Abstract
Immune checkpoint inhibitors are a new class of oncologic drugs that act via the inhibition of checkpoints, thereby unlocking the immune system to attack cancer cells. Their emergence has radically changed the concept of therapy in oncologic patients. However, despite their overall favorable profile, their use has been associated with specific toxicities that may potentially affect treatment. The so-called immune-related adverse events (irAEs) mostly correspond to dysimmune reactions that can affect nearly every organ system, in theory, notably with the development of colitis, hepatitis, pneumonitis, or thyroiditis. Dermatologic irAEs are also among the most common, reaching a rate of approximately 40%. They are characterized by a wide phenotypic range, including mainly eczematous or lichenoid rashes, psoriasis, or autoimmune bullous disorders. Pruritus may accompany the aforementioned rashes or develop as an isolated symptom without the presence of skin changes. Depigmentation and hair/nail changes can be also observed in association with immune checkpoint inhibitor treatment. In the current article, we present an overview of the clinical spectrum of irAEs and provide tips for early recognition and management of dermatologic irAEs. We highlight the role that dermatologists can play in relieving patients and allowing for oncologic treatment to be maintained and administered more safely.
Keywords: Immune checkpoint inhibitors, skin toxicity, adverse events, rash, pruritus
What is known about this subject in regard to women and their families?
-
•
Dermatologic immune checkpoint inhibitor–related adverse events are among the most frequent immune checkpoint inhibitor–related toxicities.
-
•
Widely accepted guidelines for diagnosis and management of dermatologic immune-related adverse events (dirAEs) are lacking.
-
•
However, diagnosis and early management of dirAEs are crucial for maintaining an acceptable quality of life for patients and their families.
-
•
Unfortunately, no specific data are available for women who have developed dirAEs.
What is new from this article as messages for women and their families?
-
•
This current literature review provides evidence-based data concerning early recognition and better classification of dermatologic immune-related adverse events (dirAEs).
-
•
This literature review provides evidence-based data concerning adequate management of dirAEs and optimal patient monitoring.
-
•
This literature review highlights the lack of data available to measure the specific impact of these dirAEs on female patients, which would allow for management to be optimized in this population.
Introduction
The emergence of immune checkpoint inhibitors (ICIs) has radically changed the concept of therapy in oncology. ICIs inhibit the regulatory cytotoxic T lymphocyte protein 4 (CTLA-4), programmed death (PD)-1 and PD-ligand (L)1, resulting in enhanced immunologic activity against neoplastic cells (Esfahani et al., 2020). ICIs are generally well tolerated and significantly less toxic than chemotherapy (Rapoport et al., 2020). However, despite their overall favorable safety profile, their use has been linked to certain immune-derived toxicities (so-called immune-related adverse events [irAEs]) that are triggered by overactivation of the immune system. Although irAEs remain typically low grade and manageable (especially with the use of a single agent), potentially life-threatening toxicities can occur occasionally (Cooksley et al., 2020; Cuzzubbo et al., 2017; Delanoy et al., 2019; Dougan et al., 2020; Rapoport et al., 2017; Shannon et al., 2020; Suarez-Almazor et al., 2020). Gastrointestinal, hepatic, and endocrine toxicities are more common, but irAEs can theoretically affect nearly every organ system (Cooksley et al., 2020; Cuzzubbo et al., 2017; Delanoy et al., 2019; Dougan et al., 2020; Rapoport et al., 2017; Shannon et al., 2020; Suarez-Almazor et al., 2020).
Dermatologic irAEs (dirAEs) are also among the most frequent and occur in approximately 30% to 40% and 50% of patients receiving PD-1/PD-L1 and CTLA-4 inhibitors, respectively. They have a wide phenotypic range, including eczematous maculopapular rashes, psoriasis, lichenoid reactions, and autoimmune bullous disorders (Ellis et al., 2020; Sibaud, 2018). Pruritus may accompany the aforementioned rashes or develop as an isolated symptom without the presence of skin changes (Phillips et al., 2019a). Disturbed melanogenesis, as well as hair and nail changes, can also be observed in association with ICI treatment. However, reports of potentially life-threatening skin toxicities, such as toxic epidermal necrolysis, are limited (Choi et al., 2020).
In terms of pathogenesis, several theories have been proposed, mostly involving the production of autoreactive CD4+/CD8+ T cells, stimulation of humoral immunity and B cells, the increased release of proinflammatory cytokines that are involved in immune-related damage in specific tissues/organs, and the potential exposure of host antigens from tumor cells due to cytotoxic attacks (Flatz et al., 2019; Hasan et al., 2020; Postow et al., 2018).
Clinical examination usually is sufficient to establish a correct diagnosis of dermatologic toxicity. Similarly, inducing treatment can be continued in the vast majority of cases despite dermatologic toxicity. However, early recognition and proactive management by clinicians remain crucial to lowering morbidity associated with dirAEs. Moreover, in atypical, persistent, or severe cases, a dermatologic consultation with skin biopsy should be performed to remove any diagnostic doubts and propose optimal management (Sibaud, 2018). Finally, in case of necessary interruption of immunotherapy, reintroduction should be cautious and discussed in a multidisciplinary approach on a case-by-case basis.
Appropriate dermatologic management of patients with dirAEs was recently reported to be associated with a reduction in the use of immunosuppressive drugs or treatment discontinuation and could even lead to an improved survival outcome (Chen et al., 2020; Thompson et al., 2021). Cumulative data also suggest that the development of skin toxicities may serve as a prognostic factor of better oncologic response. However, this issue is still under investigation (Chan et al., 2020; Hua et al., 2016; Matsuya et al., 2020; Postow et al., 2018; Quach et al., 2019).
In the current article, we present an overview of the clinical spectrum of irAEs and provide tips for the early recognition and management of dirAEs, highlighting the role that dermatologists can play in providing relief to patients and ensuring unimpeded oncologic treatment (Apalla and Sibaud, 2020).
Dermatologic immune-related adverse events
Eczematous rash (syn. maculopapular rash)
Eczematous rash (ER) appears in up to 68% of patients on anti-CTLA-4 and up to 20% of patients on anti-PD-1/PD-L1 treatment (Postow, 2015). Even though its pathogenesis is still unclear, the prevailing theory is based on the cross-recognition by the activated CD4+/CD8+ T lymphocytes of antigens shared by the neoplastic cells and otherwise healthy skin (Flatz et al., 2019). The onset of ER usually ranges from 3 to 6 weeks after ICI initiation. However, there have been reports of ER developing as early as a few days and as late as several months after the first dose of immunotherapy (Belum et al., 2016; Sibaud, 2018). ER is characterized by the sudden appearance of erythematous macules and papules, almost always pruritic, coalescing into larger plaques (Fig. 1). Sites of predilection include the trunk and the extensor parts of the extremities (Belum et al., 2016; Geisler et al., 2020; Sibaud, 2018). Histopathologic findings mainly include focal epidermal spongiosis, with minimal lymphocytic exocytosis, mostly perivascular lymphocytic infiltrates (CD-4 predominance), admixed with eosinophils in the papillary and mid-dermis, and papillary dermal edema (Ellis et al., 2020; Geisler et al., 2020; Lacouture et al., 2014; Sibaud, 2018).
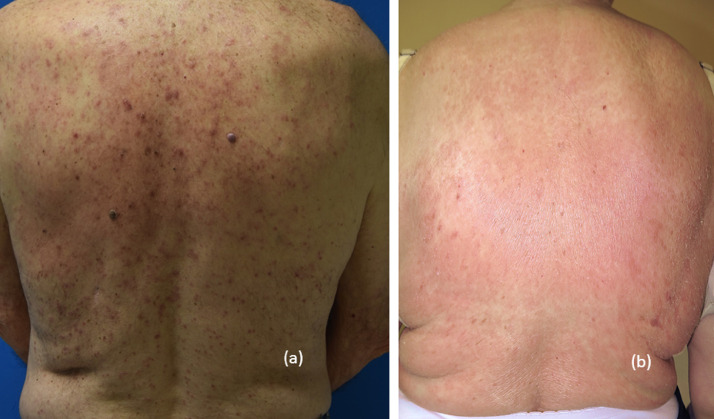
Fig. 1.
