Graphical abstract
Keywords: Perfluorooctanoic acid, Liver, Metabolomic, Metabolism, Vitamin C
Highlights
-
•
Vitamin C reduces signs of PFOA-induced liver damage.
-
•
Protective role of vitamin C is associated with signaling networks control, suppressing linoleic acid metabolism.
-
•
Vitamin C reduces thiodiglycolic acid, and elevating glutathione in the liver.
-
•
The findings demonstrate the utility of vitamin C for preventing PFOA-induced hepatotoxicity.
Abstract
Introduction
Perfluorooctanoic acid (PFOA) is a compound used as an industrial surfactant in chemical processes worldwide. Population and cross-sectional studies have demonstrated positive correlations between PFOA levels and human health problems.
Objectives
Many studies have focused on the hepatotoxicity and liver problems caused by PFOA, with little attention to remediation of these problems. As an antioxidant, vitamin C is frequently utilized as a supplement for hepatic detoxification.
Methods
In this study, we use a mouse model to study the possible role of vitamin C in reducing PFOA-induced liver damage. Based on comparative transcriptomic and metabolomic analysis, we elucidate the mechanisms underlying the protective effect of vitamin C.
Results
Our results show that vitamin C supplementation reduces signs of PFOA-induced liver damage including total cholesterol and triglyceride levels increase, liver damage markers aspartate, transaminase, and alanine aminotransferase elevation, and liver enlargement. Further, we show that the protective role of vitamin C is associated with signaling networks control, suppressing linoleic acid metabolism, reducing thiodiglycolic acid, and elevating glutathione in the liver.
Conclusion
The findings in this study demonstrate, for the first time, the utility of vitamin C for preventing PFOA-induced hepatotoxicity.
Introduction
Perfluorooctanoic acid (PFOA), a common component of industrial and consumer products including food-containing and food-associated materials is frequently detected in humans [1]. Several population studies reveal its presence in human serum [2], [3], with cross-sectional studies linking it to human health problems such as metabolic, liver, coronary, immune system, and bone diseases [4], [5], [6], [7], [8]. As a detoxification venue, the liver is a reported PFOA target and a major site for PFOA accumulation [9]. Many studies have demonstrated the hepatotoxic effect of PFOA and its association to liver diseases like the non-alcoholic fatty liver disease (NAFLD) [10], [11]. These harmful effects of PFOA to liver are mainly attributed to elevated hepatic triglyceride and altered hepatic lipid oxidation, causing liver steatosis [12].
Vitamin C, also known as ascorbic acid, is an essential micronutrient for humans, vital for the functioning of body systems including collagen formation, iron absorption, and immune system maintenance. More importantly, it is an antioxidant, with numerous studies on mammals demonstrating its protective role in the liver. For example, in a study involving rats, vitamin C was reported to prevent NAFLD [13]. Similar results were obtained from guinea pigs; a high dose of vitamin C improved non-alcoholic steatohepatitis symptoms [14]. In addition, vitamin C supplementation was suggested to provide a protective effect on toxicant-induced hepatotoxicity [15]. Moreover, a study on mice showed that vitamin C retarded triptolide-induced acute hepatotoxicity through oxidative stress mitigation [16]. A similar protective effect of vitamin C was reported for fipronil-induced oxidative stress in mouse liver [16]. Furthermore, vitamin C exhibited protective effects on arsenic-induced hepatic anomalies and dexmedetomidine-induced liver injury in rats [18], [19].
In this study, we used a mouse model and integrative omics analysis including the transcriptomics and metabolomics to investigate the utility of vitamin C for relieving hepatotoxic effects of PFOA. Our results show that vitamin C consumption protects the liver from PFOA-induced triglyceride and total cholesterol elevation. In addition, vitamin C compensates for PFOA- mediated-reduction of high-density lipoprotein in mouse liver. More importantly, vitamin C attenuates PFOA-induced liver enlargement and liver damage. These data highlight a protective effect of vitamin C on PFOA-induced hepatotoxicity. Based on the transcriptomic and metabolomic analysis, followed by systematic bioinformatics analysis, we elucidate metabolic alterations caused by PFOA and the underlying mechanisms of the protective role of vitamin C. We show that PFOA can interfere with the synthesis of primary bile acids, steroid hormones, and unsaturated fatty acids by inducing the acyl-coenzyme A gene family in the liver. More importantly, we discovered that vitamin C also retards linoleic acid metabolism and reduces thiodiglycolic acid, while elevating glutathione levels, thereby explaining its protective effect on PFOA-induced hepatotoxicity.
Materials and methods
Animal maintenance and treatment
This animal study was conducted following the guidelines and regulations of the Animal Center Laboratory in Guilin Medical University (Approval No. GLMC201503003). The ICR mice (7-weeks of age) were purchased from Hunan Slark Jingda Experimental Animal Co., Ltd. (Changsha, China). The mice were acclimatized for a week before the 7 days exposure experiments. The mice were randomly divided into 3 groups (n = 8), with a PFOA group fed with 10 mg/kg PFOA per day as previously described [20], a PFOA + vitamin C group fed with 10 mg/kg of PFOA per day and treated with 100 mg/kg of vitamin C as previously reported [21], and a control group fed with DMSO. The mice were allowed free access to food and filtered water, and after feeding for 7 days, they were dissected and their liver tissues collected, snap-frozen with liquid nitrogen, and stored at −80 °C [22], [23].
Biochemical assay of serum samples
The blood levels of enzymes involved in liver functioning (alanine aminotransferase and aspartate aminotransferase), lipids (triglyceride, total cholesterol, low density lipoprotein, and high-density lipoprotein), and hepatic lipase for the samples were measured using commercially available kits (Nanjing Jiancheng Bioengineering Institute, China; Shanghai Elisa Biotech, China), as previously described [24], [25].
RNA isolation and transcriptomic analysis
The RNA samples were extracted from the liver tissues using the TRIzol reagent according to the manufacturer’s instruction. The quality of each sample was assessed by an Agilent 2100 Bioanalyzer system, and samples (n = 3 from each treatment group) with an RNA Integrity Number (RIN) > 8 were subjected to the transcriptome library construction as previously described [26]. Briefly, poly-T oligo-attached magnetic beads were used to enrich the mRNA (poly(A)-mRNA). The purified mRNA samples were fragmented and submitted to the cDNA library construction using Illumina mRNA Seq sample preparation kits. The library was sequenced on an Illumina Hiseq 4000 sequencer with 150 bp paired-end sequencing following the manufacturer's instructions. After sequencing, the adaptor sequence and sequencing primer were trimmed, and the low-quality reads (q quality scores lower than 20) were eliminated. The clean sequence reads were mapped to the UCSC (http://genome.ucsc.edu/) reference genome of Mus musculus using the HISAT package. The StringTie and edgeR were used for determining the expression levels of all genes. Genes with a log2 (fold change) > 1 and a statistical significance (p value < 0.05) were defined as differentially expressed genes (DEGs). The DEGs were then exposed to the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses, and the Ingenuity Pathways Analysis (IPA), to evaluate system-wide effects of PFOA in the liver and the protection potential of vitamin C.
Metabolomic analysis by ultra-performance liquid chromatography-high-resolution tandem mass spectrometry
Metabolites were extracted from the collected liver tissue samples as previously described [8]. Initially, the samples were extracted using 120 μL of prechilled 50% methanol, vortexed for 1 min, and incubated at room temperature for 10 min. After centrifugation at 4000 g for 20 min, the supernatants were analyzed by LC-MS following the manufacturer’s instructions. First, chromatographic separations were performed using an ultra-performance liquid chromatography (UPLC) system (SCIEX, UK) with an ACQUITY UPLC T3 column (100 mm × 2.1 mm, 1.8 µm, Waters, UK) involving reversed-phase separation. A TripleTOF 5600+ high-resolution tandem mass spectrometer (SCIEX, UK) was used to detect metabolites eluting from the column in the positive and negative ion modes (PIM and NIM). The MS data were acquired in the IDA mode with TOF mass ranging from 60 to 1200 Da. The acquired MS data were analyzed using the XCMS software, as previously described [8], with each ion identified based on the retention time (RT) and m/z data. An online KEGG and a human metabolome database (HMDB) were employed for annotating the metabolites by molecular mass-to-charge (m/z) matching of the samples. If the mass difference between the measured sample result and the database value was < 10 ppm, the metabolite was annotated, followed by confirming the metabolite’s molecular formula through isotopic distribution measurements. The peak intensities were further processed by metaX, with features that were detected in < 50% of QC samples or 80% of biological samples removed, while the remaining peaks with missing values were input with the k-nearest neighbor algorithm to enhance data quality. The Student’s t-test was performed for the samples to assess metabolite concentrations differences between the treatment and control groups. The p-value was adjusted for multiple tests using an FDR (Benjamini–Hochberg), and a supervised PLS-DA was conducted with metaX to discriminate variables between the groups. A p-value < 0.05 and a log2 (fold change) > 1 were set as the thresholds for significant differential change of the metabolites. The VIP value was then calculated, with a cut-off value of 1.0 used for selecting important features. The deregulated metabolites were further assessed using the web-based analytical tool MetaboAnalyst [27].
Results and discussion
Liver protection from PFOA-induced damage by vitamin C supplementation
Although PFOA is banned in many countries, its detection in human population is still common due to its high persistence in the environment. The association between PFOA levels and human diseases, especially liver steatosis is reported by many studies [28], [5]. Therefore, identifying a supplement for reducing the hepatotoxicity of PFOA can be helpful for the public. Vitamin C, for example, is an antioxidant commonly for boosting liver function [29], [30]. In this study, we sought to evaluate the possible protective role of vitamin C on PFOA-induced hepatotoxicity using a mouse model. In the experimental setting, we used a relative high dose of PFOA (10 mg/kg PFOA per day) to make sure the induction of hepatotoxicity in our mouse model as the previous studies [31], [32], [33], [34]. Our data for measured lipids parameters related to liver diseases show that PFOA increases the total cholesterol and triglyceride levels, while reducing high-density lipoproteins in the liver (Table 1).
