Abstract
This review article focuses on the dermatologic manifestations of selected nutrient deficiencies, including protein-energy and micronutrient-related malnutrition. The various nutrient deficiencies presented may share common features. However, distinctive cutaneous signs may prompt clinicians to consider a nutritional cause and help distinguish a nutrient deficiency from other common dermatologic conditions. The recent reemergence of forgotten nutritional deficiencies, such as scurvy and pellagra, in the context of predisposing risk factors that may uniquely affect women more than men makes this topic timely. Recognition of nutritional disorders is important because appropriate treatment may reverse cutaneous signs and prevent irreversible sequelae.
Keywords: Nutritional disorders, vitamin deficiency, protein-energy malnutrition, mineral deficiency
What is known about this subject in regard to women and their families?
-
•
Historical teachings on the role of sex in nutrition-related dermatologic diseases has been that there is generally no sex predilection, with a few notable exceptions.
-
•
These exceptions include deficiencies related to reproductive health, such as iron deficiency due to menses and pregnancy, as well as folate deficiency during pregnancy due to increased requirements.
What is new from this article as messages for women and their families?
-
•
Recently, there has been reemergence of so-called forgotten nutritional deficiencies, such as scurvy and pellagra, that were thought to be obsolete in developed countries.
-
•
Recognizing the cutaneous signs and symptoms of nutritional deficiencies, as well as predisposing risk factors (e.g., food faddism, restricted diets, and bariatric surgery) that may uniquely affect women more than men, is important.
Introduction
Malnutrition encompasses an imbalance of nutrient intake and physiological needs. This review article focuses on the dermatologic manifestations of selected nutrient deficiencies, including protein-energy and micronutrient-related malnutrition, the latter of which includes vitamin and mineral deficiencies.
Role of sex in nutrition and skin diseases
The historical teaching on the role of sex in nutrition-related dermatologic disease has been that there is generally no sex predilection, with a few notable exceptions. These exceptions include deficiencies related to reproductive health, such as iron deficiency due to menses and pregnancy, as well as folate deficiency during pregnancy due to increased requirements. However, our understanding of the effects of nutrient deficiencies beyond cutaneous manifestations in at-risk female groups has become more nuanced in recent years. For example, a recent study found that even mild iron deficiency among young women, a group at particular risk of depleted iron stores, may be associated with decreased cognitive endurance (Dziembowska et al., 2019).
On a global scale, micronutrient deficiencies have been linked to poor health outcomes, and women in developing countries may bear a disproportionate burden of micronutrient deficiencies, although this is culturally specific (Darnton-Hill et al., 2005). For example, in cultures where women receive less sun exposure due to head coverings and more time spent indoors, vitamin D deficiency disproportionally affects women (Elsori and Hammoud, 2018; Prentice, 2008; van Schoor and Lips, 2011). Similarly, even when women in some areas of Asia have their energy and protein needs met, they may be at risk of micronutrient malnutrition due to a lower intake of nutrient dense and more expensive foods (Rao et al., 2001).
Additionally, there has been reemergence of so-called forgotten nutritional deficiencies, such as scurvy and pellagra, that were thought to be obsolete in developed countries. These entities are seen in populations with predisposing risk factors that may uniquely affect women more than men, such as restricted diets, food faddism, and bariatric surgery. For example, pellagra has been reported in the context of a severely restricted, primarily vegan diet in an otherwise healthy young woman (Ng and Neff, 2018). Similarly, a retrospective review at Mayo Clinic found that women comprised the majority of patients with scurvy between 1976 and 2002. Among common associated causes, such as gastrointestinal disease, poor dentition, and alcoholism, food faddism was noted (Olmedo et al., 2006).
Finally, micronutrient deficiencies are prevalent in the morbidly obese female population, despite an excess of macronutrients (Lee et al., 2019; Sánchez et al., 2016). These deficiencies are often exacerbated after bariatric surgery, and the majority (∼80%) of patients undergoing bariatric surgery in the United States are female (Kochkodan et al., 2018; Shankar et al., 2010).
Protein-energy malnutrition
Marasmus, kwashiorkor, and marasmic kwashiorkor
Marasmus occurs with prolonged inadequate intake of protein and calories, leading to stunted growth. Kwashiorkor is traditionally associated with inadequate protein intake in the setting of relative adequate caloric intake, resulting in the distinctive feature of edema (Fig. 1; Dipasquale et al., 2020). Marasmic kwashiorkor is an overlap syndrome wherein the growth retardation of marasmus and the edema of Kwashiorkor are both present.
Fig. 1.
Kwashiorkor (photograph credit: Albert Yan, MD).
In marasmus, loss of subcutaneous fat leads to dry, loose, and wrinkled skin. On the face, loss of the buccal fat pads results in a prematurely aged appearance. Excess lanugo-like hair can be seen, as well as fissured, poorly growing nails and thin hair (Castellani, 1947). Unlike Kwashiorkor, there is no edema or dermatitis.
