Abstract
Background and objective: E6 and E7 proteins in human papillomavirus (HPV) 16 are major oncogenes in several types of tumors, including lung cancer. Previous studies have demonstrated that both E6 and E7 oncoproteins can upregulate GLUT1 protein and mRNA expression levels in lung cancer cells. Thus, the present study aimed to investigate the main differences in the molecular mechanisms of GLUT1 expression regulated by E6 and E7. Methods: The double directional genetic manipulation and immunofluorescence were performed to explore the molecular mechanism of E6 or E7 upregulating the expression of GLUT1 in H1299 and A549 cell lines. Results: The overexpression of E6 in well-established lung cancer cell lines upregulated thioredoxin (Trx) protein expression. Notably, plasmid transfection or small interfering RNA transfection with E7 had no regulatory effect on Trx expression. As an important disulfide reductase of the intracellular antioxidant system, Trx plays important role in maintaining oxidative stress balance and protecting cells from oxidative damage. The overexpression of Trx increased the activation of NF-κB by upregulating p65 expression and promoting p65 nuclear translocation, and further upregulated GLUT1 protein and mRNA expression levels. The results of the present study demonstrated that E6, but not E7, upregulated GLUT1 expression in lung cancer cells by activating NF-κB due to the participation of Trx. Conclusion: These results suggest that Trx plays an important role in the pathogenesis of HPV-associated lung cancer, and propose a novel therapeutic target for HPV-associated lung cancer.
Keywords: human papillomavirus, thioredoxin, NF-κB, glucose transporter 1, lung cancer
Introduction
Human papilloma virus (HPV) is a circular double stranded DNA virus, and its infection is associated with different types of human diseases. The persistent infection of high-risk HPV is closely associated with the occurrence of cervical cancer.1,2 With the rapid development of molecular biology, several epidemiology studies have confirmed that high-risk HPV16 is the most common type of infection in the occurrence of cervical cancer.3,4 The E6 and E7 oncoproteins in HPV16 are the main carcinogens of the occurrence and development of cervical cancer. 5 E6 mRNA-expression in HPV16, but not E7, is positively associated with the severity of cervical intraepithelial lesions, 2 whereas E7 protein expression is able to differentiate high-grade lesions from low-grade lesions via p16 expression. 6 Whether the pathogenesis of HPV-associated lung cancer is consistent with that of cervical cancer remains to be further studied. Although it has not been confirmed that HPV infection is related to the occurrence of lung cancer in other states so far,7,8 a considerable number of infection rates (37.22%) in Asia, especially in China and Japan, are closely related to the occurrence of lung cancer.9,10 It has been reported that the tumor suppressor gene, p53, is the target gene of E6 protein 11 and has a regulatory association with thioredoxin (Trx). 12 Thus, it was speculated that there may be upstream and downstream regulatory associations between E6 and Trx. There are similar theories in the pathogenesis of HPV-related lung cancer and cervical cancer.13,14 Recently, we found that HPV16 E6/E7 in lung cancer cells upregulated GLUT1 protein and mRNA expression levels. 15 However, the molecular mechanism underlying the regulation of GLUT1 expression via HPV16 E6/E7 remains unclear.
Trx is an important disulfide reductase in the intracellular antioxidant system, which plays important roles in maintaining oxidative stress balance and protecting cells from oxidative damage. 16 Increasing evidence suggests that Trx is a key molecule in the pathogenesis of different diseases, including lung cancer, and it has the potential to be a therapeutic target for these diseases.17–19 It has been reported that serum Trx expression is significantly increased in patients with NSCLC, and thus may be used as a novel diagnostic and prognostic marker for NSCLC.17–21 Another study demonstrated that Trx can activate the NF-κB complex by regulating the subunit p65 of NF-κB in the respiratory epithelium and initiation of the inflammatory response. 22 However, whether Trx has a regulatory effect on p65 in lung cancer cells remains unknown. In recent studies, we have made several findings: GLUT1 enhances NSCLC cell proliferation, invasion, and migration but inhibits cell apoptosis; 23 GLUT1 is a downstream target gene of p65 in lung cancer cells, 24 and its expression is related to[ 18 F]FDG uptake in nonsmall cell lung cancer, 25 playing an important role in the glycolysis process of cancer cells26,27—namely, the Warburg effect. In vitro studies have confirmed that both GLUT1 and p16 have regulatory effects on EGFR expression, but the effects are opposite. 28 In patients with lung cancer, p16 significantly affects the prognosis, especially in the early stage. 29 It is well known that even under aerobic conditions, cancer cells will consume more glucose as energy supply through glycolysis. 30
The present study aimed to investigate that the molecular mechanisms of GLUT1 expression regulated by E6 via the HPV-Trx-NF-κB-GLUT1 axis. In particular, we explored the difference between E6 and E7 in the regulation of Trx, and the factors of Trx triggering the activation of p65, a subunit of NF-κB in lung cancer cells.