Grade 2/3 nonspecific maculopapular rash.
ER should be graded and treated in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events. Rash of mild or moderate intensity (grade 1–2) can be easily managed with skin-directed therapy, including moisturizers and mild- and super-potent topical steroids, without interrupting immunotherapy. In the event of pruritus, oral antihistamines can be prescribed (Geisler et al., 2020; Phillips et al., 2019a; Postow, 2015; Sibaud, 2018). If a grade 3 rash occurs, systemic steroids (0.5–1 mg/kg/day of prednisone equivalent) may be introduced with the temporary discontinuation of ICI treatment. Another reasonable option could be anti-IL4 dupilumab, although data concerning its use in the context of ICI-induced ER remain limited. Immunotherapy can be reinitiated after tapering the oral steroids at a dose of ≤10 mg of prednisone equivalent per day.
The patient should be referred for dermatologic consultation in the event of atypical, refractory, or high-grade ER to exclude the initial phase of a potentially life-threatening adverse event or the atypical presentation of a more specific condition, such as bullous pemphigoid, lichenoid reaction, or induced psoriasis.
Pruritus
Pruritus in the context of ICI treatment can present either as an isolated symptom or in association with other dirAEs (Phillips et al., 2019a; 2019b). As shown in a systematic review, individuals treated with nivolumab and pembrolizumab report pruritus at a rate of 20.2% and 13.2%, respectively. Interestingly, severe (grade 3) pruritus is comparatively uncommon, seen in only 0.5% and 2.3%, respectively (Belum et al., 2016). Unlike anti-PD-1/anti-PD-L1, the rate of pruritus with anti-CTLA4 drugs is higher, reaching an incidence of 47% (Sibaud, 2018). In general, pruritus is an early dirAE, usually appearing within the first 3 to 10 weeks of treatment (Weber et al., 2017). Severity of pruritus is graded according to its impact on quality of life (QoL) as grade 1, 2, or 3. For example, Phillips et al. (2019a) recorded higher Itchy-QoL scores in individuals with ICI-triggered pruritus than in hemodialysis patients with uremic disease. Apart from clinical inspection, a routine laboratory examination including eosinophil counts, peripheral IgE, and renal and hepatic biochemistry profile is recommended. In addition, a prebullous phase of a bullous pemphigoid triggered by ICI should always be excluded.
In terms of treatment, regular use of topical moisturizers, combined with medium- to high-potency topical steroids (if needed) are usually enough to adequately control grade 1 pruritus. Additionally, a third-generation antihistamine and/or a GABA agonist, such as pregabalin or gabapentin, may be required for grade 2 pruritus. Immunotherapy is maintained in patients with grade 1–2 disease, but temporary discontinuation of ICIs might be needed in those with grade 3 pruritus until improvement to at least grade 2. Reports in the literature suggest the potential effect of aprepitant, a neurokinin 1 receptor agonist, in this context (Ito et al., 2017). Low doses (≤10 mg/day of prednisone or equivalent) should only be used in refractory cases. Biologic agents, such as omalizumab and dupilumab, may prove beneficial in refractory patients (Barrios et al., 2021).
Lichen planus-like rash
Lichen planus-like rash (LPLR) is typically considered one of the most common dirAEs (Coleman et al., 2019; Hwang et al., 2019; Shi et al., 2016). However, in our experience, LPLR accounts for only approximately 10% of dirAEs and develops less frequently than ER (Chan et al., 2020; Geisler et al., 2020; Rovers and Bovenschen, 2020; Sibaud, 2018). LPLR occurs more commonly with the use of anti-PD-1/PD-L1 agents than with anti-CTLA-4 agents (Coleman et al., 2019; Geisler et al., 2020; Hwang et al., 2019; Schaberg et al., 2016; Shi et al., 2016). Unlike ER, LPLR is characterized by a delayed onset, ranging from several weeks to months after the initial dose of ICI treatment. Unusual events of LPLR developing after ICI discontinuation have also been reported in the literature (Chan et al., 2020; Geisler et al., 2020; Schaberg et al., 2016; Wang et al., 2018).
The phenotypic variation in LPLR is wide, including papulosquamous, atrophic, hypertrophic, pigmented, bullous, and erosive lesions (Fig. 2). As in the classic form of lichen planus, the sites of predilection are the limbs, wrists, palms, and soles. Disseminated variants or atypical cases with predominant hair/nail, palmoplantar, mucosal, or inverse involvement have also been described (Chan et al., 2020; Geisler et al., 2020; Shi et al., 2016; Sibaud, 2018; Sibaud et al., 2017). The lesions are often intensively pruritic, significantly impairing patients’ QoL (Coleman et al., 2019; Geisler et al., 2020). Of note, the hypertrophic variant of LPLR should be differentiated from squamous cell carcinoma, especially because squamous cell carcinomas can be observed in the context of ICI treatment. In diagnostically ambiguous lesions, histologic examination is strongly recommended (Freites-Martinez et al., 2017).
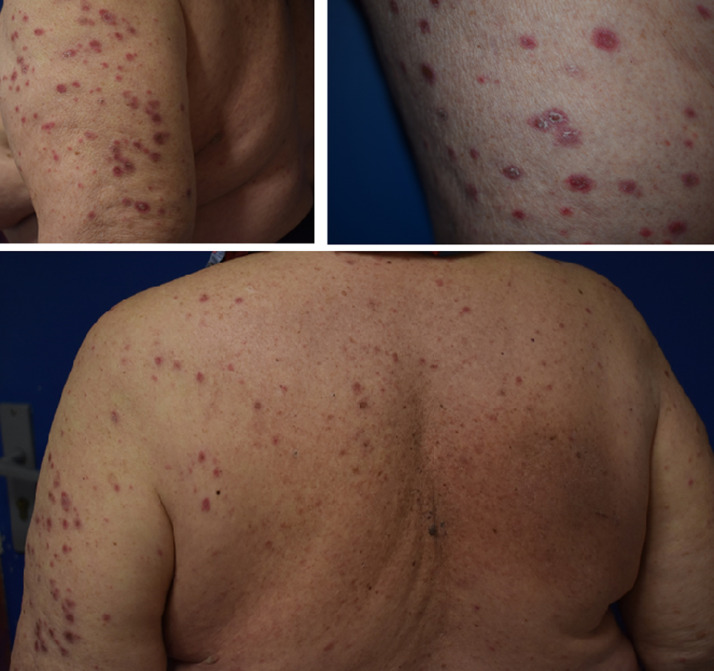
Fig. 2.
Diffuse lichen planus-like eruption in a woman treated with nivolumab.
The histopathology of LPLR may be indistinguishable from idiopathic lichen planus, displaying classic criteria such as band-like lymphohistiocytic infiltrates obscuring the dermo-epidermal junction, focal vacuolar changes of the basal cell layer, and Civatte bodies (Ellis et al., 2020; Geisler et al., 2020; Schaberg et al., 2016; Shi et al., 2016; Sibaud, 2018; Sibaud et al., 2017). Unlike classic lichen planus, however, immunostaining is characterized by a mixed CD4+/CD8+ or predominantly CD4+ T-cell infiltrate (Ellis et al., 2020; Schaberg et al., 2016; Shi et al., 2016).
As in idiopathic lichen planus, in low-grade cases (grade 1–2), topical treatment with mid- and super-potent topical steroids is efficacious and safe without the need to change the ICI treatment (Coleman et al., 2019; Geisler et al., 2020; Shi et al., 2016; Sibaud, 2018). Systemic steroids (0.5–1 mg/kg/day) and/or oral retinoids (20–30 mg/day, alitretinoin or acitretin) should be reserved for severe (grade 3) or refractory lesions, as well as lichenoid reactions involving other sites, such as the oral mucosal mucosae or nails (Coleman et al., 2019; Geisler et al., 2020; Schaberg et al., 2016; Weber et al., 2017). There is also some evidence of the beneficial effect of methotrexate, cyclosporine, and narrowband ultraviolet B (NB-UVB), although the evidence is insufficient to support their routine use. NB-UVB should be used with caution in patients with a history of melanoma.
