Abstract
Objectives
Idiopathic pulmonary fibrosis (IPF) has been defined as a distinctive type of chronic fibrotic disease, characterised by a progressive decline in lung function and a common histological pattern of interstitial pneumonia. To analyse the efficacy and safety of pirfenidone in the treatment of IPF, a systematic review and meta-analysis was performed.
Design
This is a meta-analysis study.
Participants
Patients were diagnosed as IPF.
Interventions
Use of pirfenidone.
Primary and secondary outcome
Progression-free survival (PFS), acute exacerbation and worsening of IPF and Impact on adverse events.
Measures
The inverse variance method for the random-effects model was used to summarise the dichotomous outcomes, risk ratios and 95% CIs.
Results
A total of 9 randomised controlled trials with 1011 participants receiving pirfenidone and 912 controls receiving placebo were summarised. The pooled result suggested a statistically significant difference inall-cause mortality after pirfenidone use, with a summarised relative ratio of 0.51 (p<0.01). Longer PFS was observed in patients receiving pirfenidone compared with those who were given placebo (p<0.01). The IPF groups presented a high incidence of adverse events with a pooled relative ratio of 3.89 (p<0.01).
Conclusions
Pirfenidone can provide survival benefit for patients with IPF. Pirfenidone treatment was also associated with a longer PFS, a lower incidence of acute exacerbation and worsening of IPF.
Keywords: respiratory medicine (see thoracic medicine), thoracic medicine, adult thoracic medicine
Strengths and limitations of this study.
Larger sample size studies were included compared with previous meta-analyses, and the finding were concluded based on more representative populations.
Most studies had a limited number of patients.
Most studies did not report the severity degree of the patients, and also, the follow-up period varied, causing heterogeneity of the results.
Potential language bias might exist because the literature search considered only studies published in English.
Introduction
Idiopathic pulmonary fibrosis (IPF) has been defined as a distinctive type of chronic fibrotic disease, characterised by progressive decline in lung function and a usual histological pattern of interstitial pneumonia.1 2 The prevalence of IPF was estimated to be 2–43/100 000 people in the general population based on studies adopting various designs and a wide range of populations.3–5 The median survival time of IPF patients was approximately 3–5 years after diagnosis,6 and the course of the disease was unpredictable and highly variable.6 However, the pathogenesis of IPF remains unknown, and multiple genetic and environmental factors may play a role in the occurrence and progression of pulmonary fibrosis.7 8
Recent national guidelines on IPF diagnosis and treatments from Spain, Germany, Denmark, Sweden, Austria and Ireland have recommended pirfenidone as the therapeutic agent for IPF patients.9 In addition, the UK National Institute for Health and Care Excellence recommends pirfenidone as a treatment option for IPF patients with a predicted forced vital capacity (FVC) value of between 50% and 80%.10
The choice of first-line treatment is best addressed by a direct comparison of treatment regimens in high-quality studies. Several randomised controlled trials (RCTs) compared the clinical outcomes of patients treated with pirfenidone vs placebo and confirmed that the balanced use of pirfenidone was beneficial for IPF patients.11 12 A meta-analysis encompassing five studies highlighted significant differences in physiological and clinically relevant outcomes.13 However, several recent RCTs have provided novel evidence and suggested that the use of pirfenidone may cause more adverse events in comparison to the placebo control.11 14 The newly published studies were included in the present meta-analysis to conduct an updated systematic review and meta-analysis.
This systematic review and meta-analysis based on well-conducted and adequately powered RCTs aimed to explore the efficacy and safety of pirfenidone in treating IPF.
Methods
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Literature search
PubMed, Medline, Cochrane Library and Embase were employed to search targeted studies published up to 29 July 2020. Target studies were selected following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.15 The joint and individual keywords comprising “pirfenidone’, “Anti-Inflammatory Agents”, “idiopathic pulmonary fibrosis OR IPF” AND “randomized controlled trial OR RCT” were used for literature search. We also conducted a manual search of reference lists of the eligible studies to identify other potential relevant articles.
Eligibility criteria
The inclusion criteria were as follows: (1) RCTs; (2) patients diagnosed as IPF; (3) use of pirfenidone as an intervention and use of placebo as the control; (4) reporting at least one or more clinical outcomes; (5) necessary data extracted from original studies; (6) studies published in English and (7) for studies with duplicate population, only the one providing detailed information were selected.
