Graphical abstract
Keywords: Trochlear dysplasia, Patellar instability, PI3K/AKT, Cartilage degeneration, Osteoarthritis
Highlights
-
•
Established a new experimental rat model of the developmental trochlear dysplasia;
-
•
Using the macroscopic morphological and micro-CT to assess trochlear dysplasia;
-
•
Using Histological staining to investigate the cartilage degradation of the model;
-
•
Investigated the relationship of the PI3K/AKT signaling pathway with trochlear dysplasia cartilage degeneration;
-
•
Using immunohistochemistry and qPCR to investigate the PI3K/AKT and the marker of the cartilage degeneration.
Abstract
Introduction
Trochlear dysplasia is a commonly encountered lower extremity deformity in humans. However, the molecular mechanism of cartilage degeneration in trochlear dysplasia is unclear thus far.
Objectives
The PI3K/AKT signaling pathway is known to be important for regulating the pathophysiology of cartilage degeneration. The aim of this study was to investigate the relationship of the PI3K/AKT signaling pathway with trochlear dysplasia cartilage degeneration.
Methods
In total, 120 female Sprague-Dawley rats (4 weeks of age) were randomly separated into control and experimental groups. Distal femurs were isolated from the experimental group at 4, 8, and 12 weeks after surgery; they were isolated from the control group at the same time points. Micro-computed tomography and histological examination were performed to investigate trochlear anatomy and changes in trochlear cartilage. Subsequently, expression patterns of PI3K/AKT, TGFβ1, and ADAMTS-4 in cartilage were investigated by immunohistochemistry and quantitative polymerase chain reaction.
Results
In the experimental group, the trochlear dysplasia model was successfully established at 8 weeks after surgery. Moreover, cartilage degeneration was observed beginning at 8 weeks after surgery, with higher protein and mRNA expression levels of PI3K/AKT, TGFβ1, and ADAMTS-4, relative to the control group.
Conclusion
Patellar instability might lead to trochlear dysplasia in growing rats. Moreover, trochlear dysplasia may cause patellofemoral osteoarthritis; cartilage degeneration in trochlear dysplasia might be associated with activation of the PI3K/AKT signaling pathway. These results provide insights regarding the high incidence of osteoarthritis in patients with trochlear dysplasia. However, more research is needed to clarify the underlying mechanisms.
Introduction
Trochlear dysplasia (TD) of the femur is a common deformity of the lower extremities; it is regarded as an abnormal anatomical morphology of the depth or angle of the medial or lateral groove facets [1]. In 1964, Brattström first proposed a relationship between TD and patellar instability [2]. Approximately 85–90% of patients with patellar instability reportedly exhibit TD [3], [4]. Consequently, many scholars thought that TD was a pathogenic factor of patellar instability [5], [6]. Recent studies have shown that the development of TD might be related to excessive femur anteversion, patella alta, and greater tibial tubercle to trochlear groove distance [7], [8], [9], [10], [11], [12]. In addition, TD can lead to patellar instability, patellar maltracking, and abnormal pressure load distribution; the most common long-term complication is osteoarthritis (OA) [13], [14]. Some scholars consider TD to be a pathogenic factor of early patellofemoral OA [15]. To the best of our knowledge, there have been few studies regarding TD-induced cartilage degeneration at the molecular level.
Articular cartilage has a very low matrix and cell turnover rate, because of its permanent nature [16], [17]. Because chondrocytes comprise the only cell type in articular cartilage, an imbalance between chondrocyte proliferation and apoptosis is an important factor associated with onset of OA [18]. Consequently, maintenance of the balance between chondrocyte proliferation and apoptosis can reduce cartilage degeneration. Many signaling pathways are known to be involved in cartilage degeneration; regulation of the PI3K/AKT signaling pathway is an important contributor to the pathogenesis of cartilage degeneration, because of its key roles in several characteristic changes in cartilage (e.g., expression of aggrecanases [ADAMTS-4 and ADAMTS-5] and matrix metalloproteinases) [19]. Furthermore, some studies have shown that activation of the PI3K/AKT signaling pathway can promote osteogenic differentiation of pre-osteoblasts and mesenchymal stem cells; targeted inhibition of the PI3K/AKT signaling pathway can induce bone loss and reduce bone formation [20], [21], [22]. Transforming growth factor β1 (TGFβ1) is known to be an important contributor to chondrocyte development; it can regulate extracellular matrix biosynthesis and has been extensively studied as a regulator of cartilage metabolic activity [23]. Previous studies have shown that TD can cause articular cartilage degeneration [24], [25]. However, the molecular mechanism of TD-induced cartilage degeneration has been uncertain thus far. Moreover, the effects of the PI3K/AKT signaling pathway in TD-induced cartilage degeneration have not been reported.
Here, to study the association of PI3K/AKT signaling with TD-induced cartilage degeneration, we investigated the expression pattern of the PI3K/AKT pathway in cartilage at different stages in a growing rat model of experimental TD by immunohistochemistry and quantitative real-time polymerase chain reaction (qPCR). We speculated that TD might lead to overexpression of PI3K/AKT, thus resulting in cartilage degeneration. To the best of our knowledge, this is the first study regarding the association between PI3K/AKT and cartilage degeneration in a growing rat model of experimental TD.
Materials and methods
Study design
Four-week-old female Sprague–Dawley rats were obtained from the Laboratory Animal Center of Hebei Medical University. Rats have been shown to require 12 weeks of bone development to reach a mature state [26]. In this study, 120 rats were randomly separated into control and experimental groups (n = 60 per group). In the control group, the rats’ left knees did not undergo any surgery. In the experimental group, the rats’ left knees underwent surgery to induce patellar instability. Rats were sacrificed by overdose anesthesia at 4, 8, and 12 weeks after surgery. Left distal femur tissues were collected after surgery (n = 20 knees/time point in each group).
