Graphical abstract
Keywords: Anal sphincter relaxation, Anorectal physiology, Bionics, Defecation, Fecobionics
Highlights
-
•
The physiology of defecation and the pathophysiology of defecation disorders have been described in numerous studies.
-
•
Disagreement exists between results of anorectal tests and they correlate poorly with symptoms.
-
•
We tested a novel wireless Fecobionics device, a simulated integrated stool in normal subjects.
-
•
Novel analysis of distensibility indices is presented based on preload-afterload diagrams and key parameters describing defecation are derived.
-
•
Fecobionics obtained reliable data under physiological conditions. The study suggests that Fecobionics is safe and effective in evaluation of key defecatory parameters.
-
•
Novel parameters were generated that may be important for subtyping of patients with anorectal disorders.
Abstract
Introduction
Defecation is a complex process that is difficult to study and analyze directly. In anorectal disease conditions, the defecation process may be disturbed, resulting in symptoms including fecal incontinence and constipation. Current state-of-the-art technology measures various aspects of anorectal function but detailed analysis is impossible because they are stand-alone tests rather than an integrated multi-dimensional test.
Objectives
The need for physiologically-relevant and easy-to-use diagnostic tests for identifying underlying mechanisms is substantial. We aimed to advance the field with integrated technology for anorectal function assessment.
Methods
We developed a simulated stool named Fecobionics that integrates several tests to assess defecation pressures, dimensions, shape, orientation and bending during evacuation. A novelty is that pressures are measured in axial direction, i.e. in the direction of the trajectory. Using this novel tool, we present new analytical methods to calculate physiologically relevant parameters during expulsion in normal human subjects.
Results
Data are reported from 28 human subjects with progressively more advanced versions of Fecobionics. A new concept utilizes the rear-front pressure (preload-afterload) diagram for computation of novel defecation indices. Fecobionics obtained physiological data that cannot be obtained with current state-of-the-art technologies.
Conclusion
Fecobionics measures well known parameters such as expulsion time and pressures as well as new metrics including defecation indices. The study suggests that Fecobionics is effective in evaluation of key defecatory parameters and well positioned as an integrated technology for assessment of anorectal function and dysfunction.
Introduction
Defecation is a complex physiological process through which stools are eliminated via the anus [1], [2], [3]. Defecation is initiated by an urge to defecate predominantly resulting from filling of stool in rectum. During evacuation, the abdominal pressure increases, the anal sphincter relaxes, and the anorectal angle straightens. The evacuation process may be altered in disease conditions, resulting in symptoms such as pain (proctalgia), fecal incontinence and constipation [4]. Defecatory disorders affect 25% of the population with rising incidence [1], [4], [5], [6]. These disorders including fecal incontinence and chronic constipation pose a major health care burden but are poorly recognized and treated [4]. Especially fecal incontinence is more frequent in women and elderly [4], [6].
Anorectal physiology and defecatory disorders can be assessed using specialized investigation including anorectal manometry (ARM), balloon expulsion test (BET), and defecography [4], [7], [8], [9], [10], [11]. BET is a test where a bag is distended with 50 ml followed by attempts to expel the bag [10], [11]. Physiological evacuation phenomena such as the opening characteristics of the anal sphincter during defecation cannot be described in detail with current technology. For example, defecography does not measure anorectal pressures, BET does not assess geometry, and ARM is not done during defecation. Furthermore, considerable disagreement exists between the results of various anorectal tests and they correlate poorly with symptoms and treatment outcomes [4], [12], [13]. The need for physiologically-relevant and easy-to-use diagnostic tests for identifying underlying mechanisms is substantial.
A paradigm shift is needed in anorectal functional testing to provide a better mechanistic understanding of defecation in health and disease. Anorectal function has been studied for more than a century. There is general agreement that rectal volume and sensitivity, the tone of the anal sphincters and the puborectalis muscle and their ability to relax in a coordinated way are important. We developed a device termed “Fecobionics” (simulated stool) that measures pressures, orientation, bending, and shape in a single examination of simulated defecation. Hereby, it integrates the balloon expulsion test (BET) and elements of other technologies including ARM, defecography and the functional luminal imaging probe (FLIP) [7], [8], [9], [10], [11]. Fecobionics makes it possible to describe the opening characteristics during entry into the relaxing anal canal without disturbing the defecation process. Technological validation [14] and pilot human studies on normal subjects and presumed normal subjects with abnormal phenotypes [15], [16], [17] have been published, along with modeling studies [18], [19]. One of the findings was that the pressure signatures distinguished five distinct defecatory phases [17]. A distinct feature of Fecobionics is that two of the pressure sensors are pointing in axial direction; i.e., in the direction of the trajectory. A plot of the front pressure as function of the rear pressure, a proxy of preload force and afterload resistance (analogous to the heart), provides graphically useful data representation [17].
The preload-afterload concept is modeled from cardiology, where left ventricle pressure-volume measurements provide substantial insights into heart contractility, preload (heart filling) and afterload (vascular resistance). This type of analysis may be beneficial for understanding anorectal function. In cardiac physiology, preload is the amount of sarcomere stretch at the end of ventricular filling during diastole [20]. Sarcomere length can be approximated by the volume of the ventricle. An alternative to estimating the end-diastolic volume of the heart is to measure the end-diastolic pressure. Afterload is the pressure that the heart must work against to eject blood during systole. As aortic pressure increase, the afterload increases on the left ventricle. Afterload changes to adapt to the continually changing demands on the cardiovascular system. Afterload is proportional to mean systolic blood pressure.
The preload-afterload concept from cardiology translates to Fecobionics studies in gastroenterology the following way [14], [15], [16], [17], [18], [19]: the filling of the bag inside rectum until the subject feels urge corresponds to the preload. At this point, the subject initiates abdominal contractions to generate the propulsive force needed to expel the device. Afterload is the resistance that the propulsive force must work against to evacuate feces. The resistance depends on several factors including anal diameter, anal pressure, anorectal angle and friction. Fecobionics is uniquely designed to quantify these preload-afterload properties.
The objectives of this study are to present novel analysis of Fecobionics data and technological evolution of the device and system from wired to wireless and the addition of impedance measurements for bag cross-sectional area profile. The aims of this study were as follows: 1) Develop new defecation indices computed from the defecatory preload-afterload diagram based on pressure measurements, 2) Further advance the defecatory analysis using flow equations and preload-afterload diagrams based on pressure-diameter and pressure-volume measurements, and 3) Demonstrate the feasibility of the latest wireless Fecobionics (wireless device with pressure, bending and cross-sectional area measurements) with new graphical user interface. The analysis is based on data obtained in normal human subjects where normal ranges of parameters are defined for later comparison with patient studies. The analysis applies to in-depth research studies with computation of more advanced flow and mechanical expulsion parameters which may provide useful clinical endpoints and insights.
