleviating endoplasmic reticulum (ER) stress and restoring mitochondrial function.30 FTO overexpression leads to decreased m6A leves and contributes to fat deposition in HepG2 cells.31 Furthermore, FTO overexpression causes triacylglycerol (TG) accumulation and the upregulation of lipogenic genes in human hepatocytes, aligning with the abnormal characteristics of lipid metabolism observed in the context of NAFLD.29,31 Interestingly, a mutant FTO lacking demethylase activity fails to induce lipid deposition in HepG2 cells.31 These findings support that aberrant levels of FTO expression and FTO-catalyzed m6A demethylation results in excessive lipid deposition in hepatocytes.
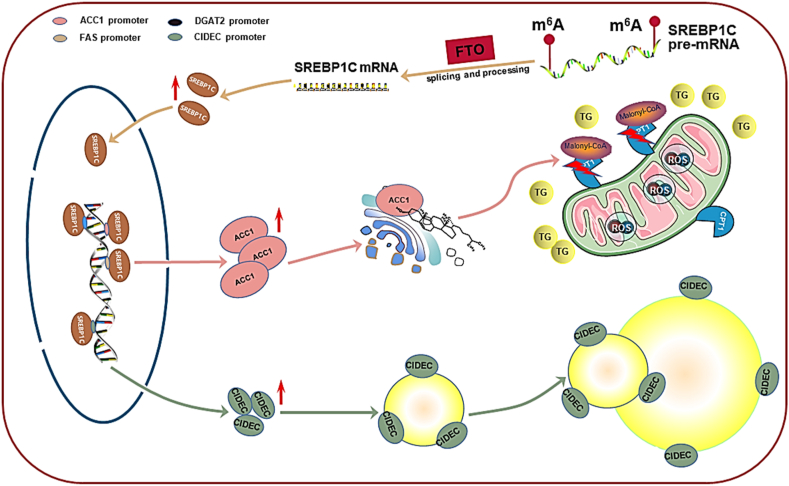
FTO enhances hepatocellular lipid synthesis and lipid droplet (LD) enlargement through the sterol regulatory element binding protein-1c (SREBP1c) pathway (Fig. 3). FTO-dependent m6A demethylation promotes the expression of these key genes including SREBP cleavage-activating protein (SCAP), site-1 proteases (S1P), and site-2 proteases (S2P), which are involved in the processing and nuclear translocation of SREBP1c mRNA in HepG2 cells, and indirectly elevates SREBP1c expression. SREBP1c is a key transcription factor that initiates the transcription of downstream genes involved in lipid synthesis. SREBP1c overexpression was reported to facilitate the development of extensive hepatic steatosis in mice.32 Mature SREBP1c binds to the promoter and increases the expression of target lipogenic genes, including fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD), acetyl-CoA carboxylase 1 (ACC1) and diacylglycerol acyltransferase 2 (DGAT2).33 FAS and SCD1 are known to participate in the de novo synthesis, esterification and desaturation of fatty acids, and DGAT2 catalyzes the final step in TG generation, greatly accelerating hepatic lipid synthesis.31 SREBP1c also binds with the promoter sequence of the cell death-inducing DFFA (DNA fragmentation factor α)-like effector C (CIDEC) gene to enhance its transcription.34 CIDEC often localizes on the surface of LDs and accelerates LD enlargement. The CIDEC-mediated LD growth process requires a net directional transfer of lipids from smaller to larger LDs at LD–LD contact sites (LDCSs), which is conducive to excessive lipid storage in HepG2 cells.28
Figure 3.
The roles and underlying mechanisms of FTO in hepatocellular lipid metabolism. FTO-dependent m6A demethylation promotes the mRNA processing, translation and nuclear translocation of SREBP1C. SREBP1C binds to the promoters of lipogenic genes, including FAS, SCD, ACC1 and DGAT, to enhance their expression and facilitate lipid synthesis in the liver. SREBP1c-upregulated ACC1 catalyzes the synthesis of malonyl COA which inactivates CPT1 activity and suppresses CPT1-mediated β-oxidation of long-chain fatty acids. The failure of fatty acid oxidation in mitochondria triggers oxidative stress and leads to high ROS generation in hepatocytes, which further aggravates mitochondrial dysfunction and hinders TG decomposition. SREBP1C also promotes the transcription and expression of CIDEC which often resides on the surface of LDs to accelerate the expansion of hepatocellular LDs.
