Abstract
Background:
Primary breast cancer (BC) has shown a higher immune infiltration than the metastatic disease, justifying the optimal scenario for immunotherapy. Recently, neoadjuvant chemotherapy (NAC) combined with immune checkpoint inhibitors has demonstrated a gain in pathological complete responses (tpCR) in patients with BC. The aim of our study is to evaluate the safety, feasibility, and efficacy of the addition of dendritic cell vaccines (DCV) to NAC in HER2-negative BC patients.
Methods:
Thirty-nine patients with early BC received DCV together with NAC conforming the vaccinated group (VG) and compared with 44 patients as the control group (CG). All patients received anthracyclines and taxanes-based NAC (ddECx4→Dx4) followed by surgery ± radiotherapy ± hormonotherapy.
Results:
The tpCR rate was 28.9% in the VG and 9.09% in the CG (p = 0.03). Pathological CR in the triple negative (TN) BC were 50.0% versus 30.7% (p = 0.25), 16.6% versus 0% in luminal B (p = 0.15), and none among luminal A patients in VG versus CG, respectively. Impact of DCV was significantly higher in the programmed cell death ligand 1 (PD-L1) negative population (p < 0.001). PD-L1 expression was increased in patients with residual disease in the VG as compared with the CG (p < 0.01). No grade ⩾3 vaccine-related adverse events occurred. With a median follow-up of 8 years, no changes were seen in event-free survival or overall survival. Phenotypic changes post DCV in peripheral blood were observed in myeloid-derived suppressor cells (MDSC), NK, and T cells. Increase in blood cell proliferation and interferon (IFN)-γ production was detected in 69% and 74% in the VG, respectively. Humoral response was also found. Clonality changes in TCR-β repertoire were detected in 67% of the patients with a drop in diversity index after treatment.
Conclusion:
The combination of DCV plus NAC is safe and increases tpCR, with a significant benefit among PD-L1-negative tumors. DCV modify tumor milieu and perform cellular and humoral responses in peripheral blood with no impact in outcome.
Trial registration:
ClinicalTrials.gov number: NCT01431196. EudraCT 2009-017402-36.
Keywords: dendritic cell vaccines, early breast cancer, immunotherapy, neoadjuvant
Introduction
Breast cancer (BC) is the most common oncologic disease in women. 1 Survival grew strikingly due to an improvement in early detection and new targeted systemic therapies, but lately has got stuck in the early high-risk scenario when chemotherapy is mandatory, highlighting the need for more individualized therapeutic strategies added to neoadjuvant chemotherapy (NAC). In fact, neoadjuvant trials are the most efficient way to get institutional fast approvals for new therapies taking the total pathological complete response (in breast and lymph nodes, tpCR) as the main endpoint; 2 or if residual disease (RD) persists, adding maintenance therapy to improve survival. 3
Immunotherapy (IMT) has emerged as one of the newest therapeutic strategies in cancer. Although BC is not a highly immunogenic tumor, an appropriate immune activation could improve disease outcome by strengthening the immune response especially in naïve BC patients.4,5 Chemotherapy and radiation facilitate tumor antigen release, dendritic cell vaccines (DCV) improve cancer antigen presentation and induce immune responses, anti-VEGF therapies increase tumor-infiltrating lymphocytes (TILs) infiltration, and check point inhibitors (CPI) potentiate cytotoxic role and block immunosuppressor milieu. 6 Programmed cell death ligand 1 (PD-L1) expression has a prognostic and predictive role in metastatic BC patients;7,8 however, CPI in the neoadjuvant scenario could increase tpCR regardless of PD-L1 expression with a better trend on event-free survival (EFS) with an unknown impact on overall survival (OS).9–11 Dendritic cells (DC) are suppressed by tumors through the release of cytokines such as IL-10, conditioning these DC to form suppressive T cells. Active therapy with DCV has shown tumor growth inhibition and T-cell memory activation in preclinical models 12 as well as clinical improvement in patients with BC without further toxicity. 13 The addition of DCV to NAC seems to be very suitable in early, non-immunosuppressed and naïve-of-therapy BC patients. Selection of a neoadjuvant scenario brings information about biological changes in the tumor and stroma added to the main tpCR endpoint. Our group has already demonstrated an additional ~20% tumor shrinkage in breast tumors measured dynamically with imaging techniques when DCV were added to standard NAC. 14
We present the results of our final study of NAC plus autologous DCV in HER2-negative early BC patients. Our main aims are to determine the clinical benefit in terms of tpCR, survival, and safety profile. Translational studies of PD-L1 expression in residual disease (RD) as well as the results of the systemic immune response in the VG are also presented.
Methods
Patients
Thirty-nine treatment-naïve patients with early BC and without overexpression/amplification of Her2/erbB2 were selected from February 2011 to September 2015 for NAC combined with DCV, 21 of them recruited in the multicenter phase II pilot clinical trial (EudraCT 2009-017402-36, NCT01431196), and 18 additional patients included under compassionate use. Thirty-five patients were recruited at Clínica Universidad de Navarra (CUN) and four patients at Complejo Hospitalario de Navarra.
Main inclusion criteria were patients 18 years and older, diagnosed with non-overexpressing operable Her2 BC who could benefit from NAC and with availability to get enough tumor sample and blood derived monocytes from leukapheresis to elaborate the vaccines. An Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate bone marrow status, kidney and liver functions were required.
Main exclusion criteria were pregnancy, severe diseases, diagnosis of HIV or hepatitis, and to be on immunosuppressant drugs.
Pathologic response evaluation system to assess tpCR as per routine clinical practice was Miller&Payne.
All patients provided an informed consent consistent with the International Conference on Harmonization of technical Requirements for Registration of Pharmaceuticals for Human Use–Good Clinical Practice and local legislation. The study was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Ethics Committee from Comité Ético de Investigación Clínica de la Comunidad Foral de Navarra 03/2010.
We compared our results with an historic cohort of 44 patients with same features treated at our institution with the same schedule but without DCVs, diagnosed from December 2007 to July 2015.
One patient from the clinical trial was excluded from the analysis of tpCR and survival due to withdrawal (no completion of NAC, vaccines, neither surgery nor radiation), but she was included in the safety and survival analysis.
Study design and treatment
Chemotherapy
All patients received sequential NAC consisting of four cycles of dose-dense epirubicin plus cyclophosphamide (ddEC) with G-CSF support followed by a second schedule of four cycles each 21 days of docetaxel according to standard protocols. Changes to the original protocol in terms of drugs or dose administration were allowed due to toxicity or specific patients’ requirements and were recorded.