Materials and Methods
This study was approved by the Ethics Committee of the First Hospital of China Medical University.
Cell culture. Human lung adenocarcinoma cell lines (H1299 and A549), large cell carcinoma cell line (H460), and squamous cell carcinoma cell line (SK) were purchased from the American Type Culture Collection. H1299, A549, and H460 cells were maintained in RPMI-1640 medium (cat.no.12633012) supplemented with 10% FBS (cat.no.16140071; both purchased from Gibco, Thermo Fisher Scientific, Inc.), at 37 ˚C with 5% CO2. SK cells were maintained in MEM medium (cat. On. SH30265.01; purchased from Hyclone) supplemented with 10% FBS at 37 ˚C with 5% CO2.
Cell transfection. The pEGFP-N1-HPV16 E6, pEGFP-N1-HPV16 E7, and pEGFP-N1 plasmids 13 were kindly provided by Professor Xudong Tang from the Institute of Biochemistry and Molecular Biology, Guangdong Medical College, (Guangzhou, China). pCMV6-entry Trx was purchased from OriGene Technologies, Inc. Empty vector and mock transfections served as appropriate controls. HPV16 E6 small interfering (si) RNA and HPV16 E7 siRNA were purchased from Guangzhou RIBOBIO Co., Ltd, while Trx siRNA and p65 siRNA were purchased from Santa Cruz Biotechnology, Inc. Disordered siRNA was used as a nonspecific siRNA control.
Plasmids with the respective target sequences were transiently transfected into cells using the Lipofectamine® 3000 transfection kit (Invitrogen P3000™; Thermo Fisher Scientific, Inc.; cat.no.L3000075). Briefly, dissociated cells that had reached 80% to 90% confluence were seeded into a T-75 flask 24 h before transfection. Cells were counted using standard trypan blue exclusion to guarantee cells were >90% viable and had reached 70% confluence on the day of transfection. Cells were seeded into a 24-well plate at a density of 1.05 × 105 cells/well in 500 μL growth medium. Transfection was performed according to the manufacturer’ protocol. A total 50 μL DNA-lipid complex with 500 ng DNA was added to each/well. Reverse transcription-polymerase (RT-q) PCR analysis was performed 24 h post-transfection, while western blot analysis was performed 48 h post-transfection.
For siRNA transfection, A549 cells were seeded at a density of 5 × 104 cells/35-mm well and cultured until they reached 60% to 80% confluence. Following incubation for 24 h, siRNA was transfected using Lipofectamine™ RNAiMAX reagent (Invitrogen; Thermo Fisher Scientific, Inc.; cat.no.13778100). A total of 50 μL siRNA-lipid complex with 5 pmol siRNA was added into each well, according to the manufacturer's protocol. Cells were further incubated for 48 h and subjected to various analyses. The siRNA sequences of HPV16 E6 small interfering (si) RNA (cat.no. siB08523161420; RiboBio) and HPV16 E7 siRNA (cat.no. siG1379163826; RiboBio) are GAGCTGCAAACAACTATAC and GCTTCGGTTGTGCGTACAA, respectively.
Western blotting. Western blot analysis was performed as previously described. 15 The following primary antibodies were used: HPV16 E6 (cat.no.bs-0990R; 1:100; Bioss Biotechonlogy Co., Ltd), HPV16 E7 (cat.no.bs-0714R; 1:100; Bioss Biotechonlogy Co., Ltd), Trx (cat.no.ab-109385; 1:1000; Abcam), p65 (cat.no. #8242; 1:1,000, Cell Signaling Technology), GLUT1 (cat.no. WL03141; 1:500, Wanleibio), and GAPDH (cat.no. #5174; 1:1000; Cell Signaling Technology).