In addition, FTO overexpression damages hepatocellular mitochondria and inhibits the decomposition of lipids, resulting in lipid accumulation in the liver (Fig. 3). FTO-upregulated SREBP1c increases ACC1 expression, which subsequently inhibits carnitine palmitoyltransferase 1 (CPT-1)-mediated fatty acid oxidation. ACC1 catalyzes the synthesis of malonyl coenzyme A, which is an allosteric inhibitor of CPT1. CPT1 is a rate limiting enzyme localized onto the outer mitochondrial membrane and conveys long chain fatty acids into the mitochondrial compartment for β-oxidation. The failure of mitochondrial fatty acid β-oxidation induces the generation of reactive oxygen species (ROS) under CPT1 deficiency, which triggers oxidative stress in hepatocytes.35 FTO overexpression was confirmed to lead to the accumulation of fatty acids and increased levels of oxidative stress in HepG2 cells.31 Interestingly, the levels of the oxidative stress biomarker malondialdehyde (MAD) were shown to be dramatically increased, while those of the antioxidant enzyme superoxide dismutase (SOD) were significantly inhibited in the context of hepatocellular overexpression of FTO in humans.35 Oxidative stress further exacerbates mitochondrial dysfunction and hinders the decomposition of TG. Moreover, FTO overexpression has been shown to reduce the biogenesis and quantity of hepatocellular mitochondria by upregulating specific genes implicated in mitochondrial fusion while downregulating that of genes involved in mitochondrial biogenesis and fission, ultimately weakening mitochondrial function and impeding hepatic lipid decomposition.31
FTO and lipid metabolism in adipose tissue
FTO is closely associated with adipogenesis and increased fat mass. The results of an epidemiological investigation demonstrated that FTO mRNA levels in adipose tissue are positively correlated with body mass index (BMI).36 FTO mRNA levels were higher in the adipose tissue of obese individuals than in that of subjects with normal BMI.37 In C57BL/6N mice, FTO deficiency was shown to cause a significant reduction in body weight and fat mass, especially a marked decrease in white but not brown adipose tissue. FTO deficiency was also observed to promote the transformation of white adipocytes into brown or beige adipocytes.38 Impaired FTO expression in the hypothalamus has been demonstrated to result in a decrease in overall energy consumption in obese populations.39,40 In another study, the systemic knockout or a limited inhibition of hypothalamic FTO led to a lean phenotype in C57BL/6N mice.41 These findings indicate that FTO ubiquitously participates in fat metabolism and accelerates adipogenesis.
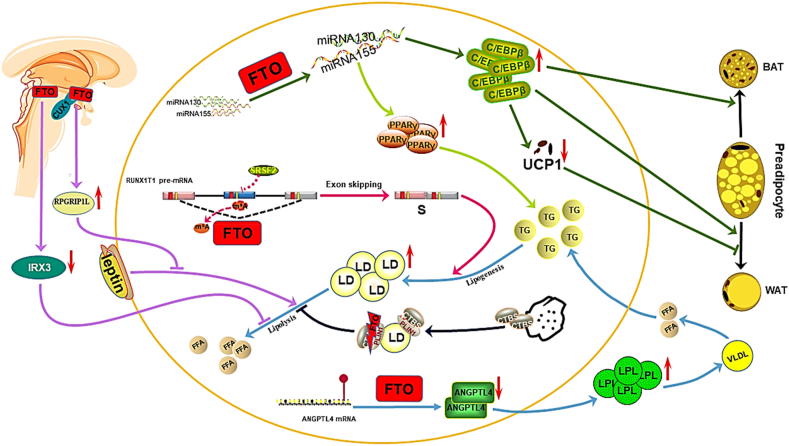
FTO-dependent m6A demethylation promotes the differentiation and transformation of adipose tissue (Fig. 4).42 The early differentiation of preadipocytes into white or brown adipocytes is promoted by CCAAT/enhancer–binding protein β (C/EBPβ), which is typically suppressed by miRNA130 and miRNA155. FTO was shown to downregulate the expression of miRNA130 and miRNA155 expression via m6A demethylation, resulting in an upregulation of C/EBPβ in 3T3-L1 preadipocytes and enhanced preadipocyte differentiation. FTO inhibits the expression of uncoupling protein (UCP)-1 in brown adipocytes, inhibiting the transformation of adipose tissue into brown adipose tissue. C/EBPβ was also observed to reduce adipocyte UCP-1 expression and inhibit the UCP-1-mediated transformation to the brown adipocyte phenotype.43 In another study, FTO was shown to upregulated C/EBPβ levels and inhibit the differentiation of adipose tissue into brown adipose tissue, which was proven to heighten the susceptibility of individuals to being obese and overweight by inhibiting the browning of white adipose tissue.44
Figure 4.
The roles and underlying mechanisms of FTO in lipid metabolism in adipose tissue. FTO-dependent m6A demethylation downregulates the expression of miRNA130 and miRNA155, causing the upregulation of C/EBPβ and the induced differentiation of preadipocytes. FTO-upregulated C/EBPβ expression reduces UCP-1 expression and inhibits the yield of brown adipocytes. FTO-dependent m6A demethylation alters the alternative splicing of RUNX1T1 and increases the production of the spliceosome subtype, which stimulates adipogenesis in preadipocytes and increases body fat mass. FTO-downregulated miRNA130 expression enhances PPARγ expression and adipogenesis in preadipocytes. FTO disrupts the binding of CTSB with LDs and inhibits phosphorylation of plin1 by CTSB, resulting in the failure of TG hydrolysis in preadipocytes. FTO-dependent m6A demethylation reduces ANGPTL4 protein levels, accelerating LPL release and extracellular TG hydrolysis which provides the material for the synthesis of TGs and LDs in adipocytes. The long-range enhancer of the FTO gene downregulates hypothalamic IRX3 and inhibits lipolysis in peripheral adipocytes. In specific subsets of hypothalamic neurons, the binding site in FTO intron 1 interacts with the transcription factor CUX1 to increase RPGRIPLl expression, which reduces leptin signal transduction and leptin-induced lipolysis in adipocytes.