The dermatitis of Kwashiorkor begins as hypopigmented to erythematous-violaceous patches in areas of friction and pressure, such as intertriginous areas, and may be misdiagnosed as atopic dermatitis Table 1 (Rogers et al., 2014). Restrictive diets for presumed atopic dermatitis may lead to a concomitant protein-energy malnutrition dermatosis (Henrique de S B Xavier et al., 2017). Over time, the skin darkens, with a shiny, varnished appearance, and the fragile skin peels away to reveal hypopigmentation. This is often referred to as enamel paint or flaky paint (Cox et al., 2014). Pigment changes are striking with dyschromia. The hair becomes dry, lusterless, and hypopigmented, and curly hair becomes straight. The distinctive flag sign is typically observed in long dark hair and described as a red-brown or pale color change of the hair during periods of undernutrition, resulting in alternating pale and dark bands of hair (McLaren, 1987). The skin may gradually repigment with increased protein intake.
Table 1.
Distinctive skin changes of nutritional disorders and selected differential diagnosis
| Skin change | Nutritional disorder | Selected differential diagnosis |
|---|---|---|
| Exfoliative erythroderma | Kwashiorkor | Atopic dermatitis, psoriasis, staphylococcal scalded skin syndrome |
| Phrynoderma | Vitamin A deficiency, vitamin B-complex deficiencies, vitamin C deficiency, essential fatty-acid deficiency | Keratosis pilaris, pityriasis rubra pilaris |
| Petechiae, purpura | Vitamin K deficiency, vitamin C deficiency (perifollicular petechiae) | Consumptive coagulopathy, coagulation disorder, thrombocytopenia, vascular fragility syndrome (Ehlers–Danlos), cutaneous vasculitis (e.g., Henoch–Schonlein purpura) |
| Yellow-orange discoloration | Carotenemia | Jaundice, quinacrine-induced |
| Hyperpigmentation | Vitamin B12 deficiency, folate deficiency | Addison disease |
| Photo-distributed dermatitis | Vitamin B3 deficiency (pellagra), vitamin B6 deficiency | Connective tissue disease (systemic lupus erythematosus, dermatomyositis), polymorphous light eruption |
| Seborrheic dermatitis-like changes | Vitamin B-complex deficiencies | Seborrheic dermatitis, psoriasis |
| Angular stomatitis | Vitamin B-complex deficiencies, iron deficiency | Angular cheilitis with candidal overgrowth |
| Glossitis | Vitamin B-complex deficiencies, zinc deficiency, iron deficiency | Sjogren syndrome, oral lichen planus, syphilis |
| Diaper dermatitis | Zinc deficiency, vitamin B-complex deficiencies | Irritant or allergic contact dermatitis, seborrheic dermatitis, inverse psoriasis, Langerhans cell histiocytosis |
| Lightening of hair | Kwashiorkor, marasmus, vitamin B-complex deficiencies, copper deficiency | Poliosis, vitiligo |
| Corkscrew hairs | Vitamin C deficiency | |
| Nail changes | Koilonychia: iron deficiency Longitudinal hyperpigmented streaks: vitamin B3 deficiency (pellagra) |
Koilonychia: chronic irritant, lichen planus, psoriasis, Plummer–Vinson syndrome, transient in young children Longitudinal hyperpigmented streaks: physiologic, drug-induced, trauma |
Vitamins
Vitamin A
Deficiency
Vitamin A deficiency is the most common preventable cause of blindness in children worldwide (Smith and Steinemann, 2000), and prompt recognition and treatment is critical (McLaughlin et al., 2014). Vitamin A deficiency may result in a spectrum of ocular disease known as xeropthalmia (Smith and Steinemann, 2000). Early signs include diminished dark adaptation and night blindness (nyctalopia). Later manifestations include conjunctival and corneal xerosis, Bitot spots (white grey plaques on the conjunctiva), corneal ulceration, keratomalacia, and retinopathy (Smith and Steinemann, 2000).
Cutaneous findings of vitamin A deficiency include generalized xerosis and phrynoderma or toad skin (Miller, 1989). Phrynoderma describes firm, follicular, hyperkeratotic papules, characteristically distributed on the extensor extremities and buttocks; the shoulders, posterior neck, back, and abdomen may also be involved. In the majority of patients, the papules are asymptomatic (Ragunatha et al., 2011).
Phrynoderma must be distinguished from other entities with follicular papules, such as keratosis pilaris and pityriasis rubra pilaris. Although classically associated with vitamin A deficiency, phrynoderma is not a specific finding and has been described in other vitamin deficiencies (B-complex, C, and E), as well as essential fatty acid deficiency (Maronn et al., 2005).
Excess
Excess vitamin A may present as vitamin A toxicity, which manifests initially with systemic symptoms, such as fatigue, anorexia, nausea, vomiting, myalgias, and arthralgias. Hypervitaminosis A may progress to xerosis, cheilitis, desquamation, pruritus, and alopecia. Pseudotumor cerebri and skeletal hyperostosis may also occur (Bollag, 1983). Carotenemia is excessive beta-carotene, the natural provitamin of vitamin A. Notably, carotenemia is not associated with vitamin A toxicity (Maharshak et al., 2003).