Table 1.
Liver Parameters Affected by PFOA Hepatotoxicity for the Control, PFOA Treatment, and PFOA + VC Treatment Sample Groups.
| Parameters | Control | PFOA | VC + PFOA |
|---|---|---|---|
| Body mass (g) | 45.65 ± 2.06 | 44.83 ± 2.16 | 43.23 ± 3.72 |
| Liver mass (g) | 1.56 ± 0.12 | 3.71 ± 0.18a | 2.35 ± 0.24b |
| Liver index (%) (liver/body weight × 100%) | 3.67 ± 0.21 | 8.9 ± 0.4a | 5.73 ± 0.4b |
| Fasting blood glucose (mmol/L) | 4.7 ± 0.5 | 6.4 ± 1.2 | 4.8 ± 0.8 |
| Total cholesterol (mmol/L) | 3.11 ± 0.15 | 6.6 ± 0.14a | 3.25 ± 0.26b |
| Triglyceride (mmol/L) | 0.68 ± 0.06 | 1.07 ± 0.25a | 0.74 ± 0.05b |
| Alanine aminotransferase (U/L) | 8.71 ± 0.76 | 17.26 ± 1.18a | 11.48 ± 0.45b |
| Aspartate aminotransferase (U/L) | 12.67 ± 1.93 | 29.28 ± 1.52a | 19.02 ± 1.38b |
| Low density lipoprotein (mmol/L) | 0.22 ± 0.03 | 0.35 ± 0.05 | 0.15 ± 0.05b |
| High density lipoprotein (mmol/L) | 7.99 ± 0.86 | 3.24 ± 0.72a | 4.94 ± 0.24b |
| Hepatic lipase (μg/mL) | 126.16 ± 15.82 | 129.85 ± 21.9 | 161.93 ± 6.97b |
Note: Paired Student’s t test (GraphPad Prism 3.02); aP<0.05 vs control; bP<0.05 vs PFOA.
These results are concordant with previous reports, with a cross-sectional study of a middle-aged Danish population highlighting a positive correlation between plasma PFOA levels and total cholesterol [35]. In addition, a study on mice demonstrated that PFOA consumption altered the expression of hepatic sterol genes, causing hypercholesterolemia [36]. Further, PFOA treatment also induced cholesterol and triglyceride in liver. PFOA-induced triglyceride has been reported to interfere with mitochondrial beta-oxidation, causing liver steatosis [12], [11]. In our study, liver damage is highlighted by two indicators, aspartate transaminase (AST) and alanine aminotransferase (ALT) (Table 1) [37]. Moreover, liver mass and liver index increases were observed in the PFOA treatment group compared to the control group (Table 1). Interestingly, vitamin C supplementation could attenuate all these indicator inductions in the liver caused by PFOA treatment (Table 1). Although many studies have demonstrated the positive effects of vitamin C on chemical-induced hepatotoxicity and liver injury [17], [18], [19], our study is the first to suggest a protective role of this vitamin in PFOA-induced liver damage. Therefore, to better understand the mechanisms underlying this protective role of vitamin C, integrative omics analyses including comparative transcriptomic and metabolomic analyses for determining the profile changes of genes and metabolites, respectively, was conducted.
PFOA hepatoxicity by alteration of fatty acids and lipids metabolism
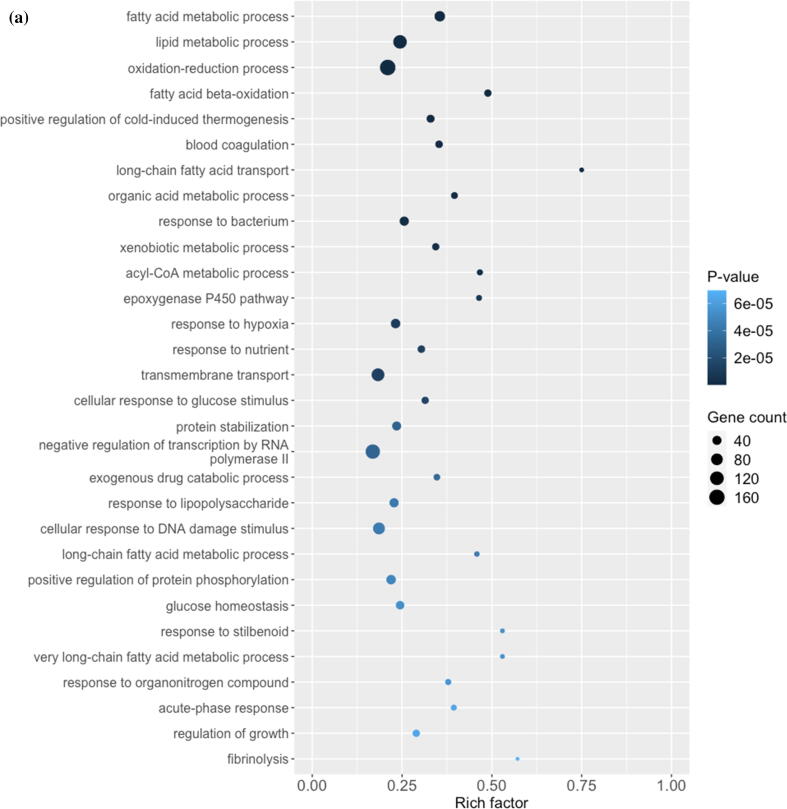
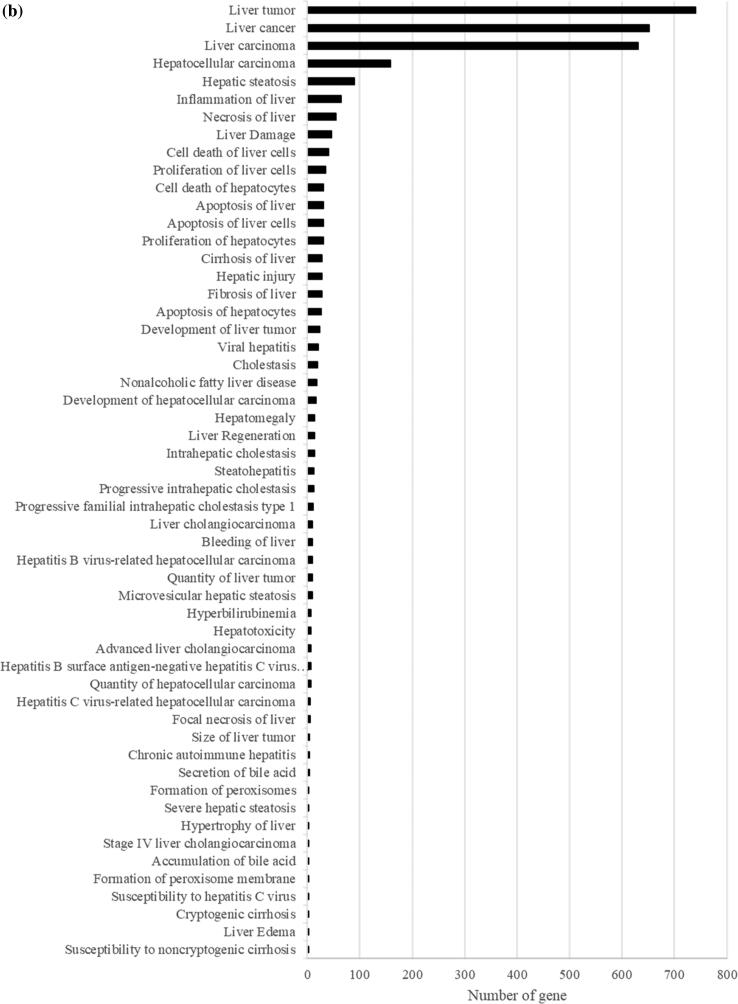
From the transcriptomic analysis, we obtained at least 31 million quality-trimmed clean reads per sample, producing 61.05 Gb of quality-trimmed bases in total (Supplementary Table 1), with a mapping rate of over 90% relative to the mouse reference genome (Supplementary Table 1). By comparing the gene expression profiles of PFOA and the control group, 2426 differentially expressed genes (DEGs) including 1066 upregulated and 1360 downregulated are found (Supplementary Fig. 1 and Supplementary Table 2). The DEGs were then subjected to the GO analysis and IPA to understand the biological functions alterations and hepatotoxicity caused by the PFOA treatment, respectively. The GO analysis shows that the PFOA exposure significantly interferes with the metabolism of many fatty acids and lipids (p < 0.05), particularly fatty acids beta-oxidation, long chain fatty acids transport, and the acyl-CoA metabolic process (Fig. 1a). Further, the IPA uncovers the critical biological processes and key toxicological responses to PFOA exposure. The Tox Functions analysis (IPA-TOX) suggests that PFOA causes liver and hepatic toxicity through the alteration of live cell proliferation and apoptosis of hepatocytes (Fig. 1b and Supplementary Table 3). These changes induce variable levels of liver inflammation including liver necrosis, hepatic steatosis, and steatohepatitis (Fig. 1b and Supplementary Table 3), with liver cancer and hepatocellular carcinoma as possible outcomes (Fig. 1b and Supplementary Table 3). Our results support previous reports that PFOA induces hepatotoxicity, particularly liver steatosis, through imbalanced fatty acids and lipids homeostasis as well as inducing β-oxidation [38], [39]. However, the detail molecular mechanisms underlying the hepatoxicity of PFOA remain largely unknown. In this study, we employ comparative transcriptome, followed by GO analysis and IPA in identifying key genes and delineating molecular mechanisms for PFOA-induced liver diseases. We observed that the acyl-coenzyme A-related gene family including acyl-CoA oxidase and acyl-CoA thioesterase were identified in PFOA-treated liver. This gene family is closely associated to hepatic oxidative stress, with our data demonstrating acyl-CoA oxidase induction such as ACOX1 and ACOX2. A liver cancer study showed that peroxisomal ACOX1 inhibition prevents oxidative damage [40]. The other acyl-CoA oxidase, ACOX2, is involved in bile acid production, a process that is associated with oxidative stress and triglyceride levels regulation [41]. In fact, overexpression of ACOX2 is reported to cause oxidative stress and death in HepG2 cells [42]. Beside the acyl-CoA oxidase, our results also demonstrate that PFOA treatment caused alterations in many acyl-CoA thioesterases (ACOTs) including ACOT1, ACOT2, ACOT3, ACOT4, ACOT6, ACOT8, ACOT9, and ACOT13 in mouse liver. These ACOTs play important cellular roles in fatty acids metabolism through the hydrolysis of acyl-CoA ester molecules, yielding coenzyme A and non-esterified fatty acid [43]. Most ACOTs are found in the mitochondrial matrix and are responsible for mitigating β-oxidation overload [44]. The ACOT2 is prominent in highly oxidative tissues such as the liver, and mitochondrial ACOT2 is reported to enhance fatty acid oxidation within those tissues [45]. Thus, identifying these candidates may provide biomarkers for PFOA-induced hepatotoxicity risk assessment.