FTO-dependent m6A demethylation promotes lipogenesis and fat deposition in adipose tissue. FTO controls the exogenous splicing of Runt–related transcription factor 1 (RUNX1T1) by reducing the number of m6A modifications around the splicing sites and reducing the binding affinity of the RUNX1T1 RNA strand with the splicing factor serine/arginine–rich splicing factor 2 (SRSF2). RUNX1T1 is an adipogenesis-related transcription factor that has two splice variants, including long (L) and short (S) isoforms. FTO increases the S isoform levels of RUNX1T1 and stimulates lipogenesis in 3T3-L1 preadipocytes, resulting in an increase in fat mass.45 FTO-dependent demethylation suppresses miRNA130 expression, increasing the level of peroxisome proliferator-activated receptor γ (PPARγ) and fat production in 3T3-L1 preadipocytes, with PPARγ being a key effector of miRNA130 that supports lipogenesis.46 FTO augments PLIN1 expression by inhibiting cathepsin B (CTSB)-mediated phosphorylation. CTSB is secreted by the lysosome and contacts the PLIN1 protein on the surface of LDs. PLIN1 localizes to the surface of mature LDs and promotes the accumulation and fusion of LDs in adipocytes. FTO interrupts the binding of CTSB with LDs and inhibits PLIN1 phosphorylation by CTSB, resulting in an inability of TG in LDs to be hydrolyzed in 3T3-L1 preadipocytes.47 FTO binds to angiopoietin–like protein 4 (ANGPTL4) mRNA in a m6A modification-mediated manner and reduces ANGPTL4 protein levels.48,49 ANGPTL4 is an endocrine signal in white adipose tissue that is involved in lipid metabolism, and FTO promotes lipoprotein lipase (LPL) expression by reducing the level of ANGPTL4, resulting in the hydrolysis of extracellular TG.50 Subsequently, free fatty acids enter adipocytes and become adipogenic substrates for the synthesis of TG and LD,49 which promotes the accumulation of intracellular lipids. Therefore, plin1 promotes fat deposition by distributing excessive fatty acids to the TG library.51
FTO inhibits the lipolysis of adipocytes (Fig. 4) and downregulates the expression of UCP-1, leading a decreased mitochondrial uncoupling and lipolysis in 3T3-L1 preadipocytes.43 The mediobasal hypothalamus is an important brain region involved in homeostatic control of caloric intake and lipolysis.52 Iroquois–class homeobox protein 3 (IRX3), which belongs to the homologous frame gene family Iroquois, is highly expressed in the hypothalamus and inhibits the occurrence of obesity.41 The long-range enhancer of the FTO gene downregulates hypothalamic IRX3 levels and inhibits lipolysis in peripheral adipocytes.53 Inhibition of hypothalamic IRX3 was shown to reduce thermogenesis and increase body weight and white adipose tissue (WAT) mass in mice and humans.41 Specific subsets of hypothalamic neurons also inhibit lipolysis by regulating FTO expression.54 In vitro and in vivo experimental results suggest that the binding site of FTO intron 1 interacts with the transcription factor CUX1, upregulating the adjacent RPGRIP1L to inhibit leptin receptor transport and leptin signaling.18,55,56 RPGRIP1L is located >100 bp in the opposite transcriptional orientation of FTO and is involved in anchoring the complex to promote leptin receptor aggregation to control food intake and regulate leptin sensitivity at the cell level.55 The increased activity of FTO in the hypothalamus results in a progressive resistance to leptin in the hypothalamus, resulting in increased caloric intake and reduced steatolysis.57 Thus, CUX1 upregulates RPGRIP1L by binding to FTO intron 1, thereby reducing leptin sensitivity and lipolysis, and promoting the occurrence and development of obesity.
