Abstract
Background:
Tendons are primarily acellular, limiting their intrinsic regenerative capabilities. This limited regenerative potential contributes to delayed healing, rupture, and adhesion formation after tendon injury.
Purpose:
To determine if a tendon’s intrinsic regenerative potential could be improved after the application of a purified exosome product (PEP) when loaded onto a collagen scaffold.
Study Design:
Controlled laboratory study.
Methods:
An in vivo rabbit Achilles tendon model was used and consisted of 3 groups: (1) Achilles tenotomy with suture repair, (2) Achilles tenotomy with suture repair and collagen scaffold, and (3) Achilles tenotomy with suture repair and collagen scaffold loaded with PEP at 1 × 1012 exosomes/mL. Each group consisted of 15 rabbits for a total of 45 specimens. Mechanical and histologic analyses were performed at both 3 and 6 weeks.
Results:
The load to failure and ultimate tensile stress were found to be similar across all groups (P ≥ .15). The tendon cross-sectional area was significantly smaller for tendons treated with PEP compared with the control groups at 6 weeks, which was primarily related to an absence of external adhesions (P = .04). Histologic analysis confirmed these findings, demonstrating significantly lower adhesion grade both macroscopically (P = .0006) and microscopically (P = .0062) when tendons were treated with PEP. Immunohistochemical staining showed a greater intensity for type 1 collagen for PEP-treated tendons compared with collagen-only or control tendons.
Conclusion:
Mechanical and histologic results suggested that healing in the PEP-treated group favored intrinsic healing (absence of adhesions) while control animals and animals treated with collagen only healed primarily via extrinsic scar formation. Despite a smaller cross-sectional area, treated tendons had the same ultimate tensile stress. This pilot investigation shows promise for PEP as a means of effectively treating tendon injuries and enhancing intrinsic healing.
Clinical Relevance:
The production of a cell-free, off-the-shelf product that can promote tendon regeneration would provide a viable solution for physicians and patients to enhance tendon healing and decrease adhesions as well as shorten the time required to return to work or sports.
Keywords: tendon healing, exosomes, tendon biomechanics, growth factors/healing enhancement, tissue engineering, injury prevention
Achilles tendon injuries can be acute (traumatic) or chronic (degenerative). 34 Tendon healing is known to be a slow process secondary to the tissues’ low metabolic rate, limited cellularity, and poor vascularity when compared with highly metabolic tissues such as muscle and bone. 4,19,34,47 Scar formation is mechanically inferior to native tendon, leading to tendon rerupture, persistent pain, and decreased functional capacity. 8,36,39,52 The rate of Achilles rerupture has been reported as approximately 3% after operative repair and 5% to 8% after nonoperative treatment. 3,13,23,29,35,45,49
Current clinical practice for Achilles tendon tears consists of either nonoperative or operative treatment. Nonoperative intervention consists of functional bracing or casting with the ankle in a resting equinus position with early range of motion and weightbearing protocols. 35,44,46 Operative treatment varies, but in general consists of sutured repair followed by approximately 4 to 6 weeks of immobilization with the ankle in slight plantarflexion followed by a variety of early range of motion and weightbearing protocols. 35,44,46 In both of these options, the tendon heals via an extrinsic process that favors adhesion formation.
Current methods for treating tendon injuries focus on accelerating extrinsic healing and optimizing early motion protocols. Although these have been shown to be beneficial, they do not address the underlying issue of poor inherent intrinsic healing. The Achilles tendon receives blood supply peripherally from the paratenon, proximally at the musculotendinous junction, and distally at the osteotendinous junction. 34 This leaves a watershed area at the midsubstance of the tendon that is susceptible to rupture. After rupture at this junction, tendon primarily heals via an extrinsic process (Table 1). In this process, cells are produced from the paratenon, and adhesions form in order to provide vascular and cellular support. This healing process produces a higher ratio of fibroblasts compared with collagen and glycosaminoglycan. 34
TABLE 1.
Stages of Tendon Healing 34 a
| Inflammatory Stage | Proliferative Stage | Remodeling: Consolidation Stage | Remodeling: Maturation Stage |
|---|---|---|---|
|
|
|
|
a GAG, glycosaminoglycan.
In contrast, intrinsic healing occurs via the proliferation of tenocytes from the epitenon and endotenon producing mature collagen fibers; thus, adhesions do not form. Intrinsic healing is known to result in superior biomechanics and collagen structure closer to normal tendon (Table 2). 34 Methods to increase the degree of intrinsic, regenerative healing remain under investigation, 11,12,15,17,40,43 but a clinically applicable option is still pending. Both platelet-rich plasma (PRP) and mesenchymal stem cells (MSCs) have been investigated as a means to enhance tendon healing. PRP contains granules with growth factors necessary for healing, including cell proliferation and angiogenesis, platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, epidermal growth factor, and insulin-like growth factor, but further studies are needed to demonstrate intrinsic healing and tendon regeneration. 16,18,33,42 MSCs have been utilized to facilitate tendon regeneration, as these multipotent cells are known to be anti-inflammatory and support collagen production favoring intrinsic “scarless” healing. 6,16,26,51 Although there are several benefits in using MSCs, they are known to have a short half-life with a large operational cost, and they require stringent manufacturing processes. Additionally, MSCs bear an increased cost, with need for preservation of cell viability, storage, and handling. 51 An off-the-shelf, cell-free regenerative product not only would broaden the accessibility of intervention but also may prompt earlier application of therapy to avoid severe injury and protracted periods of rehabilitation.
TABLE 2.
| Variable | Extrinsic Healing | Intrinsic Healing |
|---|---|---|
| Predominant cell types | Inflammatory cells, fibroblasts | Tenocytes |
| Mechanism | Invasion of inflammatory cells from paratenon | Proliferation and migration of tenocytes from epitenon and endotenon |
| Angiogenesis | Increased | Decreased |
| Tensile strength | Inferior | Superior |
| Adhesions | Present, affects gliding | None |
Exosomes are nanoscale bilayer-enclosed extracellular vesicles (30-150 nm in diameter) that contain DNA, mRNA, microRNA, lncRNA, and multiple proteins, which can cross cellular boundaries and affect physiologic and pathologic processes of recipient cells. 10,21,41,43,50 MSCs have the capacity to mass produce exosomes; thus, exosomes have emerged as a cell-free strategy promoting tissue regeneration. In comparison with current cell-based and growth factor treatment options, exosomes are cell-free and have been shown to promote regeneration. However, scaling of MSC-derived exosomes and their purification is difficult and results in costs that are similar to those of stem cell therapy.
