COVID-19 is a disease caused by severe acute respiratory syndrome related coronavirus 2 (SARS-CoV-2 virus), which is a positive single stranded RNA virus belonging to the genus of ‘Betacoronavirus’, which includes the notorious SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV). Over a million cases (1.29 million) have been reported in Pakistan so far with at least 28 000 deaths with a mortality rate of 2.23%.1 While COVID-19 affects various organs of the body, it has also been found to involve the neuraxis, ranging from mild anosmia or dysgeusia to life threatening stroke, meningoencephalitis and Guillain-Barre syndrome.2
The first case report linking cerebral venous sinus thrombosis (CVST) and COVID-19 was published on 29 April 2020.2 Since then a great many cases have been reported globally as we write. CVST secondary to viral infections is usually associated with a complete recovery in up to 80% of the patients.3 This, however, does not appear to reflect in COVID-19 related CVST due to associated multiorgan failure. Here we describe an unusual case of CVST in a patient infected lately with COVID-19 who failed to respond to medical treatment and therefore underwent mechanical thrombectomy and showed remarkable recovery. We have reviewed the current literature in detail, and to the best of our knowledge, there are no reported cases yet with successful thrombectomy results in CVST associated with COVID-19.
Case presentation
A 29-year-old man with no prior comorbidities presented to the emergency department with headache for 6 days and left-sided jerky movements of the body, followed by left-sided body weakness for 2 hours. Headache had a sudden onset, mostly at the frontal region, was intermittent, moderate in intensity, non-radiating and relieved by taking non-steroidal anti inflammatory drugs (NSAIDS). There was one episode of left-sided jerky movements of the body for around 1 min associated with uprolling of the eyes and frothing from the mouth followed by unconsciousness that lasted for around 10–15 min. After this episode, the patient developed left-sided weakness. He denied any history of fever, shortness of breath, cough, vertigo, nausea, vomiting or blurring of vision.
Prior to this presentation, he had developed anosmia 20 days ago and was diagnosed with COVID-19 through nasal swab PCR testing. He was vitally and clinically stable during this entire period hence was managed at home, in isolation. His medical and surgical history was otherwise unremarkable. Family history was not significant. He is married, working in a postgraduate programme and denied any addictions. On examination, he was vitally stable. His higher mental functions, speech and cranial nerve examination were intact. The muscle strength was assessed to be of Medical Research Council grade 4/5 power in the left upper and lower limb, while the power on right side was 5/5. The planters showed bilateral flexor responses. Cerebellar and sensory examination were unremarkable, and there were no signs of meningeal irritation.
Investigations
The baseline workup showed haemoglobin of 11.6 gm/dL and white cell count of 13600/mm3. Peripheral smear revealed mild hypochromic microcytic anaemia, and ferritin levels were low at 9.8 ng/mL (normal: 22–232 ng/mL). Inflammatory markers including procalcitonin and C reactive protein were normal, while D-dimer (6.3 mg/L; normal <0.5 mg/L) and lactate dehydrogenase (265 U/L; normal 120–246 U/L) were raised. Chest X-ray was normal. Cerebrospinal fluid (CSF) analysis was unremarkable. Hypercoagulable workup revealed slightly low levels of protein C activity at 55% IU/dL (normal range: 65–135), while protein S, anti-thrombin III, factor V leidin, homocysteine, ANA profile, lupus anticoagulant and anticardiolipin antibody were unremarkable.
MRI brain with contrast showed a right frontal lobe haemorrhagic infarct with surrounding oedema (figure 1). MR venogram with contrast depicted a filling defect in the torcular Herophili (confluence of the sinuses) extending as partial filling defect in distal portion of the superior sagittal sinus along with another filling defect in left sigmoid sinus extending along left distal transverse sinus (figure 2). MR angiography was normal. Electroencephalography was only suggestive of mild encephalopathy.
Figure 1.