Psoriasis-like rash
Exacerbation of preexisting psoriasis is a comparatively common dirAE (Bonigen et al., 2017; Nikolaou et al., 2021; Voudouri et al., 2017), but de novo psoriasis is rarer. Psoriasis typically occurs within 5 to 12 weeks from ICI initiation (Nikolaou et al., 2021; Weber et al., 2017). Plaque-type psoriasis is the most common ICI-related phenotype. However, less common presentations, such as pustular, scalp, and inverse psoriasis, can also occur (Fig. 3, Fig. 4; Nikolaou et al., 2021). In this scenario, a dermatologic consultation for diagnosis and skin-directed management is advisable.
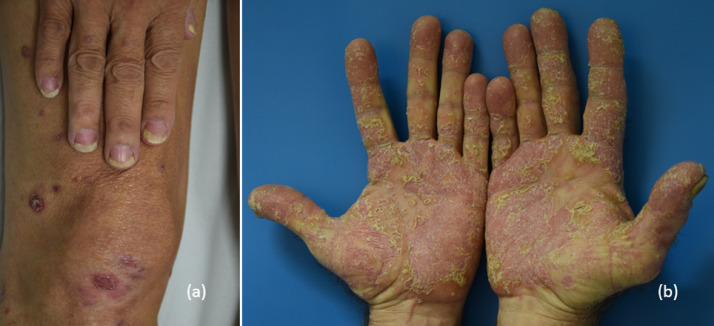
Fig. 3.
(A) Nummular psoriasis with apparent onycholysis and (B) severe psoriasis with palmar involvement.
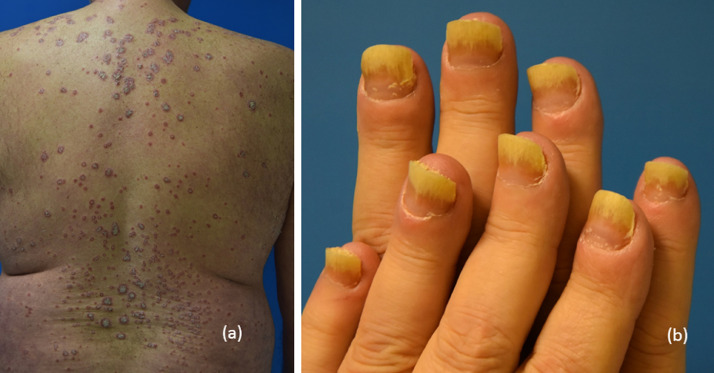
Fig. 4.
(A) Diffuse psoriasis vulgaris (B) with onycholysis occurring with atezolizumab.
In terms of pathogenesis, whether overexpression of Th1/Th17-specific cytokines (IL-17, IL-22) in the context of PD1-blocking is responsible for the exacerbation or induction of psoriasis in these individuals remains unclear (Dolus et al., 2012). Grades 1 and 2 eruptions can be successfully managed with topical steroids or vitamin D analogs depending on the site of involvement. Systematic treatments, including acitretin, methotrexate, and apremilast, are reserved for grade 3 or recalcitrant eruptions (Nikolaou et al., 2021). Cyclosporine is not recommended due to its tumor-promoting effects. Phototherapy (NB-UVB) can be considered, especially when pruritus is present, but should be used with caution in patients with melanoma (Amatore et al., 2019; Apalla et al., 2019; Bonigen et al., 2017; Nikolaou et al., 2021; Salopek, 2017; Voudouri et al., 2017).
Regarding biologic treatments, anti-tumor necrosis factors (TNFs), anti-IL23, and anti-IL12/23 have been used with positive outcomes in many cases. Anti-TNFs may prove particularly useful in the scenario of simultaneous induction of psoriatic arthritis and psoriasis due to immunotherapy (Nikolaou et al., 2021). However, before any treatment decision, physicians should consider the vastly unknown effects of biologics on ICI treatment and the label warnings for their use in oncologic patients. Τhe cost–benefit ratio for the patient must be thoroughly estimated on a case-by-case basis, optimally in the context of a multidisciplinary consultation (Apalla and Sibaud, 2020).
Vitiligo-like rash
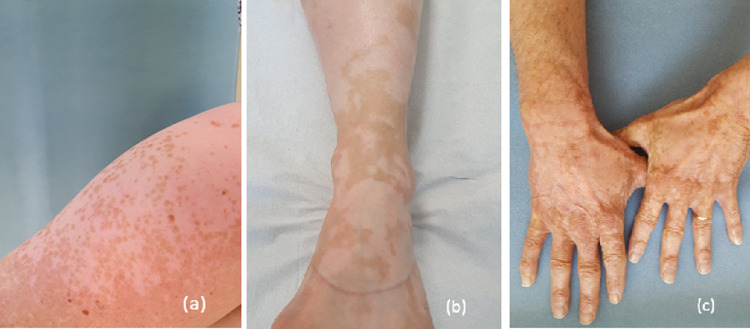
Vitiligo-like rash (VLR) develops mostly in patients with melanoma treated with anti-PD-1/anti-PD-L1. Clinically, the lesions appear as bilateral, multiple, or hypo- or depigmented macules (Fig. 5; Geisler et al., 2020). Even though prospective studies reported a higher incidence of approximately 25% (Hua et al., 2016; Matsuya et al., 2020), meta-analyses of patients with melanoma revealed an incidence of approximately 8.3% for pembrolizumab and 7.5% for nivolumab (Belum et al., 2016; Geisler et al., 2020). VLR is less common with anti-CTLA-4 (Matsuya et al., 2020).
Fig. 5.
(A–C) Progressive skin depigmentation with anti-PD-1 monoclonal antibodies.
VLR is characterized by a late onset, usually several months after ICI initiation (Geisler et al., 2020; Hua et al., 2016; Larsabal et al., 2017). Treatment with the combination of anti-PD1/PD-L1 and ipilimumab has been associated with earlier onset and more disseminated forms (Hwang et al., 2019) Both large confluent white patches and en-confetti, asymmetric, grouped macules of depigmentation can be observed (Larsabal et al., 2017; Sibaud, 2018). Hair depigmentation and poliosis can also occur at the same time. Although the pathogenesis is not clearly determined, a cross-reaction against common antigens expressed in melanoma cells and normal melanocytes (e.g., GP100, MART-1, TRP1–2, or tyrosinase) has been hypothesized (Hua et al., 2016; Postow, 2018). Development of VLR does not demand discontinuation or the temporary withdrawal of ICI treatment because vitiligo may persist after treatment cessation. However, patients should be clearly informed before starting treatment, especially in an adjuvant setting. Intense photoprotection is highly recommended, and camouflage or self-tanning products are particularly useful to limit the impact on QoL.
Autoimmune bullous disorders
Although rare, the development of autoimmune bullous disorders (AIBDs) with ICI is well understood (Juzot et al., 2021). The overall incidence ranges from 1% to 5% and is higher with the use of anti-PD-1/PD-L1 agents (Lopez et al., 2018; Siegel et al., 2018). Bullous pemphigoid (BP) is by far the most common ICI-induced AIBD, and lichen planus pemphigoides, pemphigus, dermatitis herpetiformis, linear IgA bullous dermatosis, and mucous membrane pemphigoid are extremely scarce in this context (Apalla et al., 2021; Lopez et al., 2018; Molina et al., 2020; Nelson et al., 2020; Sibaud et al., 2019; Siegel et al., 2018).