Carotenoderma describes yellow-orange pigmentation of the skin, resulting from excretion of carotenes from the sebaceous and eccrine glands and deposition in the stratum corneum. Not surprisingly, carotenoderma characteristically involves areas of the face with abundant sebaceous glands, such as the nasolabial folds and forehead, as well as areas with a thick stratum corneum (palms and soles; Maharshak et al., 2003). Lack of mucous membrane involvement helps distinguish carotenoderma from jaundice. Additional causes of yellow skin pigmentation include lycopenemia (an isomer of carotenoid), riboflavinemia, and drug-induced pigmentation (e.g., quinacrine; Maharshak et al., 2003). Pigmentary changes typically resolve within weeks to months of normalization of beta-carotene intake.
Vitamin B2 deficiency
Specific groups at risk of vitamin B2 (riboflavin) deficiency include breastfed infants of mothers with deficiency and patients who have undergone bariatric surgery. Deficiency can also be seen in the setting of alcohol use disorder, hypothyroidism, phototherapy for neonatal hyperbilirubinemia, and medications such as chlorpromazine, probenicid, and tricyclic antidepressants (Miller, 1989). Acute vitamin B2 deficiency, which may be induced by acute borate ingestion (Yan and Jen, 2012), presents with deep-red erythema, epidermal necrolysis, and mucositis. These findings may resemble the clinical features of Kwashiorkor.
In contrast, chronic vitamin B2 deficiency may lead to oral-ocular-genital syndrome. Oral findings include cheilitis and angular stomatitis with papules at the oral commissures that may bleed (Miller, 1989). Glossitis may be observed, initially as prominent lingual papillae giving a pebbly appearance and eventually as an atrophic, smooth, and magenta-colored tongue (Jen and Yan, 2010). Ocular features include conjunctivitis and photophobia. Genital involvement often presents as a scrotal dermatitis in men, sparing the midline, and may spread to the inner thighs and perianal area. Initially the dermatitis is pruritic; it then becomes painful with possible associated fissuring and swelling, as well as balanitis and phimosis. Vulvar dermatitis is less common (Lakdawala and Grant-Kels, 2015).
Facial dermatitis of vitamin B2 deficiency may resemble seborrheic dermatitis and is similarly distributed on the nasolabial folds, nasal ala, forehead, cheeks, and postauricular skin (Miller, 1989). Areas of friction, such as the perineum of infants, are commonly involved.
Vitamin B3 deficiency
Chronic vitamin B3 (niacin) deficiency results in pellagra, the features of which are often quoted as the four Ds: dermatitis, diarrhea, dementia, and (if left untreated) death. The characteristic dermatitis is a photosensitive eruption. Initially, there is erythema and edema after sun exposure, which may be painful, burning, or pruritic and may resemble a sunburn (Wan et al., 2011). If severe, vesicles or bullae may be seen, sometimes termed wet pellagra. Repeated eruptions lead to fixed, well-demarcated, hyperpigmented, and hyperkeratotic plaques. Later, the skin develops a glassy or shellac-like appearance with parchment-like consistency. As with other photodistributed eruptions, common sites of involvement include the dorsal hands (seen in up to 97% of patients; Wan et al., 2011), butterfly distribution on the face, and the neck/upper chest in a broad band or collar, termed Casal necklace. The glove and boots of pellagra refer to the sharply demarcated plaques at the distal margin of clothing at the wrist and ankles (Jen and Yan, 2010).
Although photosensitive dermatitis is most commonly seen (Hegyi et al., 2004), three other variants of cutaneous pellagra have also been described (Wan et al., 2011): perineal, genital, and mucosal erosions, which can have associated atrophic glossitis, cheilitis, and vaginitis; hyperkeratosis and hyperpigmentation over bony prominences (e.g., knees and elbows) that is usually symmetric and gradual in onset; and sebaceous gland prominence, which may resemble seborrheic dermatitis. Hartnup disease and drug-induced pellagra tend not to present with features of genital and mucosal erosions or skin thickening and pigmentation over bony prominences (Wan et al., 2011).
Vitamin B6 deficiency
The most common cutaneous presentation of vitamin B6 (pyridoxine) deficiency is a seborrheic eruption on the face, scalp, neck, shoulders, buttocks, and perineum. Features of other vitamin B deficiencies include atrophic glossitis, angular stomatitis, cheilitis, and oral mucosa ulcerations (Jen and Yan, 2010). More rarely, a pellagra-like dermatitis on the dorsal extremities is observed (Miller, 1989).