Fig. 1.
Plots showing the effect of PFOA on biological processes and functions of mouse liver through interference of gene expression. This comparative transcriptomic analysis demonstrates differential gene expression caused by PFOA (Control vs PFOA group). (a) DEGs evaluated by the Gene Ontology (GO) enrichment analysis. The rich factor plot exhibits the PFOA-altered biological processes (p < 0.05). (b) IPA-Tox of Ingenuity Pathway Analysis (IPA) data demonstrating the hepatotoxicity from PFOA (p < 0.05).
Liver protection by vitamin C against PFOA-induced hepatotoxicity
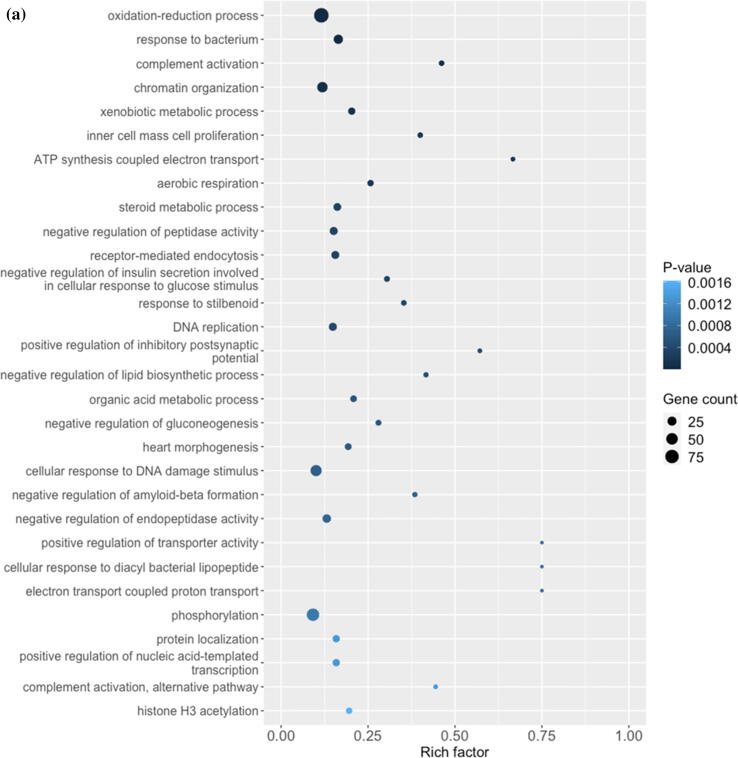
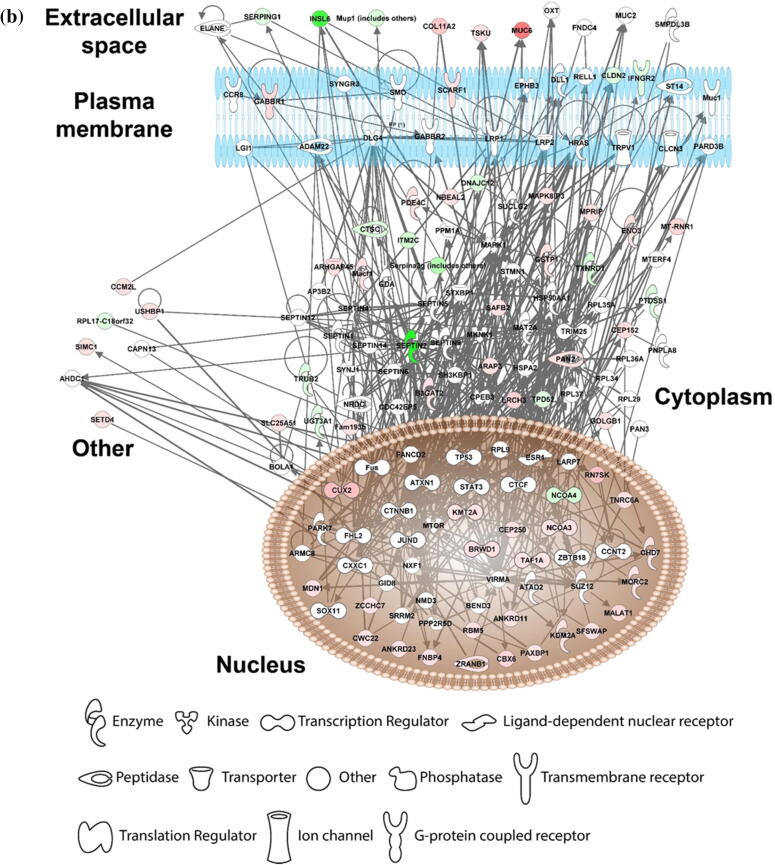
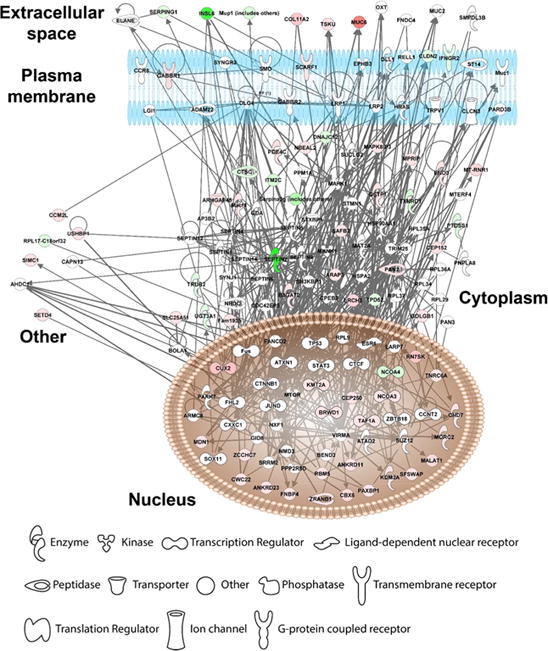
Our in vivo mice data show that vitamin C supplementation relieves PFOA-induced hepatotoxicity; thus, we compare the gene profiles of the vitamin C + PFOA and PFOA groups to further understand the mechanisms underlying this protective role of vitamin C. We found 306 DEGs including 185 upregulated and 121 downregulated (Supplementary Fig. 2 and Supplementary Table 4), with the GO analysis revealing oxidation–reduction as the principal biological process (Fig. 2a). More importantly, the vitamin C supplementation exhibits an inverse relationship to the PFOA level, reflecting negative regulation of lipid biosynthesis that can protect the liver against hepatotoxicity (Fig. 2a). Vitamin C is used as a supplement in our daily life [46], with its anti-oxidative and inflammatory properties exploited for treating liver diseases such as the alcoholic liver disease and liver cancer [47], [48]. So, our study, for the first time, provides data indicating vitamin C supplementation can protect the liver against environmental pollutants toxicity. Focusing on genes showing reversed expression under vitamin C supplementation, we found 87 genes that are upregulated by PFOA but downregulated by vitamin C or vice versa (Table 2). These genes were then processed to understand the liver protection mechanisms using IPA. Our results show that vitamin C controls the gene function expression at different cellular levels including the extracellular space, plasma membrane, cytoplasm, and nucleus (Fig. 2b). In the cellular matrix, vitamin C alters the SERPING1, TSKU, MUC6, and COL11A2 expression (Fig. 2b). The SERPING1, also called C1 inhibitor, interacts with some infection agents to influence inflammation suppression [49]. It is also reported to overcome many diseases including sepsis and bacterial infections [50]. The TSKU is a newly identified target of the peroxisome proliferator-activated receptor alpha (PPARα), playing an important role in lipids metabolism [51]. More importantly, PPARα activation is a major hepatocarcinogenic action of the PFOA [52]. Conversely, the MUC6, belonging to the mucin family, a large family of O-glycoproteins with high carbohydrate content [53], is critical in maintaining cellular functions, especially through epithelial surfaces. Its aberrant expression influences tumor growth and oxidative stress-induced apoptosis for many cancer types [54], [55]. In addition, vitamin C induces COL11A2 expression, causing activation of the scavenger receptor SCARF1 (Fig. 2b). The SCARF1 is responsible for recognizing and engulfing apoptotic cells via the complement component. A SCARF1-deficient mice study demonstrated that losing SCARF1 impairs apoptotic cells uptake [56]. So, vitamin C induced SCARF1 likely promotes unwanted cells removal in the liver. Beside SCARF1, vitamin C supplementation reduces the expression of the cell–cell adhesion molecules CLDN2 and cytokine receptor (IFNGR2), which are associated with liver diseases (Fig. 2b). The CLDN2 belongs to the claudin protein family [57], and a knockout (Cldn2(-/-)) mice study suggested that its depletion limits bile formation and secretion, which are essential functions of the hepatobiliary system [58]. In addition, CLDN2 is associated with oxidative stress in type 1 diabetic nephropathy [59]. Conversely, IFNGR2 encodes the non-ligand-binding beta chain of the gamma interferon receptor [60], and a clinical study highlighted its strong link to fibrosis progression in patients with chronic hepatitis C virus [61]. Our data also suggest that vitamin C relieves PFOA-induced hepatic steatosis. Therefore, vitamin C may play this role through the reduction of IFNGR2. Overall, our transcriptomic analysis demonstrates that vitamin C protects the liver from PFOA toxicity through the modulation of a series of cell signaling pathways.
