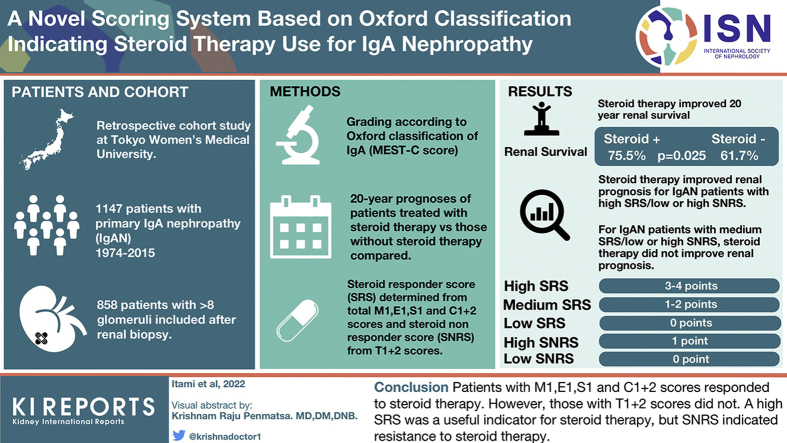
Abstract
Introduction
The usefulness of the Oxford classification (MEST-C score) for deciding the management approach for IgA nephropathy (IgAN) remains unclear.
Methods
Effects of steroid therapy on the long-term prognosis for all 858 patients with IgAN and patients classified according to each MEST-C score were evaluated using Kaplan-Meier and Cox regression analyses. Steroid responder score (SRS) and steroid nonresponder score (SNRS) were determined using individual pathology scores when steroids were found to be independently associated, or not, with clinical benefits. In addition, the effects of steroid therapy according to the total SRS/SNRS were analyzed.
Results
Steroid therapy improved the 20-year renal survival rates of patients with IgAN after matching (steroids[+] vs. steroids[−]; estimated glomerular filtration rate [eGFR] [ml/min per 1.73 m2]: 79.4 vs. 77.0, not significant; proteinuria [g/d]: 0.80 vs. 0.62, not significant; renal survival rate: 75.5% vs. 61.7%; P = 0.025) and of patients with M1, E1, S1, C1+2, and T0 scores. Therefore, we considered the total of the M1, E1, S1, and C1+2 scores (point 0: low, 1–2: medium, and 3–4: high) as the SRS and the total of the T1+2 scores (0: low and 1: high) as the SNRS. Multivariate Cox regression analyses revealed that steroid therapy improved the renal prognosis of patients with IgAN with high SRS and any SNRS, unlike patients with IgAN with medium SRS and any SNRS.
Conclusion
Patients with M1, E1, S1, and C1+2 scores responded to steroid therapy; however, those with T1+2 scores did not. Although a high SRS was a useful indicator for steroid therapy, SNRS indicated resistance to steroid therapy.
Keywords: IgA nephropathy, indication, Oxford classification, prognosis, steroid, treatments
Graphical abstract
See Commentary on Page 3
The first report of IgAN was published by Berger more than 50 years ago.1 Since then, the beneficial effects of treatment strategies for the prevention of progression to end-stage renal disease (ESRD) have been reported.2, 3, 4, 5, 6 Indications for these therapies are generally decided based on disease activity, such as proteinuria and hematuria levels, renal function, and the background of the patient, including age, presence of hypertension, and comorbidities.7, 8, 9 Histologic findings should also be considered, and several validation and classification systems that predict the renal prognosis have been reported.10, 11, 12, 13, 14 In 2009, the International IgAN Network and Renal Pathology Society established the Oxford classification, which considers 4 predictive lesions as follows: mesangial hypercellularity (M), endocapillary hyper cellularity (E), segmental sclerosis (S), and tubule-interstitial fibrosis (T).15,16 There have been several validation studies of the Oxford classification system (MEST score) as a prognostic tool,17,18 and the Oxford classification has been widely recognized to be useful for predicting prognoses; however, only a few studies have investigated the indications for steroid therapy according to the MEST score.19, 20, 21 The European Validation Study of the Oxford Classification of IgAN (VALIGA) indicated that steroid/immunosuppression blunted the predictive abilities of MST scores of the Oxford classification.19 Furthermore, other studies have revealed the responses of IgAN to steroid/immunosuppressants in patients with each MEST score.20,21 In 2017, crescent formations (C) were added to the original Oxford classification (MEST-C).22 Because E and C scores were added to the Oxford classification to indicate steroid treatment for improving renal outcomes.15,16,22,23 the Oxford classification should be used more frequently to confirm indications for steroid therapy. A recent analysis of VALIGA indicated an association between the C score and the rate of eGFR loss only in patients not treated with steroids/immunosuppressants, indicating steroids/immunosuppressants improved the status of patients with C scores.24 Pathologic findings are indications for steroid therapy; however, how to use the Oxford classification to determine indications for steroid therapy remains unknown. Therefore, we analyzed the clinical utility of the Oxford classification for making decisions regarding steroid therapy. First, we confirmed the beneficial effects of steroid therapy for IgAN in our cohort and the responses of patients with each MEST-C score to steroid therapy. Subsequently, based on those results, we introduced a new method of determining indications for steroid therapy using the total MEST-C score based on the responses to steroid therapy using the Oxford classification.
