Graphical abstract
Keywords: Esophageal varices, Biomedical informatics, Naïve Bayes, Binary logistic regression, Liver disease diagnosis
Highlights
-
•
Esophageal Varices is one complication of chronic liver disease that leads to deaths globally due to hemorrhage.
-
•
The prediction of presence the Esophageal Varices is essential to avoid bleeding for patients.
-
•
Now the only diagnostic method for Esophageal Varices by the upper gastrointestinal endoscopy but it has many disadvantages.
-
•
Only ten variables are the most significant for diagnosing the varices: PLT, Stiffness, PC, liver texture, spleen, HCV-RNA, Albumin, gender, Total bilirubin, and PV diameter.
-
•
We Evaluated the effectiveness of several noninvasive markers for predicting Varices.
-
•
We Introduced a novel (EVP) index with acceptable performance for diagnosing Varices and compared with the exist, it could save operating the upper endoscopic by nearly 46.5%.
Abstract
Introduction
Esophageal Varices (EVs) is one of the major dangerous complications of liver fibrosis. Upper Gastrointestinal (UGI) Endoscopy is necessary for its diagnosis. Repeated examinations for EVs screening severely burden endoscopic units in terms of cost and other side implications; moreover, the lack of public health resources in rural areas and primary hospitals should be considered, particularly in developing countries. So, an accurate noninvasive marker for EV is highly needed for liver disease patients.
Objectives
This study sought to evaluate the values of several indices to determine how adequate are they in predicting EV and build a novel accurate prediction index.
Methods
Five thousand and thirteen patients were enrolled. The laboratory tests, abdominal ultrasonography, liver stiffness measurement using Fibro-scan, and UGI endoscopy were performed. Ten common indices: Fib-4 score, AST-to-platelet ratio index, Fibrosis index, AST/ALT ratio Varices Prediction Rule, Baveno VI, APRI-Fib4 Combo, King score, “Model for End-Stage Liver Disease”, and Lok Score were calculated. The significant predictors for EVs were identified by using “P-value Correlation-based Filter Selection” method, where a novel Egyptian Varices Prediction (EVP) index was developed using binary logistic regression. The diagnostic performance was evaluated by some parameters and the Area Under Curve (AUC).
Results
EVP Index was correlated to EVs at 0.5; it achieved higher performance (AUC 0.788, accuracy 73.3%, and sensitivity 78%) than the other indices at a cutoff point of 0.423.
Conclusion
EVP Index was a good noninvasive predictor. It had an acceptable performance for diagnosing EVs and it was only required regular laboratory tests and imaging data. It can provide a tool for classifying or arranging the patients according to the degree pre-emptive for selective endoscopy and the degree of severity. Also, it will enable clinicians to concentrate on one marker instead of a wide set of parameters.
Introduction
Liver cirrhosis is the 13th leading cause of mortality rate worldwide [1]. EV is a major dangerous complication of liver cirrhosis. The severity of cirrhosis, the size of varices, and the presence of red spots are the most indicators of variceal bleeding. The screening for EV is recommended for cirrhotic patients because it is closely related to the treatment program with endoscopic or beta-blockers prophylaxis to prevent variceal bleeding [2].
Nowadays, UGI is the standard diagnostic method of EVs which is performed regularly on liver cirrhosis patients [3], [4], [5]. Nevertheless, there are disadvantages of this routine containing the complications related to endoscopy, particularly the need for intravenous sedation. Also, it is frequently related to complications such as perforation and bleeding.
Therefore, the noninvasive methods are vital to identify the patients who would benefit from UGI. In 2015, the experts at the Baveno VI Consensus Workshop suggested some criteria for EVs diagnosis [6]; they recommend canceling UGI in patients with platelet>150,000/mm3 and liver stiffness <20 kPa due to low risk of bleeding related to these patients, it has acceptable performance for keeping the use of UGI. The new expanded Baveno VI of classification rule was developed in 2017 with platelet count > 110 × 109 cells/L and Liver Stiffness (LS) < 25 kPa [3].
Lately, several noninvasive predictors of EV have been explored in HCV patients. Some studies evaluated the ability of several previous noninvasive markers which were used with liver fibrosis to predict the presence of EVs. These indices were Lok score, Fib-4 score, “Aspartate Aminotransferase-To-Platelet Ratio Index” (APRI), Fibrosis Index (FI), APRI-Fib-4 combo, Aspartate Aminotransferase-To-Alanine Aminotransferase Ratio (AAR), Varices Prediction Rule (VPR), King Score, and “Model for end-stage liver disease” (MELD). However, the feedback from these previous studies were dialectical and their usefulness in clinical practice was uncertain as described in the next section.
In this context, the objective of our study is twofold: The first objective sought to assess (evaluate) the predictive values of the existing noninvasive markers that were introduced in the previous studies to predict the presence of EVs in patients. The second objective is to build and to assess a novel accurate index (Egyptian Varices Prediction Index) using logistic regression for the prediction of EVs to avoid the use of endoscopy.
There are several important points for our study; we used noninvasive markers that depended on regular laboratory tests and imaging required without the need for extra cost for additional biochemical tests or specialized devices. It varies from other noninvasive markers that could prove to be harder to attain. This study could be vital to developing countries with limited resources. Nevertheless, future large potential studies are ensured to increase the diagnostic performance of predictors in diagnosing EV in countries where there is a shortage of endoscopy. This could prevent unnecessary UGI procedures that are linked to high costs and complications. Repeated upper endoscopy examinations have a great burden on endoscopic units and are costly. Also, it can help the clinicians to restrict UGI to those who are highly suspected to have EVs.
The layout of this paper is as follows: Section 2 discusses the background and literature review on the prediction of EVs. Section 3 discusses the biomedical dataset used in our study and explanation of our methodology step by step, from preprocessing to the evaluation of the performance and comparison with others. In Section 3, the classification results of our experiments were reported. Section 4 discusses the performance, advantages, and main limitations. Finally, conclusions of the paper with an outlook for our future work are presented in Section 5.
Literature review
In recent years, researchers have been attempting to selecting non-invasive predictors for predicting EVs in patients with HCV based on clinical data. These studies have shown that biochemical, clinical, and ultrasonographic parameters alone or together have some predictive power for assessing the presence of EV noninvasively [7]. Previous studies can be classified as three noninvasive categories for predicting the presence of EV.
The first was based on using one parameter such as using Transient Elastography (TE) to measure liver stiffness (LS) as studies [8], [9], [10], [11], [12], [13]. It was concluded that the stiffness score was only correlated to EV. Some other studies proved that Albumin (ALB) was a significant predictor for EVs as Amer Gomaa et al. [14] where they compared Child-Pugh scores, ALB, and MELD in terms of the effective prediction of EVs, and their results concluded that ALB score warrants are the most effective noninvasive marker for EVs at a cutoff exceeding − 2.2 with 96.7% sensitivity. This score was higher than that of MELD, allowing it to be used at a cutoff exceeding 8.5. Other studies concluded that the Platelet count (PLT) could be used as the only predictor for EVs as Abd-Elsalam et al. [15]. They concluded that the platelet count represented a noninvasive marker at a cutoff>149,000, with 59% for accuracy, 54% for NPV, 72% for PPV, 82% for specificity, and 39% for sensitivity.
The second type of studies was based on a combination of more than one predictor such as Chandail et al. in 2017 [7] where they observed that spleen diameter and Portal Vein were significant for the prediction of EV where they used 51 patients in their study. Combination of Liver Stiffness (LS) and platelet count such as Baveno VI criteria [3]. Since then, some studies evaluating these recommendations have been published such as Maurice et al. [16], Adjeka Stanislas et al. (2016)[17], and Sousa et al. (2017) [18]. Tosetti et al. [19] proved that “Expanded Baveno VI” saved screening UGI up to 45%. The “PLT > 150,000/MELD 6” was less accurate; it was saving the UGI endoscopies to 41% and the original Baveno VI saved the screening endoscopies by 21%. Then, expanded Baveno criteria reduced the proportion of unnecessary endoscopies. Llop et al. [12] proved that liver stiffness was the best noninvasive marker. Also, Baveno VI was considered as the best mixed method.
