Graphical abstract
Keywords: Tumor immunotherapy, Cancer cell vaccine, Vaccine adjuvants, Immunogenic cell death, Thymidine analog
Highlights
-
•
Effective tumor immunotherapy with in vitro NAP-UVA treated cancer cells per se
-
•
Marked survival improvement with CpG as the specific adjuvant
-
•
Enhanced tumor specific and infiltrating active T cells by treatment vaccine
-
•
Validated efficacy on established tumors with increased dosages
-
•
Potential personalized immunotherapy applications
Abstract
Introduction
Cancer cells induced into immunogenic cell death (ICD) in vitro can be directly used as a whole cell vaccine for tumor immunotherapy with many advantages, especially enacting immediate and intense ‘eat me’ signals to engage immune system. Unfortunately, there have been few successes with in vitro ICD cancer cells as a treatment vaccine.
Objective
To demonstrate that cancer cells treated in vitro with a new class of potent ICD inducer, naphthylquinoxaline thymidine conjugate (NAP) followed by UVA irradiation would be able to act as an effective tumor immunotherapy directly.
Methods
The therapeutic potentials of treated cancer cell plus different vaccine adjuvants were assessed by in vivo liver tumor model and in vitro mixed lymphocyte reaction studies. The elicited activated T cells were determined with immunohistochemistry and T cell induced cytotoxicity studies.
Results
Treatment of established H22 tumor with in vitro NAP and UVA treated cancer cell vaccine led to significantly improved survival. Further mixed lymphocyte reaction study implied that adjuvants alum and CpG would improve the therapeutic potential whereas poly IC would not be as effective. Subsequent in vivo validation of alum and CpG adjuvants indicated that only CpG in NAP and UVA treated cell vaccine resulted in markedly enhanced survival (median at 71 days and 50% tumor-free) as compared with PBS group (14.5 days, 0%) and CpG alone (36 days, 0%). It was revealed that the enhanced efficacy by CpG was specific to NAP and UVA treated cells. Moreover, the effective tumor immunotherapy was achieved through the infiltration of active CD4 and CD8 T cells in tumors and acquisition of cancer cell-specific cytotoxic CD8 T cells.
Conclusion
In vitro NAP and UVA treated cancer cells plus CpG adjuvant are effective tumor therapeutic vaccines per se.
Introduction
Immunogenic cell death (ICD) was first reported with conventional anticancer anthracyclines including mitoxantrone and doxorubicin on cancer cells as a secondary effect to the cytotoxic mechanism and then also demonstrated with photodynamic irradiation, γ-irradiation and oncolytic peptides [1], [2], [3], [4], [5]. Cancer cells in immunogenic death release a unique damage-associated molecular pattern that serves as the ‘eat me’ signal to activate dendritic cells and subsequently specific T help and cytotoxic T lymphocytes, which has been considered as a great potential for tumor immunotherapy [6], [7], [8]. While some of recent applications involving ICD are promising [9], [10], [11], the contributions by ICD are mainly secondary that requires first the killing of enough tumor cells on site to subsequently engage host immune system and in most cases, has to be modulated by other components in the treatment assembly or formulations to demonstrate sufficient effectiveness. On the other hand, cancer cells induced into ICD in vitro could act as a whole cell vaccine per se to active host immune responses for the control or even elimination of tumors, which is considered as the gold standard to determine whether a chemical entity/method is an effective ICD inducer [1], [12]. The vaccine application of in vitro ICD cancer cells has many unique advantages, namely, it can utilize a large number of treated cells in the vaccination to enact immediate and intense ‘eat me” signals to engage immune system; it contains multiple matched tumor specific and/or associated antigens that do not necessarily need to be characterized; and it can directly use the heterogenous cancer cells derived from patient tumor tissues to provide a personalized and precision vaccine treatment [13], [14], [15].
In vitro ICD cancer cells have been investigated as a tumor treatment vaccine with multiple additives and so far, with few successes as compared to prophylactic tumor vaccine [6], [7], [8], [13], [14], [15], [16], [17]. Alternatively, cancer cells induced into ICD were used to generate dendritic cell vaccines for tumor immunotherapy but still, the clinical outcome has been suboptimal [18], [19]. The inadequacy of in vitro ICD cancer cells as a tumor treatment vaccine might be attributed to the limited potency of ICD inducers and the associated mechanisms [1], [2], [3], [4], [5]. Naphthylquinoxaline thymidine conjugate (NAP) is a potent ICD inducer in vitro after UVA irradiation [20], which was originally designed as thymidine kinase inhibitors [21], [22], [23], [24]. Cancer cells treated with NAP followed by UVA irradiation exhibited a burst release profile of ICD markers including ATP and HMGB1 and detection of calreticulin only after 2 h as compared to mitoxantrone control [20]. Most importantly, the prophylactic vaccination of naïve mice with so treated cancer cells elicited a full 100% rejection of a later tumor cell challenge whereas mitoxantrone treated cancer cell vaccine only had 50% rejection under the same condition [20]. On the other hand, the potential of in vitro NAP and UVA treated cancer cells as a tumor therapeutic vaccine has not been established yet might be feasible considering its high ICD potency. In this study, we reveal that in vitro NAP and UVA treated cancer cells plus CpG adjuvant are effective tumor therapeutic vaccines per se on a mouse liver tumor model.
Material and methods
Murine liver cancer H22 cells were obtained from Shanghai Institute of Life Science Cell Culture Center (Shanghai, China). H22 cells were grown via intraperitoneal passages in mice and maintained in complete RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen, CA, USA) at 37 °C with 5% CO2. Compound trans-naphthylquinoxaline thymidine conjugate (NAP) used was previously reported [20].
Ethics statement
The animal protocol complies with the ARRIVE guidelines and was approved by the Animal Care and Use Committee of Huazhong University of Science and Technology. Animal studies were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines.