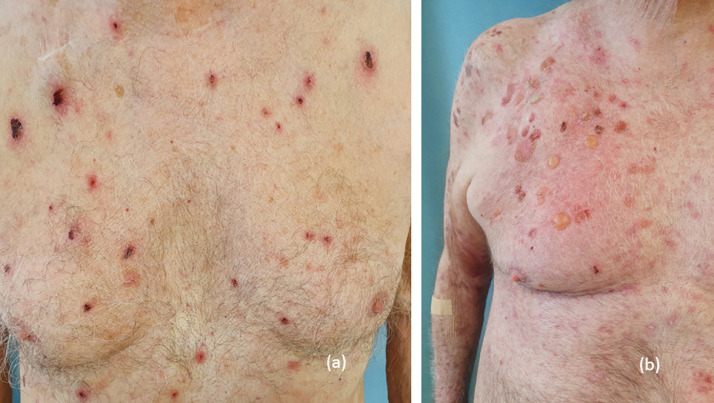
The pathogenesis of ICI-triggered BP is largely unknown, but more likely to result from the activation of antibody-secreting B cells and inhibition of immunosuppressive B regulatory cells. In addition, a possible cross-reaction against BP180-shared antigens of the skin and tumor cells cannot be excluded (Hasan et al., 2020). BP in the context of ICI treatment usually develops weeks to several months after the first infusion and is clinically characterized by tense vesicles/bullae mostly affecting the trunk and extremities (Fig. 6; Juzot et al., 2021; Lopez et al., 2018; Siegel et al., 2018). A prebullous stage, with intense pruritus and erythematous plaques reminiscent of ER or urticaria, may precede the classic bullous eruption (Apalla et al., 2021b; Molina et al., 2020). Mucosal involvement can be seen in up to 40% of cases (Juzot et al., 2021; Molina et al., 2020).
Fig. 6.
Bullous pemphigoid: (A) postbullous lesions and (B) blisters.
As in classic autoimmune BP, histology reveals subepidermal clefting with fibrin and eosinophils, as well as dermal infiltrates composed of lympho-histiocytes, eosinophils, and scarce neutrophils (Apalla et al., 2021b; Ellis et al., 2020). Immunofluorescence studies (direct immunofluorescence and salt-split direct immunofluorescence) demonstrating linear depositions of IgG and C3 at the epidermal site of the clefting and enzyme-linked immunosorbent assay indicating the presence of BP180 and less often BP230 antibodies are mandatory in this setting (Juzot et al., 2021; Molina et al., 2020; Siegel et al., 2018). The development of BP may profoundly affect ICI treatment, resulting in permanent discontinuation in many cases (Apalla et al., 2021b; Juzot et al., 2021; Lopez et al., 2018; Siegel et al., 2018). Early recognition and adequate management may facilitate subsequent control of the disease and minimize treatment modifications. Since there have been reports in the literature of BP emerging or persisting for several months after ICI discontinuation, long-term monitoring is advisable (Lopez et al., 2018).
Grade 1 and 2 eruptions can be managed with low doses of prednisolone and potent topical steroids without affecting ICI treatment. The maintenance of immunotherapy must be discussed after multidisciplinary discussion and should be adapted to the oncologic situation, given the risk of developing potentially life-threatening forms. The use of monoclonal antibodies targeting IL4 or CD20/IgE receptors may offer a therapeutic alternative (dupilumab, omalizumab, and rituximab; Barrios et al., 2021).
Hair and nail immune-related adverse events
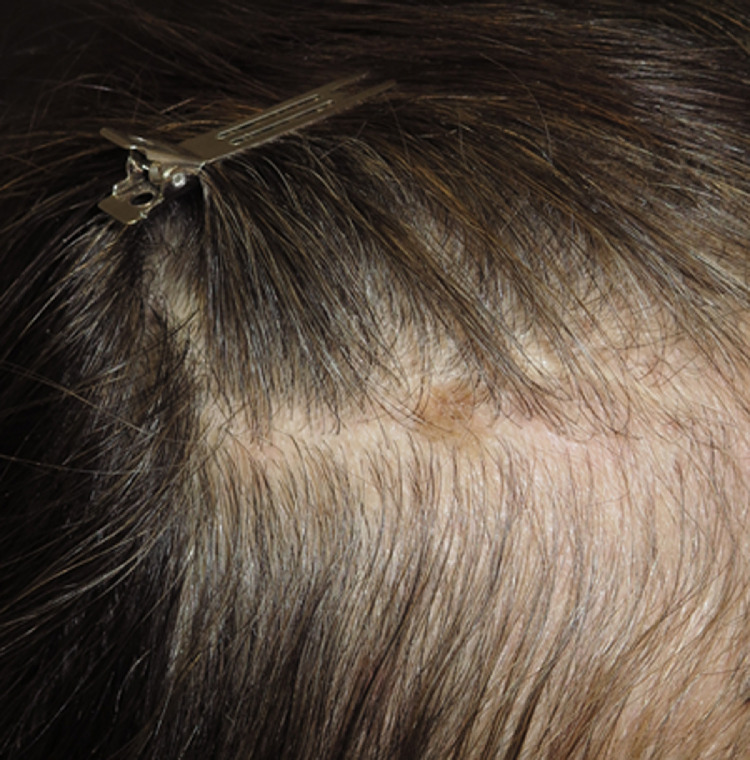
Although ICI-induced alopecia is rare (1.03% for PD-1/PD-L1 and ∼5.1% for anti-CTLA-4; Li et al., 2020), it can significantly impact patients’ QoL (Freitez-Martinez et al., 2019). The most common ICI-triggered hair disorders are alopecia areata (Fig. 7; Antoury et al., 2020), changes in hair color (e.g., hair depigmentation or poliosis; Sibaud, 2018), and much more rarely hair repigmentation (Rivera et al., 2017), as well as persistent alterations in the texture of the hair shaft (Dasanu et al., 2017). The diagnosis is established on clinical grounds with the aid of trichoscopy. Biopsy is reserved for ambiguous cases that are difficult to diagnose.
Fig. 7.
Anti-PD-1–induced alopecia areata.
Management of ICI-induced alopecia areata follows the same recommendations as for the idiopathic form, including intralesional and topical high-potency steroids, as well as systemic immunomodulators (Strazzulla et al., 2018). However, since immunomodulating drugs may lessen the efficacy of ICI, the overall cost–benefit ratio for the patient must be assessed. Crayons, colored powders, and hair dyes can be suggested as camouflage to limit the impact on patients’ QoL (Freites-Martinez et al., 2019a).
Nail irAEs usually occur as late as several months after ICI initiation, with possible persistence after ICI cessation (Di Altobrando et al., 2020). The nail changes observed with ICI have been postulated to be primarily of lichenoid or psoriatic origin (Figs 3 and 4; Sibaud et al., 2018; van Damme et al., 2021). Although rarely seen, the most common ICI-related nail changes include thinning of the nail plate with associated fragility, onycholysis, lunular erythema, longitudinal fissures, onychorrhexis, splitting in layers, and onychomadesis. The nail changes may involve few to several fingernails or toenails. In terms of management, topical hygiene measures and emollients are usually beneficial. In the event of diffuse or recalcitrant/painful lesions, systemic therapy should be considered. Oral retinoids, such as acitretin or alitretinoin, should be offered as a first-line option (van Damme et al., 2021).
Oral mucosal immune-related adverse events
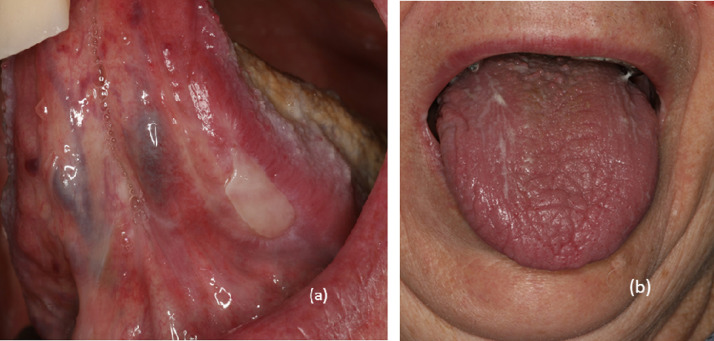
Oral irAEs occur in almost 7% of patients treated with ICI (Vigarios et al., 2017; Xu et al., 2021), usually within the first year of treatment (Shah et al., 2020; Xu et al., 2021. Oral lichenoid reactions and sicca syndrome with xerostomia (Fig. 8) are the most frequent oral toxicities and have been reported in association with both anti-PD-1 and PD-L1 regimens (Ortiz Brugués et al., 2020; Schaberg et al., 2016; Shi et al., 2016; Sibaud et al., 2017; Vigarios et al., 2017; Warner et al., 2019; Xu et al., 2021). Oral lichenoid reactions often co-occur with skin, genital, and/or nail lichenoid reactions (Sibaud, 2018; Sibaud et al., 2017; Vigarios et al., 2017). Classic reticulated white streaks together with papular, plaque, erosive, or erythematous lesions can be seen (Sibaud et al., 2017; Vigarios et al., 2017). Good oral hygiene and topical steroids (e.g., dexamethasone 0.1 mg/ml solution or fluocinonide 0.05% gel) are usually effective (Nikolaou et al., 2021; Shah et al., 2020; Shazib et al., 2020). Systemic steroids (0.5–1 mg/kg/day) should be reserved only for severe cases (Ortiz Brugués et al., 2020). Sicca syndrome with xerostomia is very common but usually remains of mild intensity (Ortiz Brugués et al., 2020; Warner et al., 2019). Basic oral hygiene and supportive measures, including hydration and lubrication with artificial saliva, are useful (Rapoport et al., 2017; Shah et al., 2020). Oral steroids (0.5 mg/kg/day) are recommended only in recalcitrant or severe cases.
