Abstract
Introduction:
Although hand temperature and electromyograph biofeedback have evidence for migraine prevention, to date, no study has evaluated heartrate variability (HRV) biofeedback for migraine.
Methods:
2-arm randomized trial comparing an 8-week app-based HRV biofeedback (HeartMath) to waitlist control. Feasibility/acceptability outcomes included number and duration of sessions, satisfaction, barriers and adverse events. Primary clinical outcome was Migraine-Specific Quality of Life Questionnaire (MSQv2).
Results:
There were 52 participants (26/arm). On average, participants randomized to the Hearthmath group completed 29 sessions (SD=29, range: 2–86) with an average length of 6:43 minutes over 36 days (SD=27, range: 0, 88) before discontinuing. 9/29 reported technology barriers. 43% said that they were likely to recommend Heartmath to others. Average MSQv2 decreases were not significant between the Heartmath and waitlist control (estimate = 0.3, 95% CI = −3.1 – 3.6). High users of Heartmath reported a reduction in MSQv2 at day 30 (−12.3 points, p=0.010) while low users did not (p=0.765).
Discussion:
App-based HRV biofeedback was feasible and acceptable on a time-limited basis for people with migraine. Changes in the primary clinical outcome did not differ between biofeedback and control; however, high users of the app reported more benefit than low users.
Keywords: Migraine, mHealth, biofeedback, heartrate variability, smartphone, behavioral therapy
1. Introduction
Migraine is a costly neurologic pain condition that affects over 40 million Americans and is the second most disabling condition worldwide,1,2 accounting for 5.6% of years lived with disability.3 Biofeedback, cognitive behavioral therapy, and relaxation therapies are safe and well tolerated with level A evidence for migraine prevention4 and long lasting benefits.5 However, patient adherence to these therapies is often suboptimal.6,7 Mobile health (mHealth) tools may provide an avenue to improve access to evidence-based behavioral interventions for migraine.
Biofeedback is a behavioral intervention that uses visual and/or auditory feedback from autonomic physiologic processes to allow a person to gain insight into how his or her body is impacted by thoughts, feelings, and conscious physical actions like breathing and exercise.8 Early investigations demonstrated efficacy of thermal biofeedback for migraine.9 Recent evidence and advances in biofeedback have led many practitioners to embrace heart rate variability (HRV) biofeedback for the treatment of stress-related disorders.10 People with higher and more complex HRV oscillation are more emotionally and physically resilient, while those with low HRV have generally impaired functioning.11 Although data suggests people with migraine demonstrate lower HRV than controls,12 no randomized clinical trials have evaluated HRV biofeedback for migraine.
Mobile health (mHealth) tools deliver behavioral healthcare interventions via mobile devices such as smartphones and tablets.13 The mHealth delivery modality can overcome systematic barriers to accessing behavioral interventions for migraine, and provide a gateway to health behavior change. Time, cost and difficulty finding providers are the most common barriers to initiating behavioral therapy for migraine.7,14–18 mHealth can address these barriers for a variety of behavioral targets and health conditions, including migraine.13,19–23
The current study aimed to evaluate whether an 8-week program of application (app)-based HRV biofeedback (HeartMath) was feasible and acceptable, and attained a significant signal for superiority compared to wait list control in improving migraine quality of life (Migraine-Specific Quality of Life Questionnaire; MSQv2).24 Secondary outcomes were validated MIDAS,25 depression (Patient Health Questionnaire-8; PHQ-8),26 anxiety (Generalized Anxiety Disorder-7; GAD-7),27 and insomnia (Insomnia Severity Index; ISI).28 We hypothesized that the slope of reduction in each outcome from baseline to 3 months would be larger in the HRV group than the waitlist group.
2. Methods
We conducted a two-arm randomized clinical trial comparing HRV biofeedback using HeartMath to waitlist control. The study team was trained in using HeartMath.[Supplement 1] The study was approved by the Institutional Review Board of NYU Langone Health. All patients gave written informed consent. Clinical Trials registry can be found at http://clinicaltrials.gov; NCT04077658.
2.1. Participants
Using consecutive recruitment, we screened in the electronic health records and approached potentially eligible patients presenting at an urban headache center from June 17, 2019-October 17, 2019. Inclusion criteria included subjects who were 18+, were diagnosed with migraine (in accordance with ICHD3 criteria), had 4–20 headache days/month, had not utilized behavioral therapy for migraine within the past year, had access to a smartphone and wifi, spoke English, reported an education level of high school completion or higher, and agreed to not change migraine preventative treatments during the course of the study period. Figure 1 shows flow of participant recruitment.
Figure 1:

Recruitment Flow Diagram
June 17-October17 2019
2.2. Procedures
Participants were enrolled and completed baseline questionnaires on Day 0. Block randomization29 determined study arm designation. A study team member called patients on days 10, 30, 45, and 60 to troubleshoot problems and administer the follow-up assessment questionnaires. Primary and secondary outcomes data were captured on days 0, 30 and 60 via phone calls with a study team member who inputted dictated responses into REDCap Participants not reached by phone were sent REDCap email surveys with the questionnaires.29
2.3. Arms
HeartMath.
HeartMath is an mHealth intervention that trains people to regulate their breathing to increase HRV. The HeartMath Innerbalance Sensor secures to the earlobe and reports HRV to a smartphone app which displays the information in a sine-wave-like pattern; a smoother wave indicated a higher level of “heart rhythm coherence,” which is the internal measure of quality use for the Inner Balance technology developed by the HeartMath Institute. A study team member downloaded and demonstrated the HeartMath app on the participant’s personal smartphones [Supplement 2]. Participants were instructed to do biofeedback at least two times a day for five minutes, or once a day for ten minutes every day for the 60-day study period. Email reminders every three days provided migraine education and encouraged adherence.
