Abstract
Aim
1) To investigate the pharmacokinetic profile of sildenafil citrate in Middle Eastern males and, 2) To highlight the impact of ethnicity on its pharmacokinetics parameters through comparing Middle Eastern data to the data estimated from different ethnic groups.
Method
The study was conducted on 24 Middle Eastern healthy male volunteers. Pharmacokinetic data including Cmax, Tmax, t1/2, AUC0-t, AUC0-∞ were estimated from blood samples collected at several time points within 24 h post-administration of a single 100-mg tablet of sildenafil citrate (Viagra®). Pharmacokinetic data of sildenafil generic 100-mg tablet (product B) was determined in the volunteers using the same analytical method. Pharmacokinetic data of other studies published on different ethnicities were obtained and compared to our Viagra®-related data.
Results
Analysis of Middle Eastern data (mean ± SD) revealed Cmax = 398.9 ± 107.7 ng/ml; Tmax = 1.84 ± 0.22 h; t1/2 = 2.66 ± 0.97 h; AUC0–24 = 1475 ± 515.3 ng.h/ml; AUC0-∞ = 1556 ± 567.58 ng.h/ml. There was no significant difference between Viagra® and product B, confirming the bioequivalence of the two preparation as well as the reliability of utilized analytical method. Data comparisons between Middle Eastern and other ethnicities indicated that Iranian, Mexican, and Thai would potentially have twice the effect observed in Arabs and Caucasians, considering the same prescribed drug formulation and dose.
Conclusion
There is a considerable difference in the pharmacokinetic profile of sildenafil citrate between Middle Eastern and other ethnic groups. Ethnicity may predispose individuals to unwanted prolonged activity of sildenafil and adverse events. Thus, it should be taken in consideration by clinicians when recommending sildenafil dose.
Keywords: Sildenafil, Pharmacokinetics, Middle Eastern, Ethnicity
1. Introduction
Sildenafil citrate, a selective phosphodiesterase type 5 (PDE5) inhibitor, is a widely prescribed medication in Saudi Arabia (AlKhamees et al., 2018) for the treatment of impotence and male erectile dysfunction (Hatzimouratidis, 2006). It functions by competing with the hydrolysis of cyclic guanosine monophosphate (cGMP) by PDE5, as it resembles cGMP’s structure. This results in an elevation of cGMP level, increasing smooth muscle relaxation and inflow of blood into the corpus cavernosum, hence promoting penile erection (Bruzziches et al., 2013).
Sildenafil is rapidly absorbed after oral administration, with a mean absolute bioavailability of ≈40% (Boolell et al., 1996, Loprete et al., 2018). Maximum plasma concentrations (Cmax) are achieved within 30 to 120 min (median 60 min), with a mean of 450 ng/mL after a single oral dose of a 100-mg tablet in healthy, fasting male volunteers (Pfizer-Laboratories., 1998, Nichols et al., 2002, Loprete et al., 2018). However, a lower therapeutic concentration of approximately 127 ng/mL is reached after a 25-mg oral dose (Nichols et al., 2002). It has been shown that fatty meal can slow the absorption rate of sildenafil, resulting in a delay in the time to reach Cmax (Tmax) and a reduction of Cmax by approximately 60 min and 29%, respectively (Nichols et al., 2002). The average steady-state volume of distribution (Vss) of sildenafil citrate is 105 L, indicating high tissue distribution (Nichols et al., 2002, Loprete et al., 2018). This may explain the enhanced platelet antiaggregatory effect of nitric oxide in vitro and increased peripheral arterial-venous dilatation and inhibition of platelet thrombus formation in vivo observed with its use that is attributed entirely to lower PDE5 levels in tissues including vascular and visceral smooth muscle as well as skeletal muscle (Pfizer-Laboratories 1998). After either oral or intravenous administration, the drug is metabolized mainly by the liver, primarily via CYP3A4 and to a lower extent CYP2C9 (Bruzziches et al., 2013, Loprete et al., 2018). Both sildenafil and its major circulating active metabolite, N-desmethyl sildenafil, which is further subjected to hepatic metabolism, have terminal half-lives (t1/2) of ≈4 h (Nichols et al., 2002). Interestingly, both are 96% bound to the plasma proteins, which is independent of the total drug concentration (Walker et al., 1999). Using a population pharmacokinetic approach, the same pharmacokinetic parameters have been observed among normal volunteers and patients (Pfizer-Laboratories 1998).
