Graphical abstract
Keywords: Cancer-induced bone pain, Central sensitization, Neuroinflammation, Acidic environment, TRPV, P2X Family
Highlights
-
•
The development of scientific research in CIBP was elaborated in a complete and clear perspective.
-
•
The possible mechanisms, including the new achievements in central sensitization, glial cell activation, acid environment, of CIBP were reviewed.
-
•
Several key molecules and treatments as well as the potential targets and promising therapies were also briefly reviewed.
-
•
The challenges we faced and the future directions are also summarized and elaborated.
Abstract
Background
Cancer-induced Bone Pain (CIBP) is an important factor affecting their quality of life of cancer survivors. In addition, current clinical practice and scientific research suggest that neuropathic pain is a representative component of CIBP. However, given the variability of cancer conditions and the complexity of neuropathic pain, related mechanisms have been continuously supplemented but have not been perfected.
Aim of Review
Therefore, the current review highlights the latest progress in basic research on the field and proposes potential therapeutic targets, representative drugs and upcoming therapies.
Key Scientific Concepts of Review
Notably, factors such as central sensitization, neuroinflammation, glial cell activation and an acidic environment are considered to be related to neuropathic pain in CIBP. Nonetheless, further research is needed to ascertain the mechanism of CIBP in order to develop highly effective drugs. Moreover, more attention needs to be paid to the care of patients with advanced cancer.
Introduction
Cancer-induced Bone Pain (CIBP) refers to the pain caused by bone metastases or severe and common pain caused by primary bone tumors. It is a complex state of pain that is usually persistent, sudden, spontaneous and often accompanied by hyperalgesia. In addition, it is not only the biggest source of pain in patients with advanced cancer but also the most important reason for reduced quality of life as well as self-confidence in cancer survivors.
Although different rates of CIBP are reported around the world, it is generally believed to occur in one-third of patients. However, given the current advances in cancer treatment, the prevalence of CIBP among cancer survivors may be greater than the reported numbers. This therefore suggests that the urgent problem of cancer-related pain should be the focus of more studies.
In addition, CIBP may run through the entire cancer diagnosis and treatment process and is therefore not only related to the disease itself but also closely associated with the treatment. A prospective study on patients with bone metastases and receiving radiation therapy showed that 25.8% of them had significant neuropathic pain [1]. Moreover, Chemotherapy Induced Peripheral Neuropathy (CIPN) has become important in the study of CIBP although it is not the main subject of the current review. Nonetheless, further details and related progress on CIPN have been highlighted [2].
The mechanisms underlying CIBP are largely unclear although a few have been proposed. Such include elements commonly associated with neuropathic, traumatic and inflammatory pain. Among these, neuropathic pain is the most important and serious cause. In 2008, the Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP) defined neuropathic pain as that caused by a lesion or disease of the somatosensory system. Under the combined effects of various factors, the tissues and nerves around the area of pain often undergo unique modifications and neurochemical changes at the spinal cord level. These changes often occur in more than one site.
Furthermore, an effective grading system for neuropathic pain was established in 2008 [3] and has been continuously updated ever since [4]. However, diagnosis of neuropathic pain mainly relies on a detailed medical history (including the cause of disease, location and the nature of pain as well as the factors that induce and reduce it), a comprehensive and detailed physical examination (especially examination of the sensory system) and the necessary auxiliary examination which is sometimes also based on the patients’ response to treatment. There are also guidelines that effective pain management requires algorithmic screening of patients as well as reliable and repeatable assessments of suspected patients using comprehensive and effective tools to provide tailored treatments.
Among the numerous auxiliary inspection and evaluation methods, a careful examination of the nervous system should include a detailed investigation of sensory, motor and autonomic functions. The evaluation of sensory nerve function is important, and quantitative analysis is recommended as an optimal choice. In addition, the painful and abnormal sensory areas in neuropathic pain should conform to the anatomical distribution of somatosensory nerves, consistent with the identified lesions. Necessary auxiliary examinations, including neuroelectrophysiological examination, Positron Emission Tomography (PET) and Functional Magnetic Resonance (fMRI) can reveal the mechanisms of neuropathic pain [5], [6]. Notably, in the presence of complex diseases or when neuropathic and nociceptive pain coexist (for example, bone metastases caused by cancer) the bias caused by grading system dependence on clinical experience and physical examination can be avoided.
Therefore, the importance of CIBP-related scientific research is increasingly evident with improvements in clinical diagnosis and treatment of the condition. Furthermore, it is crucial to elucidate the pathogenesis, preventive measures and comprehensive treatment plans against CIBP in cancer patients.
Given this background, the current review aimed to;
-
1.
Summarize the latest progress in basic research on CIBP.
-
2.
Describe and explain the mechanism of CIBP at the cellular and molecular levels.
-
3.
Identify potential therapeutic targets and possible drugs for future use.
Bone resorption and formation
Bone homeostasis
CIBP is related to imbalances in the bone microenvironment. Bone homeostasis is maintained by the synergistic actions of bone-resorbing osteoclasts and bone-forming osteoblasts [7]. Osteoclasts are for the key regulators of bone resorption which modulate bone development, bone growth, repair and reconstruction. They are often abnormally activated in bone-destructive diseases such as osteoporosis, rheumatoid arthritis, bone tumors, and bone metastases. The main markers of osteoclasts are tartrate resistant acid phosphatase and cathepsin K. RANKL is considered the most important contributor to the pathological process of bone resorption. In addition, MCP-1 [8], macrophage-inducible C-type lectin (Mincle)[9], MADS box transcription enhancer factor 2, polypeptide C (MEF2C),[10] have also been implicated in the regulation of bone microenvironment. When bone tumors or bone metastases occur, local tumor-related molecules interact with resident cells in the bone marrow, causing abnormal differentiation of osteoclasts and osteoblasts (especially abnormalities in the activity of osteoclasts). This in turn leads to increased bone absorption. In addition, calcium ions and growth factors released during bone resorption are fed back to tumor cells and promote their growth [11].
Different type of CIBP
Generally, osteogenic bone tumors are characterized by pain emanating from sores and swelling. For example, osteosarcoma often damages the bone cortex due to the expanded tumor tissue, stimulating the nerve endings of the periosteum and causing severe pain. This type of pain can gradually progress from intermittent model in the early stage to continuous model after several weeks, and the degree of pain increases accordingly. In addition, pain-avoidance claudication may accompany lower limb pain. It has been reported that the severe pain osteolytic type is mainly generated by the tumor itself, i.e., pain in the nerve endings of the bone tissue caused by the invading tumor. For example, Ewing's sarcoma is characterized by extensive osteolytic destruction, generating worm-eaten-like structural changes in medical images. Ewing sarcoma in the sacrum is more painful and may be accompanied by neurological symptoms. Furthermore, pain associated with bone metastasis is caused by osteolytic lesions or osteolytic invasion, especially in patients with lung cancer or breast cancer bone metastasis. Although the degree of pain may be vary, the nature of the pain is similar and is often worse at night.
Other mechanism
Moreover, it was confirmed that the major sensory afferent and sympathetic neurons are densely distributed in the periosteum and bone marrow. Therefore, in the case of bone resorption, large amounts of inflammatory factors are secreted hence stimulating the sensitized nerve fibers, resulting to intolerable pain.
In addition to imbalances in the bone microenvironment, there exists a series of local acidic surroundings, inflammatory factors and destruction of the anatomical structure of tissues or nerves that promote and affect each other. Moreover, this is exacerbated by bone destruction and in turn also accelerates bone destruction. Therefore, a “vicious circle” which affects the normal function of the nervous system is formed between the primary tumor, osteoclasts and growth factors. Consequently, methods of effectively interrupting the cycle and stabilizing the bone microenvironment to maintain the normal function of the body should be explored.
Furthermore, based on this theory, anti-bone resorption drugs (such as bisphosphonates and denosumab as well as anti-RANKL humanized neutralizing antibodies) will significantly reduce bone complications and reduce bone pain.
Peripheral sensitization & central sensitization
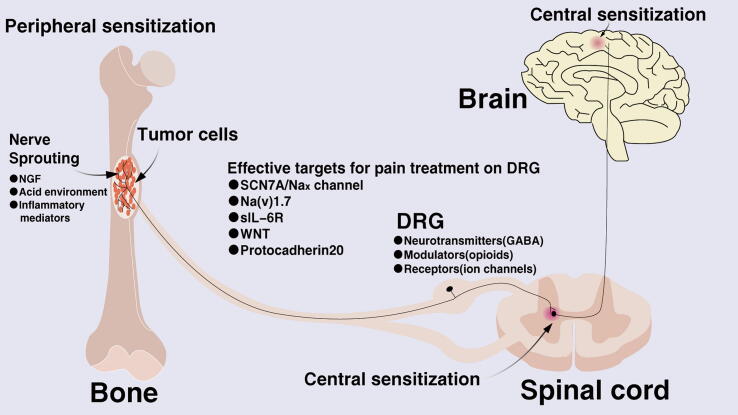
The pathogenesis of neuropathic pain is complex as it is often a combination of anatomical changes and impaired function. The possible pathological changes include; nerve damage, neuroinflammation, abnormalities in peripheral nerve excitability, anomalies in the sympathetic nervous system and changes in neuroplasticity. Additionally, current research shows that the specific pathological mechanisms include peripheral sensitization, central sensitization, abnormalities in the descending inhibitory system, activation of spinal glial cells and changes in ion channels. However, peripheral and central sensitization are the principal mechanisms of pathogenesis in neuropathic pain (Fig. 1).