The third type of studies was based on evaluating the previous markers that were used to predict the fibrosis and which of them could be used for predicting EVs. Bledar Kraja et al. [1] used six different markers, AAR, APRI, Fibrosis Index (FI), MELD, King’s Score, and Fib-4 to predict varices. They found that the Fib-4 was the only significant marker with a specificity of 58%, a sensitivity of 72%, and an AUC of 0.66. There was no proof of any significant association of the common indices with the EVs such as King’s score, (FI), AAR, and APRI. Stefanescu et al. [8] evaluated four markers Fib-4, APRI, liver stiffness, and Lok Score to predict the EVs. They found that Lok Score was the best of the other three indices at a value of a cutoff point higher than 0.62; it had 49.3% for NPV, 77.2% for PPV, and 69% for AUC in the prediction of varices. Also, it was found that LS was a significant predictor at cutoff higher than 19 KPa with 81% for NPV, 47.3% for PPV, and 68% for the diagnostic accuracy. A mixture of Lok Score with LS was used, which achieved a better diagnostic accuracy of 74.66%. Sedrak et al. [20] concluded that the APRI is a poor predictor of EVs and bleeding of EV. Also, De Mattos et al. [21] concluded that APRI was not an independent factor for the prediction of EV. Sarkar et al. [13] used APRI and LS value of 18 kpa to predict EVs in cirrhotic patients. They found that APRI had fewer values in NPV that showed there are no satisfactory cutoff values for APRI to be used as a marker. Deng et al. [22] evaluated the diagnostic accuracy in predicting EVs. The results proved that FI had the largest AUC at “0.60”, followed by Fib-4 at “AUC = 0.544”, then AST-ALT Ratio at “AUC = 0.538”, then King score at “AUC = 0.526”, finally APRI score at “AUC = 0.506”. Deng and Guo [23] evaluated values of the diagnostic accuracy for (King score, AAR, FI, APRI, Lok, Forns index, and Fib-4) predicting EVs. They found that AAR, Forns index, APRI, Fib-4, and Lok were less accurate in predicting EVs. They recommended further studies to confirm these findings in liver cirrhosis. Kamel A. Ahmed et al. [24] found that Kings Score has the highest sensitivity and specificity in EVs prediction, followed by APRI Score while AAR score has the least sensitivity and specificity. A. Isted et al. [25] examined five indices Clinical Prediction Rule (CPR), APRI, Spleen Size Z Score (SSAZ), Hepatic Artery Resistance Index (HARI), “Platelet count-to-SSAZ ratio” (P/SSAZ). CPR and APRI scores were the best overall with AUC ranging from 0.74 and 0.72, respectively. Also, a novel predictor “Varices Prediction Rule” (VPR) was developed that yielded AUC of 0.75, sensitivity 86%, sensitivity 71%. Other studies concluded that liver and spleen MR elastography analyses are significant in the diagnosis of EVs such as Hayato Abe et al. [26]. In this study, the diagnostic performance of MR elastography in predicting EVs was assessed. It was found that the measurement of liver and spleen stiffness using elastography was a significant method to predict patients of EVs. From the previous studies, multiple or binary logistic regression were commonly used to evaluate the noninvasive markers for EVs.
The performance of the existing indices that were proposed in the predictive models was neither sensitive nor specific and used a small dataset as shown in Table 1. Despite the presence of many studies up till now, there were no noninvasive markers that could be widely used for predicting EVs. Thus, we evaluated the effectiveness of various non-invasive indices and proposed a novel prediction index using the logistic regression algorithm based on the clinical data for Egyptian HCV patients.
Table 1.
The previous studies that used some markers for the prediction of varices.
| Marker [used] | Location | Year | Dataset | Cutoff value | Specificity | Sensitivity | AUC |
|---|---|---|---|---|---|---|---|
| PLT [15] | Egypt | 2016 | 110*9 | >149,000 | 82 | 39 | 0.627 |
| LS [8] | Romania | 2011 | 231*14 | >19 | 32.39 | 84 | 0.656 |
| LS[9] | Egypt | 2013 | 32*14 | 29.7 | 67 | 95 | – |
| LS[11] | KSA | 2017 | 75*5 | 25.3 | – | – | 0.67 |
| LS [13] | Bangladesh | 2018 | 65*9 | >18 | 75 | 88.7 | 0.769 |
| LS [12] | Spain | 2017 | 161*20 | >= 20 | 70.6 | 76 | |
| ALB [14] | Egypt | 2018 | 80*11 | >-2.2 | 100 | 96.7 | 0.90 |
| FI Score [22] | China | 2015 | 650*30 | 0.612 | 56 | 62.3 | 0.61 |
| VPR [25] | London | 2015 | 195*6 | 0.72 | 71 | 86 | 0.75 |
| Fib-4 Index [1] | Albania | 2017 | 139*21 | > 3.23 | 58 | 72 | 0.66 |
| LOK Score [8] | Romania | 2011 | 231*14 | >0.62 | 50.7 | 76.16 | 0.69 |
| LOK Score [27] | USA | 2016 | 2233 | 0.86 | 77 | 73 | 0.80 |
| King Score [22] | China | 2015 | 650*30 | 0.55 | 44.3 | 69.8 | 0.55 |
| King Score[24] | Egypt | 2019 | 91*19 | 12.11 | 90 | 88.3 | 0.95 |
| MELD Score [14] | Egypt | 2018 | 80*11 | >8.5 | 95 | 90 | 0.90 |
| BavenoVI [16] | United Kingdom | 2016 | 310 | LS < 20, PLT > 150 | 0.34 | 0.87 | 0.746 |
| Extend Baveno VI [19] | Italy | 2018 | 471*6 | LS < 25, PLT > 110 | 48 | 100 | – |
| Child-Score[28] | China | 2015 | 145*35 | >9 | 79.1 | 63.6 | 0.796 |
| APRI [21] | Brazil | 2013 | 164*7 | 1.3 | 72.7 | 64.7 | – |
| APRI Score [13] | Bangladesh | 2018 | 65*9 | 1.00 | 83.3 | 63.3 | 0.779 |
Patients and methods
Patient’s dataset
Data of all chronic HCV patients (n = 453,465) treated under the umbrella of “The Egyptian National Committee for Control of Viral Hepatitis in the National Treatment Program of HCV patients” from (Sep. 2006 To Aug. 2017) in Egypt were retrieved. This dataset was collected from twenty-six hospital centers distributed in distinct territories of North, Central, and South Egypt. Only 46,014 patients have upper gastrointestinal (UGI) endoscopy done within 6 months from starting antiviral therapy for chronic hepatitis C. All participants of this study signed a written informed consent and the ethics review committee. This study was carried out according to Helsinki ethical guidelines. UGI Endoscopy was performed on patients included in this study with a comment on the presence or absence of EVs. It was performed in a single endoscopy unit by an experienced endoscopist. The patients were classified into two main groups according to the presence varices(n = 27,427) and absence of varices (n = 18,587). The ages of males ranged beteen 19–74 years and ages of females within (25–70) years in our study.
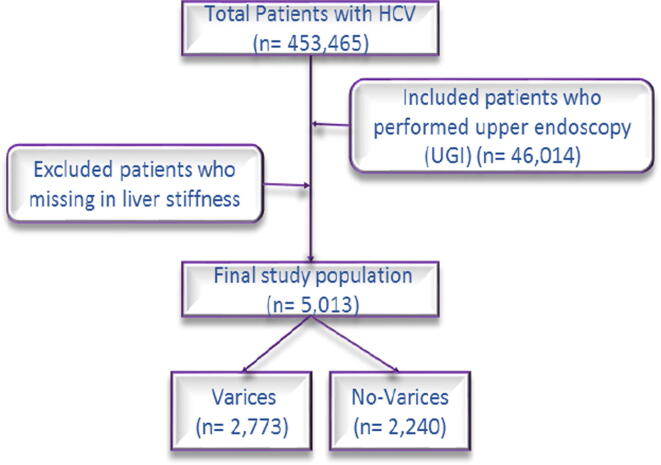
This retrospective study was conducted by 5013 from 46,014 patients with HCV because the other cases did not undergo Transient Elastography (TE) to measure Liver Stiffness (LS) using Fibro-Scan as shown in Fig. 1. Moreover, not all patients had chronic Hepatitis C cirrhotic save for dataset of 64,014 there were 95.3% of patients with chronic Hepatitis C cirrhotic and 98.8% of patients with chronic Hepatitis C cirrhotic for a dataset of 5013. All participants were subjected to the following: history taking, thorough clinical examination, laboratory investigations including complete blood picture, liver function tests including age, gender, and Body Mass Index (BMI), Platelet Count (PLT), Aspartate Aminotransferase (AST), White Blood Cell (WBC), International Normalized Ratio (INR), Alanine Aminotransferase (ALT), Albumin (ALB), Total Bilirubin (T.BIL), Creatinine (Cr), Prothrombin Time Containing Prothrombin Concentration (PC%). Ultrasonography was performed for patients and recorded; spleen (enlarged, average, or removed), Portal Vein Diameter (PVD), and liver texture (cirrhotic, abnormal, or normal). Also, the quantity of HCV viremia (HCV-RNA).