Fig. 2.
Diagrams showing vitamin C relieves FOA-induced hepatotoxicity by controlling gene expression. The comparative transcriptomic analysis reveals differential gene expression induced by vitamin C (PFOA vs vitamin C + PFOA group). (a) The DEGs were assessed by the Gene Ontology (GO) enrichment analysis. The rich factor plot shows that the vitamin C-relieved PFOA-altered biological processes (p < 0.05). (b) Data from gene network analysis using IPA, demonstrating cell signaling involved in the protective action of vitamin C.
Table 2.
Data summarizing the effect of Vitamin C on PFOA-induced gene expression.
| Gene symbol | Gene name | PFOA/Ctrl (up/down) | VC + PFOA/PFOA (up/down) | change |
|---|---|---|---|---|
| Ankrd11 | ankyrin repeat domain 11 | down | up | down-up |
| Ankrd23 | ankyrin repeat domain 23 | down | up | down-up |
| Arap3 | ArfGAP with RhoGAP domain, ankyrin repeat and PH domain 3 | down | up | down-up |
| Arhgap45 | Rho GTPase activating protein 45 | down | up | down-up |
| B3gat2 | beta-1,3-glucuronyltransferase 2 (glucuronosyltransferase S) | down | up | down-up |
| Brwd1 | bromodomain and WD repeat domain containing 1 | down | up | down-up |
| Cbx6 | chromobox 6 | down | up | down-up |
| Ccm2l | cerebral cavernous malformation 2-like | down | up | down-up |
| Cep152 | centrosomal protein 152 | down | up | down-up |
| Cep250 | centrosomal protein 250 | down | up | down-up |
| Chd7 | chromodomain helicase DNA binding protein 7 | down | up | down-up |
| Cldn2 | claudin 2 | up | down | up-down |
| Col11a2 | collagen, type XI, alpha 2 | down | up | down-up |
| Ctsc | cathepsin C | up | down | up-down |
| Cux2 | cut-like homeobox 2 | down | up | down-up |
| Cwc22 | CWC22 spliceosome-associated protein | down | up | down-up |
| Cyp2j9 | cytochrome P450, family 2, subfamily j, polypeptide 9 | down | up | down-up |
| Dnajc12 | DnaJ heat shock protein family (Hsp40) member C12 | up | down | up-down |
| Doc2g | double C2, gamma | down | up | down-up |
| Eno3 | enolase 3, beta muscle | down | up | down-up |
| Fnbp4 | formin binding protein 4 | down | up | down-up |
| Gabbr1 | gamma-aminobutyric acid (GABA) B receptor, 1 | down | up | down-up |
| Gk | glycerol kinase | up | down | up-down |
| Golgb1 | golgi autoantigen, golgin subfamily b, macrogolgin 1 | down | up | down-up |
| Gstp2 | glutathione S-transferase, pi 2 | down | up | down-up |
| Gtf2ird2 | GTF2I repeat domain containing 2 | down | up | down-up |
| Hspd1-ps3 | heat shock protein 1 (chaperonin), pseudogene 3 | up | down | up-down |
| Ifi47 | interferon gamma inducible protein 47 | up | down | up-down |
| Ifngr2 | interferon gamma receptor 2 | up | down | up-down |
| Insl6 | insulin-like 6 | up | down | up-down |
| Itm2c | integral membrane protein 2C | up | down | up-down |
| Kcnq1ot1 | KCNQ1 overlapping transcript 1 | down | up | down-up |
| Kdm2a | lysine (K)-specific demethylase 2A | down | up | down-up |
| Kmt2a | lysine (K)-specific methyltransferase 2A | down | up | down-up |
| Lrch3 | leucine-rich repeats and calponin homology (CH) domain containing 3 | down | up | down-up |
| Macf1 | microtubule-actin crosslinking factor 1 | down | up | down-up |
| Malat1 | metastasis associated lung adenocarcinoma transcript 1 (non-coding RNA) | down | up | down-up |
| Mapk8ip3 | mitogen-activated protein kinase 8 interacting protein 3 | down | up | down-up |
| Mdn1 | midasin AAA ATPase 1 | down | up | down-up |
| Mfsd2b | major facilitator superfamily domain containing 2B | down | up | down-up |
| Mir99ahg | Mir99a and Mirlet7c-1 host gene (non-protein coding) | down | up | down-up |
| Morc2a | microrchidia 2A | down | up | down-up |
| Mprip | myosin phosphatase Rho interacting protein | down | up | down-up |
| mt-Rnr1 | mitochondrially encoded 12S rRNA | down | up | down-up |
| mt-Rnr2 | mitochondrially encoded 16S rRNA | down | up | down-up |
| mt-Ti | mitochondrially encoded tRNA isoleucine | down | up | down-up |
| Muc6 | mucin 6, gastric | down | up | down-up |
| Mup18 | major urinary protein 18 | up | down | up-down |
| Nbeal2 | neurobeachin-like 2 | down | up | down-up |
| Ncoa3 | nuclear receptor coactivator 3 | down | up | down-up |
| Ncoa4 | nuclear receptor coactivator 4 | up | down | up-down |
| Oaz1-ps | ornithine decarboxylase antizyme 1, pseudogene | up | down | up-down |
| Ovgp1 | oviductal glycoprotein 1 | down | up | down-up |
| Pakap | paralemmin A kinase anchor protein | down | up | down-up |
| Pan2 | PAN2 poly(A) specific ribonuclease subunit | down | up | down-up |
| Paxbp1 | PAX3 and PAX7 binding protein 1 | down | up | down-up |
| Pde4c | phosphodiesterase 4C, cAMP specific | down | up | down-up |
| Pgam1-ps2 | phosphoglycerate mutase 1, pseudogene 2 | up | down | up-down |
| Pgk1-rs7 | phosphoglycerate kinase-1, related sequence-7 | up | down | up-down |
| Ptdss1 | phosphatidylserine synthase 1 | up | down | up-down |
| Rapgef4os2 | Rap guanine nucleotide exchange factor (GEF) 4, opposite strand 2 | down | up | down-up |
| Rbm5 | RNA binding motif protein 5 | down | up | down-up |
| Rmrp | RNA component of mitochondrial RNAase P | down | up | down-up |
| Rn7s1 | 7S RNA 1 | down | up | down-up |
| Rn7s2 | 7S RNA 2 | down | up | down-up |
| Rn7sk | RNA, 7SK, nuclear | down | up | down-up |
| Rpl28-ps3 | ribosomal protein L28, pseudogene 3 | up | down | up-down |
| Rpl31-ps9 | ribosomal protein L31, pseudogene 9 | up | down | up-down |
| Safb2 | scaffold attachment factor B2 | down | up | down-up |
| Scarf1 | scavenger receptor class F, member 1 | down | up | down-up |
| Sept2 | septin 2 | up | down | up-down |
| Serpina3f | serine (or cysteine) peptidase inhibitor, clade A, member 3F | up | down | up-down |
| Serping1 | serine (or cysteine) peptidase inhibitor, clade G, member 1 | up | down | up-down |
| Setd4 | SET domain containing 4 | down | up | down-up |
| Sfswap | splicing factor SWAP | down | up | down-up |
| Simc1 | SUMO-interacting motifs containing 1 | down | up | down-up |
| Slc25a51 | solute carrier family 25, member 51 | down | up | down-up |
| Taf1a | TATA-box binding protein associated factor, RNA polymerase I, A | down | up | down-up |
| Tnrc6a | trinucleotide repeat containing 6a | down | up | down-up |
| Tpd52 | tumor protein D52 | up | down | up-down |
| Trub2 | TruB pseudouridine (psi) synthase family member 2 | up | down | up-down |
| Tsku | tsukushi, small leucine rich proteoglycan | down | up | down-up |
| Txnrd1 | thioredoxin reductase 1 | up | down | up-down |
| Ugt3a1 | UDP glycosyltransferases 3 family, polypeptide A1 | up | down | up-down |
| Ushbp1 | USH1 protein network component harmonin binding protein 1 | down | up | down-up |
| Zcchc7 | zinc finger, CCHC domain containing 7 | down | up | down-up |
| Zranb1 | zinc finger, RAN-binding domain containing 1 | down | up | down-up |
Classification of metabolites identified in mice livers
Since the liver is an important organ for toxicants metabolism, we measured changes in liver metabolites through metabolomic analysis to elucidate mechanisms underlying the protective role of vitamin C. MetaX analysis enabled metabolites identification based on the retention time (RT) and m/z data; the untargeted metabolomic analysis yielded 9451 features in the PIM and 8122 features in the NIM. The features were evaluated through an HMDB search. In single-stage mass spectrometry (MS1) analysis, we identified 5091 metabolites in the PIM (Supplementary Table 5) and 3326 metabolites in the NIM (Supplementary Table 6) in the liver tissue samples. Based on the identified metabolites classification, the most abundant metabolites in the samples are lipid molecules, phenylpropanoids, and polyketides (Supplementary Fig. 3).