Vitamin B12 and folate deficiency
The classic triad of vitamin B12 (cobalamin) deficiency includes megaloblastic anemia, glossitis, and neuropsychiatric symptoms. Folate deficiency shares the features of hematologic abnormalities and oral manifestations but lacks neurologic symptoms. Pregnant women are at risk of folate deficiency due to increased requirements (Solano and Councell, 1986). Hunter's glossitis (or glossitis of Moeller–Hunter) is classically associated with vitamin B12 deficiency and describes diffuse erythema and atrophy of lingual papillae, affecting more than half of the tongue (Greenberg, 1981). Hunter's glossitis is a nonspecific finding and is present in only 25% of cases of vitamin B12 deficiency.
The broader term “atrophic glossitis”, which describes a smooth beefy-red tongue, can be a manifestation of other nutritional deficiencies (folate, iron, niacin, riboflavin, and zinc), as well as candidiasis, Helicobacter pylori infection, xerostomia, and diabetes mellitus (Chiang et al., 2020). Atrophic linear lesions on the tongue and hard palate may be a more specific early clinical sign of vitamin B12 deficiency that precedes the onset of macrocytic anemia (Graells et al., 2009). Symptoms of glossitis include a generalized sore mouth, burning, and taste disturbance (Brescoll and Daveluy, 2015). Angular cheilitis and aphthous stomatitis have also been associated with vitamin B12 deficiency (Brescoll and Daveluy, 2015; Vigarios et al., 2019).
The most common cutaneous manifestation of vitamin B12 deficiency is hyperpigmentation. This is typically accentuated on the face, palmar creases, and flexures (Brescoll and Daveluy, 2015), similar to what is observed in Addison disease. Pigmentation in the nails may appear as longitudinal hyperpigmented streaks (Noppakun and Swasdikul, 1986). Depigmentation of the hair and skin (vitiligo) may also be seen (Brescoll and Daveluy, 2015).
The mucocutaneous findings generally resolve with replacement therapy; however, early recognition and treatment is critical to prevent the development of advanced neurologic disease, which may be irreversible.
Vitamin C deficiency
Scurvy develops over at least 1 to 3 months of sustained severe vitamin C deficiency (Deirawan et al., 2020) and can be summarized by the four Hs: hemorrhagic signs, hyperkeratosis of the hair follicles, hypochondriasis, and hematologic abnormalities (anemia). The onset of scurvy is marked by systemic symptoms, such as fatigue, malaise, and musculoskeletal pain, as well as weight loss, emotional lability, arthralgias, anorexia, and diarrhea. Easy bleeding, bruising, and poor wound healing are also seen.
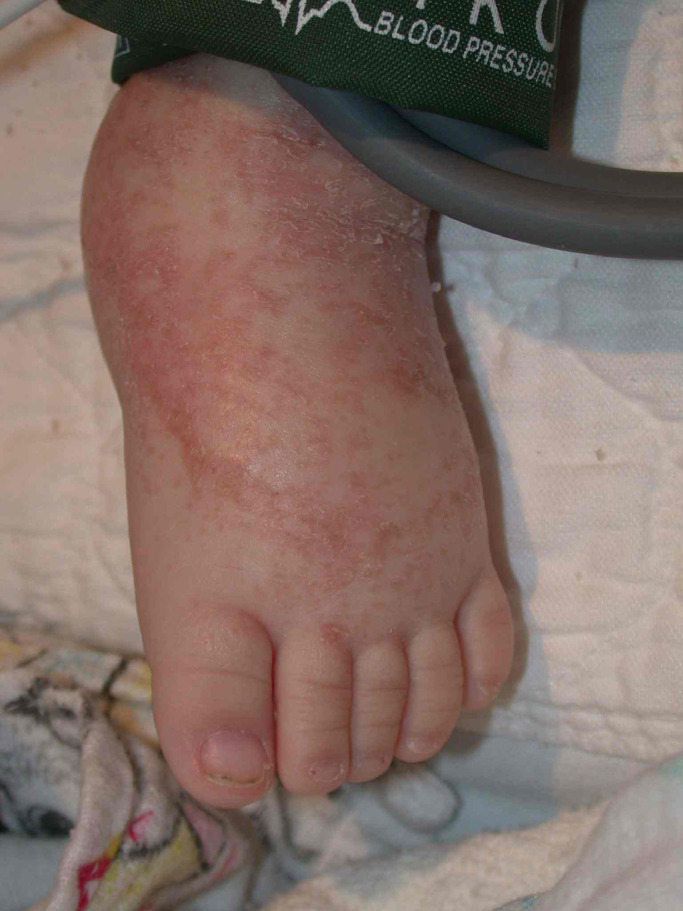
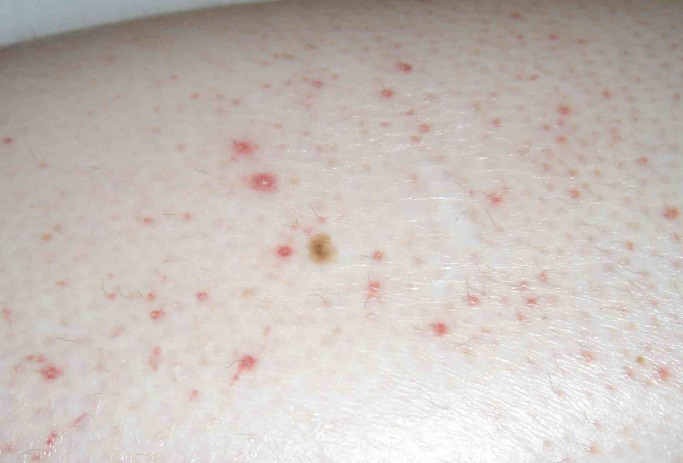
As in vitamin A deficiency, there can be follicular hyperkeratosis, especially of the posterolateral arms. Corkscrew hairs, resulting from impaired keratin-disulfide bond crosslinks, are often seen in association with these keratotic plugs (Walters et al., 2007). Helpful features on dermoscopy include a white area (perifollicular fibrosis) with a violaceous peripheral halo (red blood cell extravasation) surrounding the corkscrew hairs (Cinotti et al., 2015). Vascular congestion leads to lower extremity edema, which may be mistaken for cellulitis, and perifollicular erythema, hemorrhage, and purpura (Fig. 2). Of note, the purpura of scurvy can occasionally be palpable, mimicking a cutaneous vasculitis, such as Henoch–Schonlein purpura. Subcutaneous and intramuscular hemorrhage can result in tender nodules and ecchymoses, and subperiosteal hemorrhage presents as bone pain (Deirawan et al., 2020). Splinter hemorrhages of the nail bed may also be seen.
