Abstract
Background
Transferring the hypoglossal nerve to the facial nerve using an end-to-end method is very effective for improving facial motor function. However, this technique may result in hemitongue atrophy. The ansa cervicalis, which arises from the cervical plexus, is also used for facial reanimation. We retrospectively reviewed cases where facial reanimation was performed using the ansa cervicalis to overcome the shortcomings of existing techniques of hypoglossal nerve transfer.
Methods
The records of 15 patients who underwent hypoglossal nerve transfer were retrospectively reviewed. Three methods were used: facial reanimation with hypoglossal nerve transfer (group 1), facial nerve reanimation using the ansa cervicalis (group 2), and sural nerve interposition grafting between the hypoglossal nerve and facial nerve (group 3). In group 1, the ansa cervicalis was coapted to neurotize the distal stump of the hypoglossal nerve in a subset of patients. Clinical outcomes were evaluated using the House-Brackmann (H-B) grading system and Emotrics software.
Results
All patients in group 1 (n = 4) achieved H-B grade IV facial function and showed improvements in the oral commissure angle at rest (preoperative vs. postoperative difference, 6.48° ± 0.77°) and while smiling (13.88° ± 2.00°). In groups 2 and 3, the oral commissure angle slightly improved at rest (group 2: 0.95° ± 0.53°, group 3: 1.35° ± 1.02°) and while smiling (group 2: 2.06° ± 0.67°, group 3: 1.23° ± 0.56°). In group 1, reduced tongue morbidity was found in patients who underwent ansa cervicalis transfer.
Conclusion
Facial reanimation with hypoglossal nerve transfer, in combination with hypoglossal nerve neurotization using the ansa cervicalis for complete facial palsy patients, might enable favorable facial reanimation outcomes and reduce tongue morbidity. Facial reanimation using the ansa cervicalis or sural nerve for incomplete facial palsy patients did not lead to remarkable improvements, but it warrants further investigation.
Keywords: Cervical plexus, Facial paralysis, Hypoglossal nerve
INTRODUCTION
Numerous strategies are used to manage facial palsy. Various surgical treatments have been utilized as dynamic procedures for facial paralysis [1], including cross-face nerve grafting; transfers of the hypoglossal nerve, masseter nerve [2], and ansa cervicalis [3]; temporalis muscle transfer [4]; and functional muscle transpositions. In 1903, Korte [5] introduced hypoglossal nerve transfer with end-to-end coaptation, which has been considered as the traditional method. Although directly transferring the hypoglossal nerve to the facial nerve improves facial tone, it has an inherent disadvantage of hemitongue atrophy [6]. Using the hypoglossal nerve in the “babysitter” procedure can resolve hemitongue morbidity, but may result in weak facial tone [7].
The ansa cervicalis is a less commonly used option for nerve reconstruction. The ansa cervicalis is primarily used for recurrent laryngeal nerve reconstruction, and has the advantage of minimizing donor morbidity [8,9]. Furthermore, several studies have reported favorable results for the use of the ansa cervicalis in facial reanimation [3,10]. With this background, we conducted a retrospective analysis of cases wherein we attempted to transfer the hypoglossal nerve to the facial nerve with neurotization of the ansa cervicalis onto the distal stump of the hypoglossal nerve in order to reduce donor morbidity. Not only we used the ansa cervicalis to minimize donor morbidity, but also we used the nerve, as well as sural nerve, to improve the motor function of the facial nerve. The present study evaluated the clinical outcomes of these methods.
METHODS
Patient selection
Fifteen patients who underwent facial reanimation with hypoglossal nerve transfer from 2005 to September 2019 were retrospectively reviewed. Those who did not have sufficient medical data or had a follow-up period shorter than 2 years were excluded. The following data were collected from patients’ medical records: age, sex, underlying disease, follow-up period, etiology, duration of palsy, surgical method, adjuvant surgical method, House-Brackmann (H-B) grade, tongue atrophy, and tongue deviation. The Institutional Review Board of our institution approved this study (IRB No. 2020-11-032).
