Abstract
The diagnosis and therapy of Helicobacter pylori infection have undergone major changes based on the use the principles of antimicrobial stewardship and increased availability of susceptibility profiling. H. pylori gastritis now recognized as an infectious disease, as such there is no placebo response allowing outcome to be assessed in relation to the theoretically obtainable cure rate of 100%. The recent recognition of H. pylori as an infectious disease has changed the focus to therapies optimized to reliably achieve high cure rates. Increasing antimicrobial resistance has also led to restriction of clarithromycin, levofloxacin, or metronidazole to susceptibility-based therapies. Covid-19 resulted in the almost universal availability of polymerase chain reaction testing in hospitals which can be repurposed to utilize readily available kits to provide rapid and inexpensive detection of clarithromycin resistance. In the United States, major diagnostic laboratories now offer H. pylori culture and susceptibility testing and American Molecular Laboratories offers next-generation sequencing susceptibility profiling of gastric biopsies or stools for the six commonly used antibiotics without need for endoscopy. Current treatment recommendations include (a) only use therapies that are reliably highly effective locally, (b) always perform a test-of-cure, and (c) use that data to confirm local effectiveness and share the results to inform the community regarding which therapies are effective and which are not. Empiric therapy should be restricted to those proven highly effective locally. The most common choices are 14-day bismuth quadruple therapy and rifabutin triple therapy. Prior guidelines and treatment recommendations should only be used if proven locally highly effective.
Keywords: adherence, antibiotics, bismuth, culture, global antimicrobial resistance, Helicobacter pylori, molecular susceptibility testing, proton pump inhibitors, test-of-cure, therapy, vonoprazan
Introduction
Initially, Helicobacter pylori gastritis was largely considered in relation to its role in peptic ulcer disease, which was then one of the major and most important gastrointestinal diseases. H. pylori treatment studies done for approval by the US Food and Drug Administration (FDA) required the presence of peptic ulcer and were assigned to the Gastroenterology section. As with other ulcer studies, the focus was on healing of ulcers rather than cure of the infection.1,2 A comparator was required because ulcers healed and recurred spontaneously (i.e. a definite placebo response was present). Only during the recent development of the rifabutin triple therapy, Talicia, did the FDA permit H. pylori therapy studies based only on the presence of the infection.3,4 Although a comparator was required, approval did not include the requirement of the optimized regimen. It appears that the FDA is slowly making the transition to dealing with H. pylori as an infectious disease of the gastrointestinal tract.
H. pylori infections are typically lifelong and rarely spontaneously disappear until the gastric mucosa replaced by intestinal metaplasia. There is no placebo response to therapy. With optimized current therapies and adherent patients with susceptible infections, one can reliably achieve ⩾95% cure rates.5–7 The ability to obtain cure rates of nearly 100% and lack of a placebo response obviates the need for a comparator other than the theoretically possible, 100% cured. This differs from the majority of gastrointestinal diseases where the etiology is unknown, cure is not possible, and there is a strong placebo effect that requires that therapies for typical gastroenterology diseases include a comparator and often a placebo as the outcome is often based on a change in symptoms or disease activity scored using a validated scoring instrument. Treatment success is determined by comparison with a poorly performing legacy therapy and often a placebo. Cures are not expected and techniques such as meta-analysis are needed to compare regimens. In contrast, with infectious diseases, cures are expected, there is no placebo response, and outcomes are scored in terms of comparison with the theoretical 100% cure rate. Comparisons are typically restricted to highly successful therapies using tests of noninferiority. We hope that soon both the FDA and Gastroenterology will embrace treatment of H. pylori as an infectious disease and focus on results with susceptible infection using therapies optimized to achieve cure rates near the theoretically possible cure rate of 100%.
Although H. pylori was formally declared an infectious disease in 2015, 8 this change in status has yet to be reflected in treatment recommendations or conduct and analyses of clinical studies. In infectious diseases, antibiotics are never administered to patients known to be resistance to them and additional antibiotics are not added to a therapy with the hope that the infection will be susceptible to at least one of them. 9 Current H. pylori treatment guidelines still deal with H. pylori gastritis as if it were a typical gastroenterology disease such as constipation (i.e. of largely unknown etiology, impossible to cure, treatable but with relative low expectations, and with a high placebo response). 10 This has resulted in a focus on randomized comparative trials where differences in cure rate rather than actual cure rates are considered the most important outcome measure. 9 In reality, most comparative studies compare therapies with the same name (e.g. bismuth quadruple therapy rather than on their actual components) and ignore the differences in details of drug administration that allows factors such as the prevalence of resistance, duration of therapy, and relative potency of the antisecretory drug to greatly influence outcome. An example, in a recent network meta-analysis, the cure rates with most of the clarithromycin triple therapy comparator ranged were clinically unacceptable, ranging from 32% to 92%, with 76% below 85%. 11 Comparative trials also typically do not take antimicrobial resistance or its effects on outcome into account. 9 As such, the results of most clinical trials and meta-analyses fail to provide useful information or to provide material from which to derive meaningful guidance for the management of specific infections in specific patients. As noted above, this problem is a residual of the history of attempting to force an infectious disease into a gastroenterology mold.
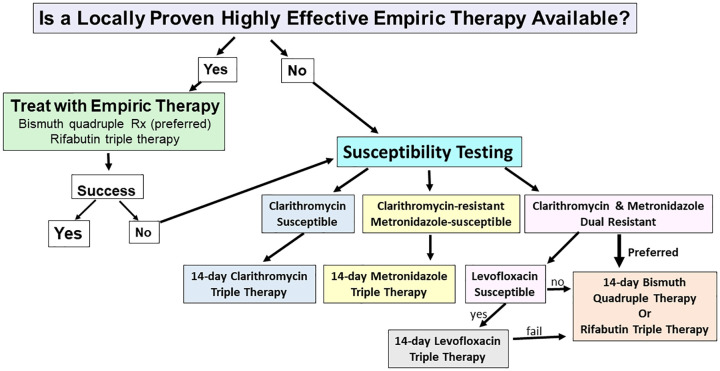
The recent rapid increase in the availability of susceptibility testing has taken us the cusp of integrating susceptibility-based H. pylori therapy into our daily practice. We anticipate that practice will rapid evolve to a combination of use of proven locally highly effective empiric therapies and susceptibility-based therapy (Figure 1). 12 The goal of this article is to review the issues involved in the choice of an effective therapy of patients with H. pylori infections and provide specific guidance that will reliably produce high cure rates. We begin with the problem of antimicrobial resistance.
Figure 1.
Proposed algorithm for selection of Helicobacter pylori regimen based upon knowledge of the results of empiric first-line therapies, and the results of susceptibility testing.
Source: Adapted from Graham and Moss 12 , with permission.
Emergence of resistance during therapy
The earliest trials to find an effective therapy identified that emergence of antimicrobial resistance during H. pylori therapy as a significant problem (i.e. with ofloxacin 13 ). Emergence of resistance was soon a major problem preventing effective therapy with metronidazole14–16 and, subsequently, with clarithromycin.17,18 This early period was one of experimentation especially omeprazole with the antibiotics amoxicillin, metronidazole/tinidazole, and clarithromycin (reviewed in Axon 19 ). Amoxicillin was noted to enhance the effectiveness of clarithromycin and metronidazole, but the general lack of susceptibility testing before and after therapy prevented recognition that one of its major effects was prevention of emergence of resistance during therapy. The problem of emergence of clarithromycin resistance was evident in the trials of clarithromycin monotherapy.20,21 Clarithromycin plus omeprazole 22 was the subject of an FDA submission that failed largely because of emergency of clarithromycin resistance.2,6,23 A study of clarithromycin and amoxicillin (plus ranitidine for ulcer healing) showed that emergence of resistance was either prevented or greatly reduced by the addition of amoxicillin. 24 The benefit of the addition of amoxicillin was confirmed in clinical trials of clarithromycin plus amoxicillin and omeprazole or lansoprazole, both of which became FDA-approved regimens. 23 This experience eventually resulted in H. pylori triple therapies consisting of an antisecretory drug, amoxicillin, and the second antibiotic such as clarithromycin, metronidazole, levofloxacin, or rifabutin triple therapies.