In vivo study of NAP and UVA treated H22 cells as a treatment vaccine
SPF level male BALB/c mice (8 weeks old) were used to establish the subcutaneous liver tumor model because of the high successful rate and obtained from Beijing HFK Bioscience Co. Ltd., China. Mouse tumors were established by subcutaneous injection of mouse liver cancer H22 cells (3 × 106 cells, 100 µL each in phosphate buffered saline (PBS)) at the left back flank. Once tumors reached to the size of 60 mm3 (approximately 12 days), mice were randomly divided into one control group (n = 9) and three treatment groups (16 mice each) including NAP-UVA, liposomal NAP-UVA and mitoxantrone treated H22 cells as therapeutic vaccines. Liposomal NAP was prepared as reported [24], and the experimental details were provided in the supplementary materials. For the generation of the NAP-UVA treatment vaccines, non-adherent H22 cells were seeded at 2 × 106 cells/well on 6-well plates and then treated with 250 nM NAP (DMSO-PBS dilution, final DMSO at 0.1%) [20] or liposomal NAP [24]. After 5 h, the treatment media were replaced with sterile PBS after centrifugation at 300 g × 10 min. UVA irradiation of the cell suspension was carried out with 400 nm LED light at 2 mW/cm2 for 20 min [20]. Cells were then collected by centrifugation and resuspended in PBS at a density of 3 × 107 cells/mL. Mitoxantrone treated H22 cells were carried out similarly with 2 µM mitoxantrone for 5 h without UVA irradiation. The resulting treated H22 cell suspensions (100 µL per each) were subcutaneously injected at right axilla twice 7 days apart. The body weights, tumor size and health of mice were checked daily over the entire period of treatment study. If the tumor continued to grow over the size of 500 mm3, anticancer drug mitoxantrone (2 mg/kg bodyweight) was injected intraperitonially four times every 3 days as a standard care of chemotherapy to mimic clinical treatment option. Mice were euthanized if the tumor size was over 1,500 mm3.
Study of impacts of vaccine adjuvants by in vitro T cell profiling through mixed lymphocyte reaction
Vaccine adjuvants used in the study included high molecular weight polyinosine-polycytidylic acid (poly IC, InvivoGen, CA, USA), alum (Imject®, Thermo Fisher Scientific, MA, USA) and phosphorothioate CpG oligonucleotide 1826 (VacciGradeTM, InvivoGen). NAP-UVA Vax was the NAP and UVA treated H22 cells (3 × 106 cells per 100 µL injection) as described in the above in vivo study. For the study, naïve male BALB/c mice (8 weeks old) were randomly divided into 8 groups (5 mice each) as PBS, NAP-UVA Vax, poly IC only, poly IC + NAP-UVA Vax, alum only, alum + NAP-UVA Vax, CpG only, and CpG + NAP-UVA Vax groups. Adjuvant poly IC (25 µg), alum (33 µL) or CpG (25 µg) was freshly mixed with 100 µL NAP-UVA Vax or PBS at 30 min before the subcutaneous injection at the right axilla of mice. After 4 weeks, spleens of these mice were harvested and passed through a cell strainer (70 µm, BD Biosciences, CA, USA). Splenocytes were collected after centrifugation at 400 g × 3 min and then labeled with 1 µM carboxyfluorescein diacetate succinimidyl ester (CFSE, eBioscience, Thermo Fisher Scientific, MA, USA) for 10 min in PBS. The CFSE labeled splenocytes were then co-incubated without or with live H22 cells at 3:1 ratio. After 72 h, cells were collected by centrifugation at 300 g × 5 min and resuspended in 2% FBS in PBS. For T cell profiling analysis, the resulting cell suspensions were incubated with anti-mouse PE/Cy7-CD3 (Cat#100220), PE-CD4 (Cat#100512), PE/Cy5-CD8α (Cat#100710) antibodies (BioLegend, CA, USA) in 1:200 dilution at 4 °C in dark for 30 min. Cells were then washed twice with 2% FBS in PBS and analyzed with a CytoFlex flow cytometer (Beckman Coulter, IN, USA).
In vivo study of therapeutic potential of NAP Vax with adjuvants
Male mice with H22 tumors (approximately 60 mm3) were randomly divided into 7 groups (8 mice per group) including PBS, alum only, alum + NAP-UVA Vax, alum + mitoxantrone Vax, CpG only, CpG + NAP-UVA Vax, and CpG + mitoxantrone Vax treatment groups. Alum (33 µL) or CpG (15 µg) was freshly mixed with treated H22 cells or PBS per 100 µL at 30 min before the subcutaneous injection at the right axilla of mice twice 7 days apart. The body weights, tumor size and health of mice were checked daily over the entire period of treatment study. Mice were euthanized if the tumor size was over 1,500 mm3. In addition, treatment and control groups (8 mice per group) of increased amount of CpG (25 µg per injection) in NAP Vax and dosage (3 injections) were carried out similarly.
Immunohistochemistry of selected tumor tissues
IHC control staining of CD4 (Cat# PA1049, Hubei Bais Biotechnology, China), CD8 (Cat# PA1050, Hubei Bais Biotechnology), FOXP3 (Cat# 98377S, Cell Signaling Technology, MA, USA), Ki67 antibodies (Cat#PA1007, Hubei Bais Biotechnology) was performed and optimized on mouse spleen tissue with dilutions of 1:1000, 1:2000, 1:50 and 1:200, respectively. Tumor tissues in the adjuvant treatment study from the PBS group, the group of 25 μg CpG alone and the group of 25 μg CpG plus NAP-UVA Vax with delayed growth were collected. Immunohistochemical staining of CD4, CD8, FOXP3, Ki67 or HE were performed on these fixed tissues followed by capture and analysis with an image viewing software NDP.view2 (Hamamatsu Photonics K.K., Hamamatsu, Japan).