Fig. 1.
Flowchart of the study population.
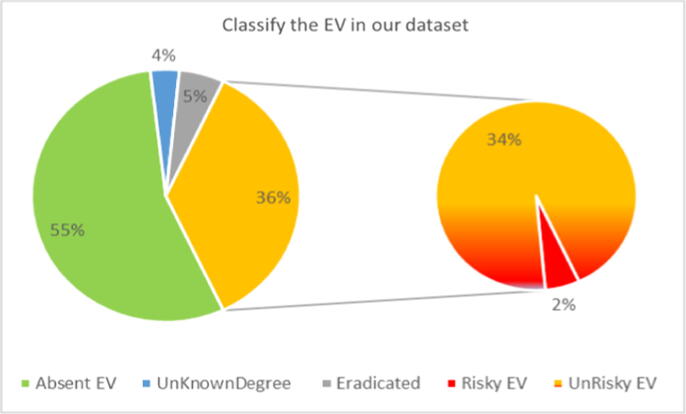
According to UGI endoscopy, the patients were classified into 2,773 (55.32%) patients who had no varices and 2,240 (44.68%) patients had varices as shown in Fig. 1. The group of EVs had only 2% risky varices, 3.5% an unknown degree, 5.4% eradicated varices, and 34% non-risky varices as shown in Fig. 2.
Fig. 2.
Classification of the esophageal varices in the dataset.
Inclusion and exclusion criteria
Inclusion criteria: We included consecutive adult patients (>18 years) with documented compensated liver cirrhosis secondary to chronic HCV. Liver cirrhosis was diagnosed based on having F4 METAVIR stage in liver biopsy, positive HCV antibodies and detectable HCV RNA by PCR, normal complete blood count, hepatitis B surface antigen negativity, total bilirubin > 1.2, INR > 1.2, and serum albumin < 3.5.
Exclusion criteria: Patients with any of the following conditions were excluded from the study; Ascites, hepatic encephalopathy, patients with previous endoscopic intervention for primary prevention of variceal bleeding and patients refusing to be enrolled in the study, missing data regarding complete blood count and liver stiffness, serious co-morbid conditions such as severe arterial hypertension, concomitant liver disease as chronic hepatitis B, autoimmune hepatitis, and alcoholic liver disease.
Ethics statement
The study was approved by the National Research Centre ethics committee with number 1458.
Methodology
Since our objectives of this study were to evaluate the predictive values of the common markers and indexes that were proposed in earlier studies, select the most significant predictors for predicting the presence of EVs disease, build (establish) a simple novel and accurate index using logistic regression, and compare the performance of our index with other indexes for prediction of EVs to avoid the use of endoscopy.
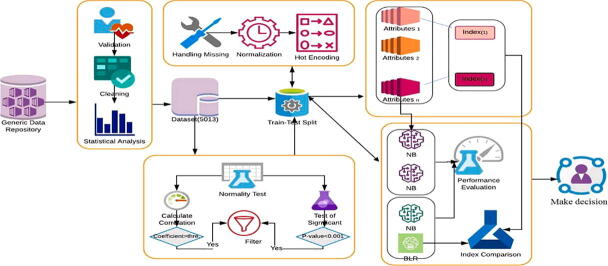
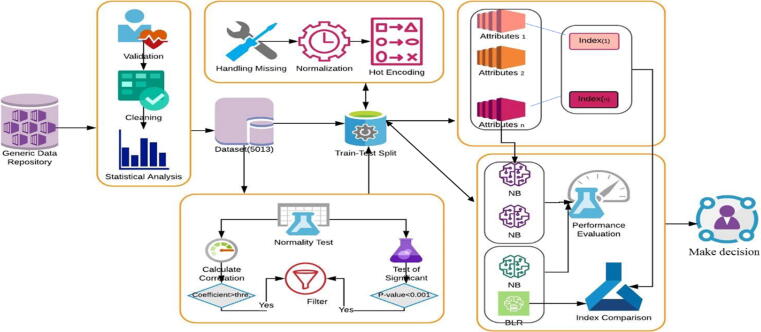
In this study, our methodology was divided into the following steps: 1) data preprocessing, to repair the data and obtain more accurate results in less time; 2) calculation and evaluation of the existing scores from our dataset, to assess their predictive values for predicting EVs, 3) attributes selection, to select the significant subset of the attributes, 4) model implementation, to implement and derive a novel accurate predictive index for helping the physicians to concentrate on only one instead of the set of parameters for prediction of EVs, 5) evaluation of the diagnostic performance, to assess the performance of the predictive models. A comparison of the performance of our index and attributes with other indexes and attributes was conducted. The main processes are illustrated in Fig. 3 and were explained in detail in the following subsections.
Fig. 3.
The proposed system for predicting EV.
Data preprocessing and statistical analysis
Data preprocessing was an important step that included data validation, cleaning, resolving missing data, statistical analysis, normalization, and data transformation. For each field, data validation is comprised of removing noise and inconsistent data by deletion. From the observations, some attributes contained “0″, though the medical knowledge denied being this value; therefore, we decided to consider them as a missing value. Besides, the attributes that contained more than fifty percent of missing values were removed. The normalization process was applied for some attributes by calculating Z-score for each attribute depending on the values of the standard deviation and mean. We transformed some numeric attributes into a nominal attribute of values 0 and 1. The hot encoding for categorical attributes was applied to help simplify the analysis of the dataset.
The data were statistically analyzed using IBM SPSS (Statistical Package for Social Science) version 23.0 “SPSS, Chicago, IL, USA” and MedCalc version 15.8 “MedCalc Software BVBA, Ostend, Belgium”.
The test of normality for the quantitative attributes was performed using the Kolmogorov-Smirnov test. The not-normally distributed attributes were reported as the median and interquartile range, the normally distributed attributes were expressed as mean ± standard deviation, and the categorical attributes were expressed as frequency. The degree of significance between attributes and varices class was calculated. For the quantitative attributes, the independent sample t-test was used for normally distributed data and the Mann-Whitney U test was performed for not-normally distributed [29]. A chi-square test was performed to compare qualitative attributes. The p-value was calculated to identify the significance attributes. Attributes were considered as statistically significant at the two-tailed p-value < 0.05. Pearson and Spearmen correlation coefficient was calculated for normal and not-normal attributes, respectively, to rank them with the classes of varices [30]. The attribute was correlated if the absolute value of correlation coefficient |r| between each attribute and varices class was more than the threshold value.
Calculation and evaluation of the common indices
By using available biological parameters in our dataset, several noninvasive markers were calculated for patients because EVs relates to liver fibrosis, according to previously published formulas. These indices were calculated according to questions are shown in Table 2: AST-ALT ratio (AAR), Fibrosis-4 (Fib-4) index, Child-Pugh score, Model for End-Stage Liver Disease (MELD), APRI, LOK score, FI, and King scores. There are three established scores for assessment of EV including Varices Prediction Rule (VPR), Baveno VI, and Expanded Baveno VI, which were accordingly calculated. The correlation coefficients for each index was calculated with varices class and ranked according to their significance.
Table 2.
The formulas of the common different indices.
| No. | Index [Ref.] | Year | Equation (Formula) |
|---|---|---|---|
| 1 | AAR [31] | 2003 | =AST(U/L)/ALT(U/L) |
| 2 | APRI [32] | 2003 | = [(AST/upper limit AST) × 100] /PLT (109/l) |
| 3 | LOK Score [33] | 2005 | = [exp (log odds)]/ [1 + exp (log odds)] log odds = -5.56–0.0089 × PLT (103/mm3) + 5.27 × INR + 1.26 × AAR |
| 4 | FI Score [34] | 2006 | = 8–0.01 × PLT (109/L) - ALB (g/dl) |
| 5 | Fib-4 [35] | 2007 | =[age(years)] × AST(U/L)]/ [PLT (109/L)] × ALT(U/L) (½)] |
| 6 | MELD Score [36] | 2007 | = 9.57 × ln (Cr) + 3.78 × ln (TBIL) + 11.2 × ln (INR) + 6.43 |
| 7 | King Score [37] | 2009 | = Age × AST (U/L) × INR/PLT (109/L) |
| 8 | BavenoVI* [3] | 2015 | =LS < 20 (kPa) + PLT > 150 000(/mm3) |
| 9 | VPR Score* [25] | 2015 | =(ALB × PLT)/1000 |
| 10 | Expanding BavenoVI* [6] | 2017 | =LS < 25(kPa) + PLT > 110 000(/mm3) |
| 11 | APRI-Fib4 Combo [38] | 2019 | = APRI / Fib-4 |
*A Marker was established to diagnose the EVs and others to diagnose the liver fibrosis.