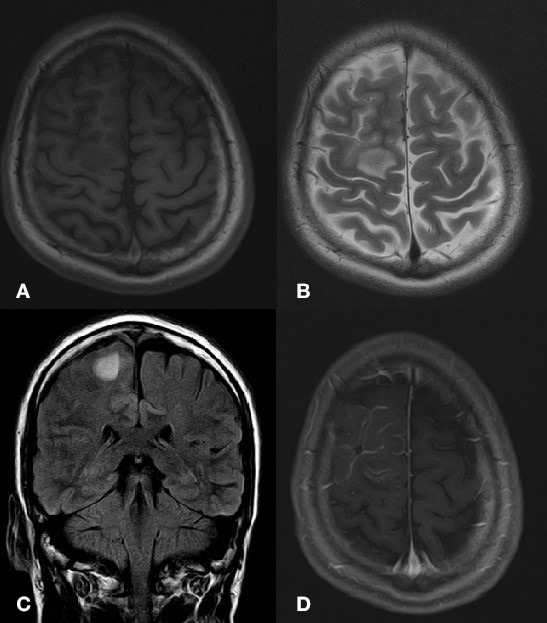
There is a focal area of T1 hypointensity (A) and T2/Fluid attenuated inversion recovery (FLAIR) hyperintensity (B and C) identified in the cortical and subcortical location of the right frontal lobe near the high vertex, parasagittal location. On postcontrast sequences, there is mild meningeal enhancement (D).
Figure 2.
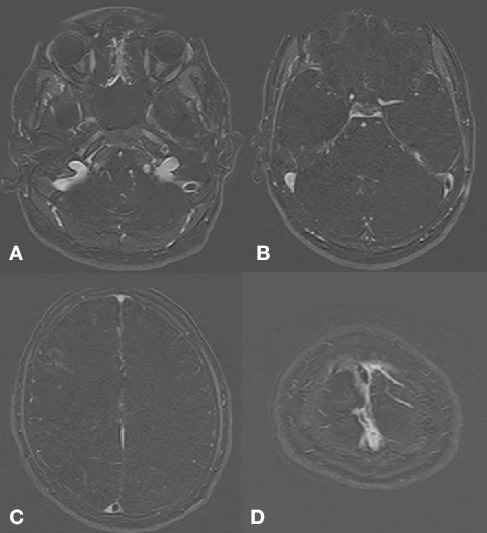
Filling defect is identified in the left sigmoid sinus (A) extending into the distal transverse sinus (B). There is also a filling defect in the torcula Herophili (C) extending into the distal portion of the superior sagittal sinus. Partial filling defect is also identified in the superior sagittal sinus near the vertex (D). Findings are suggestive of cerebral venous sinus thrombosis.
Serial CT head done 48 hours later showed interval increase in the haemorrhagic component of infarct as well as oedema in the right frontal area with increasing mass effect (figure 3).
Figure 3.
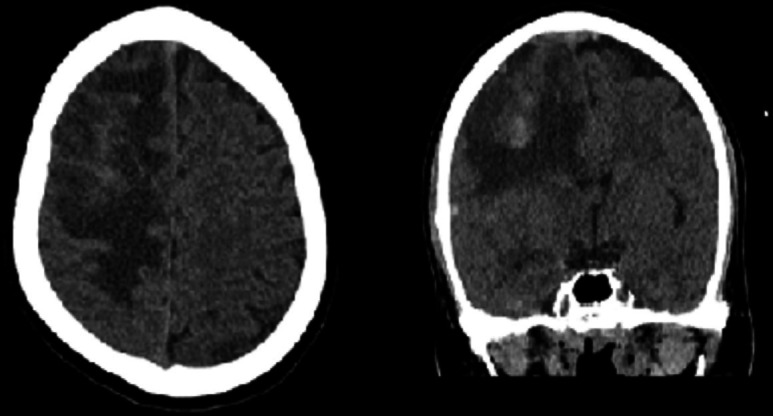
Interval increase in oedema in right frontal lobe. Interval development of few hyperdense foci in right frontal lobe, suggestive of rebleed.