Waitlist Control.
Control participants received the HearthMath app and sensor with instruction after the study concluded.
2.4. Measures
Details of the measures can be found in Supplement 3. Brief descriptions are as follows:
Baseline.
A detailed questionnaire about participants’ headache and other medical/psychiatric history.
Feasibility and Acceptability.
Length and number of sessions completed was collected in-app. Coherence was assessed as a measure of treatment fidelity. Coherence was defined as follows: 0.5 basic–good beginner level, 1.0 good, 2.0 very good, and 3.0+excellent. A higher level of coherence would indicate more precise and appropriate engagement, whereas a lower level of coherence may indicate breathing that is out-of-time with the app pacer or an unconnected device. A 5-item questionnaire assessed likelihood of recommending Heartmath to others, adverse events and barriers to using Heartmath.
Primary Clinical Outcome.
The Migraine-Specific Quality of Life Questionnaire Version 2.1 (MSQ), assesses the impact of migraine on quality of life.24 Fourteen items assess role restriction, role prevention and emotion function. Response options range from 1 (none of the time) to 6 (all of the time).
Secondary Clinical Outcomes.
The MIDAS is a 5-item survey assessing the number of days in the past 90 days participants reported role restriction or prevention.25 The GAD-727 and PHQ-826 assessed anxious and depressive symptoms. The Insomnia Severity Scale (ISI)28 assessed insomnia symptoms.
Treatment Facilitators.
Four 10-point rulers assessed self-efficacy and importance of behavioral treatment for managing migraine/headache.30,31
2.5. Analysis
A priori power analysis for the primary efficacy outcome indicated an n of 52 would provide a power of 0.95 a minimally important difference of d = 0.6 with moderate intraclass correlation estimates and alpha set at .05.
Demographics and primary and secondary outcomes at baseline were evaluated and visually inspected for distribution and outliers. Descriptive statistics were reported for all demographics and primary and secondary outcomes. Safety data and study flow were described. Categorical data were described using N and percentages; continuous data were described using means and standard deviations. Demographics and all primary and secondary outcomes were evaluated between treatment groups using t-tests (continuous, normal) Mann-Whitney U (continuous, non-normal) or chi-square (categorical). For all outcomes, random intercept mixed models for repeated measures evaluated changes over time in the intention to treat (ITT) (included all participants randomized to the Heartmath intervention group) and completer samples (all participants who completed the 8-week treatment or waitlist protocol).
Given the data on adherence issues in mHealth, we conducted a post-hoc latent class analysis.32–34 In the intervention group (n =26), weekly use was calculated using latent class analysis to group subjects into high and low users. Mixed models were used to evaluate group differences and changes over follow-ups in outcomes, app usage, coherence scores and attitudes toward behavioral therapy and the app.
A priori, a decision was made to set alpha at 0.05, two-tailed, for all inferential tests. SPSS v 25, SAS v 9.4, and R were used for all analyses.
3. Results
3.1. Background Data/Demographics
We enrolled 52 participants; the majority were White, Not-Hispanic and Female (Table 1). A substantial minority (38.5%, 20/52) of participants had sought care for migraine at the emergency room. Eight (16%) tried behavioral therapy (>1 year ago) and 17 (33%) had previously been recommended behavioral therapy but had never attended.
Table 1:
Demographic and baseline characteristics
| Participant Characteristics | Total N(%) or M(SD) (n=52) | Intervention Group N(%) or M(SD) (n=26) | Waitlist Control N(%) or M(SD) (n=26) | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Gender (n = 51) | ||||
| Female | 46 (88.5) | 24 (92.3) | 22 (84.5) | 0.605 |
| Male | 5 (9.6) | 2 (7.7) | 3 (11.5) | |
| Age | 42.2 (2.8) | 38.6 (13.2) | 45.7 (11.6) | 0.048 |
| Ethnicity | 0.149 | |||
| Hispanicor Latino | 2 (3.8) | 0 | 2 (7.7) | |
| Not Hispanicor Latino | 50 (96.2) | 26 (100) | 24 (92.6) | |
| Race | ||||
| White | 41 (78.8) | 20 (76.9) | 21 (80.8) | 0.734 |
| Non-White | 11 (21.2( | 6 (23.1) | 5 (19.2) | |
| Headache Clinical Characteristics | ||||
| Number HA days last month | 12.6 (5.8) | 11.9 (6.0) | 13.3 (5.5) | 0.380 |
| Acute Medication Days/Month | 10.1 (7.7) | 9.7 (7.8) (0–30) | 10.5 (7.8) | 0.722 |
| MSQL Role Function – Restrictive | 52.4 (15.1) | 48.9 (15.2) | 55.9 (14.2) | |
| MSQL Role Function – Preventive | 63.0 (16.6) | 62.5 (17.7) | 63.5 (15.4) | |
| MSQL Emotional Function | 43.8 (12.2) | 44.2 (13.9) | 43.3 (10.6) | |
| MIDAS | 0.778 | |||
| Severe Disability | 31 (59.6) | 16 (61.6) | 15 (57.7) | |
| Not Severe | 21 (40.4) | 10 (38.5) | 11 (42.3) | |
| Generalized Anxiety Disorder (GAD)-7 | 7 (4.7) | 8.5 (5.0) | 5.5 (3.9) | 0.022 |
| Patient Health Questionnaire (PHQ)-9 | 7.1 (5.5) | 7.8 (5.7) | 6.3 (5.3) | 0.327 |
| Insomnia Sleep Index (ISI) | 12.6 (6.9) | 13.3 (6.3) | 12 (7.5) | 0.511 |
| Self-reported psychiatric symptoms and Overlapping Pain Conditions | 15 (28.9) | 7 (26.9) | 8 (30.8) | 0.760 |
| Depression | ||||
| Anxiety (including OCD and PTSD) | 25 (48.1) | 14 (53.9) | 11 (42.3) | 0.405 |
| Insomnia | 16 (30.8%) | 6 (23.1%) | 10 (38.5%) | 0.229 |
| Irritable Bowel Syndrome (IBS) | 5 (9.6%) | 1 (3.8%) | 4 (15.4%) | 0.158 |
| Temporomandibular disorder | 7 (13.5%) | 5 (19.2%) | 2 (7.7%) | 0.223 |
| Fibromyalgia | 4 (7.7%) | 2 (7.7%) | 2 (7.7%) | 1 |
| Chronic Fatigue/Myalgic Encephalomyelitis | 3 (5.8%) | 1 (3.8%) | 2 (7.7%) | 0.552 |
| Endometriosis | 4 (7.7%) | 0 | 4 (15.4%) | 0.037 |
Note. MSQL = Migraine Specific Quality of Life; subscale scores range from 0–100. (do for all scales); OCD=Obsessive Compulsive Disorder; PTSD=Post-traumatic Stress Disorder
3.2. Feasibility and Acceptability
On average, there were 29 sessions (SD=29, range: 2–86) per person over the study period and it was used for 36 days (SD=27, range: 0, 88) before being discontinued. The average session length was 6 minutes and 43 seconds, with mean coherence scores of 3 (SD=1.5, range: 0.3–7) per session. Coherence scores did not significantly change over time (p=0.237), or with the length of sessions (p=0.072).