FTO and lipid metabolism in other tissues
FTO participates in lipid disorders in diabetic patients and lipid metabolism in skeletal muscle and macrophages. The FTO locus was shown to be closely involved in hyperlipidemia in patients with T2DM in a GWAS.58,59 FTO expression has been observed to be elevated and accompanied by increased VLDL-TG secretion from the liver to the plasma in T2DM patients, causing mild to moderate hypertriglyceridemia.60 In another study, FTO mRNA and protein levels were shown to be significantly higher in the lateral femur in T2DM patients than in normal weight individuals.35 FTO overexpression leads to excessive lipid storage in the skeletal muscles of T2DM patients.61 In addition, FTO has been confirmed to inhibit macrophage uptake of extracellular lipid and facilitate intracellular lipid efflux, suppressing macrophage lipid accumulation and foam cell formation.62
FTO overexpression leads to the dysfunction of islet cells, the inhibition of insulin secretion and the occurrence of diabetic hyperlipidemia. FTO overexpression was shown to cause a cascade of ROS production in C57BL/6 mice, activating the NF-κB pathway and triggering the inflammatory response and ER stress in pancreatic β cells,63 reducing insulin secretion.35 FTO overexpression was observed to delay the dephosphorylation of cAMP response element binding protein (CREB) and repress the CREB signaling pathway, resulting in an increase in hepatic secretion of VLDL-TG into the circulation in diabetic patients.64 The CREB pathway promotes the expression of fatty acid oxidation–related genes and accelerates the hydrolysis and clearance of circular VLDL-TG. FTO inhibits the CREB pathway, increases VLDL-TG secretion from the liver and postpones VLDL-TG clearance, eventually causing hypertriglyceridemia in T2DM patients. Furthermore, the decrease in insulin secretion contributes to disturbances in lipid metabolism in the periphery, especially in skeletal muscles. FTO was shown to impair lipid uptake in skeletal muscles by suppressing PPARβ/δ expression.65 In another study, FTO-dependent m6A demethylation was shown to inhibit inhibited lipid utilization by skeletal muscles by inhibiting the adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) pathway.66
Skeletal muscles are the primary peripheral organs associated with lipid utilization under physiological conditions. When insulin deficiency or resistance occurs, there are obstacles to skeletal muscles taking up and utilizing plasma lipids. PPARβ/δ overexpression was shown to enhance lipid uptake in skeletal muscles in mouse myogenic C2C12 cells.65 Interestingly, FTO inhibited the expression of PPARβ/δ, leading to impaired lipid uptake and metabolism in skeletal muscle and the occurrence of hyperlipidemia. AMPK is considered to be a key energy sensor and plays a crucial role in lipid metabolism in skeletal muscles, and FTO-dependent m6A demethylation has been demonstrated to be closely involved in these regulatory processes. AMPK normally prevents excessive lipid storage in skeletal muscle fibers by upregulating the m6A methylation of FTO mRNA.66,67 During insulin deficiency, AMPK activity is evidently inhibited, which contributes to the upregulation of FTO expression, and leading to the suppression of adipose TG lipase (ATGL), hormone-sensitive triglyceride lipase (HSL) and PGC1α expression C2C12 cells. ATGL and HSL are two key enzymes in lipolysis and lipid utilization in skeletal muscles.68 PGC1α promotes mitochondrial biosynthesis, promoting lipid oxidation in skeletal muscles.69 FTO expression also inhibits the CPT1-and CPT2-mediated transfer of fatty acids from the cytoplasm to the mitochondrial matrix for β-oxidation in C2C12 cells.70 The downregulation of these lipid oxidation-related factors severely disrupts lipid utilization in skeletal muscle. In addition, the increase in FTO expression promotes the expression of C/EBPα and lipid synthesis in skeletal muscle when AMPK activity is inhibited.
FTO inhibits macrophage lipid uptake and accelerates cholesterol efflux. The scavenger receptor CD36 is the primary transporter that mediating the uptake of extracellular lipids by macrophage and is directly targeted by PPARγ. FTO-dependent m6A demethylation has been observed to decrease PPARγ protein expression, resulting in the downregulation of CD36 expression and a decrease in lipid uptake in RAW264.7 cells. FTO overexpression was shown to decrease CD36 mRNA levels and dramatically lessen the rate of lipid uptake by mouse peritoneal macrophages.71 In addition, FTO promotes AMPK phosphorylation and upregulated ATP-binding cassette transporter A1 (ABCA1) in mouse macrophages. ABCA1 consumes ATP to mediate intracellular cholesterol efflux, which strongly prevents excessive lipid accumulation in macrophages.72 Decreased AMPK activity was shown to block FTO-induced ABCA1 upregulation.71,73,74 Therefore, FTO increasesABCA1 expression in an AMPK activity-dependent manner. inhibiting lipid ingestion and enhancing cholesterol efflux, suppressing foam cell formation and atherosclerosis development.
Conclusion and prospective
As an m6A eraser, FTO catalyzes the demethylation of m6A and abrogates its subsequent binding to with reader proteins, which alters the splicing, maturation, translation or degradation of substrate mRNAs, including those of lipid-related genes. FTO overexpression promotes lipogenesis and lipid storage and suppresses lipolysis via the SREBP1c pathway, promoting the accumulation of excessive lipids in the liver. FTO enhances preadipocyte differentiation through the C/EBPβ pathway. Moreover, FTO not only facilitates adipogenesis and LD formation, but also inhibits lipolysis in adipose tissue. FTO upregulation reduces insulin secretion via the inflammatory NF-κB pathway and disrupts lipid utilization in skeletal muscles by suppressing the PPARβ/δ and AMPK pathways, leading to the occurrence of diabetic hyperlipidemia. FTO-catalyzed m6A demethylation inhibits macrophage lipid influx by downregulating PPARγ, and accelerates cholesterol efflux through the phosphorylation of AMPK, thereby impeding foam cell formation and atherogenesis (Table 1). These findings suggest that FTO-catalyzed m6A demethylation controls the expression of lipid-related genes, participating in lipid metabolism and lipid disorder diseases.
Table 1.