Surgical indication and methods
We performed electromyography and nerve conduction studies on patients for a 2-year period at 3-month intervals. Group 1 contained patients with complete facial palsy that had lasted for less than 2 years. This group underwent surgery using hypoglossal nerve transfer with end-to-end coaptation.
In this study, we coined the term “booster reanimation” to refer to facial reanimation in patients with incomplete facial palsy who had some degree of facial nerve function.
Group 2 contained patients with incomplete facial palsy with no improvement during the follow-up period. This group underwent booster reanimation using the ansa cervicalis as the donor nerve. Similarly, group 3 contained patients with incomplete facial palsy who received booster reanimation with the sural nerve as the donor nerve.
A single experienced surgeon performed all procedures. Group 1 underwent facial reanimation with hypoglossal nerve transfer, as well as hypoglossal nerve neurotization using the ansa cervicalis. Facial nerve dissection was proximally performed to secure 2–3 cm of the facial nerve, which was cut in front of the stylomastoid foramen and turned over for coaptation with the hypoglossal nerve. The hypoglossal nerve was identified and dissected along the posterior belly of the digastric muscle. The bifurcation of the hypoglossal nerve to the ansa cervicalis was identified. The ansa cervicalis was dissected distally from the hypoglossal nerve to expose the primary branch, which is usually the nerve running to the superior belly of the omohyoid muscle. The ansa cervicalis with the primary branch was ligated and cut just distal to this branch. Therefore, it was possible to harvest the ansa cervicalis with a length of 6 cm. If necessary, it could be harvested with a length of 7 cm by dissecting the fascicle of the proximal nerve root on the bifurcation area of the superior root of the ansa cervicalis and hypoglossal nerve. The main trunk of the hypoglossal nerve was ligated and cut just before it entered the mylohyoid muscle. The whole complex was then rotated superiorly, and the proximal stump of the hypoglossal nerve was neurotized end-to-end with the distal stump of the facial nerve. Subsequently, the distal stump of the hypoglossal nerve was neurotized with the proximal stump of the ansa cervicalis (i.e., the branch running to the superior belly of the omohyoid muscle) in the same manner.
In group 2 (i.e., patients who underwent facial nerve booster reanimation using the ansa cervicalis), the facial nerve and ansa cervicalis were exposed in the same manner as described above. The ansa cervicalis was cut at the first or second branch depending on the length necessary to transpose the nerve to the facial nerve. The distal end and its branches were ligated and cut to mobilize the nerve, and the nerve was transposed to the facial nerve. Subsequently, the cut distal end of the ansa cervicalis was sutured to the epineural window of the facial nerve in an end-to-side fashion.
In group 3 (i.e., patients who underwent facial nerve booster reanimation with sural nerve interposition grafting between the hypoglossal nerve and facial nerve), the facial and hypoglossal nerves were exposed in the same manner as described above. The sural nerve was harvested with a length of 6–7 cm and the nerve’s both ends were sutured by end-to-side technique the epineural window of the hypoglossal and facial nerves (Fig. 1).
Fig. 1.