Fig. 1.
Schematic diagram showing the mechanism of cancer-induced bone pain. The cell body of the primary afferent neurons innervating the body is in the dorsal root ganglia and it transports sensory information from the periphery to the spinal cord and brain. Unmyelinated C fibers are involved in the detection of a variety of harmful stimuli. When bone tumors occur, or when bone destruction occurs, sensory neurons will behave as sprout and produce toxic irritants. Besides, DRG activation, microglia activation, acidic environment production, inflammatory factor expression, ion channel changes and many other factors participate in the generation and maintenance of cancer pain.
Peripheral sensitization refers to increased sensitivity of nociceptive neurons to incoming signals. After peripheral nerve injury, damaged as well as inflammatory cells (such as mast cells and lymphocytes) release chemicals, such as norepinephrine, bradykinin, histamine, prostaglandins, potassium ions, cytokines, serotonin and neuropeptides. These cellular mediators can in turn sensitize nociceptors and amplify their incoming nerve signals.
Central sensitization is a state of hypersensitivity to pain or itch, defined as excessive somatosensory and increased excitability or enhanced synaptic transmission of neurons in the dorsal horn of the spinal cord after intense stimulation, severe tissue damage, or continuous noxious input (for example, C-fiber activation) [12], [13] Specifically, this includes increased spontaneous discharge activity of neurons, enlarged receptive fields, a lowered threshold for external stimulation and increased pathological changes, thereby amplifying the transmission of pain signals. It is worth noting that CIBP often manifests as persistent chronic pain and recent studies support the relationship between chronic pain and the cingulate cortex, prefrontal cortex and ventral striatum [6]. In addition, the main feature of chronic pain is hyperalgesia and maintenance of neuropathic pain mainly depends on central sensitization. Therefore, central and peripheral sensitization both exhibit unique characteristics and play different roles in the various periods and mechanisms of CIBP.
DRG activation
The sensory neuroblasts in the spinal ganglion derived from the neural crest generate bundle-shaped axons, which enter from the back of the neural tube symmetrically. These axons receive all nerve impulses from body receptors, including general somatosensory and visceral sensation. The spinal ganglion is called Dorsal Root Ganglion (DRG). The DRG is the primary neuron for sensory conduction and has become an important target for research and treatment of neuropathic pain [14], [15]. DRG neurons not only contain neurotransmitters and modulators that transmit nociceptive sensations but also have receptors that play a role in presynaptic modulation. Such receptors include the Gamma Aminobutyric Acid (GABA), opioids, purines and some ion channels. These substances not only play an important role in the mechanism of pain but some may also become effective targets for pain treatment.
Additionally, the SCN7A/Nax channel, a sodium-level sensor in body fluids, was found to be associated with the excitability of DRG neurons [16]. In addition, electrophysiological changes happened in SCN7A / Nax positive neurons (including sub-threshold oscillations, depolarized resting membrane potential and more negative action potential thresholds) hence increasing ectopic peak discharge, which is considered to be an important cause of chronic pain[16]. The SCN7A / Nax channel also controls sodium intake by changing the excitability of neurons [17], [18]. Another crucial sodium channel is Na(v)1.7 although it has various links to the pain process. In addition, given the many specific mechanisms involved in pain regulation, the theory has constantly been updated. Recently, immunohistochemistry results showed that Na(v)1.7 was present in both the presynaptic terminals of sensory afferents and the postsynaptic terminals of interneurons [19]. Moreover, it was shown that Na(v)1.7 located in dorsal horn neurons originated from sensory neurons. As a result, excitability changes in sensory neurons and the dorsal horn can be attributed to Na(v)1.7. Furthermore, pain was significantly alleviated after treatment with specific Na(v)1.7 inhibitors.
Moreover, expression of IL-6 and its soluble receptor sIL-6R is significantly increased in DRG neurons. It was reported that IL-6 combined with sIL-6R could induce the dimerization of gp130 on the DRG neuron cell membrane. This subsequently activated the function of TRPV1 by activating the JAK/PI3K/AKT signalling pathway, which in turn led to an increase in calcium influx and sensitivity of DRG [20].
WNT is a key protein found in the brain and is thought to be involved in the development of the nervous system. Additionally, it was discovered that nerve damage as well as bone cancer could cause rapid and sustained expression of WNT[21]. In the spinal cord, the WNT signaling pathway was found to be involved in the activation of astrocytes and microglia. The pathway was also reported to enhance the sensitivity of DRG through NR2B activation and subsequent Ca2 +-dependent signaling in the dorsal horn neurons. Moreover, a recent study on the rat bone cancer pain model confirmed that intrathecal injection of recombinant IL-18 induced phosphorylation of the NR2B receptor and mechanical allodynia. On the contrary, blocking the IL-18 signaling pathway could inhibit the activation of GluN2B and subsequent Ca2 + -dependent signaling as well as glial cell over activity [22]. Furthermore, the proinflammatory cytokines IL-18 and TNF were shown to be generated through the WNT/frizzled/β-catenin pathway [21].
Additionally, Protocadherin20 was found to be associated with enhanced synaptic plasticity, which is considered to be one of the important mechanisms of central sensitization. Moreover, suppression of Protocadherin20 was reported to inhibit neurite outgrowth and formation of excitatory synapses in dorsal neurons, resulting to bone pain relief [23].
DRG is the primary sensory neuron of sensory afferents. Therefore, knowledge on receptors and ion channels that are unique or mainly expressed on DRG will be helpful in exploring the mechanism of pain and providing new insights on the choice of targets for pain medication. Consequently, there is increasing research on these receptors and ion channels as targets for drug development. In addition, several studies exist with regard to many aspects of CIBP and will be explained in the subsequent sections.
The activity of microglia
Microglia are important immune and defense cells in the nervous system. They quickly turn into a polarized state in response to external stimuli and injuries. Recent research also showed that microglia play an important role in central hyperactive states as well as in multiple alterations in dorsal horn neurons following nerve injury during the pathophysiological process of CIBP [24], [25], [26].
A large number of neuroactive and inflammatory factors are released when tumor-related biomolecules cause neuronal damage or inflammation and after microglia are activated. Such include Reactive Oxygen Species (ROS), IL-1β, IL-6, TNF-a, MMPs, chemokines, ATP and NO. On one hand, these active substances act on their corresponding receptors on neurons, resulting to pain. On the other hand, they activate more microglia and astrocytes resulting to a positive feedback and ultimately participate in the induction as well as maintenance of chronic pain. Among the substances, ATP is released from damaged cells and affects the surrounding cells (especially neuronal cells) with the cell surface P2 receptor as an intercellular mediator [27]. Moreover, some studies showed that ROS can maintain pain (not initiate) by participating in the activation of microglia. As a result, ROS scavengers (N-tert-Butyl-α-phenylnitrone, PBN and 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl, Tempol) were shown to significantly relieve CIBP [28]. Additionally, it was reported that the occurrence of tactile allodynia after nerve injury was related to p38 mitogen-activated protein kinase (p38 MAPK), one of the four subgroups of the MAPK family [29], [30]. Besides, alendronate was found to be able to inhibit the activation of microglia and p-p38, thus preventing the development of hyperalgesia [31].
Furthermore, the Brain-derived Neurotrophic Factor (BDNF) was confirmed to be widely present in the nervous system [32] and nourish nerves in a paracrine or autocrine fashion. It was also shown to increase the sensitivity of the nervous system by activating microglia. Moreover, Wang et al. found that BDNF enhances the T-type Ca2+ currents by stimulating TrkB coupled to the PI3K-p38-PKA signal, thereby inducing excessive excitability of sensory neurons [33]. Zhou et al. also reported that CSF1 activates microglial cells to release BDNF through the CSF1R / p38 MAPK signaling pathway, then BDNF leads to an increase in CGRP terminals through TrkB/CREB signaling [34]. This can explain why chronic primary pain occurs without injury or illness.