Summary of the role of FTO in lipid metabolism.
| FTO | Tissue/Cell | m6A of substrate RNA | expression ofsubstrate mRNA | Effectors | Lipid metabolism | Reference |
|---|---|---|---|---|---|---|
| ↑ | HepG2 cells | ↓ | SREBP1C↑ | FAS、SCD、ACC1↑ | lipid synthesis↑ | Conte et al (2010)33 |
| ↑ | HepG2 cells | ↓ | SREBP1C↑ | DGAT2、CIDEC↑ | lipid store↑ | Yabe et al (2003)34 |
| ↑ | HepG2 cells | ↓ | SREBP1C↑ | CPT1↓ | fatty acid oxidation↑ | Bravard et al (2011)35 |
| ↑ | 3T3-L1 preadipocytes | ↓ | MiRNA130↓ | PPARγ、C/EBPβ↑ | pre-adipocytes differentiation↑ | Lee et al (2011)46 |
| ↑ | 3T3-L1 preadipocytes | ↓ | MiRNA130↓ | UCP1↓ | brown adipocytes↑ | Kajimura et al (2009)43 |
| ↑ | 3T3-L1 preadipocytes | ↓ | – | RUNX1T1↑ | fat mass↑ | Zhao et al (2014)45 |
| ↑ | 3T3-L1 preadipocytes | ↓ | CTSB↓ | PLIN1↑ | triglyceride hydrolysis↓ | Marcinkiewicz et al (2006)47 |
| ↑ | 3T3-L1 preadipocytes | ↓ | – | ANGPTL4↓ | lipid accumulation↑ | Wang et al (2015)49 |
| ↑ | Mice and human hypothalamus | ↓ | IRX3↓ | – | lipolysis↓ | de Araujo et al (2020)53 |
| ↑ | Mice and human hypothalamus | ↓ | RPGRIP1L↑ | Leptin↓ | lipolysis↓ | Tung et al (2015)57 |
| ↑ | C57BL/6 mice | ↓ | CREB↓ | VLDL-TG↑ | Lipid circulation clearance↓ | Lin et al (2014)64 |
| ↑ | C2C12 cell | ↓ | AMPK↓ | ATGL、HSL、PGC1↓ | lipid oxidation↓ | Haemmerle et al (2002)68 |
| ↑ | C2C12 cell | ↓ | PPARβ/δ↓ | – | lipid uptake and metabolism↓ | Dressel et al (2003)65 |
| ↑ | C2C12 cell | ↓ | – | CPT1、CPT2↓ | β-oxidation↓ | Bonnefont et al (2004)70 |
| ↑ | RAW264.7 cells | ↓ | PPARγ↓ | CD36↓ | lipid uptake↓ | Mo et al (2017)71 |
| ↑ | J774.A1 cells | ↓ | AMPK↑ | ABCA1↑ | cholesterol efflux↑ | Wu et al (2019)72 |
Remarks: SREBP1C, sterol regulatory element binding protein 1c; FAS, fatty acid synthase; SCD, stearoyl-CoA desaturase; ACC1, acetyl-CoA carboxylase 1; DGAT2, diacylglycerol acyltransferase 2; CIDEC, cell death–inducing DFFA (DNA fragmentation factor-α)–like effector C; CPT1, carnitine palmitoyltransferase1; PPARγ, peroxisome proliferators–activated receptors γ; C/EBPβ, CCAAT/enhancer–binding protein β; UCP1, uncoupling protein1; RUNX1T1, Runt–related transcription factor 1; CTSB, cathepsin B; ANGPTL4, angiopoietin–like protein 4; IRX3, Iroquois–related homeobox 3; RPGRIP1L, regulatory factor interaction protein-1-like; CREB, cAMP response element binding; AMPK, adenosine 5′-monophosphate (AMP)–activated protein kinase; ATGL, adipose triglyceride lipase (ATGL); HSL, hormone–sensitive triglyceride lipase; PGC1, PPARγ coactivator 1α; CPT1, carnitine palmitoyltransferase 1; ABCA1, ATP–binding cassette transporter A1. ↑, increased; ↓, decreased; ---, non-observed result.
Although research on the role of FTO in lipid metabolism has made excellent progress, there are still many problems worthy of further study. One substrate mRNA stand typically possesses multiple sites for m6A modification, and binds various reader proteins to produce distinct biological effects. However, it remains unclear how FTO selects specific sites with m6A modifications and regulates the expression of substrate mRNA. The m6A erasers includes FTO, ALKBH5, and ALKBH3 as well as and novel unknown demethylases awaiting discovery in the future. The function or expression of FTO may be altered under medical intervention, and the side effects on other m6A demethylases or whether other demethylases will compensate for FTO intervention is not known. The upstream mechanism manipulating FTO expression and downstream molecules targeted by FTO requires further investigation, which will be helpful in identifying several strategies to effectively manipulate the expression and function of FTO. Although FTO protein expression is distributed in the hypothalamus and peripheral tissues, the results of multiple studies have shown that hypothalamic FTO appears to exhibit a powerful and extensive effect on intracorporeal lipid metabolism and energy consumption.54,75 Therefore, the modulation of hypothalamic FTO would be valuable in preventing imbalances in lipid metabolism and lipid disorder diseases. The modulation of peripheral FTO is a seemingly suitable therapeutic approach for specific lipid disorder diseases.