RT-qPCR. Total RNA was extracted from cells using TRIzol® reagent (Takara, Biotechnology Co., Ltd; cat.no.9108), following treatment. qPCR was subsequently performed using SYBR® Premix Ex Taq II (Takara, Biotechnology Co., Ltd; cat.no.RR820A.), according to the manufacture's protocol. All subsequent steps were performed as previously described. 24 The primer sequences used for qPCR are listed in Table 1.
Table 1.
Sequences and Features of Primers Used for RT-qPCR.
| Gene | Forward/Reverse | Sequence | Size(bp) | mRNA |
|---|---|---|---|---|
| E6 | 270 | GTATGGAACAACATTAGAACAGCAA | 81 | NC_001526.4 |
| 350 | AGTGGCTTTTGACAGTTAATACAC | |||
| E7 | 482 | GCATGGAGATACACCTACATTG | 273 | KX545363 |
| 754 | TGGTTTCTGAGAACAGATGG | |||
| Trx | 350 | CCACCTTTTGCGTCTTTCGG | 83 | XM_005758078.1 |
| 432 | CTCCACCAACGCCATAAGCG | |||
| P65 | 870 | GCAGGCTCCTGTGCGTGTCT | 286 | NM_021975.3 |
| 1155 | GGTGCTCAGGGATGACGTAAA | |||
| GLUT1 | 1071 | CTGGCATCAACGCTGTCTTC | 167 | NM_006516.3 |
| 1237 | GCCTATGAGGTGCAGGGTC | |||
| GAPDH | 50 | TTCTTTTGCGTCGCCAGCCGAG | 71 | XM_019023188.1 |
| 120 | CCAGGCGCCCAATACGACCAAA |
Abbreviations: mRNA, messenger RNA; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction.
Immunofluorescence. Immunofluorescence was performed as previously described. 18 H1299 cells were transiently transfected with pCMV6-entry Trx vector. After Trx transfection, the nuclear translocation of p65 was detected via immunofluorescence. The cover slips were observed under confocal microscope (Carl Zeiss, AG, × 200).
Statistical analysis. Statistical analysis was performed using SPSS 22.0 software (IBM Corp). All experiments were performed in triplicate and data are presented as the mean ± standard deviation. Analysis of variance and least significant difference test were used to compare differences between two groups. P < .05 was considered to indicate a statistically significant difference.
Results
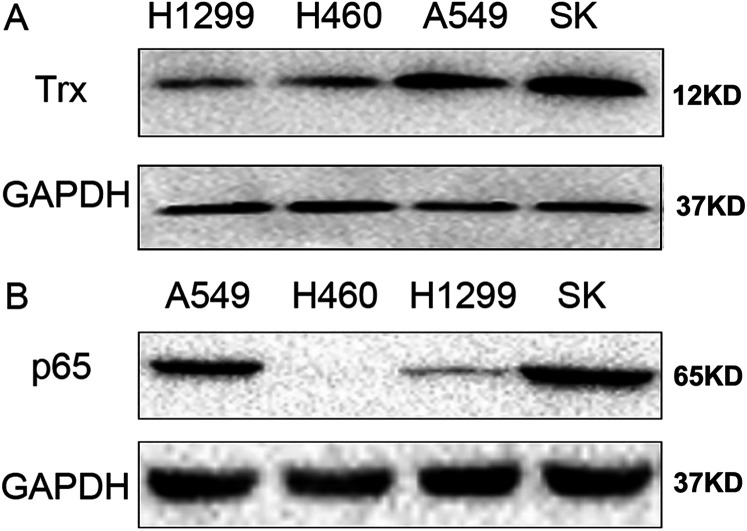
Screening of lung cancer cell lines. According to our previous screening results, H1299 cells express E6 and E7 at low levels, while A549 cells express E6 and E7 at high levels. 15 The present study detected Trx and p65 protein expression levels in H1299, H460, A549, and SK cells. The results demonstrated that Trx and p65 were expressed at low levels in H1299 and H460 cells, and at high levels in A549 and SK cells (Figure 1A and B).
Figure 1.
Expression levels of Trx and p65. Western blot analysis was performed to detect Trx and p65 expression levels in H1299, H460, A549, and SK lung cancer cell lines. GAPDH was used as the internal control. Trx, thioredoxin.