Treatment
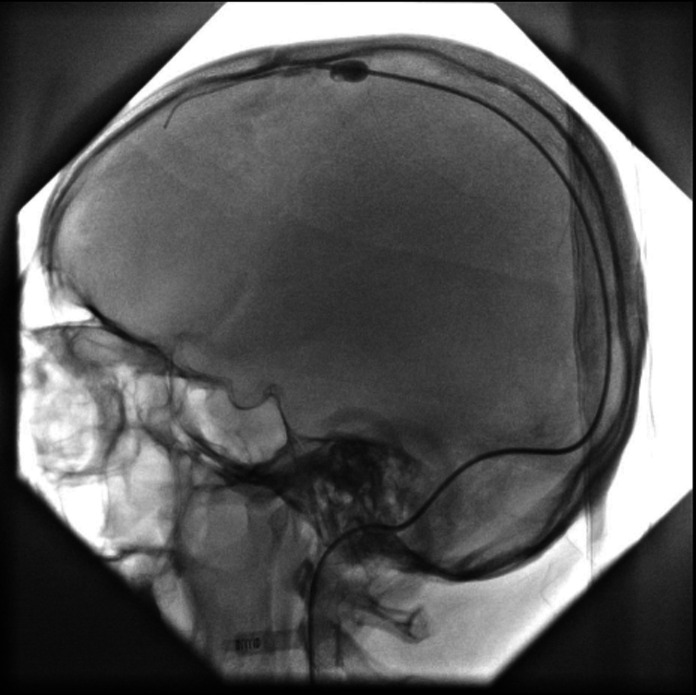
On arrival at the emergency department, the patient was given levetiracetam injection, intravenous hydration, hypertonic saline infusion and enoxaparin injection 60 mg. Initially antibiotics were given in meningitic doses but were stopped once the CSF studies came out to be normal. Inj. Enoxaparin was continued at 60 mg/kg; however, there was further deterioration, and he became drowsy, developed slurring of speech and the power in his left upper and lower limbs decreased from 4/5 to 1/5. In addition, he continued to have multiple episodes of left-sided focal seizures. Serial CT head done 48 hours later showed worsening as seen in figure 3. The antiepileptic medications and their doses were optimised, and the regimen included levetiracetam, topiramate, lamotrigine, lacosamide, phenytoin and clonazepam. Due to progressive deterioration, on day 5 of admission, mechanical thrombectomy of superior sagittal thrombosis was eventually done. During the procedure, an 8 Fr sheath was placed in the right internal jugular vein (figure 4). Through this access, multiple passes of Fogarty catheter were made, which resulted in complete clearance of superior sagittal sinus with good flow.
Figure 4.
Subsequently an 8 Fr sheath was placed in the right internal jugular vein. Through this access, multiple passes of Fogarty catheter were made, which resulted in complete clearance of superior sagittal sinus with good flow.
Outcome and follow-up
The patient’s conscious level showed immediate improvement post-thrombectomy and the frequency of seizures reduced. However, he had severe dysarthia and the power in left upper and lower limb remained the same, that is, 1/5. He was managed with speech therapy, physiotherapy and rehabilitation. By the time he was discharged home on day 18 of admission, he only had mild residual slurring of speech and the power in left upper and lower limb had improved to 3/5. He was discharged on injection enoxaparin 60 mg twice a day and the antiepileptics regimen included levetiracetam, topiramate, lamotrigine, lacosamide and phenytoin.
He was followed up in the clinic on weekly and then fortnightly basis, and the antiepileptics were gradually tapered off. He was switched to oral anticoagulation, that is, tablet rivaroxaban 20 mg once a day with a plan to continue it for a year. At 6 months post-thrombectomy, he is able to ambulate independently with powers of +4/5 and 5/5 in left upper and lower limb, respectively.
Discussion
Neurological manifestations are present in at least one-third of all COVID-19 cases.4 The underlying pathophysiology has been quoted as direct invasion, immune dysregulation and/or acquired thrombophilia. It is presumed that consumptive coagulopathy predisposes the individual to occlusion in variety of vascular beds including deep veins of legs, pulmonary and cerebral vasculature. Hence, thromboembolic complications like CVST are also on the rise. The main mechanism by which COVID-19 causes thrombophilia and hence CVST is via proinflammatory cytokines like interleukin (IL) -2, IL-6, IL-7, IL-10, Granulocyte colony-stimulating factor (G-CSF), Interferon-gamma-inducible protein 10 (IP-10) and Tumor necrosis factor-α, along with a decrease in anti-inflammatory cytokines like interferon gamma. This in turn activates the coagulation cascade, causing platelet activation and endothelial damage, eventually leading to disseminated intravascular coagulation (DIC).5 Furthermore, it has been speculated that the viral particle also binds to ACE-II receptors present on the neurons and the glial cells of the brain and cerebral vascular endothelium, thereby gaining access into the brain and causing endothelial dysfunction.6
In non-COVID CVST, around 9%–13% of the patients may worsen despite being on anticoagulation, requiring invasive catheter based thrombolysis or mechanical thrombectomy.7 A literature review of 17 studies concluded that out of 235 patients with CVST that underwent emergency mechanical thrombectomy, 76% had either complete neurological recovery or only mild deficit with a recurrence rate of 1.2%.8 Hence, the procedure appears safe and can be considered in refractory CVST patients who are unresponsive to anticoagulation. Literature review indicates ever-increasing cases of CVST associated with COVID-19. While majority of these patients received anticoagulation, six underwent surgical and/or endovascular procedure due to clinical deterioration.9–11 Two patients underwent surgical decompression alone, while two had an external ventricular drain placed. Mechanical thrombectomy along with on-site chemical thrombolysis was performed in the rest of the two patients as explained further in detail.