The aberrant expression and function of FTO protein causes abnormalities in lipid metabolism and the development of lipid disorder diseases, including obesity, NAFLD, T2DM hyperlipidemia, and atherosclerotic cardiovascular diseases. As an essential factor in the developmental process, FTO knockout leads to death at a young age in mice. Thus, it is necessary to identify a new means of thoroughly and comprehensively evaluating the regulatory roles and underlying mechanisms of FTO in intracorporeal lipid metabolism, and to obtain a theoretical basis for FTO-mediated regulation of lipid metabolism. Further studies are warranted to identify promising upstream or downstream target molecules and feasible treatments to effectively alter the expression and activity of FTO, which will correct FTO regulation in lipid metabolism and suppress the progression of lipid disorder diseases in pathogenic circumstances.
Conflict of interests
The author declares no conflict of interest.
Acknowledgement
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (No. 81770460); the Natural Science Foundation of Guangxi Zhuang Autonomous Region, China (No. 2019JJA140728): the Natural Science Foundation of Hunan Province, China (No. 2020JJ4532); Scientific Research Foundation for the Excellent Youth of the Education Department of Hunan province, China (No. 18B264); Aid Program (No. 2017KJ268) and Key Lab for Clinical Anatomy & Reproductive Medicine, China (No. 2017KJ182) from the Science and Technology Bureau of Hengyang City, China.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Tian-hong Peng, Email: thpeng67@163.com.
Yun-cheng Lv, Email: anthony0723@163.com.
References
- 1.Tirronen A., Hokkanen K., Vuorio T., Ylä-Herttuala S. Recent advances in novel therapies for lipid disorders. Hum Mol Genet. 2019;28(R1):R49–R54. doi: 10.1093/hmg/ddz132. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno T.M. Fat mass and obesity associated (FTO) gene and hepatic glucose and lipid metabolism. Nutrients. 2018;10(11):1600. doi: 10.3390/nu10111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung U.J., Choi M.S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerken T., Girard C.A., Tung Y.C., et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318(5855):1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bi Z., Liu Y., Zhao Y., et al. A dynamic reversible RNA N(6) -methyladenosine modification: current status and perspectives. J Cell Physiol. 2019;234(6):7948–7956. doi: 10.1002/jcp.28014. [DOI] [PubMed] [Google Scholar]
- 6.Speakman J. The 'fat mass and obesity related' (FTO) gene: mechanisms of impact on obesity and energy balance. Curr Obes Rep. 2015;4(1):73–91. doi: 10.1007/s13679-015-0143-1. [DOI] [PubMed] [Google Scholar]
- 7.Bartosovic M., Molares H.C., Gregorova P., Hrossova D., Kudla G., Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res. 2017;45(19):11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravind L., Koonin E.V. The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. 2001;2(3) doi: 10.1186/gb-2001-2-3-research0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia G., Fu Y., Zhao X., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M., Zhang Y., Ma J., et al. The demethylase activity of FTO (fat mass and obesity associated protein) is required for preadipocyte differentiation. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0133788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati P., Cheung M., Antrobus R., et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Nat Acad Sci USA. 2013;110(7):2557–2562. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkestein M., Laber S., McMurray F., et al. FTO influences adipogenesis by regulating mitotic clonal expansion. Nat Commun. 2015;6:6792. doi: 10.1038/ncomms7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frayling T.M., Timpson N.J., Weedon M.N., et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church C., Moir L., McMurray F., et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet. 2010;42(12):1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Shen F., Huang W., et al. Glucose is involved in the dynamic regulation of m6A in patients with type 2 diabetes. J Clin Endocrinol Metabol. 2019;104(3):665–673. doi: 10.1210/jc.2018-00619. [DOI] [PubMed] [Google Scholar]
- 16.Peeters A., Beckers S., Verrijken A., et al. Variants in the FTO gene are associated with common obesity in the Belgian population. Mol Genet Metabol. 2008;93(4):481–484. doi: 10.1016/j.ymgme.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Price R.A., Li W.D., Zhao H. FTO gene SNPs associated with extreme obesity in cases, controls and extremely discordant sister pairs. BMC Med Genet. 2008;9:4. doi: 10.1186/1471-2350-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratigopoulos G., Padilla S.L., LeDuc C.A., et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1185–R1196. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fawcett K.A., Barroso I. The genetics of obesity: FTO leads the way. Trends Genet. 2010;26(6):266–274. doi: 10.1016/j.tig.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tung Y.C.L., Yeo G.S.H., O'Rahilly S., Coll A.P. Obesity and FTO: changing focus at a complex locus. Cell Metabol. 2014;20(5):710–718. doi: 10.1016/j.cmet.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Chang J.Y., Park J.H., Park S.E., Shon J., Park Y.J. The fat mass- and obesity-associated (FTO) gene to obesity: lessons from mouse models. Obesity. 2018;26(11):1674–1686. doi: 10.1002/oby.22301. [DOI] [PubMed] [Google Scholar]