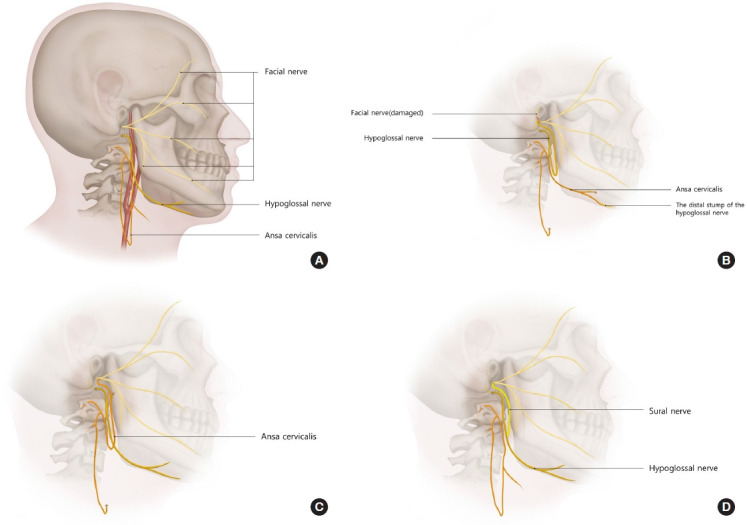
Schematic illustration of the surgical methods. (A) Normal anatomy. (B) Facial reanimation with hypoglossal nerve transfer and the distal stump of hypoglossal nerve neurotization using the ansa cervicalis. (C) Facial nerve booster reanimation using the ansa cervicalis with end-to-side coaptation. (D) Facial nerve booster reanimation with sural nerve interposition graft between the hypoglossal nerve and facial nerve.
Evaluation and data analysis
Clinical outcomes were evaluated at 3-month intervals from before surgery to 24 months after surgery. Photographs and videos at rest and while smiling were used for this evaluation. Preoperative and postoperative facial nerve function was measured using the H-B grading system and Emotrics software. Postoperative tongue function was evaluated using the Martins score (Table 1) [11]. Adjuvant surgery was performed if necessary after the 24-month evaluation.
Table 1.
Scale describing the grade of tongue dysfunction after hypoglossal-facial neurorrhaphy
| Grade | Description |
|---|---|
| 1 | Normal |
| 2 | Discrete hemiatrophy, no deviation |
| 3 | Mild hemiatrophy, tongue deviation < 30˚ |
| 4 | Severe hemiatrophy, tongue deviation > 30˚ |
Reprinted from Martins et al. Neurosurgery 2008;63:310-6, with permission from Oxford University Press [11].
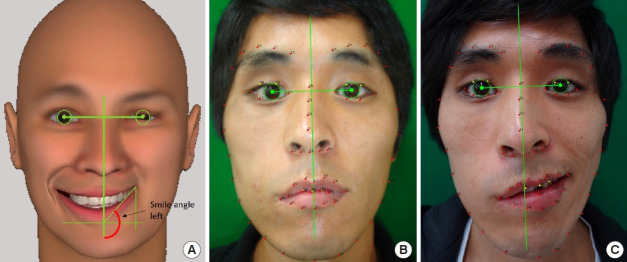
To analyze frontal-view photographs of each patient, we used Emotrics software, which automatically placed facial landmark dots on an uploaded image (Fig. 2) [12]. This method was applied to calculate the oral commissure angle both at rest and while smiling.
Fig. 2.
(A) An example of the oral commissure angle while smiling in a left facial palsy patient virtually measured using the Emotrics software. The following is an example of a 26-year-old man with right facial palsy after trauma. (B) Analysis of a clinical photograph at rest. (C) Analysis of a clinical photograph while smiling.
Statistical analysis was performed using SPSS version 26 (IBM Corp., Armonk, NY, USA), with statistical significance set at p < 0.05. The Kruskal-Wallis test was used to compare the groups, and post-hoc testing was performed using the Bonferroni method.
RESULTS
Table 2 summarizes patients’ baseline characteristics. In total, 15 patients (4 men, 11 women) with a mean age of 43.7 ± 20.3 years (range, 16–73 years) were evaluated. The average duration of preoperative palsy was 16.2 months (range, 6–24 months) in group 1, 112.16 months (range, 12–240 months) in group 2, and 114.8 months (range, 17–329 months) in group 3. The etiologies of palsy were vestibular schwannoma (seven cases), congenital facial palsy (one case), trauma (two cases), Bell’s palsy (one case), brain tumor-related (two cases), and Ramsay Hunt syndrome (two cases). In group 1, only one patient underwent hypoglossal nerve transfer without ansa cervicalis nerve transfer (patient 4).