Methods
Study Design and Participants
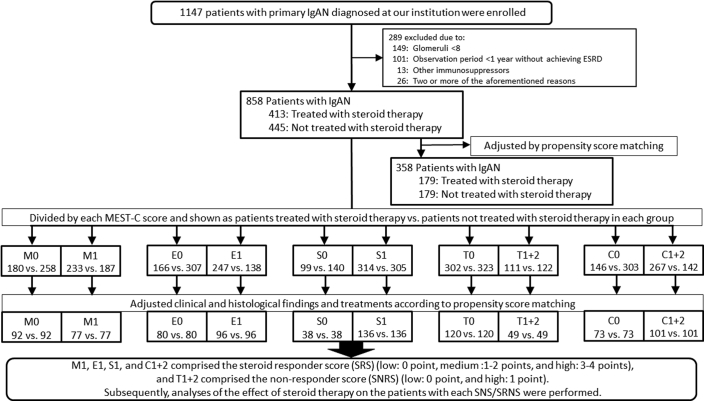
In this study, 1147 patients diagnosed with having primary IgAN at the Tokyo Women’s Medical University between 1974 and 2015 were enrolled. Of these patients, we selected 858 patients with >8 glomeruli, according to renal biopsy results, who consequently underwent observation for at least 1 year unless ESRD occurred within 1 year. Patients with IgAN treated with immunosuppressive agents other than steroid therapy and with systemic diseases, such as systemic lupus erythematosus, liver cirrhosis, and IgA vasculitis with nephritis, were excluded. Furthermore, for all patients and for each patient with M0, M1, E0, E1, S0, S1, T0, T1+2, C0, and C1+2 scores, the 20-year prognoses of patients treated with and without steroid therapy were compared using the Kaplan-Meier analysis. Steroid therapy for each patient with M0, M1, E0, E1, S0, S1, T0, T1+2, C0, and C1+2 scores was additionally analyzed to determine the relation of its use with renal prognosis according to univariate and multivariate Cox regression analyses. On the basis of the results of those analyses and the indications of each MEST-C score for steroid therapy, the SRS and SNRS were determined. The effects of steroid therapy on the renal prognoses of patients based on both the total SRS and total SNRS were analyzed using univariate and multivariate Cox regression analyses (Figure 1).
Figure 1.
Flow diagram of patient recruitment and enrolment. C, crescent formations; E, endothelial hypercellularity; ESRD, end-stage renal disease; IgAN, IgA nephropathy; M, mesangial hypercellularity; S, segmental sclerosis; SNRS, steroid nonresponder score; SRS, steroid responder score; T, tubule-interstitial fibrosis.
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Tokyo Women’s Medical University (reference #5104-R). All patients were able to view the announcements of our study by visiting our institution’s website, and they were provided the opportunity to ask questions and discuss the study with us.
Diagnosis of IgAN and Histologic Evaluation of Renal Biopsy Specimens
Histologic findings were graded according to the Oxford classification,15,16,22 which scores 5 key pathologic features of each specimen. Mesangial hypercellularity was scored as M0 if >50% of the glomeruli had fewer than 3 cells per mesangial area or as M1 if >50% of glomeruli had >3 cells per mesangial area. Segmental glomerulosclerosis was scored as absent (S0) or present (S1). Endocapillary hypercellularity was scored as absent (E0) or present (E1). The tubular atrophy/interstitial fibrosis score was based on the ratio of tubular atrophy to interstitial fibrosis in the total interstitium and scored as T0 (0%–25%), T1 (26%–50%), or T2 (>50%). The crescent score was based on the ratio of the number of glomeruli with cellular or fibrocellular crescents to the total number of glomeruli and scored as C0 (0%), C1 (>0% and <25%), or C2 (≥25%). The number of patients with T2 and C2 was very low to evaluate; therefore, T2 and C2 were combined with T1 and C1, respectively. All histologic findings were obtained from the pathology reports of each renal biopsy evaluated by renal pathologists; some of the reports were repeatedly reviewed by pathologists to confirm the findings.
Clinical and Laboratory Data
Each patient’s sex, age, body mass index, systolic, diastolic, and mean arterial pressure were recorded. Laboratory data included serum total protein, creatinine, eGFR, uric acid, total cholesterol, triglycerides, urinary protein excretion, and urinary red blood cells at the time of renal biopsy; these were evaluated as baseline data. eGFR was calculated using the modified isotope dilution mass spectrometry Modification of Diet in Renal Disease study results for Japanese individuals (eGFR = 194 × serum creatinine−1.094 × age−0.287 × 0.739 [if female]).25 The time to progression to ESRD, defined as requiring dialysis or renal transplantation, was evaluated as the end point. Similarly, risk factors associated with progression to ESRD were evaluated.
Treatments
Steroid therapy involved the following 2 protocols: oral prednisolone and steroid pulse therapy. At our institution, patients with IgAN were mainly treated with oral prednisolone before 2006; however, they were treated with steroid pulse therapy after 2007 based on the report of Pozzi et al.26 regarding the effects of steroid pulse therapy. The oral prednisolone protocol involved the administration of a daily dose of 0.5 to 0.8 mg/kg (body weight)/d for the first 4 weeks, gradual tapering by 2.5 to 5 mg every 4 weeks, and continued administration for at least 2 years. The steroid pulse therapy protocol was revised according to the methods of Pozzi et al.26 Patients were treated with i.v. methylprednisolone pulse 500 mg/d for 3 consecutive days and 2 additional steroid pulse therapies during the next 6 months; during those 6 months, 0.5 mg/kg (body weight) oral prednisolone was also administered every other day and gradually tapered off within the next 2 months. These 2 steroid therapy protocols had similar effects on the renal prognoses of our cohorts (Supplementary Table S1). Therefore, we uniformly used these 2 steroid therapy protocols during this analysis. The administration of renin-angiotensin system inhibitors and the performance of tonsillectomy were based on the decisions of the physicians.