- 22.Gerken T., Girard C.A., Tung Y.C.L., et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318(5855):1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han Z., Niu T., Chang J., et al. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature. 2010;464(7292):1205–1209. doi: 10.1038/nature08921. [DOI] [PubMed] [Google Scholar]
- 24.Rajecka V., Skalicky T., Vanacova S. The role of RNA adenosine demethylases in the control of gene expression. Biochimica et biophysica acta Gene regulatory mechanisms. 2019;1862(3):343–355. doi: 10.1016/j.bbagrm.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhong S., Li H., Bodi Z., et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20(5):1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y., Jia G., Pang X., et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters T., Ausmeier K., Rüther U. Cloning of Fatso (Fto), a novel gene deleted by the Fused toes (Ft) mouse mutation. Mamm Genome : Off J Int Mammalian Genome Soc. 1999;10(10):983–986. doi: 10.1007/s003359901144. [DOI] [PubMed] [Google Scholar]
- 28.Chen A., Chen X., Cheng S., et al. FTO promotes SREBP1c maturation and enhances CIDEC transcription during lipid accumulation in HepG2 cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(5):538–548. doi: 10.1016/j.bbalip.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Guo J., Ren W., Li A., et al. Fat mass and obesity-associated gene enhances oxidative stress and lipogenesis in nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58(4):1004–1009. doi: 10.1007/s10620-012-2516-6. [DOI] [PubMed] [Google Scholar]
- 30.Lim A., Zhou J., Sinha R., et al. Hepatic FTO expression is increased in NASH and its silencing attenuates palmitic acid-induced lipotoxicity. Biochem Biophys Res Commun. 2016;479(3):476–481. doi: 10.1016/j.bbrc.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 31.Kang H., Zhang Z., Yu L., Li Y., Liang M., Zhou L. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J Cell Biochem. 2018;119(7):5676–5685. doi: 10.1002/jcb.26746. [DOI] [PubMed] [Google Scholar]
- 32.Ritov V.B., Menshikova E.V., He J., Ferrell R.E., Goodpaster B.H., Kelley D.E. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54(1):8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 33.Conte G., Mele M., Chessa S., et al. Diacylglycerol acyltransferase 1, stearoyl-CoA desaturase 1, and sterol regulatory element binding protein 1 gene polymorphisms and milk fatty acid composition in Italian Brown cattle. J Dairy Sci. 2010;93(2):753–763. doi: 10.3168/jds.2009-2581. [DOI] [PubMed] [Google Scholar]
- 34.Yabe D., Komuro R., Liang G., Goldstein J.L., Brown M.S. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Nat Acad Sci USA. 2003;100(6):3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bravard A., Lefai E., Meugnier E., et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes. 2011;60(1):258–268. doi: 10.2337/db10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tews D., Fischer-Posovszky P., Wabitsch M. Regulation of FTO and FTM expression during human preadipocyte differentiation. Horm Metab Res. 2011;43(1):17–21. doi: 10.1055/s-0030-1265130. [DOI] [PubMed] [Google Scholar]
- 37.Grunnet L.G., Nilsson E., Ling C., et al. Regulation and function of FTO mRNA expression in human skeletal muscle and subcutaneous adipose tissue. Diabetes. 2009;58(10):2402–2408. doi: 10.2337/db09-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronkainen J., Huusko T.J., Soininen R., et al. Fat mass- and obesity-associated gene Fto affects the dietary response in mouse white adipose tissue. Sci Rep. 2015;5:9233. doi: 10.1038/srep09233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneeberger M., Dietrich M.O., Sebastián D., et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155(1):172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seoane-Collazo P., Martínez de Morentin P.B., Fernø J., Diéguez C., Nogueiras R., López M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155(5):1679–1689. doi: 10.1210/en.2013-1839. [DOI] [PubMed] [Google Scholar]
- 41.de Araujo T.M., Razolli D.S., Correa-da-Silva F., et al. The partial inhibition of hypothalamic IRX3 exacerbates obesity. EBioMed. 2019;39:448–460. doi: 10.1016/j.ebiom.2018.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berulava T., Rahmann S., Rademacher K., Klein-Hitpass L., Horsthemke B. N6-adenosine methylation in MiRNAs. PloS One. 2015;10(2) doi: 10.1371/journal.pone.0118438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kajimura S., Seale P., Kubota K., et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460(7259):1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tews D., Fischer-Posovszky P., Fromme T., et al. FTO deficiency induces UCP-1 expression and mitochondrial uncoupling in adipocytes. Endocrinology. 2013;154(9):3141–3151. doi: 10.1210/en.2012-1873. [DOI] [PubMed] [Google Scholar]
- 45.Zhao X., Yang Y., Sun B.F., et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24(12):1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee E.K., Lee M.J., Abdelmohsen K., et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2011;31(4):626–638. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcinkiewicz A., Gauthier D., Garcia A., Brasaemle D.L. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem. 2006;281(17):11901–11909. doi: 10.1074/jbc.M600171200. [DOI] [PubMed] [Google Scholar]