The importance of understanding the mechanism of treatment failure
While clarithromycin-containing therapy was being developed for FDA approval, others were experimenting with it.19,25 This included an abstract using the triple combination of clarithromycin, amoxicillin, and omeprazole that tested antimicrobial susceptibility only before treatment and reported that the therapy was successful for susceptible infections. 25 In contrast, as shown above, the keys to understanding a therapy include testing susceptibility both before and in treatment failures as was done in the FDA submissions reported above. The general lack of susceptibility testing both before therapy and in the treatment failures has largely been responsible for failure of early development of highly effective regimens. The most common causes of treatment failure include the presence of antimicrobial-resistant organisms, emergence of resistance during treatment, ineffectiveness of antisecretory therapy, improper drug doses or dosing intervals, too short a duration of therapy, and poor patient adherence to therapy. Failure to attempt to identify the cause of treatment failures and to simply to score outcomes by comparison with another therapy has largely been responsible for long delay in identifying uniformly highly effective therapies or being able to improve poorly performing regimens. Low-dose amoxicillin largely eliminated the emergence of resistance during therapy with clarithromycin, metronidazole, and levofloxacin. Whether it also enhanced therapy by acting as an additional antibiotic is unclear, but if so, the contribution was small as shown by examination of outcome using the H. pylori nomogram.26,27 In the presence of clarithromycin resistance, the additional benefit of amoxicillin was shown to be primarily related to the degree of acid suppression that improves the effect on amoxicillin on both the proton pump inhibitor (PPI) or vonoprazan (VPZ) dual therapy component.28,29 As seen in the original VPZ trial, both PPI and VPZ triple therapies achieved 98% cure rates with susceptible infection.28–30
Although clarithromycin is still widely used to treat H. pylori, by 2000 (i.e. more than 20 years ago), increasing resistance resulted in clarithromycin triple therapy becoming generally ineffective.31,32 Despite its poor clinical effectiveness, however, it remains one of the most utilized combinations.33,34 Currently, the prevalence of resistance precludes the use of triple therapy with clarithromycin, fluoroquinolones, or metronidazole except as susceptibility-based therapies. 6
Choice of therapy
A basic rule in the treatment of infectious disease is to prescribe only therapies empirically in which high local effectiveness has been both proven and confirmed. The three approaches to identifying such therapies are antibiotic use history, susceptibility testing, and personal or local experience. Only the results obtained locally are important and national and society guidelines for H. pylori should be ignored unless their recommendations coincide with local experience.
Role of antibiotic use history in antimicrobial selection
Antimicrobial resistance is the main reason for failure of otherwise successful antimicrobial therapies. The present of antibiotic resistance within a population is reflected on the amount of its use within that population.35–44 Fluoroquinolone, macrolide, and metronidazole resistance are now almost universal, whereas resistance remains rare for amoxicillin, tetracycline, rifabutin, and furazolidone. The same principles used to identify resistance in populations are also applicable for individuals making review of each individual’s antimicrobial use history and pharmacy records especially useful for identifying which antibiotics to avoid.35,40,44
These recommendations were initially useful for ensuring successful empiric use of clarithromycin, metronidazole, or fluoroquinolones. The prevalence of resistance to these antibiotics, however, has increased to the point that it is now best to restrict their use to susceptibility-based therapy. Treatment success should be always be confirmed for each individual by a test-of-cure to confirm these drugs remain highly effective (e.g. cure rates of ⩾90%, locally).6,7,45–47
Therapy should also be preceded by a formal patient education/counseling regarding the therapy, potential side effects, and importance of completing the entire course.48–52 Written instructions are particularly helpful. Based in part on the successful program used by Wink de Boer for prescribing bismuth quadruple therapy, 53 we recommend establishing a trust-based doctor–patient relationship as outlined in Table 1.49,53
Table 1.
| Take a detailed medical history and adequate time for follow-up visits |
| Explain in simplistic terms the effects of the infection on the stomach, the potential outcomes of the infection, and how cure of the infection results in healing of the damage and prevention of ulcers and ulcer recurrences and reducing or eliminating the risk of gastric cancer. |
| Provide a description of the complexities of the regimen chosen, the necessity for adherence to the treatment schedule, and completing the regimen, including a commitment to try to complete the regimen. |
| Provide a clear written description of the medications and plan for dosing and, if possible, providing appropriate containers (pill boxes or blister packs) arranged according to the dosing plans in relation to meals and bedtime. |
| Describe the adverse effects, such as feeling unwell (nausea, headaches, taste disturbances, loose stools, etc.), which are expected as a consequence of the treatment. |
| Provide a contact available after hours and weekends that can answer questions to ensure adherence. |
| Adherence should be monitored by discussion and by counting the remaining pills, which preferably should be returned by the patient at the end of the treatment. |
| A test-of-cure should be done 4 or more weeks after therapy ensure cure and provide feedback on the local effectiveness of the therapy utilized. |
Test-of-cure
The classic three-step approach to H. pylori therapy is accurate diagnosis of an active infection, therapy, and confirmation of cure. It is critical that each course of therapy be followed by a test-of-cure, preferably using noninvasive testing such as the stool antigen or urea breath test (UBT). To prevent false negative results, care must be taken to follow the recommendations regarding stopping PPIs, antibiotics, and bismuth for an adequate time for testing. The test-of-cure provides direct and immediate feedback regarding outcome and informs clinicians regarding which therapies are successful and which are failing. 40 Ideally, test-of-cure data would also be shared with colleagues to expand the local and regional knowledge base. It retrospect, it is amazing that this simple approach was not immediately recognized as a critical element able to provide immediate and local feedback to clinicians. As such, decades of local treatment failures have been recorded and published as observations rather than as critical opportunities for providing better care.6,7,33,54
Susceptibility testing
With most other infectious diseases, the local susceptibility patterns are known, susceptibility testing is readily available at local laboratories and hospitals, and the data are collected and updated local/regional lists of antibiotic of choice are made available. Susceptibility testing for H. pylori has recently become increasingly available.
In Europe
As noted by Francis Megraud, the Covid-19 epidemic resulted in establishing polymerase chain reaction (PCR) testing in essentially all local hospitals and testing laboratories. In Europe, a variety of inexpensive kits are available for testing gastric biopsies or stools for clarithromycin resistance [e.g. Amplidiag H. pylori + ClariR (Mobidiag; Espoo, Finland), the RIDA GENE Helicobacter pylori assay (R-Biopharm AG, Darmstadt, Germany), H. pylori ClariRes (Ingenetix, Vienna, Austria), Allplex H. pylori, ClariR (Seegene, Seoul, Korea), the Lightmix H. pylori (TIB; Molbiol, Germany), and the H. pylori TaqMan real-time PCR assay (Meridian Bioscience, Cincinnati, Ohio, USA)].55–61 The Genotype HelicoDR assay (Hain Lifescience, Nehren, Germany) has been used for both clarithromycin and levofloxacin resistance of gastric biopsies but proved less effective for levofloxacin testing of stools.62–64 Clinicians should encourage the local hospital/laboratory to incorporate PCR-based susceptibility testing at least for clarithromycin. Culture and susceptibility is also available internationally from Microbiology Specialists Inc (Houston, Texas, USA). Next-generation sequencing (NGS) of stools for six antibiotics (amoxicillin, clarithromycin, metronidazole, tetracycline, rifabutin, and levofloxacin) is also available by post from American Molecular Laboratories (Vernon Hills, Illinois, USA).
The United States
Culture and susceptibility testing for H. pylori is now available in the Unites States from major diagnostic laboratories, including Mayo Clinic Laboratories (HELIS), ARUP laboratories (MC HPYL), Labcorp (180885), and Quest Diagnostics (36994) and from Microbiology Specialists Inc. Molecular susceptibility testing using NGS is available from American Molecular Laboratories that provides resistance testing for six commonly used antibiotics: clarithromycin, amoxicillin, tetracycline, metronidazole, rifabutin, and levofloxacin. Importantly, NGS susceptibility testing is available for gastric biopsies, formalin-fixed gastric biopsies, or stools. The turnaround time for culture and susceptibility testing is about 2 weeks, whereas it is up to 5 working days for NGS.
Recently, Mayo Clinic Laboratories has added molecular testing for clarithromycin resistance as part of its H. pylori stool testing service. With this service, if the stool test is positive, they will do automatic (reflexive) testing for clarithromycin resistance using PCR. It behooves clinicians to query their local laboratories about the possibility of offering reflexive stool susceptibility testing at least for clarithromycin. As noted above, reflexive stool testing using NGS is available from American Molecular Laboratories and is not limited to clarithromycin as it provides NGS results for amoxicillin, clarithromycin, metronidazole, tetracycline, rifabutin, and levofloxacin. Molecular testing of stools is theoretically ideal as it is noninvasive (i.e. endoscopy is not required) and the results are rapidly available. Susceptibility testing takes the guess work out of selection of a patient-specific regimen.