In this study, we utilized a novel approach to lyophilize purified exosome product (PEP) derived from activated platelets to treat an animal model of Achilles tendon rupture to investigate the effect of exosomes on tendon regeneration using mechanical and histological analysis. PEP has previously been shown to promote dermal regeneration 7 and, in a recent ex vivo study, has been shown to enhance tenocyte proliferation, migration, and tenogenesis. 31
Methods
Study Design
The current study was performed in the Comparative Medicine Laboratory at Mayo Clinic in Rochester, Minnesota. It was performed in young adult (age, 10-12 weeks) New Zealand White female rabbits with weight ranging from 2.7 to 3.0 kg. The study design included 3 groups, each consisting of 15 rabbits, for a total of 45 rabbits. Endpoints were at both 3 and 6 weeks based on predicate studies showing histological differences between these time points. 7,28 It has been shown that the transition from type 3 to type 1 collagen does not begin until the remodeling stage (6-10 weeks), which supports the selected time points. 28,36
Group 1 was the control group in which an Achilles tenotomy was performed, followed by standard suture repair. Group 2 underwent an Achilles tenotomy, followed by standard suture repair with a type 1 collagen scaffold applied at the repair site. Group 3 underwent an Achilles tenotomy, followed by standard suture repair with a type 1 collagen scaffold loaded with 20% PEP applied at the repair site. For each rabbit, only 1 Achilles tendon underwent surgery. Rabbits were not randomized to each group, and the investigator (E.P.W.) performing each surgery was not blinded to the rabbit’s assigned group. The order of surgical treatments was not randomized, as multiple vials of collagen and/or PEP were used for multiple rabbits in a row; therefore, it made more economical sense to perform surgeries from the same group sequentially.
Three weeks after surgery, 6 rabbits in each group were sedated and sacrificed via intravenous injection of Fatal Plus (Vortech). In each group, 3 specimens were used for histologic testing, and the remaining 3 were used for mechanical testing. The nonoperative contralateral Achilles tendons were also collected for comparative analysis. Six weeks after surgery, the remaining 9 rabbits in each group were sacrificed. In each group, 3 specimens were used for histologic testing, and the remaining 6 were used for mechanical testing. All rabbits who survived the entire assigned time point (3 or 6 weeks) were included for data analysis.
Scaffold Preparation
The scaffolds utilized for groups 2 and 3 were made from Research and Development grade type 1 fibrillar pH neutral collagen (50 mg/mL; Collagen Solutions). The scaffold for group 2 was made of collagen only, while the scaffold for group 3 was combined with PEP (Rion LLC) to achieve 1 × 10 11 exosome/mL concentration as previously described. 7,31 PEP has been shown to contain fibroblast growth factor–2, platelet-derived growth factor-BB, insulin-like growth factor–1, and transforming growth factor-β. 7
The scaffold preparation technique was designed so that it could be performed via a sterile technique intraoperatively to mimic the anticipated clinical scenario. For group 2, the scaffolds were created using an 80:20 ratio of type 1 fibrillar collagen (Collagen Solutions) and normal saline, and for group 3, the type 1 fibrillar collagen (Collagen Solutions) was mixed with PEP at a ratio of 80:20. This provided a scaffold that had a paste consistency, allowing it to be applied over the repaired tenotomy site.
Surgical Procedure
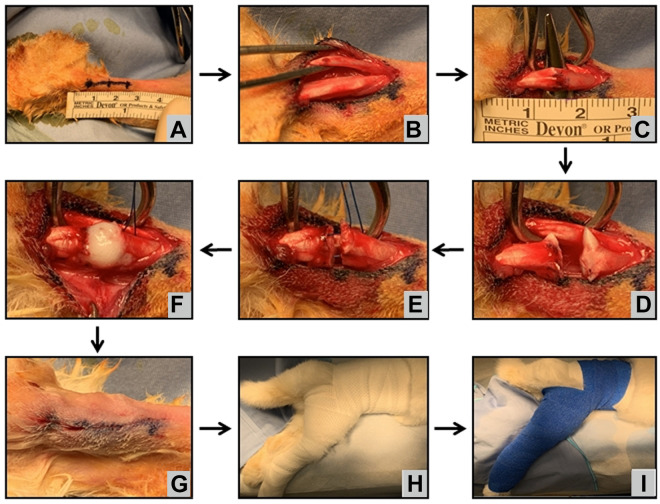
On the day of surgery, the rabbit received preoperative antibiotics and analgesia and was anesthetized via inhaled isoflurane gas, which was provided throughout the entire procedure. The Achilles tenotomy was performed through a 2-cm longitudinal incision that was marked starting approximately 0.5 cm proximal to the calcaneal tubercle (Figure 1A). Dissection was performed down to the flexor digitorum superficialis (FDS). The paratenon surrounding the FDS was incised. The FDS was isolated and retracted laterally to expose the Achilles tendon (Figure 1B). The Achilles tendon bundle was isolated and tenotomized approximately 1.5 cm proximal to the calcaneal tubercle (Figure 1, C and D). Care was taken not to cut the FDS, as the FDS was to act as an internal splint for the repair site. The 2 ends of the tendon were then repaired at approximately 150° of plantarflexion utilizing a modified Kessler core suture technique (Figure 1E). Minimal suture repair was desired, as the goal was to assess the effects of the scaffold rather than the strength of the suture repair, but the suture would help prevent initial gap formation. Suture repair was performed in the same fashion across all groups using a 5-0 polydioxanone suture (Ethicon) to allow for clean histologic assessment of specimens. In groups 2 and 3, approximately 0.2 mL of scaffold was placed topically at the tenotomy site before final tightening of the suture repair (Figure 1F). The solution rapidly gelled after application. The paratenon was not repaired. Skin was closed using 3-0 absorbable Vicryl suture (Ethicon) (Figure 1G). The hindlimb was placed in a hip spica–like cast from the toes to high into the groin, molding the ankle at 150° of plantarflexion (Figure 1H). The cast was then overwrapped using veterinary wrap from the toes up the leg and figure-8 wrapping around the abdomen (Figure 1I).
Figure 1.
Surgical technique demonstrating the rabbit positioned prone with the hindlimb prepared and draped. (A) A 2-cm incision centered 1.5 cm proximal to the calcaneal tubercle was performed. The paratenon was incised, and the (B) flexor digitorum superficialis and (C) Achilles tendon were identified and isolated. (D) Tenotomy was made through the Achilles tendon. (E) A modified Kessler core suture was performed in all groups. (F) The scaffold was placed at the tenotomy site for groups 2 and 3 before final suture tightening. (G) The incision was closed using absorbable suture, and (H, I) the hindlimb was placed in a hip spica–like cast at 150° for 3 to 6 weeks.
Mechanical Testing
Testing was performed on a servohydraulic testing machine (MTS Systems). 30 After sacrifice of the rabbit, the Achilles tendon was dissected from the hindlimb approximately 3 cm proximal to the myotendinous junction and sawed off distally at the calcaneal tubercle, ensuring a 1 × 1–cm bone block distally. The FDS was removed from the specimen as it would interfere with mechanical results.