Racial differences have been reported as one of the factors that correlates with the variability observed in the pharmacokinetic, pharmacodynamic, and overall response to medical therapies (Yasuda et al., 2008). For example, Southern Asians administered triazolam were found to have a significantly higher Cmax, which was achieved earlier as compared with Caucasians (Kiniron et al., 1996). Mexicans showed a significant increase in F% and Cmax of midazolam in comparison with Caucasians (Cháez-Teyes et al., 1999). On the other hand, and despite the absence of a difference between white and Latin American individuals, the F% and Cmax of tacrolimus among African Americans were significantly lower as compared with the former two groups (Mancinelli et al., 2001). These differences paved the way for pharmacogenetics studies, which attributed them to genetic polymorphism found in different ethnic groups. For example, Chinese population with allelic variants of CYP2C9 and CYP3A4 were shown to have reduced sildenafil clearance compared to the wild type carriers (CYP2C9*2 and *60 vs. wild type CYP2C9*1; CYP3A4*2 and CYP3A4*24 vs. wild type CYP3A4*1, respectively). Whereas those with CYP3A4*3, CYP3A4*10 or CYP3A4*14 variants had higher clearance compared to wildtype carriers (Tang et al., 2020). Another study with a majority of Caucasians demonstrated that CYP3A4*22 carriers had higher sildenafil concertation, that was more prevalent in this ethnicity (8%) (de Denus et al., 2018) compared to African American and Asians (4%) (Werk and Cascorbi, 2014, de Denus et al., 2018). Despite the lack of Clinical Pharmacogenetics Implementation Consortium (CPIC), the Royal Dutch Association for the Advancement of Pharmacy - Pharmacogenetics Working Group (DPWG), the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) of sildenafil dosing guidelines, genetic polymorphisms of CYP2C9 and CYP3A4 showed an influence on the pharmacokinetics of drugs such as sildenafil, which may potentially impact its clinical outcomes, therapeutic activity and its toxicity.
Although compelling evidence has highlighted the influence of ethnicity on the pharmacokinetics of medications, there is a paucity of research on its impact in Middle Eastern individuals (Shilbayeh and Tutunji 2006, Abou-Auda 2014). This study aims to investigate the pharmacokinetic profile of sildenafil citrate in Middle Eastern males and to highlight the impact of ethnicity on its pharmacokinetics parameters through comparing Middle Eastern data to the data obtained from different ethnic groups.
2. Methodology
2.1. Subjects
The study was conducted in King Khalid University Hospital (KKUH) after receiving the approval from the institutional review board at King Saud University College of Medicine (IRB.21–5783). All volunteers were Arabs of the Middle East living in Saudi Arabia for at least 10 years. Data from all participants were included in the study. Study participants consisted of 24 healthy male volunteers who aged between 20 and 32 years (mean ± SD; 24.29 ± 3.43 years) and had a mean body weight of 68.0 ± 9.27 kg and height of 172.9 ± 5.2 cm that did not deviate more than 10% from the values in the Metropolitan Scale. The average participants’ body mass index (BMI) was 22.7 ± 2.6 kg/m2, with a range of 18.7–27.8 kg/m2. Volunteers underwent a comprehensive physical examination, medical history, kidney and liver function tests, creatinine, Blood urea nitrogen (BUN), serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), glucose, uric acid, total proteins, albumin, lactate dehydrogenase, alkaline phosphatase, and cholesterol tests prior to drug administration. Hematological tests were also performed (complete blood count and differentials). Clinical evaluation revealed no history of cardiovascular, renal, hepatic, gastrointestinal, or respiratory disorders. None of the participants was allergic to the medications in the study. The study was performed according to the recommendations of the Declaration of Helsinki. All volunteers were required to provide informed written consent after receiving detailed instructions concerning the study performance, restrictions, and possible adverse effects. Copies of the signed and dated consent forms were given to each subject. The volunteers were free to withdraw from the study at any time.
2.2. Study plan
Volunteers were prohibited from taking any medication 14 days before and during the study. They were also restricted from ingesting caffeine-containing food or beverages 24 h prior to the study commencement. In the early morning (7:00 A.M.) of the study day, the overnight-fasting volunteers reported to the site of the study, and their vital signs were checked. An indwelling venous cannula was inserted into an antebrachial vein and two blood samples were taken, the first was 30 min before drug administration and the second was just before drug administration (0.0 h). A single 100-mg tablet of Viagra® (sildenafil citrate tablet) was administered. Administration of the drug was immediately followed by the ingestion of 240 ml of water. To measure the rate of absorption, blood samples (total of 7 ml) were drawn immediately pre-drug administration (already collected at 0.0 h) and at 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0, 14.0, 16.0, and 24.0 h post-drug administration. A total of 21 blood samples were collected from each participant [one sample 30 mins before drug administration, one just before drug administration (0.0 hr), and 19 samples after drug administration]. The samples were collected in heparinized tubes and immediately centrifuged. Two aliquots were transferred to labeled polypropylene tubes and stored at − 20 °C pending sildenafil assay. Food intake during the study period was permitted in the form of standardized meals at 5 and 10 h after drug administration for lunch and supper, respectively. Each volunteer received 240 ml of tap water at 2, 4, 6, and 8 h after drug administration. In addition, tea was served after 8 h of drug administration. The volunteers remained ambulatory, and no smoking or strenuous activity was permitted on the study day. Pulse rate and blood pressure were recorded before drug administration and immediately before each blood sample. For the period of 16 h following drug administration, volunteers were under direct medical supervision at the clinical study site. After the end of the study, volunteers were subjected to the same medical evaluations as outlined previously.