Immunotherapy
In addition to the NAC treatment described above, 39 patients received vaccination with monocyte-derived autologous DC loaded with autologous tumor lysate. The vaccination plan included at least six vaccines being the first one administered between the last ddEC and the first taxane-based cycle. Vaccines were administered intradermally every 3 weeks in the first five doses. The sixth dose was administered the day after surgery. When radiation therapy was completed, four vaccines were administered every 2 months and finally, quarterly until the end of the vaccines. DCV were prepared according to the standard procedure under good manufacturing practices at the CUN’s Cell Therapy Unit.
Translational studies
Positive PD-L1 expression was defined as a value ⩾1% of tumoral cells (membrane staining) with the monoclonal rabbit 28.8 anti PD-L1 (DAKO, Agilent Technologies) in FFPE samples from diagnostic and surgical specimens.
Phenotypic characterization of peripheral blood mononuclear cells by flow cytometry was performed in samples from 18 patients. Different subpopulations of T lymphocytes (naive, effector memory, central memory, effector and regulatory T cells), their activation status (HLA-DR, CD69), and the level of expression of immune checkpoints (PD1, CTLA-4, TIM3, LAG3) were evaluated. In addition, other populations such as NK cells, B lymphocytes, and suppressor and non-suppressor myeloid cells were assessed. Data acquisition was performed in a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) using the FACSDiva 6.1 software (BD Biosciences). Data analysis was performed using the Infinicyt software (Cytognos SL, Salamanca, Spain).
Cellular immune response was evaluated by T-cell proliferation assay and IFN-γ producing cells by ELISPOT (IFN-γ enzyme-linked immunospot). The proliferation of T cells after stimulation with tumor lysate-pulsed DC was evaluated by a radioactive assay based on incorporation of [ 3 H]thymidine in 16 patients. PBMCs obtained before and after DCV resuspended in complete culture medium (RPMI 1640 with 10% heat-inactivated human AB serum, 2 mM glutamine, 100 UI/ml penicillin and 100 µg/ml streptomycin) were plated in 96-well plates at 2 × 105 per well alone or with 2 × 104 tumor lysate-pulsed DC. After incubation at 37°C and 5% CO2 for 5 days, cells were pulsed with 0.5 μCi/well of [ 3 H]thymidine for 18 h and harvested. [ 3 H]Thymidine incorporation was determined in a scintillation counter (Topcount; Packard, Meridan, CT, USA).
The amount of IFN-γ producing cells was measured by IFN-γ ELISPOTs (Mabtech; San Diego, CA, USA) according to manufacturer’s instructions in 15 patients. 2 × 105 PBMCs obtained before and after DCV and resuspended in complete medium were plated in 96-well plates coated with anti-IFN- γ antibody and blocked with RPMI 1640 supplemented with 10% SAB for 30 min at room temperature and 2 × 104 tumor lysate pulsed DC were added. PBMCs alone or with phytohemagglutinin were used as negative or positive control, respectively. Cells were incubated at 37°C and 5% CO2 during 48 h and then cells were removed by six washings with PBS-Tween (0.05%). ELISPOT was carried out according to manufacturer’s instructions. Spots quantification was performed using an automated ELISPOT reader (CTL, Aalen, Germany).
According to cytokine profile, simultaneous determination of GM-CSF, IFN-y, TNF-a, IL-1β, lL-2, lL-4, lL-5, IL-6, IL-7, lL-8, IL-10, IL-12p70, and IL-13 was performed in serum samples of 20 patients obtained before and after vaccination by Multiplex Bead Immunoassay Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions and using Luminex® xMAP® system (Luminex, Austin, Texas).
To assess humoral response, the presence of tumor-specific antibodies in pre- and post-vaccination samples was evaluated by flow cytometry in 20 patients. Three commercial lines of breast cancer (CRL-2314, CRL-2335, CRL-2336; ATCC; Manassas, VA, USA) were selected as target. The cell lines were incubated with serum samples and then were labeled with two fluorochromes-conjugated monoclonal antibodies [IgG-FITC (DAKO) and IgM-APC (BD Biosciences, San Jose, CA)], using standard protocols, to evaluate the presence of antibodies IgG or IgM which target tumor cell lines antigens. Data acquisition was performed in a Navios flow cytometer (Beckman Coulter; Brea, CA, USA) using the Cytometry list mode data acquisition and analysis software (Beckman Coulter; Brea, CA, USA). Data analysis was performed using the Infinicyt software (Cytognos SL, Salamanca, Spain). To evaluate the humoral response, we calculated a ratio between the median fluorescence intensity (MFI) of the post-treatment and the pretreatment serum and established a positive result when the ratio was greater than 1.2 for IgG and greater than 1.5 for IgM.
TCR repertoire study was performed by flow cytometry using the Human IOTest Beta Mark TCR Vβ Repertoire Kit (Beckman Coulter; Brea, CA, USA) according to the manufacturer’s instructions. Data acquisition was performed in a Navios flow cytometer (Beckman Coulter; Brea, CA, USA) using the Cytometry list mode data acquisition and analysis software (Beckman Coulter). Data analysis was performed using the FlowJo software (BD Biosciences, San Jose, CA). The study of the TCR repertoire was carried out in 12 patients in whom an immune response had been detected.
Endpoints
The primary endpoint was tpCR in the breast and axilla (ypT0/Tis ypN0) defined as the absence of any invasive component in the resected breast and lymph nodes 15 in all patients that completed NAC ± DCV + surgery. Secondary endpoints included safety of the combination schedule as well as the DCV, EFS (time from diagnosis to disease recurrence or death), and OS (time from diagnosis to death from any cause). Research endpoints explore tumor PD-L1 expression and immune response in peripheral blood induced by DCV in paired samples. Safety and survival were evaluated in all patients who received at least one DCV, underwent surgery, or both. Results of systemic immunity were shown according to pathologic response. Very good clinical responders (VGCR) were those who achieved Miller&Payne 4–5 in the tumor with N0 after NAC.
Statistical methods
A descriptive statistical analysis was performed on the study variables including calculation of measures of central tendency and dispersion for quantitative variables, and frequencies and valid percentages for qualitative variables. Categorical variables were compared using the chi-square or Fisher’s exact test. Unpaired Student’s t-tests or Mann–Whitney U tests were performed to analyze unpaired data. Paired Student’s t-tests or Wilcoxon tests were performed to analyze paired data. Survival curves were compared with the Wilcoxon test. The software used for statistical analysis was R version3.5.3 (R foundation for statistical Computing, Vienna, Austria) and STATA/SE16.1 (StataCorp, College Station, TX, USA).