Table 2.
Patient summary
| No. | Age (yr) | Sex | Etiology | Group | Preoperative H-B grade | Postoperative H-B grade | Additional reanimation surgery | Minor adjuvant surgery | Duration of preoperative palsy (mo) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | Female | Vestibular schwannoma | 1 | VI | IV | 6 | ||
| 2 | 56 | Female | Ramsay Hunt syndrome | 1 | VI | IV | Fascia lata sling | 13 | |
| 3 | 72 | Female | Vestibular schwannoma | 1 | VI | IV | 22 | ||
| 4 | 66 | Male | Vestibular schwannoma | 1 | VI | IV | 24 | ||
| 5 | 21 | Female | Vestibular schwannoma | 2 | IV | III | Fascia lata sling | 27 | |
| Lateral epicanthoplasty | |||||||||
| 6 | 57 | Female | Bell’s palsy | 2 | III | III | Fascia lata sling | 204 | |
| 7 | 49 | Female | Trauma | 2 | III | III | Brow lift | 240 | |
| 8 | 45 | Male | Vestibular schwannoma | 2 | IV | IV | Fascia lata sling | 51 | |
| 9 | 31 | Female | Vestibular schwannoma | 2 | III | III | Brow lift | 132 | |
| 10 | 16 | Male | Ramsay Hunt syndrome | 2 | II | II | Brow lift | 12 | |
| 11 | 27 | Female | Congenital | 3 | II | II | 329 | ||
| 12 | 71 | Female | Vestibular schwannoma | 3 | IV | IV | Temporalis transfer | Brow lift | 21 |
| 13 | 26 | Male | Trauma | 3 | IV | III | 17 | ||
| 14 | 21 | Female | Brain tumor | 3 | IV | IV | Fascia lata sling | 27 | |
| 15 | 25 | Female | Brain tumor | 3 | III | III | 180 |
H-B grade, House-Brackmann grade.
All patients in group 1 displayed improvements in the H-B grade from VI to IV. One patient in group 2 showed improvement from grade III to grade II, whereas one patient in group 3 exhibited improvement from grade IV to grade III. In group 1, the oral commissure angle significantly improved after surgery (at rest, 9.96° ± 1.36° before surgery vs. 3.47° ± 0.68° after surgery; while smiling, 18.02° ± 1.79° before surgery vs. 4.14° ± 0.76° after surgery). Group 2 showed slight improvements in the oral commissure angle (at rest, 7.68° ± 1.27° before surgery vs. 6.73° ± 1.48° after surgery; while smiling, 10.18° ± 1.03° before surgery vs. 8.12° ± 0.72° after surgery), as did group 3 (at rest, 8.25° ± 1.05° before surgery vs. 6.89° ± 0.67° after surgery; while smiling, 9.15° ± 0.94° before surgery vs. 7.92° ± 0.70° after surgery) (Table 3).
Table 3.
Angle of oral commissure
| Variable | Group 1 | Group 2 | Group 3 | p-valuea) |
|---|---|---|---|---|
| At rest (˚), mean±SD | ||||
| Preoperative | 9.96 ± 1.36 | 7.68 ± 1.27 | 8.25 ± 1.05 | |
| Postoperative | 3.47 ± 0.68 | 6.73 ± 1.48 | 6.89 ± 0.67 | |
| Change after surgery | 6.48 ± 0.77b) | 0.95 ± 0.53c) | 1.35 ± 1.02b),c) | 0.02 |
| While smiling (˚), mean±SD | ||||
| Preoperative | 18.02 ± 1.79 | 10.18 ± 1.03 | 9.15 ± 0.94 | |
| Postoperative | 4.14 ± 0.76 | 8.12 ± 0.72 | 7.92 ± 0.70 | |
| Change after surgery | 13.88 ± 2.00b) | 2.06 ± 0.67b),c) | 1.23 ± 0.56c) | 0.01 |
Group 1, facial reanimation with hypoglossal nerve transfer; group 2, facial reanimation using the ansa cervicalis; group 3, sural nerve interposition grafting between the hypoglossal nerve and facial nerve.