In vitro apoptosis study of H22 cells induced by isolated CD8+ T cells
Spleens of tumor-free mice from the 25 μg CpG plus NAP-UVA Vax group were collected and digested with collagenase II (100 U/mL, Thermo Fisher) at 37 °C for 2 h. Splenocytes were isolated as described above and cultured at a density of 1 × 106 cells/mL in the complete RPMI growth media containing mouse recombinant IL-2 (300 IU/mL, BioLegend) and IL-7 (5 ng/mL, BioLegend) for 7 days. The resulting cells were collected and resuspended in 2% FBS in PBS solution at a density of 1 × 108 cells/mL. Mouse CD8 FlowComp ™ Dynabeads ® (Thermo Fisher) were then added at a ratio of 50 μL per mL of cells, and the resulting mixture was incubated at 4 °C for 10 min. CD8+ T cells captured on dynabeads were collected by a magnet, washed twice by PBS and resuspended in 2 mL 2% FBS in PBS. The concentration of CD8+ T cells captured on dynabeads was determined by the microscopic cell counting method with trypan blue staining of a sample solution, in which CD8 T cells were released from dynabeads by a modified biotin competitor provided by manufacturer. Typically, a 200 μL dynabeads suspension yielded about 1–5 × 106 free T cells. As a control, CD8 T cells from naïve mice were obtained similarly. For apoptosis study, H22 cells were first CFSE labeled as described above and then plated at 50,000 cells per well on a 48 well plate. The suspension of CD8 T cells captured on dynabeads was then added at the ratio of H22 to T cells at 1:1, 1:2, 1:5 or 1:10 based on the determined concentration of T cells on dynabeads. In addition, H22 cells alone and H22 cells with dynabeads control (no T cells) were included as controls. After incubation for 24 h, dynabeads in the media were removed with a magnet, and CFSE labeled H22 cells were collected as the supernatant. The resulting H22 cells were then incubated in 2% FBS in PBS with PE/Cy7-Annexin V (Cat#640951, 1:200 dilution, BioLegend) at 4 °C for 30 min followed by staining with a propidium iodine solution. The percentage of apoptotic cells was analyzed similarly with a CytoFlex cytometer as described above.
Statistical analysis
Statistical analysis was carried out with GraphPad Prism program (GraphPad Software, CA, USA). Statistically significant difference (*P < 0.05) was determined with multiple group comparison using one-way ANOVA followed by a post hoc test (Tukeýs method). Survival plots of treatment and control groups were carried out with methods provided by the GraphPad software. Principal component analysis (PCA) was performed in the free R software for statistical computing and graphics (version 4.0.2, https://www.r-project.org).
Results and discussion
NAP and UVA treated H22 cells as a tumor treatment vaccine led to improved overall survival.
The effectiveness of NAP and UVA treated cancer cells as a tumor treatment vaccine was assessed in vivo in mice with subcutaneous H22 liver cancer tumors of a size of 60 mm3. The liver cancer tumor has a rapid growth rate in immunocompetent BALB/C mice that could be conveniently monitored by the size measurement. The treatment vaccine solutions were produced from the same H22 cancer cells used to establish the tumor model, which were treated with two formulations of NAP compound at 250 nM followed by UVA irradiation (Fig. 1a) [20]. The formulations of NAP compound included a standard DMSO-PBS dilution system and a liposome delivery system of nanoparticles with hydrodynamic diameter of 108 nm (supporting Fig. S1) that exhibited increased tumor inhibition through enhanced induction of immunogenic cell death [24]. In addition, a standard care of tumor chemotherapy with mitoxantrone injected intraperitoneally [2] was implemented in this study once the tumor size was larger than 500 mm3 (Fig. 1a). Moreover, in addition to the PBS negative control, cells treated with 2 µM mitoxantrone were included as a positive control because of the reported tumor growth inhibition through ICD mechanism [1], [20].
Fig. 1.
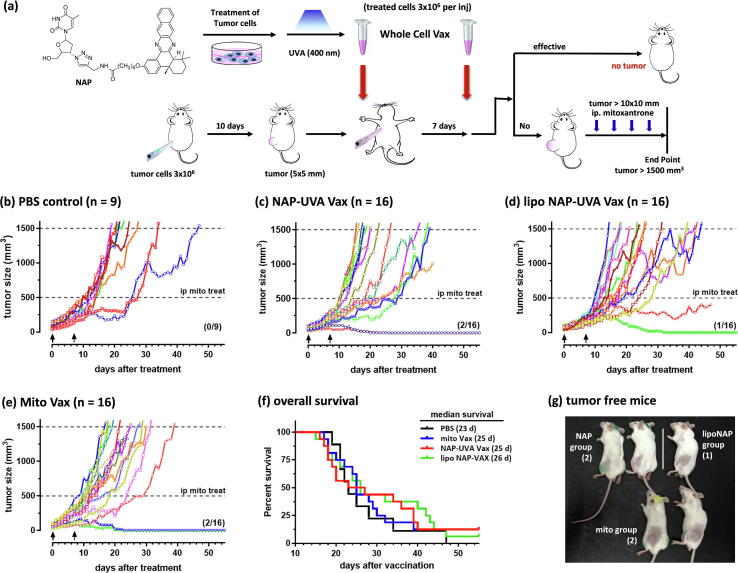
NAP and UVA treated H22 cells as a tumor treatment vaccine resulted in improved overall survival in mice with pre-existing tumors. (a) Scheme of the treatment study. H22 cell vaccines were subcutaneously injected at right axilla twice 7 days apart when the H22 tumor at the left back flank reached the size of 60 mm3. If the tumor continued to grow over the size of 500 mm3, chemotherapy with mitoxantrone (2 mg/kg) was injected intraperitonially four times every 3 days; (b) - (e) spider plots of individual tumor growth profile of the PBS control, NAP-UVA Vax, liposomal NAP-UVA Vax and mitoxantrone treated H22 vaccine (Mito Vax) groups, respectively; (f) survival plot of all the treatment groups; (g) images of the resulting tumor-free mice.