Perfluorooctanoic acid-altered liver metabolism
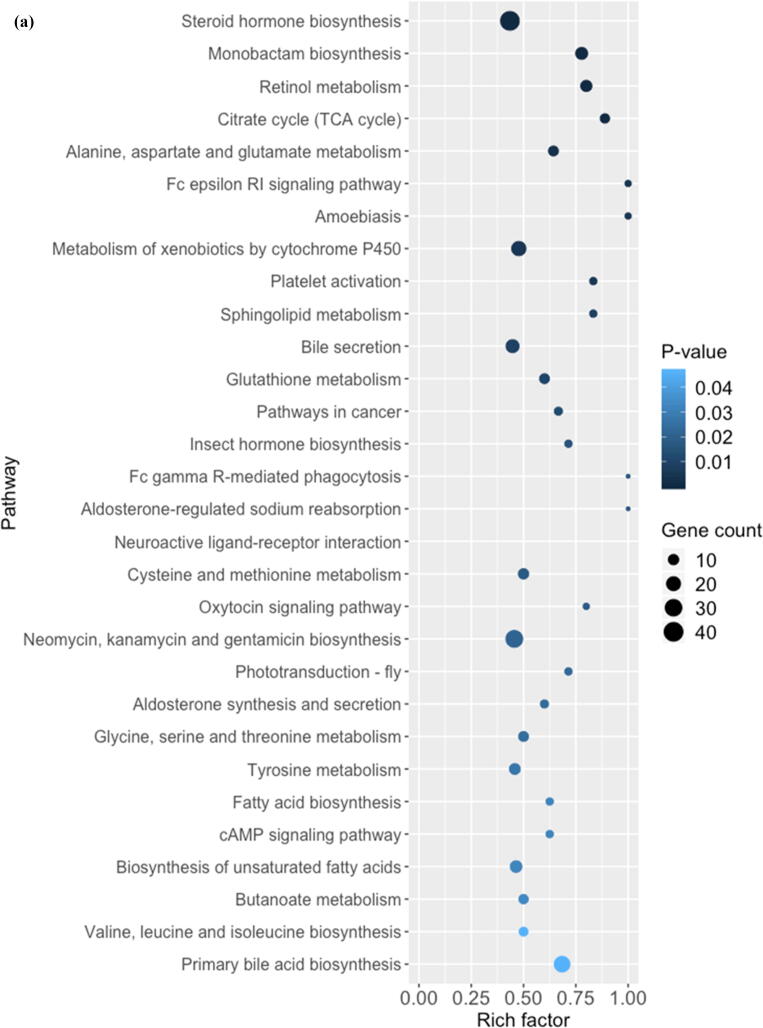
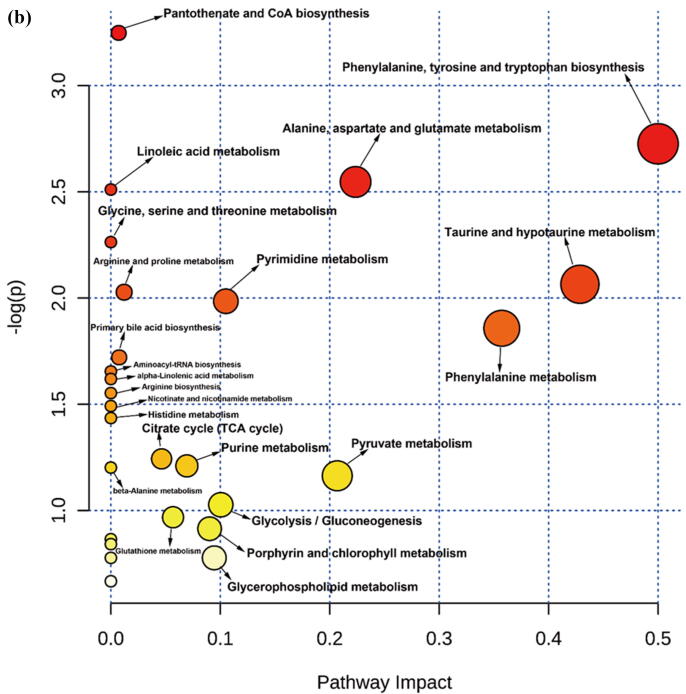
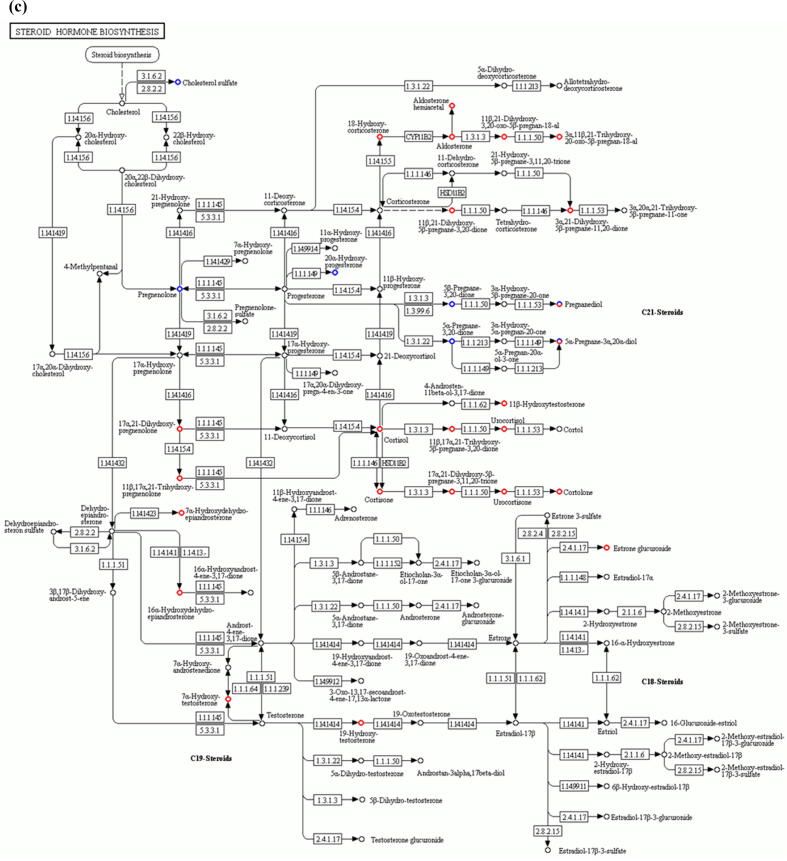
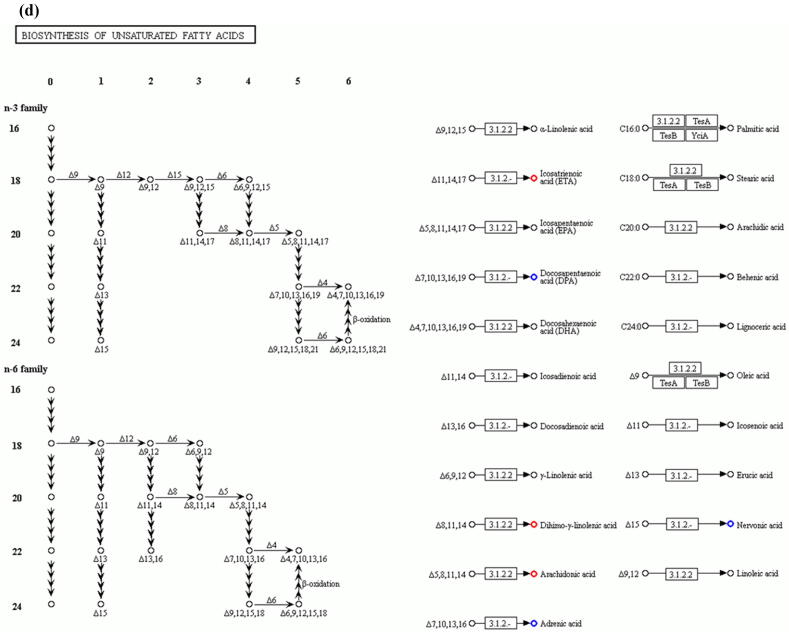
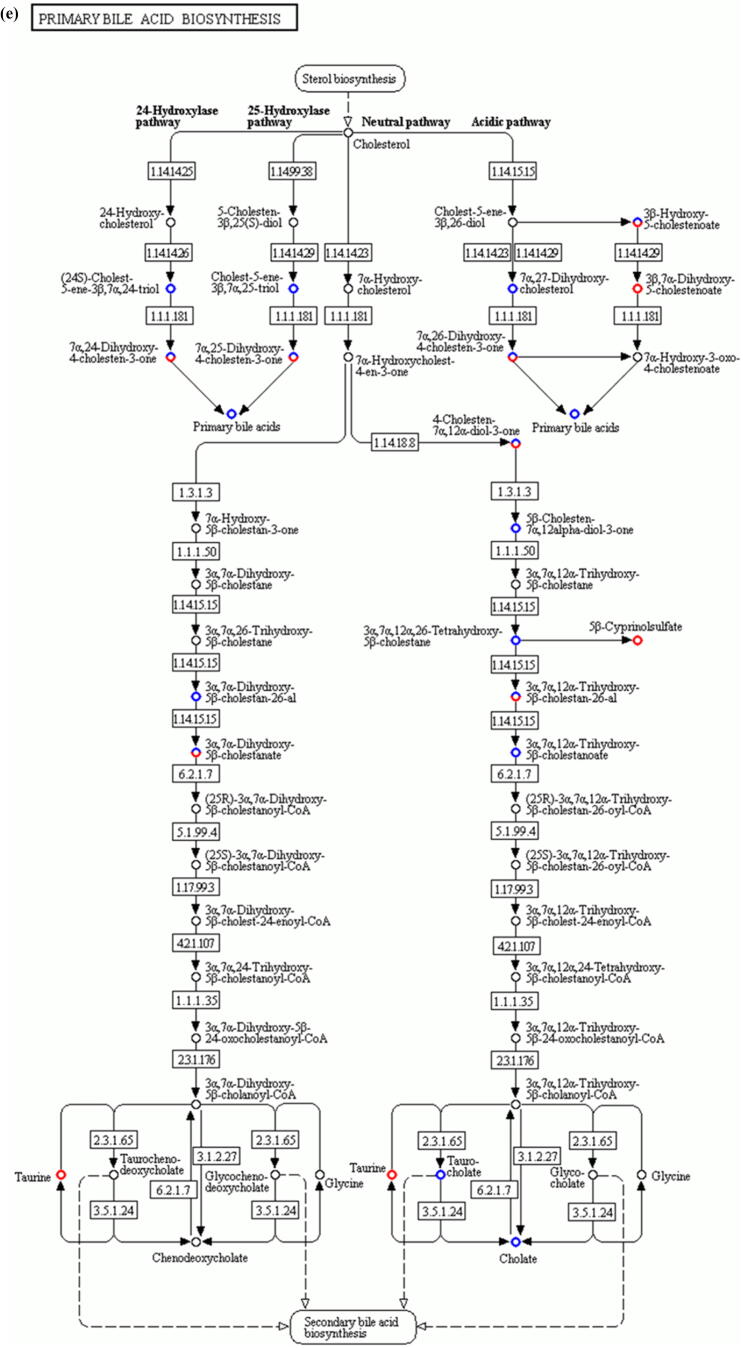
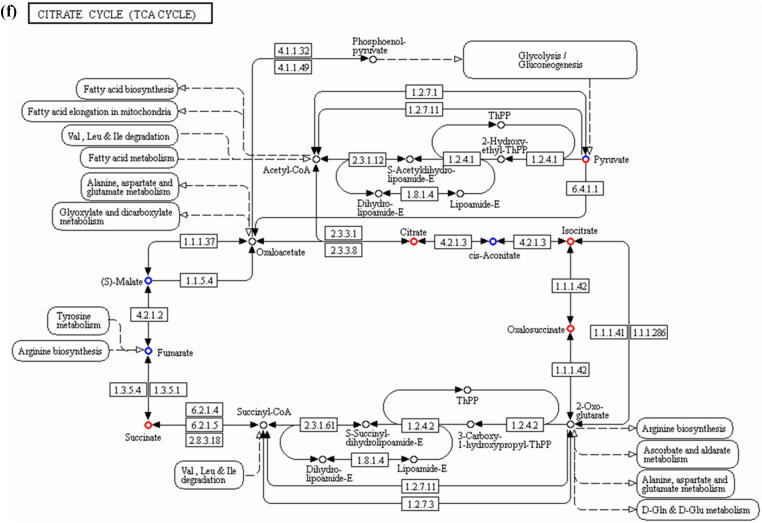
Comparative metabolomic analysis is then used to determine the hepatic toxicological effects of PFOA. In the MS1 analysis, we compare the metabolites levels in the PFOA treatment with those of the control group; we found 741 upregulated and 736 downregulated metabolites in the PIM (Supplementary Fig. 4 and Supplementary Table 7) and 759 upregulated and 643 downregulated metabolites in the NIM (Supplementary Figure 5 and Supplementary Table 8). The deregulated metabolites are then examined by the KEGG pathway analysis and MetaboAnalyst online tool for understanding the harmful effects of PFOA. Our results reveal that the PFOA treatment alters many biological processes and the biosynthesis of essential products in the liver (Fig. 3a and b). Many of these processes such as steroid hormones (Fig. 3c) and unsaturated fatty acids (Fig. 3d) biosynthesis are affected by liver disorders. Additionally, the PFOA treatment interrupted primary bile acid biosynthesis (Fig. 3e) and energy production through the TCA cycle (Fig. 3f). Bile acids are steroid acids that are synthesized and conjugated with taurine or glycine in mammalian and other vertebrate livers. A major function of bile acid is facilitating biliary cholesterol secretion, which is essential for liver protection from cholesterol toxicity [62]. The bile acid metabolism alteration by PFOA probably explains the elevated observed liver cholesterol (Table 1). Steroid hormones derived from cholesterol are synthesized mainly in endocrine glands including the gonads, with circulating steroids extensively metabolized in the liver before conversion and catabolism to active forms [63]. Glucocorticoids, a kind of steroid hormones, are responsible for diverse metabolic functions and driving the NAFLD [64]. In addition, unsaturated fatty acids levels are closely associated with hepatic steatosis [65]. Therefore, the bile acids, steroid hormones, and unsaturated fatty acids alterations by PFOA treatment likely contributed to the observed liver damage.
Fig. 3.
Diagrams demonstrating PFOA altered biological functions of the liver through metabolites interference. The altered metabolites are evaluated by the Gene Ontology (GO) enrichment analysis and MetaboAnalyst online tool. (a) Rich factor plot showing the ratio of changed metabolites to the number of metabolites annotated in the altered biological processes (p < 0.05). (b) Pathway analysis data for metabolic pathways altered by the PFOA hepatotoxicity. (c) Illustration of PFOA alteration of the metabolites involved in steroid hormone biosynthesis. (d) Display of PFOA alteration of the metabolites connected with biosynthesis of unsaturated fatty acid. (e) Evidence of PFOA alteration of the metabolites involved in primary bile acid biosynthesis. (f) Diagram showing PFOA also affected the metabolites involved in the citrate cycle (TCA cycle). The red circles represent the metabolites upregulation, while blue circles denote downregulation.
Protective effect of vitamin C on PFOA-induced liver damage
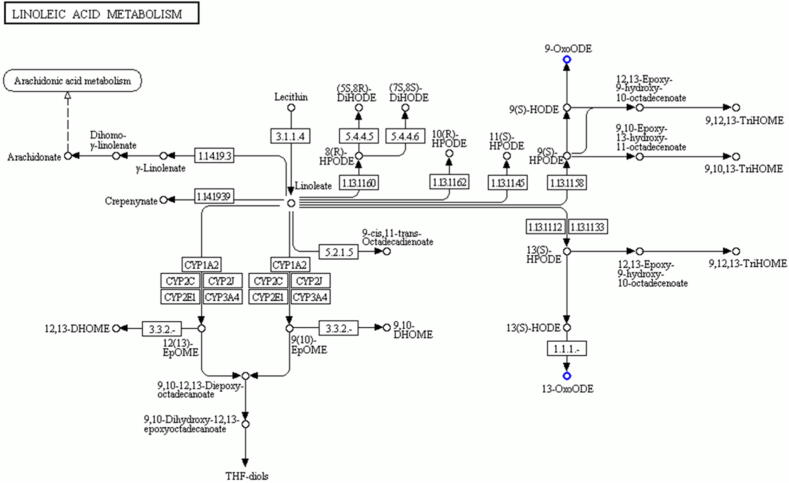