Another sildenafil citrate preparation (product B) was tested. This was performed after a one-week washout period (Al-Ghazawi et al., 2010, Loprete et al., 2018) from Viagra® administration to assess the bioequivelance of both preparations. As described below in the analytical method details, a sensitive, selective, and accurate HPLC method was used for the measurement of sildenafil concentration in the plasma and two sildenafil preparations were used to assess the validity of this method. The lower limit of quantification was 10 ng.ml−1. The rate of absorption was evaluated through the following ratio: Cmax/AUC0-∞ (Schall and Luus 1992).
2.3. Analytical method
A previously published HPLC–UV method (Abou-Auda 2014) was adopted to quantify sildenafil in human plasma after oral adminsteration in Middle Eastern healthy adult males. HPLC was performed using a liquid chromatography system (Shimadzu, Japan) that contained the following units: model LC-10Advp solvent delivery pump, a model SCL-10 Avp system controller, and a model SPD-10Avvp UV–VIS detector. The chromatographic system and peak data handling were managed by a class VP-5 software package version 5.03. The stationary phase, which provided a satisfying resolution and run time, was a Waters Novapak C18 (3.9 × 150 mm), 4-µm particle size, HPLC cartridge analytical column, protected by a sentry guard column, Novapak RP (3.9 × 20 mm), 4-µm particle size (Waters Associates, Milford, MA, USA). The mobile phase consisted of 33% acetonitrile in 0.05 M potassium dihydrogen phosphate. A flow rate of 2.0 ml.min−1 was used to separate sildenafil and internal standard (diazepam) using a UV detector operated at λ225 nm, with an injection volume of 100 µl.
Sample preparation for injection into the HPLC system involved the addition of 100 µl of the internal standard (1 µg/ml in deionized water) to 0.5 ml plasma sample, followed by vortexing for 30 s; 7 ml of extraction solvent (ethyl acetate) were added and centrifuged at 3000 rpm for 5 min. The supernatant was evaporated to dryness at 40 °C under nitrogen gas, reconstituted with 200 µl of mobile phase, transferred to an Eppendorf tube, and recentrifuged at 13,000 rpm for 2 min. A 100-µl aliquot sample (standard, control, or volunteer sample) was injected into the system.
2.4. Pharmacokinetic analysis
We used the WinNonlin computer program (Scientific Consulting Inc, Gaithersburg, MD, USA) to determine the pharmacokinetic parameters of sildenafil from the plasma concentration–time profiles. All parameters were determined from the true (actual) sample collection times and assayed plasma concentrations at these times. Concentration values below the lower limit of quantification were considered as not detected. We calculated the following model-independent parameters: area under the plasma concentration–time curves up to the last measurable concentration (AUC0-t) and up to time infinity (AUC0-∞), the maximum plasma concentration (Cmax), the time to maximum plasma concentration (Tmax), and the half-life (t1/2). We computed the elimination t1/2 from the first-order elimination rate, which was estimated by least-squares linear regression of the plasma terminal log-linear phase of the log concentration–time curve. AUC0-t was determined by the linear trapezoidal method. AUC0-∞ was calculated by adding to the AUC0-∞ the quotient resulting from dividing the last measurable sildenafil plasma concentration by the negative slope of the final log-linear phase of the plasma concentration–time curve.
The mean residence time (MRT) was calculated using the following relationship:
where AUMC0-∞ is the area under the first moment curve and calculated by the trapezoidal rule from a plot of the product of sildenafil plasma concentration and time versus time.
In addition, we digitized the sildenafil plasma concentration–time curves from the published literature on different ethnic groups and extracted their data. All concentrations were normalized to a 100-mg dose, assuming linear pharmacokinetics. The weighted average concentrations were calculated for each ethnic group based on the number of subjects included in each study.
2.5. Statistical analysis
The analysis of variance (ANOVA) for crossover design was used to assess the effect of formulations, sequences, and subjects within sequence on the raw (untransformed) and logarithmically transformed data of AUC0-t, AUC0-∞, Cmax, Kel, t1/2, and Cmax/AUC0-∞ parameters. Based on the ANOVA of the mean test/reference ratios of AUCs parameters, Cmax and Cmax/AUC0-∞, parametric 90% confidence intervals were computed under the assumption of the additive and multiplicative model. All effects of the analyses were considered statistically significant if the probability associated with F was ≤ 0.05. All analyses of the data were performed with SAS using the GLM procedure.