Kruskal-Wallis test;
Post-hoc test was performed using the Bonferroni method. There was a significant difference between group 1 and group 2 at rest, and group 1 and group 3 while smiling.
Minor adjuvant surgery was only considered in one case in group 1 (33.3%). Lifting procedures were relatively common in group 2 and group 3 (six [100%] and two [40%] cases, respectively). In group 3, additional temporalis muscle transfer was performed in one case. Three patients in group 1 achieved a grade 3 Martins score, indicating mild tongue atrophy and deviation. There were no problems with swallowing and speech (Fig. 3). In contrast, the patient who underwent only hypoglossal nerve transfer to the facial nerve attained a grade 4 Martins score, with moderate tongue atrophy and deviation. Representative preoperative and postoperative photographs for each group are presented in Figs. 4-6.
Fig. 3.
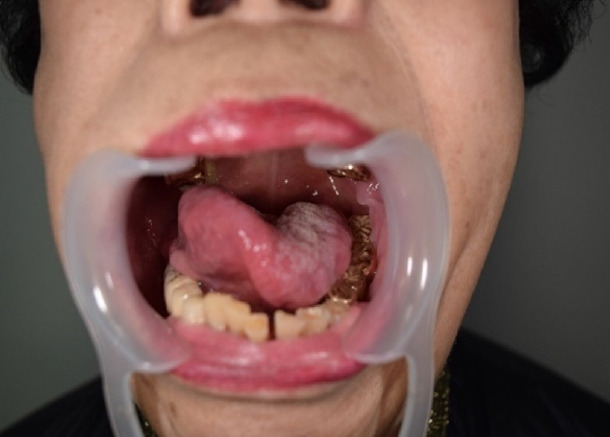
Postoperative tongue movement in group 1 (facial reanimation with hypoglossal nerve transfer). A 56-year-old woman (Patient 2) underwent hypoglossal nerve transfer, as well as hypoglossal nerve neurotization using the ansa cervicalis. This patient achieved a grade 3 Martins score with some remaining tongue motor function on the ipsilateral side of facial palsy.
Fig. 4.
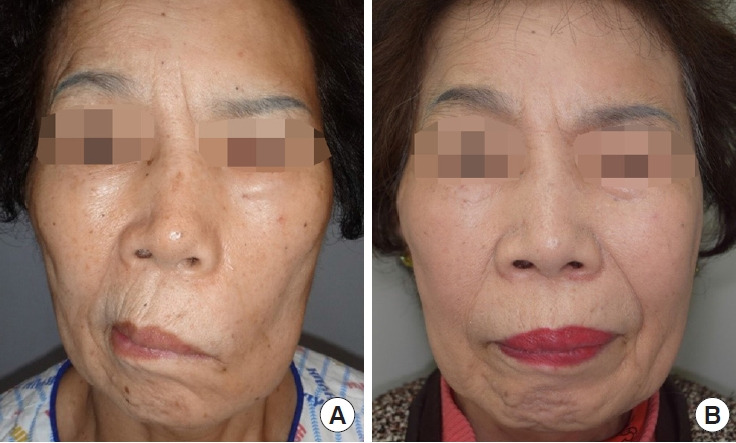
Patient 3: a 72-year-old woman in group 1 (facial reanimation with hypoglossal nerve transfer) with left hemifacial palsy. (A) Preoperative photograph at rest. (B) Two-year postoperative photograph at rest.
Fig. 5.