Fig. 8.
(A) Lichenoid reaction with ulceration and reticular streaks and (B) grade 2 xerostomia.
Rare dermatologic immune-related adverse events
This group includes various dermatologic manifestations that can be roughly grouped as follows:
-
1)
Cutaneous granulomatous/sarcoid-like eruptions (Apalla et al., 2021a; Mobini et al., 2019). A recent literature review reported on 80 cases of ICI-induced granulomatous reactions with both anti-CTLA-4, and anti-PD-1 used alone or in combination. Exclusive cutaneous/subcutaneous involvement is highly uncommon (8 of 80 patients; Apalla et al., 2021a; Mobini et al., 2019). The disease usually manifests after approximately 6 months of treatment (Mobinie et al., 2019), and the sites of predilection are the upper and lower extremities. Histologic examination is mandatory for correct diagnosis and typically shows noncaseating epithelioid granulomas (Ellis et al., 2020).
-
2)
Suprabasal acantholytic dermatoses are rarely reported in the context of ICI treatment (Chen et al., 2018) and are clinically characterized by the presence of intensively pruritic, erythematous papules or papulovesicular lesions involving the trunk. The lesions are reminiscent of Grover's disease, both clinically and histologically (i.e., suprabasal clefts with acantholysis and dyskeratosis). ICI can be maintained, and topical steroids should be prescribed as first-line therapy (Chen et al., 2018).
-
3)
There are a few reports of scleroderma and scleroderma-like rashes, mostly occurring with anti-PD-1 (De Simone et al., 2021; Herrscher et al., 2019). Although they are mainly of the Morphea type, either limited or diffuse cutaneous systemic sclerosis has also been reported (Herrscher et al., 2019). Topical and systemic steroids are the mainstay of treatment and should be prescribed depending on disease severity and rate of progress.
-
4)
Eosinophilic fasciitis is extremely rare, but all patients had to discontinue ICI treatment.
-
5)
ICI-related cutaneous vasculitis with/or without systemic involvement has been rarely reported in the literature and mostly in the context of anti-PD-1 treatment (Tomelleri et al., 2018). Oral steroids should be considered depending on the severity (Tomelleri et al., 2018).
-
6)
Potentially life-threatening dirAEs, including Stevens–Johnson syndrome/toxic epidermal necrolysis, drug reactions with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome, as well as acute generalized exanthematous pustulosis, are rarely seen (Choi et al., 2020; Di Palma-Grisi et al., 2019; Maloney et al., 2020; Raschi et al., 2019). They are characterized by a delayed onset and may initially appear as a nonspecific maculopapular or urticarial rash. Early diagnosis, hospitalization for close monitoring, and cessation of the responsible drug are the mainstay of treatment. ICI should be discontinued, and systemic steroids should be considered, depending on the severity and disease progress.
ICI reinitiation after the occurrence of a severe dirAE is a very challenging decision and should be made on a case-by-case basis, depending on the type and severity of toxicity, as well as considering other therapeutic options for the patient.
Immune-related adverse events
Gastrointestinal and hepatic immune-related adverse events
irAEs affecting the gastrointestinal (GI) tract are among the most severe inflammatory toxicities due to ICIs. GI irAEs include mucositis, ulcers, gastritis, abdominal pain, and colitis. With or without blood or mucus stool, the presence of diarrhea is the most frequent GI toxicity and is usually associated with enterocolitis. Inflammation of the colon and small intestines is the single most common GI irAE and is the main reason for stopping treatment with ICIs (Dougan et al., 2020).
Hepatotoxicity is reported to occur in 2% to 10% with monotherapy. Onset is within the first 6 to 12 weeks after treatment initiation. The severity of these irAEs can vary from symptomatic treatment to life-threatening complications, such as perforation, megacolon, or liver failure. The frequency and severity of hepatic and GI irAEs depend on the specific immunotherapy administered. For instance, colitis is more frequently seen in patients receiving treatment with CTLA-4 blockade compared with blockade of either PD-1 or PD-L1 (Dougan et al., 2020). Additionally, the combination of anti-CTLA-4 with anti-PD-1/PDL-1 is associated with the highest frequency of hepatitis and colitis. Clinical diagnoses of GI irAEs are frequent, although biopsy remains the standard of care for the diagnosis of severe immune-related enterocolitis and hepatitis. Systemic corticosteroids are the mainstay of treatment, and GI/hepatic irAEs typically respond to high-dose corticosteroids. However, a substantial number of patients require secondary immune suppression. There is limited prospective data regarding the appropriate diagnosis and management of GI irAEs. Most recommendations are based mainly on retrospective data and expert opinion. For colitis, both TNF-alpha blockade with infliximab and integrin inhibition with vedolizumab have proven highly effective in corticosteroid-refractory cases (Dougan et al., 2020).
Pneumonitis
Although uncommon, pulmonary toxicity from ICIs (most significantly immune-related pneumonitis) is one of the most severe adverse events associated with ICIs. The median time to pneumonitis is generally 3 months (Eigentler et al., 2016; Shannon et al., 2020). Pneumonitis is more commonly reported with PD-1 or PD-L1 monoclonal antibodies compared with CTLA-4 or a combination of anti-CTLA-4/anti-PD-1 regimens (Eigentler et al., 2016; Shannon et al., 2020). Grade 3 and 4 events have been reported in <5% of patients treated with anti-PD-1/PD-L1 monotherapy and <2% of those receiving combination ICI therapy (Eigentler et al., 2016; Shannon et al., 2020). Diagnostic evaluation of immune-related pneumonitis includes clinical assessment and radiologic investigation. However, in some patients, radiologic evidence may be present before clinical signs occur while others remain asymptomatic despite pneumonitis evidence on computed tomography scan (Eigentler et al., 2016; Shannon et al., 2020).
Common pneumonitis symptoms include dyspnea, decreased effort tolerance, a dry cough with or without wheeze, and less commonly fever and chest pain. In some patients, hypoxia can occur rapidly, leading to respiratory failure (Eigentler et al., 2016; Shannon et al., 2020). Diagnostic tests include lung function test and pulse oximetry performed during rest and exertion, as well as high-resolution computed tomography scan that should be urgently performed because chest x-ray is not sufficiently informative. The radiologic appearance of pneumonitis varies from patient to patient, and includes ground-glass opacities, patchy nodular infiltrates, or an aspect similar to cryptogenic organizing pneumonia. Additionally, pneumonitis can also resemble radiologic patterns of nonspecific interstitial pneumonitis, hypersensitivity, acute interstitial pneumonia, and acute respiratory distress syndrome (Eigentler et al., 2016; Shannon et al., 2020).
Lung biopsy is not usually necessary and not routinely recommended to confirm diagnosis. However, bronchoscopy should be considered to exclude differentials, such as infection in patients with grade ≥2 pneumonitis (Eigentler et al., 2016; Shannon et al., 2020). The differential diagnosis includes infective causes, such as bacterial pneumonia, tuberculosis, fungal infection, pneumocystis jirovecii, cytomegalovirus, COVID-19 infection, and atypical infections, but also lymphangitis and disease progression.