Attribute selection
It was important to reduce the dimensionality of attributes for patients' dataset and select the most significant attributes. Attributes selection is a technique in data mining that reduces the dimensionality of data. It was an important step for choosing the relevant subset of attributes for building robust learning models. Moreover, if the most significant attributes were selected, the complexity of a model decreases which makes it easier to interpret, minimize tests required from patients, diminishes cost, increases accuracy, enables the machine learning algorithm to train faster, and reduces overfitting [39]. The P-value Correlation-based Filter Selection” (PC-FS) method which evaluates the attributes by using heuristics based on general characteristics of the data was used as in [40], [41].
Derivation of a novel predictive varices index
Our goal was to find a simple index. We derived a novel predictive index by Binary Logistics Regression (BLR). The input for this model was a set of significant attributes that were chosen from the attribute selection step.
BLR [42] is the most common and widely used with medical data. It was oftentimes used to compare with machine learning algorithms. It has several advantages including high accuracy and power. The goal of BLR is to find the best fit model that describes the relationship between the varices class and a set of independent attributes.
Univariate BLR had been used to individually evaluate the significance of each attribute for implying in the predictive model and to obtain odds ratios with a 95% confidence interval. The odd ratio is usually the parameter of interest derived from a fitted logistic regression due to its ease of interpretation [43]. We summarized the associations using OR, e.g., if the OR for LS was 1.2, this means that the odds for cases with varices are 1.2 times higher than that in cases with no varices. BLR produced the coefficients, their standard errors, and significance levels of the mathematical formula. Values of cutoff within 0 and 1 will be used as values of classification.
| (1) |
| (2) |
Usually, Eq. (1) is not efficient to predict the binary values {0, 1}; therefore, the Sigmoid function was used to keep the value of Y within the [0, 1] range as in Eq. (2). BLR chooses and estimates the parameters that maximize the likelihood of observing the sample values rather than choosing parameters that minimize the sum of squared error. The predicted probability that a given patient (with given attributes) belongs to the “1″ (present) was compared against the probability that it belongs to the “0” (absent).
Evaluation of the diagnostic performance
To validate our results, we randomly split the dataset to 80: 20 for training and validation, we selected an internal derivative cohort of 1003 patients for validation with maintaining the ratio of (varices: no-varices) as found in the training cohort.
Multiple types of evaluations were performed after evaluating the common indices by ranking them by correlation.
First, the diagnostic performance of the entire prediction model was evaluated with the performance of our index. We used several parameters like accuracy, sensitivity, specificity, Area Under ROC (AUC), Likelihood Ratio (LR), Negative Predictive Value (NPV), and Positive Predictive Value (PPV). AUC was used to quantify the power of the model's predicted values to discriminate between positive and negative cases. It was an efficient measure of the authenticity of a diagnostic test. To obtain the cutoff points for our index, the optimal cutoff value was chosen so that the sum of specificity and sensitivity would be at the maximum.
Second, a comparison between using the indexes or their attributes by using Naïve Bayes to find the degree of tolerance between them was conducted. We applied the Naive Bayes classifier to implement a model using a set of significant attributes that were chosen from the attribute selection step. We considered this algorithm due to its following characteristic. The Naive Bayes algorithm [44] is a simple probabilistic classifier, a very powerful model that helps make an inference on a new sample while being simple to understand and implement. It only requires a small amount of training data to estimate the means and variances of the attributes necessary for classification. It is based on applying Bayes' theorem. It predicts a class label given an attributes vector that is used to return the prediction. The values of AUC for NB were used to rank the models that are used as an index or their attributes.
Third, a comparison of the predictive performance of our index with other indices and markers was conducted. The correlation coefficient was used to rank all indices for predicting EVs. The Receiver Operating Curve (ROC) [30] was plotted for the best five indices. ROC curve analysis used the predicted probabilities that were saved in our dataset as a new attribute.
Results
The statistical analysis of the patients’ characteristics
Our analysis included only 5013 patients with chronic hepatitis without any missing values. According to UGI endoscopy results, patients were classified into two groups: group I included 2,773 (55.3%) patients who had no-varices and group II included 2,240 (45%) patients who had varices. A descriptive overview of the patients’ basic data was shown in Table 3. Patient characteristics were compared between patients with and without EV. The quantitative attributes were represented as mean and Standard Deviation (SD) except age which was represented as median and range as shown in Table 3.
Table 3.
Baseline characteristics of patients in the dataset.
| Characteristic | With EV (2,242) | Without EV (2,771) | Significance | Correlation Coefficient |
|---|---|---|---|---|
| Male: Female | 1697(75.7): 545(24.3) | 1755(63.3): 1016(36.7) | < 0.0001* | −0.133 |
| Age, year | 54 (19–74) | 54 (22–73) | 0.037 | 0.036 |
| Body Mass Index, Kg/m2 | 29 ± 4.5 | 30 ± 5 | < 0.0001 | −0.077 |
| Alanine Aminotransferase, IU/L | 60 ± 38 | 62.5 ± 40 | 0.212 | −0.018 |
| White Blood Cell x103 mm3 | 5.8 ± 5 | 6.4 ± 6.3 | < 0.0001 | −0.055 |
| ALB, g/dL | 3.65 ± 0.6 | 3.9 ± 0.6 | < 0.0001 | −0.225 |
| Aspartate Aminotransferase, IU/L | 72 ± 40 | 69.5 ± 46 | 0.001 | 0.05 |
| T.Bil, mg/dL | 1.2 ± 0.68 | 0.95 ± 0.53 | < 0.0001 | 0.210 |
| PLT*x103mm3 | 123.5 ± 68.7 | 147.6 ± 60.4 | < 0.0001 | −0.247 |
| PV Diameter, mm | 13.66 ± 2.1 | 13 ± 1.77 | < 0.0001 | 0.187 |
| LS, kPa | 30.8 ± 16 | 24.5 ± 13.3 | < 0.0001 | 0.242 |
| HCV RNA | 5.3 ± 0.9 | 5.5 ± 0.95 | < 0.0001 | −0.110 |
| International Normalized Ratio | 1 ± 0.2 | 1.2 ± 0.3 | < 0.0001 | 0.086 |
| Creatinine, mg/dL | 0.9 ± 0.3 | 0.9 ± 0.4 | 0.002 | 0.043 |
| Prothrombin Concentration | 77 ± 14.77 | 82.7 ± 13.97 | < 0.0001 | −0.201 |
| Liver Texture | < 0.0001* | 0.160 | ||
| “Cirrhotic” | 1483 (66%) | 1414 (51%) | ||
| “Abnormal” | 685(30.5%) | 1154 (42%) | ||
| “Normal” | 74 (3.4%) | 203 (7%) | ||
| Spleen | <0.0001* | −0.190 | ||
| “Average” | 595(26.5) | 1238(44.7) | ||
| “Enlarged” | 1592(71.0) | 1502(54.2) | ||
| “Removed” | 55(2.4) | 31(1.1) |
Data was represented as “mean ± SD”, frequency (percentage), *p-values were calculated by chi-square test.
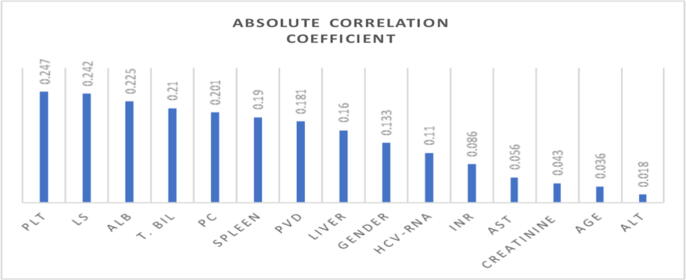
The dataset contains 69% of patients who were males and 30.1% of patients who were females. In this research, we selected only fifteen attributes from more than thirty attributes from our dataset and selected eleven markers. The quantitative attributes were found to be not-normal distribution according to the Kolmogorov-Smirnov test. So, the Mann-Whitney test was used to compare quantitative attributes. A chi-square test was performed with categorical attributes. Table 4 showed the demographic characteristics of patients in the training and validation in a coherent dataset. The correlation coefficient was calculated between all attributes and target (varices class). The absolute values of the correlation coefficient were ranked as shown in the chart of Fig. 4, at a threshold of 0.110 for the absolute correlation coefficient. The result of attributes selection was PLT, ALB, LS, T.Bil, PC, Spleen, PVD, HCV RNA, liver texture, and gender.