With two injections of the treatment vaccine solutions in the axilla region, two out of 16 mice in the standard formulation of NAP-UVA group elicited complete rejection of existing tumors at back flank within 20 days (Fig. 1). The liposomal formulation resulted in only one tumor free mouse and one mouse with significant delayed growth yet demised on day 48. The positive control group of 2 µM mitoxantrone treated H22 cell group also produced two tumor-free out of 16 mice studied. The standard care of intraperitoneal injection of anticancer drug was found not necessary for the mice with full rejection of tumors because the size of tumors never reached to the required volume for chemotherapy. The resulting five tumor-free mice from treatment groups (Fig. 1g) remained healthy over 2 months. While the NAP-UVA group and mitoxantrone control group both resulted in the same number of tumor-free mice, the NAP-UVA group showed significantly delayed growth of tumors and better survival profile than the mitoxantrone group or PBS group (Fig. 1f). Similar trend was also exhibited by the liposomal NAP/UVA group. These results suggested that liposomal formulation was not needed for the NAP compound, possibly due to its high potency of IC50 at 30 nM under UVA irradiation as compared with other thymidine conjugates [20], [24]. This was further confirmed by a similar cytotoxicity of NAP versus liposomal NAP as well as the induced ATP release profile after treatment (supporting Fig. S2). We also found that treatment with a different cell line-based vaccine such as treated lung cancer A549 cells did not inhibit the growth of the H22 liver cancer tumor, which was consistent with the specificity of the photodynamic irradiated ICD cancer cell vaccine [16]. All these results suggested that H22 cancer cells treated with 250 nM NAP compound followed by UVA irradiation were possibly an effective and specific treatment option for existing H22 tumors. On the other hand, the percentage of full tumor rejection by the treatment vaccine solutions was quite low at 12.5%, and further optimization was necessary for an effective clinical translation.
Vaccine adjuvants CpG and alum significantly altered T cell distribution of immunized mice.
Adjuvants have been demonstrated with significant advantages in vaccine formulations to elicit boosted immune responses, e.g., phosphorothioate oligonucleotide CpG through activation of toll-like receptor 9 [17], [25], [26], poly IC as a synthetic analog of double stranded RNA through toll-like receptor-3 signaling [27], and alum for adsorption and enhanced antigen presentation [28]. While CpG, poly IC and alum have all been used to enhance immune responses in the cell-based vaccine against cancer, it was not clear which of these might be a better choice for the NAP-UVA treated cancer cell-based vaccine as a treatment. Therefore, the effectiveness of CpG, poly IC or alum adjuvant was first assessed with the in vitro mixed lymphocyte reaction study post a single shot of NAP-UVA Vax plus one of these three adjuvants (Fig. 2a). Mixed lymphocytes reaction has been proved an invaluable tool for the cells-based vaccine development to assess the elicited anti-tumor immune responses in mice [25], [26], [29], [30]. We also found that in vitro co-incubation of splenocytes of vaccine-treated tumor-free mice with live H22 cells at a ratio of 3:1 resulted in a significantly different T cell distribution profile as compared with naïve mice (supporting Fig. S3). In addition, no significant difference of T cell distribution was observed with other stimulants such as cancer cell lysate or fixed cancer cells, nor did the B cell distribution with any of those stimulants (supporting Figs. S3,S4).
Fig. 2.
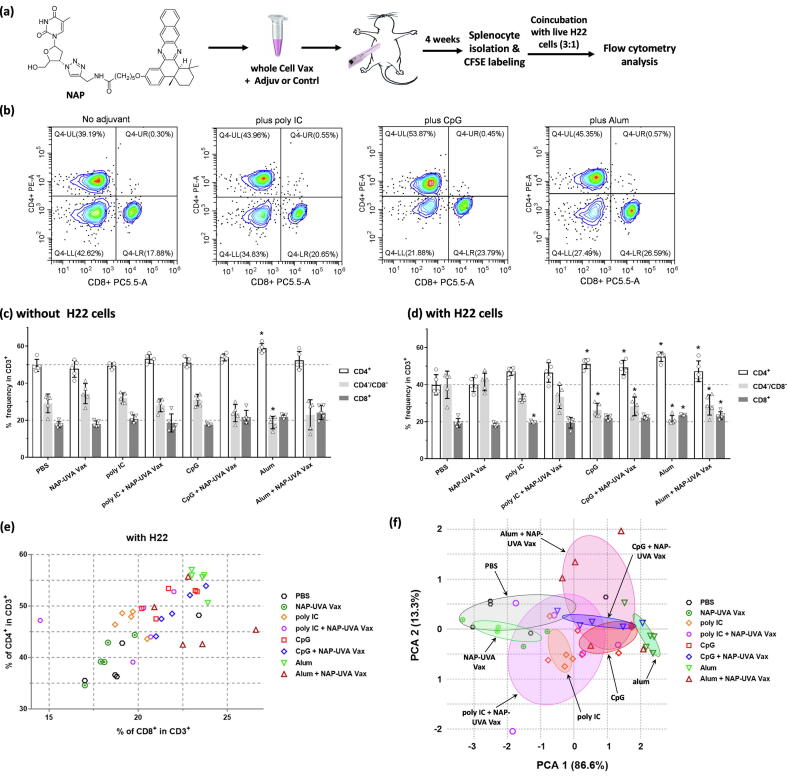
Impacts of vaccine adjuvants on T cell distribution of splenocytes post in vitro live cancer cell stimulation. Mice were vaccinated once with NAP and UVA treated H22 vaccine plus PBS, poly IC, CpG or alum as an adjuvant or controls (n = 5 per group). (a) Scheme of the adjuvant study; (b) representative flow cytometry analysis diagrams of CD4+ and CD8+ T cell subpopulations of the isolated splenocytes with in vitro live H22 cell stimulation and controls; (c) - (d) percent bar graphs of CD4+, CD8+ and CD4-CD8- cells in isolated CD3+ splenocytes without or with live untreated H22 cells (*P < 0.05 as compared with corresponding PBS control within the panel); (e) scatter plot of percentage of CD4+ and CD8+ subpopulations of individual mouse of all adjuvant groups and controls post in vitro H22 cell stimulation; (f) scatter plot of individual coordinates from principal component analysis of subpopulations of CD3+ splenocytes post in vitro H22 cell stimulation. Confidence ellipse of each group was overlaid as indicated by colors.