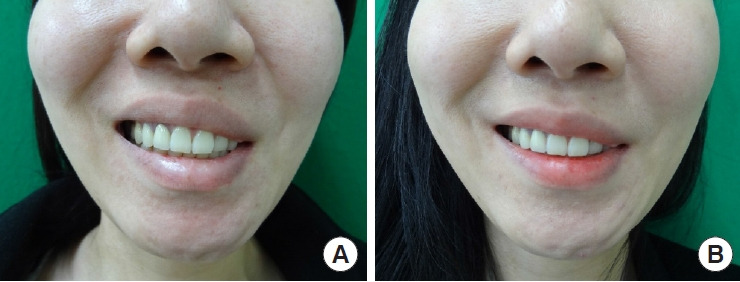
Patient 5: a 21-year-old woman in group 2 (facial reanimation using the ansa cervicalis) with left hemifacial palsy. (A) Preoperative photograph while smiling. (B) Two-year postoperative photograph while smiling.
Fig. 6.
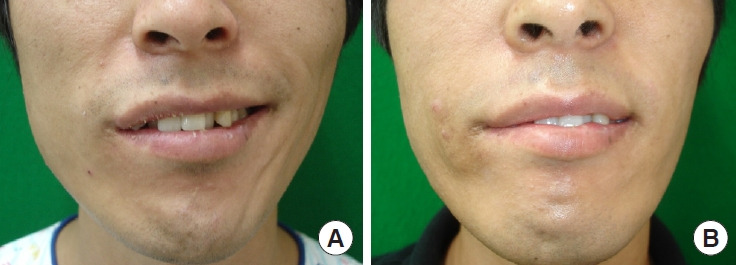
Patient 13: a 26-year-old man in group 3 (facial reanimation with sural nerve interposition grafting between the hypoglossal nerve and facial nerve) with right hemifacial palsy. (A) Preoperative photograph while smiling. (B) Two-year postoperative photograph while smiling.
DISCUSSION
Debate continues regarding which cranial nerves should be used for neurotization of the damaged facial nerve. Three methods have been conventionally used: (1) cross-face nerve grafting, (2) hypoglossal nerve transfer, and (3) masseteric nerve transfer. A benefit of the cross-face nerve graft technique is the production of truly spontaneous emotive movement using the native facial nerve. The masseter nerve transfer technique is associated with earlier nerve function recovery and minimal morbidity, whereas hypoglossal nerve transfer results in a more natural resting tone [6]. Although each method has its own advantages, we focused on hypoglossal nerve transfer to achieve powerful facial tone and movement. We also attempted to transfer the ansa cervicalis because its sacrifice results in less donor morbidity [13,14].
In our study, all patients in group 1 showed improvement in the H-B grade from VI to IV, whereas only one patient in each booster reanimation group displayed improvement in the H-B grade. As evaluated by the postoperative changes in the oral commissure angle, the effects were weaker in groups 2 and 3 than in group 1. The difference between these groups was statistically significant (p < 0.05). All patients in the booster reanimation groups had incomplete facial palsy with some remaining facial nerve function, but the improvement was insufficient in comparison to group 1. Adjuvant surgery was performed in nine patients, eight of whom belonged to the booster reanimation groups. This suggests that facial tone was not adequately improved in the booster reanimation groups.
The ansa cervicalis has been used for recurrent laryngeal nerve reconstruction after the resection of thyroid malignancies with nerve invasion. It has the advantage of avoiding donor morbidity because it has multiple anastomoses within the cervical plexus [13,14]. Considering the topographical and morphological variations in the ansa cervicalis, we selected the superior root of the ansa cervicalis, which is anatomically stable and easier to approach. It is also closest to the facial nerve and hypoglossal nerve [15]. Other studies also reported that excellent surgical outcomes could be achieved by using the superior root of the ansa cervicalis as a donor nerve, although few cases have been reported with reinnervation of the facial nerve [8-10]. Therefore, we attempted to boost the facial nerve using the ansa cervicalis. The improvement in facial tone was similar to that in group 3, but surgery in group 2 was simpler because the donor nerve was in the same operative field. Moreover, while there were no cases of donor morbidity when the ansa cervicalis was sacrificed in group 2, donor morbidity (e.g., loss of sensation) occurred in group 3.