We attempted to elucidate the protective effect of vitamin C on PFOA-induced hepatic toxicity by conducting a subtractive metabolomic analysis. By comparing the metabolic profiles of the PFOA group samples and the PFOA + vitamin C group using the MS1 analysis, we identified 132 (36 upregulated and 96 downregulated) (Supplementary Table 9) and 90 (19 upregulated and 71 downregulated) (Supplementary Table 10) metabolites that changed between the groups under the PIM (Supplementary Figure 6) and the NIM (Supplementary Figure 7), respectively. The deregulated metabolites were then subjected to the KEGG pathway analysis. Our results show that vitamin C supplementation reduces the levels of many metabolites including 9-OxoODE and 13-OxoODE, that are involved in linoleic acid metabolism (Table 3 and Fig. 4). Dietary conjugated linoleic acid is reported to induce severe hepatic steatosis in mice [66]. More importantly, 9(S)-HODE and 13(S)-HODE, the precursors of 9-OxoODE and 13-OxoODE, respectively, were elevated under oxidative stress and contributed to a hepatic proinflammatory response, thereby exacerbating liver injury [67]. Therefore, the linoleic acid metabolites reduction by vitamin C supplementation can retard PFOA-induced hepatic inflammatory response and hepatotoxicity. Additionally, vitamin C supplementation can cause downregulation of thiodiglycolic acid (TDGA) by the cytochrome P450 system (Table 3). An epidemiological study conducted in Taiwan demonstrated that urinary TDGA is associated with an increased risk of non-alcoholic fatty liver disease in children [68]. Furthermore, urinary TDGA levels are also linked with hepatotoxicity susceptibility [69]. Therefore, the TDGA levels reduction further supports our finding that vitamin C provides protection against PFOA-induced liver damage. Lastly, our data highlight glutathione upregulation under vitamin C treatment (Table 3). Glutathione is an antioxidant that can also be elevated in red blood cells by vitamin C [70] A similar protective effect was reported in a study on rat, in which combining vitamins E and C decreased ethanol-induced hepatic glutathione peroxidase activity and hepatic fibrosis [71]. For the limitation of this study, we used a relative high dose of PFOA (10 mg/kg PFOA per day), which is much higher than the environmentally relevant concentrations. Although the dose is similar to many previous PFOA studies in mouse [31], [32], [33], [34], dose-dependent study would further secure the finding of our work.
Table 3.
Summary of Metabolic Pathways for Vitamin C Associated with PFOA-Induced Toxicity.
| Pathway | number of metabolites | p-value | metabolites |
|---|---|---|---|
| Metabolism of xenobiotics by cytochrome P450 | 4 | 0.0035 | Thiodiacetic acid, (1R)-Hydroxy-(2R)-N-acetyl-L-cysteinyl-1,2-dihydronaphthalene, (1R)-N-Acetyl-L-cysteinyl-(2R)-hydroxy-1,2-dihydronaphthalene, (1S)-Hydroxy-(2S)-N-acetyl-L-cysteinyl-1,2-dihydronaphthalene |
| ABC transporters | 4 | 0.037 | Glutathione, Raffinose, Maltotriose, Isomaltotriose |
| Inflammatory mediator regulation of TRP channels | 2 | 0.025 | 1,8-Cineole, Phorbol |
| Linoleic acid metabolism | 2 | 0.0087 | 13-OxoODE, 9-OxoODE |
| Renal cell carcinoma | 1 | 0.014 | (S)-Malate |
Fig. 4.
Evidence of vitamin C relief of PFOA-induced hepatotoxicity via changes of metabolites in the liver. The comparative metabolomic analysis used for determining the metabolites changes involved in the vitamin C protection on PFOA-induced hepatotoxicity (PFOA vs vitamin C + PFOA group). GO analysis highlighting reduced linoleic acid metabolism involvement in the vitamin C-relief of PFOA-induced hepatotoxicity.
In conclusion, this study identified PFOA-responsive genes and metabolites as biomarkers for risk assessment. Also, our results provided novel detail mechanism underlying the protective role vitamin C supplementation on PFOA-induced hepatotoxicity. The hepatic protective effect of vitamin C against PFOA was through the mediation of linoleic acid metabolism, thiodiglycolic acid suppression, and glutathione induction.