Statistical Analyses
Data are presented as mean ± SD for normally distributed data and as median and interquartile range for skewed data. Data were analyzed using JMP Pro 15.0.0 (SAS Institute Inc., Cary, NC). The 20-year renal survival rates until ESRD were calculated using the Kaplan-Meier method and compared using the log-rank test. Clinical findings were compared using the unpaired Student’s t test for normally distributed data and the Mann–Whitney U test for skewed data. The χ2 test was used to compare sex distribution, number of patients with each urinary red blood cell grade at the time of renal biopsy, and the Oxford classification. Propensity score matching was performed to adjust for differences in clinical and histologic findings for each patient with each MEST-C score and for each patient with SRS/SNRS, regardless of the steroid therapy. One-to-one digit matching (nearest neighbor matching with calipers) was performed using the propensity score and logistic regression model. Univariate and multivariate Cox regression analyses were performed to evaluate the risk of ESRD. Results of the univariate and multivariate analyses are presented as hazard ratios with 95% CIs. For all analyses, values of P < 0.05 were considered statistically significant.
Results
Renal Prognosis for Patients With IgAN With or Without Steroid Therapy With Each MEST-C Score
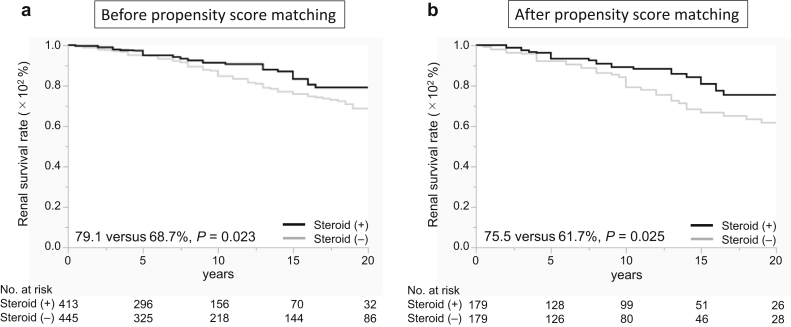
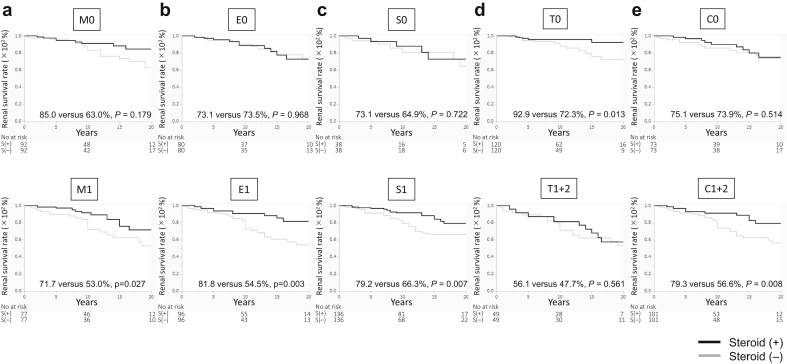
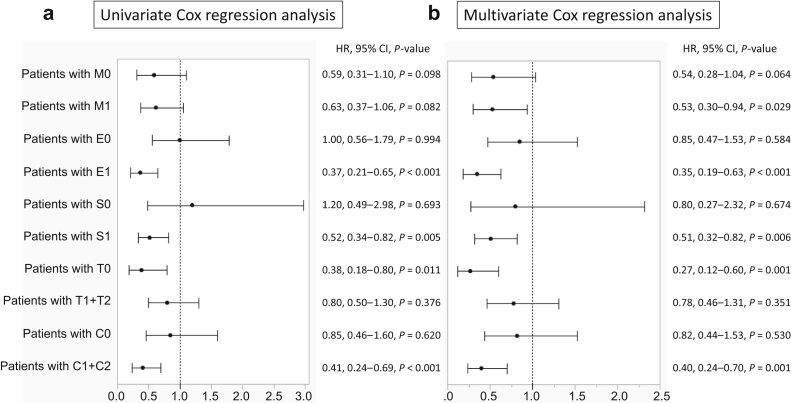
The baseline data and renal prognosis before propensity score matching are summarized for all patients (Table 1 and Figure 2a). The systolic blood pressure was significantly lower in the steroid group than in the nonsteroid group. The eGFR, urinary protein excretion, frequency of high levels of urinary red blood cells, and total cholesterol levels were significantly higher, and total protein levels were significantly lower in the steroid group than in the nonsteroid group. The frequencies of M1, E1, S1, C1, and C2 scores were significantly higher in the steroid group than in the nonsteroid group. The number of patients treated with tonsillectomy and renin-angiotensin system inhibitors was significantly higher in the steroid group than in the nonsteroid group. After matching based on these significantly different factors between the groups (caliper a, 0.20; caliper c, 0.23), the clinical and laboratory data, histologic findings, and treatments were similar between patients treated and not treated with steroid therapy (Table 1). The 20-year renal survival rates for patients treated with steroid therapy were significantly higher than those of the patients treated without steroid therapy (75.5% vs. 61.7%; P = 0.025) (Figure 2b). The 20-year renal survival rates of patients treated with steroid therapy were significantly higher than those of patients treated without steroid therapy among patients with M1 (71.7% vs. 53.0%; P = 0.027), E1 (81.8% vs. 54.5%; P = 0.003), S1 (79.2% vs. 66.3%; P = 0.007), T0 (92.9% vs. 72.3%; P = 0.013), and C1+2 (79.3% vs. 56.6%; P = 0.008) scores after propensity score matching (Figure 3a-e). The 20-year renal survival rates before matching are found in Supplementary Figure S1. Results of the univariate Cox regression analyses and multivariate analyses adjusted for MEST-C are summarized in Figure 4a and b and Supplementary Table S2. For patients with M1, steroid therapy was not an independent factor that prevents progression, according to the univariate analysis; however, the multivariate analysis adjusted for E, S, T, and C scores revealed that steroid therapy was an independent factor that prevents progression (hazard ratio, 0.53; P = 0.029). For patients with E1, S1, C1+2, and T0 scores, steroid treatment was an independent factor that prevented progression, according to the univariate and multivariate analyses adjusted for other MEST-C scores. Nevertheless, for patients with M0, E0, S0, T1+2, and C0 scores, steroid therapy was not an independent factor that prevented progression, according to the univariate and multivariate Cox regression analyses (Supplementary Table S2).
