Abstract
Practical relevance:
Human allergy to cats affects a substantial and growing proportion of the global population, and cat allergy is regarded as the third most common cause of human respiratory allergies, and the second most common indoor cause. Veterinarians will frequently encounter owners who are cat-allergic, and having an understanding of this disease and the methods available to help control the allergy will assist them in giving appropriate advice, alongside human healthcare professionals.
Aim:
The aim of this review is to summarise currently available data on the prevalence, causes, symptoms and control of human allergy to cats. In terms of managing cat allergy, the emphasis is on reviewing current and emerging modalities to reduce environmental exposure to cat allergens rather than on pharmacotherapy or immunotherapy, as it is in these areas in particular that the veterinarian may be able to offer help and advice to complement that of human healthcare professionals.
Evidence base:
The information in this review is drawn from the current and historical literature on human allergy to cats, and approaches to reduce exposure to cat allergens and manage symptoms of cat allergy.
Keywords: Allergy, human, management, diet
Prevalence of human allergy to cats
Inhaled aeroallergens are typically divided into outdoor (such as pollens and moulds) and indoor (such as house dust mite, cat, dog and certain insect allergens). 1 Climate and environmental conditions cause substantial geographical variations in the relative importance of different aeroallergens but globally domestic cats (Felis catus) are considered the second most common cause of indoor respiratory allergies and the third most common overall (after pollens and house dust mites).2-8
Respiratory and other allergies have become more common over recent decades for reasons that are complex and not fully understood, but are likely to include increased rates of allergen sensitisation along with genetics and environmental factors such as pollutants, irritants and infectious diseases.1,5,7,9-11
Sensitisation (production of allergen-specific IgE9) is necessary for signs of respiratory allergy to develop, but not all sensitised individuals develop allergy symptoms since this depends on many factors, including the level of sensitisation (amount of allergen-specific IgE produced), the ‘allergenicity’ and amount of exposure to the antigen, and other environmental factors.1,7,9,12–14 Cat allergy is reported to be approximately twice as common as dog allergy, 10 and many studies have reported a higher prevalence of sensitisation and/or allergy to cats.4,15–21 Even in studies showing similar rates of sensitisation to dogs and cats,7,11 cats may still be a more important cause of allergic symptoms due to quantitative and qualitative differences in the level of sensitisation, the allergenicity of cat antigens, and in exposure to allergens (which may be influenced by pet populations and differences in the physicochemical properties of the allergens).9,10,14,15,17,19,20,22–25
The prevalence of sensitisation to cats in different studies has typically been reported to be around 5–20%,6–8 ,10,21,26 and in patients with respiratory allergies may be as high as 20–30% or more,7,8,10,21,26 with pet allergies affecting an estimated 10–20% of the population worldwide.11 Cat allergy is therefore a major global problem.
Causes of cat allergy
Eight cat allergens are currently recognised by the World Health Organization/International Union of Immunological Societies (Table 1, allergen.org). 2 ’ 6 ’ 7 ’ 9 ’11,27 However, Fel d 1 is the only major antigen, and is by far the most important and potent allergen. Fel d 1 shares no significant cross-reactivity with other mammalian proteins’ 11 although it is also produced by other members of the Felidae family. 28 Around 90-96% of cat-allergic individuals are sensitised to Fel d 1’ and it is responsible for 60-90% of the total allergic reactivity seen in affected individuals. 2 ’ 6 ’9,11’19,29-31 The prevalence of reactivity to the other seven antigens in cat-allergic individuals is variable and typically 10-40%’6,11’14,29 often with lower levels of IgE. 6 Sequence homology between lipocalins of different species means that cross-reactivity is seen, for example between Fel d 4 and Can f 6, and Fel d 7 and Can f 1,11,32,33 which can result in cross-sensitivity in allergic individuals. Similarly, Fel d 2 is a minor cat allergen, but cross-reactivity with pork albumin (‘pork-cat syndrome’) means occasional individuals sensitised to Fel d 2 react to eating pork meat. 11
Table 1.
| Allergen | Protein family | Major source | Molecular mass |
|---|---|---|---|
| Fel d 1 | Secretoglobin | Dander, saliva | 38 kDa |
| Fel d 2 | Serum albumin | Dander, serum, urine | 69 kDa |
| Fel d 3 | Cystatin A | Dander | 11 kDa |
| Fel d 4 | Lipocalin | Saliva | 22 kDa |
| Fel d 5 | IgA | Saliva, serum | 400 kDa |
| Fel d 6 | IgM | Saliva, serum | 800-1000 kDa |
| Fel d 7 | Lipocalin | Saliva | 17.5 kDa |
| Fel d 8 | Latherin-like | Saliva | 24 kDa |
Fel d 1 is a secretoglobin and is a 38 kDa tetramer glycoprotein formed from two linked heterodimers.2,6,11 The major sources of Fel d 1 are the saliva and sebaceous glands (Figure 1),34-41 and some is also present in lacrimal and anal gland secretions and in urine.37,41,42 Skin production of Fel d 1 varies according to anatomical site, with the facial region reported to produce higher amounts than the chest. 43 Salivary Fel d 1 is distributed during grooming, 6 with papillae on the cat’s tongue efficiently wicking and depositingsaliva and Fel d 1 through the haircoat. 44 Grooming presumably also assists in distribution of Fel d 1 from sebaceous glands.
Figure 1.