Representative figures of flow cytometry analysis of the mixed lymphocyte reaction were shown in Fig. 2b. Significantly increased CD4+ and CD8+ T cell percentages were found in the presence of live H22 cells with CpG or alum as the adjuvant (Fig. 2b). Further statistical analysis of these groups (5 mice per group) indicated that without any cancer cell stimulant, most of vaccinated groups were quite similar with CD4+ at 50%, CD8+ at 20%, except that of the alum control (Fig. 2c). In the presence of live H22 cell stimulant, PBS control or the NAP-UVA Vax alone had equal distribution of CD4+ and CD4-/CD8- at 40% and CD8+ not changed at 20%. On the other hand, the CpG only, alum only, and NAP-UVA Vax plus CpG or alum groups maintained a statistically significant high level of CD4+ at 50% while CD8+ over 20% (Fig. 2d). In addition, the CD4/CD8 ratio seemed to align approximately at 2.2 in the scatter plot (Fig. 2e), while NAP-UVA Vax plus different adjuvants clustered at different locations from that of PBS or NAP-UVA Vax alone. Thus, a principal component analysis (PCA) was carried out on the distribution of T cells with live H22 cell stimulant [31]. PCA revealed that most of data were well presented by the projected PCA dimension 1 that represented 86.6% variables and was composed of almost equal contributions by CD4+, CD8+, CD4-CD8- (Fig. 2f, supporting Table S1). The distribution of variables was further scattered along the PCA dimension 2 only by 13.3% that was mainly of CD8+ plus CD4+. Hence, the T cell distributions of alum only, CpG only and the NAP-UVA Vax plus CpG or alum were shown to be distant from those of PBS or NAP-UVA Vax alone as indicated by confidence eclipses overlaid in colors. On the other hand, poly IC adjuvants were much closer to that PBS group (Fig. 2f). All these results implied that adjuvant alum or CpG in the NAP-UVA Vax could significantly impact the distribution of T cell in the presence of live cancer cell stimulant as well as by themselves without any vaccines, which was then assessed in vivo on tumor-bearing mice.
CpG adjuvant in NAP-UVA Vax treatment resulted in a marked enhancement of tumor survival
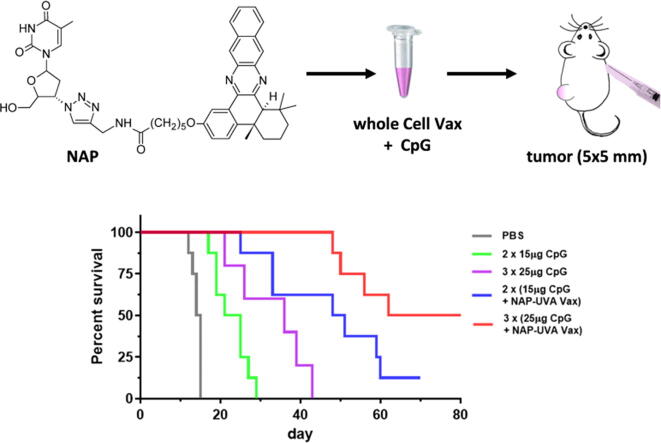
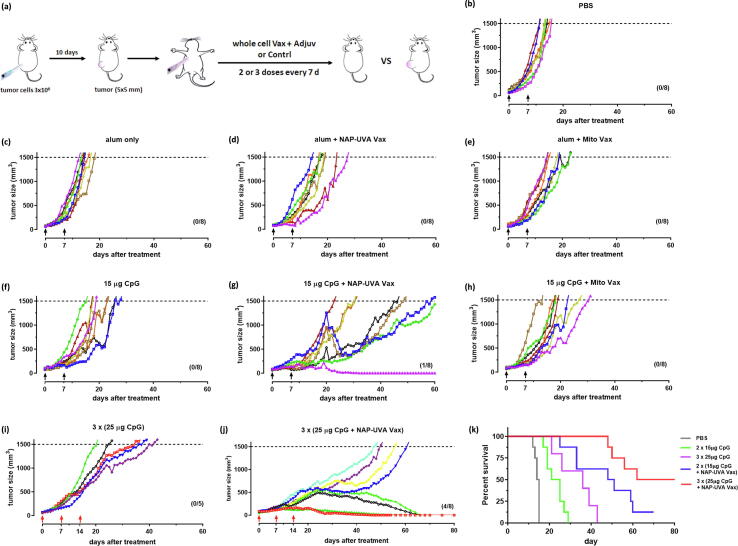
The in vivo treatment study with adjuvants was carried out similarly as described above. The standard care of intraperitoneal injection of anticancer drug was not used because of the rapid rejection of tumor expected in the tumor-free mice (Fig. 3a). Besides NAP-UVA Vax, 2 µM mitoxantrone treated cell vaccines were also included as controls. Our results indicated that adjuvant alum disappointingly produced no improvement by itself as compare to the PBS group or with either cell vaccine (Fig. 3b-e). However, two injection of CpG at 15 µg alone led to a delayed tumor growth with median survival of 23 days versus 14.5 days in PBS group. More excitingly, a significantly delayed growth of tumors was observed in the CpG plus NAP-UVA Vax group (median survival of 49.5 days) and also one tumor-free mouse out of 8 (Fig. 3f,3g). In contrast, the CpG plus mitoxantrone group has a similar tumor growth rate as that of CpG alone with no tumor-free mice (Fig. 3f,3h), suggesting specific enhancement of CpG adjuvant for NAP-UVA Vax. With these encouraging results, we further increased the amount of CpG to 25 µg per injection and dosage to 3 injections. It was found that increased CpG alone indeed further delayed the tumor growth to a mean survival of 36 days yet no tumor-free mice, likely due to enhancement of non-specific immune responses. On the other hand, the increased dosage of CpG plus NAP-UVA Vax markedly resulted in an impressive 50% tumor-free mice (4 out of 8) and an overall median survival of 71 days (Fig. 3j,3k). The delayed tumor growth and the progression to tumor-free were evident in the representative photos of mice from the treatment group of increased dosage of CpG plus NAP-UVA Vax (supporting Fig. S5).