The MTS fixture setup consisted of a toothed clamp distally and a slotted plate for the bone block proximally (Figure 2). The distal clamp was enhanced using dry ice to increase friction between the clamp and the specimen. 39 Before each test, the cross-sectional area of each specimen was measured using a calibrated linear caliper to obtain an anterior-to-posterior and medial-to-lateral measurement. The initial length of the specimen (L 0) was obtained after the specimen was mounted on the fixture as a clamp-to-clamp distance.
Figure 2.

MTS testing fixture. The calcaneal end of the tendon was seated in the slotted plate at the proximal end, while the musculotendinous junction was clamped distally. The distal clamp was frozen via dry ice to help increase friction between the clamp and the tissue. The flexor digitorum superficialis tendon was cut before testing, as it acted as an internal splint in vivo but would interfere with the mechanical testing ex vivo.
Outputs included ultimate tensile stress, load to failure, method and location of failure, strain, stiffness, and the Young modulus for all operative and multiple nonoperative samples. Ultimate tensile stress was defined as the maximum stress (force per unit area) before failure of the specimen. Load to failure was the maximum force applied that caused failure. Stiffness was a property based on the specimen structure and was based on the load applied and associated displacement. Strain was calculated based on the length of the specimen via clamp-to-clamp distance and displacement (ΔL) calculated from the MTS program.
Histologic Analysis
Tendons for the sacrificed rabbit specimens were then placed in 10% formalin solution before being embedded in paraffin. Tissue samples were cut longitudinally between 8 and 10 µm using a rotating microtome (Cryocut 1800; Leica Microsystems) and fixed on glass slides. After deparaffinization, specimens were stained with hematoxylin and eosin to assess cellularity as well as Mason trichrome to assess collagen content and organization. All slides were analyzed under light microscopy between ×10 and ×20 (Olympus DP25; Olympus America), and digital images were obtained (cellSens version 1.9; Olympus America). Tendon structure, collagen density, and cellularity were characterized from these stains. Tendon was graded both macroscopically and microscopically for adhesions using a validated adhesion grading scale as described by Tang et al 38 ; this scale was based on quantity (number of filaments) and quality (regularity and density) of adhesions. The grading was performed separately by 3 physicians (J.L., T.C.T.H., and E.P.W.). Scores for each specimen were then averaged across the 3 reviewers.
Immunohistochemistry
Specimens were prepared, paraffin embedded, cut, and deparaffinized. Antigen retrieval was not performed, as it was found to significantly alter the integrity of the tissue. Primary and secondary antibodies were applied in multiple combinations. Primary antibodies included anti–type 1 collagen (mouse monoclonal, 1:400; AB90395; Abcam), anti–type 3 collagen (goat polyclonal, 1:400; Southern Biotech), Ki-67 (mouse monoclonal, 1:100; Novus Biologicals), and P-selectin (sheep polyclonal, 1:100; R&D Systems). Secondary antibodies included Cy3 (goat anti-mouse polyclonal, 1:100; A10521; Invitrogen), Alexa Fluor 680 (donkey anti-sheep polyclonal, 1:100; A21102; Invitrogen), Alexa Fluor 555 (donkey anti-goat polyclonal, 1:400; ab150130; Invitrogen), and Alexa Fluor 647 (goat anti-mouse polyclonal, 1:400; ab150115; Invitrogen). Slides were mounted using a DAPI-enhanced glue (ProLong Gold; Invitrogen). Slides were analyzed under a contrast fluorescent microscope (Axio Observer Z1; Carl Zeiss Microscopy) using ×25 magnification. The stain intensity of type 1 and type 3 collagen, cellular proliferation, and presence of PEP were characterized from these slides.
Sample Size Justification
The sample size justification was based on the biomechanical outcome of failure load with variability estimates obtained from similar studies. 7,9 Predicting a similar variability in the current study, sample sizes of 3 per group at 3 weeks and 6 per group at 6 weeks would provide 80% power to detect differences between any 2 of the 3 study groups of at least 100 and 171 N, respectively.
Statistical Analysis
The statistical analysis focused primarily on comparing the 3 study groups separately at each of the 2 time points. Data comprising continuous variables were analyzed using 1-way analysis of variance. If the overall F test was significant, further analysis was conducted using an appropriate multiple-comparisons procedure to maintain the overall type 1 error rate. Categorical data were analyzed using chi-square tests. The interrater reliability for tendon adhesion grading was calculated using a simple coefficient of variance (σ/µ), which is a measure of the extent of variability in relation to the mean of the population that demonstrated the variability of a rater’s values relative to all raters’ values combined. The greater the value, the greater the variability from the mean. All statistical tests were 2-sided, and P values <.05 were considered statistically significant. Statistical analysis was performed using JMP for Windows version 14 (Cary, NC).
Results
Surgical Technique
Forty-four (98%) rabbits survived to their respective endpoints. One rabbit was euthanized on postoperative day 8 because of pain; during autopsy, it was found to have a contralateral dislocated patella. Eighteen (40%) rabbits required cast revisions (12 for slippage, 6 for swollen toes). Four rabbits from group 2 were found to have postoperative hematuria on postoperative day 1 that self-resolved. These 4 rabbits were part of a group of 9 rabbits from group 2 operated on the same day. The average weight loss was 0.21 ± 0.14 g without any significant difference among treatment groups (P = .49).
Mechanical Testing
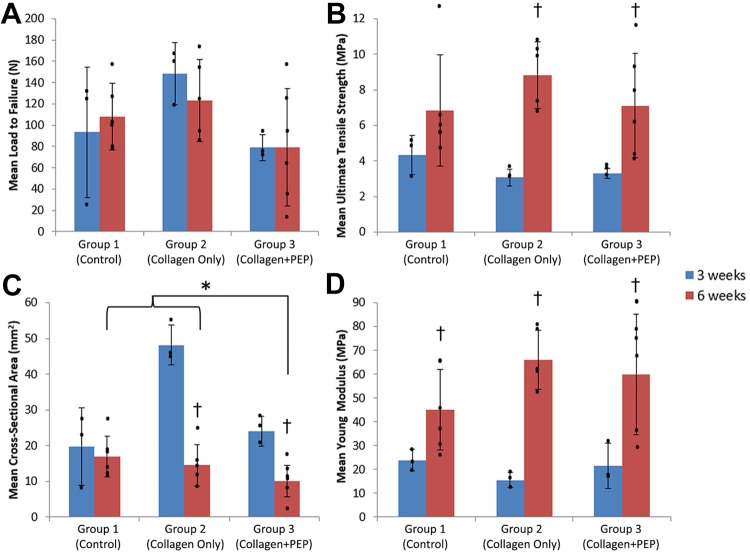
The failure load and ultimate tensile stress were found to be similar (P ≥ .15) across the 3 groups, although the ultimate tensile stress at 6 weeks was significantly higher than that at 3 weeks in the collagen and collagen+PEP groups (P < .05) (Table 3 and Figure 3, A and B). The cross-sectional area measured before MTS testing was found to be less (P = .04) for specimens in group 3 compared with groups 1 or 2 by the 6-week time point (Table 3 and Figure 3C). The Young modulus increased (P ≤ .03) over time for all groups (Table 3 and Figure 3D). There was a trend toward an increase in the Young modulus in PEP- and collagen-treated tendons relative to control tendons, but this was not significant. The most common failure mode was at the repair site (65%, n = 17) (Table 4). Other failure modes included calcaneal avulsion (n = 6) and slippage at the distal tooth clamp (n = 3).