During follow-up data collection, some participants (9/26) reported a range of technical issues using the device from not realizing the device’s battery was low to more serious technical issues like device hardware breaking. There were no adverse effects reported related to the intervention; one participant reported that s/he had had pneumonia and it was difficult to take big breathes without feeling uncomfortable. At the final study visit, the most important reasons app/sensor was used or not used as reported during final study visit were not having the sensor at all times; forgetting to do the sessions; finding time in the day; unable to practice the exercise while having a headache/migraine due to symptoms like photophobia; feeling self-conscious using the sensor in public.[Supplement 4]
Regarding recommending this device to others, between 21–28% of participants responded “Not likely” and “Definitely not” at any given follow-up, 29–42% reported “Neutral”, and 31–43% reported “likely” or “very likely”. There also were no significant differences in responses to this question based on frequency of use (p=0.3158). At day 60, 33% of low users and 14% of the high users responded “Never” or “Rarely” to “I would recommend this device to others”. In the high users, 43% recommend it often or almost always, and another 43% would sometimes recommend inner balance sometimes whereas 25% of low users recommended inner balance almost always or often while 42% recommend it sometimes (Fishers exact p-value=0.890).
3.3. Primary and Secondary Outcomes
In the intention to treat (ITT) Cohort (n=52), on average, MSQv2 scores decreased by 1.6 per month in the waitlist group and 1.3 per month in the Heartmath group, however there was no change over time (p=0.1612); these slope differences were not significant (estimate = 0.3, 95% CI = −3.1 – 3.6). There were also no differences in the slopes of reduction for GAD (estimate = −0.4, 95% CI = −1.6 – 0.8), PHQ (estimate = −0.6, 95% CI = −1.8 – 0.6), or ISI (estimate = −0.6, 95% CI = −1.9 – 0.8). The odds ratio for the rate of severe MIDAS between groups was not significant (estimate=1.2 95%CI 0.3 – 4.3).
In the completer cohort (our sensitivity analysis) (n=46), MSQv2 scores were similar, 45(SD=15, n=22) in the intervention group and 44(SD=11, n=24) in the control group (p=0.834). On average, scores decreased by 1.6 per month in the waitlist group in and 1.4 in the Heartmath group however these differences in slopes were not significant (estimate = 0.2, 95% CI = −3.2 – 3.6). There were also no differences in the slopes of reduction for GAD (estimate = −0.4, 95% CI = −1.6 – 0.8), PHQ (estimate = −0.6, 95% CI = −1.8 – 0.6), or ISI (estimate = −0.5, 95% CI = −1.9 – 0.8). The odds ratio for the rate of severe MIDAS between groups was not significant (estimate=1.1 95%CI 0.3 – 4.1).
3.4. Latent Class Analysis to determine potential differences in outcomes between high and low users
App users averaged 6 sessions (SD=3, range: 1–17) over 4 days (SD=2) per week. A latent trajectory model was used to divide participants into two latent classes based on the patterns of days used each week (Figure 2a). Of the 19 participants with app usage data, 7 were placed into group 1, using the app 6 or more times per week in the first 2 weeks, 3.6 days/week throughout the 12 weeks, and averaged 64 sessions (SD=18) total and 12 participants were assigned to group 2 with much lower use throughout the study, averaging 0.5 days/week.
Figure 2a:

Latent Class Analysis of High versus Low HeartMath Users
As seen in Figure 2b, compared to baseline, MSQv2 scores were lower by an average of 12.3 points (p=0.010) at day 30 and 8.4 points (p=0.063) at day 60 in the high users than the low users, which did not see significant changes in scores at either day 30 (p=0.765) or day 60 (p=0.665).