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Third Hospital of Hebei Medical University. (Approval no. Z2019-005-1).
Surgical technique
In this study, pentobarbital sodium (30 mg/kg, intraperitoneal injection) was used to induce anesthesia in all rats; they were then shaved and disinfected. An incision was then made along the midline of the skin; the skin and subcutaneous tissue were separated, exposing the joint capsule through the medial approach to the knee. The procedure to induce patellar instability was performed as described in our previous studies [24], [25]. Specifically, a 0.5-cm longitudinal incision was made along the patella at the medial capsule and retinaculum. In this manner, patellar instability could be observed during surgery. All procedures were carefully performed to avoid cartilage damage. The incision was carefully flushed, then sutured. Finally, the wound was bandaged. To control postoperative pain, rats were administered acetaminophen (30 mg/kg, daily) for 5 days.
Macroscopic morphological and micro-computed tomography (CT) assessment
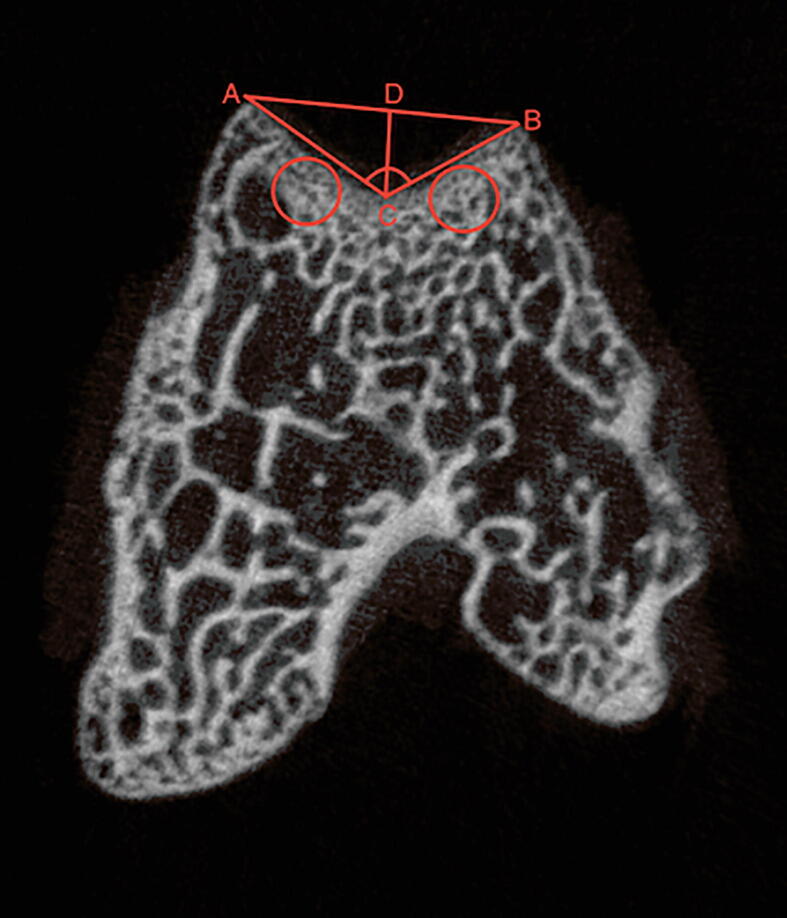
All distal femurs were carefully harvested and scanned via micro-CT (SkyScan model 1076, SkyScan, Kontich, Belgium; parameters: 10 μm voxel size at 50 kV and 800 μA) at 4, 8, 12 week after surgery, respectively. As described previously [12], [24], axial slices of the trochlea were identified; trochlear depth and sulcus angle were then calculated (Fig. 1). Using the diagnostic criteria defined by Dejour et al. [4], TD was diagnosed in micro-CT images.
Fig. 1.
Measurement diagram. DC, trochlear depth; ACB, sulcus angle.
For analysis of microstructural parameters, the region of interest was located transversely below the lateral and medial facets of the trochlea with two red cylinders of 3-mm diameter (Fig. 1); micro-CT scanning data were transformed into a three-dimensional model by using Mimics software, version 19.0 (Materialise, Leuven, Belgium). Microstructural parameters analyzed in this study included bone volume to total volume fraction (%), trabecular number (1/mm), trabecular thickness (mm), trabecular separation (mm), and bone mineral density (mg/cm3).
Histological staining
Samples were isolated at each time point, fixed with 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid until completely demineralized, and embedded in paraffin. Five-micrometer slices were cut along the femoral axis to obtain axial images of the trochlear groove. Glycosaminoglycans in cartilage were assessed using Safranin O; evaluation of cartilage degradation was performed by fast green counterstaining of protein. The modified Mankin scale was used to determine the grade of cartilage degeneration [27].
Immunohistochemistry
Slices were deparaffinized in xylene and rehydrated. At room temperature, slices were washed three times with phosphate-buffered saline (5 min per wash). Endogenous peroxidase activity was blocked by incubation of slices in 3% hydrogen peroxide for 10 min. Antigen retrieval was performed by microwave treatment of slices for 10 min in 10 mm sodium citrate (pH 6.0). Slices were incubated overnight at 4℃ with anti-PI3K (BoAoSen, Beijing, China), anti-AKT (Servicebio, Wuhan, China), anti-TGFβ1 (BoAoSen), or anti-ADAMTS-4 (BoAoSen) primary antibodies at a dilution of 1:50. For the negative control, the primary antibody step was omitted. Subsequently, the objective magnification was adjusted to 20 × 100, five regions were randomly selected for all slices in each group, and the entire area of each region was imaged. During microscopy, the tissue covered the entire field of view; the background light was maintained at a consistent level. Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) was used for analysis of all microscopy images. All images were analyzed to acquire data regarding cumulative optical density and tissue area. The areal density was defined as cumulative optical density divided by tissue area. The areal density value is positively correlated with positive protein expression.