Table 1.
Baseline characteristics of IgAN patients treated with or without steroid therapy before and after propensity score matching
| All patients (n = 858) | Before propensity score matching |
After propensity score matching |
|||||
|---|---|---|---|---|---|---|---|
| Steroid group (n = 413) | Nonsteroid group (n = 445) | P value | Steroid group (n = 179) | Nonsteroid group (n = 179) | P value | ||
| Clinical findings | |||||||
| Age (yr) | 31.0 (24.0–41.0) | 30.0 (24.0–40.5) | 30.0 (24.0–41.0) | 0.911 | 29.0 (24.0–41.0) | 29.0 (24.0–41.0) | 0.774 |
| Male sex | 349 (42.4%) | 175 (39.3%) | 174 (39.1%) | 0.330 | 82 (45.8%) | 75 (41.8%) | 0.456 |
| BMI (kg/m2) | 21.3 (19.6–23.5) | 21.4 (19.6–23.7) | 21.3 (19.6–23.3) | 0.963 | 21.5 (19.4–23.5) | 21.7 (19.8–25.7) | 0.330 |
| SBP (mm Hg) | 120.0 (110–132.0) | 118.0 (110.0–130.0) | 120.0 (110.0–132.0) | 0.046 | 119.0 (110.0–132.0) | 120.0 (111.0–134.0) | 0.346 |
| DBP (mm Hg) | 74.0 (66.0–83.0) | 74.0 (66.0–82.0) | 75.0 (66.0–84.0) | 0.530 | 75.0 (67.0–82.0) | 75.0 (67.0–86.0) | 0.724 |
| MAP (mm Hg) | 89.7 (81.3–99.0) | 88.3 (81.7–98.0) | 90.3 (80.7–100.0) | 0.263 | 90.3 (82.7–99.0) | 90.7 (81.3–101.1) | 0.626 |
| Laboratory findings | |||||||
| TP (g/dl) | 6.8 (6.3–7.2) | 6.7 (6.2–7.1) | 6.9 (6.5–7.3) | <0.001 | 6.6 (6.3–7.1) | 6.8 (6.3–7.2) | 0.106 |
| eGFR (ml/min/1.73 m2) | 77.0 (60.0–95.6) | 78.7 (61.6–96.0) | 73.3 (58.8–91.4) | 0.032 | 78.5 (62.5–96.7) | 75.2 (59.1–100.3) | 0.708 |
| UA (mg/dl) | 5.5 (4.5–6.7) | 5.6 (4.6–6.7) | 5.4 (4.4–6.8) | 0.429 | 5.8 (4.7–6.7) | 5.6 (4.5–7.1) | 0.895 |
| T-Cho (mg/dl) | 192.0 (168.0–225.0) | 201.0 (173.9–233.0) | 187.5 (164.0–212.0) | <0.001 | 195.0 (171.0–226.0) | 189.0 (168.0–225.0) | 0.415 |
| TG (mg/dl) | 100.0 (73.0–144.0) | 99.0 (75.0–142.0) | 101.0 (71.0–146.0) | 0.791 | 102.5 (78.5–149.3) | 106.0 (74.0–165.0) | 0.652 |
| U-Prot (g/d) | 0.68 (0.3–1.4) | 0.85 (0.37–1.78) | 0.55 (0.24–1.10) | <0.001 | 0.90(0.44-1.67) | 0.73 (0.31–1.4) | 0.109 |
| U-RBC (counts/HPF) <5, 5–25, 26–49, 50–99, ≤100 |
84, 360, 138, 117, 154 | 27, 174, 81, 58, 72 | 57, 186, 57, 59, 82 | 0.005 | 16, 70, 35, 22, 36 | 20, 71, 21, 27, 40 | 0.318 |
| Histologic findings, n | |||||||
| M0/M1 | 438/420 | 180/233 | 258/187 | <0.001 | 96/83 | 99/80 | 0.750 |
| E0/E1 | 473/385 | 166/247 | 307/138 | <0.001 | 77/102 | 81/98 | 0.670 |
| S0/S1 | 239/619 | 99/314 | 140/305 | 0.015 | 41/138 | 42/137 | 0.900 |
| T0/T1/T2 | 625/183/50 | 302/89/22 | 323/94/28 | 0.831 | 125/46/8 | 128/40/11 | 0.628 |
| C0/C1/C2 | 448/366/43 | 145/230/37 | 303/136/6 | <0.001 | 75/94/10 | 67/106/6 | 0.338 |
| Treatment, n | |||||||
| Steroid therapy (−/+) | 445/413 | —/413 | 445/— | — | —/179 | 179/— | — |
| Tonsillectomy (−/+) | 669/189 | 239/174 | 430/15 | <0.001 | 166/13 | 165/14 | 0.841 |
| RAS inhibitors (−/+) | 575/283 | 246/167 | 329/116 | <0.001 | 116/63 | 114/65 | 0.825 |
BMI, body mass index; C, crescent formations; DBP, diastolic blood pressure; E, endocapillary hypercellularity; eGFR, estimated glomerular filtration rate; HPF, high-power field; M, mesangial hypercellularity; MAP, mean arterial pressure; RAS, renin-angiotensin system; S, segmental sclerosis; SBP, systolic blood pressure; T, interstitial fibrosis/tubular atrophy; T-Cho, total cholesterol; TG, total glycerides; TP, total protein; UA, urinary albumin; U-Prot, urinary protein; U-RBC, urinary red blood cell.