3. Results
3.1. The impact of ethnicity on the pharmacokinetic profile of sildenafil citrate (Viagra®)
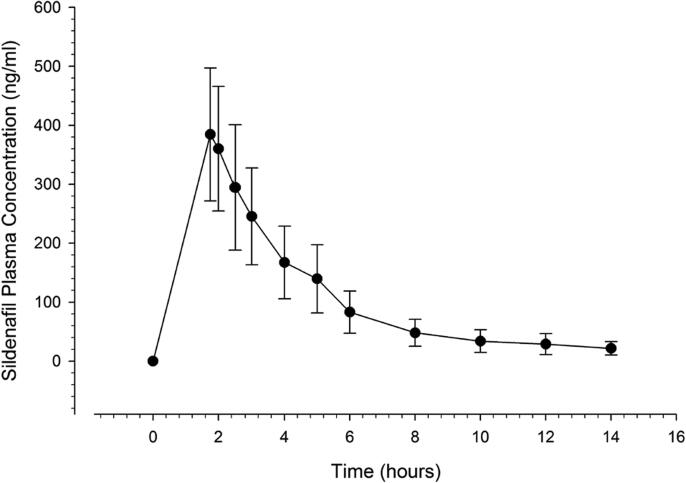
Sildenafil citrate (100-mg tablet) was well tolerated by our Middle Eastern Arab volunteers (n = 24) living in Saudi Arabia, and we did not observe any significant adverse events or protocol violations during the study period. All volunteers completed the study and were discharged in a good health. Despite undetectable concentrations (<10 ng.ml−) in some samples at different time points (9, 1, 1, 2, 9, 12, and 17 samples at 0.25, 1.5, 8, 10, 12, 14, and 16 h, respectively), the drug concentrations in all samples withdrawn between 1.75 and 6 h were quantifiable. The plasma concentration–time curve of sildenafil exhibited a two-compartment pattern after oral administration (Fig. 1). The estimated pharmacokinetic parameters (mean ± SD) of our volunteers are shown in Table 1. Sildenafil was absorbed rapidly, reaching a maximum plasma concentration of 398.9 ± 107.7 ng/ml in about 1.84 ± 0.22 h. As a good parameter for evaluation of absorption rates in comparative pharmacokinetics, absorption rate constant of sildenafil was calculated using the ratio Cmax/AUC0-∞ and shown to range between 0.1743 and 0.3919 h−1 (0.267 ± 0.048 h−1). The volume of distribution at steady state and the clearance rate were 261.5 ± 96.68 L (3.85 ± 1.27 L/kg) and 72.92 ± 27.19 L/h (1.09 ± 0.43 L.kg−1h−1), respectively.
Fig. 1.
Mean plasma concentration–time curve of sildenafil after administration of 100 mg as a single oral dose.
Table 1.
Pharmacokinetic parameters of sildenafil citrate (Viagra®) after a single oral administration of a 100-mg tablet (n = 24).
| Cmax (ng/ml) |
Tmax (h) |
AUC0-t (ng.h/ml) |
AUC0-∞ (ng.h/ml) |
t1/2 (h) |
Vss/F (L) |
CL/F (L/h) |
Vss/F per kg (L/kg) |
CL/F per kg (L.kg-1h−1) |
MRT (h) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 398.9 | 1.84 | 1474.47 | 1556.14 | 2.66 | 261.50 | 72.92 | 3.85 | 1.09 | 4.45 |
| ±SD | 107.7 | 0.22 | 515.30 | 567.58 | 0.97 | 96.68 | 27.19 | 1.27 | 0.43 | 0.94 |
| Median | 392 | 1.75 | 1438.82 | 1493.7 | 2.49 | 236.57 | 67.05 | 3.62 | 0.96 | 4.21 |
| Minimum | 207 | 1.75 | 697.13 | 727.18 | 1.04 | 141.18 | 32.82 | 1.96 | 0.46 | 2.70 |
| Maximum | 569 | 2.50 | 2682.0 | 3046.75 | 5.19 | 573.49 | 137.52 | 7.07 | 2.18 | 6.76 |
Cmax maximum plasma concentration; Tmax time to maximum plasma concentration; AUC0-t area under the plasma concentration–time curve up to the last measurable concentration; AUC0-∞ area under the plasma concentration–time curve up to time infinity; t1/2 the half-life; Vss/F apparent volume of distribution at steady state; CL/F apparent clearance; MRT mean residence time; SD standard deviation.
3.2. Validation of the analytical method for determining sildenafil pharmacokinetics in plasma samples
To test the validity of our analytical method, another single oral dose of sildenafil generic (product B; 100 mg tablet) was administered to the same volunteers after a 1-week washout period. We measured the drug concentrations in the plasma samples obtained at 1.75, 2, 2.5, 3, 4, and 5 h. Following the same protocol and method explained earlier, the data showed no significant difference in the mean concentrations between product B and Viagra® at each time point (Supplemental Fig. 1). This confirms the bioequivalence of both preparations and reliability of our results. In addition, it provides an evidence that the analytical method and protocol used in our study can be adopted in other pharmacokinetic studies.
4. Discussion
Differences in ethnicity have been linked to the variability in pharmacokinetics of different drugs and have been shown to affect the overall response to medical therapies (Yasuda et al., 2008). Although multiple studies have investigated this matter in the context of sildenafil citrate and erectile dysfunction (details discussed below), none have explored this topic in Middle Eastern Arabs living in Saudi Arabia (including both Saudis and non-Saudi residents). Moreover, the current knowledge lacks studies comparing multiple ethnicities, including Middle Eastern, with respect to sildenafil citrate pharmacokinetics and its implication on drug use.
In this study, a single 100-mg oral dose of sildenafil citrate resulted in a maximum mean plasma concentration of 398.9 ± 107.7 ng/ml, which was reached in about 2 h in healthy young participants. Under similar conditions (except for the unknown age of the volunteers), another study conducted on 11 healthy Iranians (Mahmoudian et al., 2010) demonstrated an increase in Cmax and systemic exposure of the drug (AUC0–24) by about 111% and 89%, respectively, as compared with the Middle Eastern Arab individuals in our study (Table 1, Table 2; Fig. 2). This could be driven by the enhanced absorption rate, as the Tmax in Iranians was about 71% of that estimated in our participants (1.13 vs. 1.84 h), and the potential reduction in sildenafil metabolism in Iranians as well.