qPCR
Samples were analyzed by qPCR to determine mRNA expression levels at 4, 8, and 12 weeks after surgery. Trizol reagent (Servicebio) was used to extract RNA from chondrocytes and cartilage. The RevertAid™ first-strand cDNA synthesis kit (Cat. No. K1622, Thermo Fisher Scientific, Waltham, MA, USA) was used for reverse transcription of mRNA into cDNA. Primers for PI3K, AKT, TGFβ1, and ADAMTS-4 were used, with a sequence detection system for gene analysis. mRNA expression of target genes was determined with reference to GAPDH and calculated using the formula 2-ΔΔCt (cycle threshold method). All primers used in this study are listed in Table 1. Each experiment was performed three times and mean values were used for further analyses.
Table 1.
Primers for amplification of target genes and GAPDH.
| Forward primer sequence | Reverse primer sequence | |
|---|---|---|
| PI3K | ACAGGCACAACGACAACATCAT | AGGTAAGCCCTAACGCAGACAT |
| AKT | TGAGACCGACACCAGGTATTTTG | GCTGAGTAGGAGAACTGGGGAAA |
| TGFβ1 | CTAATGGTGGACCGCAACAAC | GTAACGCCAGGAATTGTTGCTAT |
| ADAMTS-4 | GTACCTACCTGACTGGCACCATC | TGCTGCCATCTTGTCATCTGC |
| GAPDH | CTGGAGAAACCTGCCAAGTATG | GGTGGAAGAATGGGAGTTGCT |
Statistical analysis
Mean and standard deviation were used for descriptive statistical analysis. The Shapiro–Wilk test was used to determine normality for each variable, while Levene’s test was used to assess homogeneity of variance. Student’s t-test was used for comparisons between two groups; one-way analysis of variance was used for comparisons among ≥ 3 groups. SPSS Statistics, version 19.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. Differences with p < 0.05 were considered statistically significant. Preliminary analysis suggested that at least six rats were needed at each time point and in each group, to achieve 80% efficacy (1-β) and 90% confidence [28].
Results
Macroscopic morphology and micro-CT assessment of patellar instability model of TD
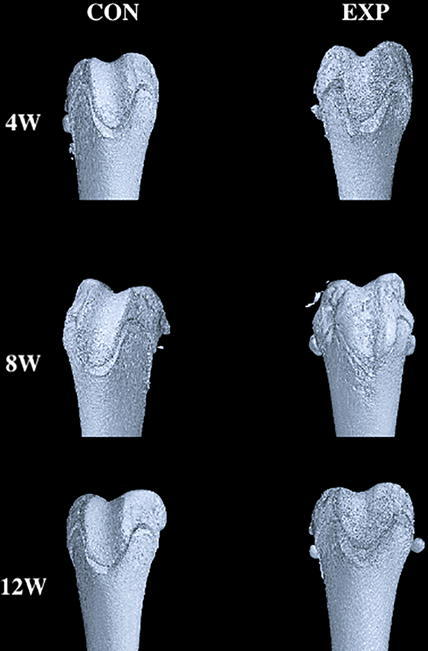
The TD model was successfully established, characterized by a larger groove angle and flatter trochlear groove (Table 2, Table 3). We regard the trochlear depth and sulcus angle of the control group at different time points as normal values. The trochlear depth and sulcus angle did not exhibit significant differences between the control and experimental groups (P > 0.05) at 4 weeks after surgery; however, they exhibited significant differences between the two groups (P < 0.05) at 8 and 12 weeks after surgery. Over time, the trochlear groove became significantly flatter and sulcus angle became significantly larger, compared with the control group (Fig. 2). In the experimental group, the trochlear depth was 0.6 ± 0.2 mm, which was lower than the trochlear depth of the control group (0.9 ± 0.2 mm, P = 0.039) at 8 weeks after surgery. Similarly, in the experimental group, the trochlear depth was 0.9 ± 0.2 mm, which was lower than the trochlear depth of the control group (1.5 ± 0.2 mm, P = 0.029) at 12 weeks after surgery. In the experimental group, the trochlear sulcus angle was 131.0° ± 2.9°, which was higher than the trochlear sulcus angle of the control group (126.0° ± 3.1°, P = 0.038) at 8 weeks after surgery. Similarly, in the experimental group, the trochlear sulcus angle was 152.0° ± 3.0°, which was higher than the trochlear sulcus angle of the control group (132.0° ± 3.4°, P = 0.026) at 12 weeks after surgery.
Table 2.
Sulcus angle measurements compared between the two groups.
| Control group | Experimental group | P value | |
|---|---|---|---|
| 4 weeks | 120.0° ± 3.0° | 118.0° ± 2.6° | 0.739 |
| 8 weeks | 126.0° ± 3.1° | 131.0° ± 2.9° | 0.038 |
| 12 weeks | 132.0° ± 3.4° | 152.0° ± 3.0° | 0.026 |
Mean ± standard deviation.
Table 3.
Trochlear depth measurements compared between the two groups.
| Control group | Experimental group | P value | |
|---|---|---|---|
| 4 weeks | 0.5 ± 0.1 mm | 0.4 ± 0.2 mm | 0.913 |
| 8 weeks | 0.9 ± 0.2 mm | 0.6 ± 0.2 mm | 0.039 |
| 12 weeks | 1.5 ± 0.2 mm | 0.9 ± 0.2 mm | 0.029 |
mean ± standard deviation.