Figure 2b:
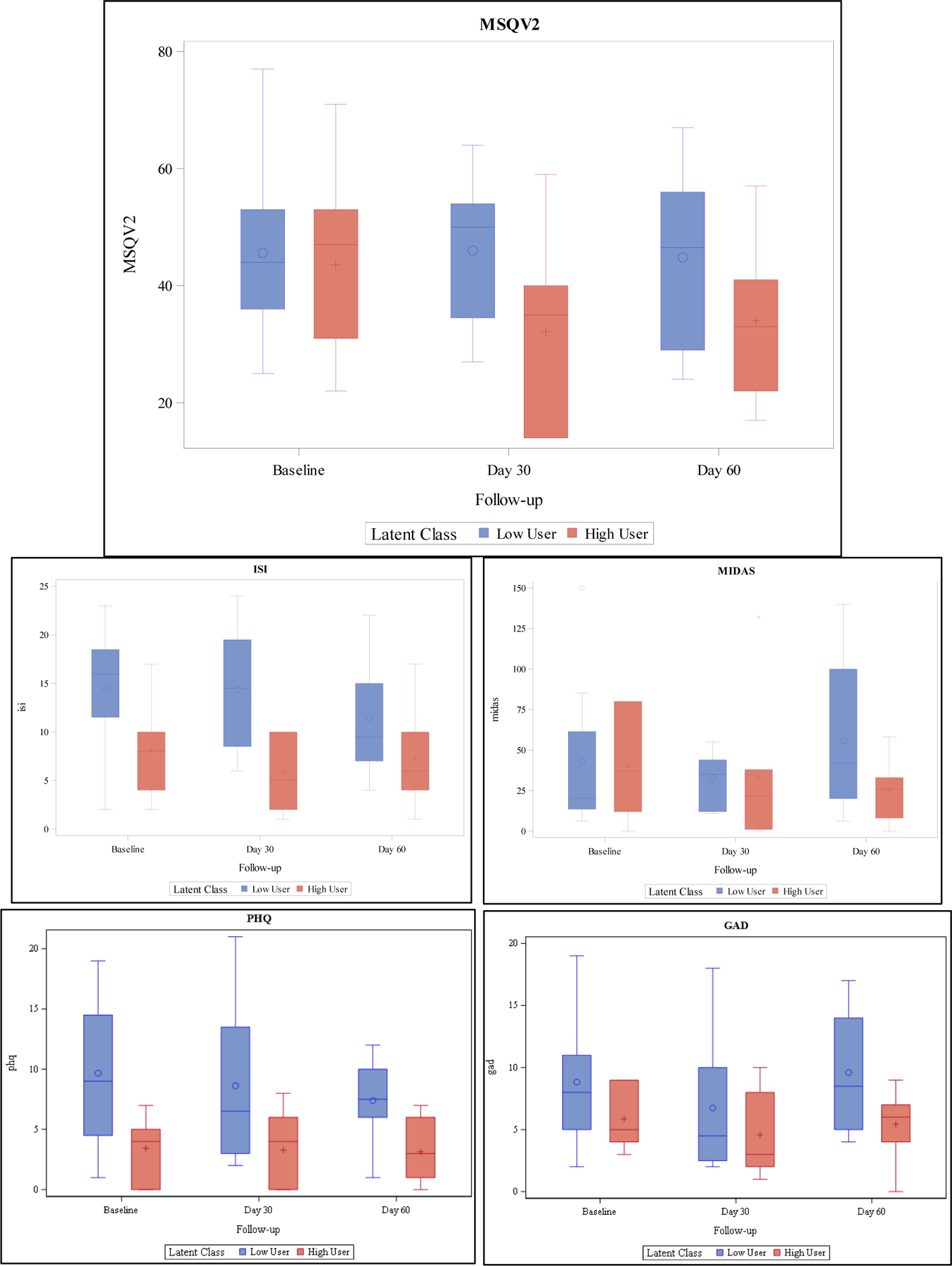
Primary and Secondary Outcomes Based on Latent Class Analysis
PHQ 8 scores were on average about 6.2 points (p=0.008) lower in high users than low users. The was no change in scores at day 30 compared to baseline in high users (p=0.749) or low users (p=0.556). At day 60 there was a significant 5-point decrease compared to baseline in low users scores (p=0.033) but high users scores had still not changed compared to baseline (p=0.215).
Compared to low users, ISI scores were an average 6.3 points (p=0.024) lower in high users overall. This effect did not significantly change at day 30 (p=0.40) or day 60 (p=0.510). Low users also did not see a significant change in scores at day 30 (p=0.942) or day 60 (p=0.121). There were no significant effects of either frequency of use or time on GAD or MIDAS scores.
3.5. Other migraine questionnaire outcomes
In the Heartmath group, the mean self-efficacy response was 6.0 (SD=1.8, range: 2–9) in the group at baseline and 5.6 (SD=2.1, range: 2–8) at the 60-day follow-up. In the control group, the average score was 6.1 (SD=2.4, range: 0–10) at baseline and 6.3 (SD=1.8, range: 1–10) at day 60. There was no significant difference in self-efficacy between groups (p=0.643) or over follow-ups (p=0.379). The means of differences in responses to self-efficacy based on app use at baseline were similar, 5.7 (SD=2.4, range: 2–8) and 5.8 (SD=1.7, range: 3–9) for high and low users, respectively. By day 60 high users mean self-efficacy increased to 5.9 (SD=2.3, range: 2–8) while low users decreased to 5 (SD=2, range: 2–8) however neither the group differences (p=0.6113) between high users, low users, and the control, or changes over time (p= 0.410) were significant.
The mean outcome expectancy in the Heartmath group was 5.5 (SD=1.9, range:0–8) at baseline and 5.9 (SD=2.6, range 0–10) at day 60 and in the control group it was 6 (SD=1.7, range:3–9) at baseline and 5.8 (SD=2.2, range 1–10) at day 60. Similar to self-efficacy, there was no significant difference in outcome expectancy between groups (p=578) or over follow-ups (p=104). Similar to what was seen for self-efficacy confidence in management, there were no significant differences in outcome expectancy based on high use, low use, and control groups (p=0.338) or over time (p=0.105). However, there was a significant increase in outcome expectancy at the 10 day follow-up (p=0.038) across all groups that attenuated by the end of the study. In the control group this increase was 0.3 points, in high users it was 1.7 points and in low users it was 0.6 points.