Fig. 2.
Scurvy (photograph credit: Albert Yan, MD).
In individuals with preexisting poor dentition, hemorrhagic gingivitis with spongy, red, shiny gingiva is followed by bleeding and necrosis. Teeth are soft, poorly formed, loose, and prone to infection. Untreated scurvy can be fatal, usually secondary to bleeding or infection (Deirawan et al., 2020).
Vitamin D deficiency
Vitamin D deficiency has no primary cutaneous manifestation other than alopecia. Globally, adequate vitamin D is present in <50% of the world's adult population during the winter. Particular groups at risk include pregnant women and older persons (van Schoor and Lips, 2017).
Minerals
Copper deficiency
Copper deficiency is marked by sensory and motor neuropathy. The primary dermatologic feature is hypopigmentation of the hair and skin because tyrosinase, a key enzyme in melanin synthesis, is copper-dependent (Finner, 2013). Menkes disease (kinky hair disease) is an X-linked recessive condition of defective copper absorption. The disease is named for the twists of hair (pili torti) that are light or ivory in color, sparse, and wiry, resembling steel wool (Aguilar et al., 1966). Infants have a characteristic cherubic face with pudgy cheeks, Cupid's bow upper lip, and doughy skin. Skeletal abnormalities, such as osteoporosis, as well as renal and urologic disease, are common. Neurologic deficits are severe, and patients may experience convulsive seizures, hypothermia, and vascular complications leading to death at a young age (Droms et al., 2017).
Zinc deficiency
The inherited autosomal recessive form of zinc deficiency, acrodermatitis enteropathica, is caused by a mutation in an intestinal zinc transporter gene. This disease often presents in infants shortly after transitioning from breastmilk to formula due to the higher absorption of zinc from human milk. Alternatively, a maternal mutation may decrease secretion of zinc into breast milk and lead to deficiency in breastfed infants, which is termed transient neonatal zinc deficiency (Watson et al., 2018). Pregnant women and children are at greatest risk of zinc deficiency (Berhe et al., 2019). Acquired zinc deficiency can also be seen in anorexia nervosa, alcoholism, inflammatory bowel disease, celiac disease, chronic diarrhea, gastric bypass surgery, and medications.
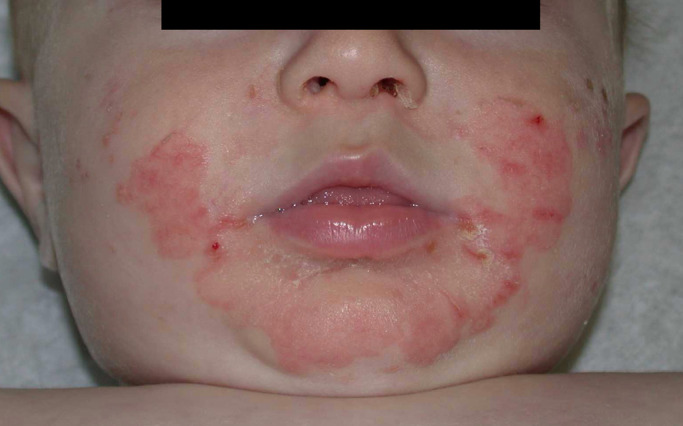
The complete classic triad of periorificial and acral dermatitis, diarrhea, and alopecia is seen in only 20% of patients with acrodermatitis enteropathica (Nistor et al., 2016). The dermatitis is characterized by sharply demarcated erythematous and eczematous plaques that become vesicular, bullous, pustular, or erosive with peripheral scale-crust and involve the perioral, acral, and anogenital skin. This disease must be considered when assessing chronic diaper rash in an infant with diarrhea. The perioral distribution often spares the upper lip, giving a U-shaped appearance (Fig. 3).