Figure 2.
Renal survival rates of patients with IgA nephropathy treated with or without steroid therapy. The renal survival rates of all patients treated with steroid therapy were significantly higher than those of patients who were not treated with steroid therapy before (a) and after (b) propensity score matching.
Figure 3.
Renal survival rates of patients with IgA nephropathy treated with or without steroid therapy in relation to the Oxford classification. The 20-year renal survival rates of patients with IgA nephropathy with or without steroid therapy are presented. (a) M scores: M0, 85.0% versus 63.0% (P = 0.179); M1, 71.7% versus 53.0% (P = 0.027). (b) E scores: E0, 73.1% versus 73.5% (P = 0.968); E1, 81.8% versus 54.5% (P = 0.003). (c) S scores: S0, 73.1% versus 64.9% (P = 0.722); S1, 79.2% versus 66.3% (P = 0.007). (d) T scores: T0, 92.9% versus 72.3% (P = 0.013); T1+2, 56.1% versus 47.7 (P = 0.561). (e) C scores: C0, 75.1% versus 73.9% (P = 0.514); C1+2, 79.3% versus 56.6% (P = 0.008). C, crescent formations; E, endothelial hypercellularity; M, mesangial hypercellularity; S, segmental sclerosis; T, tubule-interstitial fibrosis.
Figure 4.
Univariate and multivariate Cox regression analyses of steroid treatment for patients with each MEST-C score. (a) According to the univariate analysis, steroid therapy was an independent factor for the prevention of IgAN progression in patients with E1, S1, T0, and C1+2 scores. (b) According to the multivariate analysis, after adjustment for other MEST-C scores, steroid therapy was an independent factor for the prevention of IgAN progression in patients with M1, E1, S1, T0, and C1+2 scores. C, crescent formations; E, endothelial hypercellularity; HR, hazard ratio; M, mesangial hypercellularity; S, segmental sclerosis; T, tubule-interstitial fibrosis.
On the basis of these findings of the effects of steroid therapy on the renal prognosis for patients with each MEST-C score, we designated M1, E1, S1, and C1+2 scores as the SRS and T1+2 scores as the SNRS. The total points of SRS were 0 to 4, and we considered 0 point, 1 to 2 points, and 3 to 4 points to indicate low, medium, and high SRS, respectively. The total points of SNRS were 0 and 1, and we similarly considered 0 point and 1 point to indicate low and high SNRS, respectively. Therefore, to determine the feasibility of the Oxford classification in terms of making decisions regarding indications for steroid therapy, we analyzed whether the combination of the low, medium, or high SRS and the low or high SNRS was related to the renal prognosis after steroid therapy.
Univariate and Multivariate Cox Regression Analyses of Steroid Therapy for Patients With Each SRS and SNRS
The number and ratio of patients exhibiting progression to ESRD with each SRS/SNRS were evaluated (Table 2). Model 1 included the univariate Cox regression analysis. Model 2 included the multivariate Cox regression analysis with the clinical and laboratory findings. Model 3 included the multivariate Cox regression analysis with the clinical and laboratory findings and the treatments. For patients with high SRS/low or high SNRS, steroid therapy was a significant independent factor that prevented progression according to the univariate and multivariate analyses (high SRS/low SNRS, models 1, 2, and 3; high SRS/high SNRS, models 1 and 2). For patients with medium SRS/low or high SNRS, steroid therapy was not an independent factor according to the univariate and multivariate analyses. For patients with low SRS/low SNRS, none treated with steroid therapy experienced progression to ESRD; however, this was not evaluated statistically. The number of patients with low SRS/high SNRS (n = 10) was not statistically sufficient.
Table 2.
Ratio of progression to ESRD and univariate and multivariate Cox regression analyses of steroid therapy for patients with IgA nephropathy with each SRS/SNRS
| Cox analysis | Steroid nonresponder score (T1+2) |
||||
|---|---|---|---|---|---|
| low (0): T0 (n = 625) |
high (1): T1+2 (n = 233) |
||||
| ESRD/total (ratio) | HR, 95% CI, P value | ESRD/total (ratio) | HR, 95% CI, P value | ||
| Steroid responder score (M1, E1, S1, C1+2) | |||||
| Low (0) (n = 106) | Model 1 Model 2 Model 3 |
4/96 (4.2%) | Not evaluated (No patient progressed to ESRD in the steroid group) | 3/10 (30.0%) | Not evaluated (The number of patients was too small) |
| Medium (1–2) (n = 384) | Model 1 Model 2 Model 3 |
24/273 (8.8%) | 0.04, 0.11–1.28, P = 0.119 0.31, 0.08–1.18, P = 0.086 0.32, 0.08–1.36, P = 0.122 |
33/111 (29.7%) | 0.68, 0.42–2.82, P = 0.375 1.00, 0.45–2.23, P = 0.998 1.02, 0.45–2.29, P = 0.963 |
| High (3–4) (n = 368) | Model 1 Model 2 Model 3 |
15/256 (4.7%) | 0.33, 0.12–0.92, P = 0.034 0.08, 0.02–0.32, P < 0.001 0.13, 0.03–0.52, P = 0.004 |
34/112 (30.4%) | 0.47, 0.24–0.94, P = 0.032 0.40, 0.20–0.82, P = 0.013 0.51, 0.24–1.04, P = 0.065 |
C, crescent formations; E, endothelial hypercellularity; ESRD, end-stage renal disease; M, mesangial hypercellularity; S, segmental sclerosis; SNRS, steroid nonresponder score; SRS, steroid responder score; T, tubule-interstitial fibrosis.
