Abstract
Objectives
Danzhi Jiangtang capsule (DJC) is widely used for preventing and treating diabetic cardiomyopathy (DCM). However, the underlying mechanisms of the anti-inflammatory and antiapoptotic activities are unclear.
Methods
In the in vivo diabetic cardiomyopathy rat model, cardiac function was measured through echocardiography, histological changes in the myocardium were visualized using HE staining, and cardiomyocyte apoptosis was detected using TUNEL. The serum levels of anti-inflammatory cytokines were detected using ELISA. Finally, TLR4, MyD88, and NF-κB mRNA expressions were analyzed using RT-qPCR. In the in vitro experiments, the apoptosis rate of the H9c2 cells was detected using FCM; moreover, TLR4, MyD88 and NF-κB mRNA expressions were measured using RT-qPCR and related protein levels were investigated using Western blotting.
Results
In vivo, DJC effectively improved cardiac function, alleviated the pathological changes, and reduced the apoptosis rate. Moreover, DJC reduced TNF-α, IL-1β, and IL-6 activities, with significant inhibition of the TLR4, MyD88 and NF-κB p65 mRNA expression. Moreover, in vitro, DJC effectively inhibited high-glucose-induced H9c2 apoptosis-an effect similar to that for TAK242. Finally, both the DJC and TAK242 considerably reduced TLR4, MyD88, NF-κB, Bax, and caspase-3 protein expression but increased that of BCL-2.
Conclusions
DJC prevented the overactivation of the TLR4/MyD88/NF-κB signaling pathway and regulate cardiomyocyte apoptosis against DCM.
Keywords: Danzhi Jiangtang capsule, Diabetic cardiomyopathy, TLR4/MyD88/NF-κB signaling pathway, Apoptosis
1. Introduction
The prevalence of diabetes mellitus (DM), a chronic disease with severe effects on human health, and its incidence is increasing significantly worldwide (Mannino et al., 2019). The International Diabetes Federation (IDF) has predicted that by 2040, the number of patients with diabetes in the world will increase by > 50%, reaching 642 million (Jairoun et al., 2021). Diabetes-related microvascular complications increase risk of cardiovascular complications such as ischemic heart disease, peripheral vascular disease, and cardiomyopathy (Orchard and Costacou, 2017).
Diabetic cardiomyopathy (DCM), caused by extensive focal myocardial necrosis based on metabolic disorders and microvascular lesions, leads to heart failure, arrhythmia, cardiogenic shock, and even sudden death in patients with severe condition (Ali et al., 2020, Tan et al., 2020). The main pathological manifestations of DCM are cardiomyocyte necrosis, apoptosis, microvascular lesions and interstitial fibrosis (Borghetti et al., 2018). Moreover, its pathogenesis mainly includes insulin resistance, abnormal renin–angiotensin–aldosterone system (RAAS) activation, elevated inflammatory mediator levels, increased oxidative stress, and immune system dysfunction (Guo et al., 2016, Kolpakov et al., 2019). Studies have recently shown that inflammation is central in DCM occurrence and development (Wu et al., 2019a, Wu et al., 2019b). Although some modern drugs are used for DCM prevention and treatment more newer and safer prognostic drugs are required. Here, cardioprotective traditional Chinese medicine (TCM) drugs may have great potential.
DCM is closely related to the overactivation of the toll-like receptor 4 (TLR4)/nuclear factor (NF)-κB signaling pathway. TLR4 is usually overexpressed in diabetic cardiomyocytes, can activate proinflammatory factor and chemokine expression by regulating the myeloid differentiation factor 88 (MyD88)-dependent pathway. This activation can lead to many conditions, including cardiovascular, inflammatory, neurodegenerative, and autoimmune disorders (Huang et al., 2018, Tang et al., 2019, Kim et al., 2020). In particular, DCM pathogenesis involves an inflammatory response possibly triggered by this mechanism (Zhang et al., 2020).
The Danzhi Jiangtang capsule (DJC) is a hospital preparation for treating DM created by the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine (Fang et al., 2013, Zhao et al., 2015). DJCs can attenuate the toxicity induced by increased glucose levels by reducing apoptosis through the GLP-1/Akt signaling pathway (Wu et al. 2019). Its clinical application, spanning over 30 years, has shown that DJC is effective in preventing and treating diabetic complications, including diabetic nephropathy (DN), cardiovascular complications, and DCM. DJCs can significantly improve diabetic renal dysfunction, and its mechanism may be related to its antioxidant capacity and to it being able to inhibit the JAK2–STAT/STAT3 cascade, which can be used for DN treatment (Sun et al., 2019). DJCs also demonstrated protective effects on vascular endothelial injury, inflammation, and apoptosis induced by high-fat diet (HFD) via the reduction of lipid deposition, oxidative and endoplasmic reticulum stress damage (Lu et al., 2018). When used for DCM treatment, DJC can effectively alleviate myocardial injury and reduce TGF-β1, Smad2, and Smad3 protein expression in DM rats; here, the underlying mechanism may be related to the regulation of TGF-β/Smad signaling pathway and the alleviation of myocardial fibrosis (Liu et al., 2019). In our previous studies, DJCs could significantly improve cardiac hemodynamics and regulate myocardial pathological changes in DM rats, and their action was related to the inhibition of the TLR4/MyD88/NF-κB signaling pathway overexpression (Shi et al., 2019). However, the specific mechanism of DJC in the treatment of DCM is still unclear and needs further study.
In this study, we hypothesized that DJCs alleviate DCM through anti-inflammatory and antiapoptotic mechanisms. Accordingly, we evaluated the anti-inflammatory and antiapoptotic effects of DJC in vivo and in vitro, all to provide theoretical and experimental basis for the clinical application.
