Abstract
Background and aims:
Tofacitinib is a Janus kinase inhibitor (JAKi) recently approved for the treatment of moderate to severe ulcerative colitis (UC) based on robust efficacy and safety data derived from OCTAVE clinical trials. Evidence on the outcomes of tofacitinib therapy in real-world UC patients is needed, as a number of these patients would be deemed ineligible for clinical trials. We have therefore summarised data derived from observational, real-world evidence (RWE) studies on the effectiveness and safety of tofacitinib in moderate to severe UC patients.
Methods:
We searched the PubMed, EMBASE, Scopus, Web of Science and Cochrane databases for observational studies on the use of tofacitinib in UC patients, published between 30 May 2018 and 24 January 2021. Pooled induction (8–14 weeks) and maintenance (16–26 weeks) clinical response and remission rates were calculated, as well as the proportion of reported adverse events using random effects models.
Results:
Nine studies were included, comprising 830 patients, of which 81% were previously treated with anti-tumour necrosis factor (TNF) and 57% with vedolizumab. Induction of clinical response and remission were achieved in 51% (95% confidence interval, 41–60%) and 37% (26–45%) of patients, after a median follow-up of 8 weeks. At the end of a median follow-up of 24 weeks, maintenance of clinical response and remission were met in 40% (31–50%) and 29% (23–36%) of patients, respectively. Thirty-two percent of the patients had at least one adverse event, the most commonly reported being mild infection (13%) and worsening of UC, requiring colectomy (13%). A third of the patients (35%) discontinued tofacitinib, most frequently due to primary non-response (51%).
Conclusion:
Tofacitinib is a safe and effective therapy in real-world UC patients, as previously reported by clinical trials.
Keywords: clinical trials, IBD, JAKi, new therapies, real-world evidence
Introduction
The range of therapeutic agents for inflammatory bowel disease (IBD) management is rapidly expanding. Tofacitinib, a pan-Janus-kinase inhibitor (JAKi), is the first of the new, small molecule drugs to be released for the management of moderate to severe ulcerative colitis (UC). Compared to monoclonal antibodies directed against specific cytokines, JAKi target multiple pathways suggested to modulate intestinal inflammation in UC, such as interleukin (IL)-6, IL-12, IL-15, IL-23 and interferon (IFN)-γ. 1
First evidence for tofacitinib efficacy as induction therapy in moderate to severe UC was reported in an 8-week double-blind, placebo-controlled phase 2 trial. 2 This was a dose-defining study in 194 patients who had as primary outcome clinical response at 8 weeks (defined as a decrease in total Mayo score by at least 3 points from baseline and a relative decrease by at least 30%, a decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding score of 0 or 1). This was achieved by 32–78% of patients, depending on the tofacitinib dose [0.5, 3, 10 and 15 mg twice daily (BD)] versus 42% receiving placebo. Secondary outcomes including clinical remission at 8 weeks (Mayo score ⩽2, no subscore > 1) and endoscopic remission (Mayo endoscopic subscore of 0) were achieved in a greater proportion by patients on a 10 mg dose.
This was followed by two parallel group induction (OCTAVE-1 and OCTAVE-2) and one maintenance (OCTAVE Sustain) phase 3 registration studies, 3 comprising a total of 1139 patients. OCTAVE 1 and 2 demonstrated both efficacy and safety in achieving clinical remission at 8 weeks (defined as a total Mayo score ⩽(2, no subscore > 1 and a rectal bleeding score of 0) by 18.2% (OCTAVE Induction 1) and 16.6% (OCTAVE induction 2) of patients on a 10 mg dose, versus 8.2% and 3.6% in the placebo group. Patients who completed the OCTAVE-1 or OCTAVE-2 induction trials and met the primary endpoint of clinical response at week 8 were eligible for inclusion in the OCTAVE Sustain trial. Here, the primary endpoint (clinical remission at 52 weeks) was met in 34.3% and 40.6% of patients on a 5 and 10 mg dose, respectively, as compared to 11.1% in the placebo group. In addition, data from an open-label extension of the OCTAVE study (OCTAVE Open) offered evidence-based support for dose escalation after disease flare and showed the possibility of recapturing response after therapy discontinuation, 4 as well as providing long-term safety data.
Rigorously controlled, randomised clinical trials (RCTs) are mandatory to assess treatment efficacy. Importantly, however, a number of patients seen in routine clinical practice would not meet inclusion criteria for these studies, particularly those who are older, with refractory disease or significant comorbidity. 5 Indeed, a retrospective analysis of landmark IBD RCT enrolment suggests that only 34% of Crohn’s disease patients and 26% of UC ‘real-world’ patients would have been eligible for inclusion. 6 Common reasons for RCT ineligibility in UC trials were use of topical rectal therapy, being immunomodulator naive, patients with new diagnoses or needing colectomy due to age, comorbidity or concomitant advanced dysplasia. 7
Uncontrolled, observational data derived outside clinical trial research are often described as real-world evidence (RWE). They collect and use information from daily clinical practice, such as electronic health records, patient registries and surveys or digital health data. By studying a population more representative of clinical practice, RWE may offer additional insights into predictors of effectiveness, drug positioning and reveal unappreciated safety signals, particularly infection and malignancy. 8 While RWE data has some advantages, it is also often limited by recall bias and the non-protocolised data collection from uncontrolled treatment groups.
In this systematic review and meta-analysis, we aimed to summarise available data on the effectiveness and safety of tofacitinib, derived from early RWE in moderate to severe UC.
Methods
Data sources and searches
This study was conducted according to the PRISMA checklist 2020 for reporting systematic reviews and meta-analyses (Figure 1; Supplementary file). 9 A systematic search of MEDLINE (via PubMed), Embase, Scopus, Web of Science and Cochrane library was performed. We aimed to identify records that reported real-world experience with tofacitinib from 30 May 2018, date of drug approval until 24 January 2021. The search strategy included the term ‘ulcerative colitis’ in combination with ‘tofacitinib’ OR ‘JAK inhibitors’, AND/OR ‘real-world’. The fields ‘title, abstract, keyword’ and ‘all fields’ were used alternatively. Restrictions for non-English language studies and paediatric population were applied. Additional systematic search through the references of included records was performed to identify articles potentially missed by the search.
Figure 1.