Fig. 3.
Acrodermatitis enteropathica (photograph credit: Albert Yan, MD).
Superinfection with bacteria (Staphylococcus aureus) and yeast (Candida albicans) is common due to immune dysregulation. Patients are often irritable and emotionally labile. Impaired wound healing, growth retardation, and hypogonadism in male patients may be observed (Perafán-Riveros et al., 2002). Other features include diffuse alopecia, ocular manifestations (blepharitis, conjunctivitis, abnormal dark adaptation), and mucosal findings (stomatitis, cheilitis, and glossitis; Maverakis et al., 2007). Paronychia may also be commonly seen (Yan and Jen, 2012). In older children and adolescents, zinc deficiency can present as psoriasiform dermatitis of the hands, feet, and knees (Maverakis et al., 2007).
Iron deficiency
Iron deficiency is common worldwide and prevalent among women and young children in industrialized countries (Zimmermann and Hurrell, 2007). Patients with gastrointestinal disease or post Roux-en-Y gastric bypass surgery are also at high risk. In addition to microcytic anemia and fatigue, nail changes are characteristically described. Koilonychia (i.e., spoon-shaped nails) describes the upward eversion of the lateral and distal nail plate and usually affects the first three digits (Walker et al., 2016). Although classically associated with iron deficiency anemia, koilonychia is seen in only 5% of cases (Razmi et al., 2018) and can be observed in other conditions, including systemic amyloidosis, hemochromatosis, chronic irritant/detergent use, lichen planus, psoriasis, and Plummer–Vinson syndrome (a clinical triad of dysphagia, iron deficiency anemia and esophageal webs) (Walker et al., 2016).
Koilonychia can also be a transient physiologic finding in young children age <4 years, uniquely affecting the second to fourth toes, unlike other forms of koilonychia (Chinazzo et al., 2017). Other mucocutaneous findings of iron deficiency include glossitis, angular cheilitis, pruritus, and telogen effluvium. Iron deficiency as a cause of hair loss remains controversial. Ferritin is the most widely used screening test for iron deficiency contributing to alopecia, and supplementation is often considered for a level <70 ng/ml, although concomitant chronic inflammation can make ferritin unreliable (Elston, 2010).
Conclusion
The various nutrient deficiencies presented may share common features. However, distinctive cutaneous signs may prompt clinicians to consider a nutritional cause and help distinguish a nutrient deficiency from other common dermatologic conditions. Recognition of nutritional disorders is important because appropriate treatment may reverse cutaneous signs and prevent irreversible sequelae.
Footnotes
Funding: None.
Conflicts of interest: None.
Study approval: N/A
References
- Aguilar MJ, Chadwick DL, Okuyama K, Kamoshita S. Kinky hair disease. I. Clinical and pathological features. J Neuropathol Exp Neurol. 1966;25(4):507–522. doi: 10.1097/00005072-196610000-00001. [DOI] [PubMed] [Google Scholar]
- Berhe K, Gebrearegay F, Gebremariam H. Prevalence and associated factors of zinc deficiency among pregnant women and children in Ethiopia: A systematic review and meta-analysis. BMC Public Health. 2019;19(1):1663. doi: 10.1186/s12889-019-7979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag W. Vitamin A and retinoids: From nutrition to pharmacotherapy in dermatology and oncology. Lancet. 1983;1(8329):860–863. doi: 10.1016/s0140-6736(83)91394-6. [DOI] [PubMed] [Google Scholar]
- Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16(1):27–33. doi: 10.1007/s40257-014-0107-3. [DOI] [PubMed] [Google Scholar]
- Castellani A. Hypertrichosis of the lanugo hair in malnutrition. Br Med J. 1947;2(4517):188. doi: 10.1136/bmj.2.4517.188-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CP, Chang JY, Wang YP, Wu YH, Wu YC, Sun A. Atrophic glossitis: Etiology, serum autoantibodies, anemia, hematinic deficiencies, hyperhomocysteinemia, and management. J Formos Med Assoc. 2020;119(4):774–780. doi: 10.1016/j.jfma.2019.04.015. [DOI] [PubMed] [Google Scholar]
- Chinazzo M, Lorette G, Baran R, Finon A, Saliba É, Maruani A. Nail features in healthy term newborns: A single-centre observational study of 52 cases. J Eur Acad Dermatol Venereol. 2017;31(2):371–375. doi: 10.1111/jdv.13978. [DOI] [PubMed] [Google Scholar]
- Cinotti E, Perrot JL, Labeille B, Cambazard F. A dermoscopic clue for scurvy. J Am Acad Dermatol. 2015;72(1 Suppl):S37–S38. doi: 10.1016/j.jaad.2014.05.061. [DOI] [PubMed] [Google Scholar]