Table 4.
Demographic characteristics of patients in the training and validation in a coherent dataset.
| Selective attributes | Training Dataset (n = 4010) | Validation Dataset (n = 1003) | Significance |
|---|---|---|---|
| Male: Female | 69.5: 30.5 | 66.2: 33.8 | < 0.0001 |
| ALB, g/dL | 3.81 ± 0.64 | 3.74 ± 0.58 | < 0.0001 |
| T.Bil*, mg/dL | 1.06 ± 0.61 | 1.05 ± 0.63 | < 0.0001 |
| PLT*x103mm3 | 137.77 ± 65.42 | 136.45 ± 62.83 | < 0.0001 |
| PVD, mm | 13.30 ± 1.99 | 13.26 ± 1.77 | < 0.0001 |
| LS, kPa | 27.18 ± 14.3 | 28.4 ± 14.18 | < 0.0001 |
| HCV-RNA | 5.41 ± 0.95 | 5.49 ± 0.90 | < 0.0001 |
| PC | 80.31 ± 14.73 | 79.71 ± 14.02 | < 0.0001 |
| Liver Texture | < 0.0001 | ||
| Abnormal | 36.8% | 36.3% | |
| Cirrhotic | 57.3% | 59.7% | |
| Normal | 5.9% | 4.0% | |
| Spleen | <0.0001 | ||
| Average | 36.2% | 38.1% | |
| Enlarged | 62.1% | 60.3% | |
| Removed | 1.7% | 1.6% |
Fig. 4.
Ranking the attributes based on the correlation coefficient.
Performance of the novel predictive index
We used logistic regression to derivative the index. We chose the Enter method to enter the independent attributes into the model in one single step without checking. The nominal categorical attributes were identified to be encoded as numerical values by hot encoding. The attribute was included in the model if its significance was P-value < 0.05.
We found that the cutoff value of 0.423 for a classification table was the optimal point to evaluate the logistic regression model. If the value of the Index was>0.423, the class was varices; else it is no varices. The P-value was<0.0001 for the overall model; therefore, these attributes contribute to the prediction of the varices. The model correctly predicted 73.33% of the cases and 0.788 for AUC with a 95% confidence interval (0.76 to 0.813).
Since our objective was to find a simple, accurate, and easy index, a feature selection method was used to select the significant attributes for implication in a predictive model. If P-value for attributes was<0.001, the attribute was considered suitable for the model. Then the logistic regression equation for prediction was composed;
Table 5 shows the univariate binary logistic regression analyses for variables associated with EVs. Analysis of ten attributes with their coefficients and Odds Ratios (ORs) with 95% Confidence Intervals (CIs) were shown in Table 5. The values of regression coefficients were automatically calculated by the statistics software to establish a new equation for predicting EVs. The performance of logistic regression for EVP Index in the prediction of esophageal varices is illustrated in Table 6.
Table 5.
Analysis of the independent attributes of the equation with Coefficients in logistic regression.
| Coef. (B) | P-Value | ORs Exp(B) | 95% C.I. for Exp(B) | |
|---|---|---|---|---|
| Gen. (F.) | −0.488 | < 0.0001 | 0.614 | 0.514–0.675 |
| ALB | −0.387 | < 0.0001 | 0.679 | 0.597–0.746 |
| T.BiL | 0.201 | 0.001 | 1.222 | 1.089–1.364 |
| LS | 0.026 | < 0.0001 | 1.026 | 1.021–1.03 |
| PLT | −0.003 | < 0.0001 | 0.997 | 0.996–0.998 |
| PC | −0.008 | 0.003 | 0.992 | 0.988–0.997 |
| Liver Texture | < 0.0001 | |||
| L(Abnormal) | -0.219 | 0.085 | 0.803 | 0.568–1.136 |
| L(Cirrhosis) | 0.307 | 0.026 | 1.359 | 0.978–1.889 |
| PVD | 0.225 | < 0.0001 | 1.252 | 1.201–1.306 |
| Spleen | < 0.0001 | |||
| SPL(Avg.) | −1.098 | < 0.0001 | 0.334 | 0.216–0.596 |
| SPL(Enlg.) | −0.944 | 0.001 | 0.389 | 0.222–0.68 |
| HCV_RNA | −0.108 | 0.004 | 0.897 | 0.834–0.966 |
| Constant | 0.01 | 0.706 | 1.01 |
Table 6.
Diagnostic performance of models for prediction of EVs
| NPV | PPV | Specificity | Sensitivity | LR- | LR+ | AUC | Accuracy | |
|---|---|---|---|---|---|---|---|---|
| Binary Logistic Regression | 0.792 | 0.70 | 0.693 | 0.78 | 0.32 | 2.55 | 0.788 | 73.3% |
| Naïve Bayes | 0.715 | 0.726 | 0.81 | 0.61 | 0.48 | 3.21 | 0.77 | 72% |
Evaluating the performance of the common indexes with its attributes
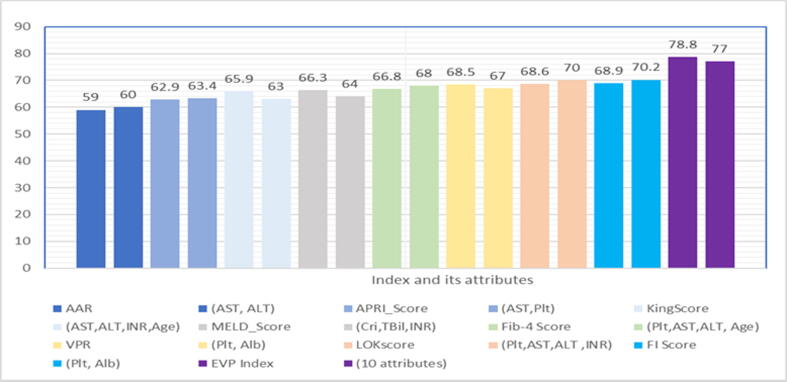
Noninvasive indices for EVs were mostly derived from the noninvasive estimation of liver fibrosis, i.e., the APRI index was first produced to identify the presence of fibrosis for HCV patients [32]. Also, Fib-4, King scores, AAR, and FI were primarily used for liver fibrosis and its severity as shown in Table 1. These indices were calculated based on regular laboratory data. Therefore, we compared between using an index and their attributes to assess the performance of each of them to diagnose the EVs. The NB algorithm was applied to their attributes and results of AUC were used to measure the performance. It was found that the value of tolerance with using an index or its attributes ranged from 0 to ± 3 as shown in Fig. 5. Also, the value of tolerance of EVP index and its attributes in this range.
Fig. 5.
Analysis of AUC for indices with their attributes by the Naïve Bayes algorithm.
Evaluation of the common indices with EVP index
The results of our study showed AAR and APRI were lower in efficiency when predicting the presence of varices, with no statistical differences between the classes. So, FI and VPR were the best and showed the better in PPV, NPV, and specificity sensitivity as shown in Table 7. The Pearson correlation coefficient was calculated across the previous indexes and the class of varices. These indices were ranked based on the absolute value of the correlation coefficient. The AUC values for the best five indexes (EVP, FI, VPR, Fib-4, and Lok score) are shown in Fig. 6. The new index (EVP) had 0.788 of AUC (95% CI, 0.761–0.813) at the optimal cutoff > 0.423, 79% for NPV, 78% for sensitivity, 72% for PPV, and 69% for specificity. VPR, Fib-4, LOK scores, and FI had AUCs of 0.685 (95% CI, 0.655–0.713), AUC of 0.668 (95% CI, 0.637–0.697), AUC of 0.686 (95% CI 0.656–0.715), AUC of 0.69 (95% CI 0.660–0.718), respectively.
Table 7.