Reviews, case reports, observational studies, non-randomised experimental studies, studies focused on animals or in vitro experiments, and studies in languages other than English were excluded from this meta-analysis.
Data extraction
All relevant studies from the databases were reviewed, and the data from included studies were extracted independently by two investigators using a standardised form, and the consensus was reached on all items by a discussion with a third reviewer. The extracted information included the following: study characteristics (authors, year of publication, sample size, follow-up period and general information of the study population), intervention characteristics and outcomes. The study outcomes included mortality, progression-free survival (PFS), distance change in 6 min walk test (6-MWT), aminotransferase secondary to treatment, and >10% of FVC.
Acute exacerbation of IPF was defined as unexplained worsening of dyspnoea in the past 30 days among cases with previous or concurrent diagnosis of IPF, with evidence of new bilateral ground glass opacities or consolidation excludingalternative causes. The worsening of IPF was defined as shortened time to acute exacerbation, death, lung transplantation or admission to hospital for respiratory problems.
Risk-of-bias assessment
The seven-category Review Manager risk-of-bias tool from RevMan (V.5.3, The Cochrane Collaboration, Oxford, UK) was used to assess the risk of bias of the included studies as either high, unclear or low, according to the Cochrane Handbook for RCTs.16
Quality of studies assessment
Two reviewers evaluated the quality of evidence using the Jadad scale, which also named the Oxford quality scoring system, to independently assess the methodological quality of a clinical trial.17 Similarly, the consensus was reached by discussion with another reviewer. The studies were scored according to the presence of three key methodological features: randomisation, blinding and accountability of all patients. One point or two points were added for a ‘yes’ answer to each of the randomisation and blinding domain, and one point was added for a ‘yes’ to the accountability of all patients domain. The overall score ranged from 0 to 5, and the score >3 was deemed as high quality.
Statistical analysis
The inverse variance method for the random-effects model was used to summarise the dichotomous outcomes, risk ratios (RRs) and 95% CIs. The heterogeneity among included studies was assessed using the I2 and Q tests and defined as low, moderate and high when the values of I2 were 25%, 50% and 75%, respectively.18 The publication bias was assessed using the Begg rank correlation19 and Egger weighted regression methods.20 A funnel plot was generated, and the Begg’s and Egger’s tests were carried out using Stata V.15.0 (Stata). Statistical analyses were performed using RevMan. A p<0.05 was considered statistically significant for all analyses.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Results
Study selection
As shown in online supplemental figures 1, 774 studies were identified by database searching using different keywords combinations after eliminating overlaps. Following the above inclusion and exclusion criteria, 479 abstracts and titles were reviewed initially. After retrieving 25 full-length manuscripts, ultimately, 9 RCTs11 12 14 21–25 were included in this meta-analysis.
bmjopen-2021-050004supp001.pdf (280.1KB, pdf)
Study characteristics
The characteristics of the included studies are summarised in table 1. A total of 9 studies with 1011 participants receiving pirfenidone and 912 placebo controls were summarised. These studies were published between 2005 and 2019, and the sample size varied from 20 to 555. The majority of the studies (n=6) were conducted in Western countries and three in Asian countries. The study period was 36–72 weeks, and the majority of the studies showed the change in the predicted FVC from baseline to week 52 as the primary outcome.
Table 1.