The P38 MAPK pathway
The above-mentioned members of the MAPK family, especially ERK (p42 / 44), p38 and JNK were shown to be able to participate in the development of inflammation and pain during neuropathy through different signaling mechanisms. Notably, research on P38 has been on the rise in the past decade. P38MAPK is a protein with a molecular weight of 38kD and is made up of 360 amino acids. In addition, it has 6 subtypes including; p38α1, p38α2, p38β1, p38β2, p38γ and p38δ. P38α is mainly distributed in the dorsal root ganglia while p38β is mainly present in brain tissue particularly in the microglia. The P38-related MAPK cascades are the central links to multiple signaling pathways. When the cell is externally stimulated, the P38MAPK signaling pathway can be activated after which it enters the nucleus and regulates the activation of multiple transcription factors or the switching or growth of ion channels. Additionally, the factors are produced to regulate a variety of important physiological functions in cells including proliferation, autophagy, apoptosis and inflammatory responses.
Under certain circumstances, P38MAPK in DRG is phosphorylated and phosphorylated P38 then activates the Na (v) 1.8 particle channels, resulting to an increase in neuron current density, causing neuron excitement or pain sensitization. Such circumstances include when bone tumors invade nerves or inflammation causes nociceptors to generate pain signals and transmit them to the spinal dorsal root ganglion sensory neurons through axons.
P38MAPK is also involved in the central mechanism of neuropathic pain, mainly through changes in plasticity and central sensitization of neurons. Fan et al. reported that increased levels of phosphorylated cAMP Response Element Binding protein (CREB) was observed in the prelimbic cortex (PL) of rats experiencing chronic inflammatory pain. In addition, inhibition of p38 phosphorylation in PL was able to down-regulate phosphorylated CREB in PL with increased expression of serotonin, thereby reversing the nociceptive response aggravated by the formalin test [35].
The role of p38 in the process of inflammation-involved microglia activation and hyperalgesia has been the focus of many studies. Zhao et al. pointed out that IL-33/ST2 signaling plays a vital role in cancer related pain. Additionally, they showed that levels of phosphorylated p38MAPK increased in the spinal cord and inhibition of p38 phosphorylation could reduce pain in a CIBP rat model. However, whether IL-33 mediates CIBP through activation of MAPKs remains largely unknown [36].
Most experimental results indicate that the ERK/MAPK, JNK/MAPK and P38MAPK pathways are involved in the process of central sensitization. However, it is possible that continuous activation of the p38 mitogen in chronic neuronal changes caused by inflammatory Protein kinases (MAPK; p38) is the cause of central sensitization and not over-phosphorylation of extracellularly regulated protein kinases (ERK) or c-JunN-terminal kinase (JNK).
Consequently, accurately inhibiting the continued activation of p38 MAPK can prevent abnormal signal transduction in neurons thus reducing the sensitivity of the central nervous system and minimizing pain in patients. This might therefore be a promising therapeutic target.
Acidic environment
Hyperalgesia is usually accompanied by an acidic microenvironment. Decreased local tissue pH was previously associated with musculoskeletal pain [37]. Additionally, the pH value of synovial fluid harvested from patients with rheumatoid arthritis was reported to be lower than that of normal individuals [38]. An acidic microenvironment also plays an important role in the process of CIBP. Two acidic environments are extremely active during the progression of CIBP namely; the primary tumor acidic microenvironment and secondary acidic environment produced by osteoclasts.
Primary acidic microenvironment
Tumor tissues produce the primary acidic microenvironment. Tumor cells will convert energy metabolism into glycolysis, and try to use this abnormal metabolism behavior to escape the apoptosis process and enhance the proliferation and migration ability. This unique metabolic pattern in tumor tissues is known as the “Warburg effect” [39]. However, excessive metabolites of glycolysis in cells make the intracellular environment acidic and this is not conducive for the growth of cancer cells. Therefore, cancer cells actively expel protons and lactic acid out of cells through Monocarboxylate transporters 1 and 4 (MCT1 and MCT4), the carbonic anhydrase and protons pump (VH + -ATPase) and TRPV. This is the origin of the pathological acidity of the bone metastasis microenvironment. [40]. Moreover, hypoxia in the bone microenvironment leads to high expression of HIF-1 in cancer cells, which upregulates the plasma membrane proton/lactate transporters and exacerbates pathological acidosis in bone metastases[41]. A recent study showed that JJN3 human multiple myeloma cells injected in mice tibiae could cause CIBP with acidosis. However, the inhibitor of V-H+-ATPase was able to reverse acidosis and alleviate CIBP [42]. These results suggested that proton release via the VH+-ATPase pathway on JJN3 cells is critical to the induction of CIBP.
Secondary acidic microenvironment
Moreover, the secondary acidic microenvironment produced by activated osteoclasts is a principal element to the development and progression of bone metastasis [11], [43]. In healthy adults, there is often a balance between osteoclast-mediated bone resorption and osteoblast-mediated osteogenesis. However, during bone cancer, tumor cells and their associated stromal cells express the Receptor Activator of Nuclear Factor j-B ligand (RANKL), which binds to its receptor RANK, expressed by osteoclasts[44], [45]. Activation of the RANKL/RANK pathway in turn promotes the proliferation and hypertrophy of osteoclasts which secrete elevated levels of protons into the resorption lacunae to degrade bone minerals through the a3 isoform V-H+-ATPase (a3V-H + -ATPase) on plasma membrane clustered in the ruffled border[46]. Consequently, sensory nerves are activated and CIBP is triggered by increased secretion of protons and decreased pH in the resorption lacuna [47]. Therefore, interfering with the binding of RANKL to RANK which will reduce the number of osteoclasts there reducing tumor-induced osteoclast bone resorption. This can not only reduce osteoclast-induced acidosis but also maintain the mechanical strength of bones against bone resorption [48]. In addition, a previous study reported that application of specific inhibitors of osteoclasts, bisphosphonates and denosumab in clinical treatment could relieve CIBP in patients with bone metastases in solid cancers and bone disease in multiple myeloma. This also demonstrated that osteoclasts are responsible for inducing CIBP [49].
All in all, the pathological acid microenvironment created by bone-resorbing osteoclasts and bone-colonizing cancer cells jointly contribute to the activation of sensory nerves and CIBP.
Additionally, an acidic microenvironment can evoke multiple currents in primary afferent neurons, mediated by several acid-sensitive ion channels. Among these, acid-sensitive ion channels and receptors including the TRPV ion channels, Acid-sensing Ion Channels (ASICs) and P2x are the most widely studied.
Trpv
TRPV1 is an acid sensing nociceptor widely expressed in mammalian sensory nerve fibers, especially unmyelinated C-fiber nociceptive afferent sensory nerves [50]. At the same time, it is a highly Ca2+-permeable non-selective cation channel that is excited by the vanilloid capsaicin, acid (<pH 6.0) and noxious heat (greater than43 °C)[51] and this kind of activation will be sensitized by inflammatory mediators.[52].
Notably, TRPV1 is the bridge between the acidic microenvironment and CIBP. Its calcium channel activity was shown to be activated at low pH levels and this allowed calcium ions to flow into sensory cells with the subsequent initiation of an action potential. Through this, pain signals were generated before being transmitted to the brain by excited sensory nerves through DRG [53]. Moreover, the slow proton-activated conductance in DRG is similar to the TRPV1-mediated current stimulated by acidosis. In preclinical models, TRPV1-knockout mice suffered from cancer-induced acidosis although they could successfully avoid CIBP [50], [54].
Furthermore, treatment with selective TRPV1 antagonists, 5-iodo-resiniferatoxin (I-RTX) [54] and SB366791 [55], effectively reduced sensory nerve activation and induction of CIBP in mice. CIBP could also be exacerbated by the upregulation and activation of TRPV1 on sensory nerves with increased osteoclastic bone resorption by several growth factors and cytokines such as interlukin-6 (IL-6), IGF-1, PTHrP and TGF-β1. Moreover, IL-6 and PTHrP produced in bone metastases are reported to stimulate cancer growth in the bone and activated TRPV1 on sensory nerves causing mechanical hypersensitivity [56], [57]. Additional research also found that growth factors such as TGF-β and IGF-1 are released during bone resorption [20], [58]. Therefore, activation of TRPV1 in the acidic bone microenvironment increases the excitability of sensory nerves and induces CIBP. Given the acidic nature of the bone environment and exacerbation of acidosis during bone metastases, inhibition of the acid-sensing nociceptor TRPV1 on sensory nerves innervating the bone may be a potential mechanism-based therapy for CIBP. Based on this theory, multi-target compounds with MOR ligand and TRPV1 antagonist activities were investigated. They were found to potentially block pain transmission and minimize the side effects associated with single targeting [59]. Besides, specific TRPV inhibition through the precise removal of the unique PKC phosphorylation site (TRPV1 S801) with CRISPR/Cas9 editing provided the same effect as the overall inhibitor of TRPV1 but without its side effects [60]. Furthermore, bisphosphonates which relieve bone pain by decreasing osteoclast-induced acidosis, in turn decrease the activation of the ion-sensing TRPV1 or ASIC3 receptors that are expressed by sensory nerve fibers [61]. Interestingly, the latest research found that activating TRPV1 can prevent β arrestin2-biased signaling via MOR, and thus enhance analgesia. Moreover, TRPV1 deficiency promoted peripheral opioid receptor desensitization. In other words, TRPV1 interacts with MOR, indicating that TRPV1 agonists can be effective analgesics for preventing opioid tolerance.