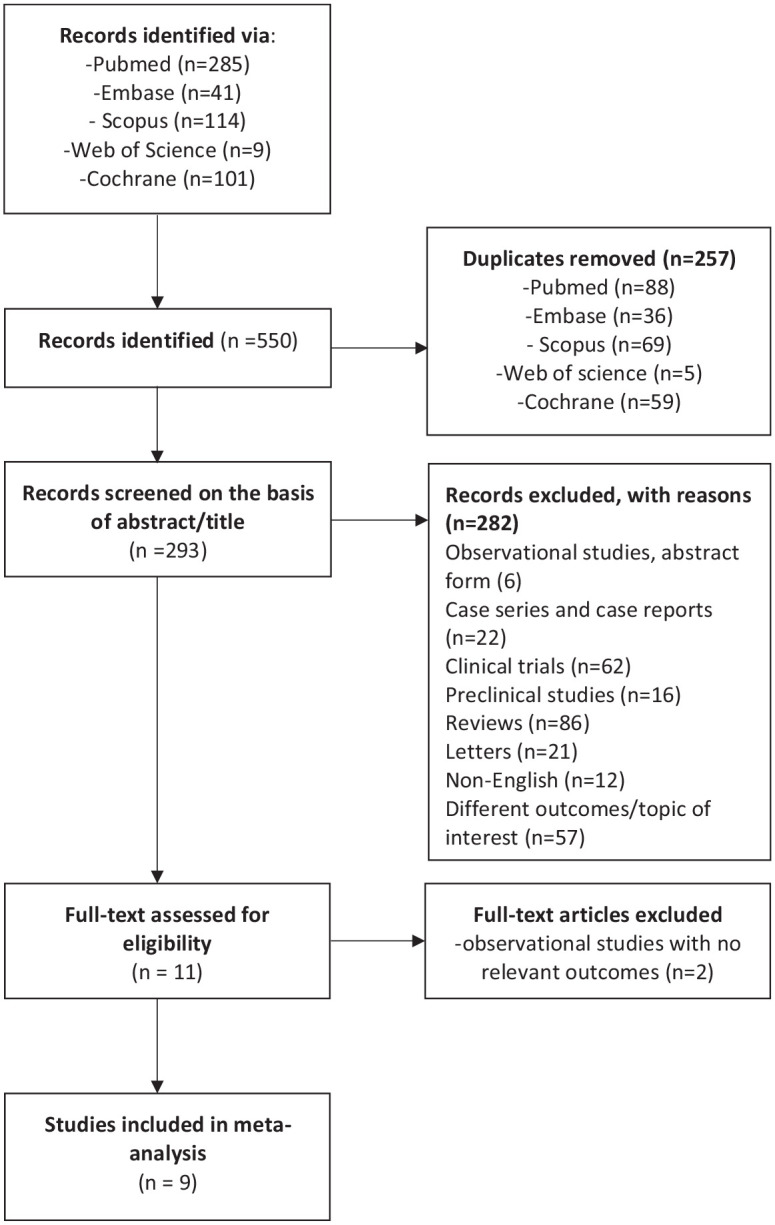
Flow diagram showing results of literature search and study selection.
Study selection and eligibility criteria
After the systematic import of all references into a reference management software (Mendeley), all duplicates were removed. The remaining records were screened for eligibility based on the title and abstract. Only studies that were published in complete form in peer-reviewed literature were considered for the pooled analysis of effectiveness and safety.
We included records that fulfilled the following criteria: (1) study type – observational studies that reported the effectiveness of tofacitinib in clinical practice; (2) population – adult patients with a diagnosis of UC, with active disease; (3) outcomes – the proportion of patients treated with tofacitinib achieving at least one of the following: clinical response, clinical remission, steroid-free remission, endoscopic remission; and (4) minimum follow-up – 24 weeks.
For topics that were covered in narrative form, data derived from conference abstracts presented at the European Crohn’s and Colitis Organisation (ECCO), United European Gastroenterology Week (UEGW), Digestive Disease Week (DDW), British Society of Gastroenterology (BSG) and American College of Gastroenterology (ACG) were referenced. Furthermore, we have excluded records classified as case series and case reports.
Data extraction
The outcomes of interest were extracted by two independent authors (L.A.L. and N.C.-C.), and disagreements were resolved by consensus. Effectiveness data were grouped under two different temporal phases, according to the time point reported by each study: ‘induction’, which covered outcomes reported at weeks 8, 12 and 14, and ‘maintenance’, which included the outcomes reported at weeks 16, 24 and 26. Data on remission at week 52 was reported only in two studies; therefore, it was not included in the analysis. Where both outcomes for weeks 16 and 26 were available, we have included the furthest follow-up time point for maintenance data.
Safety outcomes included the overall number of adverse events, namely infections, venous-thromboembolism, malignancies, need for colectomy and alteration of the lipid profile.
Study-related descriptors included the name of the first author, study design, the number of participants and the median follow-up. Patient demographics that included median age, gender, disease duration (years) and the proportion of patients with extensive disease were collected, as well as disease-related variables [disease activity scores, median levels of faecal calprotectin (FCAL) and C-reactive protein (CRP)], and treatment-related variables, such as tofacitinib dose at induction, bio-naïve status, previous biologics, concomitant steroids and immunosuppressants.
Descriptors of outcomes
Outcome effectiveness definitions are summarised in Table 1. Established clinical scores such as Partial Mayo Score (PMS) and Simple Clinical Colitis Activity Index (SCCAI) were used to assess clinical response and remission, and Mayo endoscopic subscore to assess endoscopic response and remission.
Table 1.
Descriptors of tofacitinib efficacy outcomes across real-world studies.
| Clinical response | Decrease of ⩾2 points,10,11 ⩾3 PMS/decrease > 30% from baseline12–14
Decrease ⩾1 point on RBS 10 /absolute RBS = 0/1 from baseline12,13 Reduction in SSCAI of ⩾315 Symptomatic improvement but not resolution 16 >50% reduction of symptoms |
| Clinical remission | PMS ⩽1, ⩽2,13 without individual subscores > 110
PMS < 3 with combined stool frequency and rectal bleeding subscore < 112 SCCAI < 3 Complete resolution of clinical symptoms 16 |
| Steroid-free remission | PMS ⩽2, 11 PMS P1 or SCCAI o215 without concomitant use of any steroid therapy at that time point, irrespective of the steroid use at start of tofacitinib11,15 |
| Endoscopic remission/ Mucosal healing |
Mayo endoscopic subscore = 0, 14 0–111 or absence of erosions/ulcerations within 6 months of treatment initiation 12 |
| Relapse | Recurrence of symptoms after an initial response that require therapeutic change12,16
Worsening of symptoms with endoscopic, radiological, or serological (CRP or FCAL) evidence of inflammation that led to escalation/change in medication 13 |
| Failure | Interruption of tofacitinib before the end of follow-up
12
Tofacitinib discontinuation due to symptom recurrence in patients in remission, non-response to tofacitinib treatment and serious adverse events 10 |
CRP, C-reactive protein; FCAL, faecal calprotectin; PMS, partial Mayo score; RBS, rectal bleeding subscale; SCCAI, Simple Clinical Colitis Activity Index.