Ozsoy et al. [16] reported that the axons in the hypoglossal nerve do not travel in a strict linear path through the epineurium. Sequential neuronal cross-sectional studies have often reported twisting axonal pathways through the nerve [17]. Therefore, splitting the hypoglossal nerve is likely to damage fascicles, leading to the loss of axons, which may in turn result in unpredictable outcomes related to functional aspects of both the facial muscles and the tongue [18]. For these reasons, the split type of hypoglossal-to-facial nerve coaptation was not considered. Instead, the entire nerve was transferred to obtain a more predictable result.
To reduce tongue morbidity, we proposed end-to-end coaptation of the distal stump of the hypoglossal nerve with the ansa cervicalis superior root. The patients who underwent this procedure achieved a grade 3 Martins score with slight movement on the ipsilateral side of facial palsy. We attempted to use the ansa cervicalis as a booster, but there was no significant improvement. In our opinion, it is better to use the ansa cervicalis with hypoglossal nerve neurotization than it is to perform facial nerve booster reanimation.
This study had some limitations. First, the duration of facial palsy in our cases ranged from 12 months to 27 years, which far exceeded the appropriate time for surgery and made it difficult to expect favorable recovery [19,20]. Second, as this was a retrospective study, it was not possible to control the number of patients per group. The small number of patients in this study also hinders the generalizability of the findings. Third, we were unable to quantify the clinical relationship between the axon fiber number and facial movement.
In conclusion, end-to-end hypoglossal-to-facial nerve coaptation resulted in favorable outcomes. Subsequent morbidity could be reduced through ansa cervicalis transfer. Booster reanimation using the sural nerve and ansa cervicalis slightly improved the oral commissure angle; however, the effect of booster reanimation was not significant.
Abbreviations
- H-B grade
House-Brackmann grade
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB No. 2020-11-032) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this study design is a retrospective review.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: Seong Oh Park, Hee Chang Ahn. Data curation: Won Young Koo, Seong Oh Park. Formal analysis: Won Young Koo, Seong Oh Park. Methodology: Seong Oh Park. Writing - original draft: Won Young Koo. Writing - review & editing: Won Young Koo, Seong Oh Park, Hee Chang Ahn. Software: Soo Rack Ryu. Supervision: Hee Chang Ahn. All authors read and approved the final manuscript.
REFERENCES
- 1.Oh TS, Kim HB, Choi JW, Jeong WS. Facial reanimation with masseter nerve-innervated free gracilis muscle transfer in established facial palsy patients. Arch Plast Surg. 2019;46:122–8. doi: 10.5999/aps.2018.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park H, Jeong SS, Oh TS. Masseter nerve-based facial palsy reconstruction. Arch Craniofac Surg. 2020;21:337–44. doi: 10.7181/acfs.2020.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipper J, Arndt S, Maier W, Spetzger U, Ridder GJ. Paralyzed face. Ansa-cervicalis-nervi-hypoglossi. Chirurg. 2005;76:47–53. doi: 10.1007/s00104-004-0883-z. [DOI] [PubMed] [Google Scholar]
- 4.Kwon BS, Sun H, Kim JW. Modified temporalis tendon transfer extended with periosteum for facial paralysis patients. Arch Craniofac Surg. 2020;21:351–6. doi: 10.7181/acfs.2020.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korte W. A case of nerve transfer from the facial nerve to the hypoglossal nerve. Dtsch Med Wochenschr. 1903;29:293–5. [Google Scholar]