4. Discussion:
In this feasibility/acceptability study, participants with migraine were willing to engage in a time limited manner in smartphone/wearable HRV biofeedback. Additionally, while improvements in clinical outcomes were not different between the two groups, there was a significant difference in improvement in the primary clinical outcome, migraine quality of life scores, in high users compared to low users of the HeartMath device.
4.1. Feasibility
On average, participants completed 29 sessions for near 7 minutes/session (duration they had been instructed to achieve during enrollment). Many participants completed multiple sessions/day, with the mean number of days when more than one session was completed was 21. This uptake is remarkable given the strong need for preventive therapy in this sample; 85% had been on at least one type of preventive medication or supplement previously and about one third (32.7%, 17/52) had been previously referred for behavioral therapy but had not followed through (<12% had tried one of the top evidence-based behavioral therapies).
Coherence scores did not significantly change over time, or with the length of sessions. Since coherence is thought to be a mechanism of change for HRV, we had expected to see improvement in coherence over time. In this sample, participants appeared to quickly pick up the technique, but then demonstrated no further improvements over time. It is also important to note that coherence score is a criteria of utilization specific to this technology, and future studies might consider external measures from other HRV technologies and practitioners. Future studies might also consider utilizing a therapist in combination with the HRV biofeedback to ascertain whether the addition of a therapist to contextualize and optimize use of HRV can improve outcomes.
In this low cost, low touch study, migraine participants engaged in an mHealth HRV app at higher rates than often observed for mHealth therapies. Across mobile applications, app use falls to 23% within 3 days, and to less than 10% over 90 days.35 However, despite participants’ willingness to use the intervention in this study, engagement with the intervention was still suboptimal - a minority (37%, 7/19) were high users, or used the app 6 or more times per week in the first week, 3.6 days per week throughout the study period, and averaged 64 sessions total, and the majority of participants were low users who did not see any improvement in migraine quality of life. Further, engagement with the app dropped off for many users after one month: the average number of days between the first and last session was 36. Adherence was low likely because this was a low-touch, self-guided, smartphone-based study. As there was no therapist present during these practice sessions, this study did not capitalize on the common factors that enhance the therapeutic effect of most biofeedback interventions for headache, such as the therapeutic alliance.36 While attempts have been made to understand this lack of a traditional therapeutic relationship in self-guided programs, specifically to determine whether patients develop an attachment to the program or application,37 it is unclear if this connection is enough of a relationship to affect outcomes in a comparable way to traditional therapeutic relationships.36 Future work should enrich the application by employing other engagement factors (such as those used in behavioral economics) in an attempt to improve adherence.
The obstacles reported in this study (self-consciousness, difficulty finding time, having a migraine limits use, etc.) are not uncommon in wearable/app use. Prior research examining wearable technologies has identified feeling self-conscious and concerns over device durability as factors that may affect adherence.34,38–41 In our study, participants cited difficulty finding time in the day as a barrier, and reported higher levels of success with the intervention if a routine was formed. Having a migraine has been previously cited as a barrier towards practicing mHealth delivered behavioral therapy.42
4.2. Preliminary Efficacy
Our primary efficacy outcomes were examining the slope for delta MSQv2, and our secondary efficacy outcomes were examining the slope for delta PHQ 8, GAD 7 and ISI. These outcomes were not significant.
However, when we examine the latent class analysis data (usage data) as part of the secondary analyses to better understand how feasibility (usage) might play a role in efficacy, compared to baseline, MSQv2 scores were lower by an average of 12.3 points (p=0.01) at day 30 and 8.4 points (p=0.06) at day 60 in the high users than the low users, which did not see significant changes in scores at either day 30 (p=0.76) or day 60 (p=0.66). Thus, similar to other smartphone based behavioral intervention (PMR) for migraine prevention, high users of the behavioral intervention had better outcomes.22 In neither our ITT, completer analysis nor our latent class analyses were anxiety, depression or insomnia scores changed. This contrasts with previous HeartMath studies in other populations which have observed positive effects on emotional regulation/mood and anxiety.43–45
Baseline self-efficacy and outcome expectancies were not significantly associated with high or low use of the intervention. These cognitive factors are thought to be crucial for engagement in, and success of, behavioral treatments for headache. Indeed, prior studies of biofeedback for headache have demonstrated that self-efficacy is a key change mechanism.46,47 A core difference between these studies is the lack of therapist. Future studies should consider randomizing participants to biofeedback with and without a therapist to evaluate in what ways the addition of a therapist modifies the cognitive, affective, and physiologic benefits of biofeedback for migraine.
4.3. Role of psychiatric comorbidities
In our study population, 28.8% reported a prior history of depression, 44.4% reported a prior history of anxiety, and 30.8% reported a prior history of insomnia. There was no group difference in changes in PHQ8, GAD7 or ISI scores in our study. However, those who were low users were more likely to have more symptoms of depression; PHQ 8 scores were on average about 12.5 points lower in high users than low users. These results suggest that depression may be a barrier for engaging in self-directed behavioral migraine treatments. These results are similar to another smartphone based behavioral app intervention for migraine prevention which instead of using HRV biofeedback used a PMR behavioral intervention.22 In addition, they are similar to a study using online CBT whereby those with lower rates of depression did better.48 In contrast, people with depression were highly engaged with, and actually demonstrated larger reductions in migraine days and improvements in quality of life over the course of, a minimal therapist contact behavioral migraine treatment that included four monthly sessions with a therapist.49 Future studies should attempt to further elucidate the support needed to promote adherence to behavioral treatments for people with migraine and comorbid depressive symptoms. Despite demonstrated effectiveness of HRV biofeedback in the treatment of depression,50,51 anxiety,52 and possibly insomnia,53 the lack of significant changes in these comorbidities in our study population may be due to lower baseline depression, anxiety, and insomnia measures, and thus less likely to change as compared to studies focused primary on these factors.