Material and methods
Fecobionics device description
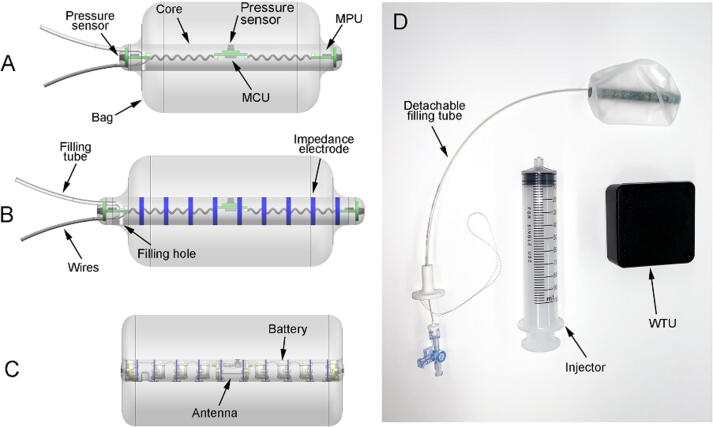
We used three versions of Fecobionics with consecutive optimization and added features. The wired device without CSA measurements has been detailed in several papers [14], [15], [16]. In brief, it was 12-mm OD, 10-cm-long and made of soft medical grade Silicone rubber to give it consistency like feces in normal persons (Fig. 1A). It contained the pressure sensors, 6-axis Motion Processing Units (MPUs) and circuit boards including the Microprogrammed Control Unit. The power source was external and data transmission was through wires exteriorizing from the front. The 2 mm OD filling tube could not be detached. A 30 μm-thick and 8 cm-long polyester-urethane bag spanned most of the core length and contained up to 80 ml without being stretched. The graphical user interface was relatively simple and used for real-time collection, computation, and display of pressure and bending angle data on the graphical user interface. Further processing was done off-line in MATLAB.
Fig. 1.
Sketches of the three Fecobionics probes used in the studies. A: First prototype was wired and contained pressure sensors and 6-axis motion processing units (MPUs). The core of the probe was 10 cm long and 12 mm diameter. B: Second prototype had impedance electrodes added to the surface of the core for cross-sectional area measurements. It was wired and the core of the probe was 12 cm long and 12.5 mm diameter. The data were collected in two programs. C: Latest stage probe is wireless and has upgraded circuitry and sensors including 9-axis MPUs. The core of the probe is 10 cm long and 10 mm diameter. The data were collected in a single program with a novel graphical user interface D: Photo of the latest stage probe. The device transmits wirelessly to the WTU (black box). The syringe is used for filling the bag and the tube can be detached after filling, making the device completely untethered.
The second stage prototype added electrodes and circuits for impedance planimetry; i.e., made simultaneous geometric profiling possible. The design of the prototype is illustrated in Fig. 1B. Due to the added electronic circuitry, the dimensions were larger (with 12 cm-long and 12.5 mm-wide bendable core). The increased dimensions allowed a bigger bag that could contain up to 120 ml. Otherwise the design was the same. Data had to be acquired in two programs due to lack of integration.
The latest stage Fecobionics compensated for deficiencies in previous prototypes. The dimensions were smaller due to optimized circuitry (Fig. 1C,D). The 6-axis MPUs were replaced with 9-axis MPUs (added 3D-magnetometer) which improves the orientation and bending angle computations. Furthermore, all electronics were embedded including batteries and wireless transmitter to make the device capable of wireless data transmission. Finally, the filling tube was detachable by a novel release mechanism. In this way, the anal canal will not be stimulated by tubes and wires after the bag is filled. Finally the graphical user interface was greatly improved, showing calibrated data as well as computed parameters.
Subjects and experiments
A total of 30 normal subjects were included. For the first aim of this paper, we reanalyzed the presumed normal subject material previously published (n = 19) [17]. Studies for the second aim with added impedance electrodes included 8 normal subjects. All procedures were the same as in the published study [17]. The third study was feasibility testing in a subject, who was studied on three occasions and two other subjects who were each studied on one occasion. In total, 20 female and 10 male subjects were included. The age of the subjects were 55 ± 4 years and none had comorbidities. All subjects scored low on fecal incontinence and constipation questionnaires.
All subjects underwent the following procedures. Interview on medical conditions, defecation habits and symptoms, and filling questionnaires. The subjects were asked to empty the rectum and bladder before the experiment. After digital exploration to insure normal anal sphincter pressure and empty lower rectum, the Fecobionics device was inserted with the subject in left lateral position. The subject moved to the commode, where the bag was filled until urge to defecate sensation. The subjects were allowed to evacuate the device in privacy after the investigators left the room. Furthermore, all subjects had ARM and BET done to ensure that they had normal anorectal function.
Ethics statement
Human experiments were done according to internationally accepted principles and adhered to the Helsinki Declaration as revised in 2000. All subjects gave written informed consent. The protocol was approved by the Joint CUHK-NT East Cluster Clinical Research Ethics Committee (ref. no. 2017.122). The trial was registered at www.clinicaltrials.gov Identifier: NCT03317938. The protocol for the third study was approved by IntegReview (ref. no. IORG0000689).
Data analysis
Advanced parameters and analyses including expulsion velocity, defecatory phases, preload-afterload, orientation, anal canal length, bending angle, tension and friction force have been described [2,15,16.18,19]. Briefly, defecation velocity was determined as the time from expulsion of the front to expulsion of the rear knowing the length of Fecobionics. The anal canal length was determined from the impedance planimetric data; i.e., the length of the narrow zone from impedance tracings and the CSA color topography. The preload-afterload diagram is a new method to express Fecobionics data. Preload-afterload diagrams have significant functional value in cardiology [2], [20] but were suggested as useful in modified forms for the GI tract [2]. In the first study, the front pressure was plotted as function of rear pressure as a proxy of preload-afterload conditions [16], [17]. To make the preload-afterload diagrams quantifiable, we developed several Defecation Indices (DIs) that are all based on the area under the curve. Four DIs are based on the front pressure integration, another four are based on rear pressure integration. Some of these DIs were normalized with respect to the defecation duration and urge-to-defecate volume. The rationale is that we observed (unpublished studies) that fecal incontinence is often associated with hypersensitivity (lower volumes) and short defecation duration whereas constipation is associated with longer duration and hyposensitivity (higher volumes). We used the following nomenclature (DI, front or rear (F or R), normalized for duration per second) and multiplied by volume (vol). For example, DI-F/volumenormalized duration means that the distensibility index is computed from the front pressure, divided by volume and normalized for the duration of the defecation. A ninth defecation index was defined as the ratio between the front and rear parameters. This is a measure of the relative contribution of defecatory work load versus anorectal resistance.