Overcoming barriers to successful therapy
Important barriers preventing effective therapy include the inoculum effect, the persister state, and intragastric acidity. The inoculum effect refers to the fact that the number of H. pylori in the stomach is truly immense such that statistically a small proportion of organisms are likely to be resistant to antimicrobial such as metronidazole, clarithromycin, and fluoroquinolones. 65 As noted above, this was observed clinically as emergence of resistance during therapy with monotherapies with these antibiotics and led to the addition of amoxicillin to reduce the overall population and thus the odds that the low prevalence populations of resistant organisms would survive to prevent effective therapy. Bismuth has a similar effect. Hybrid therapy uses a run-in of PPI plus amoxicillin dual therapy, followed by a 7-day concomitant therapy. In this instance, one can consider the run-in as aiming to reduce the low prevalence populations of clarithromycin- or metronidazole-resistant organisms and thus result in an improved outcome with shorter exposure to multiple antibiotics. The persister effect is largely related to the fact that H. pylori only replicates when the pH in its local environment is approximately 6.65,66 At lower pH, the organism becomes resistant to antibiotics that require replication to be effective such as penicillin. The traditional approach to the persister effect is to prolong the duration of therapy (an example is the treatment of tuberculosis). 67 A basic rule is that if the treatment failed and the organism is not resistant to the antibiotic used, the duration of therapy was insufficient. The ability to reliably control intragastric pH with the new potassium-competitive acid blockers (P-CABs) forms the basis for potential highly successful amoxicillin dual therapy. pH control is also important in preventing destruction of some antibiotics during passage through the stomach. 68
The current choices for effective empiric therapy are bismuth quadruple therapy, amoxicillin dual therapy, rifabutin triple therapy, and furazolidone triple or quadruple therapies. Furazolidone therapy will not be discussed here as furazolidone is no longer widely available. For details of furazolidone therapy, see Mohammad et al., 69 Song et al., 70 and Xie et al. 71
Recommendations for use of empiric therapy
All highly effective H. pylori therapies are susceptibility-based (Tables 2 and 3). The word ‘empirical’ is ‘to be based on, concerned with, or verifiable by observation or experience rather than theory or pure logic’. 72 By definition, empiric therapies should be restricted to those that reliably achieve high cure rates, which in practice implies utilization of only those antibiotics for which the prevalence of resistance is known to be low. With very few exceptions, worldwide this excludes empiric use of clarithromycin, metronidazole, and fluoroquinolones and restricts the choices to tetracycline, amoxicillin, rifabutin, and furazolidone. In addition, as discussed in detail below, metronidazole can be used empirically in bismuth quadruple therapy.
Table 2.
Currently available and effective Helicobacter pylori therapies in the United States.
| Empiric therapies | |
| Bismuth quadruple therapy Bismuth subsalicylate q.i.d. 14 days |
Bismuth (e.g. PeptoBismol) 2 tablets or 2 capsules q.i.d. 30 min before meals, tetracycline HCl 500 mg and metronidazole 500 mg 30 min after meals q.i.d. plus a PPI, 30 min b.i.d. before meals and bedtime (see PPI recommendations below) |
| Bismuth quadruple therapy Bismuth subsalicylate b.i.d. 14 days |
Bismuth (e.g. PeptoBismol) 2 tablets or 2 capsules q.i.d, 30 min before meals, tetracycline HCl 500 mg b.i.d. and metronidazole 500 mg, 30 min after meals q.i.d. plus a PPI, b.i.d. 30 min before morning and evening meals (see PPI recommendations below) |
| Pylera. 3-in-1 formulation of bismuth quadruple therapy with bismuth citrate: 14 days | Give combination tablets with meals and bedtime plus a PPI 30 min before breakfast (see PPI recommendations below) |
| Rifabutin triple therapy. 14 days | Rifabutin 150 mg b.i.d., amoxicillin 1 g t.i.d. plus 40 mg of esomeprazole or rabeprazole 30 min before breakfast and at bedtime (see PPI recommendations below). |
| Talicia 3-in-1 formulation of rifabutin triple therapy. 14 days | As directed by package insert |
| Therapies only effective as susceptibility-based therapy Do not use empirically unless proven to cure >90% locally | |
| Clarithromycin triple therapy. 14 days |
Clarithromycin 500 mg b.i.d., amoxicillin 1 g b.i.d. 30 min after meal plus a PPI b.i.d. 30 min before meals (see PPI recommendations below) |
| Metronidazole triple therapy. 14 days |
Metronidazole 500 mg b.i.d., amoxicillin 1 g b.i.d., 30 min after meal plus a PPI b.i.d. 30 min before meals (see PPI recommendations below) |
| Levofloxacin triple therapy. 14 days a |
Levofloxacin 500 mg in a.m., amoxicillin 1 g b.i.d., 30 min after meal plus a PPI b.i.d. 30 min before meals (see PPI recommendations below) |
| PPI recommendations PPI should preferably be a second generation PPI (i.e. rabeprazole or esomeprazole) and at least 20 mg, preferably 40 mg, of rabeprazole or esomeprazole b.i.d. 30 min before meals | |
| Obsolete therapies All regimens that include at least one antibiotic that offers no therapeutic benefit and only serves to increase global antimicrobial resistance: concomitant, hybrid, reverse hybrid, sequential therapies and vonoprazan, clarithromycin, and amoxicillin triple therapy. | |
Source: Table adapted from Lee et al. 73 with permission.
i.d., twice a day; FDA, US Food and Drug Administration; PPI, proton pump inhibitor; q.i.d., four times a day; t.i.d., three times a day.
The FDA recommends fluoroquinolones be used as a last choice because of the risk of serious side effects. 74
Table 3.
Combinations deemed unacceptable because each contains at least one antibiotic not required for effectiveness.
Use and optimization of bismuth quadruple therapy
Traditional bismuth quadruple therapy consists of a PPI, bismuth, metronidazole, and tetracycline. It is a complex but probably the most reliably effective therapy commonly used for H. pylori eradication. The regime is complex in terms of both medications and interpretation of the results of therapy. Treatment success in this era of increasing antimicrobial resistance requires attention to the details of therapy especially in relation to dosage and duration of therapy. Myths, misconceptions, and erroneous conclusions abound regarding bismuth quadruple therapy. 76 One of the issues relates to the effect of resistance on treatment success especially in relation to metronidazole. For example, it has often been suggested that metronidazole resistance as determined in the laboratory has limited or no ability to predict the results of therapy such that there is no reason to do susceptibility testing. This misconception is likely related to the fact that the effects of metronidazole resistance are not all or none and thus differ from what one typically expects with clarithromycin or levofloxacin. Metronidazole susceptible infections are consistently highly effective in optimized therapies.77–79 Although metronidazole resistance does not completely remove the drug for having an effect, metronidazole resistance has been conclusively shown to reduce the effectiveness of metronidazole-containing therapies, including both triple and quadruple formulations.76,78,80–82 To date, the only exception is that metronidazole resistance can be overcome using bismuth quadruple therapy but with the caveat that success is both dose- and duration-dependent (discussed below). 83
The extremely high population of H. pylori in the stomach typically results in the presence of small subpopulations of strains resistant to antibiotics such as clarithromycin, or levofloxacin called the inoculum effect. 65 With metronidazole, the odds of a resistant subpopulation are high as an analysis of multiple gastric samples from a single patient will often demonstrate the presence of both susceptible and resistant strains called hetero-resistance.84–86 Another problem is that the analysis of the claims of clinical studies in relation to resistance is often complicated by the method used to detect metronidazole resistance. For example, the Etest tends to overestimate prevalence of resistance and thus may result in erroneous conclusion regarding effectiveness in resistant infections.87,88 Possibly uniquely, the effects of metronidazole resistance can be partially or almost completely overcome by tailoring therapy in relation to the dosage of metronidazole and the duration of therapy.76,89,90 Increasing the dosage of metronidazole and/or duration of therapy is beneficial for both metronidazole triple and quadruple therapies. 78 Importantly, this benefit occurs with both resistant and susceptible infections.80,81,91,92
Metronidazole resistance is the primary impediment to successful therapy with bismuth quadruple therapy. Resistance to bismuth is vanishingly rare and tetracycline resistance is rare, but when present, resistance adversely affects outcome. 93 As such, and in contrast to metronidazole, it has been suggested that the dosage and frequency of administration of both bismuth and tetracycline may not be the keys to successful therapy. 81 Most often tetracycline is used four times daily (q.i.d.) and bismuth either twice daily (b.i.d.). Results with b.i.d. and q.i.d. administration of tetracycline and bismuth are approximately equivalent. 94 In contrast, experiments with primarily susceptible infections have reported improved outcome and adherence when the frequency of drug administration was increased allowing the individual and total doses to be decreased. 95
Unraveling the claims made in treatment studies with quadruple therapy
Bismuth quadruple therapy is highly effective in adherent patients with metronidazole-susceptible infections. High success rates have been reported with very short duration therapy (i.e. <7 days). 96 In contrast, in the presence of metronidazole resistance, high cure rates require full dosages, longer duration, and a PPI for acid suppression. For example, if the cure rate was 97% with susceptible infections and 65% with resistant infections, the overall cure rate would depend on the proportion with resistance.76,97
Until recently, the results from most studies have been based on data from studies using bismuth quadruple therapy for 14 days. Traditionally, therapy consisted of bismuth, tetracycline 500 mg, metronidazole 400 or 500 mg usually q.i.d., and a PPI given b.i.d. for 14 days. In most countries, including China, this regimen resulted in reliably high cure rates.76,79,90,97–99 Generally, however, this therapy has been less effective in Iran and Turkey. 90 It is unclear why. One possible explanation is related to the fact that in developing countries, it is more common to find drugs with reduced activity, outdated drugs, and even counterfeit drugs. 100 One of the main issues with bismuth quadruple therapy has been the relatively high frequency of side effects. It has been shown that it is possible to reduce the dosage and dosing interval (e.g. to b.i.d.) with the bismuth and tetracycline and thus reduce side effects. As noted previously, maintaining the dose of metronidazole to 1500 to 2000 mg and the duration to 14 days appears to be the critical determinants when dealing with metronidazole-resistant infections91,94,101
For susceptible infections, all drugs can be given b.i.d. It has been suggested that reducing the individual doses of metronidazole while increasing the frequency of administration may also reduce side effects.76,95,102 Studies are needed (i.e. using five administrations/day). The dosages of metronidazole available vary in different regions. For example, in the United States, metronidazole is only available as 250, 500, and 750 mg extended release version. In China, 200 mg tablets are available. It would be interesting to test 200 or 250 mg after meals and 750 or 800 mg at bedtime. There have been studies with 750 mg b.i.d. with good results but with an increase in adverse events. 103