Characteristics of study participants
| Studies included | Country | Sample size (P/C) | Age (mean±SD, years) | Males (%, P/C) | Study period (weeks) | Intervention | Primary outcome | ||
| Pirfenidone | Controls | Pirfenidone | Controls | ||||||
| Azuma et al, 200522 | Western | 72/35 | 64.0±7.1 | 64.3±7.6 | 62 (86.1%)/33 (94.3%) | 36 | Pirfenidone 1800 mg/day | Placebo pills | Change in the lowest SpO2 during 6MWT |
| Taniguchi et al, 201023 | Asian | 108/55/104* | 65.4±6.2/63.9±7.5* | 64.7±7.3 | 85 (78.7%)/47 (85.4%)/81 (77.9%)* | 52 | Pirfenidone 1800 or 1200 mg/day* | Placebo pills | Change from baseline to week 52 in predicted FVC |
| Noble et al, 2011 (PIPF 004)12 | Western | 174/87/174* | 65.7±8.2/68.0±7.6* | 66.3±7.5 | 118 (67.8%)/65 (74.7%)/128 (73.6%)* | 72 | Pirfenidone 2403 or 1197 mg/day* | Placebo pills | Change from baseline to week 72 in predicted FVC |
| Noble et al, 2011 (PIPF 006)12 | Western | 171/177 | 66.8±7.9 | 67.0±7.8 | 123 (71.9%)/124 (70.1%) | 52 | Pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 72 in predicted FVC |
| Kind et al 201424 | Western | 278/277 | 68.4±6.7 | 67.8±7.3 | 222 (80.0%)/213 (76.9%) | 48 | Pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 52 in predicted FVC |
| Huang et al, 201511 | Asian | 38/38 | 59.0±5.9 | 61.6±6.4 | 33 (86.8%)/38 (100.0%) | 48 | Pirfenidone 1800 mg/day | Placebo pills | Change from baseline to week 48 in predicted FVC |
| Furuya et al, 201721 | Asian | 20/27 | 76 (66–82) | 73 (64–84) | 18 (90.0%)/24 (88.9%) | 52 | Pirfenidone 1200–1800 mg/day | Placebo pills | 3 month survival and adverse events |
| Nathan et al, 201914 | Western | 90/80 | 70 (46–80) | 69 (40–80)† | 74 (82.2%)/59 (73.8%) | 52 | Pirfenidone 1800 mg/day | Placebo pills | Change from baseline to week 52 in predicted FVC |
| Vianello et al, 201925 | Western | 11/9 | 62 (52–78) | 74 (50–87)† | 9 (81.8%)/6 (66.7%) | 52 | Pirfenidone 2403 mg/day | Placebo pills | Change from baseline to week 52 in predicted FVC |
*Data for two trial groups and one control group.
†Range of age.
C, control group; FVC, forced vital capacity; 6MWT, 6 min walk test; NA, not available; P, pirfenidone group.
Risk of bias and quality of studies
The overall risk of bias and quality of the included RCTs is presented in online supplemental figure 2 and online supplemental table 1. None of the included RCTs was judged to have a high risk, and six studies used a randomised and double-blinded method to include the participants. Moreover, the Jadad scale score of all RCTs was ≥4 points (online supplemental table 1).
Impact on mortality
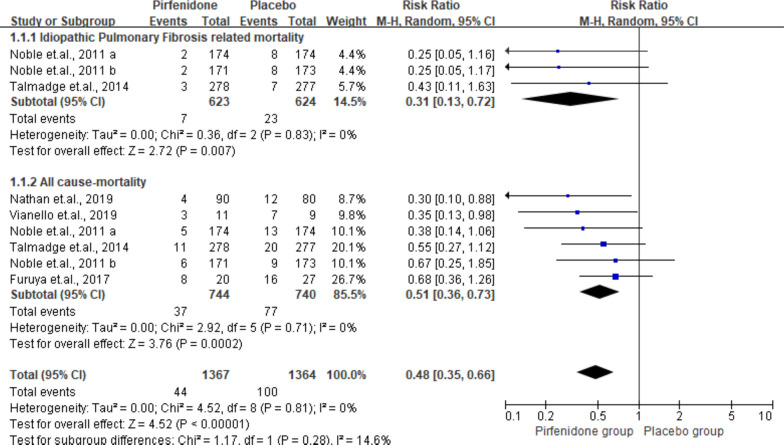
A total of 6 studies including 1484 subjects reported all-cause mortality (pirfenidone, n=744; placebo, n=740). The pooled result suggested significant differences in the effect of pirfenidone on all-cause mortality with a summarised RR of 0.51 (95% CI 0.36 to 0.73, p<0.01), without heterogeneity detected (I2=0%). Of these studies, 3 provided IPF-related mortality. Similar to all-cause mortality, the IPF-related mortality presented a significant difference with a pooled RR of 0.31 (95% CI 0.13 to 0.72, p<0.01, I2=0%). The data and forest plots are shown in figure 1.
Figure 1.
Summarised all-cause mortality and idiopathic pulmonary fibrosis-related mortality. M-H, Mantel-Haenszel.