Comparison between the performance of the noninvasive markers for prediction of EV
| Marker | Correlation Coefficient | Cutoff value | SE | SP | +LR | -LR | PPV | NPV | AUC |
|---|---|---|---|---|---|---|---|---|---|
| AAR | 0.154 | >1.09 | 64 | 51 | 1.33 | 0.7 | 52.4 | 63 | 0.59 |
| Extend Baveno VI | 0.262 | > 0 | 81.8 | 42.7 | 1.43 | 0.43 | 54 | 73.8 | 0.622 |
| APRI Score | 0.222 | >1.108 | 64.8 | 54.6 | 143 | 0.64 | 54 | 65 | 0.629 |
| LS | 0.242 | >26.4 | 59 | 62.7 | 1.58 | 0.65 | 56.8 | 64.8 | 0.64 |
| ALB | −0.249 | ≤3.5 | 49 | 75.2 | 1.97 | 0.68 | 62.1 | 64 | 0.644 |
| King Score | 0.274 | >26.77 | 71 | 54 | 1.55 | 0.53 | 56.3 | 69.4 | 0.659 |
| PLT | −0.277 | ≤100 | 47 | 80.7 | 2.43 | 0.66 | 67 | 64.7 | 0.66 |
| MELD Score | 0.281 | >5.356 | 74.5 | 51.5 | 1.53 | 0.5 | 56 | 71 | 0.663 |
| Fib-4 | 0.289 | >3.56 | 65.5 | 63.9 | 1.81 | 0.54 | 60 | 69 | 0.668 |
| VPR | −0.318 | ≤0.443 | 61.3 | 69 | 1.98 | 0.56 | 62 | 68 | 0.685 |
| LOK Score | 0.321 | >0.728 | 64 | 67 | 1.93 | 0.54 | 61.6 | 69 | 0.686 |
| FI Score | 0.285 | >3.12 | 60 | 72 | 2.17 | 0.55 | 64 | 68.6 | 0.689 |
| EVP Index | 0.496 | >0.423 | 78 | 69 | 2.55 | 0.32 | 72 | 79 | 0.788 |
SE “sensitivity”; SP “specificity”; +LR “positive likelihood ratio”; -LR “negative likelihood ratio”; NPV “Negative predictive value”; PPV “Positive predictive value”; AUC “area under curve”.
Fig. 6.
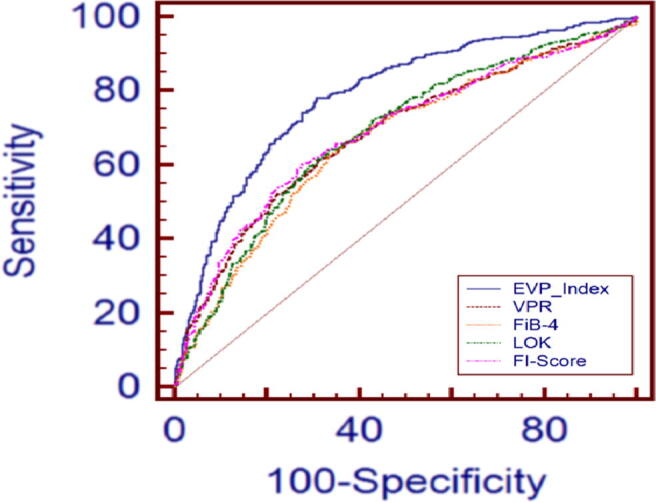
Comparative AUC analysis of different indices in predicting EVs.
Our study assessed suitability for using the proposed indexes as the predictors. They were investigated to find optimal discriminatory cutoff value points. Our study explored the diagnostic accuracy of the common established noninvasive indexes for predicting EVs for 5,013 patients, but their diagnostic performance was relatively poor as shown in Table 7.
Discussion
Noninvasive diagnosis methods of EVs are an important field concerning the treatment of liver cirrhosis. Nowadays, physicians are mindful to recognize the significant noninvasive markers which may be easier and cheaper to perform and with high specificity and sensitivity to reduce the number of UGI endoscopies. These noninvasive methods are especially needed in developing countries that lack endoscopy devices and sufficient resources.
Thus, this multicenter cross-sectional study assessed different laboratory tests, ultrasonographic parameters, and eleven indices as a noninvasive method for the diagnosis of EVs on 5,013 patients with liver cirrhosis. Thus, we attempted to examine whether the noninvasive biochemical markers such as PLT, LS, and ALB or indices such as AAR, APRI, FI, Fib-4, LOK, King’s Score, and VPR could predict EVs. We classified the indexes that were introduced in this study into two types: The first one was the indexes that were built to evaluate the severity of liver fibrosis such as Fib-4, AAR, APRI, King, Lok score, and MELD. The second group consisted of the indices that were built for predicting EVs such as VPR, Baveno VI, and extend Baveno VI.
For all these indices, we analyzed the attributes used in their formula as shown in Table 2 to select the most significant. Based on the introduction of a systematic review for previous works, we calculated these indices from our dataset and concluded the following points:
Some studies concluded that stiffness was a good predictor for EV such as [9], [11], [13], [12], [10]. Also, Albumin was used as the only predictor as study [14] while other studies suggested that PLT would suffice such as study [15]. However, they also achieved low performance in this study. The previous studies faced several limitations as described in Section 2. Our study proved that there was no single attribute that could be used alone as an efficient predictor of the presence of EVs as shown in Table 6. This agrees with Stefanescu et al. [8] where they combined Lok Score with LS to predict EV. Their results increased to 74.66% for diagnostic accuracy. In contrast, Saad et al. [9], T. Y. Kim et al. [10], Ghamdi et al. [11], Llop et al. [12], Sarkar et al. [13] proved that LS was the only effective predictor of EVs. Also, Abdel Hamed et al. [14] proved that ALB might be used as a noninvasive marker for EVs at a cutoff value greater than − 2.2, and also some studies used PLT such as Abd -Elsalam et al. [15].
Our study agreed with Sarkar et al. [13], Sedrak et al. [20], Mattos et al. [21], and Kamel Ahmed et al. [24] who concluded that AAR and APRI were poor predictors of EV. Also, Deng Qi and Guo [23] proved that Lok scores, APRI, Fib-4, and AAR had low to medium efficiency concerning diagnostic accuracy in predicting EVs.
Regarding the Fib-4 index, although some previous studies concluded that it was commonly used for predicting the progress of liver fibrosis [35], [45], [46]. Khairy et al. [46] proved that it represented the best performing ROC for diagnosing moderate and marked fibrosis among other independent factors with a PPV of 56%, NPV of 76%, a specificity of 60%, and sensitivity of 74% in Egypt. It was expected to be a significant predictor for assessing EVs such as Bledar Kraja et al. [1], who found that Fib-4 at a cutoff value of 3.23 was the only significant predictor for EVs with 66% for the AUC on 139 Albanian patients. This contradicts our study; the AUC was 0.668, so it was not enough to predict the EV. Also, Deng Qi and Guo [23] proved that Fib-4 had low to moderate diagnostic accuracy in predicting EV.
Our study proved that FI was built to predict fibrosis, which is a better index than the other indices. This agrees with Deng et al. [22] who proved that FI had the largest AUC (0.60), followed by Fib-4 (0.544), AAR (0.538), King (0.526), and APRI (0.506), while this contradicts Bledar Kraja et al. [1]. With that concluded, there was no significant association for EVs with FI.
Our study proved that Varices Prediction Rule (VPR) was built to predict the presence of EVs and is a better index than Baveno VI and expanded Baveno VI. It achieved 0.685 for AUC. This agrees with the outcomes provided by A. Isted et al. [25], who developed VPR and examined it with the other five indices. They reported that it achieved better AUC at 0.75, sensitivity at 86%, and sensitivity at 71% compared with the others. On the other hand, Tosetti et al. [19] proved that “the expanded Baveno VI” criteria allowed sparing screening endoscopies up to 45% without losing accuracy.
Our EVP index predicted nearly 2,256 True Negative (TN) outcomes (who had no varices). If we divided them on the total number of patients, 5,013, we could save operating the upper endoscopic UGI by 46.5%. Our study aims to help physicians to save costs and avoids unnecessary UGI, which could get some advantages from medical costs; however, it could be useful in daily practice to decide which patients do not need a UGI endoscopy. Our index is the most reliable predictor of EVs despite the low diagnostic accuracy. It could be used as an initial screening method for cirrhotic patients in developing countries that lack sufficient UGI facilities.