An alternative way of computing the preload-afterload parameters, following the huge amount of work in cardiology, were to display the pressure as function of the diameter change and the pressure as function of the volume change. The diameter was computed from the CSA assuming circular geometry. The volume was computed from the CSAs taking the electrode distance into account and using the data from the outer electrodes at each end to estimate the volume outside the electrode area.
Finally, flow resistance and dynamic viscosity for feces passing the anal canal was computed as devised by Faraq [21]. The anal canal length and diameter were derived from the Fecobionics measurements.
Statistical analysis
Shapiro-Wilk normality test was used to demonstrate if the data was normally distributed or not. Parametric data were plotted as mean ± SD or SEM. Non-parametric data are reported and plotted as median and quartiles. In the Box-Whisker plot, all data are shown as median, quartiles, range and outliers.
Results
Analysis of rear-front pressure plots from previously published data
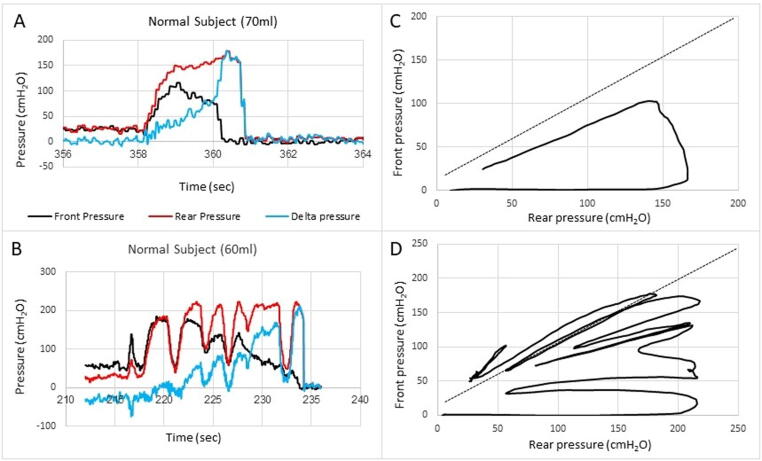
Data on healthy subjects reported in a previous publication [17] were analyzed further. The subjects were studied with the Fecobionics probe shown in Fig. 1A. The rear-front pressure plots demonstrated a typical pattern where the pressures moved along the line of pressure unity until the anal sphincter relaxed resulting in decreasing pressure at the front. Therefore, the loops were clockwise. The loop areas were small during the preparatory contractions if any such contractions occurred. The area increased when the anal sphincter relaxed. Fig. 2 shows typical phenotypes of pressure-time recordings in healthy subjects as well as the front-rear pressure loop diagram. To quantitate these diagrams, several distensibility indices (DI) were computed (Fig. 3). Data are presented in Fig. 4 as box-whisker plots of basic measures and the computed DIs for the sixteen normal subject and for the three presumed normal subjects who turned out to be abnormal in the BET (>2 min expulsion duration, which is indicative of constipation). The preload and afterload parameter with least dispersion in the normal group were the DIs based on the front pressure normalized for duration (DI-Fnormalized duration and DI-F/volumenormalized duration). However, DIs based on the rear pressure and not normalized for duration (DI-R and DI-R/volume) were the parameters that showed the most pronounced difference for the subjects with prolonged BET duration relative to the normal subjects. Clearly the abnormal subjects were different from the normal subjects and the data suggest excessive propulsive efforts in the abnormal subjects. Furthermore, the variance of the data indicates that there are other factors than the pressures at the rear and front involved in successful defecation. This constitutes a rationale for further development of the technology and analysis.
Fig. 2.
Examples of pressure recordings as function of time (A, B) and the front pressure plotted as function of the rear pressure in two normal subjects (C, D). The line of pressure unity is plotted. Defecation cannot take place if the front pressure is above the line of unity, i.e. against a positive pressure gradient. The front and rear pressures and the difference (delta pressure) between the rear and front are displayed (A, B). The defecation attempt starts with simultaneous increase in rear and front pressures. The first contraction usually follows the line of pressure unity where after the front pressure progressively decreases due to anal sphincter relaxation. In some subjects this happened in one contraction (C) whereas others use several contractions (D). Eventually the front will be outside anus and the front pressure drops to zero. The rear is expelled within 1–2 s after the front. The delta pressure is a measure of the rectoanal pressure gradient. See Fig. 3 for further explanation of the front-rear pressure plot.
Fig. 3.
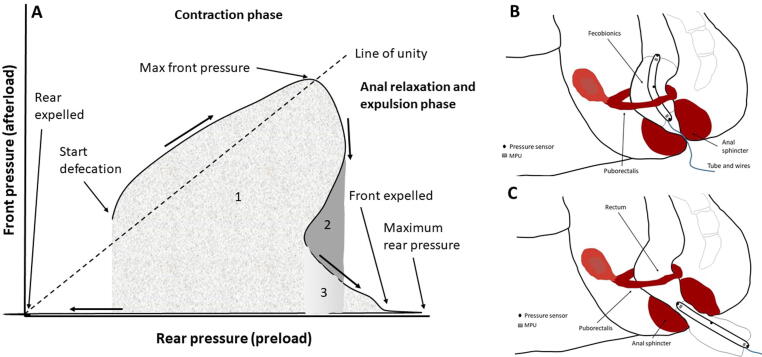
A) Graphical illustration of the front pressure as function of rear pressure (preload-afterload plot) that is used for computation of defecation indices (DIs). In this example, the front and rear pressures increase during the abdominal wall contraction initiating defecation. After reaching maximum front pressure, it declines, reflecting the anal relaxation and movement of the device front away from the high-pressure abdominal cavity into the anal canal. The rear pressure temporarily declines before the final contractions that expels Fecobionics completely. For the DIs relating to the front pressure (DI-F), the area under curve is computed, including areas that may be counted several times due to multiple contractions. The first contraction sums the areas 1, 2, and 3. The pressure decline sums area 2 and 3. The second contraction sums area 3 and 4. For the DIs based on the rear pressure (DI-R), similar areas were computed relative to the Y-axis. Some DIs were divided by the urge-to-defecate volume and some DIs were normalized with duration. B and C. The Subfigures show the Fecobionics probe and rectoanal anatomy in the pre-contraction/contraction phase (B) and in the relaxation and expulsion phase (C).