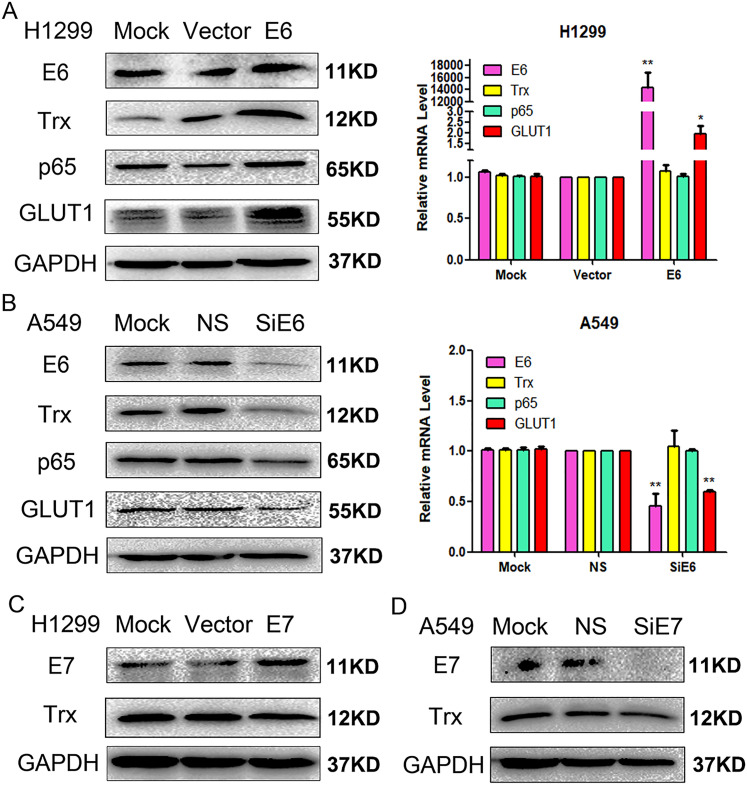
Overexpression of E6 increases the expression levels of Trx, p65 and GLUT1. Transient transfection of H1299 with pEGFP-N1-E6 vectors significantly increased E6 expression (P < .01). In addition, elevated E6 levels significantly upregulated GLUT1 protein and mRNA expression levels (P < .01), and Trx (P < .01) and p65 (P < .05) protein expression levels (Figure 2A).
Figure 2.
Effects of E6 on the regulation of Trx, p65 and GLUT1 expression via (A) transfection and (B) siRNA transfection. Effect of E7 on the regulation of Trx expression via (C) transfection and (D) siRNA transfection. Western blot and reverse transcription-quantitative PCR analyses were performed to detect E6, Trx, p65, and GLUT1 expression levels in the lung cancer cell lines. *P < .05, **P < .01. Trx, thioredoxin; si, small interfering; NS: nonspecific siRNA.
Inhibition of E6 decreases the expression levels of Trx, p65, and GLUT1. To further verify the regulatory effects of E6 on Trx, p65, and GLUT1, A549 cells were transfected with E6-specific siRNA to inhibit E6 expression. The results demonstrated that GLUT1 protein and mRNA expression levels were decreased (P < .01), but only Trx (P < .01) and p65 (P < .05) protein expression levels were decreased (Figure 2B).
E7 plasmid transfection or siRNA transfection have no regulatory effect on Trx expression. Trx expression remained unchanged following the transient transfection of H1299 cells with pEGFP-N1-E7 vectors (P > .05). Similarly, Trx expression remained unchanged following transfected of A549 cells with E7-specific siRNA (P > .05) (Figure 2C and D).
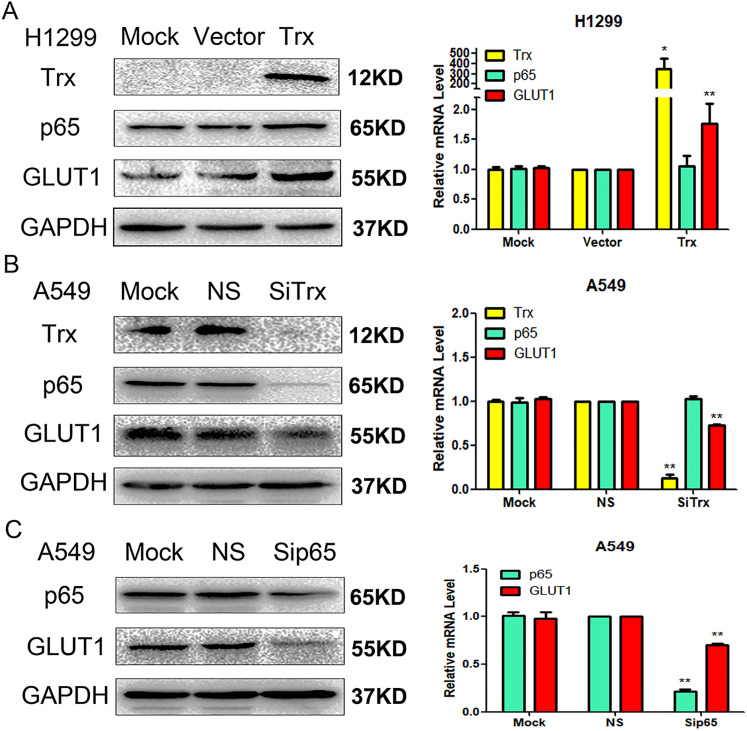
Overexpression of Trx increases the expression levels of p65 and GLUT1. Trx expression significantly increased following the transient transfection of H1299 cell with pCMV6-entry Trx vectors (P < .01). In addition, elevated Trx levels significantly increased GLUT1 protein and mRNA expression levels (P < .01) as well as p65 protein expression levels (P < .05) (Figure 3A).