Cavalcanti et al (case A in tables 1–3) described a young male who presented with headache for 1 week and altered mentation for 2 days. His nasopharyngeal swab for COVID-19 PCR came out to be positive. Once diagnosed with CVST on CT head and CT venogram, he was started on enoxaparin 70 mg SC twice a day. When he dropped GCS 7 hours into admission and developed respiratory failure, he got intubated and was kept on mechanical ventilation. A percutaneous venous mechanical thrombectomy was performed with satisfactory results in superficial venous system, but there were residual corticalvenous thrombi, hence a microcatheter was left in superior sagittal sinus for continuous infusion of alteplase at 2 mg/hour. However, the patient succumbed to respiratory failure and cardiac arrest on day 2 of presentation.12
Table 1.
Demographics and clinical features of the patients undergoing mechanical thrombectomy for COVID-19 related CVST
| Cases | Current case | Case A | Case B |
| Age (years) | 29 | 38 | 62 |
| Gender | Male | Male | Female |
| Neurological symptoms | Headache, left-sided weakness and left-sided focal seizures | Headache and altered mental status | Headache, blurred vision and left-sided hemiparesis |
| Other symptoms due to COVID-19 | Anosmia since 20 days | Vomiting and diarrhoea | None |
| COVID-19 clinical severity score13 | Mild | Moderate | Moderate |
Table 2.
Laboratory variables comparison among the patients with COVID-19 related CVST undergoing mechanical thrombectomy
| Cases | Current case | Case A | Case B |
| Complete blood picture | Haemoglobin: 11.6 gm/dL Total Leukocyte Count: 13 600/mm3 Neutrophils: 10 200/mm3 Lymphocytes: 2312/mm3 Monocytes: 924/mm3 Platelets: 285 000/mm3 Neutrophil/Lymphocyte Ratio: 4.4 |
Haemoglobin: 14.3 gm/dL Total Leukocyte Count: 16 710/mm3 Neutrophils: 14 280/mm3 Lymphocytes: 490/mm3 Monocytes: 1690/mm3 Platelets: 141 000/mm3 Neutrophil/Lymphocyte Ratio: 29.14 |
Haemoglobin: 14.9 gm/dL Total Leukocyte Count: 8000/mm3 Lymphocytes: 560/mm3 Platelets: 140 000/mm3 Estimated Neutrophil/Lymphocyte Ratio: 10.5–12.3 |
| Coagulation profile | Prothrombin Time: 10.9 s APTT: 20.1 s International normalized ratio: 1.0 |
PT: 22.5 s APTT: 30.6 s International normalized ratio: 1.9 |
Not available |
| Inflammatory markers | C-reactive Protein: 9.95 mg/L Lactate Dehydrogenase: 265 U/L Ferritin: 9.8 ng/mL Procalcitonin: 0.037 ng/mL Fibirin degradation product: >5.0 µg/mL D-dimer: 6.3 ng/mL |
Fibrinogen levels: 121 D-dimer: >55 000 |
C-reactive Protein: 40 mg/L Erythrocyte Sedimentation Rate: 12 mm/hour Lactate Dehydrogenase: 751 U/L Ferritin: 144 ng/mL D-dimer: >10 000 ng/mL |
| Liver profile | Aspartate transaminase (AST): 21 U/L Alanine transaminase (ALT): 29 U/L |
AST: 31 U/L ALT: 27 U/L |
Normal |
| CSF D/R | Total Leukocyte Count: 3 (103 /UL) Protein: 45 mg/dL Glucose: 79 mg/dL |
Not Available | Not available |
| Baseline parenchymal lesion | Haemorrhagic infarct | None (cerebral oedema only) | Haemorrhagic infarct |
| MR venography findings | Torcula Herophili, superior sagittal sinus, left transverse sinus and left sigmoid sinus | Multiple cortical veins, straight sinus, distal superior sagittal sinus, torcular and right transverse sinus | Multiple cortical veins, superior sagittal, transverse and sigmoid sinuses |
| Thrombophilic workup | Low protein C levels | Not available | Raised lupus anticoagulant and raised antiphospholipid IgM antibody |
CSF, cerebrospinal fluid.