Quality assessment of included studies
Two authors (N.P. and L.A.L.) independently assessed the quality of included studies using the Newcastle–Ottawa scale for cohort studies 17 (Supplementary file, Newcastle–Ottawa scale) (Supplementary Table 1). The following criteria were evaluated: selection (UC patients with moderate to severe disease and lack of/poor response/intolerance to biologics that received treatment with tofacitinib for at least 8 weeks) and assessment of outcomes. All studies are uncontrolled cohort studies, therefore the domain ‘comparability’ and ‘selection item 2’, describing the non-exposed cohort, were not applicable for this meta-analysis. As a result, the maximum score achievable by any of the studies reported was 7 instead of 9. Newcastle–Ottawa scores were originally defined as high (score 7–9), moderate (score 4–6) or low (score 0–3). We have graded as ‘high quality’ all studies that fulfilled a score of 6 or 7, and as ‘moderate quality’ the studies with a score of 4 or 5.
Statistical analysis
The R packages meta and metafor18–20 were used to calculate pooled proportions of patients, alongside 95% confidence intervals, responding to tofacitinib at induction and maintenance specified timelines. Pooled proportions of patients who experienced an adverse event or endoscopic remission across the entire study durations were also calculated. Due to the relatively small number of participants in the studies, logit transformations were applied before fitting random effects models using the DerSimonian and Laird method. 21 Random effects models were used as the cohort populations were not consistent between studies.
At least four studies were required for each analysis. Outliers were detected using externally standardised residuals and leave-one-out residual heterogeneity. Influential studies were found by investigating the influence deleting a study had on each individual parameter estimate using four different measures. If at least one measure found a study to be influential, the study was overall deemed to be influential. Detailed explanations of the methods used to detect outlier and influential studies can be found in the literature. 22 Evidence of publication bias was sought using funnel plots, and formally tested for using Egger’s regression test with a significance level of 5%. 23 R version 4.0.3 was used for this analysis. R code is available to reproduce the analysis at https://github.com/nathansam/tofameta. Heterogeneity was assessed using I2 values (the percentage of variation across the studies due to heterogeneity). 24
Results
Outcomes of search strategy
The search strategy identified 550 studies via PubMed, Embase, Scopus, Web of Science and Cochrane databases (Figure 1). After removing the duplicates, 293 studies were assessed against the predefined inclusion criteria. Of these, studies that did not meet the criteria for definition of outcomes or addressed different research topics, other publication types (RCTs, preclinical studies, reviews/meta-analyses, case series and case reports, comments, editorials, published erratum and letters) (n = 282) were excluded (Figure 1). Two observational studies that did not fulfil the criteria on minimum follow-up duration or reported outcomes were furthermore excluded.25,26 Nine studies, seven retrospective and two prospective, were therefore combined for qualitative analysis. The mean Newcastle–Ottawa score among the nine included studies was 6, with 8/9 studies demonstrating high quality scores (Supplementary Table 1).
Characteristics of patients across studies
A total of 830 UC patients were included in the meta-analysis, with a median age of 40 years [interquartile range (IQR), 37–45] and a median disease duration of 7 years (IQR, 5.2–9.4) (Table 2). Patients were followed up for a median duration of 31 weeks (23.5–41.5). The proportion of patients with extensive disease (Montreal classification E3) was 55% (454/830). At baseline, the median PMS was 6 (IQR, 5–6), CRP 4.5 mg/L (IQR, 2–7) and FCAL 1265 µg/g (IQR, 674–1898). Overall, 81% of patients (674/830) had received prior anti-TNF therapy. Where multiple previous anti-TNF use was reported, 118/227 (52%) patients had received 1 anti-TNF agent and 75/227 (33%) had received ⩾2 anti-TNF therapies. Prior ustekinumab use was reported by four studies, where 5% (28/534) of patients had previously been treated with ustekinumab.12,13,27,28 Only one study reported prior anti-TNF or vedolizumab use in ustekinumab-treated patients prior to tofacitinib, where 4.5% (5/113) had received all three biologic classes. 13
Table 2.
Baseline characteristics of the patients across real-world studies.
| Study | Patients, n | Gender, M, n (%) | Median age, years (IQR) | Median disease duration, years (IQR) | Median follow-up, weeks (IQR) | Extensive disease or pancolitis, n (%) | Median PMS (IQR) | Median SCCAI (IQR) | Median CRP (IQR) | Median FCAL (IQR) | Bio-naive | Previous anti-TNFα, n (%) | Previous VDZ, n (%) | Previous anti-TNFα +VDZ, n (%) | Previous UST, n (%) | Previous 1–2 biologics, n (%) | Previous ⩾ 3 biologics, n (%) | Concomitant CST, n (%) | Concomitant IMM, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weisshof et al. 16 | 58 | 36 (62) | 39.7 (29–53) | 10.4 (3.1–14.3) | 42.4 (22.4–87.2) | 29 (50) | 54 (93) | 47 (81) | 50 (40.7) | 27 (47) | 5 (9) | ||||||||
| Lair-Mehiri et al. 12 | 38 | 23 (60.5) | 41 (28–52) | 7 (5–11.8) | 41.5 (18.5–56.8) | 22 (57.9) | 6 (5–8) | 11 (5.5–19.3) | 38 (100) | 38 (100) | 38 (100) | 4 (10.5) | 11 (28.9) | 19 (50) | 20 (52.6) | 1 (2.6) | |||
| Chaparro et al. 13 | 113 | 53 (46.9) | 46 | 44 (30–66) | 79 (70) | 6 (6–8) | 113 (100) | 100 (89) | 5 (4) | 35 (31) | 78 (69) | 54 (48) | 12 (11) | ||||||
| Biemans et al. 27 | 123 | 72 (58.5) | 46.4 (32.9–55.7) | 7.6 (3.7–14.8) | 24 (12–25.9) | 63 (51.6) | 8 (5–10) | 5 (2–13) | 1730 (550–2604) | 116 (95.1) | 76 (62.3) | 73 (59.3) | 4 (3.3) | 116 (95.1) | 5 (4.1) | 44 (35.8) | 6 (4.9) | ||
| Honap et al. 15 | 134 | 86 (64) | 37 (16–81) | 5.5 (2.2–12.0) | 17 (8–26) | 66 (49) | 6 (5–8) | 6 (3–8) | 4 (2–14) | 548 (322–1198) | 24 (18) | 59 (44) | 4 (3) | 48 (36) | 68 (45) | 16 (12) | |||
| Hoffmann et al. 11 | 38 | 26 (68.4) | 33 (19–65) | 4 (0–24) | 39 (0–78) | 25 (65.7) | 6 (0–9) | 8.2 (2.0–115.1) | 800 (47–2000) | 1 (2.6) | 34 (89.5) | 26 (68.4) | 17 (44.7) | 6 (15.8) | 21 (55.3) | 1 (2.6) | |||
| Deepak et al. 28 | 260 | 109 (41.9) | 38 (27–49) | 5 (3–11) | 31 (12–50) | 140 (54.1) | 199 (76.5) | 146 (56.2) | 15 (5.8) | 149 (63.1) | 13 (5.2) | ||||||||
| Straatmijer et al. 14 | 36 | 13 (36) | 45 | 7 (3–14) | 57 (44–60) | 12 (33) | 10 (8–13) | 6 (2–19) | 2066 (395–4167) | 32 (89) | 15 (42) | 15 (42) | 0 (0) | ||||||
| Shimizu et al. 10 | 30 | 14 (46.7) | 40.5 (28.3–60) | 9.8 (6.5–11.8) | 23 (4–52) | 18 (60) | 6 (3–7) | 0.2 (0–0.8) | 29 (97) | 28 (97) | 1 (3) | 14 (46.7) | 14 (46.7) |
CRP, C-reactive protein; CST, corticosteroids; FCal, faecal calprotectin; IMM, immunomodulators; IQR, interquartile range; PMS, Partial Mayo Score; SCCAI, Simple Clinical Colitis Activity Index; TNFα, tumour necrosis factor-alpha; UST, ustekinumab; VDZ, vedolizumab.