Figure 3.
Effects of Trx on the regulation of p65 and GLUT1 expression via (A) transfection and (B) siRNA transfection. Effect of p65 on the regulation of GLUT1 expression via (C) siRNA transfection. Western blot and reverse transcription-quantitative PCR analyses were performed to detect Trx, p65, and GLUT1 expression levels in lung cancer cell lines. *P < .05, **P < 0.01. Trx, thioredoxin; si, small interfering; NS, nonspecific siRNA.
Inhibition of Trx decreases the expression levels of p65 and GLUT1. To further verify the regulatory effects of Trx on p65 and GLUT1, A549 cells were transfected with Trx-specific siRNA. The resulted demonstrated that GLUT1 protein and mRNA expression levels were decreased (P < .01), as well as p65 protein expression levels (P < .05) (Figure 3B).
Inhibition of p65 decreases GLUT1 expression. To further verify the regulatory effects of p65 on GLUT1, A549 cells were transfected with p65-specific siRNA. The resulted demonstrated that GLUT1 protein and mRNA expression levels were decreased (P < .01) (Figure 3C).
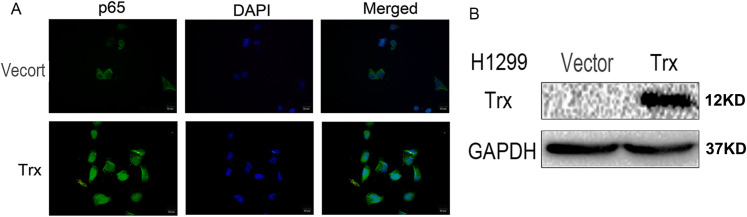
Overexpression of Trx promotes p65 nuclear translocation. To further verify the regulatory effects of Trx on p65, H1299 cells were transiently transfected with pCMV6-entry Trx, and the empty vectors served as controls. The results demonstrated that the overexpression of Trx significantly promoted p65 nuclear translocation (Figure 4).
Figure 4.
Regulatory effect of Trx on the nuclear translocation of p65. (A) Immunofluorescence demonstrated that the overexpression of Trx significantly promoted the nuclear translocation of p65 in lung cancer cells. Scale bar, 20 μm. (B) H1299 cells were transfected with Trx. Trx, thioredoxin.
Discussion
In recent studies, we found that the overexpression of E6 or E7 in HPV 16 upregulated the expression levels of both hypoxia inducible factor-1α and GLUT1 by inhibiting the activation of RRAD and NF-κB in lung cancer cells. 24 Hypoxia inducible factor-1α is a helix transcription factor that participates in carcinogenesis and tumor growth through the regulation of glycolytic metabolism and other biological mechanisms. 31 The mRNA expression levels of E6 or E7 are significantly higher in lung squamous cell carcinoma cells compared with pneumonia and tuberculosis cells. 32 We also have reported that HPV 16 E6/E7 promote the glucose uptake of GLUT1 in lung cancer through downregulation of TXNIP due to inhibition of PTEN phosphorylation 33 and that GLUT1 promotes the malignant phenotype of NSCLC via the integrin β1/Src/ focal adhesion kinase signaling pathway. 23 However, whether E6 or E7 upregulate GLUT1 expression through activation of NF-κB due to upregulate Trx expression remains unclear.