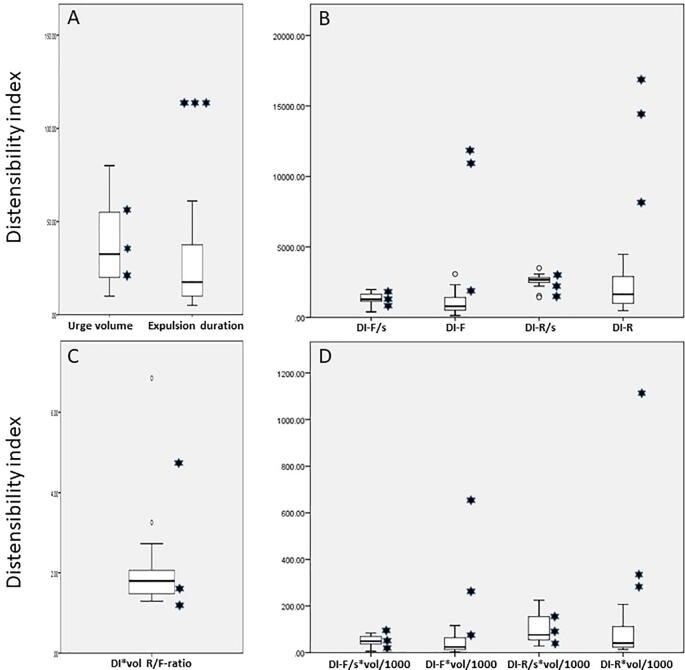
Fig. 4.
Box-Whisker plots of measured and computed Fecobionics parameters from 16 normal subjects (box and whiskers) and three presumed normal subjects with abnormal BET expulsions (•). A total of 11 parameters were tested where the two first were the urge-to-defecate volume and expulsion duration (A). Four Defecation Indices (DIs) were the integrated areas for the front and rear, respectively and normalized to expulsion duration or not (B). The lower right diagram (D) shows the same four DIs after multiplying with the urge-to-defecate volume (divided by 1000 for scaling purposes). The last DI was the ratio between the DI-R/vol and DI-F/vol, which is a proxy of the propulsive workload relative to anal resistance (C). See the main text for detailed explanation of the DIs. The box represents the interquartile range, which contains the middle 50% of the records. The line across the box indicates the median. The whiskers are lines that extend from the upper and lower edge of the box to highest and lowest values which are no greater than 1.5 times the IQ range. Outliers are cases with values between 1.5 and 3 times the IQ range; i.e., beyond the whiskers. The three subjects with BET>2 min were all abnormal on Fecobionics expulsion duration too (2 min). The urge volume is not a good parameter to differentiate patients from normal subjects. The same accounts for the defecation indices based on the DI parameters that were normalized to expulsion duration. However, the DIs not normalized to expulsion duration were quite different; i.e. generally higher for the rear sensor (indicating excessive contraction force) and with large spread indicating that they may belong to different subgroups.
Analysis of pressure-diameter and pressure-volume diagrams from wired Fecobionics device
The probe had impedance planimetry added as illustrated in Fig. 1 (middle). CSA, pressure, and bending angle data from a representative subject is shown in Fig. 5.
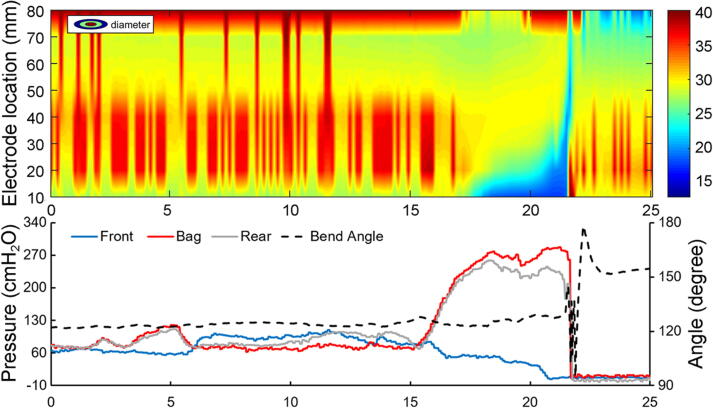
Fig. 5.
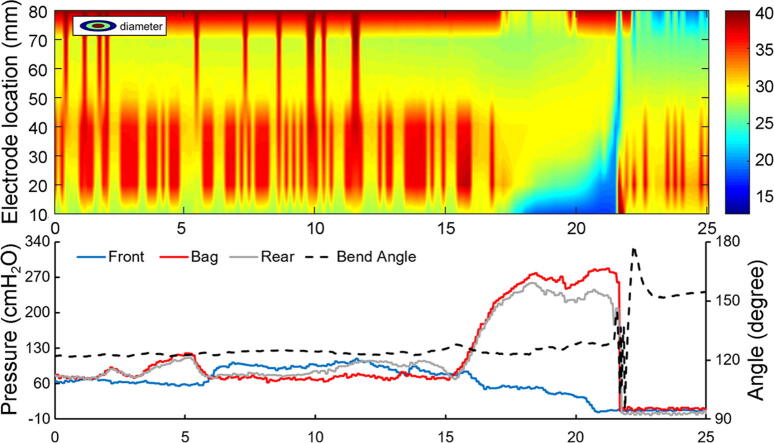
Color topography plot of diameter recordings from the multiple impedance electrodes (top). The forefront of the blue area shows the movement of the electrodes at the front into the anal canal and finally passing the anal canal. The backside of the blue area represents the rear electrodes passing the anal canal. Blue color indicates low diameter. The front, bag and rear pressures as well as the bending angle are shown in the lower diagram. The pressures are clearly associated with the changes in diameters and the bending angle is associated with the final defecation.
The derived pressure-diameter relations have different shapes which depended on which electrode set diameter data were measured from. Two examples from representative subjects are shown in Fig. 6. The derived pressure-volume curves (by integrating the diameter measurements and computing the amount of fluid in the bag still inside rectum) are also shown. The pressure-volume relations were counterclockwise with well-defined isobaric and isovolumetric phases. The maximum diameter and volume were reached when the subject felt urge. In subjects, who expelled Fecobionics with a single contraction, a well-defined loop with four distinct phases was demonstrated. If the subject used more contractions to defecate, it showed up as vertical lines in the isometric contraction phase. The median area of the loops was 8,703 (7,077 – 9,836) cmH2O*ml (n = 7).