Asics
ASICs belong to the voltage-insensitive, amiloride-sensitive epithelial Na+ channel family of cation channels and are among the molecular acid sensors [62]. According to previous reports, there was no slow and non-desensitizing proton gating current in DRG neurons in TPRV1 knockout mice, compared to the control group. However, the fast and rapid inactivation of proton gating mediated by ASICs was maintained [51], [63]. Therefore, compared to TRPV1, currents through ASICs have more similarities with the rapidly inactivating inward Na+ currents aroused by mild acidosis in DRG neurons [62], [64]. In addition, ASIC-related currents are also discovered on both DRG neurons with myelinated axons and those with unmyelinated axons. On the contrary, acidification related TRPV1-mediated currents are largely confined within unmyelinated fibers, consistent with the predominant expression of TRPV1 in unmyelinated primary afferent nerve fibers [64], [65].
ASICs including ASIC1a, ASIC1b, ASIC2a, ASIC2b and ASIC3 are directly gated by protons. However, ASIC2b does not respond to acidosis when expressed as a homomultimer although it forms functional heteromultimers with ASIC3. Additionally, it was reported that ASICs could only be activated by a limited range of stimuli, that is, an extracellular pH value of less than 7.2. The pH-sensitive subunits are located in multiple regions of the ASIC protein, particularly with His-72 and Gly-430 in the extracellular loop [65]. After being activated, ASICs start selecting cations and preferentially penetrate Na+, although the different groups can also carry other cations such as Ca2+ (ASIC1a) and K+ (ASIC1b). Moreover, ASIC3 homomultimers and ASIC2a/ASIC3 as well as ASIC2b/ASIC3 heteromultimers can generate two types of currents; one current is produced under mild acidic microenvironment or even neutral pH and another emerges only at pH values below 5 [66]. Although ASIC currents are usually fast-present and fast-inactivating, there is evidence that they can also monitor long-term acidosis.
In CIBP, ASIC3 on Calcitonin Gene-related Peptide-positive (CGRP+) sensory nerves that show pathologic sprouting in the presence of tumors are activated by the pathologic acidic microenvironment which in turn excites sensory nerves and evokes CIBP [27], [67], [68], [69]. In addition, it was shown that the selective V-ATPase inhibitor, BafA1, which deters the release of protons can alleviate the acidic environment and decrease the sprouting of CGRP+ sensory nerves innervating the bone in a murine model of multiple myeloma. This in turn led to inhibition of sensory nerve excitation and reduction of CIBP [42]. The above results therefore indicate that the following factors are critical to the pathophysiology of CIBP; (a) creation of an acidic extracellular bone microenvironment by osteoclasts and multiple myeloma cells by secreting protons through the plasma membrane V-ATPase and (b) responses of sensory nerves innervating the bone to the acidic microenvironment through ASIC3. Consequently, these could be considered as mechanism-based targets for the treatment of CIBP. Moreover, non-steroidal anti-inflammatory drugs and ASIC antagonists, regulating the expression and function of ASICs can also be potential treatments.
The P2X family
P2X Receptors (P2XR) are purinergic and ligand-gated membrane ion channels that are preferably permeable to sodium, potassium and calcium. They are commonly expressed on cells including primary afferent neurons and neuroglial cells especially on microglia, and open within milliseconds when binding to ATP. In addition, they are assembled as homomultimers or heteromultimers of P2X subunits, seven of which (P2X1–P2X7) have been identified. The P2X family is also involved in CIBP through acidification of the acidic microenvironment thus reducing the potency of ATP to gate homomultimeric P2X1, P2X3, P2X4 and P2X7 receptors. However, this sensitizes homomultimeric P2X2 receptors to the excitatory effect of ATP [70]. Among the subunits, the P2X3 and P2X4 receptors are involved in chronic pain while P2X7 plays a key role in the release of inflammatory cytokines [71], [72].
P2X receptors are preferentially expressed on DRG neurons and have been implicated in neuropathic, inflammatory and cancer pain [69]. Notably, P2X1, P2X2, P2X3, P2X4, P2X5 and P2X7 are modulated by alterations in the extracellular pH. In addition, His-319 is particularly important for the effect of protons in potentiating the agonist effect of ATP on P2X2, while the protonation of His-206 and His-286 explains the inhibitory effects on the current of P2X3 and P2X4 induced by low concentrations agonists, respectively for the reason of dual effect of acid PH on P2X receptors.[73]. Moreover, the resulting heteromultimers show pH sensitivity that is different from that of P2X homomers when different P2X subunits are co-expressed. Additionally, the P2X receptors on DRG neurons comprise of more homomultimeric P2X3 than heteromultimeric P2X2/3 receptors, whose inward currents evoked by ATP exhibit transient, persistent or biphasic components. The monitoring of acidification depends on the concomitant release and presence of ATP or related purines whose agonist action is enhanced by a decrease in extracellular pH.
Furthermore, previous studies reported the upregulation of the P2X3 receptor (P2X3R) on primary sensory afferents induced by tumor cell injection [71], [74]. In addition, it was shown that activation of P2X3Rs involved in the development of CIBP was closely related to the p65 of NF-κB. Notably, the NF-κB inhibitor, PDTC and LV-p65 shRNA which suppressed the expression of p65 could reverse the development of mechanical hypersensitivity in CIBP rats [69].
Other studies also revealed that P2X4R on activated spinal microglia is required for the expression of neuropathic pain [27], [75]. Moreover, tactile allodynia caused by ATP-stimulated microglia is connected to P2X4Rs and was relieved by the P2X4R antagonist, TNP-ATP.
P2X7R was also found to be upregulated in the Ventral Medulla Oblongata (RVM) of rats with bone cancer and was believed to be associated with abnormal mechanical pain. Additionally, injecting Brilliant Blue G (a non-competitive P2X7R antagonist) or interfering RNA into the RVM significantly reduced CIBP [76], [77]. It was also shown that P2X7R antagonism can suppress peripheral-induced neuronal responses in the dorsal horn neurons of rats with cancer. This was achieved by the attenuation of both mechanical hypersensitivity, movement and non-evoked pain behavior [76], [78]. Moreover, this therapeutic potential may be enhanced by the additional analgesic effects from both spinal and supraspinal modulation [67], [78]. In short, P2X7R antagonism has shown strong analgesic effects, and may be one of the potential options for the treatment of CIBP.
Anti-GM-CSF treatment
The Granulocyte-macrophage Colony Stimulating Factor (GM-CSF) also known as Sargramostim, is a colony stimulating component in blood that induces the production of granulocyte and macrophage populations from hematopoietic progenitor cells.
Nerve remodeling & sensitization
It is well known that GM-CSF is involved in multiple pain-related mechanisms associated with inflammation, arthritis, neuralgia and CIBP [79], [80]. Notably, GM-CSF is secreted from the cancer cells in CIBP. It regulates the interaction between tumors and nerves, remodeling of peripheral nerves and sensitization of damage-sensitive (nociceptive) nerves. It also plays an important role in the development of cancer pain [79]. A recent study showed that GM-CSF signaling is involved in pain-related behaviors that are not associated with glial and/or astrocyte responses, suggesting that it may directly activate sensory neurons [81]. Additionally, in vivo studies showed that interruption of GM-CSF signaling reduced tumor growth as well as neural remodeling and eliminated bone cancer pain, indicating the role for GM-CSF in tumor and pain.
Moreover, the GM-CSF Receptor (GM-CSFR) and signaling mediators were found to be functionally expressed on the sensory nerves next to the surface of mature granulocyte and monocyte macrophage lineages [82]. In addition, GM-CSF sensitizes nerves to mechanical stimuli in vivo and in vitro through these receptors, leading to sensory nerve sprouting. The GM-CSFR was also shown to be expressed on DRG and peripheral nerves distributed in the periosteum of mice. Additionally, these nerves had the ability to activate signaling pathways in DRG neurons, thereby enhancing neuropeptides [79].
Signaling pathways
It is reported that GM-CSFR works directly through three main signaling pathways namely; the Janus Kinase Signaling (JAK-STAT) pathway, the Phosphoinositide 3 Kinase (PI3K) pathway and the Mitogen-activated Protein Kinase (MAPK) pathway, all of which are directly or indirectly related to pain regulation.