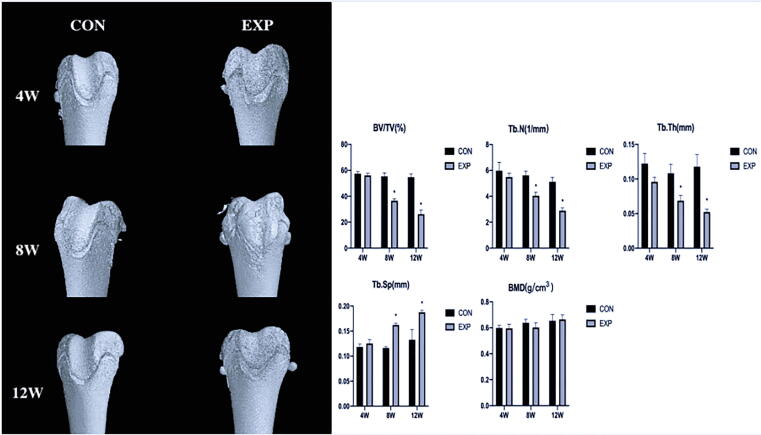
Fig. 2.
Three-dimensional reconstruction of distal femur and Histomorphometric comparison by micro-computed tomography scans at 4, 8, and 12 weeks between CON and EXP groups. We regard the trochlear depth and sulcus angle of the control group at different time points as normal values. Over time, the trochlear groove became significantly flatter and sulcus angle became significantly larger, compared with the control group at 8, 12 weeks after surgery. In the experimental group, micro-CT displayed obvious loss of subchondral bone at 8 weeks after surgery, and became more and more serious with time. *P < 0.05 (BV/TV: bone volume to total volume; TB.N: trabecular number; TB.Th: trabecular thickness; TB.Sp: trabecular separation; BMD: bone mineral density; CON, control; EXP, experimental).
Detection of subchondral bone loss by micro-CT
In the experimental group, micro-CT displayed obvious loss of subchondral bone at 8 weeks after surgery, and became more and more serious with time. Micro-CT scans of the two groups revealed that the bone volume to total volume fraction, trabecular number, and trabecular thickness decreased markedly at 8 and 12 weeks after surgery (P < 0.05); moreover, trabecular separation significantly increased at 8 and 12 weeks after surgery (P < 0.05). However, bone mineral density did not remarkably differ (P > 0.05) (Fig. 2).
Loss of cartilage proteoglycans and higher Mankin score in the TD model
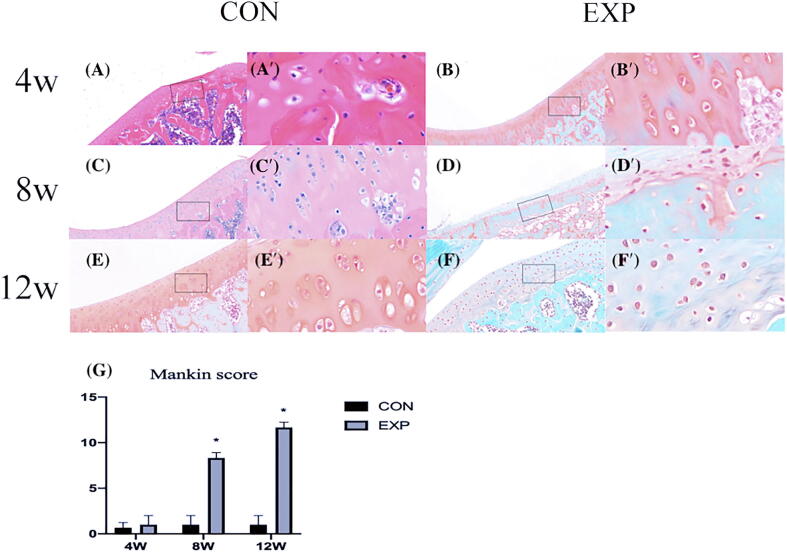
At 4 weeks after surgery, no significant differences were present in cartilage appearance between the two groups. However, the amount of matrix proteoglycans was significantly reduced, the cartilage surface was damaged (accompanied by rougher and thinner appearance), and chondrocytes were disordered in the experimental group at 8 and 12 weeks after surgery, and the number of chondrocytes was significantly reduced in the superficial and middle zone, which was consistent with OA (Fig. 3). Moreover, Mankin scores were higher in the TD model cartilage at different stages and worsened over time (Fig. 3) (P < 0.05).
Fig. 3.
Safranin O staining in rat cartilage. CON and EXP groups, respectively: (A, B) 4 weeks; (C, D) 8 weeks; (E, F) 12 weeks. A′–F′ show higher magnifications of boxed areas in A–F. The safranin O and fast green histochemical staining showed that the amount of matrix proteoglycans was significantly reduced, the cartilage surface was rougher and thinner, and the chondrocytes were disordered in the experimental group at 8 and 12 weeks after surgery. In D′, F′, we observed the number of chondrocytes in the superficial and middle zone was significantly reduced, which was consistent with OA. (G): Mankin score for trochlear cartilage: higher Mankin scores in the cartilage at different stages and worsened over time. CON, control; EXP, experimental. *P < 0.05.