For all grades of pneumonitis, it is essential to discontinue treatment with ICIs. In most grade 2 cases and all grade 3 and 4, toxicity, hospitalization, and close observation are recommended. Severe cases may require treatment in intensive care. Treatment of pneumonitis includes immunosuppressive agents, such as corticosteroids and infliximab (Eigentler et al., 2016; Naidoo et al., 2017; Shannon et al., 2020).
Endocrine-related adverse events
Endocrine function irAEs are frequently reported, mainly affecting the thyroid, pituitary, and adrenal glands. Endocrine irAEs typically occur within the first 12 weeks of starting treatment with an ICI. Endocrine irAE symptoms are nonspecific and include fatigue, mental status changes, headache, and dizziness (Cooksley et al., 2020). Hypothyroidism has emerged as the most common endocrinologic adverse event. Hypophysitis or adrenal insufficiency is more often observed with anti-CTLA-4 (Cooksley et al., 2020). Patients with type 1 diabetes may manifest with severe acute symptoms (Cooksley et al., 2020). Clinicians should screen for baseline thyroid function and monitor patients for abnormal thyroid functions regularly. Importantly, endocrine-associated irAEs persist despite treatment completion or discontinuation. These patients require long-term treatment and management with hormone replacement therapy (Cooksley et al., 2020).
Less frequent immune-related adverse events
Renal toxicity
Interstitial nephritis is the most common cause of acute kidney injury in patients who have received ICIs. Lupus-like nephritis and granulomatous nephritis have also been documented (Rapoport et al., 2017).
Musculoskeletal toxicities
Symptoms such as arthralgia and myalgia have been reported in 2% to 12% of patients receiving ICI therapy. The most frequently reported rheumatic irAEs are inflammatory arthritis, polymyalgia-like syndromes, and myositis. Patients may also present with oligoarthritis or polyarthritis that may affect both the large and small joints (Rapoport et al., 2021; Suarez-Almazor et al., 2020).
Neurologic toxicities
Neurologic irAEs are rare but potentially severe. They include myasthenia gravis, aseptic meningitis, encephalitis, motor and sensory neuropathies (including Guillain–Barre syndrome), and other rare events (e.g., enteric or autonomic neuropathies and transverse myelitis). The onset of neurologic toxicity varies from 6 to 12 weeks after treatment initiation (Cuzzubbo et al., 2017; Rapoport et al., 2021). Immunotherapy should be stopped definitively.
Cardiovascular toxicity
Although cardiovascular toxicity occurs in <0.1% of patients receiving ICI therapy, it is one of the most severe irAEs and is often associated with death. Cardiovascular toxicity in the form of myocarditis, myocardial fibrosis, cardiomyopathy, or conduction abnormalities has been reported with all ICI antibodies and is more frequent in patients receiving treatment with combination therapy (Suarez-Almazor et al., 2020). Cardiovascular toxicity can start as early as 2 weeks after treatment initiation, but may be delayed up to 32 weeks after initiation (Suarez-Almazor et al., 2020).
Hematologic toxicity
Hematologic irAEs from ICI treatment are uncommon, but reported cases include anemia, red cell aplasia, thrombocytopenia, neutropenia, myelodysplasia, and acquired hemophilia A (Delanoy et al., 2019; Rapoport et al., 2021).
Impact of sex on tolerability of immunotherapy
Several studies have recently highlighted sex-based differences in both the tumor microenvironment and the antitumor response associated with ICIs (Conforti et al., 2021a; 2021b). For example, tumor microenvironment has been individualized to be significantly enriched for specific T-cell subpopulations in women, suggesting a sex-based dimorphism of immune response (Conforti et al., 2021b).
Conversely, the putative impact of sex on tolerance to ICIs has received little attention to date (Klein and Morgan, 2020; Özdemir et al., 2018) and with conflicting data (Duma et al., 2019). Analytically, recent meta-analyses have failed to demonstrate any statistically significant sex-related differences in the development of irAEs (Jing et al., 2021), although recent comparative studies have suggested that there may still be a distinct sex-related safety profile. For example, in their multivariate analysis, Duma et al. (2019) found a higher incidence of irAEs in the female versus male population treated for non-small cell lung cancer and melanoma, especially regarding endocrinopathies and pneumonitis. Premenopausal women were particularly affected by the occurrence of the aforementioned toxicities, as well as arthralgias (Duma et al., 2019). These observations suggest that the occurrence of irAEs could be partly influenced by sex hormones (Duma et al., 2019). The role of estradiol has been mentioned in this context, notably by upregulation of CD4+ T cells and decreased TNF production. Moreover, PD-1 expression appears to be sex-dependent and regulated by estrogens (Özdemir et al., 2018). Furthermore, dermatologic toxicity is potentially more frequent in men (Duma et al., 2019). However, these data need to be confirmed prospectively and with a larger sample size.
Conclusion
Despite the undoubted benefit offered to oncologic patients by the introduction of ICIs in the therapeutic armamentarium of oncologists, irAEs still represent the major obstacle in their use. Awareness of the clinical spectrum of irAEs is extremely important, since it allows early recognition and adequate management of irAEs, facilitating unimpaired oncologic treatment for patients. More specifically, dermatologic toxicity does not usually prevent the continuation of anticancer treatment provided that early and appropriate supportive care is put in place to maintain the QoL of treated patients.
Conflicts of interest
Dr Bernardo Rapoport is supported by the Cancer Association of South Africa and the National Research Foundation of South Africa. Dr Vincent Sibaud reports a consulting or advisory role for Bristol Myers Squibb, Pierre Fabre, Novartis, Bayer, Amgen, and Incyte.
Funding
None.
Study approval
N/A
References
- Amatore F, Villani AP, Tauber M, Viguier M, Guillot B. French guidelines on the use of systemic treatments for moderate-to-severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33(3):464–483. doi: 10.1111/jdv.15340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoury L, Maloney NJ, Bach DQ, Goh C, Cheng K. Alopecia areata as an immune-related adverse event of immune checkpoint inhibitors: A review. Dermatol Ther. 2020;33(6):e14171. doi: 10.1111/dth.14171. [DOI] [PubMed] [Google Scholar]
- Apalla Z, Kemanetzi C, Papageorgiou C, Bobos M, Manoli M, Fotiadou C, et al. Challenges in sarcoidosis and sarcoid-like reactions associated to immune checkpoint inhibitors: A narrative review apropos of a case. Dermatol Ther. 2021;34(1):e14618. doi: 10.1111/dth.14618. [DOI] [PubMed] [Google Scholar]
- Apalla Z, Lallas A, Delli F, Lazaridou E, Papalampou S, Apostolidou S, et al. Management of immune checkpoint inhibitor-induced bullous pemphigoid. J Am Acad Dermatol. 2021;84(2):540–543. doi: 10.1016/j.jaad.2020.05.045. [DOI] [PubMed] [Google Scholar]
- Apalla Z, Psarakis E, Lallas A, Koukouthaki A, Fassas A, Smaragdi M. Psoriasis in patients with active lung cancer: Is apremilast a safe option? Dermatol Pract Concept. 2019;9(4):300–301. doi: 10.5826/dpc.0904a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apalla Z, Sibaud V. Immunotherapy-mediated dermatological adverse events: The urgent need for a common, clinically meaningful, management strategy. Support Care Cancer. 2020;28:5597–5599. doi: 10.1007/s00520-020-05701-9. [DOI] [PubMed] [Google Scholar]
- Barrios DM, Phillips GS, Geisler AN, Trelles SR, Markova A, Noor SJ, et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann Oncol. 2021;32(6):736–745. doi: 10.1016/j.annonc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, et al. Anti-PD1-induced psoriasis: A study of 21 patients. J Eur Acad Dermatol Venereol. 2017;31:e254–e257. doi: 10.1111/jdv.14011. [DOI] [PubMed] [Google Scholar]