Pylera
Pylera is the commercial name of a three-in-one bismuth, metronidazole, and tetracycline preparation. Pylera has no special properties or formulations. It consists of the same drugs used in generic bismuth quadruple therapy but they are put into capsules. The metronidazole is put in small capsules that are then included within a larger capsule. Pylera was marketed for 10 days to have a marketing advantage over Helicac, which was marketed for 14 days. This was done during the period when the FDA focused on H. pylori in peptic ulcer rather than as an infectious disease. Pylera is often the only form of bismuth quadruple therapy available in Europe and a head-to-head comparison of 10- and 14-day Pylera has not been attempted in patients with metronidazole-resistant infections. For metronidazole-susceptible infections, 5- to 7-day therapy is typically adequate although metronidazole triple therapy would be a better option as it is less complicated and has fewer side effects. 79 Although there have been a number of studies in unknown, but likely low, metronidazole-resistant populations with good results, studies in proven metronidazole-resistant populations suggest that 10-day therapy is insufficient as results with 14-day therapy are better. 98 In the United States, many pharmacies will fill the prescription for 14-day Pylera. In areas where metronidazole resistance is common and only Pylera is available and the local pharmacies will not provide the three-in-one therapy for 14 days, 10-day therapy is often the best available choice. Longer duration twice-a-day Pylera can also be obtained using the current dose pack by giving four caps b.i.d. plus 500 mg of metronidazole at bedtime for 15 days and achieve the full 1500 mg metronidazole dose with 500 mg of tetracycline and 560 mg of bismuth/day. Experiments are needed comparing 10- and 14-day Pylera in metronidazole-resistant strains and how to use it b.i.d. considering that no alternate formulations are available in Europe. The cost in Europe is approximately 100 Euro versus more than $1000 in the United States such that in the United States generic drugs are typically preferred. If the cure rate per protocol of less than 95% is obtained with 10-day therapy, one should consider 14-day therapy. As a general rule, a 90% cure rate is not the goal but is rather the minimally acceptable result. For example, a 6% decrease in cure rate (e.g. from 96% to 90% or from excellent to good results) results in administration of about 3000 kg of unneeded antibiotics/million treatments. The unneeded antibiotics only contribute to global resistance.
Optimization of VPZ-containing therapy
Vonoprazan is P-CAB that has advantages over traditional PPIs as it becomes fully active on day 1 and produces potent acid suppression. VPZ is stable under acidic condition and not affected by the CP2C19 genotype or meals. 104 VPZ was approved for treatment of H. pylori in 2015 in Japan. The pivotal phase III randomized controlled trial (RCT) compared a 7-day triple therapy with amoxicillin and clarithromycin plus the PPI, lansoprazole, or VPZ. In the presence of clarithromycin-susceptible infections, both the PPI and VPZ triple therapies were equivalent with a cure rate of approximately 98%. The cure rates with clarithromycin-resistant infections differ markedly in terms of cure rate: PPI triple therapy = 40% versus VPZ = 80%. In the presence of clarithromycin resistance, the patients effectively receive only a PPI or VPZ plus amoxicillin dual therapy. 30 The cure rates with clarithromycin-resistant infections are PPI triple therapy = 40% versus VPZ = 80%. Thus, the cure rate of 92% would consist of 80% from dual VPZ–amoxicillin therapy and only 12% from VPZ–amoxicillin + clarithromycin (i.e. at most 12% would benefit from receiving clarithromycin). 28 The Japanese H. pylori treatment guideline now recommends replacing PPIs with VPZ for first eradication regimens that results in more than 14,000 kg of unneeded clarithromycin/1 million treatments and contributes to the problem of increasing antimicrobial resistance. Over time, the overall cure rate of VPZ triple therapy has fallen in Japan coincident with increasing clarithromycin resistance and is now below 90% (range = 88–92%). The dictum of the Alliance to Save Our Antibiotics is that no individual, animal, or human should receive unnecessary antibiotics. 105
The overall cure rates with VPZ triple therapy in Japan currently vary between 88% and 92% with the combination of VPZ and amoxicillin dual therapy being responsible for the majority of the success. Currently, there are ongoing efforts to improve the cure rate of VPZ–amoxicillin dual therapy.
PPI–amoxicillin dual therapy
PPI–amoxicillin dual therapy was first introduced in 1998 and has a long history (reviewed in Dore et al. 90 and Graham et al. 106 ). PPI–amoxicillin dual therapy proved to be unreliable for achieving high cure rates in western populations. Recent studies in Asia where CYP2C19 rapid metabolizers are uncommon and gastric acid secretion is generally low due to corpus gastritis, have shown that the combination high dose PPI–amoxicillin can produce cure rates ⩾90%.107–109 The effectiveness of the regimen is sensitive to the total dose of amoxicillin, the frequency of amoxicillin and antisecretory drug administration, and the duration of therapy. Trials with PPI-dual therapy suggested that 3 g of amoxicillin per day, a high potency PPI b.i.d., and duration of 14 days are generally the most effective. In Asia, there have been studies using PPIs b.i.d. or q.i.d. based on Japanese studies of intragastric pH suggest it may be possible to reliably maintain the intragastric pH above 5.110–113 In Asia, PPIs are typically more effective than in the west in part related to low prevalence of CYP2C19 rapid metabolizes, an average lower body and parietal cell mass, and a higher prevalence of corpus gastritis in Asian populations.114,115
Sustaining high intragastric pH
The success achieved in maintaining a sustained high intragastric pH by increasing the frequency of PPI administration in Asia contrasts with the results of western studies where there was no advantage of increasing the frequency of administration above twice a day as increasing the dosage and/or frequency of administration has failed to reliably achieve a sustained intragastric pH ⩾6 as may be desired for the treatment of upper gastrointestinal bleeding. 116 Accurate assessment of intragastric pH is difficult and there are many technical issues such as use of antimony versus glass electrodes, the computer programs used to collect the data using Digitrapper pH-Z or similar collection devices, electrode calibration solutions with antimony electrodes, calibration pH (e.g. 1 and 4 versus 1, 4, and 7), calibration before and after testing, and so on (discussed in detail in the supplement to Graham and Tansel 116 ). Nonetheless, dual PPI–amoxicillin therapy has proven to be generally more effective in Asia than in Europe or the United States. Importantly, in the United States, dual high dose PPI–amoxicillin therapy using 40 mg of esomeprazole and 750 mg of amoxicillin every 8 h for 14 days resulted in unacceptably low cure rate (i.e. 72%)28,117,118 and reliable success independent of geography will likely require use of a more potent long-acting antisecretory agents with rapid onset and effective against nonactive proton pumps such as VPZ.27,68,119–121
VPZ–amoxicillin dual therapy
It is important to note that the Japanese government currently restricts the duration of H. pylori therapies to 7 days. Three recent trials of VPZ–amoxicillin dual therapy have been reported from Japan.122–124 One was a retrospective study using propensity score matching to improve comparability between the two regimen groups, which were dual group with VPZ 20 mg b.i.d. amoxicillin 500 mg three times daily (t.i.d.) (rather than 750 b.i.d. which is currently approved for amoxicillin therapy) for 1 week versus triple therapy with VPZ 20 mg, amoxicillin 750 mg, clarithromycin 200 mg, b.i.d. for 1 week. In the intention-to-treat analysis (ITT), the eradication rate with the dual therapy was 92.9% and not inferior to the triple therapy [92.9%; 95% confidence interval (CI) = 82.7–98.0%) versus (91.9%; 95% CI = 80.4–97.0%, p = 0.728). Importantly, that study showed conclusively that the addition of clarithromycin had no additional benefit. 122 A prospective observational study of junior high school students also indicated noninferiority of VPZ and lower dose amoxicillin dual therapy with VPZ 20 mg b.i.d. plus amoxicillin 750 mg b.i.d. for 7 days. 123 In that study the ITT, eradication rates were 85% (95% CI = 75.8–94.2%) with the dual therapy versus 82% (95% CI = 76.0–87.9%) with the triple therapy. Moreover, the same group performed a prospective, randomized clinical trial using the same regimens. 124 The dual therapy yielded the cure rate of 84.5% and 87.1% in the ITT and per protocol analysis, respectively. The VPZ dual therapy failed to achieve a 90% cure rate but was statistically noninferior to the triple therapy. 124 Together, these data confirm that the addition of clarithromycin to the triple therapy has minimal to no added benefit, which was also confirmed in the US/European trials. 125
Optimal amoxicillin dosage and frequency of administration for dual therapy
Since the introduction of penicillin, there have been disagreements about dosing and frequency of administration partially in response to the presence of microbial dormancy and the persister effect.67,126–128 H. pylori infection is associated with a marked enhancement of the gastric penetration of amoxicillin, as confirmed with a xenograft model of human H. pylori infection. 129 The fact that permeability of the large molecule, sucrose, is also enhanced during H. pylori infection and that enhancement is correlated with the density of the mucosal polymorphonuclear cell infiltration is consistent with the paracellular pathway being the site of increased permeability.130,131 Finally, Kimura et al. previously showed it was possible to cure H. pylori infections in humans with topical therapy consisting of a 1 h instillation of bismuth, amoxicillin, and metronidazole into balloon-occluded stomachs.132,133
The optimal dosage and frequency of administration of amoxicillin remain unclear. The major impediment to effective therapy is the ability to reliably obtain marked acid suppression. In Japan, dual therapy with either VPZ or PPIs has been able to achieve an approximately 93% cure with VPZ 20 mg b.i.d. and 750 mg amoxicillin t.i.d. for 7 days. 122 When 750 mg of amoxicillin was given b.i.d, the cure rate per protocol was 87.1%. 124 In contrast, in the United States and Europe when VPZ was given 20 mg b.i.d. and amoxicillin 1 g t.i.d., the cure rate was only 81.2%, per protocol. 125
The data suggest that the ability to maintain the intragastric pH at or near 6 for an extended time cannot be reliably be obtained with only 20 mg of VPZ given b.i.d. Likely, a higher dose of VPZ, such as 20 mg t.i.d., will be required. With VPZ, the frequency of administration of amoxicillin required for an effective dual therapy has been shown, at least in one study, to be similar whether 750 mg of amoxicillin was given either b.i.d. or t.i.d. 124 Based on the pharmacodynamics of amoxicillin, achieving a constant blood level above the minimal inhibitory concentration (MIC) requires 500 mg every 6 h or 750 mg every 8 h (discussed in detail in Furuta and Graham 134 ). The requirement that one maintain blood levels above the MIC, however, is not based on experimental data obtained while the intragastric pH is maintained at 6 or greater. Rather it is a largely untested hypothesis and, as noted above, the high cure rates with b.i.d. amoxicillin with VPZ antisecretory therapy suggest that more frequent administration may not be necessary. 128 Proof will only come when dual amoxicillin plus a P-CAB therapy is formally optimized, which will clarify the parameters required to reliably achieve high cure rates in particular populations.