Fifty percent of the patients (412/830) received concomitant corticosteroid therapy at tofacitinib induction, with 8% (68/825) of patients receiving concomitant immunomodulators. Tofacitinib use in biologic naive population was quantified in two studies, comprising 69 of 257 (59%) patients. 29 Tofacitinib dose was either 5 or 10 mg twice daily, according to investigator’s protocol (Table 2).
Efficacy outcomes
Outcomes were heterogeneously reported across studies, with many studies reporting the outcomes at different time points. We have pooled the results from the number of studies that had analysed the outcome of interest; therefore, the number of patients per outcome assessment may differ.
Induction of clinical response and remission
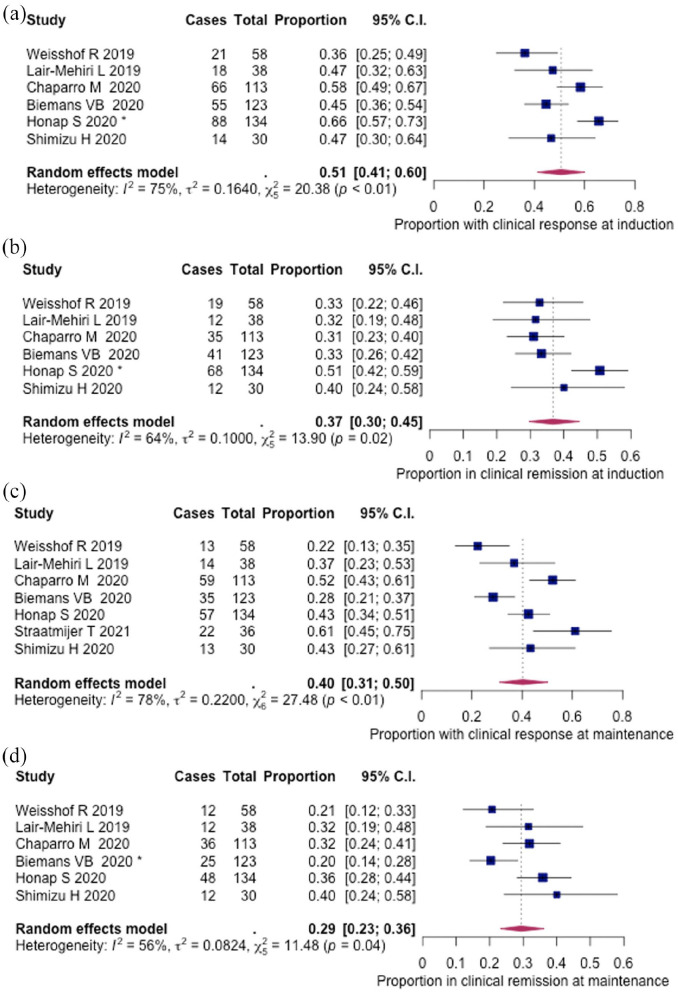
Clinical response and remission were reported in 496 patients across 6 studies (Table 3). Median time to outcome assessment in the induction group was 8 weeks (IQR, 8–12). Clinical response was achieved in 51% (262/496, 95% CI: 0.41–0.60%) and clinical remission in 37% (187/496, 95% CI: 30–45%) of patients, respectively (Figure 2(a) and (b)). Steroid-free remission was 32% (129/391, 95% CI: 25–40%), as assessed by five records (Supplementary figure 1a).
Table 3.
Efficacy of tofacitinib treatment across real-world studies in ulcerative colitis patients at induction8,12,14 and maintenance15,23,25 time points.
| Study | Data collection | Patients, n | UC, n | Tofacitinib dose at induction, mg, b.d., n (%) | Clinical response weeks, n (%) | Clinical remission weeks, n (%) | Steroid-free clinical remission, weeks, n (%) | Endoscopic remission, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15/20 mg | Induction weeks 8–14 | Maintenance weeks 16–26/36 | Induction weeks 8–14 | Maintenance weeks 16–26 | Induction weeks 8–14 | Maintenance weeks 16–26/36 | |||||
| Weisshof et al. 16 | Retrospective | 58 | 53 | 23 (40) | 35 (60) | 21/58 (36) | 13/48 (27) | 19/58 (33) | 12/48 (25) | 14/58 (24) | 10/48 (21) | 5/12 (13.2) | |
| Lair-Mehiri et al. 12 | Retrospective | 38 | 38 | 38 (100) | 18/38 (45) | 14/38 (37) | 12/38 (32) | 12/38 (32) | 12/38 32% | 13/38 (34.2) | |||
| Chaparro et al. 13 | Prospective | 113 | 113 | 6 (5) | 106 (94) | 1 (1) | 66 (60) | 59 (57) | 34 (31) | 33 (32) | |||
| Biemans et al. 27 | Prospective | 123 | 118 | 118 (100) | 10 | 55/99 (55.6) | 35/77 (45.5) | 41/99 (41.4) | 25/77 (32.5) | 35/99 (35.4) | 22/77 (28.6) | 7/33 (21.2) | |
| Honap et al. 15 | Retrospective | 134 | 134 | 134 (100) | 88/119 (74) | 57/108 (53) | 68/119 (57) | 48/108 (44) | 57/119 (48) | 47/108 (44) | 34/90 (37.7) | ||
| Hoffmann et al. 11 | Retrospective | 38 | 38 | 38 (100) | 11/38 (28.9) | 7/36 (19.4) | 2/11 (18.8) | ||||||
| Straatmijer et al. 14 | Retrospective | 36 | 36 | 1 (3) | 35 (97) | 22/36 (61) | 11/35 (31) | 12/31 (39) | |||||
| Shimizu et al. 10 | Retrospective | 30 | 30 | 30 (100) | 14 (47) | 13 (45) | 12 (40) | 12 (41) | |||||
d., twice daily; UC, ulcerative colitis.
Figure 2.
Pooled efficacy of tofacitinib in ulcerative colitis (UC) real-world patients: (a) clinical response during induction (8, 12, 14 weeks), (b) clinical remission during induction (8, 12, 14 weeks), (c) clinical response during maintenance (16, 24, 26 weeks) and (d) clinical remission during maintenance (16, 24, 26 weeks). Influential studies are denoted with *.