2. Materials and methods
2.1. Animals
We purchased Sprague Dawley (SD) rats, aged 8 to 9 weeks (Male, body weight = 200 ± 20 g) from the Experimental Animal Center of Anhui Medical University [Certificate No. SCXK (Anhui) 2011–002]. All rats were fed according to standard conditions.
All experiments met the requirements pertaining to the handling of animals and thus were approved by the Experimental Animal Ethics Committee of Anhui University of Chinese Medicine.
2.2. Chemicals and regents
Paeoniflorin was purchased from Shanghai Yuanye Biotechnology Company (Shanghai, China), and TAK-242 was from APExBio (A3850). TLR4, MyD88, NF-κB p65, and β-actin were purchased from Abcam (USA) and those against caspase-3, Bax, and BCL2 were from Abbkine (China). The H9c2 cell line were purchased from the cell resource center of Shanghai Institute of Life Sciences, Chinese Academy of Sciences.
2.3. DJC preparation
DJCs were provided by the First Affiliated Hospital of Anhui University of Chinese Medicine (patent number: ZL200310112845.1). Every DJC contained a 0.4 g of a mixture of Radix Pseudostellariae, Radix Rehmanniae, Cortex Moutan, Rhizoma Alismatis, Semen Cuscutae Chinensis, and Leech (ratio: 6:5:4:4:3:3).
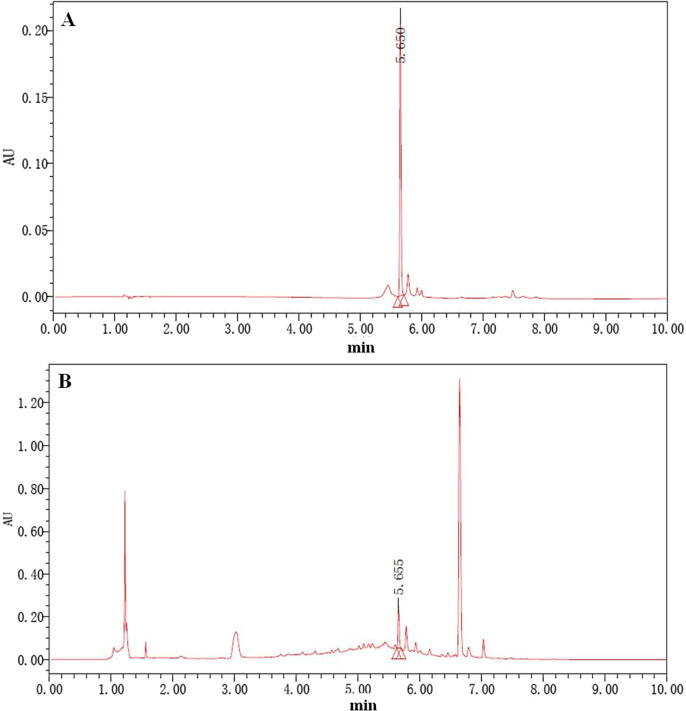
DJC preparation method has been reported by Jiang et al. (2011). For quality control, the amount of paeoniflorin was quantified, and it was found to be 0.1 mg/g (Fig. 1) (Wang et al., 2012).
Fig. 1.
UPLC-PDA analysis of DJC. The chromatograms of paeoniflorin (A) and DJC (B).
2.4. In vivo experiment
2.4.1. Rat model establishment
Control (CTL) group rats were fed a normal diet, whereas the others were fed a high-energy diet (containing 51% carbohydrate, 14% protein, and 35% fat) for 6 weeks. After the 6 weeks, all the rats (except CTL group) were intraperitoneally injected with 1% STZ (BioFroxx, EZ3414B220) at 35 mg/kg body weight. After 3 days, the random blood glucose level ≥ 16.7 mmol /L, indicating that the modeling was successful. And then the drug intervention was started immediately.
2.4.2. Drug treatment
We divided the model rats into 4 groups, with 15 rats in each group: the model group (DM + distilled water), low dose DJC (DJCL) group (DM + 270 mg/kg DJC), medium dose DJC (DJCM) group (DM + 540 mg/kg DJC), and high dose DJC (DJCH) group (DM + 1080 mg/kg DJC). DJC was dissolved in distilled water and given to the rats by intragastric administration. After 8 weeks of continuous administration, all rats were anesthetized with pentobarbital sodium, dissected under sterile conditions, and euthanized, followed by heart tissue analysis.
2.4.3. Echocardiography for cardiac function analysis
All rats were anesthetized with isoflurane and administered 2D echocardiography on (Sabatino et al., 2020) (VisualSonics, Canada) for calculating ejection fraction (EF) and fractional shortening (FS).
2.4.4. Myocardial histology
The hearts were fixed in 4% paraformaldehyde. Then, paraffin was used to embedded 2-mm-thick slices of the ventricle and then cut into 5-μm-thick sections. Histopathological changes were observed by hematoxylin and eosin (H&E).
2.4.5. TUNEL for myocardial apoptosis detection
As with the embedding and sectioning procedures of HE staining, sections (thickness = 5 μm) were obtained. The free 3 '–OH chain breaks induced by DNA degradation were detected by TdT-mediated dUTP nick-end labeling (TUNEL) technique. Under the microscope, five visual fields were randomly selected from each slice; in these fields, we counted the numbers of all cardiomyocytes and of those apoptotic. Next, the apoptosis rate = number of apoptotic cardiomyocytes/number of all cardiomyocytes × 100%.