Table 3.
Summary of Mechanical Data Performed for All Groups at Both 3 and 6 Weeks. a
| Group 1 (Control) | Group 2 (Collagen Only) | Group 3 (Collagen+PEP) | P Value (Between Groups) | ||||
|---|---|---|---|---|---|---|---|
| Mean | P Value (Between Time Points) | Mean | P Value (Between Time Points) | Mean | P Value (Between Time Points) | ||
| Load to failure, N | .73 | .34 | .99 | ||||
| 3 wk | 93.40 | 148.33 | 78.99 | .16 | |||
| 6 wk | 108.05 | 123.44 | 79.39 | .25 | |||
| Cross-sectional area, mm 2 | .71 | .0007 | .008 | ||||
| 3 wk | 19.79 | 48.19 | 23.96 | .007 | |||
| 6 wk | 16.96 | 14.54 | 10.05 | .04 | |||
| Stiffness, N/mm | .30 | .20 | .97 | ||||
| 3 wk | 16.88 | 17.96 | 18.98 | .95 | |||
| 6 wk | 26.32 | 26.19 | 18.75 | .37 | |||
| Ultimate tensile stress, MPa | .12 | .0014 | .02 | ||||
| 3 wk | 4.34 | 3.07 | 3.31 | .15 | |||
| 6 wk | 6.86 | 8.83 | 7.12 | .46 | |||
| Young modulus, MPa | .03 | .0004 | .014 | ||||
| 3 wk | 23.92 | 15.57 | 21.48 | .32 | |||
| 6 wk | 45.04 | 65.97 | 59.88 | .21 | |||
a Bolded P values indicate statistically significant differences in the data compared (P < .05). PEP, purified exosome product.
Figure 3.
(A) Load to failure and (B) ultimate tensile stress at 3 and 6 weeks for each group showed no significant difference between groups, although ultimate tensile stress increased significantly over time in groups 2 and 3 († P < .05). (C) Cross-sectional area decreased significantly in the purified exosome product (PEP)–treated group by 6 weeks (* P = .04). (D) The Young modulus increased over time for all groups († P ≤ .03). Dots indicate individual values; error bars indicate SDs.
TABLE 4.
Summary of Mechanical Data Performed and Failure Mode for Each Specimen a
| Group | Load to Failure, N | Cross-sectional Area, mm2 | Stiffness, N/mm | Ultimate Tensile Stress, MPa | Young Modulus, MPa | Failure Mode |
|---|---|---|---|---|---|---|
| 3 wk | ||||||
| Control | 122.44 | 23.80 | 15.87 | 5.14 | 28.71 | Calcaneal avulsion |
| Control | 134.70 | 28.08 | 28.71 | 4.80 | 23.09 | Tenotomy site |
| Control | 23.06 | 7.48 | 6.05 | 3.08 | 19.96 | Calcaneal avulsion |
| Collagen | 115.05 | 44.40 | 9.98 | 2.59 | 12.29 | Distal clamp |
| Collagen | 168.42 | 54.56 | 18.07 | 3.09 | 15.74 | Distal clamp |
| Collagen | 161.53 | 45.60 | 25.83 | 3.54 | 18.68 | Distal clamp |
| Collagen+PEP | 70.29 | 19.61 | 22.15 | 3.58 | 32.47 | Tenotomy site |
| Collagen+PEP | 93.02 | 27.88 | 16.00 | 3.34 | 15.79 | Tenotomy site |
| Collagen+PEP | 73.66 | 24.40 | 18.79 | 3.02 | 16.18 | Tenotomy site |
| 6 wk b | ||||||
| Control | 126.43 | 27.29 | 29.27 | 4.63 | 31.20 | Calcaneal avulsion |
| Control | 106.57 | 17.77 | 23.15 | 6.00 | 37.89 | Tenotomy site |
| Control | 100.42 | 18.36 | 15.33 | 5.47 | 25.83 | Calcaneal avulsion |
| Control | 78.39 | 12.24 | 16.76 | 6.40 | 44.76 | Calcaneal avulsion |
| Control | 77.15 | 13.95 | 29.95 | 5.53 | 64.93 | Tenotomy site |
| Control | 159.32 | 12.14 | 43.47 | 13.12 | 65.60 | Calcaneal avulsion |
| Collagen | 122.09 | 11.60 | 25.54 | 10.53 | 78.02 | Tenotomy site |
| Collagen | 91.50 | 14.14 | 23.75 | 6.47 | 60.73 | Tenotomy site |
| Collagen | 172.39 | 23.94 | 34.57 | 7.20 | 51.30 | Tenotomy site |
| Collagen | 149.96 | 14.70 | 25.28 | 10.20 | 59.92 | Tenotomy site |
| Collagen | 81.26 | 8.32 | 21.83 | 9.77 | 79.88 | Tenotomy site |
| Collagen+PEP | 157.67 | 13.76 | 36.31 | 11.46 | 88.76 | Tenotomy site |
| Collagen+PEP | 61.68 | 10.08 | 17.01 | 6.12 | 62.87 | Tenotomy site |
| Collagen+PEP | 13.34 | 3.20 | 3.11 | 4.17 | 23.59 | Tenotomy site |
| Collagen+PEP | 122.68 | 15.51 | 29.63 | 7.91 | 73.05 | Tenotomy site |
| Collagen+PEP | 89.74 | 9.90 | 18.69 | 9.07 | 76.13 | Tenotomy site |
| Collagen+PEP | 31.22 | 7.83 | 7.74 | 3.99 | 34.88 | Tenotomy site |
a PEP, purified exosome product.
b Only 5 specimens were analyzed for the 6-week collagen-only group, as 1 of the 6 was euthanized before the study time point because of pain from the contralateral dislocated patella.