The duration of therapy bias
One of the biggest problems to overcome when prescribing H. pylori therapy is the apparent physician–investigator preference for 7-day regimens. As a general rule, H. pylori therapies have proven most effective when given for 14 days compared with shorter durations. Whereas, interestingly, the eradication rates of the clarithromycin-resistant strain with the VPZ dual therapy was higher (92.3% versus 76.2%, p = 0.043) than those in the VPZ triple therapy. 124 The lower cure rate in the triple group might be because interaction of clarithromycin with VPZ and amoxicillin decreases the sensitivity to amoxicillin. The target of amoxicillin is the penicillin-binding protein (PBP), which is the enzyme involved in the biosynthesis of the bacterial cell wall. When H. pylori grows, the bactericidal effect of amoxicillin is enhanced due to the increase of PBP expression. Both VPZ and clarithromycin are metabolized by the same hepatic enzyme (cytochrome P450 3A4). Therefore, administering both drugs together increases the maximum plasma concentration of VPZ by 1.5-fold to 1.9-fold in comparison with the administration of VPZ alone. Penicillin is only effective in actively dividing H. pylori at a narrow external pH range between 6 and 7. VPZ–clarithromycin interaction might cause suboptimal gastric acid pH levels (i.e. >7), resulting in a decrease in the H. pylori sensitivity to amoxicillin in the VPZ triple therapy. Moreover, theoretically, the combination of amoxicillin and clarithromycin might be antagonistic. 135 Clarithromycin is known to affect as the inhibitor of ribosomal RNA (rRNA) and inhibit protein synthesis, including PBP, which is target to amoxicillin. 136 While this possibility is intriguing, the hypothesis remains to be tested.
Rifabutin triple therapy
The antibiotics used to treat H. pylori infections associated with a low prevalence of resistance are amoxicillin, tetracycline, furazolidone, and rifabutin. These antibiotics can thus be potentially used empirically. In western countries, this generally equates with bismuth quadruple therapy or a rifabutin-containing therapy. Rifabutin is typically administered in combination with a PPI along with amoxicillin or as a triple therapy (reviewed in Gisbert 137 ). The therapy is most often successful when combined with a high dose of PPI and amoxicillin. 137 Recently, a three-in-one combination (Talicia) has been introduced in the United States for firstline H. pylori eradication. The combination contains 150 mg rifabutin, 3 g of amoxicillin, and 120 mg of omeprazole administered as four capsules every 8 h for 14 days. 3 The cure rate was 84% (90% in those proven to have taken the drugs). It was well tolerated, and considering the short duration and low rifabutin dosage, significant side effects are unlikely to become a problem. Nietherrifabutin triple therapy nor bismuth quadruple therapy, has yet to be formally optimized and both regimens can likely be improved in terms of outcome. With bismuth quadruple therapy the reduction in side effects would likely improve often limits adherence.98,138 A generic equivalent of the Talicia formulation has not been tested. Rifabutin is packaged as 150 mg capsules. A compounding pharmacy could easily formulate fourteen 150 mg capsules into twenty-eight 75 mg or twenty-one 100 mg capsules and this along with a high potency PPI (e.g. 40 mg of rabeprazole or esomeprazole b.i.d.) and 1 g of amoxicillin every 8 h, or 750 mg every 6 h should prove effective at possibly reduced cost.
Rifabutin use and concerns about its effect on tuberculosis
In Europe, concern has been raised that the recent large influx of immigrants from countries with a high incidence of both H. pylori and tuberculosis might lead to a problem if rifabutin was widely used for H. pylori therapy. 139 This was addressed in a recent study of the effect of immigration on tuberculosis in Europe that reported that the overall incidence of tuberculosis had decreased by 25% despite the total number of immigrants increasing by 33%. 140 The finding of a strong negative correlation between the incidence of tuberculosis and the number of immigrants was considered ‘reassuring and indicated that there was not yet any cause for undue concern’. 140
A recent comprehensive review of rifabutin therapy also reported no correlation between the short-term use of rifabutin and emergence of rifabutin-resistance tuberculosis. 137 This finding was consistent with the data showing that with prolonged use of rifabutin for treatment of tuberculosis, the increase in rifabutin resistance has been negligible. 141 Studies have also reported that emergence of resistance among Mycobacterium avium and Mycobacterium tuberculosis was less likely to occur with rifabutin than with rifampicin. The emergence of resistance to rifabutin was studied by repeatedly subculturing M. tuberculosis in the presence of subinhibitory concentrations (0.05 or 0.1 MIC) of the drug and concluded that ‘these studies suggest that emergence of resistance among MAC and M. tuberculosis may be less likely to occur with rifabutin than with rifampicin’. 142 There was also a lack of acquired resistance among 36 isolates obtained from patients with rifampicin-resistant M. tuberculosis, M. xenopi, or M. avium infections treated for 12 months with rifabutin plus on other drug to which the pathogens were susceptible. 142
In summary, the European community has long been aware of the potential for tuberculosis to be reintroduced from the migrant population and most countries have instituted formal screening programs for active tuberculosis in immigrants from high tuberculosis incidence countries for tuberculosis. While it remains prudent to screen individuals from high tuberculosis incidence countries for tuberculosis before instituting therapy with rifabutin, the risk of developing resistance from short duration therapy for H. pylori is negligible and is not a reason to limit rifabutin for anti-H. pylori therapy generally.
Summary
The diagnosis and management of H. pylori continues to evolve. The recognition that H. pylori infections should be treated similarly to other infectious diseases had resulted in a reassessment how to approach therapy. The use of test-of-cure data to distinguish those therapies that are effective locally and those which are not and which should be avoided should markedly improve overall cure rates by rapid elimination of empiric use of locally ineffective therapies. Susceptibility testing is now widely available in the United States. There are now few barriers to inexpensive PCR-based clarithromycin susceptibility testing in Europe and clinicians should encourage their hospitals to offer it. We anticipate that treatment will continue to rapidly evolve and utilize a either the ‘proven highly effective empiric therapy first strategy’, followed by susceptibility-based therapy for initial treatment failures, If a proven highly effective empiric therapy is not available, initial therapy will be susceptibility-based (Figure 1). 12
Globally, resistance to clarithromycin, metronidazole, and fluoroquinolones has increased to the point that these antimicrobials should not be used empirically. The exception is metronidazole when used in bismuth quadruple therapy. Concomitant, sequential, hybrid, reverse hybrid, and VPZ triple therapy all contain at least one unneeded antibiotic and should no longer be used as they are responsible to tens of thousands of kilograms of unnecessary antibiotic use and likely contribute significantly to global antimicrobial resistance.