2.4.6. ELISA for serum levels of TNF-α, IL-1β and IL-6
The levels of tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β) and IL-6 in rats serum were detected by commercial test kits (Jiancheng Bio-engineering Institute, Nanjing, China). The wavelength was selected at 450 nm, and the data was read by a microplate reader (Biotek, USA).
2.4.7. Reverse transcription real-time polymerase chain reaction (RT-PCR) for mRNA expression measurement
Total RNA was extracted from left ventricular myocardium at 50 mg using the Trizol reagent and then reverse-transcribed to cDNA. RNA purity and quantification were determined based on its absorbance at 260 and 280 nm on a UV spectrophotometer. The cDNA was then analyzed using ABI 7500 real-time fluorescent quantitative PCR instrument (ABI, USA). Reaction conditions were set as follows: (1) 95 °C for 2 min, (2) 40 cycles of 95 °C for 5 s, (3) 60 °C for 10 s.
The mRNA expression levels of TLR4, MyD88 and NF-κB p65 were analyzed using the comparative 2–ΔΔCt method. The primer sequences used were shown in Table 1.
Table 1.
Primers used in RT-qPCR.
| Primers | Sequence (5′→3′) | |
|---|---|---|
| TLR4 | Forward | TGATCATGGCATTGTTCCTT |
| Reverse | TGATCCATGCATTGGTAGGT | |
| MyD88 | Forward | TGGTGGTTGTTTCTGACGAT |
| Reverse | GATCAGTCGCTTCTGTTGGA | |
| NF-κB p65 | Forward | TTTTGATAACCGTGCCCCCA |
| Reverse | GATCTCATCCCCACCAAGGC | |
| β-actin | Forward | CCCATCTATGAGGGTTACGC |
| Reverse | TTTAATGTCACGCACGATTTC | |
2.5. In vitro experiment
2.5.1. Preparation of DJC-containing serum
Thirty rats were randomly divided into two groups. Normal saline was given to rats in blank control group, and DJC (5.4 g/kg/day) was given to DJC group for consecutive 7 days. DJC was dissolved in distilled water and given to the rats by intragastric administration. The rats were anesthetized using intraperitoneal sodium pentobarbital (Sigma, USA), and their abdominal aorta blood was harvested from rats under aseptic conditions 1 h after DJC or saline administration, followed by serum extraction. The centrifugally separated serum was placed at 56 °C and inactivated for 30 min.The obtained serum can be used after microporous membrane filtration to eliminate any bacterial contamination and kept at −80 °C for later use.
2.5.2. Cell culture and treatment
H9c2 cells were cultured in 15% fetal bovine serum (FBS; Sigma, USA), penicillin (100 U/mL)/ streptomycin (100 U/mL) mixture in Dulbecco’s modified Eagle medium (DMEM) under 37 °C and 5% CO2. The H9c2 cells were inoculated into 6-well plates and incubated in 15% FBS-containing DMEM for 24 h, followed by starvation in serum-free low-glucose DMEM for 24 h, to induce high glucose (HG)-induced H9c2 injury model. H9c2 cells were grouped: (1) CTL (5.5 mmol/L glucose + 15% normal serum), (2) HG (30 mmol/L glucose + 15% normal serum), (3) HG + DJC (30 mmol/L glucose + 15% DJC-containing-serum), and (4) HG + TAK242 (30 mmol/L glucose + 15% normal serum + 1.0 μmol/L TAK-242) groups. All groups were treated for 48 h.
2.5.3. Flow cytometry (FCM) for apoptosis detection
In brief, H9c2 cells were washed with cold (4 °C) PBS and digested using 0.25% trypsin. Next, 400 μL digestive solution and 5 μL annexin V/FITC staining solution were incubated for 15 min, and then 5 μL propidium iodide (PI) staining fluid was added for 5 min. We measured the percentages of apoptotic cells on a DxFlex flow cytometer (Beckman, USA) and analyzed them using FlowJo (version 7.6).
2.5.4. RT-qPCR for mRNA expression measurement
For each group, a cell suspension was inoculated in a 6-well plate at 1 × 106 cells/well. Next, RT-qPCR were performed using the methods presented in in vivo RT-PCR.
2.5.5. Western blotting for protein expression measurement
The protein concentration was firstly quantitatively determined by BCA detection kit (Beyotime, China) to get the protein concentration for the preparation of subsequent experiments. Next, we performed polyacrylamide gradient gel electrophoresis to separate the proteins using a 4%-to-12% polyacrylamide gradient gel. These separated proteins were then transferred onto polyvinylidene fluoride membranes. The membranes were blocked in 5% non-fat dry milk for 1 h and incubated overnight with primary antibody at 4 °C. After they were carefully washed three times with tris-buffered saline with tween 20 (TBST), which were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. The concentrations of antibody were TLR4(1:1000), NF-κB p65(1:1000), MyD88(1:500), Caspase-3 (1:500), Bax(1:500), Bcl2 (1:500) and β-actin(1:1000), which were visualized through electrochemiluminescence, with the detected protein bands captured on a chemiluminescence image analyzer.
2.5.6. Statistical analysis
All statistical data were analyzed using SPSS (version 23.0) and GraphPad Prism (version 5.0), with the significance level set at P < 0.05. Multiple groups were compared using one-way analysis of variance and the least significant difference method.