Model 1: univariate analysis.
Model 2: multivariate analysis with age, sex, mean arterial pressure, estimated glomerular filtration rate, and urinary protein excretion.
Model 3: multivariate analysis with model 2 + tonsillectomy + renin-angiotensin system inhibitors.
Discussion
This study revealed the effects of steroid therapy on the long-term prognoses of patients with IgAN and revealed that steroid therapy is particularly effective for the patients with IgAN patients M1, E1, S1, and C1+2 scores of the Oxford classification. Moreover, higher M1, E1, S1, and C1+2 scores may be useful indicators for steroid therapy; however, patients with T1+2 scores were not responsive to steroid therapy.
We have presented the new grading scale, the O-grade, with the total score of the Oxford classification in our previous report.27 In that study, we calculated the total of each MEST-C score to predict the progression to ESRD. The O-grade was similar to the SRS/SNRS because it combined MEST-C scores; however, the O-grade did not consider the adaptation of treatments. Furthermore, we reported the renal survival rates for patients with each MEST-C score by considering treatment with immunosuppressive agents, including steroid therapy. We found that the T score was the best predictor of the renal prognosis, irrespective of the immunosuppressive agents, and that the renal prognosis of patients with E1, C1, or S1 scores improved with the administration of immunosuppressive agents.28 Nevertheless, in that study, we performed a direct comparison of patients with E1, C1, and S1 scores who were treated with or without immunosuppressive agents without matching the baseline characteristics of the groups; however, we did not perform a direct comparison of patients with other scores. Moreover, the response to immunosuppressive agents was not analyzed using a combination of other scores, as was performed with the O-grade. In that study, 13 patients treated with immunosuppressive agents other than steroids were included, and the effects of steroid therapy alone were not accurately evaluated. To resolve these issues encountered in our previous studies, after the exclusion of patients treated with immunosuppressive agents other than steroids, we conducted a direct comparison of patients with each MEST-C score who were treated with or without steroid therapy and adjusted for clinical findings and treatments in both groups (Figures 3 and 4, Supplementary Table S2, and Supplementary Figure S1). Subsequently, in this study, we analyzed the effects of steroid therapy on the total MEST-C scores of patients after considering the SRS and SNRS (Table 2). In this analysis, patients with E1, C1+2, and S1 scores responded to steroid therapy even after adjustments similar to those performed in our previous study.25 Moreover, patients with M1 scores also responded to steroid therapy after adjustment. Nevertheless, patients with T1+2 scores were resistant to steroid therapy, and T1+2 scores were found to be independent factors that determine progression to ESRD when considering other scores of the Oxford classification. Therefore, we designated M1, E1, S1, and C1+2 scores as the SRS and T1+2 scores as the SNRS. Furthermore, our results indicate that steroid therapy affected patients with IgAN with high SRS (SRS: 3–4 points) and low or high SNRS (high SRS/low SNRS [models 1, 2, and 3] or high SRS/high SNRS [models 1 and 2]), but did not affect patients with IgAN with medium SRS (SRS: 1–2 points) and low or high SNRS. Of the patients with low SRS/low SNRS, none in the steroid group progressed to ESRD. These results indicated that the SRS and SNRS of the Oxford classification are useful for guiding decisions regarding the use of steroid therapy.
The clinical usefulness of the Oxford classification for determining the indications for steroid therapy remains controversial; however, a previous study reported that corticosteroid therapy reduced proteinuria in patients with M1 scores and proteinuria of >1.0 g/g creatinine but that it did not improve the renal prognosis. Moreover, corticosteroid therapy did not improve the renal prognosis of patients with E1, S1, and T1 scores.20 Palamuthusingam et al.29 did not identify any advantages with the use of the MEST score (during an analysis performed without the C score) when predicting the efficacy of immunosuppressive agents. Nevertheless, crescents and/or necrotizing lesions are more likely to be indications of immunosuppression.29 A systematic review and meta-analysis of the MEST score (another analysis performed without the C score) involving 5 studies with 637 patients with IgAN revealed that patients with IgAN with M1, S1, and T1+2 (designated as T1/T2 in that review) scores were resistant to steroid therapy; however, patients with E1 scores exhibited a better response to steroid therapy.21 Although the results of previous studies have some discrepancies, they indicated the possibility that the findings of each Oxford classification determine the appropriate treatment for IgAN. Moreover, we suspected that the split system resulted in these discrepancies because the treatment was not decided based on the histologic findings of one lesion. In Japan, an inclusive system to evaluate the histologic findings was proposed; this combined system and the clinical findings were useful for predicting renal prognoses and making treatment decisions.30, 31, 32 With this classification, the ratio of the glomeruli with lesions to the total number of glomeruli was evaluated. Moreover, active lesions, such as cellular or fibrocellular crescent formations, and chronic lesions, such as fibrous crescents, segmental sclerosis, and global sclerosis, were considered. In this study, we used the SRS and SNRS instead of active and chronic lesions to determine the indications for steroid therapy. Our analysis indicated that steroid therapy is useful in patients with IgAN with high SRS/low SNRS and probably useful in high SRS/high SNRS, whereas steroid therapy is not useful in medium SRS/low or high SNRS. The indications for steroid therapy identified in this study might not be generalizable to other cohorts. Nevertheless, we believe that further studies should be performed to improve the validation of the Oxford classification as an indicator of appropriate treatments for IgAN. These additional studies will also provide important information regarding treatment strategies for IgAN.