4.4. Strengths
There are several strengths of this study: 1. This was a RCT assessing HRV biofeedback in a scalable, accessible manner. 2. Participants were instructed that they could not change their preventive medications so this study would only examine the efficacy of the HRV biofeedback. 3. We collected usage data directly from the phone’s application at the exit interview so the data was not based on just self-report.
4.5. Limitations
Our study was a convenience sample of people who sought care and were diagnosed with migraine in a tertiary care academic headache center. This was a pilot study and as such, the sample size was small, and the study duration was brief (2 months). The participants were not all naive to nonpharmacologic treatment for migraine as a substantial number of the participants had tried nonpharmacologic treatment for migraine previously; almost half (46.2%) had tried meditation and acupuncture, and just over a third (36.5%) had previously tried yoga. In the study, we did not assess whether changes to preventive medications did in fact occur during the study period. In addition, we did not examine whether participants incorporated these exercises into their daily lives and completed sessions without using the inner balance sensor. Finally, while coherence “levels” could be adjusted within the app to create higher levels of difficulty, we were unable to monitor this throughout the study.
4.6. Future Directions
Future work can examine more dosing considerations and methods to advance adherence. In this study, we examined whether brief sessions over time might improve our outcomes. Other studies might examine prolonged sessions to assess improvement over time. A study in perinatal depression examined the effect of two 30–60 minute sessions with the HeartMath sensor on depression.54 Another study with a HeartMath product examined HRV for craving for substance abuse. Participants were asked to practice HRV BFB for two 20-minute sessions each day, on their own for the 3-week study duration.55 Future studies might evaluate whether the incorporation of HRV in a relationship with a therapist improves outcomes for migraine. Future studies should also evaluate the effects of changing user settings for coherence difficulty affect outcomes and whether there might be methods for improving adherence to the application, such as gamification or peer support.
5. Conclusions
While this study did not demonstrate efficacy of HRV biofeedback to improve migraine quality of life compared to a waitlist control, subgroup analysis suggests adherence to the treatment could improve outcomes. Future studies should evaluate the efficacy of HRV biofeedback for migraine in the context of a relationship with a therapist, and should evaluate methods to improve adherence to biofeedback mHealth apps to improve access for people with migraine.
Supplementary Material
Acknowledgements
We would like to thank the following members of the Migraine HeartMath Study Group: Kaitlyn Toy; Jana Jaran; Tyler Gumpel, BS; Seher Ali; Talia Boyers, BA; Fatoumata Sow.
Dr. Minen received funding from the NIH NCCIH (K23 AT009706‐01) for salary support. Dr. Minen is also a recipient of the Doris Duke Fellowship to Retain Clinician Scientists.
Dr. Adhikari receives research support from the NIH and Johnson & Johnson.
Dr. Seng receives research support from the NINDS (K23 NS096107 PI: Seng) and has consulted for GlaxoSmithKline, Eli Lilly, and Click Therapeutics. Dr. Seng has received travel funds from the American Psychological Association, the American Academy of Neurology, the American Association of Pain Medicine Foundation, and the American Headache Society.
Funding:
This work was supported by the Doris Duke Charitable Foundation (Funds to Retain Clinical Scientists); the NIH NCCIH (K23 AT009706‐01).
Footnotes
Supplemental appendices: Includes recruitment flow-chart, data availability statement, consort checklist, and author credit checklist.
Clinical trial registry: http://clinicaltrials.gov; NCT04077658
Disclosures:
Ms. Corner reports no disclosures.
Dr. Berk receives honoraria from Medlink neurology and is on the advisory board for Biohaven.
Dr. Levitan reports no disclosures.
Mr. Friedman reports no disclosures.
This paper is being submitted on behalf of the Migraine HeartMath Study Group
The following are members of the Migraine HeartMath Study Group: Mia T. Minen,MD, MPH; Sarah Corner, BA; Thomas Berk, MD; Valeriya Levitan, MD; Steven Friedman, MS; Samrachana Adhikari, PhD; Elizabeth B. Seng, PhD; Kaitlyn Toy; Jana Jaran; Tyler Gumpel, BS, Seher Ali; Talia Boyers, BA; Fatoumata Sow.
Data availability statement:
De-identified data will be provided upon reasonable request from any qualified investigator in accordance with NYU Langone Health and NYU DOHMH data sharing policies.