3. Results
3.1. DJC improves cardiac function in DCM rats
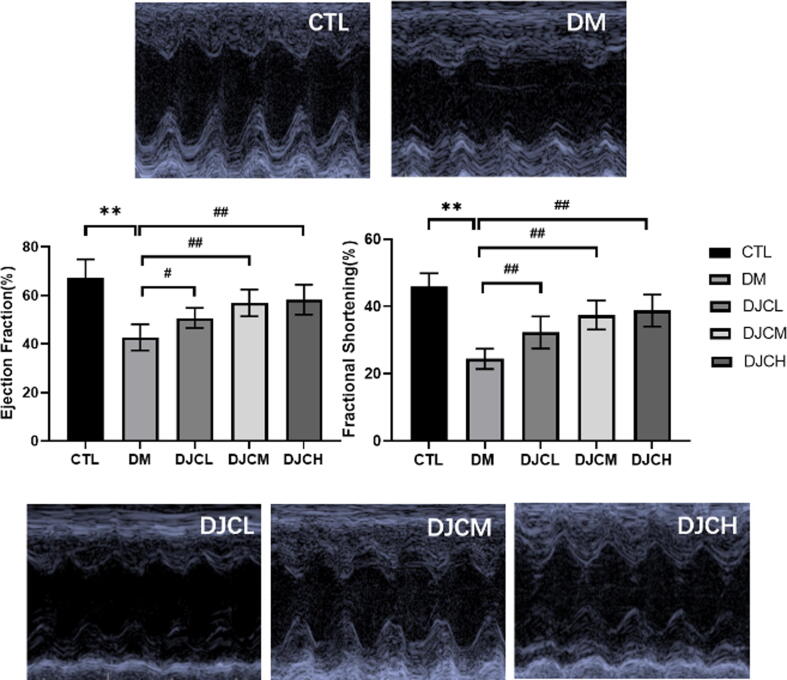
Compared with the CTL group, the DM group demonstrated significant decreases in ejection fraction (EF) and fractional shortening (FS; P < 0.01). Moreover, different DJC concentrations had varied, dose-dependent effects on EF and FS (P < 0.01); that is, the therapeutic effects of DJCs are dose-dependent (Fig. 2). Thus, DJCs could effectively improve cardiac function.
Fig. 2.
DJC improves cardiac function in rats with DCM. Representative echocardiograms of CTL, DM, DJCL, DJCM, and DJCH group rats after treatment for 8 weeks. The values are expressed as mean ± standard deviation (n = 15). **P < 0.01 vs. CTL group, ##P < 0.01 vs. DM group.
3.2. DJC alleviates morphological changes and myocardial apoptosis in DCM rats
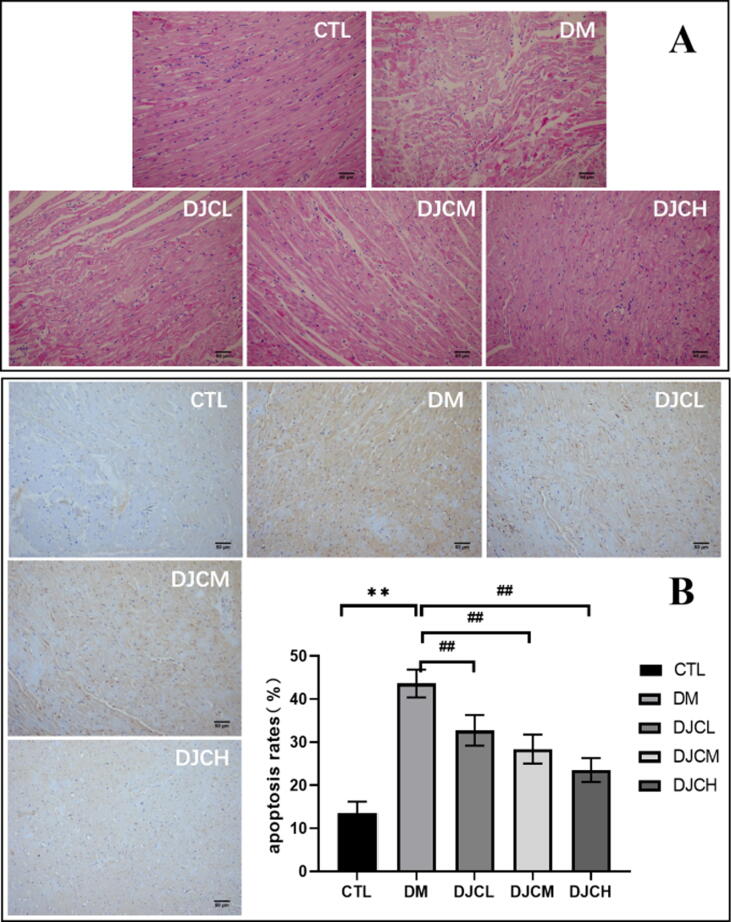
H&E staining results indicated the effect of DJCs on rats with DCM induced histopathological alterations. As shown in Fig. 3A, in the CTL group, the myocardial morphology was normal, with a clear texture, ordered arrangement, and few cardiac fibroblasts. The DM group demonstrated myolysis of cardiomyocytes and infiltration of inflammatory cells. In the DJC-treated groups, myocardial cells had a regular cell arrangement with a clear structure and attenuated pathological changes.
Fig. 3.
DJC alleviates morphological changes (A) and apoptosis (B) in the myocardium. (Scale bar = 50 μm) Values are expressed as mean ± standard deviation (n = 15). **P < 0.01 vs. CTL group, ##P < 0.01 vs. DM group.
The TUNEL results demonstrated cellular apoptosis in the myocardial tissue. As shown in Fig. 3B, many brownish yellow nuclei were found in the DM group, with the apoptosis rate being significantly higher than that in the CTL group (P < 0.01). After 8 weeks, compared with the DM group, the apoptosis rate in the DJC-treated groups were significantly reduced (P < 0.01), with the therapeutic effects being dose-dependent.