Footnotes
Author contributions: All authors have read and approved the final manuscript and each meets the criteria for authorship established by the International Committee of Medical Journal Editors and verifies the validity of the results reported.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DYG is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori. He is also a consultant for DiaSorin regarding H. pylori diagnostics and with Otsuka Japan regarding novel breath tests. He has ongoing collaborative research projects with American Molecular regarding molecular diagnostics for H. pylori. He was the Principal Investigator (PI) of an international study of the use of antimycobacterial therapy for Crohn’s disease. AS received lecture fees from Takeda. HL is a paid consultant for AstraZeneca, Takeda, and China Medical System Holdings Limited (CMS) Pharmaceuticals regarding novel H. pylori therapies and has received research support from National Natural Science Foundation of China Pharmaceutical Company. PR has nothing to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DYG is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
ORCID iDs: Hong Lu  https://orcid.org/0000-0002-3127-6048
https://orcid.org/0000-0002-3127-6048
David Y. Graham  https://orcid.org/0000-0002-6908-8317
https://orcid.org/0000-0002-6908-8317
Contributor Information
Akiko Shiotani, Department of Internal Medicine, Kawasaki Medical School, Okayama, Japan.
Priya Roy, Department of Medicine, Baylor College of Medicine, Houston, TX, USA.
Hong Lu, GI Division, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
David Y. Graham, Department of Medicine, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX 77030, USA.
References
- 1. Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology 1996; 110: 1244–1252. [DOI] [PubMed] [Google Scholar]
- 2. Hopkins RJ. Current FDA-approved treatments for Helicobacter pylori and the FDA approval process. Gastroenterology 1997; 113(Suppl. 6): S126–S130. [DOI] [PubMed] [Google Scholar]
- 3. Graham DY, Canaan Y, Maher J, et al. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: a double-blind, randomized, controlled trial. Ann Intern Med 2020; 172: 795–802. [DOI] [PubMed] [Google Scholar]
- 4. Kalfus IN, Graham DY, Riff DS, et al. Rifabutin-containing triple therapy (RHB-105) for eradication of Helicobacter pylori: randomized ERADICATE Hp trial. Antibiotics 2020; 9: 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 6. Graham DY, Liou JM. Primer for development of guidelines for Helicobacter pylori therapy using antibiotic stewardship. Clin Gastroenterol Hepatol 2021. (in press). DOI: 10.1016/j.cgh.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics 2020; 9: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015; 64: 1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graham DY. Illusions regarding Helicobacter pylori clinical trials and treatment guidelines. Gut 2017; 66: 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graham DY, Dore MP. Helicobacter pylori therapy demystified. Helicobacter 2011; 16: 343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rokkas T, Gisbert JP, Malfertheiner P, et al. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: a network meta-analysis. Gastroenterology 2021; 161: 495–507. [DOI] [PubMed] [Google Scholar]
- 12. Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: who, when, and how. Am J Gastroenterol 2021. (in press; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glupczynski Y, Labbe M, Burette A, et al. Treatment failure of ofloxacin in Campylobacter pylori infection [Letter]. Lancet 1987; 1: 1096–1096. [DOI] [PubMed] [Google Scholar]
- 14. Glupczynski Y, Burette A, De Koster E, et al. Metronidazole resistance in Helicobacter pylori [Letter]. Lancet 1990; 335: 976–977. [DOI] [PubMed] [Google Scholar]
- 15. Bell GD, Powell K, Burridge SM, et al. Experience with ‘triple’ anti-Helicobacter pylori eradication therapy: side effects and the importance of testing the pre- treatment bacterial isolate for metronidazole resistance. Aliment Pharmacol Ther 1992; 6: 427–435. [DOI] [PubMed] [Google Scholar]
- 16. Results of a multicentre European survey in 1991 of metronidazole resistance in Helicobacter pylori. European Study Group on Antibiotic Susceptibility of Helicobacter pylori. Eur J Clin Microbiol Infect Dis 1992; 11: 777–781. [PubMed] [Google Scholar]
- 17. Debets-Ossenkopp YJ, Sparrius M, Kusters JG, et al. Mechanism of clarithromycin resistance in clinical isolates of Helicobacter pylori. FEMS Microbiol Lett 1996; 142: 37–42. [DOI] [PubMed] [Google Scholar]
- 18. Versalovic J, Shortridge D, Kibler K, et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother 1996; 40: 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Axon AT. The role of omeprazole and antibiotic combinations in the eradication of Helicobacter pylori – an update. Scand J Gastroenterol Suppl 1994; 205: 31–37. [PubMed] [Google Scholar]
- 20. Graham DY, Opekun AR, Klein PD. Clarithromycin for the eradication of Helicobacter pylori. J Clin Gastroenterol 1993; 16: 292–294. [DOI] [PubMed] [Google Scholar]
- 21. Peterson WL, Graham DY, Marshall B, et al. Clarithromycin as monotherapy for eradication of Helicobacter pylori: a randomized, double-blind trial. Am J Gastroenterol 1993; 88: 1860–1864. [PubMed] [Google Scholar]
- 22. Graham DY, Ramirez F, Lew GM, et al. Omeprazole as an adjuvant to antimicrobial therapy for eradication of Helicobacter pylori infection. Curr Therapeut Res 1994; 55: 213–219. [Google Scholar]
- 23. FDA. BIAXIN Filmtabs (clarithromycin tablets). FDA full prescribing information, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050662s044s050,50698s026s030,050775s015s019lbl.pdf
- 24. Al-Assi MT, Genta RM, Karttunen TJ, et al. Clarithromycin-amoxycillin therapy for Helicobacter pylori infection. Aliment Pharmacol Ther 1994; 8: 453–456. [DOI] [PubMed] [Google Scholar]
- 25. Lamouliatte HC, Cayla R, Megraud F, et al. Amoxicillin-clarithromycin-omeprazole: the best therapy for Helicobacter pylori infection [Abstract]. Acta Gastroenterol Belg 1993; 56: A139. [Google Scholar]
- 26. Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2015; 21: 85–90. [DOI] [PubMed] [Google Scholar]
- 27. Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019; 24: e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol 2021; 36: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 29. Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues. Gut 2017; 66: 384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016; 65: 1439–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janssen MJ, Van Oijen AH, Verbeek AL, et al. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther 2001; 15: 613–624. [DOI] [PubMed] [Google Scholar]
- 32. Laheij RJ, Rossum LG, Jansen JB, et al. Evaluation of treatment regimens to cure Helicobacter pylori infection—a meta-analysis. Aliment Pharmacol Ther 1999; 13: 857–864. [DOI] [PubMed] [Google Scholar]
- 33. Ginnebaugh BD, Baker J, Watts L, et al. S1348 triple therapy for primary treatment of Helicobacter pylori: a 19-year U.S. Single Center Experience. J Am Coll Gastroenterol 2020; 115: S680–S681. [Google Scholar]
- 34. Nyssen OP, Bordin D, Tepes B, et al. European Registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021; 70: 40–54. [DOI] [PubMed] [Google Scholar]
- 35. Valle Muñoz J, Muñoz Gómez P, Sierra Bernal C, et al. Tailored Helicobacter pylori eradication based on prior intake of macrolide antibiotics allows the use of triple therapy with optimal results in an area with high clarithromycin resistance. Rev Esp Enferm Dig 2019; 111: 655–661. [DOI] [PubMed] [Google Scholar]
- 36. Boltin D, Levi Z, Gingold-Belfer R, et al. Impact of previous exposure to macrolide antibiotics on Helicobacter pylori infection treatment outcomes. Am J Gastroenterol 2019; 114: 900–906. [DOI] [PubMed] [Google Scholar]
- 37. Muñoz-Gómez P, Jordán-Castro JA, Abanades-Tercero M, et al. Macrolide use in the previous years is associated with failure to eradicate Helicobacter pylori with clarithromycin-containing regimens. Helicobacter 2018; 23: e12452. [DOI] [PubMed] [Google Scholar]
- 38. Lim SG, Park RW, Shin SJ, et al. The relationship between the failure to eradicate Helicobacter pylori and previous antibiotics use. Dig Liver Dis 2016; 48: 385–390. [DOI] [PubMed] [Google Scholar]
- 39. Shiota S, Reddy R, Alsarraj A, et al. Antibiotic resistance of Helicobacter pylori among male United States veterans. Clin Gastroenterol Hepatol 2015; 13: 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62: 34–42. [DOI] [PubMed] [Google Scholar]
- 41. McNulty CA, Lasseter G, Shaw I, et al. Is Helicobacter pylori antibiotic resistance surveillance needed and how can it be delivered? Aliment Pharmacol Ther 2012; 35: 1221–1230. [DOI] [PubMed] [Google Scholar]
- 42. McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med 2003; 139: 463–469. [DOI] [PubMed] [Google Scholar]
- 43. Perez Aldana L, Kato M, Nakagawa S, et al. The relationship between consumption of antimicrobial agents and the prevalence of primary Helicobacter pylori resistance. Helicobacter 2002; 7: 306–309. [DOI] [PubMed] [Google Scholar]
- 44. Banatvala N, Davies GR, Abdi Y, et al. High prevalence of Helicobacter pylori metronidazole resistance in migrants to east London: relation with previous nitroimidazole exposure and gastroduodenal disease. Gut 1994; 35: 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16: 992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020; 69: 2093–2112. [DOI] [PubMed] [Google Scholar]
- 47. Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 2021; 160: 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Eidan FA, McElnay JC, Scott MG, et al. Management of Helicobacter pylori eradication – the influence of structured counselling and follow-up. Br J Clin Pharmacol 2002; 53: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Graham DY, Mohammadi M. Synopsis of antibiotic treatment. In: Kim N. (ed.) Helicobacter pylori. Singapore: Springer Science + Business Media, 2016, pp. 417–426. [Google Scholar]
- 50. Adherence to long-term therapies. Evidence for action. Geneva: World Health Organization, 2003. [Google Scholar]
- 51. Blaschke TF, Osterberg L, Vrijens B, et al. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol 2012; 52: 275–301. [DOI] [PubMed] [Google Scholar]
- 52. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487–497. [DOI] [PubMed] [Google Scholar]