Table 3.
Treatment and outcome details in patients with COVID-19 related CVST undergoing mechanical thrombectomy
| Cases | Current case | Case A | Case B |
| Treatment for COVID-19 | Nil | Lopinavir - Ritonavir | Lopinavir - Ritonavir |
| Treatment for CVST (pharmacological) | Enoxaparin 60 mg S/C twice daily | Enoxaparin 70 mg S/C twice daily | Unfractionated heparin 1000 units per hour as continuous infusion |
| Treatment for CVST (surgical) | Mechanical thrombectomy of superior sagittal sinus | Mechanical thrombectomy of superior sagittal sinus and subsequent catheter based continuous r-TPA infusion at 2 mg/hour | Intrasinus thrombosis with r-TPA 20 mg slowly into superior sagittal sinus followed by mechanical thrombectomy; later underwent decompressive craniotomy on 8th day |
| Time duration between symptoms and endovascular therapy for CVST | On 5th day of admission | Within 24 hours | On 4th day of admission |
| Duration of admission | 18 days | 32 hours | 10 days |
| Outcome | Discharged home with minimal deficits | Death after 32 hours of admission | Death after 10 days |
| Cause of death (if applicable) | N/A | Respiratory failure and cardiac arrest | Septic shock and DIC |
Ostovan et al10 (case B in tables 1–3) reported the case of a 64-year-old woman who presented with right-sided headache, nausea, vomiting, blurred vision and left-sided weakness. Her workup revealed a positive PCR for COVID-19. She was diagnosed with CVST on brain imaging and started on intravenous unfractionated heparin 1000 units/hour continuous infusion; however, her condition kept worsening. On day 4, she underwent mechanical thrombectomy and chemical thrombolysis with 20 mg of slow infusion of alteplase resulting in successful recanalisation of sinuses. On day 8 of admission, due to deterioration in her mental status and development of left-sided midline shift, an urgent decompressive craniectomy was done. However, she later succumbed to ongoing septic shock and DIC.
A multicentre retrospective study conducted over a period of 3 months by Al-Mufti et al13 on CVST in patients with COVID-19 in the USA showed that 2 out of 12 CVST patients were treated with endovascular thrombectomy. However, further details regarding the demographics and the outcome of thrombectomy in these patients were not provided.
Tables 1–3 summarise a comprehensive comparison of our case with the aforementioned two cases. Headache was uniformly seen in all of these patients. Superior sagittal sinus was involved in all three although other sinuses, superficial and deep veins were involved as well. There was no pulmonary involvement in our patient. According to the WHO Clinical Progression Scale,14 our patient had mild disease, while the other two had moderate disease. In comparison with our case, the other two patients had remarkably raised D-dimers and neutrophil-to-lymphocyte ratio along with moderate severity of COVID-19 disease.15 No antiviral therapy was given to our patient for COVID-19. Both of the other cases received on site chemical thrombolysis with alteplase along with mechanical thrombectomy, while ours did not. Mechanical thrombectomy led to full recanalisation of superior sagittal sinus in all of the cases. Residual thrombi persisted in cortical veins in our case as in case A, while there was no mention of cortical veins in case B. Although both patients developed multiorgan failure, neurological deterioration in these cases played a critical role.
Low ferritin and protein C levels in our case are intriguing findings. COVID-19 is considered as a part of the hyperferritinaemia syndromes. Interestingly, low iron status appears to protect against the detrimental hyperinflammatory response to COVID-19 infection.16 Women with naturally low mean haemoglobin levels are less affected with COVID-19 infection and have better outcomes compared with men,17 whereas patients taking oral iron supplementation have shown an increased risk of infection. Our patient, with better outcome than rest two cases, had mild hypochromic microcytic anaemia and low ferritin levels. This relationship between low iron stores and COVID-19 related thrombotic complications remains elusive and needs further evaluation. Protein C and S deficiency may precipitate CVST, although this may be transient.18 In 2012, two cases of CVST associated with varicella zoster infection and pre-existing protein C and S deficiency were reported.19 IL-6 and hypoxia driven protein C and S deficiency have been held responsible for thrombophillia in COVID-19.20 21 Hence, low protein C levels in our case may have had a contributory role in the development of CVST.