Compliance with Ethics Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was partially supported by the National Natural Science Foundation of China (Nos. 81973574, 81660610 and 81560134), the National Natural Science Foundation of Guangxi Province (Nos. 2019GXNSFBA185015, 2019GXNSFFA245001, 2017GXNSFDA198019, 2018GXNSFBA281025, and 2018GXNSFAA281159), and Scientific Research and Technology Development Project of Guigang City (No. 1908029). Keng Po Lai was supported by the Hong Kong SAR, Macao SAR, and Taiwan Province Talented Young Scientist Program of Guangxi.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the Guilin Medical University, China (Approval no. GLMC201503003).
Biographies
Rong Li is a researcher at Guilin Medical University. He plotted the figures based on bioinformatics analysis, using omics methods.
Chao Guo is a pharmacist of Department of Pharmacy, Guigang City People's Hospital. He conducted data analysis and performed literature searches.
Xiao Lin work in Hong Kong Bioinformatics Centre, The Chinese University of Hong Kong who contributed to data collection and figure plotting.
Ting Fung Chan work in Hong Kong Bioinformatics Centre, The Chinese University of Hong Kong who contributed to data collection and figure plotting.
Min Su is a professor of Guilin Medical University who worked on analyzing data and literature searches.
Zhiyong Zhang is a professor of Guilin Medical University who worked on analyzing data and literature searches.
Keng Po Lai is a researcher at Guilin Medical University. His research is focused on revealing mechanism networks underlying complex diseases, through deep sequencing of transcriptomes, small RNA transcriptomes, and methylomes, which is then integrated with data from environmental pollutants.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.04.003.
Contributor Information
Zhiyong Zhang, Email: glmcrapzz@163.com, rpazz@163.com.
Keng Po Lai, Email: glmu_kengplai@yeah.net.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Susmann H.P., Schaider L.A., Rodgers K.M., Rudel R.A. Dietary habits related to food packaging and population exposure to PFASs. Environ Health Perspect. 2019;127 doi: 10.1289/EHP4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner E.M., Bloom M.S., Wu Q., Kannan K., Yucel R.M., Shrestha S., et al. Occupational exposure to perfluoroalkyl substances and serum levels of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in an aging population from upstate New York: a retrospective cohort study. Int Arch Occup Environ Health. 2018;91:145–154. doi: 10.1007/s00420-017-1267-2. [DOI] [PubMed] [Google Scholar]
- 3.Ye X., Kato K., Wong L.Y., Jia T., Kalathil A., Latremouille J., et al. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health. 2018;221:9–16. doi: 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X., Liu Y., Xu B., Gu L., Tang W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci Total Environ. 2018;625:566–574. doi: 10.1016/j.scitotenv.2017.12.186. [DOI] [PubMed] [Google Scholar]
- 5.Bassler J., Ducatman A., Elliott M., Wen S., Wahlang B., Barnett J., et al. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 2019;247:1055–1063. doi: 10.1016/j.envpol.2019.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda-Kohmo K., Hutcheson R., Innes K.E., Conway B.N. Perfluoroalkyl substances are inversely associated with coronary heart disease in adults with diabetes. J Diabetes Complications. 2019;33:407–412. doi: 10.1016/j.jdiacomp.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cluett R., Seshasayee S.M., Rokoff L.B., Rifas-Shiman S.L., Ye X., Calafat A.M., et al. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: A cross-sectional study (Project Viva, United States) Environ Health Perspect. 2019;127:87006. doi: 10.1289/EHP4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R., Guo C., Tse W.K.F., Su M., Zhang X., Lai K.P. Metabolomic analysis reveals metabolic alterations of human peripheral blood lymphocytes by perfluorooctanoic acid. Chemosphere. 2020;239 doi: 10.1016/j.chemosphere.2019.124810. [DOI] [PubMed] [Google Scholar]
- 9.Darrow L.A., Groth A.C., Winquist A., Shin H.M., Bartell S.M., Steenland K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ Health Perspect. 2016;124:1227–1233. doi: 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ Int 2020; 134: 105220. [DOI] [PMC free article] [PubMed]
- 11.Hui Z., Li R., Chen L. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene. 2017;622:67–71. doi: 10.1016/j.gene.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Das K.P., Wood C.R., Lin M.T., Starkov A.A., Lau C., Wallace K.B., et al. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52. doi: 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira C.P., Gayotto L.C., Tatai C., Della Nina B.I., Lima E.S., Abdalla D.S., et al. Vitamin C and vitamin E in prevention of Nonalcoholic Fatty Liver Disease (NAFLD) in choline deficient diet fed rats. Nutr J. 2003;2:9. doi: 10.1186/1475-2891-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S.H., Han A.L., Kim N.H., Shin S.R. Liver histological improvement after administration of high-dose Vitamin C in Guinea Pig with nonalcoholic steatohepatitis. Int J Vitam Nutr Res. 2018;88:263–269. doi: 10.1024/0300-9831/a000515. [DOI] [PubMed] [Google Scholar]
- 15.Bashandy S.A.E., Ebaid H., Abdelmottaleb Moussa S.A., Alhazza I.M., Hassan I., Alaamer A., et al. Potential effects of the combination of nicotinamide, vitamin B2 and vitamin C on oxidative-mediated hepatotoxicity induced by thioacetamide. Lipids Health Dis. 2018;14(17):29. doi: 10.1186/s12944-018-0674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P., Li Y., Yu Z., Yang L., Shang R., Yan Z. Protective effect of Vitamin C on triptolide-induced acute hepatotoxicity in mice through mitigation of oxidative stress. An Acad Bras Cienc. 2019;91:20181257. doi: 10.1590/0001-3765201920181257. [DOI] [PubMed] [Google Scholar]
- 17.Badgujar P.C., Chandratre G.A., Pawar N.N., Telang A.G., Kurade N.P. Fipronil induced oxidative stress involves alterations in SOD1 and catalase gene expression in male mice liver: Protection by vitamins E and C. Environ Toxicol. 2016;31:1147–1158. doi: 10.1002/tox.22125. [DOI] [PubMed] [Google Scholar]
- 18.Arslan M., Sezen S.C., Turgut H.C., Kocabiyik M., Arpaci H., Comu F.M., et al. Vitamin C ameliorates high dose Dexmedetomidine induced liver injury. Bratisl Lek Listy. 2016;117:36–40. doi: 10.4149/bll_2016_008. [DOI] [PubMed] [Google Scholar]
- 19.Mondal R., Biswas S., Chatterjee A., Mishra R., Mukhopadhyay A., Bhadra R.K., et al. Protection against arsenic-induced hematological and hepatic anomalies by supplementation of vitamin C and vitamin E in adult male rats. J Basic Clin Physiol Pharmacol. 2016;27:643–652. doi: 10.1515/jbcpp-2016-0020. [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Xie G., Xu X., Wu W., Yang B. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res Int. 2018;25:4787–4793. doi: 10.1007/s11356-017-0872-7. [DOI] [PubMed] [Google Scholar]
- 21.Su M., Liang X., Xu X., Wu X., Yang B. Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate-induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environ Toxicol Pharmacol. 2019;65:60–65. doi: 10.1016/j.etap.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Xu X., Guo C., Liang X., Li R., Chen J. Potential biomarker of fibroblast growth factor 21 in valproic acid-treated livers. BioFactors. 2019;45:740–749. doi: 10.1002/biof.1519. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Ma X, Song Y, Zhang Y, Xiong W, Li L, et al. Anti-colorectal cancer targets of resveratrol and biological molecular mechanism: Analyses of network pharmacology, human and experimental data. 2019;11265-11273. [DOI] [PubMed]
- 24.Zhou R., Wu K., Su M., Li R. Bioinformatic and experimental data decipher the pharmacological targets and mechanisms of plumbagin against hepatocellular carcinoma. Environ Toxicol Pharmacol. 2019;70 doi: 10.1016/j.etap.2019.103200. [DOI] [PubMed] [Google Scholar]
- 25.Li R., Qin X., Liang X., Liu M., Zhang X. Lipidomic characteristics and clinical findings of epileptic patients treated with valproic acid. J Cell Mol Med. 2019;23:6017–6023. doi: 10.1111/jcmm.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R., Guo C., Lin X., Chan T.F., Lai K.P., Chen J. Integrative omics analyses uncover the mechanism underlying the immunotoxicity of perfluorooctanesulfonate in human lymphocytes. Chemosphere. 2020;256 doi: 10.1016/j.chemosphere.2020.127062. [DOI] [PubMed] [Google Scholar]
- 27.Chong J., Soufan O., Li C., et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46(W1):W486–W494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ Int. 2020;134:105220. [DOI] [PMC free article] [PubMed]
- 29.Radi A.M., Mohammed E.T., Abushouk A.I., Aleya L., Abdel-Daim M.M. The effects of abamectin on oxidative stress and gene expression in rat liver and brain tissues: Modulation by sesame oil and ascorbic acid. Sci Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134882. [DOI] [PubMed] [Google Scholar]
- 30.Xu P., Li Y., Yu Z., Yang L., Shang R., Yan Z. Protective effect of Vitamin C on triptolide-induced acute hepatotoxicity in mice through mitigation of oxidative stress. An Acad Bras Cienc. 2019;91 doi: 10.1590/0001-3765201920181257. [DOI] [PubMed] [Google Scholar]
- 31.Blake BE, Cope HA, Hall SM, Keys RD, Mahler BW, McCord J, et al. Evaluation of Maternal, Embryo, and Placental Effects in CD-1 Mice following Gestational Exposure to Perfluorooctanoic Acid (PFOA) or Hexafluoropropylene Oxide Dimer Acid (HFPO-DA or GenX). Environ Health Perspect. 2020 Feb;128(2):27006. doi: 10.1289/EHP6233. Epub 2020 Feb 13. PMID: 32074459; PMCID: PMC7064328. [DOI] [PMC free article] [PubMed]