- 48.Gray N.E., Lam L.N., Yang K., Zhou A.Y., Koliwad S., Wang J.C. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J Biol Chem. 2012;287(11):8444–8456. doi: 10.1074/jbc.M111.294124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C.Y., Shie S.S., Wen M.S., et al. Loss of FTO in adipose tissue decreases Angptl4 translation and alters triglyceride metabolism. Sci Signal. 2015;8(407):ra127. doi: 10.1126/scisignal.aab3357. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida K., Shimizugawa T., Ono M., Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43(11):1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 51.Mizunoe Y., Kobayashi M., Hoshino S., et al. Cathepsin B overexpression induces degradation of perilipin 1 to cause lipid metabolism dysfunction in adipocytes. Sci Rep. 2020;10(1):634. doi: 10.1038/s41598-020-57428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavadas C., Aveleira C.A., Souza G.F., Velloso L.A. The pathophysiology of defective proteostasis in the hypothalamus - from obesity to ageing. Nat Rev Endocrinol. 2016;12(12):723–733. doi: 10.1038/nrendo.2016.107. [DOI] [PubMed] [Google Scholar]
- 53.de Araujo T.M., Velloso L.A. Hypothalamic IRX3: a new player in the development of obesity. Trends Endocrinol Metabol. 2020;31(5):368–377. doi: 10.1016/j.tem.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Velloso L.A., Schwartz M.W. Altered hypothalamic function in diet-induced obesity. Int J Obes. 2011;35(12):1455–1465. doi: 10.1038/ijo.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stratigopoulos G., LeDuc C.A., Cremona M.L., Chung W.K., Leibel R.L. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem. 2011;286(3):2155–2170. doi: 10.1074/jbc.M110.188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stratigopoulos G., Burnett L.C., Rausch R., et al. Hypomorphism of Fto and Rpgrip1l causes obesity in mice. J Clin Invest. 2016;126(5):1897–1910. doi: 10.1172/JCI85526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tung Y.C., Gulati P., Liu C.H., et al. FTO is necessary for the induction of leptin resistance by high-fat feeding. Mol Metab. 2015;4(4):287–298. doi: 10.1016/j.molmet.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LJ S., Kl M., LL B., et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science (New York, NY) 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banales-Luna M., Figueroa-Vega N., Marin-Aragon C.I., et al. Associations of nicotidamide-N-methyltransferase, FTO, and IRX3 genetic variants with body mass index and resting energy expenditure in Mexican subjects. Sci Rep. 2020;10(1):11478. doi: 10.1038/s41598-020-67832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn F.L. Hyperlipidemia and diabetes. Med Clin. 1982;66(6):1347–1360. doi: 10.1016/s0025-7125(16)31368-2. [DOI] [PubMed] [Google Scholar]
- 61.Pan D.A., Kriketos A.D., Milner M.R., et al. Skeletal muscle triglycerid e levels are inversely related to insulin action. Diabetes. 1997;46(6):983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 62.Gleissner C.A., Leitinger N., Ley K. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension. 2007;50(2):276–283. doi: 10.1161/HYPERTENSIONAHA.107.089854. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y., Chen F. Reactive oxygen species (ROS), troublemakers between nuclear factor-kappaB (NF-kappaB) and c-Jun NH(2)-terminal kinase (JNK) Canc Res. 2004;64(6):1902–1905. doi: 10.1158/0008-5472.can-03-3361. [DOI] [PubMed] [Google Scholar]
- 64.Lin L., Hales C.M., Garber K., Jin P. Fat mass and obesity-associated (FTO) protein interacts with CaMKII and modulates the activity of CREB signaling pathway. Hum Mol Genet. 2014;23(12):3299–3306. doi: 10.1093/hmg/ddu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dressel U., Allen T.L., Pippal J.B., Rohde P.R., Lau P., Muscat G.E. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17(12):2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 66.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujii N., Ho R.C., Manabe Y., et al. Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes. 2008;57(11):2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haemmerle G., Zimmermann R., Hayn M., et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277(7):4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 69.Medina-Gomez G., Gray S., Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1) Publ Health Nutr. 2007;10(10A):1132–1137. doi: 10.1017/S1368980007000614. [DOI] [PubMed] [Google Scholar]
- 70.Bonnefont J.P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspect Med. 2004;25(5–6):495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Mo C., Yang M., Han X., et al. Fat mass and obesity-associated protein attenuates lipid accumulation in macrophage foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Hypertens. 2017;35(4):810–821. doi: 10.1097/HJH.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y.R., Shi X.Y., Ma C.Y., Zhang Y., Xu R.X., Li J.J. Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc Diabetol. 2019;18(1):146. doi: 10.1186/s12933-019-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yvan-Charvet L., Wang N., Tall A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30(2):139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li D., Wang D., Wang Y., Ling W., Feng X., Xia M. Adenosine monophosphate-activated protein kinase induces cholesterol efflux from macrophage-derived foam cells and alleviates atherosclerosis in apolipoprotein E-deficient mice. J Biol Chem. 2010;285(43):33499–33509. doi: 10.1074/jbc.M110.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tung Y.C., Yeo G.S. From GWAS to biology: lessons from FTO. Ann N Y Acad Sci. 2011;1220:162–171. doi: 10.1111/j.1749-6632.2010.05903.x. [DOI] [PubMed] [Google Scholar]