The results of the present study demonstrated that the overexpression of E6 upregulated GLUT1 expression through activation of NF-κB due to upregulated Trx expression. Notably, E7 had no regulatory effect on Trx expression. To the best of our knowledge, the present study was the first to demonstrate that Trx is involved in HPV16 E6 in lung cancer cells, but not E7, by upregulating GLUT1 expression through the activation of NF-κB. However, further studies are required to determine the specific regulatory effects of E6 on Trx. In addition, since GLUT1 overexpression is the main determinant of the Warburg effect, we will further investigate GLUT1 through the study of the genes encoding lactate dehydrogenase unit (LDHA or LDHB), and the genes encoding the main lactic acid exporters (SLC16A1 or SLC16A3) in order to solve the limitations of this study.
Recently, it has been reported that pyruvate kinase isozyme type M2 regulates the activity of p65 subunit in the NF-κB complex by promoting p65 to enter the nucleus.34,35 Another study reported that PKM2 promotes glucose metabolism through a let-7a-5p/Stat3/hnRNP-A1 regulatory feedback loop in breast cancer cells. 36 Trx can activate the NF-κB complex by regulating the subunit p65 of NF-κB in the respiratory epithelium and initiation of the inflammatory response. 22 The results of two-way regulation in the present study showed that the overexpression of Trx only significantly increased the level of p65 protein in lung cancer cells, this conclusion suggests that the oncoproteins of Trx regulate HIF-1α protein expression possibly through translational or posttranslational mechanisms. Immunofluorescence analysis indicated that the overexpression of Trx notably promoted p65 nuclear translocation in H1299 cells. Taken together, these results suggest that the activation of p65 is triggered by two factors, increasing p65 expression and promoting p65 nuclear translocation. In previous studies, we found that the expression level of p-p65 (serine 536) was closely related to the activity of p65. However, there are some other sites of phosphorylation, we do not have a comprehensive detection. Next, if we can obtain all the phosphorylated antibodies of p65, we will make a comprehensive evaluation of the expression levels of p-p65 to make up for the limitations of this study. Notably, the inhibition of p65 expression in A549 cells, downregulated GLUT1 protein and mRNA expression levels. These results were consistent with previous findings in lung cancer cells. 24
In conclusion, E6 and E7 are the two main oncoproteins in HPV16. E6 is closely associated with the inhibition of cell apoptosis, while E7 plays an important role in promoting cell proliferation.37,38 Previous studies have failed to exhibit differences in the regulatory effects of E6 and E7 proteins on the occurrence of lung cancer.15,24,39 The results of the present study demonstrated that HPV16 E6, but not E7, upregulated GLUT1 expression in lung cancer cells through the activation of NF-κB due to upregulated Trx expression. Thus, E6 regulates of GLUT1 expression in lung cancer cells via the HPV-Trx-p65-GLUT1 axis. Taken together, these results suggest that Trx plays an important role in the pathogenesis of HPV-associated lung cancer, and propose a novel therapeutic target for HPV-associated lung cancer.
Footnotes
Authors’ Note: Ethical approval was obtained for the experimental procedures by the Ethics Committee of the First Hospital of China Medical University, Shenyang, China. All procedures in this study were conducted in accordance with the First Hospital of China Medical University's (APPROVAL NUMBER/2016-125) approved protocols. This article does not contain any studies with patients and animals.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China to Guang-Ping Wu (grant number 81171650 and 81672082).
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Authors’ Contributions: Zi-Yu Gao, Hong-Tao Xu, Qing-Chang Li, and Guang-Ping Wu contributed to study design and conduct. Zi-Yu Gao, Na-Jin Gu, and Ming-Zhe Wu analyzed the data and provided statistical support. Shiyu Wang contributed to language editing in this article. All authors made substantial contributions to interpretation of results, were involved in drafting the manuscript and revising it critically for important intellectual content, approved the final version for submission and agree to be accountable for all aspects of the work.