- 32.Wu X., Liang M., Yang Z., Su M., Yang B. Effect of acute exposure to PFOA on mouse liver cells in vivo and in vitro. Environ Sci Pollut Res Int. 2017 Nov;24(31):24201–24206. doi: 10.1007/s11356-017-0072-5. Epub 2017 Sep 8 PMID: 28887612. [DOI] [PubMed] [Google Scholar]
- 33.Wen Y, Chen J, Li J, Arif W, Kalsotra A, Irudayaraj J. Effect of PFOA on DNA Methylation and Alternative Splicing in Mouse Liver. Toxicol Lett. 2020 Sep 1;329:38-46. doi: 10.1016/j.toxlet.2020.04.012. Epub 2020 Apr 19. PMID: 32320774; PMCID: PMC7533079. [DOI] [PMC free article] [PubMed]
- 34.Rosen M.B., Thibodeaux J.R., Wood C.R., Zehr R.D., Schmid J.E., Lau C. Gene expression profiling in the lung and liver of PFOA-exposed mouse fetuses. Toxicology. 2007 Sep 24;239(1–2):15–33. doi: 10.1016/j.tox.2007.06.095. Epub 2007 Jun 29 PMID: 17681415. [DOI] [PubMed] [Google Scholar]
- 35.Eriksen K.T., Raaschou-Nielsen O., McLaughlin J.K., Lipworth L., Tjønneland A., Overvad K., et al. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0056969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebholz S.L., Jones T., Herrick R.L., Xie C., Calafat A.M., Pinney S.M., et al. Hypercholesterolemia with consumption of PFOA-laced Western diets is dependent on strain and sex of mice. Toxicol Rep. 2016;3:46–54. doi: 10.1016/j.toxrep.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai K.P., Li J.W., Cheung A., Li R., Billah M.B., Chan T.F., et al. Transcriptome sequencing reveals prenatal PFOS exposure on liver disorders. Environ Pollut. 2017;223:416–425. doi: 10.1016/j.envpol.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 38.Das K.P., Wood C.R., Lin M.T., et al. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 2017;378:37–52. doi: 10.1016/j.tox.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wielsøe M., Long M., Ghisari M., Bonefeld-Jørgensen E.C. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere. 2015;129:239–245. doi: 10.1016/j.chemosphere.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 40.Chen X.F., Tian M.X., Sun R.Q., et al. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018;19(5) doi: 10.15252/embr.201745124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson A., Curran J.E., Johnson M.P., et al. Identification of ACOX2 as a shared genetic risk factor for preeclampsia and cardiovascular disease. Eur J Hum Genet. 2011;19(7):796–800. doi: 10.1038/ejhg.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monte M.J., Alonso-Peña M., Briz O., et al. ACOX2 deficiency: An inborn error of bile acid synthesis identified in an adolescent with persistent hypertransaminasemia. J Hepatol. 2017;66(3):581–588. doi: 10.1016/j.jhep.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Kirkby B., Roman N., Kobe B., Kellie S., Forwood J.K. Functional and structural properties of mammalian acyl-coenzyme A thioesterases. Prog Lipid Res. 2010;49(4):366–377. doi: 10.1016/j.plipres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Bekeova C., Anderson-Pullinger L., Boye K., et al. Multiple mitochondrial thioesterases have distinct tissue and substrate specificity and CoA regulation, suggesting unique functional roles. J Biol Chem. 2019;294(50):19034–19047. doi: 10.1074/jbc.RA119.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moffat C., Bhatia L., Nguyen T., et al. Acyl-CoA thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. J Lipid Res. 2014;55(12):2458–2470. doi: 10.1194/jlr.M046961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9(11):1211. Published 2017 Nov 3. doi:10.3390/nu9111211 [DOI] [PMC free article] [PubMed]
- 47.Ghorbani Z., Hajizadeh M., Hekmatdoost A. Dietary supplementation in patients with alcoholic liver disease: a review on current evidence. Hepatobiliary Pancreat Dis Int. 2016;15(4):348–360. doi: 10.1016/s1499-3872(16)60096-6. [DOI] [PubMed] [Google Scholar]
- 48.Alkahtane AA. Protective potency of ascorbic acid supplementation against cytotoxicity and DNA fragmentation induced by triphenyltin on human liver carcinoma cells [published online ahead of print, 2020 May 16]. Environ Sci Pollut Res Int. 2020;10.1007/s11356-020-08821-1. doi:10.1007/s11356-020-08821-1 [DOI] [PubMed]
- 49.Davis A.E., 3rd, Mejia P., Lu F. Biological activities of C1 inhibitor. Mol Immunol. 2008;45(16):4057–4063. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis A.E., 3rd, Lu F., Mejia P. C1 inhibitor, a multi-functional serine protease inhibitor. Thromb Haemost. 2010;104(5):886–893. doi: 10.1160/TH10-01-0073. [DOI] [PubMed] [Google Scholar]
- 51.Janssen AW, Betzel B, Stoopen G, et al. The impact of PPARα activation on whole genome gene expression in human precision cut liver slices. BMC Genomics. 2015;16:760. Published 2015 Oct 8. doi:10.1186/s12864-015-1969-3 [DOI] [PMC free article] [PubMed]
- 52.Filgo A.J., Quist E.M., Hoenerhoff M.J., Brix A.E., Kissling G.E., Fenton S.E. Perfluorooctanoic Acid (PFOA)-induced Liver Lesions in Two Strains of Mice Following Developmental Exposures: PPARα Is Not Required. Toxicol Pathol. 2015;43(4):558–568. doi: 10.1177/0192623314558463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasprzak A, Adamek A. Mucins: the Old, the New and the Promising Factors in Hepatobiliary Carcinogenesis. Int J Mol Sci. 2019;20(6):1288. Published 2019 Mar 14. doi:10.3390/ijms20061288 [DOI] [PMC free article] [PubMed]
- 54.Betge J., Schneider N.I., Harbaum L., et al. MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: expression profiles and clinical significance. Virchows Arch. 2016;469(3):255–265. doi: 10.1007/s00428-016-1970-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia Y., Persson C., Hou L., et al. A comprehensive analysis of common genetic variation in MUC1, MUC5AC, MUC6 genes and risk of stomach cancer. Cancer Causes Control. 2010;21(2):313–321. doi: 10.1007/s10552-009-9463-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez-Ortiz Z.G., Pendergraft W.F., 3rd, Prasad A., et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol. 2013;14(9):917–926. doi: 10.1038/ni.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakaguchi T., Gu X., Golden H.M., Suh E., Rhoads D.B., Reinecker H.C. Cloning of the human claudin-2 5'-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem. 2002;277(24):21361–21370. doi: 10.1074/jbc.M110261200. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, Imasato M, Yamazaki Y, et al. Claudin 2 deficiency reduces bile flow and increases susceptibility to cholesterol gallstone disease in mice. Gastroenterology. 2014;147(5):1134‐45.e10. doi:10.1053/j.gastro.2014.07.033 [DOI] [PubMed]
- 59.Molina-Jijón E., Rodríguez-Muñoz R., Namorado Mdel C., Pedraza-Chaverri J., Reyes J.L. Oxidative stress induces claudin-2 nitration in experimental type 1 diabetic nephropathy. Free Radic Biol Med. 2014;72:162–175. doi: 10.1016/j.freeradbiomed.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 60.Al-Muhsen S., Casanova J.L. The genetic heterogeneity of mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. 2008;122(6):1043–1053. doi: 10.1016/j.jaci.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 61.Nalpas B., Lavialle-Meziani R., Plancoulaine S., et al. Interferon gamma receptor 2 gene variants are associated with liver fibrosis in patients with chronic hepatitis C infection. Gut. 2010;59(8):1120–1126. doi: 10.1136/gut.2009.202267. [DOI] [PubMed] [Google Scholar]
- 62.Chiang J.Y.L., Ferrell J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018;18:71–87. doi: 10.3727/105221618X15156018385515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charni-Natan M., Aloni-Grinstein R., Osher E., Rotter V. Liver and Steroid Hormones-Can a Touch of p53 Make a Difference? Front Endocrinol (Lausanne). 2019;10:374. doi: 10.3389/fendo.2019.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woods C.P., Hazlehurst J.M., Tomlinson J.W. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015;154:94–103. doi: 10.1016/j.jsbmb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 65.Song L., Zhou H., Yu W., Ding X., Yang L., Wu J., et al. Effects of phytosterol ester on the fatty acid profiles in rats with nonalcoholic fatty liver disease. J Med Food. 2020 doi: 10.1089/jmf.2019.4468. [DOI] [PubMed] [Google Scholar]
- 66.Vyas D., Kadegowda A.K., Erdman R.A. Dietary conjugated linoleic Acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab. 2012;2012 doi: 10.1155/2012/932928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warner D.R., Liu H., Miller M.E., Ramsden C.E., Gao B., Feldstein A.E., et al. Dietary linoleic acid and its oxidized metabolites exacerbate liver injury caused by ethanol via induction of hepatic proinflammatory response in mice. Am J Pathol. 2017;187:2232–2245. doi: 10.1016/j.ajpath.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C.W., Chuang H.Y., Liao K.W., Yu M.L., Dai C.Y., Chang W.T., et al. Urinary thiodiglycolic acid is associated with increased risk of non-alcoholic fatty liver disease in children living near a petrochemical complex. Environ Int. 2019;131 doi: 10.1016/j.envint.2019.104978. [DOI] [PubMed] [Google Scholar]
- 69.Wang C.W., Liao K.W., Chan C.C., Yu M.L., Chuang H.Y., Chiang H.C., et al. Association between urinary thiodiglycolic acid level and hepatic function or fibrosis index in school-aged children living near a petrochemical complex. Environ Pollut. 2019;244:648–656. doi: 10.1016/j.envpol.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 70.Johnston C.S., Meyer C.G., Srilakshmi J.C. Vitamin C elevates red blood cell glutathione in healthy adults. Am J Clin Nutr. 1993;58:103–105. doi: 10.1093/ajcn/58.1.103. [DOI] [PubMed] [Google Scholar]
- 71.Soylu A.R., Altaner S., Aydodu N., Basaran U.N., Tarcin O., Gedik N., et al. Effects of vitamins E and C supplementation on hepatic glutathione peroxidase activity and tissue injury associated with ethanol ingestion in malnourished rats. Curr Ther Res Clin Exp. 2006;67:118–137. doi: 10.1016/j.curtheres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.