In the complex network of mechanisms that cause hyperalgesia associated with high levels of GM-CSF in bone cancer patients, JAK-STAT 3 is considered to be the main signaling pathway for GM-CSF to exert its effect on DRG neurons. In the JAK-STAT signaling pathway, Jak activated by GM-CSFR leads to the activation of Stat family transcription factors, which dimerize, move to the nucleus and regulate gene expression [83]. In addition, recent studies showed that Jak2 and Stat3 were selectively phosphorylated in DRG after GM-CSF treatment. Additionally, although phosphorylated Jak1 was unaffected, phosphorylated Jak3 and Stat5 were not found [84]. Coincidentally, according to data from iBrain, the Jak3 and Stat5 mRNA levels in DRG neurons were very low [85]. This evidence therefore highlights the importance of Jak2 and Stat3 in the JAK-STAT signaling pathway during bone cancer. Moreover, some targeted drugs have been discovered or developed based on these studies. Sinomenine was also reported to inhibit the activation of microglia to interrupt the process of increasing pain by suppressing the JAK2/STAT3 pathway and neuronal CAMKII/CREB cascades [86].
Additional studies also showed that GM-CSF selectively increases the expression of three voltage-gated sodium channels in DRG neurons, namely; Na(v)1.7, Na(v)1.8 and Na(v)1.9. This provides further clarification on the mechanism of CIBP [84]. Although there is no clear evidence that Na(v)1.9 can cause pain, Na(v)1.7[19], [87], [88] and Na(v)1.8[88], [89], [90] can be considered as potential targets for analgesics.
Immunomodulatory
Furthermore, GM-CSF indirectly exerts its effect by regulating immune function [91]. GM-CSF released by inflamed tissues [92] and tumor tissues can recruit and stimulate inflammatory cells such as monocytes and macrophages [93]. These cells then sensitize peripheral nerve fibers and contribute to nerve remodeling through the release of inflammatory cytokines such as TNF-α, Interleukin-1 (IL-1), Interferon (IFN) and endothelin-1[91].
Moreover, it was reported that GM-CSF enhances monocyte secretion of TNF-α while GM-CSF and TNF-α jointly increase the secretion IL-1 [94], [95]. Additionally, TNF-α and IL-1 can in turn strongly stimulate the release of many growth factors including G-CSF and GM-CSF. In addition, GM-CSF can increase the production and release of endothelin 1 in human monocytes, thereby exacerbating pain [96]. Therefore, the above findings indicate that GM-CSF signaling is functionally related to excessive pain in bone cancer.
Currently, GM-CSF is extensively used in clinical practice for the treatment of myelodysplastic syndromes, aplastic anemia, tumor radiotherapy and chemotherapy-induced neutropenia [97]. Notably, the most common side effect to these GM-CSF therapies is bone pain with an incidence of up to 90% [79]. Consequently, keeping the concentration of GM-CSF in blood can be a potential therapeutic strategy for alleviating CIBP or bone pain caused by clinical GM-CSF doses.
Anti-NGF treatment
In most cancer tissues, tumor cells and associated inflammatory (immune) cells including macrophages, T lymphocytes, mast cells and endothelial cells produce a variety of chemical mediators. Such include Prostaglandin (PGE2), the Nerve Growth Factor (NGF), Endothelin (ET), bradykinin and extracellular adenosine triphosphate. Normally, the limitation of endogenous NGF and the continuous competition between nerves for NGF are the reasons for maintaining the normal density of nerve terminals. However, treatment with exogenous NGF will stimulate nerve sprouting, partly through stimulating the TrkA + nerve fibers, which will in turn trigger hyperalgesia due to bone metastases and induced bone pain.
The pathophysiological basis of hyperalgesia involves the proliferation of nerve fibers within the bone marrow, bone and periosteum. Cancer cells induced sensory in sympathetic nerve fibers reorganization and proliferation in the periosteum. In addition, an abundance of nerve fibers in the bone marrow, bone and periosteum and cancer cells can induce sensory and sympathetic nerve fibers in the periosteum to produce and reorganize.
Moreover, neurofibromatous structures caused by the highly disordered growth of sensory and sympathetic neurons under certain conditions lead to spontaneous metastatic pain.
NGF is mainly derived from cancer tissues, tumor cells and related inflammatory (immune) cells (including macrophages, T lymphocytes, mast cells and endothelial cells). It has a high affinity to its main molecular target, Trk A, which is highly expressed in the nerve fibers of bones. According to previous studies, more than 80% of osteosensory nerves are Trk A + nerve fibers [98], [99]. Therefore, this is considered to be the reason why anti-NGF therapy gives significant relief from bone pain.
In recent studies, NGF was shown to bind p75NTR and form homodimers present in Trk A [100], [101]. Additionally, formation of heterodimers leads to activation of the secondary messenger cascade which regulates the expression of receptors and ion channels on peripheral nerve endings and causes sensitization through a variety of neurotransmitters.
In a rat spinal cord injury model, anti-NGF antibodies were shown to successfully inhibit mechanical hyperalgesia and enhance the response of neurons with a wide dynamic range [102]. In addition, the expression of NGF protein and its receptors and mRNA level was found to be upregulated in the DRG and spinal dorsal horn, whereas the expression of MOR was downregulated. In vivo and in vitro experiments have proved that anti-NGF agents can inhibit hyperalgesia by reversing the down-regulation of MOR expression [103]. Additional studies also reported that therapies that block NGF/TrkA can effectively alleviate neuralgia and inflammatory pain by inhibiting tumor-induced nerve sprouting and formation of neuroma [104], [105]. Furthermore, a recent study reported that through the intended mode of administration (Monoclonal anti-NGF antibody (mAb911)), changes in functional connectivity between the central hubs of the major pain channels caused by developing cancer pain, can successfully be prevented. Such include the gray matter around the catheter, amygdala, somatosensory area of thalamus and cortex. [106].
Comprehensive treatment
There is no doubt that CIBP is extremely painful, fortunately, treatment with opioids for pain relief is effective in more than 70% of CIBP patients. Additionally, routine medication includes the use of acetaminophen, Nonsteroidal Anti-Inflammatory Drugs (NSAIDS) and opioids. Adjuvant drugs also include corticosteroids, bisphosphonates, radiopharmaceuticals, N-methyl-D-aspartate receptor antagonists, local anesthetics and α2-adrenergic agonists. Moreover, chronic pain is often accompanied by painful psychological conditions, including depression. Therefore, antidepressants, such as tricyclic antidepressants and noradrenaline/serotonin reuptake inhibitors may be used. Nonetheless, radiotherapy is the main intervention for the treatment of CIBP and can effectively relieve pain. Further details on the use of radiotherapy have been highlighted [107], [108].
In summary, both physicians and families need to work together on strategies that help patients accept their condition and ease the pain that comes with cancer through effective pain management [109], [110]. Timely and professional psychological intervention is also necessary.
Conclusion and future directions
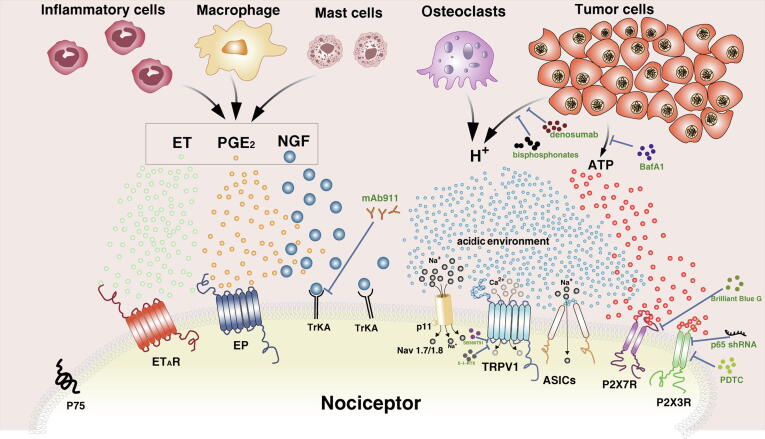
The complex mechanism underlying CIBP is concluded in Fig. 2 and extensive research on the field is currently ongoing. Existing evidence indicates that neuroinflammation plays an important role in the induction and maintenance of chronic pain. This especially occurs through the activation of glial cells and production of inflammatory mediators in the peripheral and central nervous system. In addition, the complexity of CIBP is not only due to the intricate crosstalk between key molecules or signaling pathways but also because of the pathological changes arising from the cancer itself. Moreover, there is an overlap between the advantages and disadvantages of existing pain relief methods. However, there is still a challenge on how to maximize the therapeutic effect of existing interventions. Therefore, although the targeted high-efficiency small molecule drugs are ideal, drugs that suppress the generation and persistence of neuropathic pain and accompanying neurochemical changes without affecting normal pain sensitivity and exercise ability are urgently needed.
Fig. 2.
Schematic representation of the roles of the acidic environment and inflammatory response related pathway in the mechanism of cancer-induced bone pain.
Fig. 2. Schematic representation of the roles of the acidic environment and inflammatory response related pathway in the mechanism of cancer-induced bone pain.
Funding
No funding.
Author contributions
XQZ and AMW designed the study. XQZ, YHW, JFH and AMW conducted literature collection and summary. XQZ, YHW, JFH and AMW drafted the manuscript. All authors critically revised the manuscript.