Results
Between February 2011 and February 2015, 39 patients were included in the program of DCV in combination with NAC. Baseline patient demographics and clinical characteristics are listed in Table 1. Median age of all patients was 49 years (range, 36–84). At baseline, 56.6% of patients had stage II disease, 71% nodal involvement; 36% were triple negative (TN) subtype. Cohorts were well balanced for most of the variables. Prevalence of post-menopausal women was higher in CG than in VG (56.8% versus 28.2%) although no differences were seen in the age at diagnosis. Breast-conserving surgery was more prevalent in the VG (58.9% versus 40.9%). The mean of vaccines received per patient was 12 (range, 6–26). Other features regarding manufacturing are not shown.
Table 1.
Demographic and clinical characteristics of the patients at baseline and therapeutic intervention.
| Experimental VG (N = 39) | CG (N = 44) | p-value | ||
|---|---|---|---|---|
| Median age (years, range) | 45.68 (36.15–74.48) | 55.31 (26–84.35) | 0.91 | |
| Menopausal status | Premenopause | 26 (66.66) | 17 (38.64) | 0.02 |
| Perimenopause | 2 (5.12) | 2 (4.55) | ||
| Menopause | 11 (28.20) | 25 (56.82) | ||
| ECOG performance status | 0 | 8 (20.51) | 15 (34.09) | 0.08 |
| 1 | 31 (79.49) | 29 (65.91) | ||
| Germline BRCA 1/2 | Mutated | 2 (5.12) | – | 0.38 |
| Wild-type | 8 (20.51) | 6 (16.63) | ||
| Unknown | 29 (74.35) | 38 (86.36) | ||
| Subtype | Luminal A | 10 (25.64) | 13 (29.54) | 0.40 |
| Luminal B | 12 (30.77) | 18 (40.91) | ||
| Triple negative | 17 (43.59) | 13 (29.54) | ||
| Stage | I | – | 2 (4.55) | 0.41 |
| II | 21 (53.85) | 26 (59.09) | ||
| III | 10 (25.64) | 14 (31.82) | ||
| IV | 4 (10.26) | 2 (4.55) | ||
| Longest diameter at diagnosis | ⩾30 mm | 30 (76.92) | 26 (59.09) | 0.31 |
| <30 mm | 9 (23.04) | 16 (36.36) | ||
| Unknown | – | 2 (4.55) | ||
| Tumor size | T1 | 2 (5.12) | 5 (11.36) | 0.40 |
| T2 | 27 (69.23) | 22 (50.00) | ||
| T3 | 7 (17.95) | 11 (25.00) | ||
| T4 | 1 (2.56) | 4 (6.82) | ||
| TX | – | 2 (4.55) | ||
| Lymph node status | N0 | 10 (25.64) | 14 (31.82) | 0.86 |
| N+ | 29 (74.35) | 30 (68.18) | ||
| % Ki 67 (median, range) | 33.68 (1–100) | 37.45 (1–100) | 0.31 | |
| Histological differentiation | Low (G1 + G2) | 21 (53.84) | 24 (54.54) | 0.27 |
| High (G3) | 18 (46.15) | 20 (45.45) | ||
| % TILs (median, range) | 1.01 (0.19–13.17) | 1.30 (0.03–13.29) | 0.88 | |
| % PD-L1 positive patients | 33.4 | 50.0 | 0.06 | |
| Treatment Schedule | EC → D | 36 (92.31) | 40 (90.90) | 0.93 |
| CBDCA added to D | 3 (7.69) | 4 (9.09) | ||
| Total dose | E (mean, range) | 382.46 (352–409) | 380.56 (298–406) | 0.99 |
| D (mean, range) | 333.54 (274–400) | 340.78 (282–398) | 0.98 | |
| Radiotherapy | Yes | 36 (92.31) | 42 (95.45) | 0.68 |
| No | 3 (7.69) | 2 (4.55) | ||
| Breast surgery | Mastectomy | 15 (38.46) | 26 (59.10) | 0.09 |
| Conservative | 23 (58.97) | 18 (40.91) | ||
| Non-operated | 1 (2.56) | – | ||
| Lymph node surgery | Sentinel node | 11 (28.21) | 8 (18.18) | 0.75 |
| Lymphadenectomy | 27 (69.23) | 36 (81.81) | ||
| Non-operated | 1 (2.56) | – | ||
C: cyclophosphamide; CG, control group; D, docetaxel; E, epirubicin; ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1; TILs, tumor-infiltrating lymphocytes; VG, vaccinated group.
ECOG performance status. PD-L1 positivity was defined in tumoral cells as ⩾1%. Stromal TILs were quantified by digital imaging.
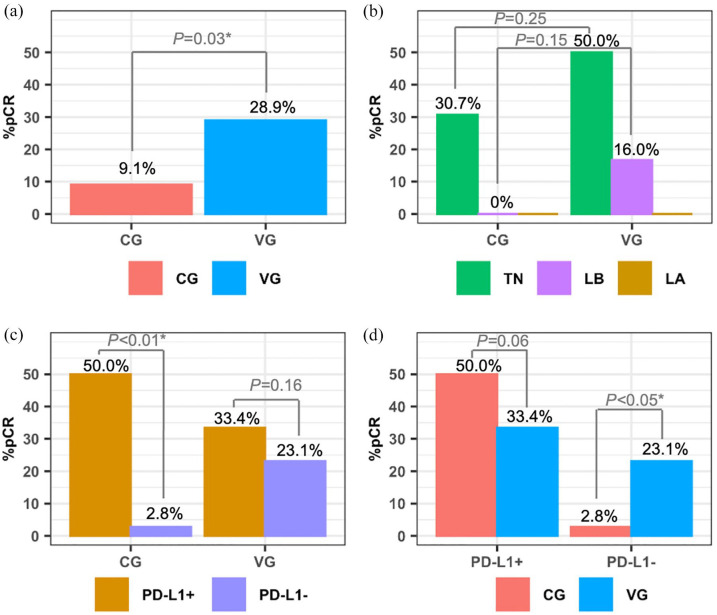
All responses were confirmed by pathological study of the surgical specimen. The experimental therapy helped to downstage from mastectomy to conservative surgery in 13.6% of the patients, as compared to none in the CG (p < 0.001). After NAC, the tpCR rate was superior among vaccinated patients (28.9% versus 9.1%, p = 0.03, absolute increment of 19%) (Figure 1(a)). According to subtype, triple negative (TN) BC patients experienced the highest tpCR (50% for VG versus 30.7% for CG, p = 0.25, absolute benefit of 19%), with modest responses for luminal B types (16.6% for vaccinated versus none in CG, p = 0.15). No tpCR were seen in luminal A tumors from any group (Figure 1(b)).