Maintenance of clinical response and remission
Median time to outcome assessment in the maintenance group was 24 weeks (IQR, 18–24) (Table 2). Clinical response and remission in the maintenance phase were reported in 532 and 496 patients across 7 and 6 studies, respectively. Clinical response was achieved in 40% (213/532, 95% CI: 31–50%) and clinical remission in 29% (145/496, 95% CI: 23–36%) of patients, respectively (Figure 2(c) and (d)). The proportion of patients in steroid-free remission was 25% (109/427, 95% CI: 18–33%), as reported by six studies (Supplementary figure 1b). Endoscopic evaluation was conducted at the end of follow-up in 177 patients across 5 studies, and endoscopic remission was achieved by 34% (60/177, 95% CI: 26–43%) of patients (Supplementary figure 2).
Predictors of response and remission
Due to significant heterogeneity in reporting patients’ characteristics and outcomes, it was not possible to perform pooled analysis for predictors of response. There were fewer than 10 studies with case data for any outcome of interest. As a result, we lacked the statistical power required to perform valid subgroup analyses. 30
Factors independently associated with clinical response at week 8 were reported in one study. 29 Multivariable analysis in this work revealed that patient’s treatment naive status (OR: 4.50, 95% CI: 1.64–12.37) and a higher albumin (OR: 2.63, 95% CI: 1.02–6.80) were associated with a greater chance of achieving clinical response at week 8, whereas the concomitant use of corticosteroids at the start of tofacitinib treatment (OR: 0.22, 95% CI: 0.08–0.58), male gender (adjusted hazard ratio = 0.25, 95% CI: 0.08–0.83) and pancolitis were associated with a lower chance of achieving week 8 response. 29 In the same study, prior exposure to two biologic classes had lower rates (16.7%) of endoscopic healing at 6 months compared with bio-naive patients (87.1%) and those with exposure to one biologic agent (57.1%). A similar finding was reported by a Dutch prospective observational registry, which showed that steroid-free clinical remission rate at week 24 was influenced by prior exposure to vedolizumab in a multivariable analysis (OR = 0.301, 95% CI: 0.100–0.907). 27
Clinical predictors, such as a higher PMS at week 4 was the only variable associated with the likelihood of achieving short-term remission at week 8 (OR: 0.2; 95% CI: 0.1–0.4), but not at week 16, as reported by Chaparro et al. 13 The multivariable analysis by Verstockt et al. 31 showed that a higher baseline albumin and a lower Mayo endoscopic subscore were independent predictors of endoscopic (OR = 1.06 and 0.59, respectively) and biological remission (OR = 1.06 and 0.57, respectively). Tofacitinib dose did not correlate with clinical response at week 8. 16
Treatment discontinuation
Treatment discontinuation was reported in 35% of patients (254/800, 95% CI: 28–43%) across eight studies (Supplementary figure 3a). The most common reason for discontinuation was PNR, reported in 51% of these patients (111/194, 95% CI: 34–68%) (Supplementary figure 3b). Other reasons for treatment discontinuation were adverse events (AEs) in 20% of patients (44/241, 95% CI: 13–30%) (Supplementary figure 3c), need for colectomy 19% (29/147, 95% CI: 6–47%) and patient request (0.8%, 3/370) (Table 3). Median time to discontinuation of therapy was 9 weeks.14,15,27
Predictors of treatment discontinuation and PNR
The only factor associated with treatment discontinuation was higher PMS at week 8 in a multivariable analysis. 13 A prospective cohort following endoscopic and histologic outcomes of tofacitinib treatment in 35 patients refractory to anti-TNF and vedolizumab 31 reported that 10 of the patients who experienced PNR to one anti-TNF discontinued tofacitinib due to same reason. Moreover, PNR to two anti-TNFs resulted in PNR to tofacitinib in four out of five patients. In contrast, for the eight patients previously treated with vedolizumab, only three were nonresponders to tofacitinib, as reported by same study. In the work of Honap et al., 15 younger age at treatment initiation [median of 28 years, IQR = 23.0–37.5, aHR = 1.04 (95% CI: 1.01–1.07)] and elevated CRP at baseline [aHR = 0.29 (95% CI: 0.12–0.66), for every 10-fold increase in baseline CRP] were independently associated with PNR at week 8. In addition, response and remission rates were not different stratified by prior biologic use, as reported by the same study. These findings were confirmed by other reports, 16 which have not detected a link between PNR and previous biologic therapy.
Safety outcomes
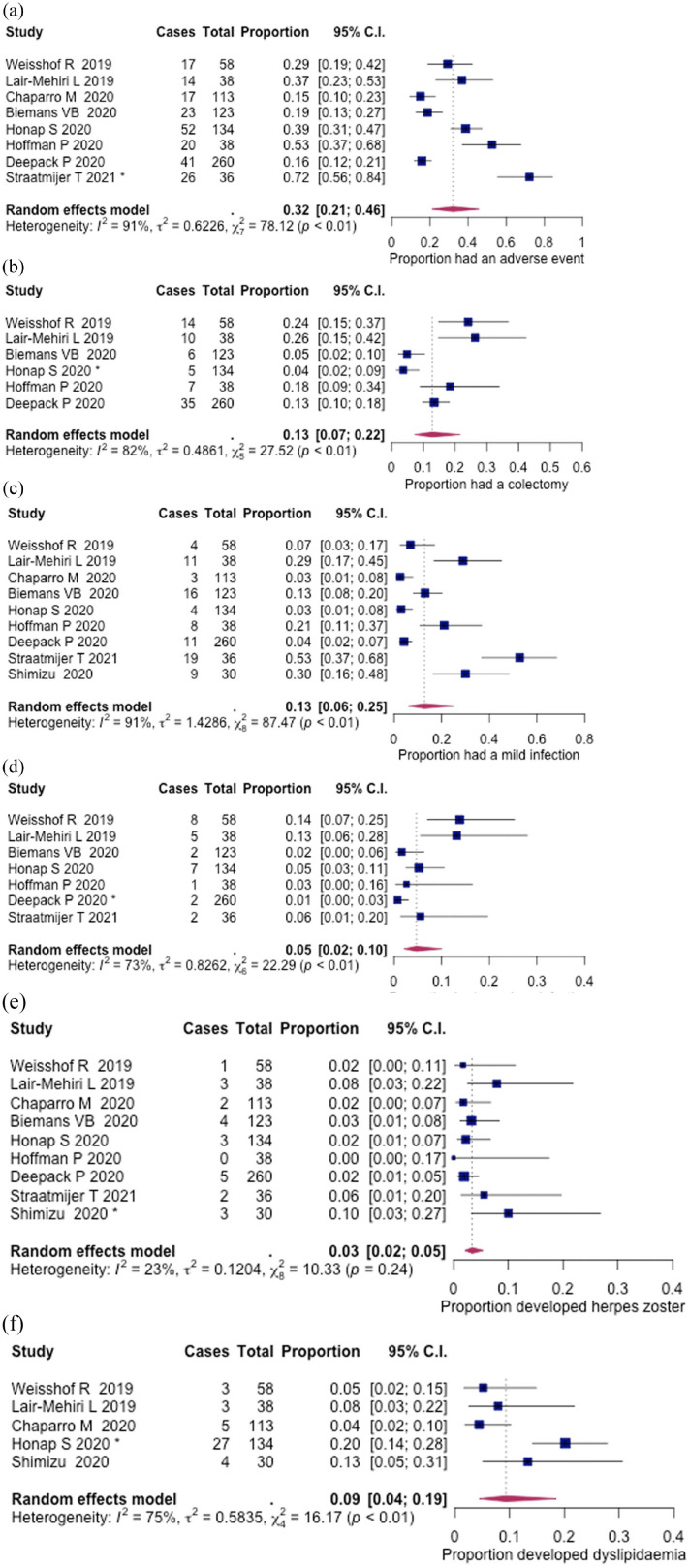
Relevant safety outcomes across all studies are summarised in Table 4. The proportion of patients that experienced at least one AE was 32% (210/800, 95% CI: 21–46%), during a median follow-up of 18 weeks (IQR, 11–42), as reported across eight studies (Figure 3(a)). The most common AEs were mild infection 13% (47/830, 95% CI: 6–25%) and colectomy 13% (77/651, 95% CI: 7–22%); other AEs were dyslipidaemia 9% (42/373, 95% CI: 4–19%), serious infection leading to hospitalisation 5% (27/687, 95% CI: 2–10%) and herpes zoster infection 3% (23/830, 95% CI: 2–5%) (Figure 3(b)–(f)). Where herpes zoster infection was reported, patients received 5 or 10 mg BD of tofacitinib, with an additional one patient who received 15 mg BD. 13
Table 4.