CRediT authorship contribution statement
XQZ and AMW: Conceptualization. XQZ and AMW: Data curation. XQZ, YHW, JFH and AMW: Formal analysis. XQZ, YHW, JFH and AMW: Investigation. XQZ, YHW, JFH and AMW: Methodology. XQZ and AMW: Project administration. XQZ and AMW: Resources. AMW: Supervision. XQZ, YHW, JFH and AMW: Writing - original draft. XQZ, YHW, JFH and AMW: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors report there are no conflicts of interest related to the present article.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Biographies

Xuan-Qi Zheng: My name is Xuan-Qi Zheng, I am a postgraduate student in Wenzhou medical, my mentor is Ai-min Wu. In June 2018, I completed my undergraduate degree from Wenzhou medical university, and in September 2018, I was admitted as a postgraduate student of Wenzhou medical university, majoring in orthopaedics in Zhejiang Provincial Key Laboratory of Orthopaedics. Currently, I am mainly engaged in bone tumor-related research and has published several papers. In August 2019, I published an article titled Incidence, prognostic factors, and a nomogram of lung cancer with bone metastasis at initial diagnosis: a population-based study on Translational Lung Cancer Research, demonstrating big data research on lung cancer bone metastasis. In the same year, we started to study the mechanism of cancer-induced bone pain.

Yu-hao Wu: I’m Yu-hao Wu from Wenzhou Medical university, Zhejiang province, China. Though I’m now in my junior year majoring in clinal medicine, courses I’ve studied could help me understand the signaling pathways and pathological mechanisms of diseases which include anatomy, biochemistry, molecular biology, cell biology, etc. In November, 2018, I joined the research group of Zhejiang Provincial Key Laboratory of Orthopaedics and start my scientific research as a member of Wu’s laboratory.

Jin-feng Huang: I’m Jin-feng Huang, a postgraduate student in Wenzhou medical, my mentor is Ai-min Wu. In June 2018, I completed my undergraduate degree from Hebei medical university, and in September 2018, I was admitted as a postgraduate student of Wenzhou medical university, majoring in orthopaedics in Zhejiang Provincial Key Laboratory of Orthopaedics. During my master's degree, I was mainly engaged in the analysis of bioinformatics based on the large-scale bone tumor database, and published several papers in related fields.

Ai-Min Wu: My name is Aimin Wu, I am an associate chief orthopaedic-spine surgeon and associate professor in The Second Affiliated Hospital & Yuying Children’ Hospital of Wenzhou Medical University. Since 2017, we have established our own laboratory in Zhejiang Provincial Key Laboratory of Orthopaedics. Our team mainly studies the mechanism and treatment of bone tumors and intervertebral disc degeneration. The relevant results have been published in well-known journals. In clinical work and scientific research, we have found that the incidence of lung cancer bone metastasis is high, and cancer-induced bone pain (CIBP) is also very significant, which has reduced quality of life as well as self-confidence in cancer survivors, and also brought huge medical burden to society. It is urgent to study the mechanism of CIBP. Therefore, we reviewed the mechanism of CIBP, tried to summarize the latest progress of basic research related to CIBP, and attracted the attention of researchers, hoping to develop specific drugs as soon as possible to reduce the pain of cancer patients.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lechner B., Chow S., Chow R., Zhang L., Tsao M., Danjoux C., et al. The incidence of neuropathic pain in bone metastases patients referred for palliative radiotherapy. Radiother Oncol. 2016;118(3):557–561. doi: 10.1016/j.radonc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Smith B.H., Raja S.N. NeuPSIG: investing in solutions to the growing global challenge of neuropathic pain. Br J Anaesth. 2017;119(4):705–708. doi: 10.1093/bja/aex276. [DOI] [PubMed] [Google Scholar]
- 3.Treede R.D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 4.Finnerup N.B., Haroutounian S., Kamerman P., Baron R., Bennett D.L., Bouhassira D., et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung C., Ichesco E., Ratai E., Gonzalez R., Burdo T., Loggia M., et al. Magnetic resonance imaging of neuroinflammation in chronic pain: a role for astrogliosis? Pain. 2020;161(7):1555–1564. doi: 10.1097/j.pain.0000000000001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehlmann D., Grandjean J., Xandry J., Rudin M. Longitudinal resting-state functional magnetic resonance imaging in a mouse model of metastatic bone cancer reveals distinct functional reorganizations along a developing chronic pain state. Pain. 2018;159(4):719–727. doi: 10.1097/j.pain.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 7.Iezaki T., Onishi Y., Ozaki K., Fukasawa K., Takahata Y., Nakamura Y., et al. The Transcriptional Modulator Interferon-Related Developmental Regulator 1 in Osteoblasts Suppresses Bone Formation and Promotes Bone Resorption. J Bone Miner Res. 2016;31(3):573–584. doi: 10.1002/jbmr.2720. [DOI] [PubMed] [Google Scholar]
- 8.Ohba T., Cole H.A., Cates J.M., Slosky D.A., Haro H., Ando T., et al. Bisphosphonates inhibit osteosarcoma-mediated osteolysis via attenuation of tumor expression of MCP-1 and RANKL. J Bone Miner Res. 2014;29(6):1431–1445. doi: 10.1002/jbmr.2182. [DOI] [PubMed] [Google Scholar]
- 9.Andreev D., Liu M., Weidner D., Kachler K., Faas M., Gruneboom A., et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J Clin Invest. 2020;130(9):4811–4830. doi: 10.1172/JCI134214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii T., Murata K., Mun S.H., Bae S., Lee Y.J., Pannellini T., et al. MEF2C regulates osteoclastogenesis and pathologic bone resorption via c-FOS. Bone Res. 2021;9(1):4. doi: 10.1038/s41413-020-00120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji R.R., Kohno T., Moore K.A., Woolf C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y.S., Chu Y., Han L., Li M., Li Z., LaVinka P.C., et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81(4):873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North R.Y., Li Y., Ray P., Rhines L.D., Tatsui C.E., Rao G., et al. Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain. 2019;142(5):1215–1226. doi: 10.1093/brain/awz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X., Liu H., Hamel K.A., Morvan M.G., Yu S., Leff J., et al. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun. 2020;11(1):264. doi: 10.1038/s41467-019-13839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke C.B., He W.S., Li C.J., Shi D., Gao F., Tian Y.K. Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience. 2012;227:80–89. doi: 10.1016/j.neuroscience.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Hiyama T.Y., Watanabe E., Okado H., Noda M. The subfornical organ is the primary locus of sodium-level sensing by Na(x) sodium channels for the control of salt-intake behavior. J Neurosci. 2004;24(42):9276–9281. doi: 10.1523/JNEUROSCI.2795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe E., Fujikawa A., Matsunaga H., Yasoshima Y., Sako N., Yamamoto T., et al. Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci. 2000;20(20):7743–7751. doi: 10.1523/JNEUROSCI.20-20-07743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alles S.R.A., Nascimento F., Lujan R., Luiz A.P., Millet Q., Bangash M.A., et al. Sensory neuron-derived NaV1.7 contributes to dorsal horn neuron excitability. Sci Adv. 2020;6(8):eaax4568. doi: 10.1126/sciadv.aax4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang D., Kong L.Y., Cai J., Li S., Liu X.D., Han J.S., et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain. 2015;156(6):1124–1144. doi: 10.1097/j.pain.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y.K., Huang Z.J., Liu S., Liu Y.P., Song A.A., Song X.J. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. J Clin Invest. 2013;123(5):2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S., Liu Y.P., Lv Y., Yao J.L., Yue D.M., Zhang M.Y., et al. IL-18 Contributes to Bone Cancer Pain by Regulating Glia Cells and Neuron Interaction. J Pain. 2018;19(2):186–195. doi: 10.1016/j.jpain.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Ke C., Li C., Huang X., Cao F., Shi D., He W., et al. Protocadherin20 promotes excitatory synaptogenesis in dorsal horn and contributes to bone cancer pain. Neuropharmacology. 2013;75:181–190. doi: 10.1016/j.neuropharm.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Pm G., Mr H., Sf M., Lr W. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.T. S, A peripheral messenger for chronic pain, Nature neuroscience 19(1) (2016) 9. [DOI] [PubMed]
- 26.I. K, T. M, Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential, Nature reviews. Neuroscience 19(3) (2018) 138-152. [DOI] [PubMed]