Table 2.
Pharmacokinetic parameters of sildenafil citrate reported in different ethnic groups after a single oral administration of a 100-mg or 50-mg tablet.
| Ethnic Group |
n | Age (years) |
Dose (mg) |
(ng/ml) |
(h) |
(ng.h/ml) |
(ng.h/ml) |
(h) |
CL/F | MRT (h) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mexican | 29 | 30.2 | 100 mg (1 tab) | 765.9 | 0.85 | 1873.3 (t = 24 h) | 1894.6 | 4.12 | 352.56 | 60.3 | 3.68 | (Marcelín-Jiménez et al., 2012) |
| Mexican | 30 | 24.4 | 100 mg (1 tab) | 657.64 | 1.42 | 1884.97 (t = 16 h) | 1983.03 | 3.06 | — | — | 3.86* | (Valenzuela et al., 2011) |
| Mexican | 24 | — | 100 mg (1 tab) | 1044 | 1.2 | 2960 (t = 12 h) | — | 4.3 | — | — | 6.2 | (Flores-Murrieta et al., 2000) |
| Iranian | 11 | — | 100 mg (2x50 mg tabs) | 840.0 | 1.13 | 2790.0 (t = 24 h) | 2870.0 | 2.90 | — | — | 3.13* | (Mahmoudian et al., 2010) |
| Thai | 15 | 28.8 | 100 mg (1 tab) | 661.54 | 0.99 | 1876.36 (t = 12 h) | — | 3.11 | — | 58.94 | 4.48 | (Kanjanawart et al., 2011) |
| Caucasian | 34 | ≈ 31.5 | 100 mg (1 tab) | 514.0 | 0.95 | 1651.0 (t = 24 h) | 1670 | 3.98 | — | — | 5.88* | (Nichols et al., 2002) |
| Caucasian | 27 | 28.8 | 100 mg (1 tab) | 302.0 | 3.10 | 1468 (t = 24 h) |
— | 3.48 | — | — | 5.03* | (Muirhead et al., 2000) |
| Caucasian | 24 | 29 | 50 (1 tab) | 254.89 | 0.75 | — (t = 24 h) |
619.88 | 3.79 | — | — | 5.47* | (Jetter et al., 2002) |
| Egyptian | 12 | 22 | 50 (1 tab) | 236.0 | 1.74 | — (t = 12 h) |
1336 | 2.39 | 134.9 | 40.1 | 2.97* | (Hedaya et al., 2005) |
| Egyptian | 10 | ≈ 29 | 50 (1 tab) | 318.35 | 1.25 | — (t = 12 h) |
484.2 | 1.92 | — | — | 2.63* | (Hegazy 2014) |
| Jordanian | 6 | 30 | 50 (1 tab) | 212.4 | 1.25 | 515.4 (t = 24 h) |
564.5 | 1.73 | — | — | 2.50* | (Al-Ghazawi et al., 2010) |
Cmax maximum plasma concentration; Tmax time to maximum plasma concentration; AUC0-t area under the plasma concentration–time curve up to the last measurable concentration; AUC0-∞ area under the plasma concentration–time curve up to time infinity; t1/2 the half-life; Vss/F apparent volume of distribution at steady state; CL/F apparent clearance; MRT mean residence time.
Estimated from references as (MRT = half-life/0.693).
Fig. 2.
Comparative digitized mean plasma concentration–time curves of sildenafil between Middle Eastern populations after a 100-mg single oral dose (All plasma concentrations from 50-mg–based studies [Hegazy S, Hedaya et al, and Al-Ghazawi et al] were normalized after digitization).
We identified three additional studies conducted in Middle Eastern Arabs using a 50-mg rather than 100-mg oral tablet of sildenafil citrate (Table 2). Among Egyptians, Hadaya et al. and Hegazy reported a Cmax of 236 and 318.35 ng/ml and a Tmax of 1.74 and 1.25 h in 12 and 10 healthy young volunteers, respectively (Hedaya et al., 2005, Hegazy, 2014). Interestingly, as compared with the previous two studies, Alghazawi and colleagues demonstrated a lower Cmax (212.4 ng/ml) and shortened Tmax (1.25 h) among six healthy Jordanian men (Al-Ghazawi et al., 2010). When the mean plasma concentrations and Cmax were digitized and normalized to a 100-mg dose, Jordanians were the closest to our participants (414.8 vs. 398.9 ng/ml), despite the overlapping plasma concentration–time curves of all Middle Eastern–based studies (Fig. 2).