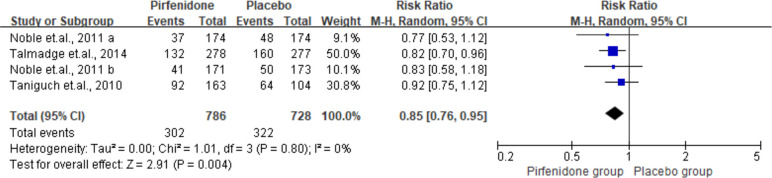
Progression-free survival
The pooled PFS is presented in figure 2. Among the studies, four RCTs reported the effect of pirfenidone and PFS. The pooled analysis included 786 cases treated with pirfenidone and 728 controls treated with placebo. Furthermore, the result indicated a higher PFS for pirfenidone as compared with placebo (RR 0.85, 95% CI 0.76 to 0.95, I2=0%).
Figure 2.
Summarisedpfs of pirfenidone among idiopathic pulmonary fibrosis cases. M-H, Mantel-Haenszel.
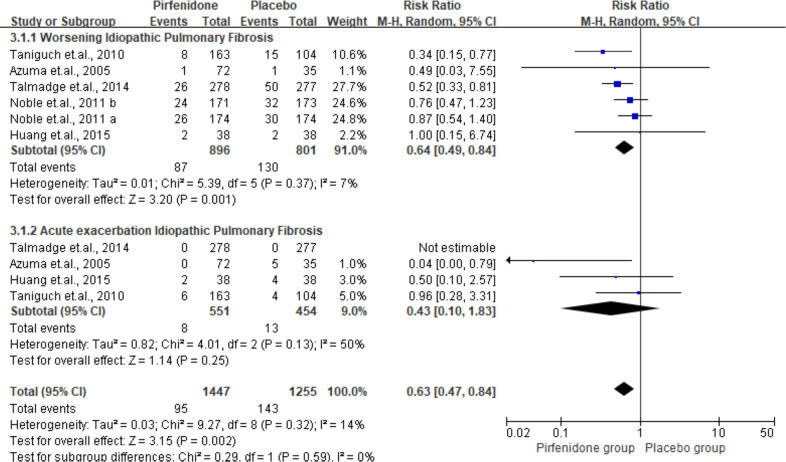
Acute exacerbation and worsening of IPF
As shown in figures 3 and 4 RCTs reported the outcome on worsening and acute exacerbation of IPF. The pooled results indicated suppression of IPF deterioration with an RRs of 0.64 (95% CI 0. 49 to 0.84, I2=7%).
Figure 3.
Summarised acute exacerbation and worsening idiopathic pulmonary fibrosis among the participants. M-H, Mantel-Haenszel.
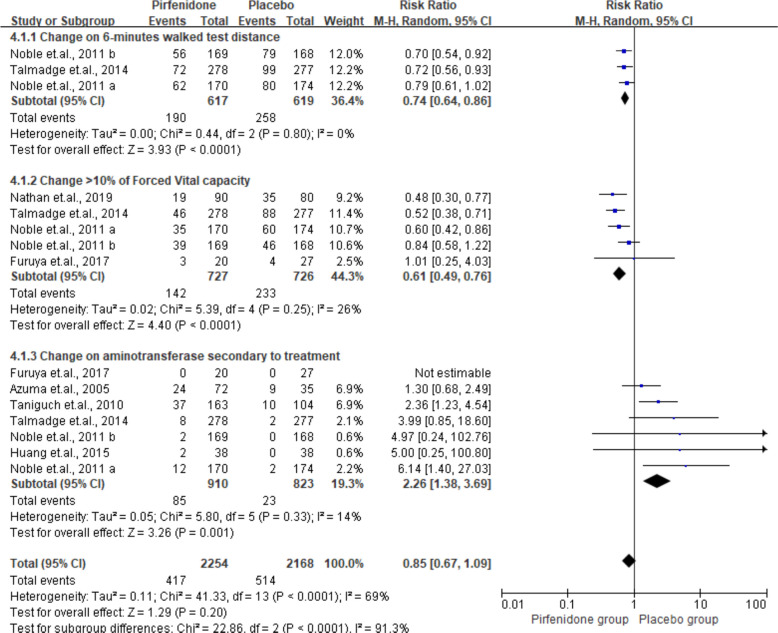
Figure 4.