- Chan L, Hwang SJE, Byth K, Kyaw M, Carlino MS, Chou S, et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82(2):311–316. doi: 10.1016/j.jaad.2019.06.035. [DOI] [PubMed] [Google Scholar]
- Chen ST, Molina GE, Lo JA, Durbin S, Cohen JV, Reynolds KL, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: A retrospective cohort study. J Am Acad Dermatol. 2020;82(4):994–996. doi: 10.1016/j.jaad.2019.09.026. [DOI] [PubMed] [Google Scholar]
- Chen WS, Tetzlaff MT, Diwan H, Jahan-Tigh R, Diab A, Nelson K, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: A spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45(10):764–773. doi: 10.1111/cup.13312. [DOI] [PubMed] [Google Scholar]
- Choi J, Anderson R, Blidner A, Cooksley T, Dougan M, Glezerman I, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors. Support Care Cancer. 2020;28(12):6119–6128. doi: 10.1007/s00520-020-05706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. 2019;80(4):990–997. doi: 10.1016/j.jaad.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Pala L, Pagan E, Corti C, Bagnardi V, Queirolo P, et al. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Pala L, Pagan E, Bagnardi V, De Pas T, Queirolo P, et al. Sex-based dimorphism of anticancer immune response and molecular mechanisms of immune evasion. Clin Cancer Res. 2021;27(15):4311–4324. doi: 10.1158/1078-0432.CCR-21-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksley T, Girotra M, Ginex P, Gordon RA, Anderson R, Blidner A, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune checkpoint inhibitor endocrinopathies and the role of advanced practice providers in the management of immune-mediated toxicities. Support Care Cancer. 2020;28(12):6175–6181. doi: 10.1007/s00520-020-05709-1. [DOI] [PubMed] [Google Scholar]
- Cuzzubbo S, Javeri F, Tissier M, Roumi A, Barlog C, Doridam J, et al. Neurological adverse events associated with immune-checkpoint inhibitors: Review of the literature. Eur J Cancer. 2017;73:1–8. doi: 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Dasanu CA, Lippman SM, Plaxe SC. Persistently curly hair phenotype with the use of nivolumab for squamous cell lung cancer. J Oncol Pharm Pract. 2017;23(8):638–640. doi: 10.1177/1078155216674355. [DOI] [PubMed] [Google Scholar]
- De Simone C, Mannino M, Sollena P, Deilhes F, Sibaud V, Peris K. Morphea-like changes in the setting of cancer immunotherapy. J Eur Acad Dermatol Venereol. 2021;35(10):e684–e685. doi: 10.1111/jdv.17388. [DOI] [PubMed] [Google Scholar]
- Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: A descriptive observational study. Lancet Haematol. 2019;6(1):e48–e57. doi: 10.1016/S2352-3026(18)30175-3. [DOI] [PubMed] [Google Scholar]
- Di Altobrando A, Bruni F, Alessandrini A, Starace M, Misciali C, Piraccini BM. Severe de-novo palmoplantar and nail psoriasis complicating Nivolumab treatment for metastatic melanoma. Dermatol Ther. 2020;33(3):e13363. doi: 10.1111/dth.13363. [DOI] [PubMed] [Google Scholar]
- Di Palma-Grisi JC, Vijayagopal K, Muslimani MA. Case reports of DRESS syndrome and symptoms consistent with DRESS syndrome following treatment with recently marketed monoclonal antibodies. Autoimmune Dis. 2019;2019 doi: 10.1155/2019/7595706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Blidner AG, Choi J, Cooksley T, Glezerman I, Ginex P, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe gastrointestinal and hepatic toxicities from checkpoint inhibitors. Support Care Cancer. 2020;28(12):6129–6143. doi: 10.1007/s00520-020-05707-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulos J, Carven GJ, Van Boxtel SJ, Evers S, Driessen-Engels LJA, Hobo W, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- Duma N, Abdel-Ghani A, Yadav S, Hoversten KP, Reed CT, Sitek AN, et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: Are we all equal? Oncologist. 2019;24(11):e1148–e1155. doi: 10.1634/theoncologist.2019-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features. J Am Acad Dermatol. 2020;83(4):1130–1143. doi: 10.1016/j.jaad.2020.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17:504–515. doi: 10.1038/s41571-020-0352-8. [DOI] [PubMed] [Google Scholar]
- Flatz L, Berner F, Bomze D, Diem S, Ali OH, Fässler M, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 2019;5(7):1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites-Martinez A, Chan D, Sibaud V, Shapiro J, Fabbrocini G, Tosti A, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155(6):724–728. doi: 10.1001/jamadermatol.2018.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites-Martinez A, Kwong BY, Rieger KE, Coit DG, Colevas AD, Lacouture ME. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153(7):694–697. doi: 10.1001/jamadermatol.2017.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255–1268. doi: 10.1016/j.jaad.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan Ali O, Bomze D, Ring SS, Berner F, Fässler M, Diem S, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol. 2020;82(4):854–861. doi: 10.1016/j.jaad.2019.08.045. [DOI] [PubMed] [Google Scholar]
- Herrscher H, Tomasic G, Castro Gordon A. Generalised morphea induced by pembrolizumab. Eur J Cancer. 2019;116:178–181. doi: 10.1016/j.ejca.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- Hwang SJE, Park JJW, Wakade D, Chou S, Byth K, Fernandez-Penas P. Cutaneous adverse events of anti-programmed death 1 antibodies combined with anti-cytotoxic T-lymphocyte-associated protein 4 therapy use in patients with metastatic melanoma. Melanoma Res. 2019;29(2):172–177. doi: 10.1097/CMR.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Ito J, Fujimoto D, Nakamura A, Nagano T, Uehara K, Imai Y, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer. 2017;109:58–61. doi: 10.1016/j.lungcan.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Jing Y, Zhang Y, Wang J, Li K, Chen X, Heng J, et al. Association between sex and immune-related adverse events during immune checkpoint inhibitor therapy. J Natl Cancer Inst. 2021;113(10):1396–1404. doi: 10.1093/jnci/djab035. [DOI] [PubMed] [Google Scholar]
- Juzot C, Sibaud V, Amatore F, Mansard S, Seta V, Jeudy G, et al. Clinical, biological and histological characteristics of bullous pemphigoid associated with anti-PD-1/PD-L1 therapy: A national retrospective study. J Eur Acad Dermatol Venereol. 2021;35(8):e511–e514. doi: 10.1111/jdv.17253. [DOI] [PubMed] [Google Scholar]
- Klein SL, Morgan R. The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ. 2020;11(1):24. doi: 10.1186/s13293-020-00301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture ME, Wolchok JD, Yosipovitch G, Kähler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161–169. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76(5):863–870. doi: 10.1016/j.jaad.2016.10.044. [DOI] [PubMed] [Google Scholar]
- Li M, Huang L, Ren X, Liu L, Shi Q, Liu L, et al. The incidence risk of programmed cell death-1/programmed cell death ligand 1 inhibitor-related alopecia for cancer patients: A systematic review and meta-analysis. Medicine (Baltimore) 2020;99(42):e22555. doi: 10.1097/MD.0000000000022555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57(6):664–669. doi: 10.1111/ijd.13984. [DOI] [PubMed] [Google Scholar]
- Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens–Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int J Dermatol. 2020;59:e183–e188. doi: 10.1111/ijd.14811. [DOI] [PubMed] [Google Scholar]
- Matsuya T, Nakamura Y, Matsushita S, Tanaka R, Teramoto Y, Asami Y, et al. Vitiligo expansion and extent correlate with durable response in anti-programmed death 1 antibody treatment for advanced melanoma: A multi-institutional retrospective study. J Dermatol. 2020;47(6):629–635. doi: 10.1111/1346-8138.15345. [DOI] [PubMed] [Google Scholar]
- Mobini N, Dhillon R, Dickey J, Spoon J, Sadrolashrafi K. Exclusive cutaneous and subcutaneous sarcoidal granulomatous inflammation due to immune checkpoint inhibitors: Report of two cases with unusual manifestations and review of the literature. Case Rep Dermatol Med. 2019;2019:1–7. doi: 10.1155/2019/6702870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina GE, Reynolds KL, ST Chen. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: A cross-sectional study. Br J Dermatol. 2020;183(6):1126–1128. doi: 10.1111/bjd.19313. [DOI] [PubMed] [Google Scholar]