Marcelin et al. investigated the pharmacokinetic profile of sildenafil citrate after a single dose of Viagra® (100-mg tablet), its generic (100-mg tablet) and chewable tablets (50 mg, two tablets) in 29 healthy Mexican volunteers, who were instructed to drink 250 ml water with all doses, except for the chewable tablets (Marcelín-Jiménez et al., 2012). Despite the nonsignificant delayed Tmax of chewable tablets as compared with other formulations (0.86 vs. 0.66 h), no appreciable difference in pharmacokinetic data was observed between the three formulations. Intriguingly, when the pharmacokinetic parameters in individuals administered Viagra® only were compared to our participants, Cmax stood out as a major concern, as it was almost doubled in Mexicans (756.9 vs. 398.9 ng/ml; Table 1, Table 2). Nonetheless, similar drug formulations, strengths, and amounts of water were administered in both groups. Thus, the difference is potentially attributed to the rapid absorption rate in Mexicans, with the Tmax, accounting for only 45% of that observed in our population (0.82 vs. 1.84 h). More importantly, given the shortened t1/2 and enhanced clearance among Middle Eastern Arabs compared with Mexicans (2.66 vs. 4.12 h and 72.9 vs. 60.3 L/hr, respectively), a reduction in the duration of activity is anticipated in our participants. On the other hand, prolonged activity in Mexicans is expected, partially because of the reduced CYP3A4 activity reported by other studies among persons of this ethnicity (Castañeda‐Hernández et al., 1996, Chávez-Teyes et al., 1999). Overall, this can explain the noticed difference in Cmax and AUC0–24 when their results are compared with ours (Fig. 1, Fig. 3; Table 1, Table 2). Additionally, the results of two studies on healthy young Mexican men using a similar strength of sildenafil reiterate the notion of an enhanced absorption rate and reduced sildenafil metabolism in this population, as indicated by the increasing Cmax by approximately 65% and 161% (658 and 1044 ng/ml; Table 1, Table 2) and decreasing Tmax by 23% and 35% (1.42 and 1.2 h; Table 1, Table 2) when compared with our participants’ data (Flores-Murrieta et al., 2000, Valenzuela et al., 2011). Refer to Fig. 1, Fig. 3 for additional details about the plasma concentration–time curves of sildenafil.
Fig. 3.
Comparative digitized mean plasma concentration–time curves of sildenafil in Mexican individuals after a 100-mg single oral dose.
Nichols and colleagues conducted a study on 34 healthy British volunteers to determine the bioavailability and the effect of food on sildenafil pharmacokinetics (Nichols et al., 2002). After comparing their results of a single oral dose of 100 mg sildenafil citrate tablet during the fasting state, we observed a close peak concentration and systemic exposure of the drug relative to ours, despite the reduced Tmax and increased t1/2 (0.95 vs. 1.84 h and 3.98 vs. 2.66 h), respectively, when compared with our participants (Table 1, Table 2). Another study published by the Muirhead group (Muirhead et al., 2000) on the pharmacokinetic interaction between sildenafil and saquinavir/ritonavir in Caucasian individuals revealed that a 100-mg single oral dose of sildenafil citrate with placebo resulted in a Cmax close to what we have reported in this study (Table 1, Table 2). Although an approximate 30% increase in t1/2 (3.48 vs. 2.66 h) was observed, an unexpected similarity in AUC0–24 and delay in Tmax by about 69% was found when compared with our population (Table 1, Table 2). In another study of 24 healthy Caucasian males who received a 50-mg single oral dose of sildenafil citrate, Jetter et al. found a relatively lower Cmax than reported in the previous two studies (Table 2), which was reached early with a Tmax of 0.75 h (Jetter et al., 2002). A comprehensive comparison of the weighted mean plasma concentration–time curves of 100 mg sildenafil citrate after digitization between Middle Eastern, Mexican, and Caucasians is depicted in Fig. 4.
Fig. 4.
Comparative digitized and weighted mean plasma concentration–time curves of sildenafil between Middle Eastern, Mexican, and Caucasian individuals based on a 100-mg single oral dose.
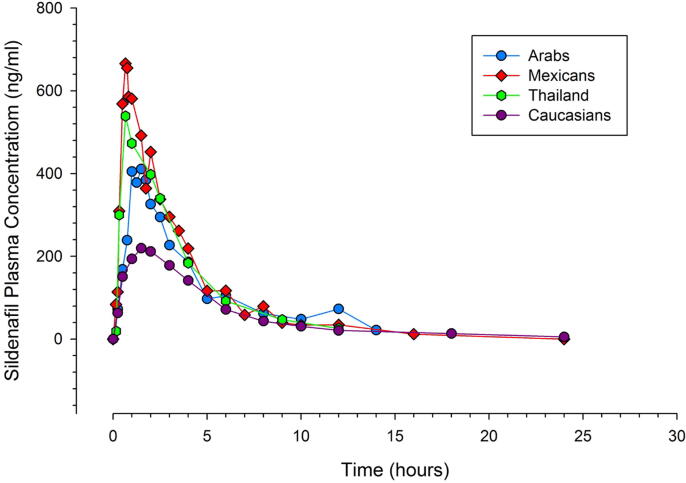
Finally, in their study of 15 healthy young Thai volunteers, Kanjanawart and colleagues focused on comparing the bioequivalence and pharmacokinetics of a single dose of 100 mg sildenafil generic formulation to Viagra® 100-mg tablet, as a reference drug (Kanjanawart et al., 2011). When we compared the Viagra® parameters obtained from the volunteers to our participants, we found a 66% increase in Cmax in the former (661.54 vs. 398.9 ng/ml) despite the close systemic concentration in both groups (Table 1, Table 2). This could mainly be related to the enhanced absorption rate among Thai volunteers, supported by a reduced Tmax of about 46% relative to our population (0.99 vs. 1.84 h). An overall comprehensive comparison of the weighted mean plasma concentrations–time of sildenafil citrate 100 mg after digitization between Middle Eastern Arabs, Mexicans, Caucasians, and Thais are presented in Fig. 5.