Histologic Analysis
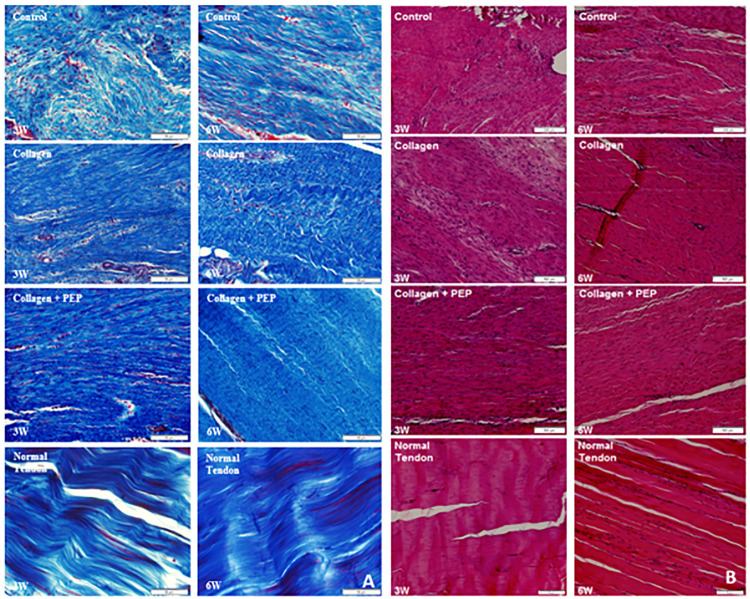
Six specimens from each group were analyzed histologically using both hematoxylin and eosin and Mason trichrome stains. Tendons treated with PEP were found to contain dense collagen fibers with parallel organization (Figure 4), more closely resembling normal tendon compared with the disorganized structure commonly found in groups 1 and 2. There appeared to be mature (flattened) nuclei in the PEP-treated groups, and over time (Figure 4) they approached the acellular-like nature of normal tendon.
Figure 4.
(A) Trichrome and (B) hematoxylin and eosin stains of specimens from each group at each time point as well as normal contralateral, untreated tendon. Images show more organized, denser collagen with less peripheral adhesions in the purified exosome product (PEP)–treated group, more closely resembling normal tendon.
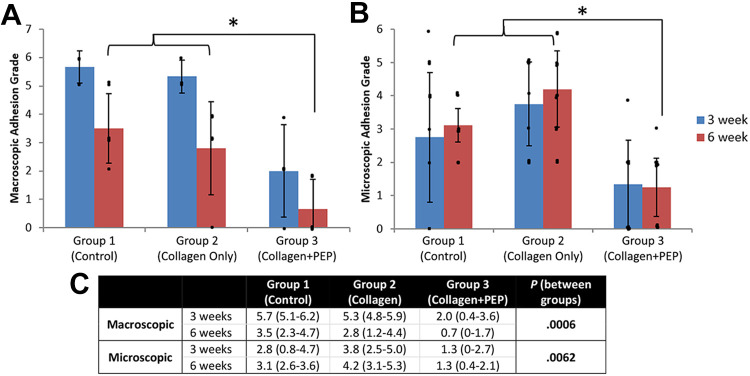
Tendon treated with PEP was found to have lower (P ≤ .006) adhesion grade both macroscopically and microscopically compared with groups 1 and 2 (Figures 5 and 6). The mean interrater variability coefficient between the 3 raters was –0.16, indicating low variance.
Figure 5.
Mean (A) macroscopic and (B) microscopic adhesion grades at 3 and 6 weeks for each group, according to the grading criteria of Tang et al. 38 Group 3 demonstrated significantly lower adhesion grade both macroscopically (*P = .0006) and microscopically (*P = .0062). Dots indicate individual values; error bars indicate SDs. (C) Data in tabular format. PEP, purified exosome product.
Figure 6.
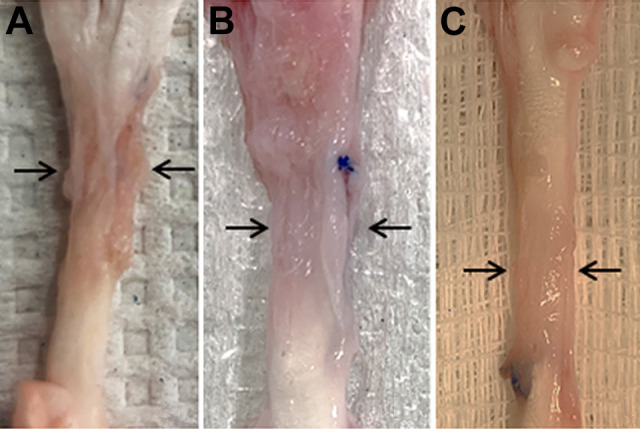
Images depicting tendon adhesions after dissection at the 6-week time point. Macroscopically, the adhesion grade was greater in the (A) control group and (B) collagen-only group compared with (C) the collagen and purified exosome product group (P = .0006).
Immunohistochemistry
Six specimens from each group were used for immunohistochemical analysis with multiple antibody combinations. After analysis under fluorescent microscopy, tendon treated with PEP was found to stain more similarly to normal tendon compared with groups 1 and 2 with regard to ratios of type 1 to type 3 collagen (Figure 7). Cellular proliferation was prominent across all groups as indicated by Ki-67 marker, while the antibody marker for PEP (P-selectin) was not visualized at either time point in group 3, indicating that exosomes had already been resorbed by the 3-week time point (Figure 8).
Figure 7.
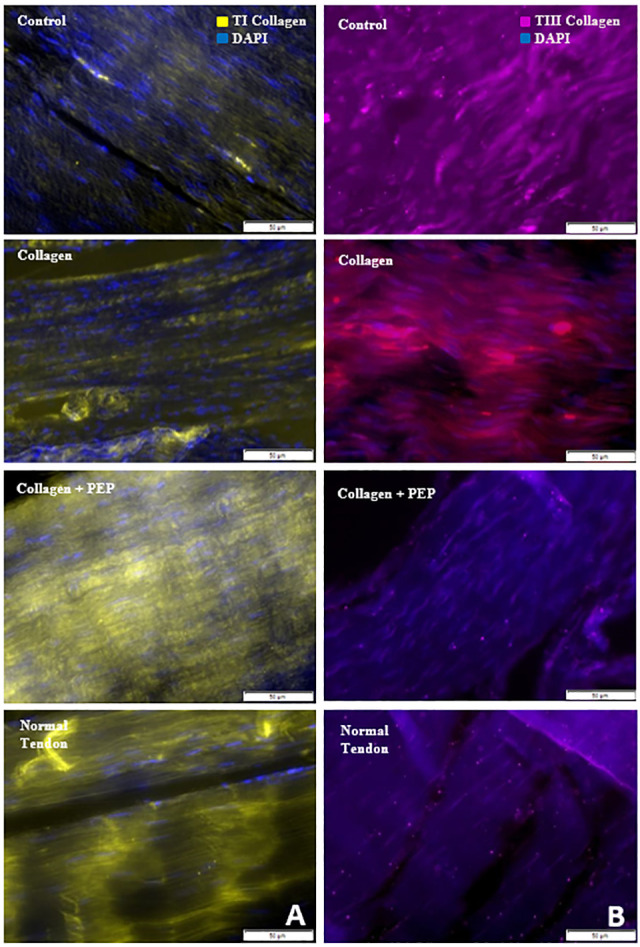
Immunohistochemical staining against (A) type 1 (TI) collagen and (B) type 3 (TIII) collagen for specimens from each group as well as normal contralateral, untreated tendon. Images show an increase in stain intensity for TI collagen and decreased stain intensity for TIII collagen with purified exosome product (PEP)–treated tendon, similar to the untreated normal tendon.