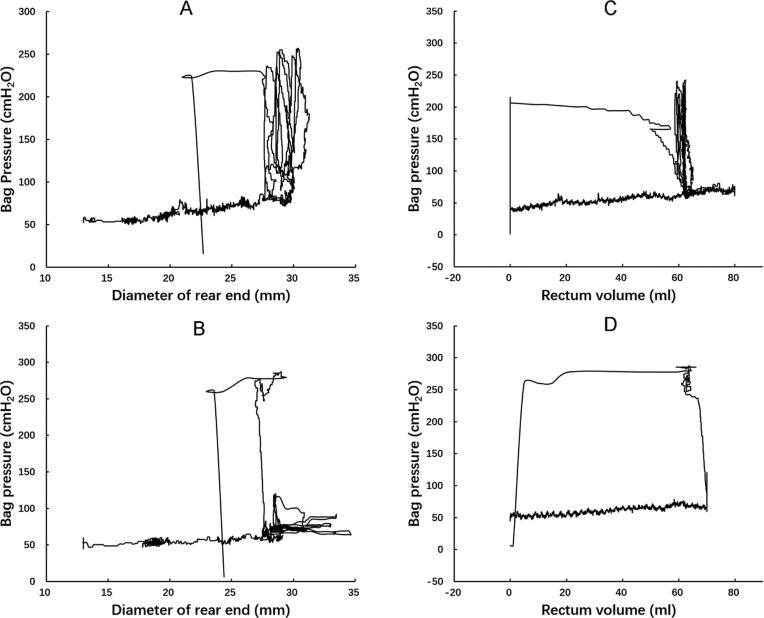
Fig. 6.
Rectal preload-afterload plots from two subjects in terms of bag pressure as function of the lowest diameter recorded by the rear electrodes (left) or as function of volume (right). The curve is counterclockwise. The first part represents filling of the bag. When urge is reached at a diameter of 30 mm, the subject initiates several contractions that are inefficient for inducing defecation. These can be considered isometric contractions though there is a slight translation to the left. The last contraction is successful, first represented by an isobaric contraction before the loop is completed when the device is finally expelled.
Analysis of anal resistance, dynamic viscosity, and friction force based on wired probe with added CSA functionality
Anal resistance and dynamic viscosity was computed on basis of Poiseuilles law flow equations provided by Faraq et al [21] and friction force was computer using previously published equations [19]. Fecobionics with the added functionality provided all variables needed for the computations. The measured median (quartiles) of filling volume, anal flow, anal canal diameter and length, and bag pressure were 75 (60–80) ml, 0.017 (0.013–0.039) L/s, 2.4 (2.3–2.5) cm, 3.2 (2.7–3.3) cm, and 12.6(10.7–13.4) kPa. The computed anal resistance and dynamic viscosity were 692.3 (316.7–1124.9) kPa L−1 s and 5.89 * 10−5 (3.73 * 10−5–17.24 * 10−5) kg m−1 s−1.
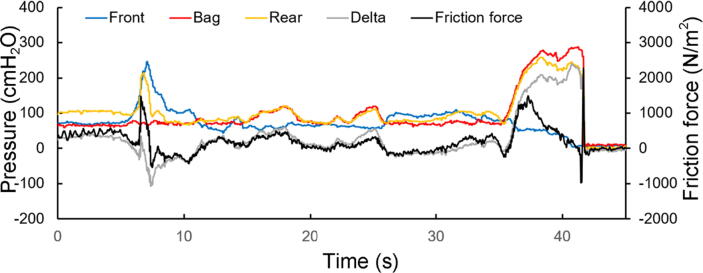
Fig. 7 shows the pressures and friction force the last 40 s before and during defecation. The pressure tracings show an anal sphincter contraction in the beginning that is followed by low-amplitude rectal contractions. The defecation starts at time 35 s and lasts 7 s. The frictional force peaks during the beginning of the contractions.
Fig. 7.
Pre-defecation and defecation tracings. The defecation attempt starts at time 35 s. The measures front, bag and rear pressures, the computed delta pressure and the friction force are shown.
Data obtained with wireless Fecobionics probe
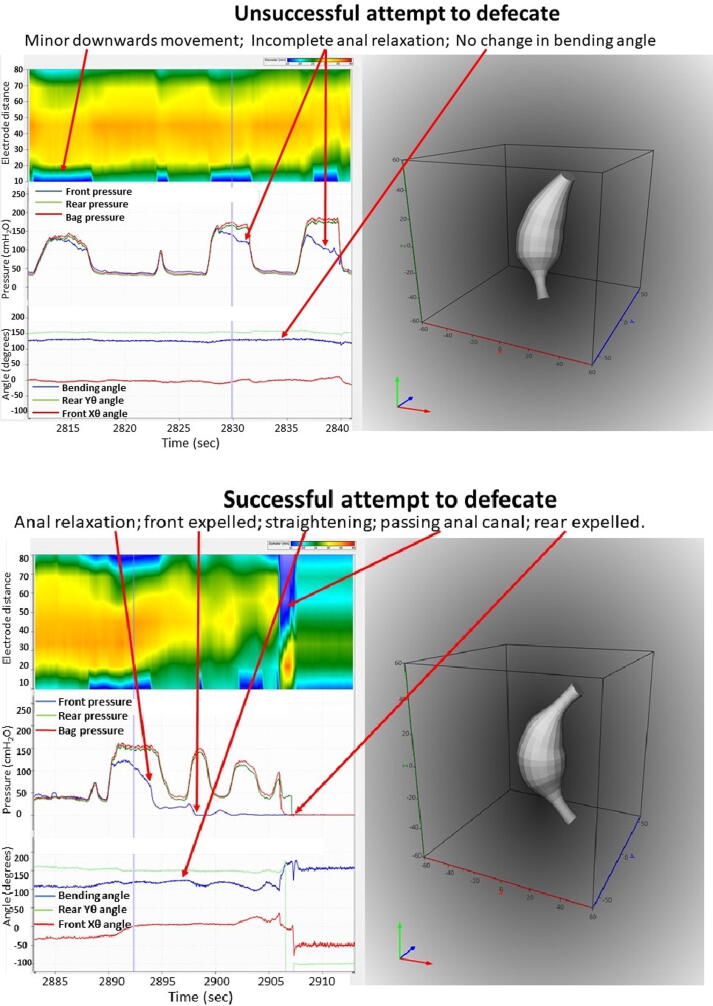
A total of five experiments were done in three subjects. A consistent defecation pattern was observed and comparable data were similar to those previously obtained as described above. The computed defecation indices were well within the limits of those presented in Fig. 4. An unsuccessful attempt to defecate and a successful defecation are shown in Fig. 8, where the GUI shows CSA topography, pressures, orientation and bending angle, and a 3D representation. The unsuccessful attempt shows less anal relaxation and minor change in bending angle compared to the successful attempt. Since the 3D plot of orientation and shape is dynamic, videos are more informative than images. A video clip of the GUI is presented in Supplementary video 1. The video shows the fine coordination between the various measures during defecation.