Summarised changes in >10% of forced vital capacity, change in 6 min walk test distance, and aminotransferase among the placebo and intervention groups. M-H, Mantel-Haenszel.
Change in 6-MWT distance, aminotransferase secondary to treatment and >10% of FVC
As shown in figure 4, two, three and eight studies provided detailed data on 6MWT, aminotransferase secondary to treatment, and >10% of FVC, respectively. All the changes differed significantly between the IPF and placebo group (6-MWT: RR 0.74, 95% CI 0.64 to 0.86, p<0.01; aminotransferase secondary to treatment: RR 2.26, 95% CI 1.38 to 3.69, p<0.01).
Impact on adverse events
All included studies provided data on adverse events, such as nausea, rash, dyspepsia, vomiting, photosensitivity, anorexia, elevation in aminotransferase level, upper airway infection, asthenia and weight loss. The pooled results on adverse events for IPF and placebo groups were 3.89 (95% CI 2.09 to 7.24, I2=47%). The result for skin-related adverse events was similar between the two groups (RR 1.04, 95% CI 0.95 o 1.14, p=0.38, I2=37%; online supplemental figure 3).
Publication bias
No potential publication bias was observed among the included trials, according to Begg’s rank correlation and Egger’s weighted regression analyses (all p>0.05, (online supplemental table 2).
Discussion
A total of nine RCTs were included and summarised in the present meta-analysis addressing the treatment efficacy and safety of pirfenidone in IPF cases. The quality of all included studies was acceptable. The use of pirfenidone was beneficial to IPF patients by lowering all-cause and IPF-related mortality. Moreover, IPF patients could exhibit prolonged PFS, low prevalence of acute exacerbation, worsening of IPF, and low proportion of overall adverse events.
One previous meta-analysis26 included five trials focusing on the same topic and observed significant differences in the physiological and clinically relevant outcomes, such as a reduction in all-cause mortality, IPF-related mortality and worsening and exacerbation of IPF and PFS. In the current meta-analysis, we includedupdated eligible studies up to 29 July 2020, doublingthe number of included trials. Based on appropriate and more representative target populations, we obtained a similar conclusion. The all-cause mortality was commonly deemed as one of the most robust endpoints in therapeutic clinical trials in IPF.27 The characterisation of mortality related to IPF was vital to supplement the analysis of all-cause mortality, as it is the most specific endpoint for treatment efficacy.6 However, deaths unrelated to IPF also occur in this patient population and could potentially confound all-cause mortality findings. Pirfenidone is a bioavailable synthetic molecule, administered orally, with antifibrotic and anti-inflammatory properties. Pirfenidone therapy was associated with an approximately one-third reduction of death for the treated and hospitalised AE-IPF patients, which was yet unexplained. Other clinically relevant outcomes in this analysis were investigated with respect to the prospective of acute exacerbation and worsening of IPF. The adverse events often occurred among patients in the control group, and similar results were observed in the high-dose and low-dose pirfenidone groups.28 This might be partially due to the phenomenon that patients were well-informed regarding the side effects of rashes. Despite the manifestation of the anticipated skin rash, pirfenidone was generally well tolerated in IPF patients.
The findings in the current study are strengthened by the high rate of study completion and treatment adherence, as well as the consistentency magnitude of treatment effects across the primary and secondary endpoints. The current study demonstrated the difference in the primary endpoint and also a significant difference in secondary endpoints including change in acute exacerbation. Pirfenidone treatment suppressed the decrease in oxygenation during exercise in the subset of patients who did not demonstrate SpO2 <80% during 6-MWT.8 29 The decrease in SpO2 of 4% or >6 min of walking has been demonstrated to predict survival in IPF patients.30 31
Nevertheless, the limitations of the present meta-analysis should be considered while interpreting the results. First, most studies included a small number of patients. Due to the limited number of patients in each study, subgroup or sensitivity analyses were difficult to perform. Second, due to small sample size, the mean age and sex ratio of the patients varied significantly, which might lead to heterogeneity and reduced stability of the results. Thirdly, most studies did not report the severity of adverse events, and also, the follow-up period varied, causing heterogeneity of the results. Fourth, the primary and secondary outcomes were strikingly different, and sufficient studies were not available to summarise the outcomes. Fifth, potential selection bias might exist because only publications in English were included.