Figure 1.
Total pathologic complete responses (stage ypT0/Tis ypN0) in both groups (a), regarding biologic subtypes (b), and according to therapeutic group and PD-L1 expression (c, d).
Assessment of PD-L1 was performed in 40 samples in the CG and 35 samples in VG at diagnosis. No differences in tpCR were seen regardless of PD-L1 expression in the VG (33.4% versus 23.1% in both PD-L1+ versus negative expression, p = 0.16). However, differences were found in the CG in those patients who achieved tpCR (50% versus 2.8%, p < 0.01) with a remarkable benefit in PD-L1+ population (Figure 1(c)). Among the PD-L1+ population, tpCR were 33.4% and 50% in the VG versus the CG (p = 0.06); and 23.1% versus 2.8% (p < 0.05) in the PD-L1 negative population, respectively (Figure 1(d)). Regarding paired samples at diagnosis and in RD, 33 patients have these results available in the CG and 27 patients in the VG. An increase in PD-L1 expression was shown in two patients (6.06%) in the CG (TN and LA subtypes, respectively; p = 0.37). Eight patients (29.62%) increased PD-L1 expression in RD as compared to diagnostic sample after NAC plus DCV (4 TN and 4 LB subtypes, respectively, p < 0.01), with significant differences among CG and VG (p < 0.01).
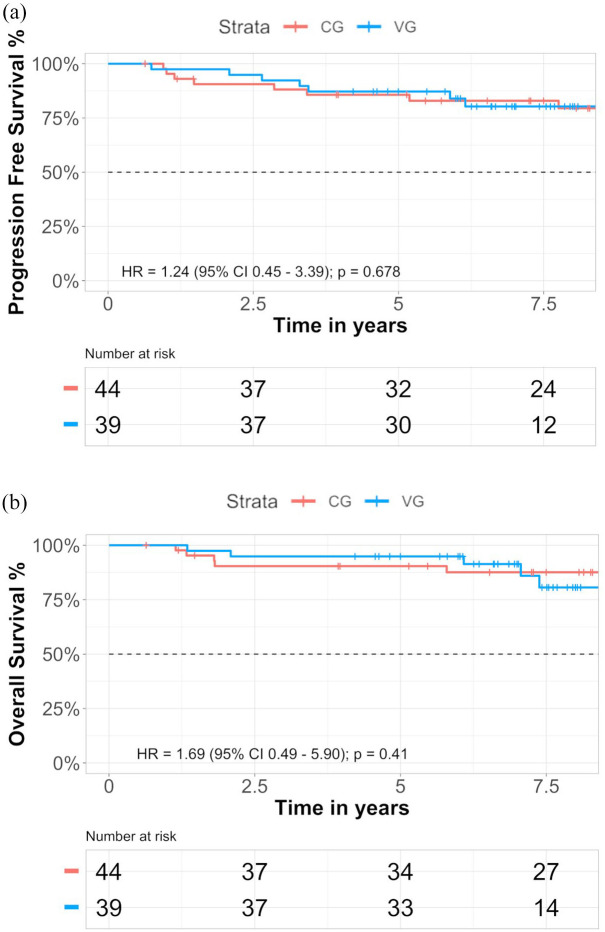
At the time of the present analysis, median follow-up was 6.96 years in the VG and 9 years in the CG. No significant differences were observed for event EFS (Figure 2(a)) or for OS (Figure 2(b)). The percentage of patients at 5-year who were alive with disease progression was 12.82% in the VG versus 14.35% in the CG, whereas after 7 years 17.08% in the CG and 19.70% in the VG (Supplemental Table S1a). At 5- and 7-year follow-up, patients alive in the CG were 90.41% and 87.58%, respectively, and 94.87% and 91.36% in the VG, respectively (Supplemental Table S1b). No significant differences were found stratifying EFS or OS by PD-L1 expression in both groups (data not shown).
Figure 2.
Kaplan–Meier estimates of EFS (a) and OS (b) according to groups in the intention-to-treat population are shown respectively.
Tick marks indicate data censored at the last time the patient was known to be alive and without event. The hazard ratio and confidence interval were analyzed with the use of a Cox regression model.
Clinically significant related adverse events (AEs) are listed in Table 2. Overall, total number of AEs was higher among VG versus CG, particularly mucositis, nausea/vomiting, and myalgia (p < 0.05). However, treatment-related grade ⩾3 toxicities were similar among both groups and only the rate of grade 3 nausea/vomiting was significantly higher in the CG versus the VG (11.3% versus 0%, p < 0.01). The most common grade 3 AEs were lymphopenia and asthenia. In the VG, one patient developed grade 3 hepatic toxicity (4.7%) related to docetaxel. The toxicity related to DCV was mild in all the 39 patients: only one patient developed fever ⩾38°C the day of intradermal injection; two patients shown erythema and one more patient showed tenderness at the site of the injection (Supplemental Table S2).
Table 2.
Main and more severe adverse events during the neoadjuvant phase are shown.
| Toxicity | VG (N = 39) | CG (N = 44) | p-value |
|---|---|---|---|
| All grades most common adverse events | |||
| Mucositis | 39 (100) | 28 (63.64) | 0.004 |
| Asthenia | 31 (79.49) | 25 (56.82) | 0.052 |
| Nausea and vomiting | 30 (79.92) | 22 (50.00) | 0.009 |
| Lymphopenia | 14 (35.90) | 17 (38.64) | 0.751 |
| Anemia | 14 (35.90) | 13 (29.54) | 0.431 |
| Diarrhea | 10 (25.64) | 6 (13.64) | 0.055 |
| Leucopenia | 6 (15.38) | 6 (13.64) | 0.747 |
| Neutropenia | 5 (12.82) | 8 (18.18) | 0.336 |
| Fever | 5 (12.82) | 7 (15.91) | 0.564 |
| Infection | 4 (10.25) | 6 (13.64) | 0.488 |
| Myalgia | 4 (10.25) | 1 (2.27) | 0.02 |
| Grade 3 or higher adverse events | |||
| Lymphopenia | 8 (20.51) | 9 (20.45) | 0.992 |
| Asthenia | 6 (15.38) | 6 (13.64) | 0.747 |
| Neutropenia | 4 (10.25) | 2 (4.55) | 0.138 |
| Leucopenia | 2 (5.12) | 2 (4.55) | 0.908 |
| Nausea and vomiting | – | 5 (11.36) | <0.001 |
| Mucositis | 2 (5.12) | 1 (2.27) | 0.294 |
| Myalgia | 1 (2.56) | 1 (2.27) | 0.895 |
| Anemia | – | 1 (2.27) | 0.132 |
| Hypertransaminasemia | 1 (2.56) | – | 0.109 |
CG, control group; VG, vaccinated group.