- 6.Jandali D, Revenaugh PC. Facial reanimation: an update on nerve transfers in facial paralysis. Curr Opin Otolaryngol Head Neck Surg. 2019;27:231–6. doi: 10.1097/MOO.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 7.Terzis JK, Tzafetta K. The “babysitter” procedure: minihypoglossal to facial nerve transfer and cross-facial nerve grafting. Plast Reconstr Surg. 2009;123:865–76. doi: 10.1097/PRS.0b013e31819ba4bb. [DOI] [PubMed] [Google Scholar]
- 8.Frazier CH. Anastomosis of the recurrent laryngeal nerve with the descendens noni: in cases of recurrent paralysis. JAMA. 1924;83:1637–41. [Google Scholar]
- 9.Frazier CH. Treatment of recurrent laryngeal nerve paralysis by nerve anastomosis. Surg Gynecol Obstet. 1926;43:134–9. [Google Scholar]
- 10.Leong SC, Lesser TH. Long-term outcomes of facial nerve function in irradiated and nonirradiated nerve grafts. Ann Otol Rhinol Laryngol. 2013;122:695–700. doi: 10.1177/000348941312201106. [DOI] [PubMed] [Google Scholar]
- 11.Martins RS, Socolovsky M, Siqueira MG, Campero A. Hemi-hypoglossal-facial neurorrhaphy after mastoid dissection of the facial nerve: results in 24 patients and comparison with the classic technique. Neurosurgery. 2008;63:310–6. doi: 10.1227/01.NEU.0000312387.52508.2C. [DOI] [PubMed] [Google Scholar]
- 12.Guarin DL, Dusseldorp J, Hadlock TA, Jowett N. A machine learning approach for automated facial measurements in facial palsy. JAMA Facial Plast Surg. 2018;20:335–7. doi: 10.1001/jamafacial.2018.0030. [DOI] [PubMed] [Google Scholar]
- 13.Prades JM, Gavid M, Dubois MD, Dumollard JM, Timoshenko AT, Peoc’h M. Surgical anatomy of the ansa cervicalis nerve: which branch to use for laryngeal reinnervation in humans? Surg Radiol Anat. 2015;37:139–45. doi: 10.1007/s00276-014-1355-x. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Chen D, Chen S, Li D, Li M, Xia S, et al. Laryngeal reinnervation using ansa cervicalis for thyroid surgery-related unilateral vocal fold paralysis: a long-term outcome analysis of 237 cases. PLoS One. 2011;6:e19128. doi: 10.1371/journal.pone.0019128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhetri DK, Berke GS. Ansa cervicalis nerve: review of the topographic anatomy and morphology. Laryngoscope. 1997;107:1366–72. doi: 10.1097/00005537-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Ozsoy U, Hizay A, Demirel BM, Ozsoy O, Bilmen Sarikcioglu S, Turhan M, et al. The hypoglossal-facial nerve repair as a method to improve recovery of motor function after facial nerve injury. Ann Anat. 2011;193:304–13. doi: 10.1016/j.aanat.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, Suh MJ, Kim JW, Cho HS, Moon IS. Facial reanimation using hypoglossal-facial nerve anastomosis after schwannoma removal. Acta Otolaryngol. 2017;137:99–105. doi: 10.1080/00016489.2016.1212398. [DOI] [PubMed] [Google Scholar]
- 18.Shipchandler TZ, Seth R, Alam DS. Split hypoglossal-facial nerve neurorrhaphy for treatment of the paralyzed face. Am J Otolaryngol. 2011;32:511–6. doi: 10.1016/j.amjoto.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Kim SH, Hwang JH, Kim KS. Sensory recovery after infraorbital nerve avulsion injury. Arch Craniofac Surg. 2020;21:244–8. doi: 10.7181/acfs.2020.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco-Vidal V, Blanchet H, Liguoro D, Darrouzet V. Side-to-end ypoglossal-facial nerve anastomosis with intratemporal facial nerve ranslocation. Long-term results and indications in 15 cases over 10 years. Rev aryngol Otol Rhinol (Bord) 2006;127:97–102. [PubMed] [Google Scholar]