ORCID iD: Guang-Ping Wu https:orcid.org/0000-0003-3478-6868
References
- 1.Guo Y, Meng X, Ma Jet al. et al. Human papillomavirus 16 E6 contributes HIF-1α induced Warburg effect by attenuating the VHL-HIF-1α interaction. Int J Mol Sci. 2014;15(5):7974‐7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu MZ, Li WN, Cha Net al. et al. Diagnostic utility of HPV16 E6 mRNA or E7 mRNA quantitative expression for cervical cells of patients with dysplasia and carcinoma. Cell Transplant. 2018;27(9):1401‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvard V, Baan R, Straif Ket al. WHO International agency for research on cancer monograph working group. A review of human carcinogens–part B: biological agents. Lancet Oncol. 2009;10(4):321‐322. [DOI] [PubMed] [Google Scholar]
- 4.Menon S, van den Broeck D, Rossi R, Ogbe E, Mabeya H. Multiple HPV infections in female sex workers in western Kenya: implications for prophylactic vaccines within this sub population. Infect Agent Cancer. 2017;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu MZ, Wang S, Zheng Met al. et al. The diagnostic utility of p16 immunostaining in differentiating cancer and HSIL from LSIL and benign in cervical cells. Cell Transplant. 2019;28(2):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva EM, Mariano VS, Pastrez PRA, et al. Human papillomavirus is not associated to non-small cell lung cancer; data from a prospective cross-sectional study. Infect Agent Cancer. 2019;14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombara DV, Manhart LE, Carter JJ, et al. Prior human polyomavirus and papillomavirus infection and incident lung cancer: a nested case-control study. Cancer Causes Control. 2015;26(12):1835‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Liu X, Yang Yet al. et al. Effect of FHIT loss and p53 mutation on HPV-infected lung carcinoma development. Oncol Lett. 2015;10(1):392‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamasa T, Miyagi J, Tsuhako K, et al. Prognostic implication of human papillomavirus infection in squamous cell carcinoma of the lung. Pathol Res Pract. 2000;196(4):209‐218. [DOI] [PubMed] [Google Scholar]
- 11.Gui S, Xie X, O’Neill WQet al. et al. P53 functional states are associated with distinct aldehyde dehydrogenase transcriptomic signatures. Sci Rep. 2020;10(1):1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajavel T, Banu Priya G, Suryanarayanan V, Singh SK, Pandima Devi K. Daucosterol disturbs redox homeostasis and elicits oxidative-stress mediated apoptosis in A549 cells via targeting thioredoxin reductase by a p53 dependent mechanism. Eur J Pharmacol. 2019;855:112‐123. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le AD. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Clin Cancer Res. 2007;13(9):2568‐2576. [DOI] [PubMed] [Google Scholar]
- 14.Zhang EY, Tang XD. Human papillomavirus type 16/18 oncoproteins: potential therapeutic targets in non-smoking associated lung cancer. Asian Pac J Cancer Prev. 2012;13(11):5363‐5369. [DOI] [PubMed] [Google Scholar]
- 15.Fan R, Hou WJ, Zhao YJet al. et al. Overexpression of HPV16 E6/E7 mediated HIF-1α upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 2016;37(4):4655‐4663. [DOI] [PubMed] [Google Scholar]
- 16.Guo NN, Sun XJ, Xie YK, Yang GW, Kang CJ. Cloning and functional characterization of thioredoxin gene from kuruma shrimp marsupenaeus japonicus. Fish Shellfish Immunol. 2019;86:429‐435. [DOI] [PubMed] [Google Scholar]
- 17.Fath MA, Ahmad IM, Smith CJ, Spence J, Spitz DR. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clin Cancer Res. 2011;17(19):6206‐6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Xu W, Sun R, Yin H, Dong C, Zeng H. Synergism between thioredoxin reductase inhibitor ethaselen and sodium selenite in inhibiting proliferation and inducing death of human non-small cell lung cancer cells. Chem Biol Interact. 2017;275:74‐85. [DOI] [PubMed] [Google Scholar]
- 19.Ceccarelli J, Delfino L, Zappia Eet al. The redox state of the lung cancer microenvironment depends on the levels of thioredoxin expressed by tumor cells and affects tumor progression and response to prooxidants. Int J Cancer. 2008;123(8):1770‐1778. [DOI] [PubMed] [Google Scholar]