Fig. 5.
Comparative digitized and weighted mean plasma concentration–time curves of sildenafil between Middle Eastern (including Iranians and Arabs), Mexican, Thai, and Caucasian individuals after a 100-mg single oral dose.
There are some limitations to our study. First, the reduced sensitivity of the analytical method, as result of samples dilution or low drug levels, initially speculated to be drawback. Nevertheless, this seemed not to be problematic to our method, as all drug concentrations greater than 10 ng/ml were detected. Second, the use of a 50-mg single dose by a few studies could hinder their utility in our comparative analysis. However, this was overcome by the use of digitized data. Third, different analytical methods used by comparative studies carry their own limitations, which might have had some impact on our comparisons. Despite all these limitations, this study is unique for the following reasons: 1) the detailed analytical method that was used could be utilized to investigate pharmacokinetics of other drugs, and 2) it revealed and compared the pharmacokinetic profile of sildenafil 100-mg oral tablet in Middle Eastern Arabs living in Saudi Arabia to individuals of other ethnicities. Overall, our study underlines the significant role of ethnicity on the pharmacokinetics of sildenafil and other similar drugs.
Based on the estimation of pharmacokinetic data in Middle Eastern Arabs and different ethnic groups from the previous studies, the use of lower-strength sildenafil citrate tablets (50 mg) by Mexican, Iranian, and Thai individuals would have an equivalent impact to 100 mg in Arabs and Caucasians. Thus, to avoid unwanted prolonged activity and adverse events, it is important to consider ethnicity of the user upon recommending sildenafil dose.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
This study is funded by Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia through the project no. (IFKSURG-2020-148).
Declarations
AA, SA, ZA, FA, BA and HA have no conflicts of interest that are directly relevant to the content of this article.
Ethics approval
The study was conducted in KKUH in accordance the ethical principles provided in the Declaration of Helsinki. The IRB Approval number is E-21-5783.
Consent to participate
All volunteers provided informed written consent before participating in the study.
Availability of Data and Material
The datasets from previous studies are publicly available. The data generated during the current study are not publicly available but can be requested from the corresponding author.
Authors’ Contributions
HA and BA designed and performed the research. HA analyzed the data. AA and SA interpreted the data, reviewed the available literature and wrote the manuscript. AA, SA, ZA, HA, FA and BA critically reviewed the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2021.11.011.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abou-Auda, H. S., 2014. Possible Interethnic Differences in Rifampin Pharmacokinetics: Comparison of Middle Eastern Arabs With Other Populations. Advanced Techniques in Biology & Medicine.
- Al-Ghazawi M.A., Tutunji M.S., AbuRuz S.M. The effects of pummelo juice on pharmacokinetics of sildenafil in healthy adult male Jordanian volunteers. Eur. J. Clin. Pharmacol. 2010;66(2):159–163. doi: 10.1007/s00228-009-0738-0. [DOI] [PubMed] [Google Scholar]
- AlKhamees O.A., AlNemer K.A., Bin Maneea M.W., AlSugair F.A., AlEnizi B.H., Alharf A.A. Top 10 most used drugs in the Kingdom of Saudi Arabia 2010–2015. Saudi Pharm J. 2018;26(2):211–216. doi: 10.1016/j.jsps.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boolell M., Allen M.J., Ballard S.A., et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996 [PubMed] [Google Scholar]
- Bruzziches R., Francomano D., Gareri P., Lenzi A., Aversa A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin. Pharmacother. 2013;14(10):1333–1344. doi: 10.1517/14656566.2013.799665. [DOI] [PubMed] [Google Scholar]
- Castañeda‐Hernández G., Palma‐Aguirre J.A., Montoya‐Cabrera M.A., Flores‐Murrieta F.J. Interethnic variability in nifedipine disposition: reduced systemic plasma clearance in Mexican subjects. Br. J. Clin. Pharmacol. 1996;41(5):433–434. [PubMed] [Google Scholar]
- Chávez-Teyes L., Casta??eda-Hern??ndez G., Flores-Murrieta F.J. Pharmacokinetics of midazolam in Mexicans evidence for interethnic variability. Clinical drug. 1999;17(3):233–239. [Google Scholar]