Increased expression of PI3K/AKT, TGFβ1, and ADAMTS-4 in the TD model cartilage
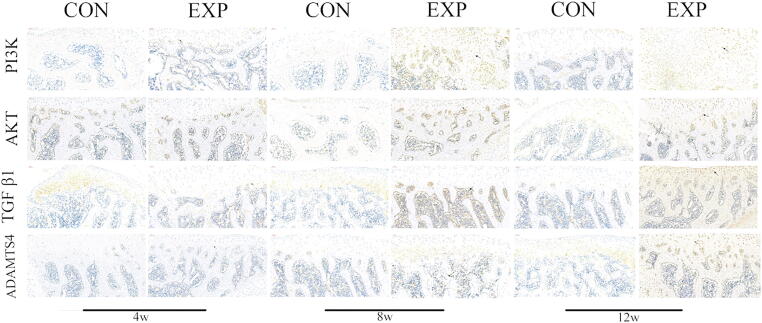
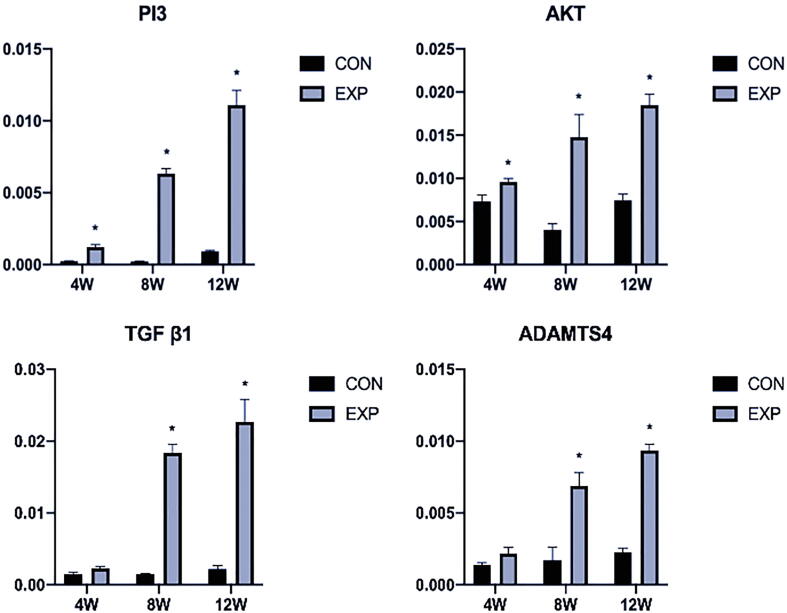
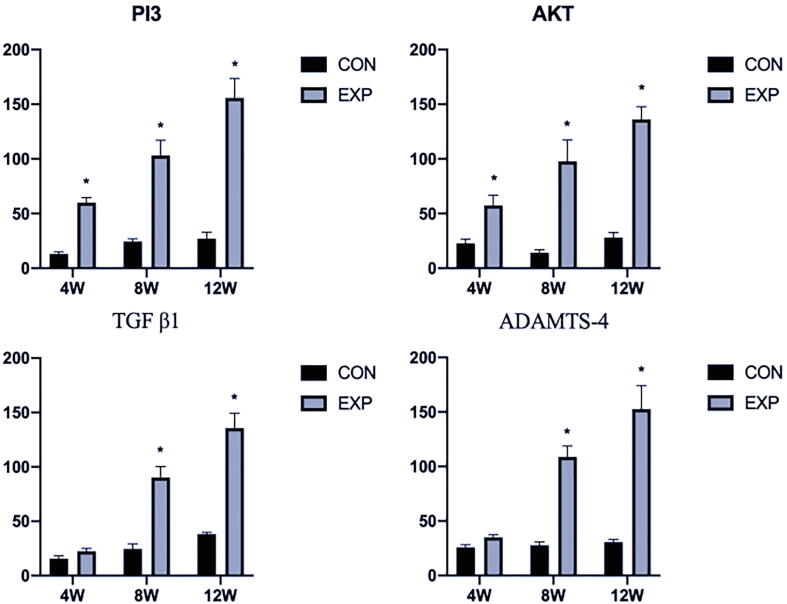
Immunohistochemistry analysis revealed that the expression of PI3K/AKT was slightly elevated at 4 weeks after surgery (P < 0.05), compared with the control group; however, the expression patterns of TGFβ1 and ADAMTS-4 showed no remarkable changes (P > 0.05). Additionally, there were remarkable differences in PI3K/AKT, TGFβ1, and ADAMTS-4 expression at 8 and 12 weeks after surgery (P < 0.05) (Fig. 4, Fig. 5). Furthermore, qPCR analysis showed expression patterns similar to those of immunohistochemical analysis (P < 0.05) (Fig. 6).
Fig. 4.
Immunohistochemical comparison between CON and EXP groups: PI3K/AKT, TGFβ1, and ADAMTS-4 at 4, 8, and 12 weeks. Arrows in EXP group indicate positive expression. Brown to black color indicates positive staining. Scale bar: 50 μm. CON, control; EXP, experimental.
Fig. 5.
The result of analyzing the staining intensity of PI3K/AKT, TGFβ1, and ADAMTS-4 in Fig. 4. There were remarkable differences in PI3K/AKT, TGFβ1, and ADAMTS-4 expression at 8 and 12 weeks after surgery. CON, control; EXP, experimental. *P < 0.05.
Fig. 6.
qPCR evaluation of PI3K/AKT, TGFβ1, and ADAMTS-4 expression levels at 4, 8, and 12 weeks in CON and EXP groups: showed higher expression of PI3K/AKT, TGFβ1, and ADAMTS-4 in the EXP Group at 8, 12 weeks after surgery. The Y-axis represents the PCR amplification multiple of the target gene. CON, control; EXP, experimental. *P < 0.05.
Discussion
In this study, we successfully established an experimental model of TD in growing rats after induction of patellar instability; the model was characterized by subchondral bone loss and cartilage degeneration, which worsened over time. Importantly, we observed remarkably elevated expression of PI3K/AKT at the protein and mRNA levels in the cartilage degeneration model, which revealed that TD-induced cartilage degeneration might be associated with activation of the PI3K/AKT signaling pathway. These results provide new insights regarding the high incidence of OA in patients with TD. To the best of our knowledge, this is the first report regarding the relationship between PI3K/AKT signaling and cartilage degeneration in the patellar instability model of TD.
