Herpesviruses have been proposed as potential mediators of vascular injury (3). In particular, indirect evidence links human cytomegalovirus (HCMV) infection with the development of vascular diseases, including coronary artery disease, vascular sclerosis, and arterial restenosis (2), but a clear role for the virus has yet to be established. In addition to HCMV, other herpesviruses can infect endothelial cells. Human herpesvirus 6 (HHV-6) has a preferential tropism for CD4 T lymphocytes but can infect endothelial cells in vitro (5).
To investigate a potential role for HHV-6 in vascular diseases, endothelial cells obtained from aorta, vasa vasorum, and heart microvessel (auricle specimen) from an immunocompetent patient with aortic insufficiency and aortic aneurysm were cultivated in vitro as already described (1). Samples were analyzed by nested PCR for the presence of HHV-6 DNA (4). HHV-6 variant B was detected in cultured cells from aorta but not from vasa vasorum and heart microvessels. Viral DNA was not detected in mononuclear cells purified from peripheral blood.
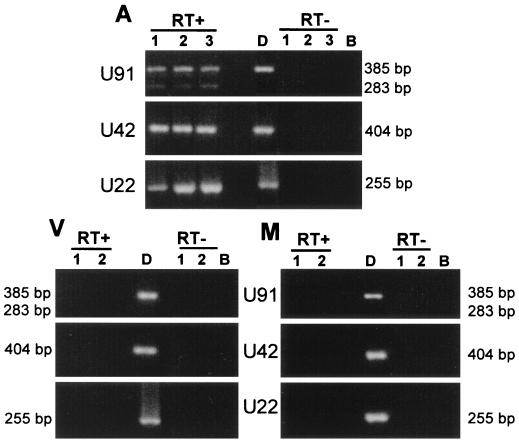
We have recently reported that HHV-6 latency is associated with the presence of U94 mRNA in the absence of other mRNAs transcribed during the immediate-early phase of infection (4). Therefore, to ascertain whether HHV-6 was latent or productively replicating in the aortic endothelium, RNA was extracted from the cell cultures 7, 22, and 64 days after explantation and was analyzed by nested reverse transcription PCR. Viral transcripts from immediate-early (U91, U42) and late (U22) genes were detected in aortic endothelial cells in all culture passages (Fig. 1). No residual DNA contamination of RNA samples was detected by analyzing the same amount of mRNA, without the initial reverse transcription reaction. Furthermore, the presence of spliced forms of U91 (283 bp) (Fig. 1) definitively ruled out the possibility that positive reactions could be attributed to contamination by residual DNA. Endothelial cells from microvessels and vasa vasorum did not harbor HHV-6 transcripts (Fig. 1). Cell cultures positive for HHV-6 mRNA were analyzed by indirect immunofluorescence with monoclonal antibodies to HHV-6 p41 antigen. No viral antigen was detected, suggesting that HHV-6 supported a low-level replication in aortic endothelial cells.
FIG. 1.
Reverse transcription-PCR analysis for HHV-6 transcripts. mRNA was extracted from cultured endothelial cells from aorta (A), vasa vasorum (V), and auricle microvessels (M), retrotranscribed and amplified with HHV-6 primers for U91, U42, and U22. PCR was performed with (RT+) and without (RT−) retrotranscription on the same samples. The sizes of the amplified bands are shown. Cells were harvested 7, 22, or 64 days after explantation (lanes 1, 2, and 3, respectively). The spliced form of U91, corresponding to 283 bp, was present in all positive samples (lanes 1, 2, and 3) but reproduced poorly in the print. Lane D represents a control of amplification reaction performed using HHV-6 DNA as a template; lane B is a blank reverse transcription-PCR reaction performed without adding any RNA.
Nested PCR specific for HCMV was performed on HHV-6-positive samples, but no viral DNA was detected.
This report highlights two important observations: (i) HHV-6 exhibits an in vivo tropism for aortic endothelium and (ii) HHV-6 can support a low-level replication in aortic cells. The possibility that HHV-6 was latent in the aortic tissue and reactivated upon in vitro cultivation needs to be addressed via further experiments. However, these observations show that aortic endothelium might represent an important reservoir for viral reactivation and that it might be a significant target for virus-induced injury.
Acknowledgments
We acknowledge financial support from the Italian Ministry of Health (AIDS Project) and from MURST.
REFERENCES
- 1.Alessandri G, Chirivi R G S, Castellani P, Nicolò G, Giavazzi R, Zardi L. Isolation and characterization of human tumor-derived capillary endothelial cells: role of oncofetal fibronectin. Lab Invest. 1998;78:127–128. [PubMed] [Google Scholar]
- 2.Melnick J L, Adam E, DeBakey M E. The link between CMV and atherosclerosis. Infect Med. 1998;15:479–486. [Google Scholar]
- 3.Nicholson A C, Hajjar D P. Herpesviruses and thrombosis: activation of coagulation on the endothelium. Clin Chim Acta. 1999;286:23–29. doi: 10.1016/s0009-8981(99)00091-1. [DOI] [PubMed] [Google Scholar]
- 4.Rotola A, Ravaioli T, Gonelli A, Dewhurst S, Cassai E, Di Luca D. U94 of human herpesvirus 6 is expressed in latently infected peripheral blood mononuclear cells, and blocks viral gene expression in transformed lymphocytes in culture. Proc Natl Acad Sci USA. 1998;95:13911–13916. doi: 10.1073/pnas.95.23.13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C A, Shanley J D. Chronic infection of human umbilical vein endothelial cells by human herpesvirus 6. J Gen Virol. 1998;79:1247–1256. doi: 10.1099/0022-1317-79-5-1247. [DOI] [PubMed] [Google Scholar]