- 53. de Boer WA. How to achieve a near 100% cure rate for H. pylori infection in peptic ulcer patients. A personal viewpoint. J Clin Gastroenterol 1996; 22: 313–316. [DOI] [PubMed] [Google Scholar]
- 54. Graham DY, El-Serag HB. European Registry on Helicobacter pylori management shows that gastroenterology has largely failed in its efforts to guide practitioners. Gut 2021; 70: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lehours P, Mégraud F. Helicobacter pylori molecular diagnosis. Expert Rev Mol Diagn 2011; 11: 351–355. [DOI] [PubMed] [Google Scholar]
- 56. Hays C, Delerue T, Lamarque D, et al. Molecular diagnosis of Helicobacter pylori infection in gastric biopsies: evaluation of the Amplidiag® H. pylori + ClariR assay. Helicobacter 2019; 24: e12560. [DOI] [PubMed] [Google Scholar]
- 57. Rolon RM, Cunningham SA, Mandrekar JN, et al. Clinical evaluation of a real-time PCR assay for simultaneous detection of Helicobacter pylori and genotypic markers of clarithromycin resistance directly from stool. J Clin Microbiol 2021; 59: e03040-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pichon M, Pichard B, Barrioz T, et al. Diagnostic accuracy of a noninvasive test for detection of Helicobacter pylori and resistance to clarithromycin in stool by the Amplidiag H. pylori+ClariR real-time PCR assay. J Clin Microbiol 2020; 58: e01787-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van den Poel B, Gils S, Micalessi I, et al. Molecular detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies: a prospective evaluation of RIDA®GENE Helicobacter pylori assay. Acta Clin Belg 2021; 76: 177–183. [DOI] [PubMed] [Google Scholar]
- 60. Jehanne Q, Bénéjat L, Mégraud F, et al. Evaluation of the Allplex™ H pylori and ClariR PCR assay for Helicobacter pylori detection on gastric biopsies. Helicobacter 2020; 25: e12702. [DOI] [PubMed] [Google Scholar]
- 61. Pohl D, Keller PM, Bordier V, et al. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol 2019; 25: 4629–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cambau E, Allerheiligen V, Coulon C, et al. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori. J Clin Microbiol 2009; 47: 3600–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Egli K, Wagner K, Keller PM, et al. Comparison of the diagnostic performance of qPCR, Sanger sequencing, and whole-genome sequencing in determining clarithromycin and levofloxacin resistance in Helicobacter pylori. Front Cell Infect Microbiol 2020; 10: 596371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brennan DE, Omorogbe J, Hussey M, et al. Molecular detection of Helicobacter pylori antibiotic resistance in stool vs biopsy samples. World J Gastroenterol 2016; 22: 9214–9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 1998; 115: 1272–1277. [DOI] [PubMed] [Google Scholar]
- 66. Scott D, Weeks D, Melchers K, et al. The life and death of Helicobacter pylori. Gut 1998; 43(Suppl. 1): S56–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Michiels JE, Van den Bergh B, Verstraeten N, et al. Molecular mechanisms and clinical implications of bacterial persistence. Drug Resist Updat 2016; 29: 76–89. [DOI] [PubMed] [Google Scholar]
- 68. Howden CW. Editorial: pharmacodynamics of potassium-competitive acid blockers: comparing apples with apples-and with oranges. Aliment Pharmacol Ther 2021; 53: 190–191. [DOI] [PubMed] [Google Scholar]
- 69. Mohammadi M, Attaran B, Malekzadeh R, et al. Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran. Dig Dis Sci 2017; 62: 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song C, Qian X, Zhu Y, et al. Effectiveness and safety of furazolidone-containing quadruple regimens in patients with Helicobacter pylori infection in real-world practice. Helicobacter 2019; 24: e12591. [DOI] [PubMed] [Google Scholar]
- 71. Xie Y, Zhang Z, Hong J, et al. Furazolidone-containing triple and quadruple eradication therapy for initial treatment for Helicobacter pylori infection: a multicenter randomized controlled trial in China. Helicobacter 2018; 23: e12496. [DOI] [PubMed] [Google Scholar]
- 72. https://www.google.com/search?q=definition+of+empirical&rlz=1C1GCEA_enUS829US829&oq=def&aqs=chrome.1.69i57j35i39j69i59l2j0i433j69i60l3.3996j1j7&sourceid=chrome&ie=UTF-8 (accessed 3 December 2021).
- 73. Lee YC, Dore MP, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. Annu Rev Med 2022; 73: 41–12. [DOI] [PubMed] [Google Scholar]
- 74. Keller A. Fluoroquinolones, 2020, https://www.consumernotice.org/drugs-and-devices/fluoroquinolones/
- 75. Graham DY, Liou JM. Primer for development of guidelines for Helicobacter pylori therapy using antimicrobial stewardship. Clin Gastroenterol Hepatol. Epub ahead of print 26 March 2021. DOI: 10.1016/j.cgh.2021.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Graham DY, Lee SY. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 2015; 44: 537–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Der Wouden EJ, Thijs JC, Van Zwet AA, et al. Review article: nitroimidazole resistance in Helicobacter pylori. Aliment Pharmacol Ther 2000; 14: 7–14. [DOI] [PubMed] [Google Scholar]
- 78. van der Wouden EJ, Thijs JC, van Zwet AA, et al. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta-analysis. Am J Gastroenterol 1999; 94: 1751–1759. [DOI] [PubMed] [Google Scholar]
- 79. Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther 2019; 49: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 80. Bardhan K, Bayerdorffer E, Veldhuyzen Van Zanten SJ, et al. The HOMER study: the effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter 2000; 5: 196–201. [DOI] [PubMed] [Google Scholar]
- 81. Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther 2007; 26: 343–357. [DOI] [PubMed] [Google Scholar]
- 82. Fischbach LA, van ZS, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther 2004; 20: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 83. Graham DY, Osato MS, Hoffman J, et al. Metronidazole containing quadruple therapy for infection with metronidazole resistant Helicobacter pylori: a prospective study. Aliment Pharmacol Ther 2000; 14: 745–750. [DOI] [PubMed] [Google Scholar]
- 84. Kao CY, Lee AY, Huang AH, et al. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect Genet Evol 2014; 23: 196–202. [DOI] [PubMed] [Google Scholar]
- 85. Matteo MJ, Perez CV, Domingo MR, et al. DNA sequence analysis of rdxA and frxA from paired metronidazole-sensitive and -resistant Helicobacter pylori isolates obtained from patients with heteroresistance. Int J Antimicrob Agents 2006; 27: 152–158. [DOI] [PubMed] [Google Scholar]
- 86. van der Wouden EJ, de Jong A, Thijs JC, et al. Subpopulations of Helicobacter pylori are responsible for discrepancies in the outcome of nitroimidazole susceptibility testing. Antimicrob Agents Chemother 1999; 43: 1484–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Osato MS, Reddy R, Reddy SG, et al. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int J Antimicrob Agents 2001; 17: 39–44. [DOI] [PubMed] [Google Scholar]
- 88. Miftahussurur M, Fauzia KA, Nusi IA, et al. E-test versus agar dilution for antibiotic susceptibility testing of Helicobacter pylori: a comparison study. BMC Res Notes 2020; 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Graham DY, Fagoonee S, Pellicano R. Increasing role for modified bismuth-containing quadruple therapies for Helicobacter pylori eradication. Minerva Gastroenterol Dietol 2017; 63: 77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016; 65: 870–878. [DOI] [PubMed] [Google Scholar]
- 91. Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016; 111: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 92. Tsay F-W, Wu D-C, Yu H-C, et al. A randomized controlled trial shows that both 14-day hybrid and bismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with moderate antibiotic resistance. Antimicrob Agents Chemother 2017; 61: e00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hsieh MT, Chang WL, Wu CT, et al. Optimizing the MIC breakpoints of amoxicillin and tetracycline for antibiotic selection in the rescue therapy of H. pylori with bismuth quadruple regimen. Eur J Clin Pharmacol 2020; 76: 1581–1589. [DOI] [PubMed] [Google Scholar]
- 94. Roghani HS, Massarrat S, Pahlewanzadeh MR, et al. Effect of two different doses of metronidazole and tetracycline in bismuth triple therapy on eradication of Helicobacter pylori and its resistant strains. Eur J Gastroenterol Hepatol 1999; 11: 709–712. [DOI] [PubMed] [Google Scholar]
- 95. Borody TJ, Brandl S, Andrews P, et al. Use of high efficacy, lower dose triple therapy to reduce side effects of eradicating Helicobacter pylori. Am J Gastroenterol 1994; 89: 33–38. [PubMed] [Google Scholar]
- 96. De Boer WA, Driessen WM, Tytgat GN. Only four days of quadruple therapy can effectively cure Helicobacter pylori infection. Aliment Pharmacol Ther 1995; 9: 633–638. [DOI] [PubMed] [Google Scholar]
- 97. Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev Anti Infect Ther 2018; 16: 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Salazar CO, Cardenas VM, Reddy RK, et al. Greater than 95% success with 14-day bismuth quadruple anti-Helicobacter pylori therapy: a pilot study in US Hispanics. Helicobacter 2012; 17: 382–390. [DOI] [PubMed] [Google Scholar]
- 99. Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013; 25: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Laxminarayan R, Bhutta Z, Duse A, et al. Drug resistance. In: Jamison DT, Breman JG, Measham AR, et al. (eds) Disease control priorities in developing countries. 2nd ed. Washington, DC: World Bank, 2006, pp. 1031–1052. [PubMed] [Google Scholar]
- 101. Liang X, Xu X, Zheng Q, et al. Efficacy of bismuth-containing quadruple therapies for clarithromycin-, metronidazole-, and fluoroquinolone-resistant Helicobacter pylori infections in a prospective study. Clin Gastroenterol Hepatol 2013; 11: 802–807. [DOI] [PubMed] [Google Scholar]
- 102. Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992; 102: 493–496. [DOI] [PubMed] [Google Scholar]
- 103. Koksal AS, Onder FO, Torun S, et al. Twice a day quadruple therapy for the first-line treatment of Helicobacter pylori in an area with a high prevalence of background antibiotic resistance. Acta Gastroenterol Belg 2013; 76: 34–37. [PubMed] [Google Scholar]
- 104. Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. J Neurogastroenterol Motil 2018; 24: 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Antibiotic misuse (2021, accessed 4 March 2021). http://www.ciwf.org.uk/our-campaigns/antibiotics-health-crisis/?gclid=CJy78I6G3c8CFQ6BaQodUtQJtw.