References
- 1.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343–349. doi: 68/5/343 [pii]. [DOI] [PubMed] [Google Scholar]
- 2.Feigin V, Abajobir A, Abate K. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the global burden of disease study 2015 2017;16(11):877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J Headache Pain 2018;19(1):17–018-0846–2. doi: 10.1186/s10194-018-0846-2 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell J, Penzien D, Wall E. Evidence-based guidelines for migraine headache: Behavioral and physical treatments. American Academy of Neurology 2000(US Headache Consortium). [Google Scholar]
- 5.Andrasik F, Blanchard EB, Neff DF, Rodichok LD. Biofeedback and relaxation training for chronic headache: A controlled comparison of booster treatments and regular contacts for long-term maintenance. J Consult Clin Psychol 1984;52(4):609–615. doi: 10.1016/0304-3959(85)90211-8. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzawa Y, Lee YSC, Fraser F, et al. Barriers to behavioral treatment adherence for headache: An examination of attitudes, beliefs, and psychiatric factors. Headache 2019;59(1):19–31. doi: 10.1111/head.13429 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minen MT, Sahyoun G, Gopal A, et al. A pilot randomized controlled trial to assess the impact of motivational interviewing on initiating behavioral therapy for migraine. Headache 2020;60(2):441–456. doi: 10.1111/head.13738 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz M, Andrasik F. Biofeedback: A Practitioner’s guide 4th ed. New York, NY: Guilford Press.; 2016. [Google Scholar]
- 9.Andrasik F Biofeedback in headache: An overview of approaches and evidence. Cleve Clin J Med 2010;77 Suppl 3:S72–6. doi: 10.3949/ccjm.77.s3.13 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Goessl VC, Curtiss JE, Hofmann SG. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol Med 2017;47(15):2578–2586. doi: 10.1017/S0033291717001003 [doi]. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer PM, Gevirtz R. Heart rate variability biofeedback: How and why does it work? Front Psychol 2014;5:756. doi: 10.3389/fpsyg.2014.00756 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenig J, Williams DP, Kemp AH, Thayer JF. Vagally mediated heart rate variability in headache patients--a systematic review and meta-analysis. Cephalalgia 2016;36(3):265–278. doi: 10.1177/0333102415583989 [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Marcolino MS, Oliveira JAQ, D’Agostino M, Ribeiro AL, Alkmim MBM, Novillo-Ortiz D. The impact of mHealth interventions: Systematic review of systematic reviews. JMIR Mhealth Uhealth 2018;6(1):e23. doi: 10.2196/mhealth.8873 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minen MT, Azarchi S, Sobolev R, et al. Factors related to migraine patients’ decisions to initiate behavioral migraine treatment following a headache specialist’s recommendation: A prospective observational study. Pain Med 2018;19(11):2274–2282. doi: 10.1093/pm/pny028 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schafer AM, Rains JC, Penzien DB, Groban L, Smitherman TA, Houle TT. Direct costs of preventive headache treatments: Comparison of behavioral and pharmacologic approaches. Headache 2011;51(6):985–991. doi: 10.1111/j.1526-4610.2011.01905.x [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrasik F Behavioral treatment of headaches: Extending the reach. Neurol Sci 2012;33 Suppl 1:S127–30. doi: 10.1007/s10072-012-1073-2 [doi]. [DOI] [PubMed] [Google Scholar]
- 17.Ernst MM, O’Brien HL, Powers SW. Cognitive-behavioral therapy: How medical providers can increase patient and family openness and access to evidence-based multimodal therapy for pediatric migraine. Headache 2015;55(10):1382–1396. doi: 10.1111/head.12605 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minen M, Shome A, Halpern A, et al. A migraine management training program for primary care providers: An overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache 2016;56(4):725–740. doi: 10.1111/head.12803 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinkamp JM, Goldblatt N, Borodovsky JT, et al. Technological interventions for medication adherence in adult mental health and substance use disorders: A systematic review. JMIR Ment Health 2019;6(3):e12493. doi: 10.2196/12493 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grau-Pellicer M, Lalanza JF, Jovell-Fernandez E, Capdevila L. Impact of mHealth technology on adherence to healthy PA after stroke: A randomized study. Top Stroke Rehabil 2019:1–15. doi: 10.1080/10749357.2019.1691816 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: A randomized clinical trial. JAMA Psychiatry 2020. doi: 10.1001/jamapsychiatry.2019.4491 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minen MT, Adhikari S, Seng EK, et al. Smartphone-based migraine behavioral therapy: A single-arm study with assessment of mental health predictors. Nature Digital Medicine 2019;46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MM, Firth J, Minen M, Torous J. User engagement in mental health apps: A review of measurement, reporting, and validity. Psychiatr Serv 2019;70(7):538–544. doi: 10.1176/appi.ps.201800519 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ version 2.1). Headache 2000;40(3):204–215. doi: hed00030 [pii]. [DOI] [PubMed] [Google Scholar]
- 25.Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia 1999;19(2):107–14; discussion 74. doi: 10.1046/j.1468-2982.1999.019002107.x [doi]. [DOI] [PubMed] [Google Scholar]
- 26.Kroenkea K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokhada AH. The PHQ-8 as a measure of current depression in the general population. Journal of affective disorders 2009;114(1–3):163–173. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166(10):1092–1097. doi: 166/10/1092 [pii]. [DOI] [PubMed] [Google Scholar]
- 28.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bandura A Guide for creating self-efficacy scales. In: Pajares U, ed. Self-efficacy beliefs of adolescents Greenwich, CT: Information Age Publication, Inc.; 2006. [Google Scholar]
- 31.Miller W, Rollnick S. Motivational interviewing: Helping people change 3rd ed. New York: Guilford Press; 2013. [Google Scholar]
- 32.Druce KL, McBeth J, van der Veer SN, et al. Recruitment and ongoing engagement in a UK smartphone study examining the association between weather and pain: Cohort study. JMIR Mhealth Uhealth 2017;5(11):e168. doi: 10.2196/mhealth.8162 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.T Minen M, Adhikari S, K Seng E, et al. Smartphone-based migraine behavioral therapy: A single-arm study with assessment of mental health predictors. NPJ Digit Med 2019;2:46–019-0116-y. eCollection 2019. doi: 10.1038/s41746-019-0116-y [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelan ME, Orme MW, Kingsnorth AP, Sherar LB, Denton FL, Esliger DW. Examining the use of glucose and physical activity self-monitoring technologies in individuals at moderate to high risk of developing type 2 diabetes: Randomized trial. JMIR Mhealth Uhealth 2019;7(10):e14195. doi: 10.2196/14195 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen A New data shows losing 80% of mobile users is normal, and why the best apps do better AndrewChen.co Web site. https://andrewchen.co/new-data-shows-why-losing-80-of-your-mobile-users-is-normal-and-that-the-best-apps-do-much-better/. Updated 2019. Accessed 7/6, 2020.