Mechanical stress is known as a major factor in the development of bone and cartilage [29]. Changes in stress distribution in the patellofemoral joint will affect cartilage metabolism; positional mismatches between the patella and femoral trochlea may lead to OA [30], [31], [32]. When patellofemoral joint pressure increases due to abnormal patellar biomechanics, cartilage is damaged more easily [33], [34]. The present results support the hypothesis that TD may be induced by mechanical stimuli, because pressure changes in the patellofemoral joint due to patellar instability; such changes worsen over time during TD progression. However, mechanical factors may not be the only means by which TD develops into OA. Because TD is a developmental disease, abnormal joint development may lead to early articular cartilage degeneration.
The PI3K/AKT signaling pathway is a key component of cell development, including cell metabolism, apoptosis, transcription, and cell cycle [35]. Additionally, abnormal expression levels of PI3K/AKT have been found in many cancers [36]. Previous studies have shown that PI3K/AKT signaling contributes to the pathogenesis of OA [37], [38]; inhibition of PI3K/AKT expression in rat chondrocytes can improve autophagy and delay the progression of OA [39], [40]. The progression of OA is known to be affected by inflammatory cytokines, and the PI3K/AKT signaling pathway can mediate NF-κB activation and the mRNA expression of TNFα in osteoblasts; inhibition of PI3K/AKT signaling can protect chondrocytes from inflammatory disruption in OA [41], [42], [43], [44]. Thus, we presumed that the PI3K/AKT signaling pathway plays an important role in TD pathology. To test this hypothesis, we examined PI3K/AKT expression in rat trochlear cartilage. Immunohistochemistry and mRNA expression analyses revealed that the levels of PI3K/AKT increased over time in the patellar instability model of TD. Thus, persistent high expression of PI3K/AKT in the TD model may explain the continued existence of developmental factors in articular cartilage. However, further studies are needed to clarify how PI3K/AKT contributes to TD pathology.
TGFβ1 is an important research focus, with potential for clinical use; it may aid in diagnosis and treatment of OA [45]. Abnormally elevated levels of TGFβ1 in cartilage can promote production of proteoglycans and lead to abnormal growth of osteophytes and synovium [46]. TGFβ1 accelerates the condensation of mesenchymal stem cells, and improves early chondrocyte differentiation, while inhibiting terminal hypertrophic differentiation; thus, it influences regular bone morphology [47]. In the present study, persistent high expression of TGFβ1 in the TD model increased in an age-dependent manner. Overall, these findings suggested that articular cartilage in the rat TD model exhibited early molecular expression of OA.
Notably, our previous study confirmed that TD can lead to cartilage degeneration [24], [25]. In the present study, we found that, with increasing age, cartilage morphology changed in the rat TD model: its surface became rough, vertical cracks appeared, and articular chondrocytes aggregated. Furthermore, qPCR analysis revealed that ADAMTS-4 [48], a marker of cartilage degeneration, was overexpressed in cartilage of the TD model. Thus, this study confirmed the presence of cartilage degeneration in a model of TD. These findings were consistent with the manifestation of OA, suggesting that TD may contribute to onset of patellofemoral OA.
As we all know, human OA is characterized by pathological changes of most joint tissues, including cartilage degradation, subchondral bone structure changes and synovial inflammation, which eventually lead to joint space narrowing and osteophyte formation, leading to serious damage of knee joint function [49], [50], [51]. Our study found that in our model of TD in growing rates after induction of patellar stability; the model was characterized by subchondral bone loss and cartilage degradation, which worsen over time. Importantly, we observed significantly elevated expression of PI3K/AKT at the protein and mRNA levels in the TD model, which were consistent with the characteristics of human OA. Through these studies, we speculate that the PI3K/AKT signaling pathway can be used as a potential therapeutic target for the treatment of human OA to prevent cartilage degradation and subchondral bone loss.
Our study had several limitations. First, findings in a rat model can not be readily translated to the clinic. Second, this study only investigated the early stage of patellofemoral OA at 12 weeks after induction of patellar instability; it did not generate long-term follow-up data. Third, further investigation is needed regarding the molecular mechanisms of PI3K/AKT signaling during TD-induced cartilage degeneration.
Conclusions
The findings in this study suggested that patellar instability may lead to TD in growing rats. Moreover, TD may cause patellofemoral OA; TD-induced cartilage degeneration might be associated with activation of the PI3K/AKT signaling pathway. These results provide new insights regarding the high incidence of OA in patients with TD. However, additional research is needed to clarify the underlying mechanisms.