Figure 8.
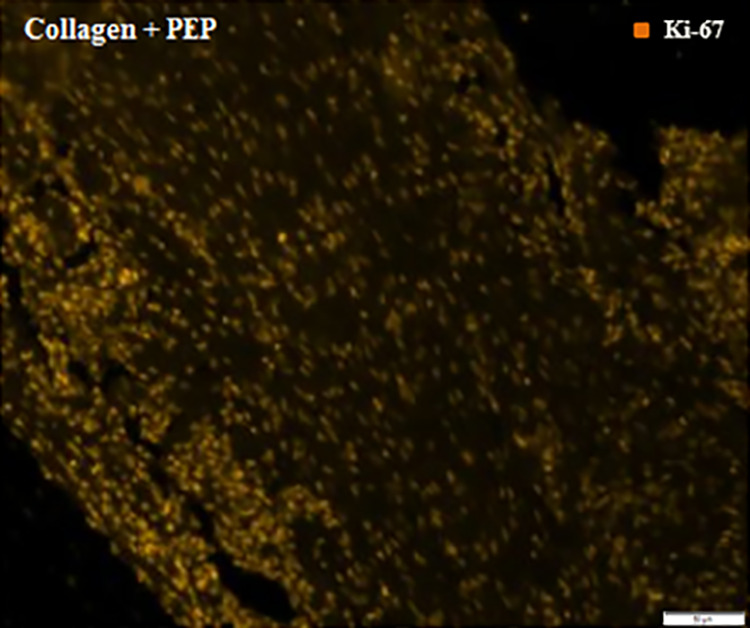
Immunohistochemical staining against P-selectin and Ki-67 for purified exosome product (PEP)–treated tendon. Images show immunoreactivity to Ki-67 but no reactivity for P-selectin, indicating all PEP exosomes had been reabsorbed by neighboring cells.
Discussion
Tendon healing can be intrinsic, extrinsic, or a combination of the 2. 34 Intrinsic healing (healing from within the tendon) provides superior outcomes in terms of mechanical strength and is more analogous histologically to native tissue. 34 Extrinsic healing, by definition, requires cells to migrate to the injury site from the surrounding tendon sheath or soft tissue. Tissues undergoing primarily extrinsic healing have shown a higher rate of rerupture due to a higher ratio of immature fibers and type 3 collagen. 8,34,39,52 In the current study, a collagen scaffold supplemented with PEP was compared with tendons treated with collagen or solely suture repair and was found to have a greater degree of intrinsic healing compared with the other groups. This finding was supported by both mechanical and histologic findings.
Native tendon has a high Young modulus and ultimate tensile stress. 34 Specifically, the human Achilles tendon is known to have an ultimate tensile stress of approximately 100 to 110 MPa. 5,25 Scar tissue formed via extrinsic healing has been shown to produce inferior material properties. 8,9,39,52 Burgisser et al 9 found a significant decrease in load to failure, ultimate tensile stress, and the Young modulus at both 3 and 6 weeks in rabbit Achilles tendons treated with primary suture repair compared with normal tendon. Studies 2,6,17,20 that have demonstrated tendon regeneration via stem cell therapy have shown relative improvement in tendon material properties. A study by Beredjiklian et al 6 investigated intrinsic versus extrinsic healing of tendon using fetal sheep tissue. They found that relative to healing in adult tissue, the ultimate stress and modulus recovered better with the fetal sheep tissue, suggesting that the presence of stem cells helped to improve the mechanical properties of the tendon. In the current study, the failure load and ultimate tensile stress were similar across all groups despite the smaller cross-sectional diameter of the PEP-treated tendons, suggesting an increase in stiffness in the PEP-treated tendons. There was a trend toward an increase in the Young modulus in tendons treated with collagen+PEP and collagen only relative to control tendons, but since both groups contained a collagen scaffold and there was no significant difference between the collagen scaffold groups, it is very likely the collagen scaffold may have positively affected the Young modulus. All these findings suggest that tendon repairs treated with PEP have the potential to favor intrinsic over extrinsic healing, but future studies providing additional supportive qualitative data are required.
Histologically, native tendon consists of approximately 80% type 1 collagen, up to 5% type 3 collagen, 2% elastin, minimal cellularity, and minimal vascularity. 4,34 Throughout the phases of extrinsic healing, studies have shown higher ratios of type 3 collagen, greater percentages of fibroblasts, and disorganized collagen architecture. 31,34 Type 3 collagen is known to be present at a higher percentage in scar tissue, while tendon undergoing intrinsic healing has a higher ratio of type 1 collagen. 8,31,34,39 A study by Qiu et al 32 found that tendon undergoing intrinsic healing had an upregulation of type 1 collagen and downregulation of type 3 collagen. In this current study, the architecture and staining of PEP-treated tendons was more analogous to that of native tendon when compared with tendons treated with collagen only and controls. This further supports the hypothesis that PEP may promote intrinsic healing.
Extrinsic tendon healing favors adhesion formation; such adhesions, while supporting tendon repair, lead to complications including limitation in motion, decreased tendon gliding, and possible pain. 22,24,34,38,40 Adhesion prevention after tendon repair has been an active area of research for several decades. Several therapies have been used to help prevent and/or treat adhesions, including nonsteroidal anti-inflammatory drugs, 37 5-fluorouracil, 1,27 and barrier sheaths, 22 but none have completely prevented adhesion formation. A study by Galatz et al 16 showed that the concept of “scarless” intrinsic healing could prevent the need for extrinsic healing and therefore adhesion formation. In this current study, it was found that tendon treated with PEP demonstrated less macroscopic and microscopic circumferential adhesions. This finding supports the hypothesis that PEP favors intrinsic healing.