- 27.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109(1–2):210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y.Q., Liu D.Q., Chen S.P., Sun J., Zhou X.R., Rittner H., et al. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018;14:391–397. doi: 10.1016/j.redox.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trempolec N., Dave-Coll N., Nebreda A.R. SnapShot: p38 MAPK signaling. Cell. 2013;152(3) doi: 10.1016/j.cell.2013.01.029. 656-656 e1. [DOI] [PubMed] [Google Scholar]
- 30.Thornton T.M., Pedraza-Alva G., Deng B., Wood C.D., Aronshtam A., Clements J.L., et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320(5876):667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao Y., Tan Y.H., Light A.R., Mao J., Yu A.C., Fu K.Y. Alendronate Attenuates Spinal Microglial Activation and Neuropathic Pain. J Pain. 2016;17(8):889–903. doi: 10.1016/j.jpain.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y. Expression of BDNF in dorsal root ganglion of rats with bone cancer pain and its effect on pain behavior. J Musculoskelet Neuronal Interact. 2018;18(1):42–46. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Wei Y., Pu Y., Jiang D., Jiang X., Zhang Y., et al. Brain-derived neurotrophic factor stimulation of T-type Ca(2+) channels in sensory neurons contributes to increased peripheral pain sensitivity. Sci Signal. 2019;12(600) doi: 10.1126/scisignal.aaw2300. [DOI] [PubMed] [Google Scholar]
- 34.Zhou L.J., Peng J., Xu Y.N., Zeng W.J., Zhang J., Wei X., Mai C.L., Lin Z.J., Liu Y., Murugan M., Eyo U.B., Umpierre A.D., Xin W.J., Chen T., Li M., Wang H., Richardson J.R., Tan Z., Liu X.G., Wu L.J. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019;27(13) doi: 10.1016/j.celrep.2019.05.087. 3844-3859 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan X.C., Fu S., Liu F.Y., Cui S., Yi M., Wan Y. Hypersensitivity of Prelimbic Cortex Neurons Contributes to Aggravated Nociceptive Responses in Rats With Experience of Chronic Inflammatory Pain. Front Mol Neurosci. 2018;11:85. doi: 10.3389/fnmol.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Zhang H., Liu S.B., Han P., Hu S., Li Q., et al. Spinal interleukin-33 and its receptor ST2 contribute to bone cancer-induced pain in mice. Neuroscience. 2013;253:172–182. doi: 10.1016/j.neuroscience.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 37.Abdelhamid R.E., Sluka K.A. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology (Bethesda) 2015;30(6):449–459. doi: 10.1152/physiol.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantyh P.W. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7(10):797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 39.Parks S.K., Chiche J., Pouyssegur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13(9):611–623. doi: 10.1038/nrc3579. [DOI] [PubMed] [Google Scholar]
- 40.Yoneda T., Hiasa M., Okui T. Crosstalk Between Sensory Nerves and Cancer in Bone. Curr Osteoporos Rep. 2018;16(6):648–656. doi: 10.1007/s11914-018-0489-x. [DOI] [PubMed] [Google Scholar]
- 41.Maes C., Carmeliet G., Schipani E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat Rev Rheumatol. 2012;8(6):358–366. doi: 10.1038/nrrheum.2012.36. [DOI] [PubMed] [Google Scholar]
- 42.Hiasa M., Okui T., Allette Y.M., Ripsch M.S., Sun-Wada G.H., Wakabayashi H., et al. Bone Pain Induced by Multiple Myeloma Is Reduced by Targeting V-ATPase and ASIC3. Cancer Res. 2017;77(6):1283–1295. doi: 10.1158/0008-5472.CAN-15-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suva L.J., Washam C., Nicholas R.W., Griffin R.J. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7(4):208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honore P., Mantyh P.W. Bone cancer pain: from mechanism to model to therapy. Pain Med. 2000;1(4):303–309. doi: 10.1046/j.1526-4637.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 45.Luger N.M., Mach D.B., Sevcik M.A., Mantyh P.W. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage. 2005;29(5 Suppl):S32–S46. doi: 10.1016/j.jpainsymman.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Qin A., Cheng T.S., Pavlos N.J., Lin Z., Dai K.R., Zheng M.H. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol. 2012;44(9):1422–1435. doi: 10.1016/j.biocel.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Maeda H., Kowada T., Kikuta J., Furuya M., Shirazaki M., Mizukami S., et al. Real-time intravital imaging of pH variation associated with osteoclast activity. Nat Chem Biol. 2016;12(8):579–585. doi: 10.1038/nchembio.2096. [DOI] [PubMed] [Google Scholar]
- 48.Lipton A. Emerging role of bisphosphonates in the clinic–antitumor activity and prevention of metastasis to bone. Cancer Treat Rev. 2008;34(Suppl 1):S25–S30. doi: 10.1016/j.ctrv.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Cleeland C.S., Body J.J., Stopeck A., von Moos R., Fallowfield L., Mathias S.D., et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119(4):832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 50.Ghilardi J.R., Rohrich H., Lindsay T.H., Sevcik M.A., Schwei M.J., Kubota K., et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caterina M.J., Leffler A., Malmberg A.B., Martin W.J., Trafton J., Petersen-Zeitz K.R., et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 52.Negri L., Lattanzi R., Giannini E., Colucci M., Margheriti F., Melchiorri P., et al. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26(25):6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niiyama Y., Kawamata T., Yamamoto J., Omote K., Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148(2):560–572. doi: 10.1016/j.neuroscience.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 55.Niiyama Y., Kawamata T., Yamamoto J., Furuse S., Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102(2):251–258. doi: 10.1093/bja/aen347. [DOI] [PubMed] [Google Scholar]
- 56.Xu Q., Zhang X.M., Duan K.Z., Gu X.Y., Han M., Liu B.L., et al. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci. 2013;33(49):19099–19111. doi: 10.1523/JNEUROSCI.4852-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Cai J., Han Y., Xiao X., Meng X.L., Su L., et al. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain. 2014;18(6):774–784. doi: 10.1002/j.1532-2149.2013.00420.x. [DOI] [PubMed] [Google Scholar]
- 58.Shepherd A.J., Mickle A.D., Kadunganattil S., Hu H., Mohapatra D.P. Parathyroid Hormone-Related Peptide Elicits Peripheral TRPV1-dependent Mechanical Hypersensitivity. Front Cell Neurosci. 2018;12:38. doi: 10.3389/fncel.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H., Ahn S., Ann J., Ha H., Yoo Y.D., Kim Y.H., et al. Discovery of dual-acting opioid ligand and TRPV1 antagonists as novel therapeutic agents for pain. Eur J Med Chem. 2019;182 doi: 10.1016/j.ejmech.2019.111634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph J., Qu L., Wang S., Kim M., Bennett D., Ro J., et al. Phosphorylation of TRPV1 S801 Contributes to Modality-Specific Hyperalgesia in Mice. J Neurosci. 2019;39(50):9954–9966. doi: 10.1523/JNEUROSCI.1064-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–S62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 62.Kellenberger S., Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82(3):735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 63.Khasabova I.A., Holman M., Morse T., Burlakova N., Coicou L., Harding-Rose C., et al. Increased anandamide uptake by sensory neurons contributes to hyperalgesia in a model of cancer pain. Neurobiol Dis. 2013;58:19–28. doi: 10.1016/j.nbd.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leffler A., Mönter B., Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139(2):699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 65.P. Holzer, Acid-sensitive ion channels and receptors, Handb Exp Pharmacol (194) (2009) 283-332. [DOI] [PMC free article] [PubMed]
- 66.Wemmie J.A., Price M.P., Welsh M.J. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29(10):578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 67.Ducourneau V.R., Dolique T., Hachem-Delaunay S., Miraucourt L.S., Amadio A., Blaszczyk L., et al. Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain. 2014;155(2):275–291. doi: 10.1016/j.pain.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Demeule M., Beaudet N., Regina A., Besserer-Offroy E., Murza A., Tetreault P., et al. Conjugation of a brain-penetrant peptide with neurotensin provides antinociceptive properties. J Clin Invest. 2014;124(3):1199–1213. doi: 10.1172/JCI70647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Y.L., Jiang G.Q., Wei J., Zhang H.H., Chen W., Zhu H., et al. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain. 2015;156(10):1892–1905. doi: 10.1097/j.pain.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.North R.A. P2X receptors. Philos Trans R Soc Lond B Biol Sci. 2016;371(1700) doi: 10.1098/rstb.2015.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilchrist L.S., Cain D.M., Harding-Rose C., Kov A.N., Wendelschafer-Crabb G., Kennedy W.R., et al. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005;1044(2):197–205. doi: 10.1016/j.brainres.2005.02.081. [DOI] [PubMed] [Google Scholar]