- 20.Duan D, Wang Y, Pan Det al. Targeting thioredoxin reductase by deoxyelephantopin from Elephantopus scaber triggers cancer cell apoptosis. Arch Biochem Biophys. 2021 Oct 30;711:109028. doi: 10.1016/j.abb.2021. Epub 2021 Sep 10. [DOI] [PubMed] [Google Scholar]
- 21.Fan J, Yu H, Lv Y, Yin L. Diagnostic and prognostic value of serum thioredoxin and DJ-1 in non-small cell lung carcinoma patients. Tumour Biol. 2016;37(2):1949‐1958. [DOI] [PubMed] [Google Scholar]
- 22.Kelleher ZT, Sha Y, Foster MW, Foster WM, Forrester MT, Marshall HE. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor κB (NF-κB) activation. J Biol Chem. 2014;289(5):3066‐3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Sun J, Shao Jet al. et al. Glucose transporter 1 promotes the malignant phenotype of non-small cell lung cancer through integrin β1/Src/FAK signaling. J Cancer. 2019;10(20):4989‐4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu NJ, Wu MZ, He Let al. et al. HPV 16 E6/E7 up-regulate the expression of both HIF-1α and GLUT1 by inhibition of RRAD and activation of NF-κB in lung cancer cells. J Cancer. 2019;10(27):6903‐6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi K, Ueda Y, Sakurai Aet al. Correlation of glut-1 glucose transporter expression with [(18)F]FDG uptake in non-small cell lung cancer. Eur J Nucl Med. 2000;27(12):1778–1785. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Menendez P, Hevia D, Mayo JC, Sainz RM. The dark side of glucose transporters in prostate cancer: are they a new feature to characterize carcinomas? Int J Cancer. 2018;142(12):2414‐2424. [DOI] [PubMed] [Google Scholar]
- 27.Long D, Wu H, Tsang AWet al. The oxidative state of cysteine thiol 144 regulates the SIRT6 glucose homeostat. Sci Rep. 2017;7(1):11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzuto A, D’Ascanio M, Ricci A, Pagliuca A, Carico E. Expression and role of p16 and GLUT1 in malignant diseases and lung cancer: a review. Thorac Cancer. 2020;11(11):3060‐3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pezzuto A, Cappuzzo F, D’Arcangelo Met al. Prognostic value of p16 protein in patients With surgically treated non-small cell lung cancer; relationship with Ki-67 and PD-L1. Anticancer Res. 2020;40(2):983‐990. [DOI] [PubMed] [Google Scholar]
- 30.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309‐314. [DOI] [PubMed] [Google Scholar]
- 31.Pezzuto A, Carico E. Role of HIF-1 in cancer progression: novel insights. A review. Curr Mol Med. 2018;18(6):343‐351. [DOI] [PubMed] [Google Scholar]
- 32.Zhao HY, Yang JH, Wang X, Sun J, Wang EH, Wu GP. Analysis of human papillomavirus 16 E6/E7 and L1 in the bronchial brushing cells of patients with squamous cell carcinoma of the lungs. Int J Clin Exp Pathol. 2018;11(8):4124‐4129. [PMC free article] [PubMed] [Google Scholar]
- 33.Tang JY, Li DY, He L, Qiu XS, Wang EH, Wu GP. HPV 16 E6/E7 promote the glucose uptake of GLUT1 in lung cancer through downregulation of TXNIP due to inhibition of PTEN phosphorylation. Front Oncol. 2020;10:559543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azoitei N, Becher A, Steinestel Ket al. et al. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer. 2016;15:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng B, Geng L, Zeng L, Liu F, Huang Q. AKT2 Contributes to increase ovarian cancer cell migration and invasion through the AKT2-PKM2-STAT3/NF-κB axis. Cell Signal. 2018;45:122‐131. [DOI] [PubMed] [Google Scholar]
- 36.Yao A, Xiang Y, Si YRet al. PKM2 promotes glucose metabolism through A let-7a-5p/Stat3/hnRNP-A1 regulatory feedback loop in breast cancer cells. J Cell Biochem. 2019;120(4):6542‐6554. [DOI] [PubMed] [Google Scholar]
- 37.Niu XY, Peng ZL, Duan WQ, Wang H, Wang P. Inhibition of HPV 16 E6 oncogene expression by RNA interference in vitro and in vivo. Int J Gynecol Cancer. 2006;16(2):743‐751. [DOI] [PubMed] [Google Scholar]
- 38.Cui X, Wang X, Zhou X, Jia J, Chen H, Zhao W. miR-106a regulates cell proliferation and autophagy by targeting LKB1 in HPV-16-associated cervical cancer. Mol Cancer Res. 2020;18(8):1129‐1141. [DOI] [PubMed] [Google Scholar]
- 39.Shao JS, Sun J, Wang Set al. et al. HPV16 E6/E7 upregulates HIF-2α and VEGF by inhibiting LKB1 in lung cancer cells. Tumour Biol. 2017;39(7):1010428317717137. [DOI] [PubMed] [Google Scholar]