In this study, PEP was applied using a collagen scaffold to aid in delayed release and structural support. Several types of grafts and scaffolds have been studied, but each with their own disadvantages. Autografts produce donor site morbidity, while allografts are decellularized, which inhibits regeneration potential and produces an inflammatory response. 17 Cell-mediated scaffolds have limited availability, can produce ectopic bone, and may be associated with tumor growth. 17 It has been well studied that tendon scaffold barriers made of type 1 collagen and glycosaminoglycan (TenoGlide; Integra) or type 1 and type 3 collagen (matrix-induced autologous chondrocyte implantation) reduce production of adhesion formation while allowing for diffusion of essential healing factors. 40,48 Collagen scaffolds loaded with PRP have been studied in dermal regeneration that improved cellular recruitment and induced stem cell differentiation. 18 Takamura et al 36 used PRP in an Achilles tendon model alone without a scaffold, and tendons were analyzed histologically at 1-week time points up to 6 weeks. They concluded that PRP shortened the inflammatory phase and accelerated healing in the proliferative phase and thus the remodeling phase began by week 3 compared with the usual week 6. 36 Although the study36 is promising, the use of PRP has yet to show evidence of inducing tendon regeneration. 16
There were several limitations to this study. Only 2 time points were chosen in order to increase sample size, specifically for mechanical testing. Using a collagen hydrogel has been shown to provide sustained release of PEP for up to 1 month 7,31 but is likely a product of how much hydrogel is placed at the site of injury. It would have been beneficial to include an earlier time point to show the early concentrations of PEP. By the 6-week time point, PEP is completely resorbed; therefore, no remaining vesicles would be seen on immunohistochemistry staining. It would be beneficial to both mechanically and histologically see the progression through the healing process by having an additional earlier and later time point to capture all stages of tendon healing. Another consideration for further studies would be to utilize rabbit tendon models that correlate with the fifth or sixth decade of a human Achilles tendon, which is the most common time period for tendon rupture. The tendon ages utilized in this study were based on predicate Achilles tendon rabbit models and weight. 7,14,36 Another limitation was that during the initial design of the study, it was not anticipated that PEP would have such a significant effect on adhesions; therefore, adhesion-specific mechanical testing to assess tendon gliding resistance was not performed. This study was performed in an Achilles tendon model that has different properties regarding adhesions compared with the more common flexor tendon model to evaluate adhesions. 52 Regarding immunohistochemistry, only a select few antibodies were chosen to characterize tendon regeneration. In future studies, it would be beneficial to include additional stains including tenomodulin and decorin as markers of tendon regeneration and to analyze PEP’s interactions with the inflammatory cascade to further characterize PEP’s mechanism of action.
Conclusion
This study has provided evidence suggesting that PEP may promote some degree of intrinsic tendon healing. This was supported by decreased adhesion production, an increased qualitative ratio of type 1 to type 3 collagen, and the demonstration of a more organized collagen architecture while maintaining an equivalent load to failure and ultimate tensile stress. Although the evidence appeared to favor intrinsic healing for PEP-treated tendons throughout the study, some of the data were subjective in nature, and a direct comparison to PEP’s effect in human tendon has not yet been fully described or supported. The production of a cell-free, off-the-shelf product that can promote tendon regeneration would provide a viable solution for physicians and patients to decrease pain, improve functional capacity, and thus accelerate return to work or sports. Given the lack of solutions for patients with tendon-related injury, the results from this study have provided a jump-start to future animal and eventual clinical studies to support clinical translation of this technology to patients with chronic disabling tendon disease.
Acknowledgment
The authors give special thanks to the Department of Comparative Medicine and Medical Sciences Surgical Center at Mayo Clinic for their assistance and care of the animals in this study. The Mayo Orthopedic Surgery Residency, under the leadership of Mark W. Pagnano, MD, Norman S. Turner, MD, Jonathon D. Barlow, MD, and Matthew P. Abdel, MD, has developed and maintained this special educational opportunity, which provided this research opportunity.
Footnotes
Final revision submitted July 16, 2021; accepted September 3, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was funded by the Orthopedic Research Review Committee (ORRC) as well as the Obaid Vascularized Composite Tissue Award (E.P.W, S.L.M., M.T.H.). All PEP vials were manufactured and supplied by Rion LLC at the Advanced Product Incubator (API) at Mayo Clinic. A.R. has received hospitality payments from Medtronics. A.B. has received hospitality payments from Abiomed. S.L.M. has received consulting fees from Ascension Orthopedics and Integra LifeSciences, nonconsulting fees from Integra LifeSciences, and royalties from Integra LifeSciences. M.T.H. has received consulting fees from Daiichi Sankyo and hospitality payments from Linkbio, Medical Device Business Services, Stryker, and Zimmer Biomet. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Mayo Clinic Institutional Animal Care and Use Committee (A00004403).
References
- 1. Akali A, Khan U, Khaw PT, McGrouther AD. Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Reconstr Surg. 1999;103(1):151–158. [DOI] [PubMed] [Google Scholar]
- 2. Angelidis IK, Thorfinn J, Connolly ID, et al. Tissue engineering of flexor tendons: the effect of a tissue bioreactor on adipoderived stem cell-seeded and fibroblast-seeded tendon constructs. J Hand Surg Am. 2010;35(9):1466–1472. [DOI] [PubMed] [Google Scholar]
- 3. Barfod KW, Bencke J, Lauridsen HB, et al. Nonoperative dynamic treatment of acute Achilles tendon rupture: the influence of early weight-bearing on clinical outcome: a blinded, randomized controlled trial. J Bone Joint Surg Am. 2014;96(18):1497–1503. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212(3):211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett MB, Ker RF, Dimery NJ, Alexander RM. Mechanical-properties of various mammalian tendons. J Zool. 1986;209:537–548. [Google Scholar]
- 6. Beredjiklian PK, Favata M, Cartmell JS, et al. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143–1152. [DOI] [PubMed] [Google Scholar]
- 7. Bowers MH, Huang TC, Sabbagh MD, Moran SL. Application of purified exosome product improved wound healing in ischemic wounds: a rabbit model. Presented at: Academic Surgical Congress; 2019. Accessed November 25, 2021. https://www.asc-abstracts.org/abs2019/66-08-application-of-purified-exosome-product-improved-wound-healing-in-ischemic-wounds-a-rabbit-model/
- 8. Bruns J, Kampen J, Kahrs J, Plitz W. Achilles tendon rupture: experimental results on spontaneous repair in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):364–369. [DOI] [PubMed] [Google Scholar]