This study had some limitations. It was a retrospective cohort analysis involving a single center in Japan. The number of patients with T2 and C2 scores was very low to evaluate; therefore, they were combined with patients T1 and C1 scores, respectively. The number of patients with low SRS/high SNRS and the number of patients who progressed to ESRD with low SRS/low SNRS were statistically insufficient. Steroid therapy involved 2 protocols (steroid pulse therapy and oral steroid); however, we used them uniformly because there was no significant difference in their renal prognoses of those 2 protocols. Patients with IgAN with proteinuria of >3 g/d or <1 g/d were included in this study; however, these patients were sometimes excluded from trials. Nevertheless, the results were similar with or without the inclusion of those patients (data not found). Moreover, we only analyzed the effects of steroid therapy in relation to the Oxford classification because we aimed to evaluate the utility of the Oxford classification as a tool for determining the indications for steroid therapy. We plan to perform additional studies to analyze other treatments and treatment combinations.
In conclusion, our results revealed the efficacy of steroid therapy for IgAN according to the new interpretation of the total SRS and SNRS of the Oxford classification. M1, E1, S1, and C1+2 scores were designated as the SRS, and T1+2 scores were designated as the SNRS. The total score might be a good indicator of whether steroid therapy should be used to treat patients with IgAN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Editage (www.editage.jp) for editing the draft of this manuscript.
Author Contributions
Conception or design: TM, YM, and KK; analysis and interpretation of data: IS, TM, and YM; drafting the article: IS and TM; revising the article: YM, KK, and KN; providing intellectual content of critical importance to the work described: YM, KK, and KN; final approval of the version to be published: IS, TM, and KN.
Footnotes
Table S1. Multivariate Cox regression analyses of treatments (steroid pulse therapy, oral prednisolone, and nonsteroid therapy) for all patients with IgAN. (a) Oral prednisolone and steroid pulse therapy against nonsteroid therapy. (b) Steroid pulse therapy against oral prednisolone. Multiple analyses were adjusted for clinical and laboratory findings (age, sex, mean arterial pressure, estimated glomerular filtration rate, and urinary protein excretion); Oxford classification (MEST-C scores); and treatments (tonsillectomy and renin-angiotensin system inhibitors).
Table S2. Univariate and multivariate Cox regression analyses of patients with each MEST-C score.
Figure S1. Renal survival rate until progression to ESRD for patients with IgAN treated with steroid therapy and patients with IgAN treated without steroid therapy for each MEST-C score (without matching).
Supplementary Material
Table S1. Multivariate Cox regression analyses of treatments (steroid pulse therapy, oral prednisolone, and nonsteroid therapy) for all patients with IgAN. (a) Oral prednisolone and steroid pulse therapy against nonsteroid therapy. (b) Steroid pulse therapy against oral prednisolone. Multiple analyses were adjusted for clinical and laboratory findings (age, sex, mean arterial pressure, estimated glomerular filtration rate, and urinary protein excretion); Oxford classification (MEST-C scores); and treatments (tonsillectomy and renin-angiotensin system inhibitors).
Table S2. Univariate and multivariate Cox regression analyses of patients with each MEST-C score.
Figure S1. Renal survival rate until progression to ESRD for patients with IgAN treated with steroid therapy and patients with IgAN treated without steroid therapy for each MEST-C score (without matching).