Immune response monitoring in peripheral blood
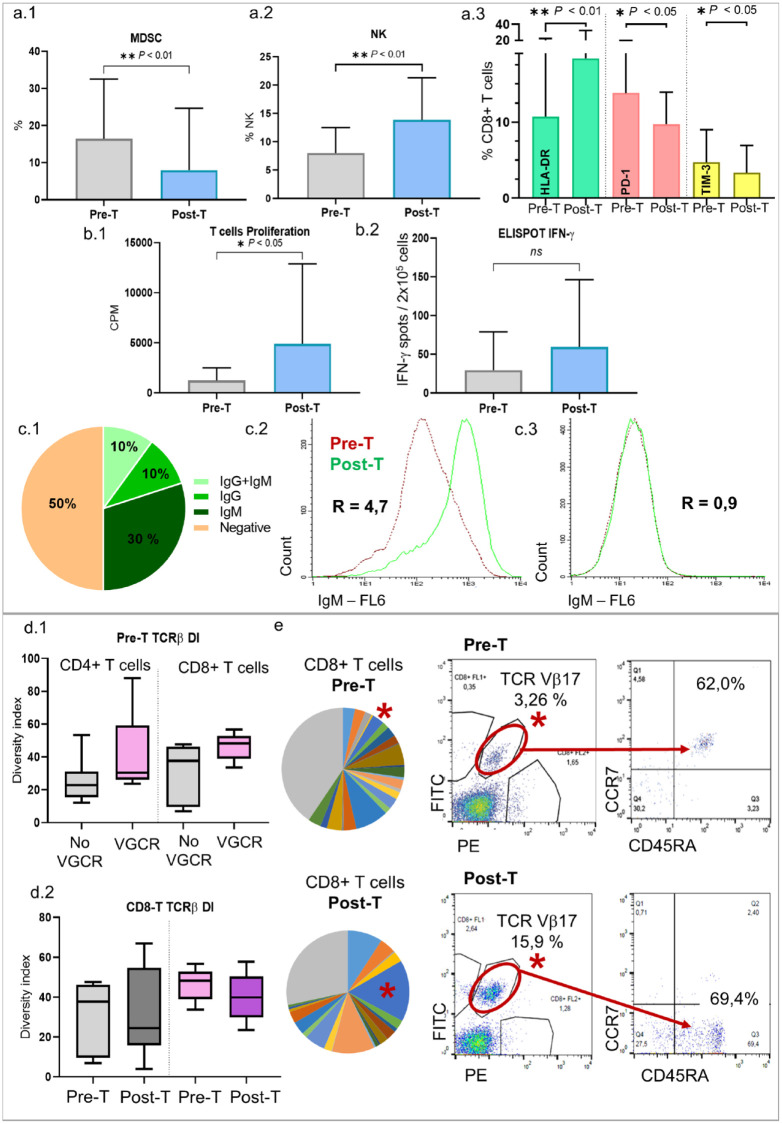
Phenotypic changes in peripheral blood have been analyzed in 18 patients in the VG. Our results shown a significant decrease of myeloid-derived suppressor cells (MDSC) (16.5% versus 7.9%, p < 0.01) and an increase in the NK cells (8% versus 14%, p < 0.01) after treatment. Moreover, we found an increase of the activation marker HLA-DR in CD4 (3.8% versus 8.3%, p = 0.002) and in CD8 T cells (10.7% versus 18.4%, p < 0.01) and a significant decrease in the expression of PD-1 (13.8% versus 9.7%, p < 0.05) and TIM-3 (4.7% versus 3.3%, p < 0.05) in CD8 T cells after DCV (Figure 3(a)).
Figure 3.
Phenotype of PBMC (a): MDSC decreased (a.1) and NK cells increased (a.2) with the treatment. There was an increase in HLA-DR and a decrease in PD1 and TIM3 expression (CD8+ T cells) (a.3). Functional studies (b): An increase in the proliferation of specific T cells (b.1) and in the number of IFN-g producing cells (b.2) after stimulation with tumor lysate pulsed DC was detected after treatment. Humoral response (c): Proportion of patients with IgG and IgM anti-breast cancer cell lines antibodies, and negative patients (c.1). Example of a patient with IgM anti-breast cancer cell lines antibodies (c.2) and a negative patient (c.3). TCR Vb clonality (d): VGCR patients had higher TCR diversity index (DI) in CD4+ and CD8+ T cells in pretreatment samples (d.1). In CD8+ T cells, DI decreased in both groups after treatment, with higher difference with the VGCR (d.2). A representative example of a patient (e): TCR Vb Pre-T and Post-T repertoire is shown (each color is a TCR Vb clone; in gray TCR not cover by the kit). TCR Vb17 clone (*) is shown clearly expanded after treatment; moreover, clone phenotype changed from naïve (CCR7+ CD45RA+) to effector/TEMRA (CCR7-CD45RA+). VGCR: Very good clinical responders; No VGCR: Rest of the patients; MDSC: Myeloid-derived suppressor cells.
Regarding functional assays, an increase in PBMC proliferation and IFN-γ production with specific tumor lysate was detected in DCV-treated samples compared to basal ones in 69% (p = 0.03) and 74% (p = 0.15) of the patients, respectively (Figure 3(b)). However, no correlation was found between the clinical response and the increase in proliferation or in IFN-γ production (data not shown). Changes in cytokines profiles were evaluated in serum samples of 20 patients after DCV highlighting a decrease in IL-6 levels (1.9 versus 1.4 pg/ml, p < 0.05).
Concerning the humoral response, we identified IgG antibodies specific for BC cell lines in the serum of 20% of the patients and IgM in 40% of the patients (Figure 3(c)). No correlation between humoral and clinical responses was found (data not shown).
A T-cell receptor (TCR)-β repertoire’s study was performed in 12 patients with outstanding cellular immune responses. The clonality was quantified using the inverse of Simpson’s diversity index (DI) 16 and changes were observed in 67% of the patients. Very good clinical responders had higher TCR-DI in CD4 (40.3 versus 26) and CD8 (46.4 versus 32.6) T cells in basal samples. Moreover, in CD8 T cells, the DI decreased in both groups after treatment, with a higher difference in the VGCR group (46.4 to 40.0, 13.7% decrease, ns) than in the remaining patients (32.6 to 30.8, 5.5% decrease, ns). In CD4 T cells, there was a decrease in the TCR-DI in the VGCR group after treatment (40.3 to 31.9, 20.8% decrease) while there was an increase in the rest of the patients (26.0 to 28.4, 9.2% increase) (Figure 3(d)). A representative example of a clone that clearly increases after vaccination is shown in Figure 3(e).