Compliance with ethics requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the staff of all participating departments. This work was supported by the National Natural Science Foundation of China (grant no. 81873983), Key Program of Natural Science Foundation of Hebei Province (grant no. H2019206694), Hebei Province Key Project of Achievement Transformation (grant no. zh2018007), and the Fund for Graduates’ Innovative Projects of the Academic Degree Office of Hebei Provincial Department of Education (grant no. CXZZBS2020114).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Sillanpää P, Mattila VM, livonen T, Visuri T, Pihlajamäki H. Incidence and risk factors of acute traumatic primary patellar dislocation. Med Sci Sports Exerc. 2008, 40(4):606-11. [DOI] [PubMed]
- 2.Brattstroem H. Shape of the Intercondylar Groove Normally and in Recurrent Dislocation of Patella: A Clinical and X-Ray Anatomical Investigation. Acta Orthop Scand Suppl. 1964;68(SUPPL 68):1–148. [PubMed] [Google Scholar]
- 3.Fithian D.C., Paxton E.W., Cohen A.B. Indications in the treatment of patellar instability. J Knee Surg. 2004;17(1):47–56. doi: 10.1055/s-0030-1247149. [DOI] [PubMed] [Google Scholar]
- 4.Dejour H., Walch G., Nove-Josserand L., Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 5.Laurin C.A., Lévesque H.P., Dussault R., Labelle H., Peides J.P. The abnormal lateral patellofemoral angle: a diagnostic roentgenographic sign of recurrent patellar subluxation. J Bone Joint Surg Am. 1978;60(1):55–60. [PubMed] [Google Scholar]
- 6.Goldthwait J.E. Slipping or recurrent dislocation of the patella: with the report of eleven cases. J Bone Joint Surg Am. 2003;85(12):2489. [PubMed] [Google Scholar]
- 7.Parikh S.N., Rajdev N., Sun Q. The growth of trochlear dysplasia during adolescence. J Pediatr Orthop. 2018;38(6):e318–e324. doi: 10.1097/BPO.0000000000001168. [DOI] [PubMed] [Google Scholar]
- 8.Franciozi C.E., Ambra L.F., Luzo M.V., et al. Increased femoral Anteversion influence over surgically treated recurrent patellar instability patients. Arthroscopy. 2017;33(3):633–640. doi: 10.1016/j.arthro.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Ferlic P.W., Runer A., Dammerer D., Wansch J., Hackl W., Liebensteiner M.C. Patella height correlates with trochlear dysplasia: a computed tomography image analysis. Arthroscopy. 2018;34(6):1921–1928. doi: 10.1016/j.arthro.2018.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Williams A.A., Elias J.J., Cosgarea A.J., et al. The relationship between Tibial tuberosity-trochlear groove distance and abnormal patellar tracking in patients with unilateral patellar instability. Arthroscopy. 2016;32(1):55–61. doi: 10.1016/j.arthro.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Dejour D., Le Coultre B. Osteotomies in Patellofemoral instabilities. Sports Med Arthrosc. 2018;26(1):8–15. doi: 10.1097/JSA.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 12.Kang H., Lu J., Wang F., et al. The effect of increased femoral anteversion on the morphological and trabecular microarchitectural changes in the trochlea in an immature rabbit. J Adv Res. 2020;23:143–149. doi: 10.1016/j.jare.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitoussi F., Akouré S., Chouteau Y., Bouger D. Hollow femoral trochlea and femoro-patellar osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 1994;80(6):520–524. [PubMed] [Google Scholar]
- 14.Dejour H., Walch G., Neyret P., Adeleine P. Dysplasia of the femoral trochlea. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45–54. [PubMed] [Google Scholar]
- 15.Jungmann P.M., Tham S.C., Link T.M., et al. Association of trochlear dysplasia with degenerative abnormalities in the knee: data from the osteoarthritis initiative. Skeletal Radiol. 2013;42(10):1383–1392. doi: 10.1007/s00256-013-1664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q., Chen D., Zuscik M.J., O'Keefe R.J., Rosier R.N. Overexpression of Smurf2 stimulates endochondral ossification through upregulation of β-catenin. J Bone Miner Res. 2008;23(4):552–563. doi: 10.1359/JBMR.071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Taboas J.M., Tuan R.S. PTHrP overexpression Partially inhibits a mechanical strain-induced arthritic phenotype in chondrocytes. Osteoarthritis Cartilage. 2011;19(2):213–221. doi: 10.1016/j.joca.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sezgin M., Barlas Í.Ö., Erdal M.E., et al. Apoptosis-related Fas and FasL gene polymorphisms' associations with knee osteoarthritis. Rheumatol Int. 2013;33(8):2039–2043. doi: 10.1007/s00296-013-2688-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang C.Y., Lin H.J., Chen H.S., Cheng S.Y., Hsu H.C., Tang C.H. Thrombin Promotes matrix metalloproteinase-13 expression through the PKCdelta c-Src/EGFR/Pl3K/Akt/AP-1 signaling pathway in human chondrocytes. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/326041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker N., Sohn J., Tuan R.S. Promotion of human mesenchymal stem cell osteogenesis by PI3-kinase/Akt signaling, and the influence of caveolin-1/cholesterol homeostasis. Stem Cell Res Ther. 2015;01(6):238. doi: 10.1186/s13287-015-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauzon M.A., Drevelle O., Daviau A., Faucheux N. Effects of BMP-9 and BMP-2 on the PI3K/Akt Pathway in MC3T3-E1 Preosteoblasts. Tissue Eng Part A. 2016;22(17–18):1075–1085. doi: 10.1089/ten.TEA.2016.0151. [DOI] [PubMed] [Google Scholar]
- 22.Xi J.C., Zang H.Y., Ma Y.Z., et al. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J Recept Signal Transduct Res. 2015;35(6):640–645. doi: 10.3109/10799893.2015.1041647. [DOI] [PubMed] [Google Scholar]
- 23.Haudenschild D.R., Chen J., Steklov N., Lotz M.K., D'Lima D.D. Characterization of the chondrocyte actin cytoskeleton in living three-dimensional culture: response to anabolic and catabolic stimuli. Mol Cell Biomech. 2009;6(3):135–144. [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y., Lu J., Li F., Yang G., Ji G., Wang F. Changes in cartilage and subchondral bone in a growing rabbit experimental model of developmental trochlear dysplasia of the knee. Connect Tissue Res. 2019;12:1–14. doi: 10.1080/03008207.2019.1697245. [DOI] [PubMed] [Google Scholar]