This study had some limitations: The number of patients included was heterogeneous through the participating centers, and physicians’ approach for managing patients with liver differed. The accuracy may be unacceptable because of the data’s heterogeneous nature and the drawback of logistic regression. In contrast, applying a boosted naive Bayes tree on these ten attributes gave a better performance that exceeded 0.86 and 78.6% for AUC and the accuracy, respectively, as in the study [40]. There were only 2% of varices classified as risky varices, 3.5% unknown degree, 5.4% eradicated, and 34% non-risky as shown in Fig. 2. Liver stiffness was evaluated by the elastography technique and portal vein diameter was useful for the prediction of EVs. However, these techniques were not readily available at all centers as there was a struggle with a large portion of missing data. Also, this study did not cover or assess any indexes that were not available with their attributes in our dataset such as Liaoning Score [47] and Forns’ index [48] for prediction of esophageal varices.
Finally, these ten markers depended on regular laboratory tests. They did not require any extra cost or additional biochemical tests and specialized devices. This fact may be beneficial in developing countries.
Conclusion
Our study sought to evaluate the diagnostic performance for several common indices in predicting the presence of EVs. We introduced a prospective study for a multicenter on HCV patients in Egypt, which was an endemic area of hepatitis C virus infection. This study evaluated the effectiveness of various noninvasive predictors for predicting EV, including liver stiffness, PLT, ALB, APRI score, Lok score, AAR, Fib-4 score, FI score, VPR, and King Score. Based on the results, PLT was the best single predictor (AUC = 0.66), but it cannot be used as the only predictor. We concluded that although stiffness, AlB, and PLT were significant in diagnosing EV, each of them was not sufficient to achieve accepted predictive performance when used alone, and, hence, cannot replace or minimize the need for UGI. “FI” was the largest AUC considering the common markers followed by the VPR and Lok index (AUC = 0.68), followed by “Fib-4” (AUC = 0.66). AAR and APRI had low diagnostic accuracy for EVs. We developed a new index “Egyptian Varices Prediction” (EVP Index) to predict EVs. EVP Index was derived using a binary logistic regression, that relied on only ten significant attributes (PLT, LS, PC, liver, spleen, HCV-RNA, ALB, gender, T.BIL, and PVD). EVP Index achieved higher performance than other predictors introduced in this study. It was significantly correlated to EVs at 0.5 at a cutoff point>0.423 and achieved 78%, 69%, 2.55, 0.32 72%, 79%, 0.788, and 73.3% for SN, SP, +LR, -LR, PPV, NPV, AUC, and diagnostic accuracy, respectively. In the future, we shall validate the EVP Index to predict the high-risk EV.
Compliance with ethics requirements
All the procedures performed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and/ or national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). the study was approved by National Research Centre ethics committee with number 1458. All the participants gave written informed consent to the participation in the study.
Authors' contributions
Shimaa Abd-ElSalam, Mohamed Ezz, and Mahmoud ElHefnawi, Prof. Shehab Gamalel Din, and Prof. Gamal Esmat contributed to the study conceptualization and design; Wafaa Elakel, data acquisition; Shimaa Abd El-Salam analyzed the data and statistical analysis; Shimaa Abd El-Salam and Mohamed Ezz performed the methodology and interpreted the results; Shimaa AbdEl-Salam drafted the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
-
•
the paper is original and has not been published in other journals or books.
-
•
the paper does not infringe upon any copyright or other proprietary right;
-
•
the manuscript has been reviewed and approved by all named authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors are thankful to “the Egyptian National Committee for Control of Viral Hepatitis” to supply the medical dataset.
Data Availability and Materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Shimaa M. Abd-Elsalam, Email: eng.shimaa_2006@yahoo.com.
Mohamed M. Ezz, Email: ezz.mohamed@azhar.edu.eg.
Shehab Gamalel-Din, Email: drshehabg@yahoo.com.
Gamal Esmat, Email: gesmat@cu.edu.eg.
Wafaa Elakel, Email: wafaaelakel@link.net.
Mahmoud ElHefnawi, Email: mahef@aucegypt.edu.
References
- 1.Bledar Kraja, Iris Mone, Ilir Akshija, Adea Koçollari, Skerdi Prifti GB, Bledar. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J Gastroenterol 2017;23:4661–846. doi:10.3748/wjg.v23.i26.4806. [DOI] [PMC free article] [PubMed]
- 2.Jakab SS, Garcia-Tsao G. Chapter 15: Esophageal Varices. In: S. M. Cohen, P. Davitkov (eds.) LD, editor. Liver Disease, Cham: Springer International Publishing; 2019, p. 195–208. doi:10.1007/978-3-319-98506-0_15.
- 3.de Franchis R. Expanding consensus in portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Elrazek A.E.M.A.A., Mahfouz H. Prediction Analysis of Esophageal Variceal Degrees using Data Mining: Is Validated in Clinical Medicine? Global J Comput Sci Technol Software Data Eng. 2013;13 [Google Scholar]
- 5.Kim D.H., Park J.Y. Prevention and management of variceal hemorrhage. Int J Hepatol. 2013;2013:434609. doi: 10.1155/2013/434609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustin S., Pons M., Maurice J.B., Bureau C., Stefanescu H., Ney M., et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980–1988. doi: 10.1002/hep.29363. [DOI] [PubMed] [Google Scholar]
- 7.Chandail V.S., Kotwal S.K., Koul S., Gupta R., Mahajan A. Non-invasive markers for prediction of varices in patients with portal hypertension. Int J Res Med Sci. 2017;5:1007. doi: 10.18203/2320-6012.ijrms20170652. [DOI] [Google Scholar]
- 8.Stefanescu H., Grigorescu M., Lupsor M., Maniu A., Crisan D., Procopet B., et al. A New and Simple Algorithm for the Noninvasive Assessment of Esophageal Varices in Cirrhotic Patients Using Serum Fibrosis Markers and Transient Elastography. J Gastrointestinal Liver Dis JGLD. 2011;20:57–64. [PubMed] [Google Scholar]
- 9.Saad Y., Said M., Idris M.O., Rabee A., Zakaria S. Liver stiffness measurement by fibroscan predicts the presence and size of esophageal varices in egyptian patients with HCV related liver cirrhosis. J Clin Diagnostic Res JCDR. 2013;7:2253–2257. doi: 10.7860/JCDR/2013/6026.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim T.Y., Kim T.Y., Kim Y., Lim S., Jeong W.K., Sohn J.H. Diagnostic Performance of Shear Wave Elastography for Predicting Esophageal Varices in Patients With Compensated Liver Cirrhosis. J Ultrasound Med. 2016;35:1373–1381. doi: 10.7863/ultra.15.07024. [DOI] [PubMed] [Google Scholar]
- 11.Hassan Al Ghamdi M, Wang L, Hu JW, Dong S, Jian YC, Hu L, et al. Transient Elastography (Fibroscan) Compared to Diagnostic Endoscopy in the Diagnosis of Varices in Patients with Cirrhosis. Science Journal of Clinical Medicine 2017;5:55–9. doi:10.11648/j.sjcm.20160506.13.
- 12.Llop E., Lopez M., de la Revilla J., Fernandez N., Trapero M., Hernandez M., et al. Validation of noninvasive methods to predict the presence of gastroesophageal varices in a cohort of patients with compensated advanced chronic liver disease. J Gastroenterol Hepatol. 2017;32:1867–1872. doi: 10.1111/jgh.13781. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar D.K., Azam G., Haque M., Rahman A. Prediction of esophageal varices in liver cirrhosis by transient elastography and aspartate aminotransferase - to - platelet ratio index (APRI) Bangladesh Critical Care J. 2018;6:16–21. doi: 10.3329/bccj.v6i1.36606. [DOI] [Google Scholar]
- 14.Amer bdel Hamid Gomaa, Sayed Farouk Mohammed, Waleed Mohamed Mousa, Nabil Fathy Esmael Hasan MAMM. Evaluation of ALBI, MELD and Child-Pugh Scores as non-Invasive Predictors of Esophageal Varices. The Egyptian Journal of Hospital Medicine 2018;73:7358–64.