Adverse events of tofacitinib treatment and therapy discontinuation in ulcerative colitis patients across real-world studies.
| Study | Patients, n | Patients with AE, n | Herpes zoster, n | Total | Infection, n | VTE, n | Dyslipidaemia, n | Malignancy, n | Colectomy, n (%) | Therapy discontinuation, n (%) | Reasons for therapy discontinuation, n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild/moderate | Serious | AE | Colectomy | PNR | LOR | Patients’ choice | ||||||||||
| Weisshof et al. 16 | 58 | 17 | 1 | 12 | 4 | 8 | 0 | 3 | 14 (24.1) | 26 (43.1) | 5/26 (19.2) | 6/26 (23.7) | ||||
| Lair-Mehiri et al. 12 | 38 | 14 | 3 | 16 | 11 | 5 | 0 | 3 | 10 (26) | 16 (42) | 5/16 (31.2) | 7/16 (43.7) | 4/16 (25) | |||
| Chaparro et al. 13 | 113 | 17 | 2 | 3 | 3 | 0 | 5 | 1 | 45 (40) | 7 /45 (15.5) | 29/45 (64.4) | 8/45 (17.7) | 1/45 (2.2) | |||
| Biemans et al. 27 | 123 | 23 | 4 | 16 | 16 | 0 | 0 | 6 (4.9) | 46 (37.4) | 7/46 (15.2) | 35/46 (76.1) | 3/46 (6.5) | 1/46 (2.2) | |||
| Honap et al. 15 | 134 | 52 | 3 | 11 | 4 | 7 | 0 | 27 | 5/134 (3.7) | 48 (35.8) | 21/48 (43.7) | 31/48 (64.5) | 14/48 (29.1) | 1/48 (2.0) | ||
| Hoffmann et al. 11 | 38 | 20 | 0 | 9 | 8 | 1 | 0 | 7 (18.4) | 13 (34.2) | 1/13 (7.6) | 13/13 (30.7) | |||||
| Deepak et al. 28 | 260 | 41 | 5 | 13 | 11 | 2 | 2 | 2 | 35 (13) | 47 (18) | 12/47 (25.5) | 1/47 (2.1) | 34/47 (72.3) | |||
| Straatmijer et al. 14 | 36 | 26 | 2 | 21 | 19 | 2 | 14/36 (33) | 6/36 (16.6) | 6/36 (16.6) | |||||||
| Shimizu et al. 10 | 30 | 3 | 9 | 9 | 0 | 4 | 16 (53.3) | |||||||||
AE, adverse events; LOR, loss of response; PNR, primary non-response; VTE, venous thromboembolism.
Figure 3.
Adverse events among real-world UC patients: (a) proportion of patients that had at least one adverse event, (b) colectomy, (c) mild or moderate infection, (d) serious infection, (e) herpes zoster infection and (f) dyslipidaemia. Influential studies are denoted with *.
Two episodes of deep vein thrombosis (DVT): 28 one in a 19-year-old patient, without venous thromboembolism (VTE) risk factors, but who developed DVT in the context of septic arthritis requiring hospitalisation. The other patient was 64 years old, with risk factors including obesity, hypertension, hyperlipidaemia and a prior VTE event.
There was one case of malignancy (metastatic breast cancer observed in this cohort of real-world patients). 13
Discussion
Tofacitinib is the first small molecule JAKi licensed for moderate to severe UC. In this meta-analysis, we have summarised the RWE for tofacitinib use in UC.
Our meta-analysis showed clinical response induction rates (51%) comparable with those reported from OCTAVE Induction trials 1 and 2 (59.9% and 55%, respectively). Clinical remission at 8 weeks was divergent: 37% across real-world cohorts versus 18.5% and 16.8% for OCTAVE Induction 1 and 2, respectively. While maintenance of clinical response at 16, 24 and 26 weeks from our RWE analysis was lower than those in OCTAVE Sustain (40.0% versus 63.6%), clinical remission rates at the same time points were broadly comparable (29.0% versus 34.3%) at 24 weeks in OCTAVE Sustain. Testing for differences in outcomes between OCTAVE and the observational studies was also not possible due to differences in outcome definitions.
There are several factors to be considered when comparing our data with results from clinical trials. First, the timing of efficacy evaluation varied widely between studies. Therefore, we pooled the results into induction (8–14 weeks) and maintenance (16–26 weeks) time points since most studies reported data points at 8 and 24 weeks. Second, there was heterogeneity among studies in reporting clinical information regarding patients’ characteristics; therefore, multivariable analysis to identify predictors of response and remission could not be performed.
The majority of RWE patients (81%) were refractory to one or two anti-TNF agents, whereas 57% and 5% had previously been exposed to vedolizumab and ustekinumab. In one of the two studies that reported tofacitinib effectiveness in biologic naive patients, clinical and endoscopic response rates were higher compared to those previously treated with biologics. 29
Overall, tofacitinib was well tolerated among patients and had a comparable safety profile with data from clinical trials. In our meta-analysis, the proportion of patients that experienced at least one adverse event was lower (32%) in comparison to 56% and 54% reported by OCTAVE induction trials; this is likely an artefact from the formal RCT AE reporting process. Furthermore, we reported lower rates for infection (mild/moderate 13%, serious infection 5% versus 23% and 18%, respectively) but higher herpes zoster infection rates (4% herpes versus 0.6% and 0.5%). Dose analysis of herpes zoster risk was not possible in this dataset due to a lack of infection-associated dosing outcome reporting.