- de Denus S., Rouleau J.L., Mann D.L., Huggins G.S., Pereira N.L., Shah S.H., Cappola T.P., Fouodjio R., Mongrain I., Dubé M.-P. CYP3A4 genotype is associated with sildenafil concentrations in patients with heart failure with preserved ejection fraction. Pharmacogenomics J. 2018;18(2):232–237. doi: 10.1038/tpj.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Murrieta F.J., Castaneda-Hernandez G., Granados-Soto V., et al. Increased bioavailability of sildenafil in Mexican men. JAMA. 2000 doi: 10.1001/jama.283.14.1825. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis K. Sildenafil in the treatment of erectile dysfunction: an overview of the clinical evidence. Clin. Interv. Aging. 2006;1(4):403–414. doi: 10.2147/ciia.2006.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedaya M., El-Afify D., El-Maghraby G. The effect of ciprofloxacin and clarithromycin on sildenafil oral bioavailability in human volunteers. Bullet Pharm Sci Assiut. 2005;28(1):137–142. doi: 10.1002/bdd.488. [DOI] [PubMed] [Google Scholar]
- Hegazy S. The effect of green tea on sildenafil pharmacokinetics in Egyptian healthy volunteers. J. Pharm. Res. 2014;4(3):289–300. [Google Scholar]
- Jetter A., Kinzig-Schippers M., Walchner-Bonjean M., et al. Effects of grapefruit juice on the pharmacokinetics of sildenafil. Clin. Pharmacol. Ther. 2002 doi: 10.1067/mcp.2002.121236. [DOI] [PubMed] [Google Scholar]
- Kanjanawart S., Gaysonsiri D., Tangsucharit P., Vannaprasaht S., Phunikhom K., Kaewkamson T., Wattanachai N., Tassaneeyakul W. Comparative bioavailability of two sildenafil tablet formulations after single-dose administration in healthy Thai male volunteers. Int. J. Clin. Pharmacol. Ther. 2011;49(08):525–530. doi: 10.5414/cp201496. [DOI] [PubMed] [Google Scholar]
- Irons M.T.K., Lang C.C., He H.B., Ghebreselasie K., Shay S., Robin D.W., Wood A.J.J. Triazolam pharmacokinetics and pharmacodynamics in Caucasians and Southern Asians: ethnicity and CYP3A activity. Br. J. Clin. Pharmacol. 1996;41(1):69–72. doi: 10.1111/j.1365-2125.1996.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Loprete L., Leuratti C., Frangione V., Radicioni M. Pharmacokinetics of a Novel Sildenafil Orodispersible Film Administered by the Supralingual and the Sublingual Route to Healthy Men. Clin Drug Investig. 2018;38(8):765–772. doi: 10.1007/s40261-018-0665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudian M., Falahatpishe H., Taiebi L., et al. Determination of pharmacokinetic parameters of sildenafil in Iranian volunteers by an HPLC method. Turkish. J. Pharm. Sci. 2010 [Google Scholar]
- Mancinelli L.M., Frassetto L., Floren L.C., et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin. Pharmacol. Ther. 2001 doi: 10.1067/mcp.2001.113183. [DOI] [PubMed] [Google Scholar]
- Marcelín-Jiménez G., Ángeles-Moreno A.P., Contreras-Zavala L., García-González A., Ramírez-San Juan E. Comparison of fasting bioavailability among 100-mg commercial, 100-mg generic, and 50-mg chewable generic sildenafil tablets in healthy male Mexican volunteers: a single-dose, 3-period, crossover study. Clin. Ther. 2012;34(3):689–698. doi: 10.1016/j.clinthera.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Muirhead G.J., Wulff M.B., Fielding A., Kleinermans D., Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br. J. Clin. Pharmacol. 2000;50(2):99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D.J., Muirhead G.J., Harness J.A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002;53:5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer-Laboratories. 1998. VIAGRA- sildenafil citrate tablet prescriping information. from http://labeling.pfizer.com/ShowLabeling.aspx?id=652.
- Schall R., Luus H.G. Comparison of absorption rates in bioequivalence studies of immediate release drug formulations. Int. J. Clin. Pharmacol. Ther. Toxicol. 1992 [PubMed] [Google Scholar]
- Shilbayeh S., Tutunji M.F. Possible interethnic differences in omeprazole pharmacokinetics : comparison of Jordanian Arabs with other populations. Clin. Pharmacokinet. 2006;45(6):593–610. doi: 10.2165/00003088-200645060-00003. [DOI] [PubMed] [Google Scholar]
- Tang P.F., Zheng X., Hu X.X., et al. Functional Measurement of CYP2C9 and CYP3A4 Allelic Polymorphism on Sildenafil Metabolism. Drug Des. Devel. Ther. 2020 doi: 10.2147/DDDT.S268796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela F., Davila G., Ibañez Y. Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100mg in Healthy Volunteers Under Fasting Conditions. J. Bioequiv. Availab. 2011;03(09) doi: 10.4172/jbb10.4172/jbb.1000087. [DOI] [Google Scholar]
- Walker D.K., Ackland M.J., James G.C., et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999 doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- Werk A.N., Cascorbi I. Functional gene variants of CYP3A4. Clin. Pharmacol. Ther. 2014;96(3):340–348. doi: 10.1038/clpt.2014.129. [DOI] [PubMed] [Google Scholar]
- Yasuda S.U., Zhang L., Huang S.-M. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin. Pharmacol. Ther. 2008;84(3):417–423. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets from previous studies are publicly available. The data generated during the current study are not publicly available but can be requested from the corresponding author.