Fig. 8.
Screen dumps of the GUI showing an unsuccessful attempt to defecate (top) and defecation (bottom). Color contours of the CSA (blue represents low diameter and red large diameter), pressures and bending angle. The diagrams to the right show 3D representation of the shape, orientation and bending (see also the Supplementary video).
Discussion and perspectives
In these human studies, we quantified the “preload-afterload” diagrams in terms of several defecation indices. Further analysis of previously obtained data [17] show that some of the DIs reveal distinct differences between normal and subjects with prolonged BET time and FI [36]. It is possible to display CSA topographies and compute key parameters such as friction force and resistance. Preload-afterload diagrams based on pressure-diameter and pressure-volume relations are generated and resemble those known in heart physiology. With the new graphical user interface, all relevant data for anorectal continence function and defecation (rectal sensitivity, propulsive force, anal relaxation and resistance, friction and anorectal angle) can be displayed and video clips generated (see Supplementary data). This paper also describes developments and advances using the novel Fecobionics. The technology has been developed in three progressive steps with the first device being wired, with fixed filling tube, and with pressure sensors and 6-axis MPUs. The next generation device added impedance planimetry for CSA or diameter measurements. However, it was fairly large, still wired, and needed data to be collected by a single software program. The latest stage device addressed these technological deficiencies since it contains batteries internal to the device probe, use wireless transmission to a data receiver, the filling tube is detachable, MPUs are 9-axis, and data are collected and displayed in a novel graphical user interface.
Design considerations
Design considerations for the electronic circuits can be found in a previous publication [14]. As mentioned above, the known factors affecting defection are rectal sensation (volume), abdominal-rectal propulsive force, anal continence (resting pressure, squeeze pressure, diameter, relaxation, and resistance), the anorectal angle, and consistency of feces [2], [3], [21]. Fecobionics was designed to measure anal pressure and relaxation with the front pressure sensor. The rear pressure sensor measures the propulsive force. It is crucial that the front and rear pressure sensors in Fecobionics point in the trajectory of fecal expulsion since flow in tubes depends on pressure gradients in flow direction. The bag pressure is used in computation of parameters such as tension, friction force, anal resistance and dynamic viscosity. The MPUs provide data on orientation in 3D space and the bending of the device that reflects the anorectal angle. Impedance planimetry allows quantification of the shape and detailed color topographies of shape changes. The shape and consistency of Fecobionics (core and bag) was designed as normal stool. With the architecture, the selected silicone hardness and the bag, Fecobionics obtained consistency that corresponds approximately to type 4 (range 3–4) on the Bristol stool form scale [22]. The range from types 3–4 is found in +60% of healthy subjects [22]. Hence, we believe that all known factors are quantifiable with Fecobionics and can be obtained in a single examination. It integrates measurements that currently are obtained with BET, HRAM, defecography and EndoFLIP. This does not mean that Fecobionics should replace those technologies as this stage; i.e., defecography provides detailed information on pathologies including rectocele and malformations. Furthermore, previous studies have demonstrated that simple measures including the expulsion duration are comparable between technologies whereas many others differ. Although such differences can be explained by differences in device geometry and location of measurement, this suggests that normal ranges must be defined for Fecobionics from larger scale studies.
Future clinical studies may require further design diversity. Fecobionics with pressure sensors only may be used in general practice clinics and tertiary hospitals for screening. If further examination is required, the patient can be referred to a specialized clinic. Furthermore, it is known that some types of constipation are associated with hard stools. Since such patients may be able to defecate the current Fecobionics, devices may be developed with different stiffness. This can be accomplished by using a different resin to construct the core or using a gel-like fluid in the bag with higher viscosity. Design changes of the graphical user interface may also be required for an intended future use as biofeedback therapy at the point-of-care in the home of patients. The graphical user interface can inform the patients about correctly or incorrectly performed therapeutic maneuvers and ultimately health care personnel can be connected in real-time to instruct the patient remotely.
Preload-afterload considerations and distensibility indices
Fecobionics pressure data are displayed as preload-afterload data. The system allows evaluation of pressure cycles without the time element where rectum or abdominal muscle contractions generate the preload and the afterload reflects anal resistance. The preload must exceed the afterload before evacuation can take place since feces movement cannot occur against an anorectal pressure gradient. Fecobionics (and feces) will be expelled when the recto-anal pressure gradient is large enough to overcome the friction between the surface and mucosa. Measurement of axial pressures at front and rear, and the bag pressure is essential in this regard. The front-rear pressure diagram is a very informative way to illustrate Fecobionics pressure data. The gradual downwards bending of the curves are due to progressive anal relaxation and movement of the device in consecutive pressure cycles (Fig. 2). Since such diagrams must be quantified, nine DIs were proposed. Four of these relate to the front pressure and another four to the rear pressure. Some indices were normalized for time, and others multiplied with the distending volume. The ninth DI was the ratio between parameters for the front and rear. Clearly, all indices may not be necessary and the developed DIs may need refinement in future studies. The abnormal subjects, who could not defecate BET and Fecobionics, showed abnormal values, especially for the DIs that were not normalized for time and for the rear pressure. Our hypothesis based on the development of the DIs is that different DIs will be necessary for diverse disorders such as fecal incontinence and constipation. Future clinical studies will provide insight into which parameters are most useful for diagnostics of various types of defecation disorders. The afterload seems especially important since obstructed (dyssynergic) defecation [23], [24], [25] and anal stricture will be associated with increased afterload. On the contrary, fecal incontinence due to anal sphincter damage or impairment [4], [26] will be associated with decreased afterload. The analogy from the cardiovascular system is that increased afterload is associated with increased vascular resistance, hypertension, and aortic stenosis [20]. The preload and afterload may be important for differentiating subtypes of patients. For example, the current dyssynergia classification [23], [24], [25] operates with a 2x2 diagram where two subtypes show abnormal expulsion pressures and two subtypes are associated with anal sphincter function. The classification is being criticized for being too simple and dyssynergic abnormality is found in 90% of healthy subjects [27]. Due to increased afterload, the rectum (or abdominal muscles) must work harder to accomplish defecation. Long-term, this may lead to dyscoordination, hypertrophy, and altered rectal sensitivity [2]. Increased feces volume and deferred defecation may be associated with increased preload and afterload.