Together, these results indicated that DJCs could effectively alleviate the pathological changes and reduce the apoptosis rate.
3.3. DJC inhibits TLR4/MyD88/NF-κB signaling pathway protein overexpression in DCM rats
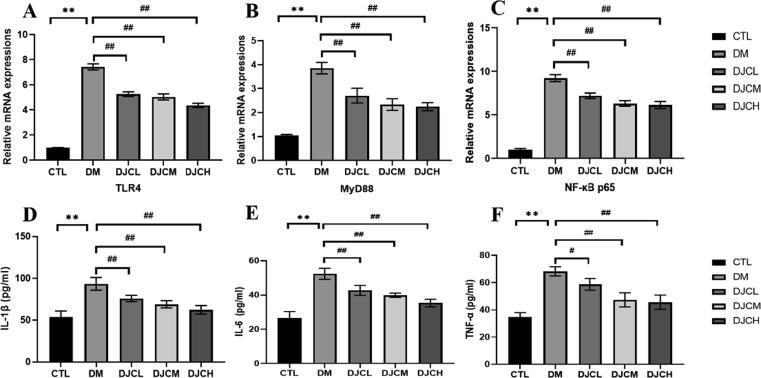
As shown in Fig. 4, the RT-qPCR indicated that TLR4, MyD88, and NF-κB p65 mRNA expression in the rat myocardium increased significantly in the DM group compared with that in the CTL group (P < 0.01). TNF-α, IL-1β, and IL-6 activities in rat serum increased significantly in the DM group compared with the CTL group (P < 0.01). Moreover, TLR4, MyD88, and NF-κB p65 mRNA expression decreased in the DJCL, DJCM, and DJCH groups compared with that in the DM group (P < 0.01). TNF-α, IL-1β, and IL-6 activities in rat serum were decreased significantly in the DJC groups compared with that in the DM group (P < 0.01, P < 0.05, and P < 0.05, respectively).
Fig. 4.
Effect of DJC on TLR4/MyD88/NF-κB signaling pathway in diabetic rats. TLR4 (A), MyD88 (B) and NF-κB p65 (C) mRNA level in the rat myocardium. TNF-α (D), IL-1β (E), and IL-6 (F) activities in rat serum. The values are expressed as mean ± standard deviation (n = 10 in each group). **P < 0.01 vs. CTL group; #P < 0.05, ##P < 0.01 vs. DM group.
3.4. DJC inhibits H9c2 cell apoptosis
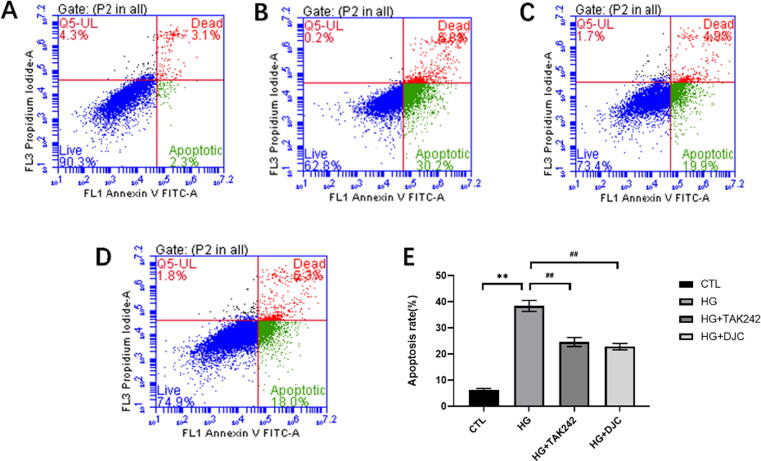
H9c2 cells were treated with 30 mmol/L high-glucose solution for 48 h, which led to a significant increase in the apoptosis rate in the HG group but decreased in the HG + DJC and HG + TAK242 compared with the CTL group (P < 0.01; Fig. 5). These results suggested that DJC effectively inhibited H9c2 cell apoptosis induced by high glucose, and the effect was similar to that of TAK242.
Fig. 5.
DJC inhibits H9c2 cell apoptosis. A: CTL group; B: HG group; C: HG + DJC group; D: HG + TAK242 group; E: H9c2 cell apoptosis rate. The values are expressed as mean ± standard deviation. **P < 0.01 vs. CTL group; ##P < 0.01 vs. HG group.
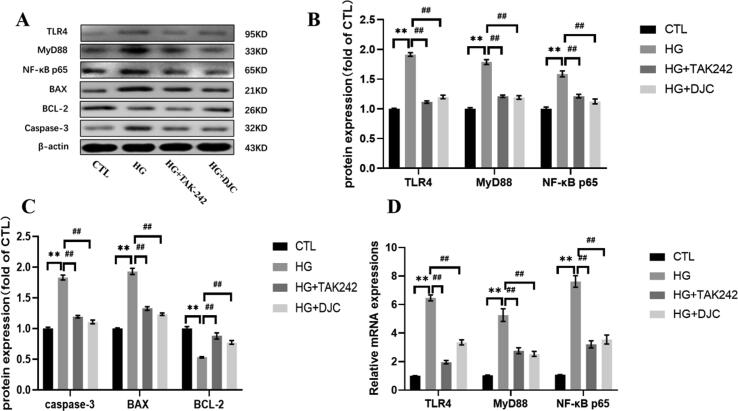
3.5. DJC regulates TLR4, MyD88, and NF-κB p65 expression in H9c2 cells
As shown in Fig. 6, in the HG group, the protein expression levels of TLR4, MyD88, NF-κB p65, Bax, and caspase-3 increased, whereas those of BCL2 decreased significantly compared with CTL group (P < 0.01). After DJC and TAK242 treatment, TLR4, MyD88, NF-κB p65, Bax, and caspase-3 expression considerably decreased, whereas that of BCL2 significantly increased compared with that in the HG group (P < 0.01). TLR4, MyD88, and NF-κB p65 mRNA expression trend was consistent with that of the protein expression (P < 0.01).