The expression of basal LAG3 in CD4 and CD8 T cells was lower in VGCR as compared to the remaining patients (1.9% versus 3.2% in CD4, p < 0.05; 2.5% versus 7.2% in CD8, p < 0.05). Moreover, there was a positive correlation between the IL-1 and IL-7 basal levels and treatment response (p < 0.05) (data not shown).
Discussion
To our knowledge, this is the first clinical trial combining active cell therapy based on DCV with standard NAC in early HER2-negative BC patients that incorporates long-term survival results and translational studies both in the tumor, its milieu and in the peripheral blood. There is enough evidence to demonstrate that early BC is more immunogenic than advanced disease due to higher TILs, PD-L1, and immune gene expression.4,17,18 In fact, FDA has just approved pembrolizumab for early-stage TNBC together with NAC on July 2021 based on pCR and EFS benefits.
Total pCR is a predictive endpoint in the neoadjuvant setting that confirms in vivo the sensitivity of BC to the administered therapy and could translate into absence of maintenance chemotherapy and survival improvement. In our study, DCV have shown to increase tpCR when combined with NAC in the whole cohort, with a not significant trend in TN and LB subtypes due to sample size limitations. The addition of CPI to NAC in TNBC shows up to 44–65% of tpCR9,10,19–21 regardless of PD-L1 expression in most of the studies. Anthracyclines were not used as NAC in the NeoTRIP study, that was unable to show benefit in tpCR when atezolizumab was added to nab-paclitaxel plus carboplatin. 19 The addition of durvalumab to NAC increased tpCR only in the windows cohort. 20 The highest tpCR seen in the KEYNOTE-522 trial may be due to the CT schedule enriched with anthracyclines and platinum salts. Platinum salts contribute to a 15% increase in tpCR in TNBC, with unknown results in long-term outcomes; but this gain is also provided when IMT is combined with NAC with lower toxicity. Therefore, the need for a de-escalation of platinum salts in non-BRCA germinal mutation carriers due to the introduction of IMT together with NAC needs a deeper analysis, but selection of anthracyclines remains mandatory based on their role in DC activation, cross-priming enhancement, depletion of MDSC, immunogenic cell death and antitumor CD4 T immunity induction and tumor cell recognition and killing.6,22–24 In addition, when anthracyclines were missed in this scenario, poorer results were found. 19
Luminal BC were only included as in our study in the I-SPY-2 trial, justifying the lower tpCR in the whole cohort (17%), and in the luminal tumors (13%) in the chemotherapy-alone arm, but showing an absolute increment in tpCR of 17% when pembrolizumab is added to NAC. 9 These results fit with our 16.6% gain in this biologic subtype when DCV are added to NAC. In that study, however, information regarding PD-L1 expression is not available.
The absolute increment in tpCR of ~19% obtained with the addition of DCV to TNBC patients is also confirmed in other studies, ranging between 9% and 38%.9–11,20 However, in the most recent interim analysis from the KEYNOTE-522, the absolute pCR improvement with pembrolizumab, although significant, decreased from 13.6% to 7.5% (unpublished data, www.fda/gov/media/145654). This benefit observed in TNBC patients with the addition of IMT could be explained by the high immunogenic profile by increased TILs, 25 high PD-L1 expression, 26 and increased nonsynonymous mutations. 27 The modest responses in luminal tumors with the combination therapy could be explained by lower rates of TILs and PD-L1/PD-1 expression,18,26,28,29 defects in antigen presentation due to lack of HLA surface molecules, and lower oncogenic mutations that impact on a lower CD8 infiltration in this subtype. 30 Nevertheless, responses in LB subtype with DCV could be explained by an improved antigen presentation together with a suitable NAC schedule. Total cumulative doses of each of the drugs are well balanced among cohorts, and so is the effect of ddEC on tpCR. Cyclophosphamide can deplete T regulatory cells and could restore effector functions of T and NK cells. 31 Higher tpCR are directly related to TILs and PD-L1 expression in patients with NAC ± IMT. Regarding our data, the additional benefit of DCV seems to be exclusive for patients with PD-L1 negative tumors at diagnosis, as compared to patients treated with CPI in which higher tpCR are achieved in the PD-L1-positive population. This could be due to (1) differences in the measurement of PD-L1 expression based on the combined positive score in both tumor and immune cells, or just in tumor expression; (2) the translation of PD-L1 expression into a more immunosuppressive niche that reflects an adaptive mechanism of resistance due to TILs infiltration; (3) PD-1/PD-L1 axis could be druggable with CPI but not with DCV that modulate the immune system in a different way; and (4) DCV could alter the tumor microenvironment (submitted) and increase sensitivity to PD-1/PD-L1 blockade.
The need for a strategy that modifies the tumor milieu with a gain in markers that predicts a higher tpCR is mandatory. In fact, PD-L1 expression in the tumor after DCV increased in up to 30% of the patients in RD. An absolute 19% improvement in the VG as compared to the CG was also found in the PD-L1-negative population. Thus, PD-L1 staining is not standardized and depends on the pathologist accuracy, the immunostaining used, as well as the method of quantification. It is therefore crucial to look for new biomarkers, standardized quantification, and improved knowledge about tumor, niche, and systemic immune fitness in order to define the best strategy for our BC patients. In this way, positive PD-L1 TNBC patients could benefit from upfront NAC plus CPI, whereas PD-L1 negative TNBC patients could start with NAC plus DCV and continue CPI as maintenance. Moreover, new phase III clinical trials need to be performed.
To date, the studies with NAC plus CPI have not shown mature outcome results.11,19 However, KEYNOTE-522 shows a benefit in EFS with an HR = 0.65 (p = 0.002) and a median follow-up of 26 months in the last interim analysis evaluated by the Oncologic Drugs Advisory Committee from the FDA (unpublished data, www.fda/gov/media/145654). Regarding our results, the immune boost produced with the DCV in the first 5 years could be lost later. Although we have seen no impact on EFS nor OS, data analyzed at different points could suggest a mild positive trend in OS in the experimental arm. Second-line chemotherapies after immunotherapy in different solid tumors could work better if the immune system has been activated previously, as described.32–35
Toxicity related to vaccines was found to be seldom and mild. CPI have higher immune-mediated toxicity than the active DCV based on infusion-related reactions, skin rash, endocrinopathies and hepatitis.9–11,19,20
DCV added to NAC induce an activation of the immune system and a decrease in some immune brakes. These results confirm previously published data and justify the combination of both strategies to achieve synergistic effects.