We extended the preload-afterload diagrams further with probes that implemented impedance planimetry. Pressure-diameter and pressure-volume diagrams resembled those known from the heart with isovolumetric and isobaric phases. These more advanced diagrams may prove more useful in research studies of anorectal physiology and for modeling anorectal behavior. The simpler pressure-based diagram, however, is likely more useful clinically.
Towards a more complete model of defecatory function
A better understanding of anorectal function is important for understanding of defecatory disorders. The biggest diagnostic and therapeutic challenge is dyssynergic defecation which is believed to be the result of pelvic floor dysfunction [4], [23], [24], [25], [27]. Improved integrated diagnostics may aid individualized treatment of subtyped patients and define those who may benefit from biofeedback training [4]. As mentioned above, continence and normal defecation function depend on a variety of parameter that, too a large extend, is measured by Fecobionics in a single examination. Fecobionics was developed in an attempt to integrate current tests and to provide a new bionics concept that will allow more physiological recordings under the same conditions. Current tests have been criticized for not reflecting defecatory physiology, for example BET, ARM, defecography, and dynamic pelvic MRI are indirect surrogates for the act of defecation, and provide incomplete and often conflicting information [12]. The problem with most tests is that they do not provide detailed physiological data during defecation, reflecting the dynamics of the defecation process.
Modeling of anorectal function is still in its infancy. Models have been proposed [3], [21], [28] but are currently based on flow equations that are too simple to describe defecatory function. In this study, we used the Poisseuille’s law model proposed by Faraq [21]. Using this model, however, it is assumed that the dynamic viscosity is fairly constant between subjects since it relates to properties of the device. On the contrary, we found that this parameter was highly variable, which indicates that other factors are involved. From a bioengineering perspective, modeling efforts should first be focused on construction of anatomically correct models, then mechano-physiological models, and finally mechanosensory models can be advanced [2], [29], [30], [31], [32], [33], [34], [35]. Such models are important for our understanding of organ function and the wide variability encountered in physiology. Fecobionics has several advantages to current technology; i.e., the simulated feces integrate a variety of measurements in a single test with less variability and fewer false positive dyssynergia phenotypes [14], [15], [17]. With the latest integrated version of Fecobionics technology, such work can start now. A recent paper showed pronounced differences in distensibility indices between fecal incontinence patients and normal subjects [36] as well as the wireless technology was recently applied to colon studies [37].
Conclusions and future aspects
We demonstrated successful development of Fecobionics with testing in normal human subjects. Fecobionics made it possible to evaluate conventional measures and novel defecation indices. Preload-after load analysis with computation of distensibility indices is not possible with any other available technology. Fecobionics provides several improvements to current anorectal functional assessment technologies, including mechanical properties that mimic stool and pressure measurements in the direction of the trajectory. Fecobionics has significant potential to shift the current paradigm since it is a simulated stool that provide novel endpoints not simultaneously assessable with current technologies. Although we have demonstrated that the device is safe and useful for assessment of anorectal physiology and evacuatory efficacy, normal ranges for the biomarker parameters have to be determined in larger scale studies
The potential translational outcome of future studies is a bionics platform for anorectal functional studies based on simulated defecations. The present study establishes the foundation for future use of Fecobionics for dyssynergia diagnostics and as a biofeedback tool, where patients based on the functional signatures visualized on the graphical user interface can learn to control and train the muscles to correct the neuromuscular dysfunction.
Financial support
Research reported in this publication was supported by the Office Of The Director, National Institutes Of Health under Award Number OT2OD025308, OT2ODO28203 and 1R44DK129097-01 (specifically the development of the wireless Fecobionics) and Hong Kong Research Council Grant #14106717.
Compliance with ethics requirements
The protocol was approved by the Joint CUHK-NT East Cluster Clinical Research Ethics Committee (ref. no. 2017.122). The trial was registered at www.clinicaltrials.gov Identifier: NCT03317938. The third study was feasibility testing in a 57-year-old male, who was studied on three occasions and two other healthy subjects. The protocol was approved by IntegReview (ref. no. IORG0000689).
CRediT authorship contribution statement
H. Gregersen: Conceptualization, Methodology, Validation, Writing - original draft, Supervision, Funding acquisition. D. Sun: Methodology, Formal analysis, Software, Writing - review & editing. S.C. Chen: Investigation, Formal analysis. W.W. Leung: Investigation, Resources. C. Wong: Investigation, Resources. T. Mak: Resources, Writing - review & editing, Supervision. S. Ng: Resources, Supervision, Writing - review & editing. K. Futaba: Investigation, Resources, Writing - review & editing. Kar Man Lo: Formal analysis, Project administration, Writing - review & editing. G.S. Kassab: Conceptualization, Validation, Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.05.005.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Barleben A., Mills S. Anorectal anatomy and physiology. Surg Clinics. 2010;90:1–15. doi: 10.1016/j.suc.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Gregersen H, Christensen J. Clinical Biomechanics in the Gut. An Introduction. Bentham Science Publishers 2016. Sharjah, United Arab Emirates. ISBN 978-1-68108-119-9 eISBN 978-1-68108-118-2.