Fig. 6.
TLR4 (A, B), MyD88 (A, B), NF-κB p65 (A, B), Bax (A, C), BCL2 (A, C), and caspase-3 (A, C) protein expression; TLR4, MyD88 and NF-κB p65 (D) mRNA levels. **P < 0.01 vs. CTL group; ##P < 0.01 vs. HG group.
4. Discussion
A DJC has been widely for preventing and treating of cardiovascular complications of DM for nearly 20 years, and they have achieved satisfactory clinical effect (Zheng et al., 2015). Our previous studies have shown that a DJC effectively reduces blood glucose levels, regulates blood lipid, improves insulin resistance, regulates neuroendocrine and inflammatory factors, and has a definite curative effect on cardiovascular complications of DM (Shi et al., 2019). The recent results suggest that DJCs can effectively protect the cardiac structure and function damage in diabetic rats, which is related to the TLR4/MyD88/NF-κB signaling pathway. Therefore, in this study, we studied the underlying mechanism of this activity, exploring how DJCs protect against cardiac structure and function damage.
Patients with type 2 DM have cardiac dysfunction and myocardial injury, both of which are manifested by the thinning of ventricular wall, dysfunction of myocardial contraction, and increase of left ventricular volume (Li et al., 2019, Mahdi et al., 2019, Li et al., 2020a, Li et al., 2020b). DCM is the primary structural and functional injury of the heart caused by disordered glucose metabolism, which is a main complication of T2DM and a main causes of heart failure and death in patients with advanced diabetes (Wang et al., 2020). Hyperglycemia, as the major feature of diabetes, can induce myocardial cell metabolism disorder and damage to the endoplasmic reticulum and mitochondria, leading to increased cardiomyocyte oxidative stress, inflammation, and apoptosis, which in turn leads to myocardial remodeling and dysfunction (Huang et al., 2016). Therefore, inflammation and apoptosis are closely related to cardiomyopathy occurrence and development.
Apoptosis is a complex network structure reaction, with caspases, Bcl2, and adaptor proteins playing an important role in its occurrence and development. Of these, caspase-3, a downstream apoptosis regulator and Bax, is a proapoptotic protein. BCL2 and MCL1 are antiapoptotic proteins, which inhibit apoptosis by blocking the permeability of mitochondrial membrane of proapoptotic proteins (Xu et al., 2020). Activation of innate immune system and chronic inflammation provide new evidence for the pathogenesis of diabetic complications. TLRs plays an important role in immune response activation and inflammation-mediated heart disease. Here, after high glucose stimulated the cardiomyocytes, TLR4 expression increased as noted based on the increase in TLR4 receptor ligand expression on the membrane surface. MyD88 is a downstream protein of TLR4. The activity of NF-κB increases via the TLR4/MyD88 pathway (Yuan et al., 2019, Li et al., 2020a, Li et al., 2020b). NF-κB is a transcription factor located downstream of TLR4. Activated by TLR4, NF-κB can regulate apoptosis and inflammatory response of cardiomyocytes by regulating downstream apoptotic genes or inflammatory mediators. In particular, when the TLR4/MyD88/NF-κB signaling pathway is activated, which causes NF-κB to migrate from cytoplasm to nucleus and thereby induces apoptosis. And activated NF-κB can further synthesize and release inflammatory cytokines such as IL-1, IL-6, and TNF-α, all of which can play the role of early immune response and induce myocardial inflammatory response (Youssef et al., 2021). TAK-242, being a specific inhibitor of TLR4, can significantly reduce the inflammatory response. TAK-242 can selectively bind to Cys747 in the TIR domain of TLR4 and inhibit TLR4 signal transduction by interrupting the interactions between TLR4 and its adaptor molecule, by directly binding to the intracellular region of TLR4, thus inhibiting inflammatory factor production (Matsumoto et al., 2020).
Our results showed that DJCs could effectively inhibit cardiomyocyte apoptosis in rats with type 2 DM induced by a high-fat diet combined with STZ. The results of in vivo experiments showed that DJC could significantly improve the heart function, alleviate the pathological changes, and reduce the apoptosis of myocardial cells, that means it has good efficacy on DCM. DJC could decrease TLR4, MyD88, and NF-κB p65 mRNA expression, and reduce the levels of downstream inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, suggested that it may be related to the TLR4/MyD88/NF-κB signaling pathway. Moreover, the results of the in vitro experiments confirmed that a DJC-containing serum can effectively reduce the apoptosis rate that was induced by 30 mmol/L glucose in H9c2 cells. The serum could also inhibit the expression of TLR4/MyD88/NF-κB signaling pathway proteins and mRNA, all of which are involved in apoptosis regulation. Thus, the mechanism underlying DJC-based DCM prevention and treatment involves prevention of TLR4/MyD88/NF-κB signaling pathway overactivation and regulation of cardiomyocyte apoptosis.
5. Conclusion
In this study, we confirmed the effects of the DJC on the DCM-related cardiomyocyte apoptosis in vitro and in vivo. Our results enrich the theoretical basis of the DJC’s mechanism of action in protecting DM-related cardiovascular complications, laying a foundation for the clinical application of this TCM drug.