- 9. Burgisser GM, Calcagni M, Bachmann E, et al. Rabbit Achilles tendon full transection model—wound healing, adhesion formation and biomechanics at 3, 6 and 12 weeks post-surgery. Biology Open. 2016;5:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain CS, Clements AEB, Kink JA, et al. Extracellular vesicle-educated macrophages promote early Achilles tendon healing. Stem Cells. 2019;37(5):652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaudhury S. Mesenchymal stem cell applications to tendon healing. Muscles Ligaments Tendons J. 2012;2(3):222–229. [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke AW, Alyas F, Morris T, et al. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med. 2011;39(3):614–623. [DOI] [PubMed] [Google Scholar]
- 13. Costa ML, Achten J, Marian IR, et al. Plaster cast versus functional brace for non-surgical treatment of Achilles tendon rupture (UKSTAR): a multicentre randomised controlled trial and economic evaluation. Lancet. 2020;395(10222):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doherty GP, Koike Y, Uhthoff HK, Lecompte M, Trudel G. Comparative anatomy of rabbit and human Achilles tendons with magnetic resonance and ultrasound imaging. Comp Med. 2006;56(1):68–74. [PubMed] [Google Scholar]
- 15. Evrova O, Kellenberger D, Calcagni M, Vogel V, Buschmann J. Supporting cell-based tendon therapy: effect of PDGF-BB and ascorbic acid on rabbit Achilles tenocytes in vitro. Int J Mol Sci. 2020;21(2):458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA. Tendon regeneration and scar formation: the concept of scarless healing. J Orthop Res. 2015;33(6):823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaspar D, Spanoudes K, Holladay C, Pandit A, Zeugolis D. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. 2015;84:240–256. [DOI] [PubMed] [Google Scholar]
- 18. Houdek MT, Wyles CC, Stalboerger PG, et al. Collagen and fractionated platelet-rich plasma scaffold for dermal regeneration. Plast Reconstr Surg. 2016;137(5):1498–1506. [DOI] [PubMed] [Google Scholar]
- 19. Josza L, Lehto MU, Jarvinen M, et al. A comparative study of methods for demonstration and quantification of capillaries in skeletal muscle. Acta Histochem. 1993;94(1):89–96. [DOI] [PubMed] [Google Scholar]
- 20. Juncosa-Melvin N, Shearn JT, Boivin GP, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12(8):2291–2300. [DOI] [PubMed] [Google Scholar]
- 21. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khanna A, Friel M, Gougoulias N, Longo UG, Maffulli N. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85–109. [DOI] [PubMed] [Google Scholar]
- 23. Lantto I, Heikkinen J, Flinkkila T, et al. Early functional treatment versus cast immobilization in tension after Achilles rupture repair: results of a prospective randomized trial with 10 or more years of follow-up. Am J Sports Med. 2015;43(9):2302–2309. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Thoreson A, Kadar A, Moran S, Zhao C. Evaluation of hollow mesh augmentation on the biomechanical properties of the flexor tendon repaired with modified Kessler technique. J Orthop Translat. 2020;20:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maganaris CN, Narici MV, Maffulli N. Biomechanics of the Achilles tendon. Disabil Rehabil. 2008;30(20-22):1542–1547. [DOI] [PubMed] [Google Scholar]
- 26. Martin P, D’Souza D, Martin J, et al. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:112–1128. [DOI] [PubMed] [Google Scholar]
- 27. Moran SL, Ryan CK, Orlando GS, Pratt CE, Michalko KB. Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg Am. 2000;25(2):242–251. [DOI] [PubMed] [Google Scholar]
- 28. Nagasawa K, Noguchi M, Ikoma K, Kubo T. Static and dynamic biomechanical properties of the regenerating rabbit Achilles tendon. Clin Biomech (Bristol, Avon). 2008;23(6):832–838. [DOI] [PubMed] [Google Scholar]
- 29. Ochen Y, Beks RB, van Heijl M, et al. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ. 2019;364:k5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Probst A, Palmes D, Freise H, Langer M, Joist A, Spiegel HU. A new clamping technique for biomechanical testing of tendons in small animals. J Invest Surg. 1999;13:313–318. [DOI] [PubMed] [Google Scholar]
- 31. Qi J, Liu Q, Reisdorf RL, et al. Characterization of a purified exosome product and its effects on canine flexor tenocyte biology. J Orthop Res. 2020;38(8):1845–1855. [DOI] [PubMed] [Google Scholar]
- 32. Qiu Y, Wang X, Zhang Y, et al. In vitro two-dimensional and three-dimensional tenocyte culture for tendon tissue engineering. J Tissue Eng Regen Med. 2016;10(3):E216–E226. [DOI] [PubMed] [Google Scholar]
- 33. Schepull T, Kvist J, Norrman H, et al. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38–47. [DOI] [PubMed] [Google Scholar]
- 34. Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. [DOI] [PubMed] [Google Scholar]
- 35. Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Joint Surg Am. 2012;94(23):2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takamura M, Yasuda T, Nakano A, Shima H, Neo M. The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop Traumatol Turc. 2017;51(1):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan V, Nourbakhsh A, Capo J, et al. Effects of nonsteroidal anti-inflammatory drugs on flexor tendon adhesion. J Hand Surg Am. 2010;35(6):941–947. [DOI] [PubMed] [Google Scholar]
- 38. Tang JB, Ishii S, Usui M, Aoki M. Dorsal and circumferential sheath reconstructions for flexor sheath defect with concomitant bony injury. J Hand Surg Am. 1994;19(1):61–69. [DOI] [PubMed] [Google Scholar]
- 39. Thermann H, Frerichs O, Biewener A, Krettek C. Healing of the Achilles tendon: an experimental study. Foot Ankle Int. 2001;22(6):478–483. [DOI] [PubMed] [Google Scholar]
- 40. Turner JB, Corazzini RL, Butler TJ, Garlick DS, Rinker BD. Evaluating adhesion reduction efficacy of type I/III collagen membrane and collagen-GAG resorbable matrix in primary flexor tendon repair in a chicken model. Hand (N Y). 2015;10(3):482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 42. Wang X, Qiu Y, Triffitt J, et al. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J Orthop Res. 2012;30(6):982–990. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, He G, Guo Y, et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med. 2019;23(8):5475–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weber M, Niemann M, Lanz R, Muller T. Nonoperative treatment of acute rupture of the Achilles tendon: results of a new protocol and comparison with operative treatment. Am J Sports Med. 2003;31(5):685–691. [DOI] [PubMed] [Google Scholar]
- 45. Wilkins R, Bisson LJ. Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am J Sports Med. 2012;40(9):2154–2160. [DOI] [PubMed] [Google Scholar]
- 46. Willits K, Amendola A, Bryant D, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92(17):2767–2775. [DOI] [PubMed] [Google Scholar]
- 47. Wu F, Nerlich M, Docheva D. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yannas IV. Studies on the biological activity of the dermal regeneration template. Wound Repair Regen. 1998;6(6):518–523. [DOI] [PubMed] [Google Scholar]
- 49. Young SW, Patel A, Zhu M, et al. Weight-bearing in the nonoperative treatment of acute Achilles tendon ruptures: a randomized controlled trial. J Bone Joint Surg Am. 2014;96(13):1073–1079. [DOI] [PubMed] [Google Scholar]
- 50. Yu H, Cheng J, Shi W, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328–341. [DOI] [PubMed] [Google Scholar]
- 51. Zhang S, Chuah SJ, Lai RC, et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. [DOI] [PubMed] [Google Scholar]
- 52. Zhao C, Ozasa Y, Reisdorf RL, et al. CORR® ORS Richard A. Brand Award for Outstanding Orthopaedic Research: engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res. 2014;472(9):2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao X, Jiang S, Liu S, et al. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials. 2015;61:61–74. [DOI] [PubMed] [Google Scholar]