- 3.Gibbons C.P. The mechanics of the anal sphincter complex. J Biomech. 1988;21:601–604. doi: 10.1016/0021-9290(88)90223-0. [DOI] [PubMed] [Google Scholar]
- 4.Rao S.S., Bharucha A.E., Chiarioni G., Felt-Bersma R., Knowles C., Malcolm A., et al. Functional Anorectal Disorders. Gastroenterology. 2016;150:1430–1442. doi: 10.1053/j.gastro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 6.Peery A.F., Crockett S.D., Murphy C.C., Lund J.L., Dellon E.S., Williams J.L., et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156(1):254–72 e11. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tirumanisett P., Prichard D., Fletcher J.G., Chakraborty S., Zinsmeister A.R., Bharucha A.E. Normal values of assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterol Motility. 2018;30 doi: 10.1111/nmo.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharucha A.E. Update on tests of colon and rectal structure and function. J Clin Gastroenterol. 2006;40:96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 9.Van Koughnett J.A.M., da Silva G. Anorectal physiology and testing Gastroenterol. Clin N Am. 2013;42:713–728. doi: 10.1016/j.gtc.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Chiarioni G., Kim S.M., Vantini I., Whitehead W.E. Validation of the balloon evacuation test: Reproducibility and agreement with findings from anorectal manometry and electromyography. Clin Gastroenterol Hepatol. 2014;12:2049–2054. doi: 10.1016/j.cgh.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Carrington EV, Heinrich H, Knowles CH, et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil 2019:e13679. doi: 10.1111/nmo.13679. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 12.Palit S., Thin N., Knowles C.P., Lunniss P.J., Bharucha A.E., Scott S.M. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterol Motility. 2016;28:1589–1598. doi: 10.1111/nmo.12859. [DOI] [PubMed] [Google Scholar]
- 13.Coss-Adame E., Rao S.S.C., Valestin J., Ali-Azamar A., Remes-Troche J.M. Accuracy and reproducibility of high-definition anorectal manometry and pressure topography analyses in healthy subjects. Clin Gastroenterol Hepatol. 2015;13:1143–1150. doi: 10.1016/j.cgh.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun D., Huang Z., Zhuang Z., Ma Z., Lo K.M., Liao D., et al. Fecobionics: A novel bionics device for studying defecation. Ann Biomed Eng. 2019;47:576–589. doi: 10.1007/s10439-018-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregersen H., Krogh K., Liao D. Fecobionics: Integrating anorectal function measurements. Clin Gastroenterol Hepatol. 2018;16:981–983. doi: 10.1016/j.cgh.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 16.Gregersen H. Fecobionics: a novel bionic test of anorectal function and defecation. Gastroenterology. 2017;152(Suppl 1):S317. doi: 10.1016/S0016-5085(17)31338-0. [DOI] [Google Scholar]
- 17.Gregersen H., Chen S.C., Leung W.W., Wong C., Mak T., Ng S., et al. Novel Fecobionics Defecatory Function Testing. Clin Transl Gastroenterol. 2019;10(12) doi: 10.14309/ctg.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao D., Chen A.S., Lo K.M., Zhao J., Futaba K., Gregersen H. Theoretical tools to analyze anorectal mechanophysiological data generated by the Fecobionics device. J Biomech Eng. 2019 doi: 10.1115/1.4044134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D, Liao D, Chen SS, Wong C, Leung WW, Futaba K, et al. Mechanophysiological analysis of anorectal function using simulated feces in human subjects. J Adv Res 2020. https://doi.org/10.1016/j.jare.2020.07.002 [in press]. [DOI] [PMC free article] [PubMed]
- 20.Takeuchi M., Odake M., Takeoka H., Hayashi Y., Hata K., Yokoyama M. Comparison between preload recruitable stroke work and he end-systolic pressure volume relationship in man. Eur Heart J. 2003;13:80–84. doi: 10.1093/eurheartj/13.suppl_e.80. [DOI] [PubMed] [Google Scholar]
- 21.Faraq A. The use of flow equation in functional coloproctology: a new theory in anorectal physiology. Pelviperineology. 2009;28:17–23. [Google Scholar]
- 22.Heaton K.W., Radvan J., Cripps H., Mountford R.A., Braddon F.E., Hughes A.O. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao S.S.C., Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J Neurogastroenterol Motility. 2016;22:423–435. doi: 10.5056/jnm16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao S.S., Tuteja A.K., Vellema T., Kempf J., Stessman M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2014;38:680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich H., Sauter M., Fox M., Weishaupt D., Halama M., Misselwitz B., et al. Assessment of obstructive defecation by high-resolution anorectal manometry compared with magnetic resonance defecography. Clin Gastroenterol Hepatol. 2015;13(1310–7) doi: 10.1016/j.cgh.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead W.E., Borrud L., Goode P.S., Meikle S., Mueller E.R., Tuteja A., et al. Fecal incontinence in US adults: epidemiology and risk factors”. Gastroenterology. 2019;137(512–517) doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossi U., Carrington E.V., Bharucha A.E., Horrocks E.J., Scott S.M., Knowles C.H. Diagnostic accuracy study of anorectal manometry for diagnosis of dysynergic defecation. Gut. 2016;65:447–455. doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bush M, Petros P, Swash M, Fernandez M, Gunnemann AA. Defecation 2: Internal anorectal resistance is a critical factor in defecatory disorders. Tech Coloproctol 2012;16:445–50. [DOI] [PubMed]
- 29.Gregersen H. Biomechanics of the Gastrointestinal Tract. London: Springer-Verlag; 2002. ISBN 1852335203.
- 30.Casares-Magaz O., Thor M., Liao D., Frøkjær J.B., Kraemer P., Krogh K., et al. An image-based method to quantify biomechanical properties of the rectum in radiotherapy of prostate cancer. Acta Oncol. 2015;54:1335–1342. doi: 10.3109/0284186X.2015.1066933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frøkjær J.B., Liao D., Bergmann A., McMahon B.P., Steffensen E., Drewes A.M., et al. Three-dimensional biomechanical properties of the human rectum evaluated with magnetic resonance imaging. Neurogastroenterol Motil. 2005;17:531–540. doi: 10.1111/j.1365-2982.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 32.Liao D, Frøkjær JB, Yang J, Zhao J, Drewes AM, Gilja OH, et al. Three-dimensional surface model analysis in the gastrointestinal tract. World J Gastroenterol. 2006;12:2870–5. doi: 10.3748/wjg.v12.i18.2870. [DOI] [PMC free article] [PubMed]
- 33.Liao D., Zhao J., Gregersen H. Gastrointestinal tract modelling in health and disease. World J Gastroenterol. 2009;15:169–176. doi: 10.3748/wjg.15.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.X., Li W., Deng H.Y., Li K., Zhou Z.R. Friction behavior of esophageal mucosa under axial and circumferential extension. Tribol Lett. 2019;67:9. [Google Scholar]
- 35.Gregersen H., Kassab G.S., Fung Y.C. Determination of membrane tension during balloon distension of intestine. Mech Chem Biosyst. 2004;1:191–199. [PubMed] [Google Scholar]
- 36.Gregersen H, Chen SC, Leung WW, Wong C, Mak T, Ng S, et al. Fecobionics Characterization of Patients with Fecal Incontinence. Clin Gastroenterol Hepatol 2020:S1542-3565(20)31501-9. doi: 10.1016/j.cgh.2020.10.043. [DOI] [PubMed]
- 37.Gregersen H., Wang Y., Guo X., Field F., Nelson M., Combs W., et al. Simulated colonic feces reveals novel contraction patterns. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.09.055. S0016-5085(20)35392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.