Cellular and humoral immune responses were found in patients after DCV. Unfortunately, a correlation with the clinical response could not be identified. Other studies with DCV in our group 36 and in other groups have confirmed immune activation against tumor, without correlation between survival and immune response.37–41 This can be explained by different factors besides the small sample size: the immune response is not powerful enough to produce a clinical response, the response was ineffective due to the immunosuppressive tumor environment, the sampling time is not the most appropriate to measure dynamic changes in host immunity, it might be more appropriate to measure the immune response in the tumor and not in the peripheral blood or even the tests used are not optimal to detect the possible changes produced. However, some immunological markers in the baseline samples were correlated with the best pathological responses. Patients with lower baseline LAG-3 levels had a better clinical response. Since LAG-3 is a negative regulator of T-cell activation that restricts the ability of these cells to generate an effective immune response against tumors, 42 the immune system could be less inhibited in patients with lower LAG-3 expression and could therefore be able to activate efficiently after treatment.
Moreover, we found a positive correlation between basal levels of IL-1 and IL-7 and treatment response. Both cytokines play a key role in modulating both the innate and adaptive immune response with antitumoral effects.43,44 Our study also showed that the treatment induced a decrease in the proinflammatory IL-6 levels, which are associated with aggressive tumor growth, poor prognosis, worst response to treatment and shorter survival.44,45 These facts together could help to get a greater therapeutic efficacy.
Regarding the study of humoral response, we used BC cell lines with a similar histology to patients’ tumors since there were no autologous tumor cells and we performed an assay adapted from flow cytometry crossmatch which is routinely used to detect preformed anti-human leucocyte antigen (HLA) antibodies in clinical transplantation. 11 Thus, we were able to detect shared antibodies against tumor antigens (they were present both in tumor samples and in cell lines). Since all patients were discarded for autoimmunity and no allosensitization or infection was reported, there is no reason for an increase in immunoglobulins from causes other than the vaccine-induced response. Therefore, we believe that this technique could serve as an approximation that may be useful in the absence of autologous tumor material.
Finally, we studied DI of TCR-β repertoire which is higher when T-cell clones are more distributed and lower when some clones are expanded. DI in pretreatment samples in VGCR was higher, concluding that immune system in patients with a higher baseline T-cell repertoire is better suited to respond to the tumoral antigens contained in the vaccine. Also, the decrease in the DI in CD4 and CD8 T cells in VGCR after treatment was greater compared to other patients, suggesting that there was a change in T-cell clonality due to an expansion of some tumor-specific T-cell clones. These findings might be concordant to Park et al.’s 16 and Tumeh et al.’s 46 work. The in-depth study of expanded clones by TCR sequencing will allow us to know the antigens against which the immune response is directed to.
The main limitations of our study encompass the small number of patients and the absence of randomization; therefore, these results must be evaluated with caution.
To conclude, the addition of DCV to NAC increases tpCR with a tolerable profile and without impact on outcome in early BC patients. A remarkable benefit of DCV has been seen in PD-L1-negative tumors. DCV could increase PD-L1 expression in tumor cells and induce immune changes in peripheral blood. Further phase III clinical trials in selected patients combining NAC together with CPI and DCV should be performed.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211064653 for Final results regarding the addition of dendritic cell vaccines to neoadjuvant chemotherapy in early HER2-negative breast cancer patients: clinical and translational analysis by Marta Santisteban, Belén Pérez Solans, Laura Hato, Amaia Urrizola, Luis Daniel Mejías, Esteban Salgado, Rodrigo Sánchez-Bayona, Estefanía Toledo, Natalia Rodríguez-Spiteri, Begoña Olartecoechea, Miguel Angel Idoate, Ascensión López-Díaz de Cerio and Susana Inogés in Therapeutic Advances in Medical Oncology
Acknowledgments
We acknowledge O.A. Fernández-Hidalgo, J. Espinós, S. de la Cruz, I. Goicoechea, S. Arrubla, and E. Navarcorena for their support and all the patients involved in the study.
Footnotes
Author Contributions: Conception and design: M Santisteban.
Development of methodology: BP Solans, L Hato, LD Mejías, MA Idoate, E Salgado, A López-Díaz de Cerio, S Inogés. M Santisteban.
Inclusion and care of patients: E Salgado, R Sánchez-Bayona, N Rodríguez-Spiteri, B Olartecoechea, M Santisteban.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computationalanalysis): BP Solans, L Hato, A Urrizola, E Salgado, R Sánchez-Bayona, E Toledo, LD Mejías, MA Idoate, A López-Díaz de Cerio, S Inogés, M Santisteban.
Writing, review, and/or revisión of the manuscript: BP Solans, L Hato, A Urrizola, R Sánchez- Bayona, E Toledo, B Olartecoechea, N Rodríguez-Spiteri, LD Mejías, A López-Díaz de Cerio, M Santisteban.
Administrative, technical, or material support (i.e. reporting or organizing data, constructing databases: BP Solans, L Hato, A Urrizola, M Santisteban.
Study supervision: A López-Díaz de Cerio, S Inogés, M Santisteban.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.S. has received honoraria from Roche, Pfizer, and Novartis and travel support from Roche, Pfizer, and Miltenyi. A.L.-D.d.C. and S.I. have travel support from Miltenyi. No other disclosures were reported. All remaining authors have declared no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Ministerio de Sanidad y Política Social in the section of Advanced Therapies (TRA-005) and by the Ministerio de Ciencia e Innovación (PI16/01245), Gobierno de España.
ORCID iD: Marta Santisteban  https://orcid.org/0000-0003-0625-686X
https://orcid.org/0000-0003-0625-686X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marta Santisteban, Department of Medical Oncology, Clínica Universidad de Navarra, Avda. Pío XII 36, 31008 Pamplona, Spain; Breast Cancer Unit, Clínica Universidad de Navarra, Pamplona, Spain; IdiSNA, Navarra Institute for Health Research, Pamplona, Spain.
Belén Pérez Solans, IdiSNA, Navarra Institute for Health Research, Pamplona, Spain; Pharmacometrics and Systems Pharmacology, Universidad de Navarra, Pamplona, Spain.
Laura Hato, Department of Immunology and Immunotherapy, Clínica Universidad de Navarra, Pamplona, Spain.
Amaia Urrizola, Medical Oncology, Clínica Universidad de Navarra, Pamplona, Spain.
Luis Daniel Mejías, Department of Pathology, Clínica Universidad de Navarra, Pamplona, Spain.
Esteban Salgado, IdiSNA, Navarra Institute for Health Research, Pamplona, Spain; Medical Oncology, Complejo Hospitalario de Navarra, Pamplona, Spain.