- Cox JA, Beachkofsky T, Dominguez A. Flaky paint dermatosis. Kwashiorkor. JAMA Dermatol. 2014;150(1):85–86. doi: 10.1001/jamadermatol.2013.5520. [DOI] [PubMed] [Google Scholar]
- Darnton-Hill I, Webb P, Harvey PW, Hunt JM, Dalmiya N, Chopra M, et al. Micronutrient deficiencies and gender: Social and economic costs. Am J Clin Nutr. 2005;81(5) doi: 10.1093/ajcn/81.5.1198. 1198S–1205S. [DOI] [PubMed] [Google Scholar]
- Deirawan H, Fakhoury JW, Zarka M, Bluth MH, Moossavi M. Revisiting the pathobiology of scurvy: A review of the literature in the context of a challenging case. Int J Dermatol. 2020;59(12):1450–1457. doi: 10.1111/ijd.14832. [DOI] [PubMed] [Google Scholar]
- Dipasquale V, Cucinotta U, Romano C. Acute malnutrition in children: Pathophysiology, clinical effects and treatment. Nutrients. 2020;12(8) doi: 10.3390/nu12082413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droms RJ, Rork JF, McLean R, Martin M, Belazarian L, Wiss K. Menkes disease mimicking child abuse. Pediatr Dermatol. 2017;34(3):e132–e134. doi: 10.1111/pde.13106. [DOI] [PubMed] [Google Scholar]
- Dziembowska I, Kwapisz J, Izdebski P, Żekanowska E. Mild iron deficiency may affect female endurance and behavior. Physiol Behav. 2019;205:44–50. doi: 10.1016/j.physbeh.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Elsori DH, Hammoud MS. Vitamin D deficiency in mothers, neonates and children. J Steroid Biochem Mol Biol. 2018;175:195–199. doi: 10.1016/j.jsbmb.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Elston DM. Commentary: Iron deficiency and hair loss: Problems with measurement of iron. J Am Acad Dermatol. 2010;63(6):1077–1082. doi: 10.1016/j.jaad.2009.09.054. [DOI] [PubMed] [Google Scholar]
- Finner AM. Nutrition and hair: Deficiencies and supplements. Dermatol Clin. 2013;31(1):167–172. doi: 10.1016/j.det.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Graells J, Ojeda RM, Muniesa C, Gonzalez J, Saavedra J. Glossitis with linear lesions: An early sign of vitamin B12 deficiency. J Am Acad Dermatol. 2009;60(3):498–500. doi: 10.1016/j.jaad.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Greenberg MS. Clinical and histologic changes of the oral mucosa in pernicious anemia. Oral Surg Oral Med Oral Pathol. 1981;52(1):38–42. doi: 10.1016/0030-4220(81)90170-5. [DOI] [PubMed] [Google Scholar]
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: Dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43(1):1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- Henrique de S B Xavier M, De Magalhães E, Ferraz Oliveira G, Keltke Magalhães M, Prates de Almeida E Oliveira C, Bragança Oliveira N. A child with kwashiorkor misdiagnosed as atopic dermatitis. Dermatol Online J. 2017;23(5) [PubMed] [Google Scholar]
- Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol. 2010;28(6):669–685. doi: 10.1016/j.clindermatol.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Kochkodan J, Telem DA, Ghaferi AA. Physiologic and psychological gender differences in bariatric surgery. Surg Endosc. 2018;32(3):1382–1388. doi: 10.1007/s00464-017-5819-z. [DOI] [PubMed] [Google Scholar]
- Lakdawala N, Grant-Kels JM. Acrodermatitis enteropathica and other nutritional diseases of the folds (intertriginous areas) Clin Dermatol. 2015;33(4):414–419. doi: 10.1016/j.clindermatol.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Lee PC, Ganguly S, Dixon JB, Tan HC, Lim CH, Tham KW. Nutritional deficiencies in severe obesity: A multiethnic Asian cohort. Obes Surg. 2019;29(1):166–171. doi: 10.1007/s11695-018-3494-3. [DOI] [PubMed] [Google Scholar]
- Maharshak N, Shapiro J, Trau H. Carotenoderma–A review of the current literature. Int J Dermatol. 2003;42(3):178–181. doi: 10.1046/j.1365-4362.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Maronn M, Allen DM, Esterly NB. Phrynoderma: A manifestation of vitamin A deficiency?... The rest of the story. Pediatr Dermatol. 2005;22(1):60–63. doi: 10.1111/j.1525-1470.2005.22113.x. [DOI] [PubMed] [Google Scholar]
- Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56(1):116–124. doi: 10.1016/j.jaad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- McLaren DS. Skin in protein energy malnutrition. Arch Dermatol. 1987;123(12) 1674–6a. [PubMed] [Google Scholar]
- McLaughlin S, Welch J, MacDonald E, Mantry S, Ramaesh K. Xerophthalmia–A potential epidemic on our doorstep? Eye (Lond) 2014;28(5):621–623. doi: 10.1038/eye.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ. Nutritional deficiency and the skin. J Am Acad Dermatol. 1989;21(1):1–30. doi: 10.1016/s0190-9622(89)70144-4. [DOI] [PubMed] [Google Scholar]