- 15.Abd-Elsalam S., Habba E., Elkhalawany W., Tawfeek S., Elbatea H., El-kalla F., et al. Correlation of platelets count with endoscopic findings in a cohort of Egyptian patients with liver cirrhosis. Medicine. 2016;95 doi: 10.1097/MD.0000000000003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurice J.B., Brodkin E., Arnold F., Navaratnam A., Paine H., Khawar S., et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65:899–905. doi: 10.1016/j.jhep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Adjeka Stanislas D., Constant A., Ndam Antonin Wilson N., Hardryt Dimitri K., Demba B., Anzouan-Kacou K., et al. Liver Transient Elastography Combined to Platelet Count (Baveno VI) Predict High Esophageal Varices in Black African Patient with Compensated Hepatitis B Related Cirrhosis. Open J Gastro-Enterol. 2016;8:192–200. doi: 10.4236/ojgas.2018.85021. [DOI] [Google Scholar]
- 18.Sousa M., Fernandes S., Proença L., Silva A.P., Leite S., Silva J., et al. The Baveno VI criteria for predicting esophageal varices: validation in real life practice. Revista Española de Enfermedades Digestivas. 2017;109:704–707. doi: 10.17235/reed.2017.5052/2017. [DOI] [PubMed] [Google Scholar]
- 19.Tosetti G, Mura VL, D’ambrosio R, Degasperi E, Mezzina N, Viganò M, et al. Screening of esophagogastric varices: Performance of the “Expanded Baveno Vi criteria” and the “platelet 150/MELD 6” strategy in all etiology compensated advanced chronic liver disease. Journal of Hepatology 2018;68:S734–5. doi:10.1016/S0168-8278(18)31730-6.
- 20.Sedrak H., Khalifa R., Elkafrawy A., Elewa H. Noninvasive predictors of large esophageal varices: is there an emerging role of aspartate aminotransferase-to-platelet ratio index in hepatocellular carcinoma? Egyptian Soc Internal Med. 2015;27:139–146. doi: 10.4103/1110-7782.174935. [DOI] [Google Scholar]
- 21.de Mattos Â.Z., de Mattos A.A., Daros L.F., Musskopf M.I. Aspartate aminotransferase-to-platelet ratio index (APRI) for the non-invasive prediction of esophageal varices. Ann Hepatol. 2013;12:810–814. doi: 10.1016/s1665-2681(19)31324-9. [DOI] [PubMed] [Google Scholar]
- 22.Deng H., Qi X., Peng Y., Li J., Li H., Zhang Y., et al. Diagnostic accuracy of APRI, AAR, FIB-4, FI, and king scores for diagnosis of esophageal varices in liver cirrhosis: A retrospective study. Med Sci Monit. 2015;21:3961–3977. doi: 10.12659/MSM.895005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng H., Qi X., Guo X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, king, lok, forns, and fibroindex scores in predicting the presence of esophageal varices in liver cirrhosis. Medicine (United States) 2015;94 doi: 10.1097/MD.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A. Ahmed K, H. Mohammed I, Alaa F, Ahmed G. Assessment of Different Non-Invasive Score Modalities in Prediction of Esophageal Varices in Patients with Liver Cirrhosis. Zagazig University Medical Journal 2019;25:261–8. doi:10.21608/ZUMJ.2019.27411.
- 25.Isted A., Grammatikopoulos T., Davenport M. Prediction of esophageal varices in biliary atresia: Derivation of the “varices prediction rule”, a novel noninvasive predictor. J Pediatr Surg. 2015;50:1734–1738. doi: 10.1016/j.jpedsurg.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Abe H., Midorikawa Y., Matsumoto N., Moriyama M., Shibutani K., Okada M., et al. Prediction of esophageal varices by liver and spleen MR elastography. Eur Radiol. 2019;29:6611–6619. doi: 10.1007/s00330-019-06230-8. [DOI] [PubMed] [Google Scholar]
- 27.Rockey D.C., Elliott A., Lyles T. Prediction of esophageal varices and variceal hemorrhage in patients with acute upper gastrointestinal bleeding. J Invest Med. 2016;64:745–751. doi: 10.1136/jim-2015-000047. [DOI] [PubMed] [Google Scholar]
- 28.Peng Y., Qi X.S., Dai J., Li H., Guo X.Z. Child-pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8:751–757. [PMC free article] [PubMed] [Google Scholar]
- 29.Lind, Douglas A, William G. SAW. statistical Techniques in business and Economics. Fifteenth. Americas, New York,: McGraw-Hill/Irwin, a bTim Vertovec. usiness unit of The McGraw-Hill Companies, Inc., 1221Avenue of the; 2012.
- 30.Nisbet R, John E, Miner G. Handbook of Statistical Analysis and Data Mining Applictions. vol. 39. Elsevier; 2009.
- 31.Giannini E., Risso D., Botta F., Chiarbonello B., Fasoli A., Malfatti F., et al. Validity and Clinical Utility of the Aspartate Aminotransferase-Alanine Aminotransferase Ratio in Assessing Disease Severity and Prognosis in Patients With Hepatitis C Virus-Related Chronic Liver Disease. Arch Intern Med. 2003;163:218. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 32.Wai C.T., Greenson J.K., Fontana R.J., Kalbfleisch J.D., Marrero J.A., Conjeevaram H.S., et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 33.Lok A.S.F., Ghany M.G., Goodman Z.D., Wright E.C., Everson G.T., Sterling R.K., et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratoiy tests: Results of the HALT-C cohort. Hepatology. 2005;42:282–292. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 34.Ohta T., Sakaguchi K., Fujiwara A., Fujioka S.I., Iwasaki Y., Makino Y., et al. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60:77–84. doi: 10.18926/AMO/30729. [DOI] [PubMed] [Google Scholar]
- 35.Vallet-Pichard A., Mallet V., Nalpas B., Verkarre V., Nalpas A., Dhalluin-Venier V., et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 36.Kamath P.S., Kim W.R. The Model for End-stage Liver Disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 37.Cross T.J.S., Rizzi P., Berry P.A., Bruce M., Portmann B., Harrison P.M. King’s Score: An accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:730–738. doi: 10.1097/MEG.0b013e32830dfcb3. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulos N., Vasileiadi S., Papavdi M., Sveroni E., Antonakaki P., Dellaporta E., et al. Liver fibrosis staging with combination of APRI and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann Gastroenterol. 2019;32:498–503. doi: 10.20524/aog.2019.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain D., Singh V. Feature selection and classification systems for chronic disease prediction: A review. Egyptian Informatics J. 2018;19:179–189. doi: 10.1016/j.eij.2018.03.002. [DOI] [Google Scholar]
- 40.Abd-Elsalam S.M., Ezz M.M., Gamalel-Din S., Esmat G., Salama A., ElHefnawi M. Early diagnosis of esophageal varices using Boosted-Naïve Bayes Tree: A multicenter cross-sectional study on chronic hepatitis C patients. Inf Med Unlocked. 2020;20:100421. doi: 10.1016/j.imu.2020.100421. [DOI] [Google Scholar]
- 41.Abd-elsalam S.M., Ezz M.M., Hashem S., Elakel W., Salama R., ElMakhzangy H., et al. Performance of machine learning approaches on prediction of esophageal varices for Egyptian chronic hepatitis C patients. Inf Med Unlocked. 2019;17:1–7. doi: 10.1016/j.imu.2019.100267. [DOI] [Google Scholar]
- 42.Dreiseitl S., Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. 2002;35:352–359. doi: 10.1016/S1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 43.Iyer R., Hosmer D.W., Lemeshow S. Applied Logistic Regression. The. Statistician. 1991;40:458. doi: 10.2307/2348743. [DOI] [Google Scholar]
- 44.Huang J., Lu J., Ling C.X. Third IEEE International Conference on Data Mining. 2003. Comparing naive Bayes, decision trees, and SVM with AUC and accuracy; pp. 553–556. 10.1109/ICDM.2003.1250975. [Google Scholar]
- 45.Kasputyt S., Petraityt M., Karu A. Non-invasive prediction of Liver fibrosis and cirrhosis. J Med Sci. 2017;1:1–4. [Google Scholar]
- 46.Khairy M., Abdel-Rahman M., El-Raziky M., El-Akel W., Zayed N., Khatab H., et al. Non-Invasive Prediction of Hepatic Fibrosis in Patients With Chronic HCV Based on the Routine Pre-Treatment Workup. Hepatitis Monthly. 2012;12 doi: 10.5812/hepatmon.6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi X., Li Y., Wang R., Lin L., Li J., Wang L., et al. Liaoning Score for Prediction of Esophageal Varices in Cirrhotic Patients Who Had Never Undergone Endoscopy: A Multicenter Cross-Sectional Study in Liaoning Province China. Adv. Therapy. 2019;36:2167–2178. doi: 10.1007/s12325-019-00967-w. [DOI] [PubMed] [Google Scholar]
- 48.Siregar R.A., Dairi L.B., Siregar G.A. Forns index as a useful noninvasive predictor of esophageal varices in liver cirrhosis. Universa Medicina. 2016;35:199. doi: 10.18051/univmed.2016.v35.199-205. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.