Conclusions
The present meta-analysis explored the efficacy and safety of pirfenidone therapy in IPF cases. Pirfenidone was found to be beneficial to IPF patients as it lowered the mortality. In addition, the IPF cases obtained a prolonged PFS, low incidence of acute exacerbation, and worsening of IPF. Pirfenidone might also be associated with a lower incidence of overall adverse events. In the future, large-size RCTs matched for age, sex and degree of severity in IPF patients should be conducted to detect critical differences in treatment outcomes.
Footnotes
Contributors: WW: study design, data collection and analysis, statistical analysis and manuscript drafting, manuscript revision. LQ: study design, data collection and analysis, statistical analysis and manuscript drafting. XL: data collection. JW: manuscript revision. GZ: study design and critical revision of the manuscript. All authors read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1.Martinez FJ, Collard HR, Pardo A, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017;3:17074. 10.1038/nrdp.2017.74 [DOI] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018;378:1811–23. 10.1056/NEJMra1705751 [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G, Chen S-Y, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J 2016;48:179–86. 10.1183/13993003.01653-2015 [DOI] [PubMed] [Google Scholar]
- 5.Strongman H, Kausar I, Maher TM. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv Ther 2018;35:724–36. 10.1007/s12325-018-0693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–40. 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 7.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014;9:157–79. 10.1146/annurev-pathol-012513-104706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 2018;19:32. 10.1186/s12931-018-0730-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the gold science Committee report 2019. Eur Respir J 2019;53:1900164. 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence . Pirfenidone for treating idiopathic pulmonary fibrosis. Available: https://www.nice.org.uk/guidance/ta504 [Accessed 23 Jul 2021].
- 11.Huang H, Dai HP, Kang J, et al. Double-Blind randomized trial of pirfenidone in Chinese idiopathic pulmonary fibrosis patients. Medicine 2015;94:e1600. 10.1097/MD.0000000000001600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (capacity): two randomised trials. Lancet 2011;377:1760–9. 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 13.Ren H, Wang K, Yang H, et al. Efficacy and adverse events of pirfenidone in treating idiopathic pulmonary fibrosis. Saudi Med J 2017;38:889–94. 10.15537/smj.2017.9.19349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan SD, Costabel U, Albera C, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis and more advanced lung function impairment. Respir Med 2019;153:44–51. 10.1016/j.rmed.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Joseph L, Fergusson D, et al. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ 2012;345:e5798. 10.1136/bmj.e5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuya K, Sakamoto S, Shimizu H, et al. Pirfenidone for acute exacerbation of idiopathic pulmonary fibrosis: A retrospective study. Respir Med 2017;126:93–9. 10.1016/j.rmed.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 22.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005;171:1040–7. 10.1164/rccm.200404-571OC [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:821–9. 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 24.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 25.Vianello A, Molena B, Turato C, et al. Pirfenidone improves the survival of patients with idiopathic pulmonary fibrosis hospitalized for acute exacerbation. Curr Med Res Opin 2019;35:1187–90. 10.1080/03007995.2019.1565530 [DOI] [PubMed] [Google Scholar]
- 26.Aravena C, Labarca G, Venegas C, et al. Pirfenidone for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. PLoS One 2015;10:e0136160. 10.1371/journal.pone.0136160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barratt SL, Creamer A, Hayton C, et al. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med 2018;7:201. 10.3390/jcm7080201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costabel U, Albera C, Lancaster LH, et al. An open-label study of the long-term safety of pirfenidone in patients with idiopathic pulmonary fibrosis (recap). Respiration 2017;94:408–15. 10.1159/000479976 [DOI] [PubMed] [Google Scholar]
- 29.Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res 2019;20:205. 10.1186/s12931-019-1161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito S, Alkhatib A, Kolls JK, et al. Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 2019;11:S1740–54. 10.21037/jtd.2019.04.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghu G, Richeldi L. Current approaches to the management of idiopathic pulmonary fibrosis. Respir Med 2017;129:24–30. 10.1016/j.rmed.2017.05.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050004supp001.pdf (280.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.