- Ng E, Neff M. Recognising the return of nutritional deficiencies: A modern pellagra puzzle. BMJ Case Rep. 2018;11(1) doi: 10.1136/bcr-2018-227454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor N, Ciontu L, Frasinariu OE, Lupu VV, Ignat A, Streanga V. Acrodermatitis enteropathica: A case report. Medicine (Baltimore) 2016;95(20):e3553. doi: 10.1097/MD.0000000000003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppakun N, Swasdikul D. Reversible hyperpigmentation of skin and nails with white hair due to vitamin B12 deficiency. Arch Dermatol. 1986;122(8):896–899. [PubMed] [Google Scholar]
- Olmedo JM, Yiannias JA, Windgassen EB, Gornet MK. Scurvy: A disease almost forgotten. Int J Dermatol. 2006;45(8):909–913. doi: 10.1111/j.1365-4632.2006.02844.x. [DOI] [PubMed] [Google Scholar]
- Perafán-Riveros C, França LF, Alves AC, Sanches JA. Acrodermatitis enteropathica: Case report and review of the literature. Pediatr Dermatol. 2002;19(5):426–431. doi: 10.1046/j.1525-1470.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- Prentice A. Vitamin D deficiency: A global perspective. Nutr Rev. 2008;66(10 Suppl 2):S153–S164. doi: 10.1111/j.1753-4887.2008.00100.x. [DOI] [PubMed] [Google Scholar]
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol. 2011;56(4):389–392. doi: 10.4103/0019-5154.84760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Yajnik CS, Kanade A, Fall CH, Margetts BM, Jackson AA, et al. Intake of micronutrient-rich foods in rural Indian mothers is associated with the size of their babies at birth: Pune Maternal Nutrition Study. J Nutr. 2001;131(4):1217–1224. doi: 10.1093/jn/131.4.1217. [DOI] [PubMed] [Google Scholar]
- Razmi TM, Nampoothiri RV, Dogra S. Koilonychia in iron deficiency. QJM. 2018;111(4):271–272. doi: 10.1093/qjmed/hcx229. [DOI] [PubMed] [Google Scholar]
- Rogers AS, Shaughnessy KK, Davis LS. Dermatitis and dangerous diets: A case of kwashiorkor. JAMA Dermatol. 2014;150(8):910–911. doi: 10.1001/jamadermatol.2013.10328. [DOI] [PubMed] [Google Scholar]
- Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26(11-12):1031–1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Smith J, Steinemann TL. Vitamin A deficiency and the eye. Int Ophthalmol Clin. 2000;40(4):83–91. doi: 10.1097/00004397-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Solano FX, Jr, Councell RB. Folate deficiency presenting as pancytopenia in pregnancy. Am J Obstet Gynecol. 1986;154(5):1117–1118. doi: 10.1016/0002-9378(86)90771-4. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Rojas P, Basfi-Fer K, Carrasco F, Inostroza J, Codoceo J, et al. Micronutrient deficiencies in morbidly obese women prior to bariatric surgery. Obes Surg. 2016;26(2):361–368. doi: 10.1007/s11695-015-1773-9. [DOI] [PubMed] [Google Scholar]
- van Schoor N, Lips P. Global overview of vitamin D status. Endocrinol Metab Clin North Am. 2017;46(4):845–870. doi: 10.1016/j.ecl.2017.07.002. [DOI] [PubMed] [Google Scholar]
- van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25(4):671–680. doi: 10.1016/j.beem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Vigarios E, Comont T, Piroth M, Cougoul P, Sibaud V. Severe aphthous stomatitis secondary to vitamin B12 deficiency with isotretinoin therapy. JAAD Case Rep. 2019;5(6):563–565. doi: 10.1016/j.jdcr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Baran R, Vélez N, Jellinek N. Koilonychia: An update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30(11):1985–1991. doi: 10.1111/jdv.13610. [DOI] [PubMed] [Google Scholar]
- Walters RW, Vinson EN, Soler AP, Burton CS. Scurvy with manifestations limited to a previously injured extremity. J Am Acad Dermatol. 2007;57(2 Suppl):S48–S49. doi: 10.1016/j.jaad.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Wan P, Moat S, Anstey A. Pellagra: A review with emphasis on photosensitivity. Br J Dermatol. 2011;164(6):1188–1200. doi: 10.1111/j.1365-2133.2010.10163.x. [DOI] [PubMed] [Google Scholar]
- Watson L, Cartwright D, Jardine LA, Pincus D, Koorts P, Kury S, et al. Transient neonatal zinc deficiency in exclusively breastfed preterm infants. J Paediatr Child Health. 2018;54(3):319–322. doi: 10.1111/jpc.13780. [DOI] [PubMed] [Google Scholar]
- Yan AC, Jen MV. Skin signs of pediatric nutritional disorders. Curr Probl Pediatr Adolesc Health Care. 2012;42(8):212–217. doi: 10.1016/j.cppeds.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]