- 72.Aby F., Whitestone S., Landry M., Ulmann L., Fossat P. Inflammatory-induced spinal dorsal horn neurons hyperexcitability is mediated by P2X4 receptors. Pain Rep. 2018;3(3) doi: 10.1097/PR9.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerevich Z., Zadori Z.S., Koles L., Kopp L., Milius D., Wirkner K., et al. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J Biol Chem. 2007;282(47):33949–33957. doi: 10.1074/jbc.M705840200. [DOI] [PubMed] [Google Scholar]
- 74.Liu M., Yang H., Fang D., Yang J.J., Cai J., Wan Y., et al. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain. 2013;154(9):1551–1568. doi: 10.1016/j.pain.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 75.Tsuda M., Shigemoto-Mogami Y., Koizumi S., Mizokoshi A., Kohsaka S., Salter M.W., et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 76.Falk S., Schwab S.D., Frosig-Jorgensen M., Clausen R.P., Dickenson A.H., Heegaard A.M. P2X7 receptor-mediated analgesia in cancer-induced bone pain. Neuroscience. 2015;291:93–105. doi: 10.1016/j.neuroscience.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 77.Huang Z.X., Lu Z.J., Ma W.Q., Wu F.X., Zhang Y.Q., Yu W.F., et al. Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain. 2014;155(4):783–791. doi: 10.1016/j.pain.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 78.Hansen R.R., Nielsen C.K., Nasser A., Thomsen S.I., Eghorn L.F., Pham Y., et al. P2X7 receptor-deficient mice are susceptible to bone cancer pain. Pain. 2011;152(8):1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schweizerhof M., Stosser S., Kurejova M., Njoo C., Gangadharan V., Agarwal N., et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15(7):802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 80.Cook A.D., Pobjoy J., Steidl S., Durr M., Braine E.L., Turner A.L., et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res Ther. 2012;14(5):R199. doi: 10.1186/ar4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicol L.S.C., Thornton P., Hatcher J.P., Glover C.P., Webster C.I., Burrell M., et al. Central inhibition of granulocyte-macrophage colony-stimulating factor is analgesic in experimental neuropathic pain. Pain. 2018;159(3):550–559. doi: 10.1097/j.pain.0000000000001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 83.Choi J.K., Kim K.H., Park H., Park S.R., Choi B.H. Granulocyte macrophage-colony stimulating factor shows anti-apoptotic activity in neural progenitor cells via JAK/STAT5-Bcl-2 pathway. Apoptosis. 2011;16(2):127–134. doi: 10.1007/s10495-010-0552-2. [DOI] [PubMed] [Google Scholar]
- 84.Zhang F., Wang Y., Liu Y., Han H., Zhang D., Fan X., et al. Transcriptional Regulation of Voltage-Gated Sodium Channels Contributes to GM-CSF-Induced Pain. J Neurosci. 2019;39(26):5222–5233. doi: 10.1523/JNEUROSCI.2204-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qiu F., Li Y., Fu Q., Fan Y.Y., Zhu C., Liu Y.H., et al. Stromal Cell-Derived Factor 1 Increases Tetrodotoxin-Resistant Sodium Currents Nav1.8 and Nav1.9 in Rat Dorsal Root Ganglion Neurons via Different Mechanisms. Neurochem Res. 2016;41(7):1587–1603. doi: 10.1007/s11064-016-1873-5. [DOI] [PubMed] [Google Scholar]
- 86.Chen S.P., Sun J., Zhou Y.Q., Cao F., Braun C., Luo F., Ye D.W., Tian Y.K. Sinomenine attenuates cancer-induced bone pain via suppressing microglial JAK2/STAT3 and neuronal CAMKII/CREB cascades in rat models. Mol Pain. 2018;14 doi: 10.1177/1744806918793232. 1744806918793232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drissi I., Woods W.A., Woods C.G. Understanding the genetic basis of congenital insensitivity to pain. Br Med Bull. 2020 doi: 10.1093/bmb/ldaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kushnarev M., Pirvulescu I.P., Candido K.D., Knezevic N.N. Neuropathic pain: preclinical and early clinical progress with voltage-gated sodium channel blockers. Expert Opin Investig Drugs. 2020;29(3):259–271. doi: 10.1080/13543784.2020.1728254. [DOI] [PubMed] [Google Scholar]
- 89.Zhang F., Zhang C., Xu X., Zhang Y., Gong X., Yang Z., et al. Naja atra venom peptide reduces pain by selectively blocking the voltage-gated sodium channel Nav1.8. J Biol Chem. 2019;294(18):7324–7334. doi: 10.1074/jbc.RA118.007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xiao Y., Barbosa C., Pei Z., Xie W., Strong J.A., Zhang J.M., et al. Increased Resurgent Sodium Currents in Nav1.8 Contribute to Nociceptive Sensory Neuron Hyperexcitability Associated with Peripheral Neuropathies. J Neurosci. 2019;39(8):1539–1550. doi: 10.1523/JNEUROSCI.0468-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuster A., Klotz M., Schwab T., Lilischkis R., Schneider A., Schafer K.H. Granulocyte-colony stimulating factor: a new player for the enteric nervous system. Cell Tissue Res. 2014;355(1):35–48. doi: 10.1007/s00441-013-1744-1. [DOI] [PubMed] [Google Scholar]
- 92.Angst M.S., Clark J.D., Carvalho B., Tingle M., Schmelz M., Yeomans D.C. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139(1):15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 93.Kasper B., Herbst A., Pilz C., Germeshausen M., Tidow N., Hadam M.R., et al. Severe congenital neutropenia patients with point mutations in the granulocyte colony-stimulating factor (G-CSF) receptor mRNA express a normal G-CSF receptor protein. Blood. 1997;90(7):2839–2841. [PubMed] [Google Scholar]
- 94.Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. PNAS. 1988;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seledtsov V.I., Malashchenko V.V., Gazatova N.D., Meniailo M.E., Morozova E.M., Seledtsova G.V. Directs effects of granulocyte-macrophage colony stimulating factor (GM-CSF) on adaptive immunogenesis. Hum Vaccin Immunother. 2019;15(12):2903–2909. doi: 10.1080/21645515.2019.1614396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salh B., Hoeflick K., Kwan W., Pelech S. Granulocyte-macrophage colony-stimulating factor and interleukin-3 potentiate interferon-gamma-mediated endothelin production by human monocytes: role of protein kinase C. Immunology. 1998;95(3):473–479. doi: 10.1046/j.1365-2567.1998.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia J.A., Elson P., Tyler A., Triozzi P., Dreicer R. Sargramostim (GM-CSF) and lenalidomide in castration-resistant prostate cancer (CRPC): results from a phase I-II clinical trial. Urol Oncol. 2014;32(1) doi: 10.1016/j.urolonc.2012.12.004. 33 e11-7. [DOI] [PubMed] [Google Scholar]
- 98.Jimenez-Andrade J.M., Mantyh W.G., Bloom A.P., Xu H., Ferng A.S., Dussor G., et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46(2):306–313. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugiura A., Ohtori S., Yamashita M., Inoue G., Yamauchi K., Koshi T., Suzuki M., Norimoto M., Orita S., Eguchi Y., Takahashi Y., Watanabe T.S., Ochiai N., Takaso M., Takahashi K. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine (Phila Pa 1976) 2008;33(19):2047–2051. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- 100.Jeannerod M., Arbib M.A., Rizzolatti G., Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18(7):314–320. [PubMed] [Google Scholar]
- 101.Barker P.A. High affinity not in the vicinity? Neuron. 2007;53(1):1–4. doi: 10.1016/j.neuron.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 102.Gwak Y.S., Nam T.S., Paik K.S., Hulsebosch C.E., Leem J.W. Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci Lett. 2003;336(2):117–120. doi: 10.1016/s0304-3940(02)01251-x. [DOI] [PubMed] [Google Scholar]
- 103.Yao P., Ding Y., Wang Z., Ma J., Hong T., Zhu Y., et al. Impacts of anti-nerve growth factor antibody on pain-related behaviors and expressions of opioid receptor in spinal dorsal horn and dorsal root ganglia of rats with cancer-induced bone pain. Mol Pain. 2016;12 doi: 10.1177/1744806916644928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jimenez-Andrade J.M., Ghilardi J.R., Castaneda-Corral G., Kuskowski M.A., Mantyh P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sevcik M.A., Ghilardi J.R., Peters C.M., Lindsay T.H., Halvorson K.G., Jonas B.M., et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1–2):128–141. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 106.Buehlmann D., Ielacqua G.D., Xandry J., Rudin M. Prospective administration of anti-nerve growth factor treatment effectively suppresses functional connectivity alterations after cancer-induced bone pain in mice. Pain. 2019;160(1):151–159. doi: 10.1097/j.pain.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 107.Thomas C. Single-Fraction Radiotherapy and Early Subjective Improvement in Pain. JAMA oncology. 2017;3(7):960. doi: 10.1001/jamaoncol.2016.6723. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen Q., Chun S., Chow E., Komaki R., Liao Z., Zacharia R., et al. Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA oncology. 2019;5(6):872–878. doi: 10.1001/jamaoncol.2019.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scarborough B., Smith C. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182–196. doi: 10.3322/caac.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zaporowska-Stachowiak I., Łuczak J., Hoffmann K., Stachowiak K., Bryl W., Sopata M. Managing metastatic bone pain: New perspectives, different solutions. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;93:1277–1284. doi: 10.1016/j.biopha.2017.07.023. [DOI] [PubMed] [Google Scholar]