References
- 1.Berger J., Hinglais N. Les dépôts intercapillaries d’IgA-IgG. [Intercapillary deposits of IgA-IgG] J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Cheng J., Zhang W., Zhang X.H., He Q., Tao X.J., Chen J.H. ACEI/ARB therapy for IgA nephropathy: a meta-analysis of randomised controlled trials. Int J Clin Pract. 2009;63:880–888. doi: 10.1111/j.1742-1241.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z., Yang Y., Jiang S.M., Li W.G. Efficacy and safety of immunosuppressive treatment in IgA nephropathy: a meta-analysis of randomized controlled trials. BMC Nephrol. 2019;20:333. doi: 10.1186/s12882-019-1519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L.L., Wang L.N. ω-3 fatty acids therapy for IgA nephropathy: a meta-analysis of randomized controlled trials. Clin Nephrol. 2012;77:119–125. doi: 10.5414/CN107244. [DOI] [PubMed] [Google Scholar]
- 5.Liu L.L., Wang L.N., Jiang Y., et al. Tonsillectomy for IgA nephropathy: a meta-analysis. Am J Kidney Dis. 2015;65:80–87. doi: 10.1053/j.ajkd.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 6.Moriyama T. Clinical and histological features and therapeutic strategies for IgA nephropathy. Clin Exp Nephrol. 2019;23:1089–1099. doi: 10.1007/s10157-019-01735-4. [DOI] [PubMed] [Google Scholar]
- 7.Okonogi H., Utsunomiya Y., Miyazaki Y., et al. A predictive clinical grading system for immunoglobulin A nephropathy by combining proteinuria and estimated glomerular filtration rate. Nephron Clin Pract. 2011;118:c292–c300. doi: 10.1159/000322613. [DOI] [PubMed] [Google Scholar]
- 8.Magistroni R., Furci L., Leonelli M., et al. A validated model of disease progression in IgA nephropathy. J Nephrol. 2006;19:32–40. [PubMed] [Google Scholar]
- 9.Xie J., Kiryluk K., Wang W., et al. Predicting progression of IgA nephropathy: new clinical progression risk score. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alamartine E., Sabatier J.C., Berthoux F.C. Comparison of pathological lesions on repeated renal biopsies in 73 patients with primary IgA glomerulonephritis: value of quantitative scoring and approach to final prognosis. Clin Nephrol. 1990;34:45–51. [PubMed] [Google Scholar]
- 11.Radford M.G., Jr., Donadio J.V., Jr., Bergstralh E.J., Grande J.P. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8:199–207. doi: 10.1681/ASN.V82199. [DOI] [PubMed] [Google Scholar]
- 12.Shigematsu H. Multiplicity of histopathologic renal lesions in IgA nephropathy: retrospective analysis of histologic grade and stage in serial biopsy specimens. Clin Exp Nephrol. 1998;2:137–141. doi: 10.1007/BF02479935. [DOI] [Google Scholar]
- 13.Katafuchi R., Kiyoshi Y., Oh Y., et al. Glomerular score as a prognosticator in IgA nephropathy: its usefulness and limitation. Clin Nephrol. 1998;49:1–8. [PubMed] [Google Scholar]
- 14.Alamartine E., Sabatier J.C., Guerin C., Berliet J.M., Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–19. doi: 10.1016/s0272-6386(12)80284-8. [DOI] [PubMed] [Google Scholar]
- 15.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Cattran D.C., Coppo R., et al. The Oxford Classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- 16.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Roberts I.S., Cook H.T., et al. The Oxford Classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 17.Lv J., Shi S., Xu D., et al. Evaluation of the Oxford Classification of IgA nephropathy: a systematic review and meta-analysis. Am J Kidney Dis. 2013;62:891–899. doi: 10.1053/j.ajkd.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Shao X., Li B., Cao L., et al. Evaluation of crescent formation as a predictive marker in immunoglobulin A nephropathy: a systematic review and meta-analysis. Oncotarget. 2017;8:46436–46448. doi: 10.18632/oncotarget.17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppo R., Troyanov S., Bellur S., et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014;86:828–836. doi: 10.1038/ki.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon Y.C., Chang T.I., Kang E.W., et al. Clinical usefulness of the Oxford classification in determine immunosuppressive treatment in IgA nephropathy. Ann Med. 2017;49:217–229. doi: 10.1080/07853890.2016.1252058. [DOI] [PubMed] [Google Scholar]
- 21.Yang P., Chen X., Zeng L., Hao H., Xu G. The response of the Oxford classification to steroid in IgA nephropathy: a systematic review and meta-analysis. Oncotarget. 2017;8:59748–59756. doi: 10.18632/oncotarget.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trimarchi H., Barratt J., Cattran D.C., et al. Oxford Classification of IgA nephropathy 2016: an update from IgA nephropathy classification working groups. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Haas M., Verhave J.C., Liu Z.H., et al. A multicenter study of the predictive value of crescents in IgA nephropathy [published correction appears in J Am Soc Nephrol. 2017;28:1665] J Am Soc Nephrol. 2017;28:691–701. doi: 10.1681/ASN.2016040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coppo R., D’Arrigo G., Tripepi G., et al. Is the long-term value of pathology scoring in immunoglobulin A nephropathy? A validation study of the Oxford Classification for IgA nephropathy (VALIGA) update. Nephrol Dial Transplant. 2020;35:1002–1009. doi: 10.1093/ndt/gfy302. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo S., Imai E., Horio M., et al. Revised equation for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Pozzi C., Andrulli S., Del Vecchio L., et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol. 2004;15:157–163. doi: 10.1097/01.asn.0000103869.08096.4f. [DOI] [PubMed] [Google Scholar]
- 27.Miyabe Y., Karasawa K., Akiyama K., et al. Grading system utilising the total score of Oxford classification for predicting renal prognosis in IgA nephropathy. Sci Rep. 2021;11:3584. doi: 10.1038/s41598-021-82967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriyama T., Karasawa K., Miyabe Y., et al. Validation of the revised Oxford classification for IgA nephropathy considering treatment with corticosteroids/immunosuppressors. Sci Rep. 2020;10:11151. doi: 10.1038/s41598-020-68087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palamuthusingam D., Castledine C., Lawman S. Outcomes immunosuppression in IgA nephropathy based on the Oxford classification. Saudi J Kidney Dis Trans. 2018;29:341–350. doi: 10.4103/1319-2442.229292. [DOI] [PubMed] [Google Scholar]
- 30.Okonogi H., Kawamura T., Joh K., et al. A grading system that predicts the risk of dialysis induction in IgA nephropathy patients based on the combination of the clinical and histological severity. Clin Exp Nephrol. 2019;23:16–25. doi: 10.1007/s10157-018-1657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T., Joh K., Okonogi H., et al. A histologic classification of IgA nephropathy for predicting long-term prognosis: emphasis on end-stage renal disease. J Nephrol. 2012;26:350–357. doi: 10.5301/jn.5000151. [DOI] [PubMed] [Google Scholar]
- 32.Yuzawa Y., Yamamoto R., Takahashi K., et al. Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol. 2016;20:511–535. doi: 10.1007/s10157-015-1223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.