- 106. Graham DY, Lu H, Shiotani A. Failure of optimized dual proton pump inhibitor amoxicillin therapy: what now? Saudi J Gastroenterol 2017; 23: 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Furuta T, Shirai N, Xiao F, et al. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology 2003; 50: 2274–2278. [PubMed] [Google Scholar]
- 108. Tai WC, Liang CM, Kuo CM, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: a prospective randomized trial. J Antimicrob Chemother 2019; 74: 1718–1724. [DOI] [PubMed] [Google Scholar]
- 109. Shirai N, Sugimoto M, Kodaira C, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol 2007; 63: 743–749. [DOI] [PubMed] [Google Scholar]
- 110. Furuta T, Shirai N, Xiao F, et al. Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin Pharmacol Ther 2001; 70: 484–492. [DOI] [PubMed] [Google Scholar]
- 111. Sahara S, Sugimoto M, Uotani T, et al. Potent gastric acid inhibition over 24 hours by 4-times daily dosing of esomeprazole 20 mg. Digestion 2015; 91: 277–285. [DOI] [PubMed] [Google Scholar]
- 112. Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther 2004; 76: 290–301. [DOI] [PubMed] [Google Scholar]
- 113. Sugimoto M, Shirai N, Nishino M, et al. Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment Pharmacol Ther 2012; 36: 627–634. [DOI] [PubMed] [Google Scholar]
- 114. Graham DY, Lu H, Yamaoka Y. African, Asian or Indian enigma, the East Asian Helicobacter pylori: facts or medical myths. J Dig Dis 2009; 10: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wirth HP, Yang M. Different pathophysiology of gastritis in east and west? A western perspective. Inflamm Intest Dis 2016; 1: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol 2018; 16: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Graham DY, Javed SU, Keihanian S, et al. Dual proton pump inhibitor plus amoxicillin as an empiric anti-H. pylori therapy: studies from the United States. J Gastroenterol 2010; 45: 816–820. [DOI] [PubMed] [Google Scholar]
- 118. Li C, Shi Y, Suo B, et al. PPI-amoxicillin dual therapy four times daily is superior to guidelines recommended regimens in the Helicobacter pylori eradication therapy within Asia: a systematic review and meta-analysis. Helicobacter 2021; 26: e12816. [DOI] [PubMed] [Google Scholar]
- 119. Gao CP, Zhang D, Zhang T, et al. PPI-amoxicillin dual therapy for Helicobacter pylori infection: an update based on a systematic review and meta-analysis. Helicobacter 2020; 25: e12692. [DOI] [PubMed] [Google Scholar]
- 120. Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology 2018; 154: 462–466. [DOI] [PubMed] [Google Scholar]
- 121. Song Z, Zhou L, Xue Y, et al. A comparative study of 14-day dual therapy (esomeprazole and amoxicillin four times daily) and triple plus bismuth therapy for first-line Helicobacter pylori infection eradication: a randomized trial. Helicobacter 2020; 25: e12762. [DOI] [PubMed] [Google Scholar]
- 122. Furuta T, Yamade M, Kagami T, et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori. Digestion 2020; 101: 743–751. [DOI] [PubMed] [Google Scholar]
- 123. Gotoda T, Kusano C, Suzuki S, et al. Clinical impact of vonoprazan-based dual therapy with amoxicillin for H. pylori infection in a treatment-naïve cohort of junior high school students in Japan. J Gastroenterol 2020; 55: 969–976. [DOI] [PubMed] [Google Scholar]
- 124. Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020; 69: 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vonoprazan, 2021, https://investors.phathompharma.com/static-files/c8885802-074c-4d03-8af2-6d7817fdc80d
- 126. Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007; 5: 48–56. [DOI] [PubMed] [Google Scholar]
- 127. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bigger JW. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 1944; 247: 497–500. [Google Scholar]
- 129. Lozniewski A, Duprez A, Renault C, et al. Gastric penetration of amoxicillin in a human Helicobacter pylori- infected xenograft model. Antimicrob Agents Chemother 1999; 43: 1909–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Goodgame RW, Malaty HM, El-Zimaity HM, et al. Decrease in gastric permeability to sucrose following cure of Helicobacter pylori infection. Helicobacter 1997; 2: 44–47. [DOI] [PubMed] [Google Scholar]
- 131. Graham DY. Pathogenesis of increased sucrose permeability in H. pylori gastritis [Letter]. Dig Dis Sci 2000; 45: 889–889. [DOI] [PubMed] [Google Scholar]
- 132. Kihira K, Satoh K, Saifuku K, et al. Endoscopic topical therapy for the treatment of Helicobacter pylori infection. J Gastroenterol 1996; 31(Suppl. 9): 66–68. [PubMed] [Google Scholar]
- 133. Kimura K, Ido K, Saifuku K, et al. A 1-h topical therapy for the treatment of Helicobacter pylori infection. Am J Gastroenterol 1995; 90: 60–63. [PubMed] [Google Scholar]
- 134. Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am 2010; 39: 465–480. [DOI] [PubMed] [Google Scholar]
- 135. Drago L, Nicola L, Rodighiero V, et al. Comparative evaluation of synergy of combinations of β-lactams with fluoroquinolones or a macrolide in Streptococcus pneumoniae. J Antimicrob Chemother 2011; 66: 845–849. [DOI] [PubMed] [Google Scholar]
- 136. Furuta T, Yamade M, Kagami T, et al. Influence of clarithromycin on the bactericidal effect of amoxicillin in patients infected with clarithromycin-resistant strains of H. pylori. Gut 2020; 69: 2056. [DOI] [PubMed] [Google Scholar]
- 137. Gisbert JP. Rifabutin for the treatment of Helicobacter pylori infection: a review. Pathogens 2021; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011; 377: 905–913. [DOI] [PubMed] [Google Scholar]
- 139. Baglio G. [Tuberculosis and immigration: the answers that epidemiology can provide (and society is waiting for)]. Epidemiol Prev 2015; 39: 73–74. [PubMed] [Google Scholar]
- 140. Boudville DA, Joshi R, Rijkers GT. Migration and tuberculosis in Europe. J Clin Tuberc Other Mycobact Dis 2020; 18: 100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li J, Munsiff SS, Driver CR, et al. Relapse and acquired rifampin resistance in HIV-infected patients with tuberculosis treated with rifampin- or rifabutin-based regimens in New York City, 1997-2000. Clin Infect Dis 2005; 41: 83–91. [DOI] [PubMed] [Google Scholar]
- 142. Brogden RN, Fitton A. Rifabutin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 1994; 47: 983–1009. [DOI] [PubMed] [Google Scholar]