Compliance with ethical standards
All experiments met the requirements pertaining to the handling of animals and thus were approved by the Experimental Animal Ethics Committee of Anhui University of Chinese Medicine.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
zio National Natural Science Foundation of China (Nos. 81202631, 82004180), Natural Science Fund of Anhui Province (No.1708085MH201), and Key Project Foundation of Natural Science Research of Anhui University of Chinese Medicine (2020zrzd06).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Zhao-hui Fang, Email: fangzh9097@126.com.
Liang Wang, Email: wangliang_01@163.com.
References
- Ali T.M., Abo-Salem O.M., El Esawy B.H., El Askary A. The potential protective effects of diosmin on streptozotocin-induced diabetic cardiomyopathy in rats. Am. J. Med. Sci. 2020;359(1):32–41. doi: 10.1016/j.amjms.2019.10.005. [DOI] [PubMed] [Google Scholar]
- Borghetti G., von Lewinski D., Eaton D.M., Sourij H., Houser S.R., Wallner M. Diabetic cardiomyopathy: Current and future therapies. Beyond glycemic control. Front Physiol. 2018;9:1514. doi: 10.3389/fphys.2018.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z.H., Liu Y., Bao T.T., Ni Y.Q., Liu J., Shi G.B., Wu J.P., Yang J.P., Zhang H. Effect of Danzhijiangtang capsule on monocyte chemoattractant protein-1 mRNA expression in newly diagnosed diabetes subclinical vascular lesions. World J. Gastroenterol. 2013;19(19):2963–2969. doi: 10.3748/wjg.v19.i19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Xue M., Li C.J., Yang W., Wang S.S., Ma Z.J., Zhang X.N., Wang X.Y., Zhao R., Chang B.C., Chen L.M. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J. Ethnopharmacol. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- Huang Z., Zhou T., Sun X., Zheng Y., Cheng B., Li M., Liu X., He C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018;25(1):180–189. doi: 10.1038/cdd.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhuang X., Xie C., Hu X., Dong X., Guo Y., Li S., Liao X. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol. Biochem. 2016;40(6):1578–1590. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- Jairoun A.A., Shahwan M.J., Khattab M.H. A comparative assessment of metabolic dyndrome and its sssociation with vitamin D and other risk factors in type 2 diabetes mellitus patients. Curr. Diabetes Rev. 2021;17:233–242. doi: 10.2174/1573399816666200716193115. [DOI] [PubMed] [Google Scholar]
- Jiang L., Wang Y.Z., Han Y.Q. Optimization of extracting process of Danzhi Jiangtang capsule. Chin. J. Inform. on TCM. 2011;18:55–57. [Google Scholar]
- Kim H.Y., Hong M.H., Yoon J.J., Kim D.S., Na S.W., Jang Y.J., Lee Y.J., Kang D.G., Lee H.S. Protective effect of Vitis labrusca leaves extract on cardiovascular dysfunction through HMGB1-TLR4-NFκB signaling in spontaneously hypertensive rats. Nutrients. 2020;12(10):3096. doi: 10.3390/nu12103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakov M.A., Sikder K., Sarkar A., Chaki S., Shukla S.K., Guo X., Qi Z., Barbery C., Sabri A., Rafiq K. Inflammatory serine proteases play a critical role in the early pathogenesis of diabetic cardiomyopathy. Cell Physiol. Biochem. 2019;53:982–998. doi: 10.33594/000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Liu Y., Shan Y.G., Gao L., Wang F., Qiu C.G. Bailcalin protects against diabetic cardiomyopathy through Keap1/Nrf2/AMPK-mediated antioxidative and lipid-lowering effects. Oxid. Med. Cell Longev. 2019;2019:1–15. doi: 10.1155/2019/3206542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zheng Z., Tang X., Zhong J., Liu X., Zhao Y., Chen L., Zhu J., Liu J., Chen Y. Impact of HbA1c variability on subclinical left ventricular remodeling and dysfunction in patients with type 2 diabetes mellitus. Clin. Chim. Acta. 2020;502:159–166. doi: 10.1016/j.cca.2019.12.006. [DOI] [PubMed] [Google Scholar]
- Li X.X., Zheng X., Liu Z., Xu Q., Tang H., Feng J., Yang S., Vong C.T., Gao H., Wang Y. Cryptotanshinone from Salvia miltiorrhiza Bunge (Danshen) inhibited inflammatory responses via TLR4/MyD88 signaling pathway. Chin. Med. 2020;15:20. doi: 10.1186/s13020-020-00303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Shi H., Wang L., Gao G., Shen A.L. Effects of Danzhi Jiangtang Capsules on myocardial injury in diabetic rats. Chin. Tradi. Patent Med. 2019;41:1616–1620. [Google Scholar]
- Lu Y., Chen Y., Li R., Liu Q., Wang N., Zhang Y.i., Li B., Fang Z. Protective effects of Danzhi jiangtang capsule on vascular endothelial damages induced by high-fat diet and palmitic acid. Biomed. Pharmacother. 2018;107:1631–1640. doi: 10.1016/j.biopha.2018.08.129. [DOI] [PubMed] [Google Scholar]