JAK inhibition has also been associated with alteration of serum lipid profile, the occurrence of major cardiovascular adverse events (MACE) and VTE. Our pooled analysis showed a 9% rate of dyslipidaemia among five studies and no MACEs reported among real-world patients. 16
A post hoc analysis of OCTAVE clinical trials comprising 1157 UC 32 patients reported one occurrence of DVT and four pulmonary emboli in the overall cohort; all were treated with tofacitinib 10 mg twice daily and had other risk factors for VTE. With regard to MACE, such as haemorrhagic stroke, aortic dissection, acute coronary syndrome and myocardial infarction, the IR was 0.2 (0.1–0.6%) based on four patients in the overall cohort.
There was one case of malignancy in our cohort of real-world patients. 13 An integrated safety analysis 33 based on all RCTs of tofacitinib in UC (phase II induction, 2 OCTAVE Induction 1, 2 and Sustain, 34 OCTAVE Open 35 ) reported similarly low rates of malignancies, with an IR of malignancies (excluding non-melanoma skin-cancer) of 0.7% (95% CI: 0.3–1.2) for 11/1157 patients.
Treatment discontinuation rate across RWE studies was 35%, mostly due to PNR (51%) and AE (20%). Other reasons for treatment discontinuation were need for colectomy (19%) and patient’s preference (0.8%). In OCTAVE induction trials, treatment discontinuation rate irrespective of the reason was 6.5% (32/492) and 7.5% (33/435) for Induction 1 and 2 trials, respectively, whereas in OCTAVE Sustain 39.8% (157/394) discontinued treatment in both 5 and 10 mg dose arms.
It is noteworthy that across our RWE dataset, the median age was 41 years. This should be borne in mind when comparing AEs, especially MACE and malignancy, with data from rheumatology with tofacitinib. Preliminary reports of the ORAL Surveillance (A3921133; NCT02092467) study in rheumatoid arthritis have shown an increase in MACE and malignancy with tofacitinib versus infliximab in patients over 50 years of age with at least one additional cardiovascular risk factor. 36
There is limited evidence on the use of tofacitinib for moderate-to-severe UC outside RCTs. To safeguard our findings against the emergence of future publications, we repeated our analyses with the inclusion of conference abstracts that have not to date been published in full.29,31,37–40 This search had yielded an additional six studies including a total of 332 patients on top of those reported here. The results from this analysis did not deviate substantially from the findings we have reported (data not shown). On the contrary, the inclusion of abstracts was shown to reduce the number of outcomes with significant publishing bias from four to two. Sensitivity analysis of full papers alone and both full papers and abstracts included showed that overall, the inclusion of abstracts augmented our findings by contributing to increased precision and comprehensiveness of the analysis (Supplementary figure 4).
Estimates of heterogeneity calculated using I2 for many of our outcomes was greater than 75% which is commonly interpreted to indicate considerable heterogeneity. However, for many of the outcomes of interest, there was a limited number of events or studies and as such, I2 is arguably not reliable in the context of this meta-analysis. 41 This was most notable when investigating treatment discontinuation due to AE which yielded a lower bound of 0 and an upper bound of 94.3% for a 95% confidence interval of I2.
This meta-analysis has two major limitations: first, the majority of studies are retrospective, leading to significant heterogeneity in study population, data collection, definitions of outcomes, tofacitinib dose, time of drug exposure, length of follow-up and endoscopic evaluation. Therefore, extracting data for predictors of response or testing for differences in outcomes between real-world patients and the OCTAVE trials was not achievable. Second, real-world studies comprising patients treated before 30 May 201812,16,39 used off-label medication or accessed it through compassionate programmes which restricted the selection criteria to severe, refractory cases. Other studies 42 exclusively included patients refractory to other biologics (anti-TNF and vedolizumab).
Taxonera et al. 43 have recently conducted a similar meta-analysis investigating the effectiveness and safety of tofacitinib. However, our work differs in a few substantial ways. First, calculated proportions of response and remission have been reported by using the number of subjects still taking tofacitinib at each time point. In contrast, we have calculated these proportions from the total number of subjects belonging to each cohort; this approach is more informative, by incorporating subjects with PNR and LOR. Second, the inclusion of conference abstracts in the quantitative analysis may have led to biased results, due to incomplete findings. This was also reflected in slightly wider confidence intervals when compared to our results.
Therefore, our study is the first to summarise available efficacy and safety evidence in the real-world population with UC, across exclusively peer-reviewed data. Tofacitinib appears to be at least as effective as it was previously demonstrated in clinical trials, with an attractive safety profile. Long-term follow-up data may provide additional information with regard to non-response, effectiveness in special populations (pregnancy, children, elderly) and adverse events (malignancies).
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-10-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-11-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-5-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-6-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-7-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-8-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-9-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: LAL, NP and SS contributed to conception and study design, data collection, analysis and interpretation, and writing of the manuscript. LAL and NP contributed to quality assessment of the studies. NC-C contributed to data collection and statistical analysis, and writing of the manuscript. G-RJ and LAAPD contributed to writing of the manuscript and critical revision of the manuscript. CWL contributed to conception and study design, writing of the manuscript and critical revision of manuscript. LAL had full access to all the data in this study and takes responsibility for the accuracy and integrity of the data analysis. All authors approved the final draft of the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: L.A.L., none declared. N.C.-C., none declared. N.P. has received consultancy fees from Takeda, and speaker fees and/or travel support from Abbvie, Takeda and Norgine. S.S., none declared. L.A.A.P.D., none declared. G.-R.J., none declared. C.W.L. has received research support from Abbvie and Gilead; consultancy fees from Abbvie, Pfizer, Janssen, Gilead, Celltrion, Pharmacosmos, Takeda, Vifor, Iterative Scopes and Trellus Health; and speaker fees and/or travel support from Janssen, Abbvie, Pfizer, Dr Falk, Ferring, Hospira and Takeda.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work has been supported by the ISSF3 Wellcome Trust clinical lecturer bridging award (to G.-R.J.), the Medical Research Council & University of Edinburgh, Precision Medicine PhD studentship (MR/N013166/1, to N.C.-C.) and the UKRI Future Leaders Fellowship (MR/S034919/1, to C.W.L.).
ORCID iD: Laura A. Lucaciu  https://orcid.org/0000-0002-6310-2384
https://orcid.org/0000-0002-6310-2384
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laura A. Lucaciu, Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK; Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Nathan Constantine-Cooke, MRC Human Genetics Unit, Institute of Genetics and Cancer, The University of Edinburgh, Western General Hospital, Edinburgh, UK.