Rodrigo Sánchez-Bayona, Medical Oncology, Clínica Universidad de Navarra, Pamplona, Spain.
Estefanía Toledo, IdiSNA, Navarra Institute for Health Research, Pamplona, Spain; Department of Preventive Medicine and Public Health, Universidad de Navarra, Pamplona, Spain.
Natalia Rodríguez-Spiteri, Breast Cancer Unit, Clínica Universidad de Navarra, Pamplona, Spain.
Begoña Olartecoechea, Breast Cancer Unit, Clínica Universidad de Navarra, Pamplona, Spain.
Miguel Angel Idoate, Department of Pathology, Clínica Universidad de Navarra, Pamplona, Spain.
Ascensión López-Díaz de Cerio, IdiSNA, Navarra Institute for Health Research, Pamplona, Spain; Department of Immunology and Immunotherapy, Clínica Universidad de Navarra, Pamplona, Spain; Cell Therapy Unit, Clínica Universidad de Navarra, Pamplona, Spain; Clínica Universidad de Navarra, Universidad de Navarra, Complejo Hospitalario de Navarra and IdisNA, Pamplona, Spain.
Susana Inogés, IdiSNA, Navarra Institute for Health Research, Pamplona, Spain; Department of Immunology and Immunotherapy, Clínica Universidad de Navarra, Pamplona, Spain; Cell Therapy Unit, Clínica Universidad de Navarra, Pamplona, Spain; Clínica Universidad de Navarra, Universidad de Navarra, Complejo Hospitalario de Navarra and IdisNA, Pamplona, Spain.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 2012; 366: 2438–2441. [DOI] [PubMed] [Google Scholar]
- 3. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376: 2147–2159. [DOI] [PubMed] [Google Scholar]
- 4. Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol 2018; 29: 2232–2239. [DOI] [PubMed] [Google Scholar]
- 5. Hutchinson KE, Yost SE, Chang CW, et al. Comprehensive profiling of poor-risk paired primary and recurrent triple-negative breast cancers reveals immune phenotype shifts. Clin Cancer Res 2020; 26: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017; 541: 321–330. [DOI] [PubMed] [Google Scholar]
- 7. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 44–59. [DOI] [PubMed] [Google Scholar]
- 8. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020; 396: 1817–1828. [DOI] [PubMed] [Google Scholar]
- 9. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 2020; 6: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020; 396: 1090–1100. [DOI] [PubMed] [Google Scholar]
- 11. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 12. Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA 1998; 95: 9482–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qi CJ, Ning YL, Han YS, et al. Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunol Immunother 2012; 61: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solans BP, López-Díaz de Cerio A, Elizalde A, et al. Assessing the impact of the addition of dendritic cell vaccination to neoadjuvant chemotherapy in breast cancer patients: a model-based characterization approach. Br J Clin Pharmacol 2019; 85: 1670–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 16. Park JH, Jang M, Tarhan YE, et al. Clonal expansion of antitumor T cells in breast cancer correlates with response to neoadjuvant chemotherapy. Int J Oncol 2016; 49: 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 2008; 14: 518–527. [DOI] [PubMed] [Google Scholar]
- 18. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018; 19: 40–50. [DOI] [PubMed] [Google Scholar]
- 19. Gianni L, Huang C-S, Egle D, et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Cancer Res 2020; 80: GS3-04. [DOI] [PubMed] [Google Scholar]
- 20. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019; 30: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 21. Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol 2020; 31: 569–581. [DOI] [PubMed] [Google Scholar]
- 22. Bracci L, Schiavoni G, Sistigu A, et al. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014; 21: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 2015; 3: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31: 860–867. [DOI] [PubMed] [Google Scholar]
- 26. Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014; 2: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luen S, Virassamy B, Savas P, et al. The genomic landscape of breast cancer and its interaction with host immunity. Breast 2016; 29: 241–250. [DOI] [PubMed] [Google Scholar]
- 28. Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018; 167: 671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res 2018; 24: 2804–2811. [DOI] [PubMed] [Google Scholar]
- 30. Sobral-Leite M, Salomon I, Opdam M, et al. Cancer-immune interactions in ER-positive breast cancers: PI3K pathway alterations and tumor-infiltrating lymphocytes. Breast Cancer Res 2019; 21: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol 2019; 5: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin-Romano P, Ammari S, El-Dakdoukti Y, et al. Chemotherapy beyond immune checkpoint inhibitors in patients with metastatic colorectal cancer. Eur J Cancer 2020; 137: 117–126. [DOI] [PubMed] [Google Scholar]
- 33. Hadash-Bengad R, Hajaj E, Klein S, et al. Immunotherapy potentiates the effect of chemotherapy in metastatic melanoma—a retrospective study. Front Oncol 2020; 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 2018; 13: 106–111. [DOI] [PubMed] [Google Scholar]
- 35. Saleh K, Daste A, Martin N, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 2019; 121: 123–129. [DOI] [PubMed] [Google Scholar]
- 36. Inoges S, Tejada S, de Cerio AL, et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med 2017; 15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 2004; 64: 4973–4979. [DOI] [PubMed] [Google Scholar]
- 38. Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 2005; 11: 4160–4167. [DOI] [PubMed] [Google Scholar]
- 39. Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 2008; 68: 5955–5964. [DOI] [PubMed] [Google Scholar]
- 40. Fadul CE, Fisher JL, Hampton TH, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother 2011; 34: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008; 14: 3098–3104. [DOI] [PubMed] [Google Scholar]
- 42. Joller N, Kuchroo VK. Tim-3, Lag-3, and TIGIT. Curr Top Microbiol Immunol 2017; 410: 127–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baker KJ, Houston A, Brint E. IL-1 family members in cancer; two sides to every story. Front Immunol 2019; 10: 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kumari N, Dwarakanath BS, Das A, et al. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol 2016; 37: 11553–11572. [DOI] [PubMed] [Google Scholar]
- 45. Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat 2013; 138: 657–664. [DOI] [PubMed] [Google Scholar]
- 46. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211064653 for Final results regarding the addition of dendritic cell vaccines to neoadjuvant chemotherapy in early HER2-negative breast cancer patients: clinical and translational analysis by Marta Santisteban, Belén Pérez Solans, Laura Hato, Amaia Urrizola, Luis Daniel Mejías, Esteban Salgado, Rodrigo Sánchez-Bayona, Estefanía Toledo, Natalia Rodríguez-Spiteri, Begoña Olartecoechea, Miguel Angel Idoate, Ascensión López-Díaz de Cerio and Susana Inogés in Therapeutic Advances in Medical Oncology