For better insight of COVID-19 related neurological manifestations and particularly thrombotic complications, a multicentre data registry and analysis is imperative. Mechanical thrombectomy should be considered as an optimistic choice in refractory CVST when response to anticoagulation remains unsatisfactory.
Learning points.
Patients with COVID-19 are prone to developing a hypercoagulable state, thereby leading to thromboembolic complications like CVST.
Mechanical thrombectomy should be considered an optimistic choice in refractory CVST, that is, failure to achieve response to anticoagulation.
Physicians must remain vigilant as CVST with severe COVID-19 infection may have fatal outcomes if treatment is delayed.
Acknowledgments
We are thankful to Dr Tanveer ul Haq for his support, advice and for carrying out the mechanical thrombectomy procedure.
Footnotes
Contributors: AS: conception, data analysis, manuscript writing and review. AFK: data collection, data analysis and manuscript writing. LJ: planning, data analysis, manuscript writing and review. AKK: conception, design and review. The final manuscript has been approved by all the authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.COVID-19 health Advisory platform by Ministry of national health services regulations and coordination, 2021. Available: https://covid.gov.pk/stats/pakistan [Accessed 30 Nov 2021].
- 2.Hughes C, Nichols T, Pike M, et al. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med 2020;7:001691. 10.12890/2020_001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudhaker B, Dnyaneshwar MP, Jaidip CR, et al. Cerebral venous sinus thrombosis (CVST) secondary to varicella induced hypercoagulable state in a adult. Intern Med Inside 2014;2:1. 10.7243/2052-6954-2-1 [DOI] [Google Scholar]
- 4.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry BM, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta 2020;507:167–73. 10.1016/j.cca.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 2020;251:228–48. 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferro JM, Canhão P, Stam J, et al. Prognosis of cerebral vein and dural sinus thrombosis: results of the International study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke 2004;35:664–70. 10.1161/01.STR.0000117571.76197.26 [DOI] [PubMed] [Google Scholar]
- 8.Ilyas A, Chen C-J, Raper DM, et al. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg 2017;9:1086–92. 10.1136/neurintsurg-2016-012938 [DOI] [PubMed] [Google Scholar]
- 9.Hameed S, Wasay M. Cerebral venous sinus thrombosis associated with coronavirus infection (covid-19). PJNS 2020;15:60–5. [Google Scholar]
- 10.Ostovan VR, Foroughi R, Rostami M, et al. Cerebral venous sinus thrombosis associated with COVID-19: a case series and literature review. J Neurol 2021;268:3549–60. 10.1007/s00415-021-10450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kananeh MF, Thomas T, Sharma K, et al. Arterial and venous strokes in the setting of COVID-19. J Clin Neurosci 2020;79:60–6. 10.1016/j.jocn.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavalcanti DD, Raz E, Shapiro M, et al. Cerebral venous thrombosis associated with COVID-19. AJNR Am J Neuroradiol 2020;41:1370–6. 10.3174/ajnr.A6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Mufti F, Amuluru K, Sahni R, et al. Cerebral venous thrombosis in COVID-19: a new York metropolitan cohort study. AJNR Am J Neuroradiol 2021;42:1196–200. 10.3174/ajnr.A7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20:e192–7. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 2020;296:E15–25. 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menshawey R, Menshawey E, Alserr AHK, et al. Low iron mitigates viral survival: insights from evolution, genetics, and pandemics—a review of current hypothesis. Egypt J Med Hum Genet 2020;21:1–4. 10.1186/s43042-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J-M, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020;8:152. 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikejiri M, Shindo A, Ii Y, et al. Frequent association of thrombophilia in cerebral venous sinus thrombosis. Int J Hematol 2012;95:257–62. 10.1007/s12185-012-1006-0 [DOI] [PubMed] [Google Scholar]
- 19.Siddiqi SA, Nishat S, Kanwar D, et al. Cerebral venous sinus thrombosis: association with primary varicella zoster virus infection. J Stroke Cerebrovasc Dis 2012;21:917.e1–917.e4. 10.1016/j.jstrokecerebrovasdis.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee S, Sengupta T, Majumder S, et al. COVID-19: a probable role of the anticoagulant protein S in managing COVID-19-associated coagulopathy. Aging 2020;12:15954–61. 10.18632/aging.103869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin JH, Lyden P. COVID-19 hypothesis: activated protein C for therapy of virus-induced pathologic thromboinflammation. Res Pract Thromb Haemost 2020;4:506–9. 10.1002/rth2.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]