- 36.Lopez A, Schwenk S, Schneck CD, Griffin RJ, Mishkind MC. Technology-based mental health treatment and the impact on the therapeutic alliance. Curr Psychiatry Rep 2019;21(8):76–019-1055–7. doi: 10.1007/s11920-019-1055-7 [doi]. [DOI] [PubMed] [Google Scholar]
- 37.Berry K, Salter A, Morris R, James S, Bucci S. Assessing therapeutic alliance in the context of mHealth interventions for mental health problems: Development of the mobile agnew relationship measure (mARM) questionnaire. J Med Internet Res 2018;20(4):e90. doi: 10.2196/jmir.8252 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher JM, Hammerla NY, Rochester L, Andras P, Walker RW. Body-worn sensors in parkinson’s disease: Evaluating their acceptability to patients. Telemed J E Health 2016;22(1):63–69. doi: 10.1089/tmj.2015.0026 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simone LK, Sundarrajan N, Luo X, Jia Y, Kamper DG. A low cost instrumented glove for extended monitoring and functional hand assessment. J Neurosci Methods 2007;160(2):335–348. doi: S0165-0270(06)00475-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 40.Cancela J, Pastorino M, Tzallas AT, et al. Wearability assessment of a wearable system for parkinson’s disease remote monitoring based on a body area network of sensors. Sensors (Basel) 2014;14(9):17235–17255. doi: 10.3390/s140917235 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson D, Malmgren K, Alt Murphy M. Wearable sensors for clinical applications in epilepsy, parkinson’s disease, and stroke: A mixed-methods systematic review. J Neurol 2018;265(8):1740–1752. doi: 10.1007/s00415-018-8786-y [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minen M, Morio K, Schaubhut K, Powers S, Lipton R, Seng E. Focus group findings on the migraine patient experience during research studies and ideas for future investigations. Cephalalgia December 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Zemon V, Lehrer P, et al. Emotion regulation after acquired brain injury: A study of heart rate variability, attentional control, and psychophysiology. Brain injury 2019;33(8). [DOI] [PubMed] [Google Scholar]
- 44.McAusland L, Addington J. Biofeedback to treat anxiety in young people at clinical high risk for developing psychosis. Early Interv Psychiatry 2018;12(4):694–701. doi: 10.1111/eip.12368 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beckham AJ, Greene TB, Meltzer-Brody S. A pilot study of heart rate variability biofeedback therapy in the treatment of perinatal depression on a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health 2013;16(1):59–65. doi: 10.1007/s00737-012-0318-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holroyd KA, Penzien DB, Hursey KG, et al. Change mechanisms in EMG biofeedback training: Cognitive changes underlying improvements in tension headache. J Consult Clin Psychol 1984;52(6):1039–1053. doi: 10.1037//0022-006x.52.6.1039 [doi]. [DOI] [PubMed] [Google Scholar]
- 47.Mizener D, Thomas M, Billings R. Cognitive changes of migraineurs receiving biofeedback training. Headache 1988;28(5):339–343. doi: 10.1111/j.1526-4610.1988.hed2805339.x [doi]. [DOI] [PubMed] [Google Scholar]
- 48.Christensen H, Griffiths K, Groves C, Korten A. Free range users and one hit wonders: Community users of an internet-based cognitive behaviour therapy program. Aust N Z J Psychiatry 2006;40(1):59–62. doi: ANP1743 [pii]. [DOI] [PubMed] [Google Scholar]
- 49.Seng EK, Holroyd KA. Psychiatric comorbidity and response to preventative therapy in the treatment of severe migraine trial. Cephalalgia 2012;32(5):390–400. doi: 10.1177/0333102411436333 [doi]. [DOI] [PubMed] [Google Scholar]
- 50.Siepmann M, Aykac V, Unterdorfer J, Petrowski K, Mueck-Weymann M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl Psychophysiol Biofeedback 2008;33(4):195–201. doi: 10.1007/s10484-008-9064-z [doi]. [DOI] [PubMed] [Google Scholar]
- 51.Karavidas MK, Lehrer PM, Vaschillo E, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z [doi]. [DOI] [PubMed] [Google Scholar]
- 52.Rozman D, Childre D, Rozman D. Overcoming emotional chaos: Eliminate anxiety, lift depression and create security in your life Jodere Group; 2002:303. [Google Scholar]
- 53.McLay RN, Spira JL. Use of a portable biofeedback device to improve insomnia in a combat zone, a case report. Appl Psychophysiol Biofeedback 2009;34(4):319–321. doi: 10.1007/s10484-009-9104-3 [doi]. [DOI] [PubMed] [Google Scholar]
- 54.Beckham AJ, Greene TB, Meltzer-Brody S. A pilot study of heart rate variability biofeedback therapy in the treatment of perinatal depression on a specialized perinatal psychiatry inpatient unit. Arch Womens Ment Health 2013;16(1):59–65. doi: 10.1007/s00737-012-0318-7 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eddie D, Kim C, Lehrer P, Deneke E, Bates ME. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Appl Psychophysiol Biofeedback 2014;39(3–4):181–192. doi: 10.1007/s10484-014-9251-z [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data will be provided upon reasonable request from any qualified investigator in accordance with NYU Langone Health and NYU DOHMH data sharing policies.