Fig. 3.
CpG adjuvant in NAP and UVA treated H22 cell vaccine markedly enhanced the overall survival of mice with pre-existing H22 tumors. (a) Scheme of the tumor treatment design; (b) - (h) spider plots of the individual tumor growth profile after treatments of NAP-UVA Vax or mitoxantrone Vax with alum or CpG adjuvant and controls. H22 cell vaccines were subcutaneously injected at right axilla twice (7 days apart) when the H22 tumor at the left back flank reached the size of 60 mm3; (i) - (j) spider plots of the tumor growth profile with increased amount of CpG adjuvant (25 µg) per injection and dosages (3 injections); (k) survival plot of the CpG vaccine treatment groups. The number of mice in each treatment group was indicated in each panel.
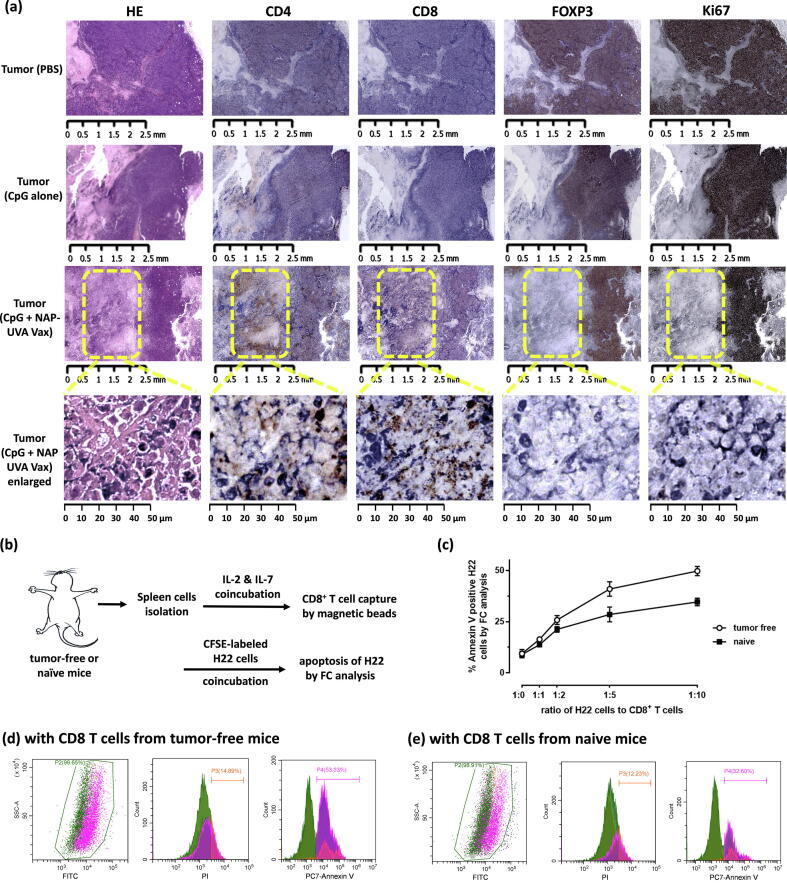
The tumor tissues with significantly delayed growth in the increased dosage of CpG plus NAP-UVA Vax (Fig. 3j) were then collected for immunohistochemistry analysis to reveal possible underlying mechanisms. As a comparison, those of PBS and increased CpG alone groups (Fig. 3b,3i) were also determined. Our analysis indicated that high proliferating tumor cells (Ki67 positive) in the PBS and increased CpG alone groups were highly correlated with the expression of FOXP3, the immune suppressive Treg cells marker that was also CD4 positive (Fig. 4a). No significant positive staining of CD8+ T cells or only weakly distinctive CD4 + positive was observed. This was in contrast to those of control IHC staining of the mouse spleen tissue with significantly low level of FOXP3, and different levels of CD4 and CD8 (supporting Fig. S6). More importantly, the tumor tissues in the increased dosage of CpG plus NAP-UVA Vax group showed CD4 and CD8 positive staining regions distinctively from FOXP3 and Ki67 positive areas at millimeter scale. Further enlargement of these highlighted regions into micrometer scale revealed clearly the high positive CD4 and/or CD8 T cell staining regions where there was full absence of Foxp3 or Ki67 staining (Fig. 4a). These data indicated that the significantly delayed tumor growth in the increased dosage of CpG plus NAP-UVA Vax was apparently due to the infiltration of active CD4+ and CD8+ T cells in the tumor to counteract the rapid proliferation of tumor cells and immunosuppressive microenvironment. With these results, we then investigated whether the tumor-free mice of the increased dosage of CpG plus NAP-UVA Vax group might acquire specific cytotoxic CD8+ T cells against H22 cancer cells [25], [26], [32], [33]. CD8+ T cells from the cultured splenocytes of tumor-free mice was first cultured in vitro and then captured on magnetic dynabeads, and then co-incubated with CFSE-labeled H22 cells (Fig. 4b). After 24 h, CD8+ T cells on beads were removed by a magnet, and the extent of apoptotic CFSE-labeled H22 was determined by a flow cytometry analysis (supporting Fig. S7). It was revealed that with the increasing ratio of CD8+ T cells of the tumor-free mice to cancer cells, more cancer H22 cells underwent early apoptosis than those by CD8+ T cells of the naïve mice (Fig. 4c). At 10 to 1 ratio, over 50% of H22 were apoptotic with CD8+ T cells from tumor-free mice whereas only about 30% were annexin V positive with those from naïve mice against H22 cells (Fig. 4d,4e). All these results indicated that the therapeutical potential of NAP-UVA Vax plus CpG adjuvant was achieved through infiltration of active CD4+ and CD8+ T cells in tumors and acquisition of cancer cell-specific cytotoxic CD8+ T cells.