- 25.Yang G., Li F., Wang F., et al. The dysplastic trochlear sulcus due to the insufficient patellar stress in growing rats. BMc Musculoskelet Disord. 2019;05;20(1):411 doi: 10.1186/s12891-019-2802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan E.F., Harjanto R., Sah R.L., et al. Structural and functional maturation of distal femoral cartilage and bone during postnatal development and growth in humans and mice. Orthop Clin North Am. 2012;43(2) doi: 10.1016/j.ocl.2012.01.005. pp. 173–85, v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mankin H.J., Johnson M.E., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar- containing macromolecules. J Bone Joint Surg Am. 1981;63(1):131–139. [PubMed] [Google Scholar]
- 28.Ranstam J. Repeated measurements, bilateral observations and pseudoreplicates, why does it matter? Osteoarthritis Cartilage. 2012;20(6):473–475. doi: 10.1016/j.joca.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Guevara J.M., Moncayo M.A., Vaca-González J.J., Gutiérrez M.L., Barrera L.A., Garzón-Alvarado D.A. Growth plate stress distribution implications during bone development: A simple framework computational approach. Comput Methods Programs Biomed. 2015;118(1):59–68. doi: 10.1016/j.cmpb.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Fujikawa K., Seedhom B.B., Wright V. Biomechanics of the patellofemoral joint. Part II: a study of the effect of simulated femorotibial varus deformity on the congruity of the patellofemoral compartment and movement of the patella. Eng Med. 1983;12(1):13–21. doi: 10.1243/emed_jour_1983_012_005_02. [DOI] [PubMed] [Google Scholar]
- 31.Harrison M.M., Cooke T.D., Fisher S.B., Griffin M.P. Patterns of knee arthrosis and patellar subluxation. Clin Orthop Relat Res. 1994;309:56–63. [PubMed] [Google Scholar]
- 32.lwano T, Kurosawa H, Tokuyama H, Hoshikawa Y. Roentgenographic and clinical findings of patellofemoral osteoarthrosis. With special reference to its relationship to femorotibial osteoarthrosis and etiologic factors. Clin Orthop Relat Res. 1990, (252):190-7. [PubMed]
- 33.Grelsamer R.P., Weinstein C.H. Applied biomechanics of the patella. Clin Orthop Relat Res. 2001;389:9–14. doi: 10.1097/00003086-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Post W.R., Teitge R., Amis A. Patellofemoral malalignment: looking beyond the viewbox. Clin Sports Med. 2002;21(3) doi: 10.1016/s0278-5919(02)00011-x. pp. 521–46, x. [DOI] [PubMed] [Google Scholar]
- 35.Malemud C.J. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7(9):1137–1147. doi: 10.4155/fmc.15.55. [DOI] [PubMed] [Google Scholar]
- 36.Aziz A.U.R., Farid S., Qin K., Wang H., Liu B. PIM kinases and their relevance to the PI3K/AKT/mTOR pathway in the regulation of ovarian cancer. Biomolecules. 2018;02 04:8(1). doi: 10.3390/biom8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cravero J.D., Carlson C.S., Im H.J., Yammani R.R., Long D., Loeser R.F. Increased expression of the Akt/PKB inhibitor TRB3 in osteoarthritic chondrocytes inhibits insulin-like growth factor 1-mediated cell survival and proteoglycan synthesis. Arthritis Rheum. 2009 Feb;60(2):492–500. doi: 10.1002/art.24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Vasheghani F., Kapoor M., et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015 Jul;74(7):1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 39.Xue J.F., Shi Z.M., Zou J., Li X.L. Inhibition of Pl3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–1261. doi: 10.1016/j.biopha.2017.01.130. [DOI] [PubMed] [Google Scholar]
- 40.Hu Z.C., Gong L.F., Ni W.F., et al. Inhibition of Pl3K/Akt/NF-kappaB signaling with leonurine for ameliorating the progression of osteoarthritis: in vitro and in vivo studies. J Cell Physiol. 2019;234(5):6940–6950. doi: 10.1002/jcp.27437. [DOI] [PubMed] [Google Scholar]
- 41.Qiu L., Zhang L., Mi X., et al. PI3K/Akt mediates expression of TNF-alpha mRNA and activation of NF-kappaB in calyculin A-treated primary osteoblasts. Oral Dis. 2008;14(8):727–733. doi: 10.1111/j.1601-0825.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen H.W., Lin A.H., Liu K.L., et al. Inhibition of TNF-alpha-Induced Inflammation by andrographolide via down-regulation of the Pl3K/ Akt signaling pathway. J Nat Prod. 2011;28 74(11):2408–13 doi: 10.1021/np200631v. [DOI] [PubMed] [Google Scholar]
- 43.Liang J., Xu L., Tu M., et al. MALAT1/miR-127-5p regulates osteopontin (OPN)-mediated proliferation of human chondrocytes through Pl3K/Akt pathway. J Cell Biochem. 2018;119(1):431–439. doi: 10.1002/jcb.26200. [DOI] [PubMed] [Google Scholar]
- 44.Rao Z., Wang S., Wang J. Peroxiredoxin 4 inhibits IL-1 beta-induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed Pharmacother. 2017;90:414–420. doi: 10.1016/j.biopha.2017.03.075. [DOI] [PubMed] [Google Scholar]
- 45.Long E., Motwani R., Seegmiller R., et al. The role of TGF-ss1 in osteoarthritis of the temporomandibular joint in two genetic mouse models. Arch Oral Biol. 2016;67:68–73. doi: 10.1016/j.archoralbio.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Bakker A.C., van de Loo F.A., van den Berg W.B., et al. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondroosteo- phyte formation. Osteoarthritis Cartilage. 2001;9(2):128–136. doi: 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- 47.Wang W., Rigueur D., Lyons K.M. TGF β signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today. 2014 Mar;102(1):37–51. doi: 10.1002/bdrc.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plaas A., Osborn B., Sandy J.D., et al. Aggrecanolysis in human osteoarthritis: Confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15(7):719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Beyer C., Zampetaki A., Kiechl S., et al. Signature of circulating microRNAs in osteoarthritis. Ann Rheum Dis. 2015;74(3) doi: 10.1136/annrheumdis-2013-204698. [DOI] [PubMed] [Google Scholar]
- 50.Loeser R.F., Collins J.A., Diekman B.O. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi: 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]