Nikolas Plevris, Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK.
Spyros Siakavellas, Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK.
Lauranne A.A.P. Derikx, Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK Inflammatory Bowel Disease Centre, Department of Gastroenterology and Hepatology, Radboud University Medical Centre, Nijmegen, The Netherlands.
Gareth-Rhys Jones, Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK; Centre for Inflammation Research, The Queen’s Medical Research Institute, The University of Edinburgh, Edinburgh, UK.
Charles W. Lees, Centre for Genomics and Experimental Medicine, Institute of Genetics and Cancer, The University of Edinburgh, Western General Hospital, Edinburgh EH4 2XU, UK; Edinburgh IBD Unit, Western General Hospital, Edinburgh, UK.
References
- 1. Perez-Jeldres T, Tyler CJ, Boyer JD, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol 2019; 10: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 2012; 367: 616–624. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 377: 496–497. [DOI] [PubMed] [Google Scholar]
- 4. Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther 2020; 51: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence – what is it and what can it tell us? N Engl J Med 2016; 375: 2293–2297. [DOI] [PubMed] [Google Scholar]
- 6. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012; 10: 1002–1007. [DOI] [PubMed] [Google Scholar]
- 7. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 8. Pugliese D, Armuzzi A. Difference in treatment outcomes between clinical trials and ‘real-life’ clinical practice: ustekinumab in ulcerative colitis. United European Gastroenterol J 2020; 8: 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimizu H, Fujii T, Hibiya S, et al. Rapid prediction of 1-year efficacy of tofacitinib for treating refractory ulcerative colitis. Intest Res 2021; 19: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann P, Globig A-M, Thomann AK, et al. Tofacitinib in treatment-refractory moderate to severe ulcerative colitis: real-world experience from a retrospective multicenter observational study. J Clin Med 2020; 9: 2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lair-Mehiri L, Stefanescu C, Vaysse T, et al. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis 2020; 52: 268–273. [DOI] [PubMed] [Google Scholar]
- 13. Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA Registry. J Crohns Colitis 2021; 15: 35–42. [DOI] [PubMed] [Google Scholar]
- 14. Straatmijer T, van Gennep S, Duijvestein M, et al. Real-world clinical and endoscopic outcomes after one year tofacitinib treatment in ulcerative colitis. Eur J Gastroenterol Hepatol 2021; 33: 1288–1297. [DOI] [PubMed] [Google Scholar]
- 15. Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis 2020; 14: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 16. Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci 2019; 64: 1945–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 18. RC Team. R: a language and environment for statistical computing. Vienna: R Core Team, 2019. [Google Scholar]
- 19. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 22. Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010; 1: 112–125. [DOI] [PubMed] [Google Scholar]
- 23. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 25. Rudrapatna VA, Glicksberg BS, Butte AJ. A comparison of the randomized clinical trial efficacy and real-world effectiveness of tofacitinib for the treatment of inflammatory bowel disease: a cohort study. medRxiv 2019: 19007195, http://medrxiv.org/content/early/2019/10/02/19007195.abstract
- 26. Kolar M, Lukas M, Malickova K, et al. Tofacitinib induction efficacy and safety in ulcerative colitis at week 8 – results from clinical practice. Gastroenterol Hepatol 2020; 74: 28–34. [Google Scholar]
- 27. Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) Registry. Aliment Pharmacol Ther 2020; 51: 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deepak P, Alayo QA, Khatiwada A. et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2021; 19: 1592–1601.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ungaro R, Fenster M, Dimopoulos C, et al. P344 real-world effectiveness of tofacitinib in ulcerative colitis: a multi-centre study. J Crohns Colitis 2019; 13(Suppl. 1): S274–S275. [Google Scholar]
- 30. Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004; 23: 1663–1682. [DOI] [PubMed] [Google Scholar]
- 31. Verstockt B, Outtier A, Lefrère J, et al. P399 endoscopic and histologic outcome in tofacitinib treated refractory moderate-to-severe ulcerative colitis: a prospective real-life cohort. J Crohns Colitis 2020; 14(Suppl. 1): S369–S370. [Google Scholar]
- 32. Sandborn WJ, Panes J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019; 50: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from Global Clinical Trials. Clin Gastroenterol Hepatol 2019; 17: 1541–1550. [DOI] [PubMed] [Google Scholar]
- 34. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 35. Feagan BG, Dubinsky M, Lukas M, et al. Efficacy and safety of an additional 8 weeks of Tofacitinib induction therapy: results of the OCTAVE open study for Tofacitinib 8-week induction non-responders. Gastroenterology 154: S-377. [Google Scholar]
- 36. Pfizer. Pfizer shares co-primary endpoint results from post-marketing required safety study of Xeljanz (tofacitinib) in subjects with rheumatoid arthritis (RA), https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-co-primary-endpoint-results-post-marketing (2021, accessed 13 February 2021).
- 37. Cremer A, Lobaton T, Vieujan S. et al. P422 tofacitinib induces clinical and endoscopic remission in biologic refractory ulcerative colitis patients: a real-world Belgian cohort study. J Crohns Colitis 2020; 14(Suppl. 1): S384–S386. [Google Scholar]
- 38. Clark-Snustad KD, Singla A, Lee SD. Su1928–tofacitinib improves clinical disease activity in a real-world population of patients with moderate-severe ulcerative colitis and Crohn’s disease. Gastroenterology 2019; 156: S-664. [Google Scholar]
- 39. Xiao Y, Lakatos PL, Bourdages R, et al. P512 efficacy of tofacitinib for the treatment of moderate-to-severe ulcerative colitis: real-world data. J Crohns Colitis 2020; 14(Suppl. 1): S444–S445. [Google Scholar]
- 40. Rutka M, Farkas K, Pigniczki D, et al. P663 efficacy of tofacitinib in patients with ulcerative colitis after previous ineffective biological therapies. J Crohns Colitis 2020; 14(Suppl. 1): S545–S546. [Google Scholar]
- 41. Thorlund K, Imberger G, Johnston BC, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 2012; 7: e39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verstockt B, Van Assche G, Vermeire S, et al. Biological therapy targeting the IL-23/IL-17 axis in inflammatory bowel disease. Expert Opin Biol Ther 2017; 17: 31–47. [DOI] [PubMed] [Google Scholar]
- 43. Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: systematic review with meta-analysis. Inflamm Bowel Dis. Epub ahead of print 15 February 2021. DOI: 10.1093/ibd/izab011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-4-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-10-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-11-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-5-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-6-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-7-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-8-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology
Supplemental material, sj-tiff-9-tag-10.1177_1687814020966927 for Real-world experience with tofacitinib in ulcerative colitis: a systematic review and meta-analysis by Laura A. Lucaciu, Nathan Constantine-Cooke, Nikolas Plevris, Spyros Siakavellas, Lauranne A.A.P. Derikx, Gareth-Rhys Jones and Charles W. Lees in Therapeutic Advances in Gastroenterology