Fig. 4.
Activated CD4+ and CD8+ T cells evidently contributed to the improved survival in the treatment of 25 µg CpG plus NAP-UVA Vax. (a) IHC staining of selected tumor tissues after treatment, including PBS group, 25 µg CpG alone and 25 µg CpG plus NAP-UVA with delayed growth; (b) scheme of in vitro apoptosis study of H22 cells induced by CD8+ T cells from tumor-free mice after treatment of 25 µg CpG plus NAP-UVA Vax; (c) plot of the percent apoptotic H22 cells as determined by flow cytometry analysis after co-incubation with CD8+ T cells from tumor-free mice for 24 h (Fig. 3j) or naïve mice at an increasing ratio; (d, e) representative flow cytometry analysis of apoptotic CFSE-labeled H22 cells after co-incubation with CD8+ T cells captured on dynabeads at 1:10 ratio from tumor-free or naïve mice for 24 h.
Our results have revealed that CpG adjuvant exhibited a specific enhancement on NAP-UVA Vax because no significant impact was observed with CpG on mitoxantrone treated cell vaccine (Fig. 3). This was possibly attributed to the unique burst release profile of ICD markers from cancer cells after NAP and UVA treatment as compared with relatively slow release of ICD markers with mitoxantrone treatment [20]. Moreover, although the in vitro mixed lymphocyte reaction study post vaccination was not accurate with alum adjuvant as shown in the in vivo study, it was consistent with CpG adjuvant alone and CpG plus NAP-UVA Vax, implying that the activation of toll-like receptor 9 would be beneficial for tumor immunotherapy [17], [25], [26]. Surely, a positive result in the in vitro tumor-specific CD8+ cytotoxicity assay would further confirm the elicited immune responses by the whole cell vaccine. Finally, our initial safety assessment of the vaccination of NAP-UVA treated cancer cells plus CpG adjuvant revealed no observable impact on the body weight of treated mice nor did any detectable damage in heart, liver, spleen, lung and kidney tissues by HE analysis (supporting Fig. S8), which implied potentially low safety risks of the vaccine.
Conclusion
The marked survival of tumor-bearing mice after vaccination with NAP and UVA treated cancer cells plus CpG adjuvant demonstrated that in vitro ICD cancer cells could directly be utilized as an effective tumors therapy per se. The therapeutic potential of in vitro NAP and UVA treated cancer cells plus CpG adjuvant was revealed evidently through infiltration of active CD4+ and CD8+ T cells in tumors and acquisition of cancer cell-specific cytotoxic CD8+ T cells. The in vitro NAP and UVA treated cancer cell vaccine will potentially be a personalized and precision tumor vaccine because the effectiveness against tumor was specifically achieved with the same cancer cells in the treatment vaccine against tumor. Certainly, further validation of the in vitro treated cancer cells as a therapeutic vaccine of a variety of tumor models would be necessary to demonstrate the potential to address the challenges of tumor heterogeneity and poor vascularity in solid tumor. It is conceivable that with the rapid advances in biotechnology for adaptive cell therapy [34], similar GMP production process would be applicable and compatible for the development of personalized whole cell therapeutic vaccines from tumor-derived cancer cells through in vitro induced immunogenic cell death mechanism.
Author’s contributions
YY performed the synthesis, in vitro and in vivo biological studies and analysis. SZ carried out part of in vivo animal study. QZ conceived of the study, and participate in the design, coordination and drafted the manuscript. All authors read and approved the manuscript.