- Mahdi A., Jiao T., Yang J., Kövamees O., Alvarsson M., von Heijne M., Zhou Z., Pernow J. The effect of glycemic control on endothelial and cardiac dysfunction induced by red blood cells in type 2 diabetes. Front Pharmacol. 2019;10:861. doi: 10.3389/fphar.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino G.C., Andreozzi F., Sesti G. Pharmacogenetics of type 2 diabetes mellitus, the route toward tailored medicine. Diabetes Metab. Res. Rev. 2019;35(3):e3109. doi: 10.1002/dmrr.v35.310.1002/dmrr.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Takayanagi K., Kojima M., Taguchi K., Kobayashi T. Toll-like receptor 4 inhibitor TAK-242 augments acetylcholine-induced relaxation in superior mesenteric arteries of the streptozotocin-induced diabetic rat. Biol. Pharm. Bull. 2020;43(8):1283–1287. doi: 10.1248/bpb.b20-00328. [DOI] [PubMed] [Google Scholar]
- Orchard T.J., Costacou T. Cardiovascular complications of type 1 diabetes: update on the renal link. Acta. Diabetologica. 2017;54(4):325–334. doi: 10.1007/s00592-016-0949-7. [DOI] [PubMed] [Google Scholar]
- Sabatino J., De Rosa S., Tammè L., Iaconetti C., Sorrentino S., Polimeni A., Mignogna C., Amorosi A., Spaccarotella C., Yasuda M., Indolfi C. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020;19:66. doi: 10.1186/s12933-020-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang L., Fang Z.H., Ni Y.Q., Shen A.L., Liu P.P., Wang X., Huang J.L. Experimental study on effect and mechanism of Danzhi Jiangtang Capsules on diabetic myocardial injury. Zhongguo Zhong Yao Za Zhi. 2019;2019(44):5159–5165. doi: 10.19540/j.cnki.cjcmm.20191015.401. [DOI] [PubMed] [Google Scholar]
- Sun M., Bu W., Li Y., Zhu J., Zhao J., Zhang P., Gu L., Zhang W., Fang Z. Danzhi Jiangtang capsule ameliorates kidney injury via inhibition of the JAK-STAT signaling pathway and increased antioxidant capacity in STZ-induced diabetic nephropathy rats. Biosci. Trends. 2019;12:595–604. doi: 10.5582/bst.2018.01255. [DOI] [PubMed] [Google Scholar]
- Tan Y., Zhang Z., Zheng C., Wintergerst K.A., Keller B.B., Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat. Rev. Cardiol. 2020;17(9):585–607. doi: 10.1038/s41569-020-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Li T., Han X., Sun J. TLR4 antagonist ameliorates combined allergic rhinitis and asthma syndrome (CARAS) by reducing inflammatory monocytes infiltration in mice model. Int. Immunopharmacol. 2019;73:254–260. doi: 10.1016/j.intimp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Wang Y.Z., Jiang L., Han Y.Q., Zuo D., Luo H., Xia L.Z. Simultaneous determination of the contents of paeonol and paeoniflorin in Danzhi Jiangtang capsule by UPLC. Chin. J. New Drugs. 2012;21:1337–1340. [Google Scholar]
- Wang Z., Zhu Y., Zhang Y., Zhang J., Ji T., Li W., Li W. Protective effects of AS-IV on diabetic cardiomyopathy by improving myocardial lipid metabolism in rat models of T2DM. Biomed. Pharmacother. 2020;127:110081. doi: 10.1016/j.biopha.2020.110081. [DOI] [PubMed] [Google Scholar]
- Wu B., Huang X.-Y., Li L.e., Fan X.-H., Li P.-C., Huang C.-Q., Xiao J., Gui R., Wang S. Attenuation of diabetic cardiomyopathy by relying on kirenol to suppress inflammation in a diabetic rat model. J. Cell Mol. Med. 2019;23(11):7651–7663. doi: 10.1111/jcmm.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.-J., Wu Y.-B., Fang Z.-H., Chen M.-Q., Wang Y.-F., Wu C.-Y., Lv M.-A. Danzhi Jiangtang capsule mediates NIT-1 insulinoma cell proliferation and apoptosis by GLP-1/Akt signaling pathway. Evid. Based Complement Alternat. Med. 2019;2019:1–7. doi: 10.1155/2019/5356825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Zhang X., Chen X., Yang S., Chen H. Inhibition of miR-223 attenuates the NLRP3 inflammasome activation, fibrosis, and apoptosis in diabetic cardiomyopathy. Life Sci. 2020;256:117980. doi: 10.1016/j.lfs.2020.117980. [DOI] [PubMed] [Google Scholar]
- Youssef M.E., Abdelrazek H.M., Moustafa Y.M. Cardioprotective role of GTS-21 by attenuating the TLR4/NF-κB pathway in streptozotocin-induced diabetic cardiomyopathy in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2021;394(1):11–31. doi: 10.1007/s00210-020-01957-4. [DOI] [PubMed] [Google Scholar]
- Yuan R., Huang L., Du L.J., Feng J.F., Li J., Luo Y.Y., Xu Q.M., Yang S.L., Gao H., Feng Y.L. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol. Res. 2019;142:102–114. doi: 10.1016/j.phrs.2019.02.017. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Y., Huang X., Zhang F., Tang L., Xu S., Liu Y., Tong N., Min W. Systemic delivery of siRNA specific for silencing TLR4 gene expression reduces diabetic cardiomyopathy in a mouse model of streptozotocin-induced type 1 diabetes. Diabetes Ther. 2020;11:1161–1173. doi: 10.1007/s13300-020-00802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.D., Ding L., Bao T.T., Wang H., Lu R.M., Cui L.Q., Xie F.T., Yang X., Fang Z.H. Meta-analysis of adjusting blood lipid of type 2 diabetes mellitus by Danzhi Jiangtang capsule. Chin. Arch. Tradi. Chin. Med. 2015;33:570–573. [Google Scholar]
- Zheng S.G., Tao S.J., Zhao M.Q., Ren Y.N., Wu Y.J. Effect of Danzhi Jiangtang capsule on myocardial fibrosis in diabetic rats. Zhong Yao Cai. 2015;38:2120–2124. [PubMed] [Google Scholar]