- Naidoo J, Wang X, Woo K, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Singer S, Chen T, Puleo AE, Lian CG, Wei EX, et al. Reply to: “Comment on Bullous pemphigoid after anti-PD-1 therapy: a retrospective case-control study evaluating impact on tumor response and survival outcomes. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.023. S0190–9622(20)30854–9. [DOI] [PubMed] [Google Scholar]
- Nikolaou V, Sibaud V, Fattore D, Sollena P, Ortiz-Brugués A, Giacchero D, et al. Immune checkpoint-mediated psoriasis: A multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J Am Acad Dermatol. 2021;84(5):1310–1320. doi: 10.1016/j.jaad.2020.08.137. [DOI] [PubMed] [Google Scholar]
- Ortiz Brugués A, Sibaud V, Herbault-Barrés B, Betrian S, Korakis I, De Bataille C, et al. Sicca syndrome induced by immune checkpoint inhibitor therapy: Optimal management still oending. Oncologist. 2020;25(2):e391. doi: 10.1634/theoncologist.2019-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir BC, Coukos G, Wagner AD. Immune-related adverse events of immune checkpoint inhibitors and the impact of sex-what we know and what we need to learn. Ann Oncol. 2018;29(4):1067. doi: 10.1093/annonc/mdx818. [DOI] [PubMed] [Google Scholar]
- Phillips GS, Freites-Martinez A, Wu J, Chan D, Fabbrocini G, Hellmann MD, et al. Clinical characterization of immunotherapy-related pruritus among patients seen in 2 oncodermatology clinics. JAMA Dermatol. 2019;155:249–251. doi: 10.1001/jamadermatol.2018.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. 2019;37(30):2746–2758. doi: 10.1200/JCO.18.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ B. 2015;(35):76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- Quach HT, Dewan AK, Davis EJ, Ancell KK, Fan R, Ye F, et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 2019;5:906–908. doi: 10.1001/jamaoncol.2019.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport BL, Anderson R, Cooksley T, DB Johnson. MASCC 2020 recommendations for the management of immune-related adverse events of patients undergoing treatment with immune checkpoint inhibitors. Support Care Cancer. 2020;28(12):6107–6110. doi: 10.1007/s00520-020-05727-z. [DOI] [PubMed] [Google Scholar]
- Rapoport BL, Cooksley T, Johnson DB, Anderson R. Supportive care for new cancer therapies. Curr Opin Oncol. 2021;33(4):287–294. doi: 10.1097/CCO.0000000000000736. [DOI] [PubMed] [Google Scholar]
- Rapoport BL, van Eeden R, Sibaud V, Epstein JB, Klastersky J, Aapro M, et al. Supportive care for patients undergoing immunotherapy. Support Care Cancer. 2017;25(10):3017–3030. doi: 10.1007/s00520-017-3802-9. [DOI] [PubMed] [Google Scholar]
- Raschi E, Antonazzo IC, La Placa M, Ardizzoni A, Poluzzi E, De Ponti F. Serious cutaneous toxicities with immune checkpoint inhibitors in the U.S. Food and Drug Administration Adverse Event Reporting System. Oncologist. 2019;24(11):e1228. doi: 10.1634/theoncologist.2019-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera N, Boada A, Bielsa MI, Fernández-Figueras MT, Carcereny E, Moran MT, et al. Hair repigmentation during immunotherapy treatment with an anti–programmed cell death 1 and anti–programmed cell death ligand 1 agent for lung cancer. JAMA Dermatol. 2017;153(11):1162–1165. doi: 10.1001/jamadermatol.2017.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovers JFJ, Bovenschen HJ. Dermatological side effects rarely interfere with the continuation of checkpoint inhibitor immunotherapy for cancer. Int J Dermatol. 2020;59(12):1485–1490. doi: 10.1111/ijd.15163. [DOI] [PubMed] [Google Scholar]
- Salopek TG. Recurrence of melanoma after starting apremilast for psoriasis. Case Rep Dermatol. 2017;9(2):108–111. doi: 10.1159/000478898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg KB, Novoa RA, Wakelee HA, Kim J, Cheung C, Srinivas S, et al. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J Cutan Pathol. 2016;43(4):339–346. doi: 10.1111/cup.12666. [DOI] [PubMed] [Google Scholar]
- Shah N, Cohen L, Seminario-Vidal L. Management of oral reactions from immune checkpoint inhibitor therapy: A systematic review. J Am Acad Dermatol. 2020;83(5):1493–1498. doi: 10.1016/j.jaad.2020.05.133. [DOI] [PubMed] [Google Scholar]
- Shannon VR, Anderson R, Blidner A, Choi J, Cooksley T, Dougan M, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune-related adverse events: Pulmonary toxicity. Support Care Cancer. 2020;28(12):6145–6157. doi: 10.1007/s00520-020-05708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shazib MA, Bin Woo S, Sroussi H, Carvo I, Treister N, Farag A, et al. Oral immune-related adverse events associated with PD-1 inhibitor therapy: A case series. Oral Dis. 2020;26(2):325–333. doi: 10.1111/odi.13218. [DOI] [PubMed] [Google Scholar]
- Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152(10):1128–1136. doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- Sibaud V, Eid C, Belum VR, Combemale P, Barres B, Lamant L, et al. Oral lichenoid reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J Eur Acad Dermatol Venereol. 2017;31:e464–e469. doi: 10.1111/jdv.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaud V, Vigarios E, Siegfried A, Bost C, Meyer N. Pages-Laurent C. Nivolumab-related mucous membrane pemphigoid. Eur J Cancer. 2019;121:172–176. doi: 10.1016/j.ejca.2019.08.030. [DOI] [PubMed] [Google Scholar]
- Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F, Sznol M, et al. Bullous disorders associated with anti–PD-1 and anti–PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79(6):1081–1088. doi: 10.1016/j.jaad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: An appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15–24. doi: 10.1016/j.jaad.2017.04.1142. [DOI] [PubMed] [Google Scholar]
- Suarez-Almazor ME, Pundole X, Abdel-Wahab N, Johnson DB, Gupta D, Glezerman I, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune-mediated cardiovascular, rheumatic, and renal toxicities from checkpoint inhibitors. Support Care Cancer. 2020;28(12):6159–6173. doi: 10.1007/s00520-020-05710-8. [DOI] [PubMed] [Google Scholar]
- Thompson LL, Li EB, Krasnow NA, Chang MS, Said JT, Molina GE, et al. Effect of dermatological consultation on survival in patients with checkpoint inhibitor-associated cutaneous toxicity. Br J Dermatol. 2021;185(3):627–635. doi: 10.1111/bjd.20074. [DOI] [PubMed] [Google Scholar]
- Tomelleri A, Campochiaro C, De Luca G, Cavalli G, Dagna L. Anti-PD1 therapy-associated cutaneous leucocytoclastic vasculitis: A case series. Eur J Int Med. 2018;57:e11–e12. doi: 10.1016/j.ejim.2018.07.023. [DOI] [PubMed] [Google Scholar]
- van Damme C, Sibaud V, André J, Richert B, Berlingin E. Anti-PD-1-induced lichenoid changes of the nail unit: Histopathologic description. JAAD Case Rep. 2021;10:110–112. doi: 10.1016/j.jdcr.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigarios E, Epstein JB, Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer. 2017;25:1713–1739. doi: 10.1007/s00520-017-3629-4. [DOI] [PubMed] [Google Scholar]
- Voudouri D, Nikolaou V, Laschos K, Charpidou A, Soupos N, Triantafyllopoulou I, et al. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer. 2017;41(6):407–412. doi: 10.1016/j.currproblcancer.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Wang LL, Patel G, Chiesa-Fuxench ZC, McGettigan S, Schuchter L, Mitchell TC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol. 2018;154(9):1057–1061. doi: 10.1001/jamadermatol.2018.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist. 2019;24(9):1259–1269. doi: 10.1634/theoncologist.2018-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wen N, Sonis ST, Villa A. Oral side effects of immune checkpoint inhibitor therapy (ICIT): An analysis of 4683 patients receiving ICIT for malignancies at Massachusetts General Hospital, Brigham & Women's Hospital, and the Dana-Farber Cancer Institute, 2011 to 2019. Cancer. 2021;127(11):1796–1804. doi: 10.1002/cncr.33436. [DOI] [PubMed] [Google Scholar]