Funding sources
This work is partially supported by the National Natural Science Foundation of China, China (81671812). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.03.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Obeid M., Tesniere A., Ghiringhelli F., Fimia G.M., Apetoh L., Perfettini J.L., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 2.Michaud M., Martins I., Sukkurwala A.Q., Adjemian S., Ma Y., Pellegatti P., et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 3.Obeid M., Panaretakis T., Joza N., Tufi R., Tesniere A., van Endert P., et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 4.Garg A.D., Krysko D.V., Verfaillie T., Kaczmarek A., Ferreira G.B., Marysael T., et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 2012;31:1062–1079. doi: 10.1038/emboj.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H., Forveille S., Sauvat A., Yamazaki T., Senovilla L., Ma Y., et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Gotwals P., Cameron S., Cipolletta D., Cremasco V., Crystal A., Hewes B., et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer. 2017;17:286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 9.Rios-Doria J., Harper J., Rothstein R., Wetzel L., Chesebrough J., Marrero A., et al. Antibody-drug conjugates bearing pyrrolobenzodiazepine or tubulysin payloads are immunomodulatory and synergize with multiple immunotherapies. Cancer Res. 2017;77:2686–2698. doi: 10.1158/0008-5472.CAN-16-2854. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q., Chen F., Hou L., Shen L., Zhang X., Wang D., et al. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano. 2018;12:7812–7825. doi: 10.1021/acsnano.8b01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Liu L., Liang R., Luo Z., He H., Wu Z., et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633–8645. doi: 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- 12.Humeau J., Lévesque S., Kroemer G., Pol J.G. Gold standard assessment of immunogenic cell death in oncological mouse models. Methods Mol. Biol. 1884;2019:297–315. doi: 10.1007/978-1-4939-8885-3_21. [DOI] [PubMed] [Google Scholar]
- 13.Keenan B.P., Jaffee E.M. Whole cell vaccines–past progress and future strategies. Semin. Oncol. 2012;39:276–286. doi: 10.1053/j.seminoncol.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang C.L., Coukos G., Kandalaft L.E. Whole tumor antigen vaccines: Where are we? Vaccines. 2015;3:344–372. doi: 10.3390/vaccines3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seledtsov V.I., Goncharov A.G., Seledtsova G.V. Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum. Vaccin. Immunother. 2015;11:851–869. doi: 10.1080/21645515.2015.1009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korbelik M., Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006;55:900–909. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bencherif S.A., Sands R.W., Ali O.A., Li W.A., Lewin S.A., Braschler T.M., et al. Injectable cryogel-based whole-cell cancer vaccines. Nat. Commun. 2015;6:7556. doi: 10.1038/ncomms8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A.D. Garg, L. Vandenberk, C. Koks, T. Verschuere, L. Boon, S.W. Van Gool, et al., Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma, Sci. Transl. Med. 8 (2016) 328ra27. [DOI] [PubMed]
- 19.Vandenberk L., Belmans J., Van Woensel M., Riva M., Van Gool S.W. Exploiting the immunogenic potential of cancer cells for improved dendritic cell vaccines. Front. Immunol. 2016;6:663. doi: 10.3389/fimmu.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan Y., Wang Z., Rong Y., Qian T., Zhou Q. Naphthyl quinoxaline thymidine conjugate is a potent anticancer agent post UVA activation and elicits marked inhibition of tumor growth through vaccination. Eur. J. Med. Chem. 2019;171:255–264. doi: 10.1016/j.ejmech.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Wei Q., Zhang D., Yao A., Mai L., Zhang Z., Zhou Q. Design, synthesis, and in vitro and in vivo biological studies of a 3́-deoxythymidine conjugate that potentially kills cancer cells selectively. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0052199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei Q., Liu H., Zhang D., Zhou H., Zhang Z., Zhou Q. Anticancer activity of a thymidine quinoxaline conjugate is modulated by cytosolic thymidine pathways. BMC Cancer. 2015;15:159. doi: 10.1186/s12885-015-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D., Liu H., Wei Q., Zhou Q. Structure-activity relationship study of anticancer thymidine-quinoxaline conjugates under the low radiance of long wavelength ultraviolet light for photodynamic therapy. Eur. J. Med. Chem. 2016;107:180–191. doi: 10.1016/j.ejmech.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Rong Y., Wang Z., Yuan Y., Qian T., Zhou Q. cRGD target liposome delivery system promoted immunogenic cell death through enhanced anticancer potency of a thymidine conjugate under UVA activation as a cancer vaccine. Eur. J. Med. Chem. 2019;169:499–509. doi: 10.1016/j.ejmech.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 25.Kooreman N.G., Kim Y., de Almeida P.E., Termglinchan V., Diecke S., Shao N.Y., et al. Autologous iPSC-based vaccines elicit anti-tumor responses in vivo. Cell Stem Cell. 2018;22:501–513. doi: 10.1016/j.stem.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conniot J., Scomparin A., Peres C., Yeini E., Pozzi S., Matos A.I., et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat. Nanotechnol. 2019;14:891–901. doi: 10.1038/s41565-019-0512-0. [DOI] [PubMed] [Google Scholar]
- 27.Ammi R., De Waele J., Willemen Y., Van Brussel I., Schrijvers D.M., Lion E., et al. Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs. Pharmacol. Ther. 2015;146:120–131. doi: 10.1016/j.pharmthera.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Didierlaurent A.M., Morel S., Lockman L., Giannini S.L., Bisteau M., Carlsen H., et al. Garçon, AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 29.Chiang C.L., Kandalaft L.E., Tanyi J., Hagemann A.R., Motz G.T., Svoronos N., et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin. Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stannard K.A., Collins P.M., Ito K., Sullivan E.M., Scott S.A., Gabutero E., et al. Galectin inhibitory disaccharides promote tumour immunity in a breast cancer model. Cancer Lett. 2010;299:95–110. doi: 10.1016/j.canlet.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Attardi E., Di Cesare S., Amodio D., Giancotta C., Cotugno N., Cifaldi C., et al. Phenotypical T cell differentiation analysis: A diagnostic and predictive tool in the study of primary immunodeficiencies. Front. Immunol. 2019;10:2735. doi: 10.3389/fimmu.2019.02735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson N., Lopez-Pelaez M., Palazon A., Poon E., De La Roche M., Barry S., et al. A cell-engineered system to assess tumor cell sensitivity to CD8+ T cell-mediated cytotoxicity. Oncoimmunology. 2019;8:1599635. doi: 10.1080/2162402X.2019.1599635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He L., Hakimi J., Salha D., Miron I., Dunn P., Radvanyi L. A sensitive flow cytometry-based cytotoxic T-lymphocyte assay through detection of cleaved caspase 3 in target cells. J. Immunol. Methods. 2005;304:43–59. doi: 10.1016/j.jim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Iyer R.K., Bowles P.A., Kim H., Dulgar-Tulloch A. Industrializing autologous adoptive immunotherapies: Manufacturing advances and challenges. Front. Med. 2018;5:150. doi: 10.3389/fmed.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.