Major sources of Fel d 1 present on the haircoat and shed into the environment are the saliva and sebaceous glands
Entire male cats (at least partially under the influence of testosterone) produce greater amounts of Fel d 1 than neutered males or females (irrespective of neuter status).45-49 Production is not affected by coat colour or hair length.40,49-51 Production of Fel d 1 varies considerably between cats and also within individual cats over time, but some cats tend to remain higher (or lower) producers compared with others.49,52-54 Production may decline in older cats. 49
The structure of Fel d 1 indicates a potential carrier function.2,6,55,56 While its biological function remains to be determined, a role in the transport of steroids, hormones or perhaps pheromones seems likely.2,57,58
Fel d 1 in the haircoat and on the skin is the main reservoir for environmental Fel d 1, being shed in substantial amounts on dander (dried saliva, cutaneous flakes and debris).9,30,59 Custovic et al 24 found that 49% of cat danderparticles were >9 µm and 23% <4.5 µm in size. The small particle size means that, as well as being present in settled dust, Fel d 1 can remain airborne for prolonged periods (several days) with minimal air disturbance. Particles readily reach smaller airways, explaining the rapid onset of clinical symptoms seen in some cat-allergic individuals following exposure.2,60-62 Moreover, the sticky nature of dander and Fel d 1 means that spread to environments outside of the home readily occurs on clothes or even human hair; 63 thus Fel d 1 isa ubiquitous allergen.2,30,50,59-62,64
Beyond houses with cats, immunologically significant levels of Fel d 1 are frequently detected in homes without cats, schools and day care centres, cars, hospitals, churches, cinemas, hotels and other public buildings, and on public transport including trains, buses and aeroplanes.2,30,50,59-62,64-80 In regions where pet cat ownership is common, detection rates in homes, schools and other public buildings are often in the order of75-100%.66,68,69,71,74-80 Generally, environmental concentrations of Fel d 1 are much higher in houses where cats are kept as pets, and in other places the amount is influenced primarily by the number of cat-owning people who use the space.9,30,50,60,61,64,71,73,81 Fel d 1presence is also affected by the physical environment, as concentrations are higher in dust from soft furnishings such as upholsteredchairs, carpets and mattresses.2,24,30,60,65,68,69,78,80,82The very widespread distribution of Fel d 1 beyond cat-owning homes is regarded as an important source of allergen for bothsensitisation and allergic symptoms.30,59,60,64,66,68,75,76,78,81,83
Clinical symptoms and diagnosis of cat allergy
Briefly, the major clinical symptoms of cat allergy are perennial rhinoconjunctivitis and asthma through type I hypersensitivity and chronic inflammation, with combinations of different symptoms (conjunctivitis, rhinitis and asthma) being seen.1,6,10,17,18,84 The severity of clinical symptoms varies considerably between individuals, but up to 20-30% of asthma sufferers may develop severe symptoms on contact with cats, 10 and cat allergy has a significant negative impact on these patients’ quality of life. 7 In addition to respiratory signs, cat allergy may also influence the severity of atopic dermatitis in affected individuals, 85 and signs of irritable bowel syndrome in atopy sufferers. 86
A diagnosis of cat allergy is generally made on the basis of history, clinical symptoms, knowledge of exposure, and reactivity to catallergens demonstrated by detection of serum-specific IgE and/or a positive skin prick test.7,84,87,88 Because Fel d 1 is the dominant cat allergen and because the vast majority of cat-allergic individuals react to this protein, recombinant Fel d 1 is often used in IgE assays or skin prick tests rather than a mixed antigen extract, and has usually proved reliable and reproducible diagnostically.2,6,7,17 Nevertheless, the use of a mixed antigen skin prick test and/or other recombinant feline allergens may allow the detection of allergen-specific IgE in the small proportion of patients with less typical sensitisation profiles (without Fel d 1 sensitisation). 89 Quantitative assessment of serum Fel d 1-specific IgE helps in the diagnosis and assessing the prognosis in cat allergy.2,6,90,91 Provocative testing can also be undertaken, although this is rarely necessary or advised.7,12,88
The rise in popularity of cats as pets and the fact that many people now spend well over 90% of their time in enclosed environments, 92 has likely contributed to the increase in exposure to cat allergens and the development of cat allergies in atopy-predisposed individuals.1,7,10,93 The relationship between genetics, environment, allergen exposure and the development of sensitisation is complex, but there is some evidence that early life (in the first year) exposure to cats may help reduce the risk of subsequent sensitisa-tion.1,7,9,60,94 However, the risk reduction of early-life exposure is not considered sufficient to recommend getting a cat to avoid IgE sensitisation.4,95
Methods for managing cat allergy
Members of the veterinary healthcare team may frequently get involved in discussions with cat owners about management of cat allergies, especially if the owner or someone in their household is allergic to cats, and working alongside human healthcare professionals may provide valuable insights on potential management strategies.
Most (but not all) individuals who develop a cat allergy will also suffer with otherallergies,1,7,8,19,26,61,62,64,76,90,96 but the focus hereis purely on the management of cat allergy. As with other allergies, the classic triad of management options is firstly avoidance (reducing exposure to cat allergens), secondly pharmacotherapy (to modify the immune response and relieve symptoms) and thirdly immunotherapy. 25 Avoiding or reducing exposure to the allergen(s) is a critical foundational management strategy, 25 but does not necessarily imply complete avoidance of all allergen exposure. The concept of a critical threshold with allergy suggests that if the degree ofinflammation (caused by exposure to allergens, irritants, etc) is kept below this threshold, the patient may remain free of symptoms, 25 and achieving this may be possible by reducing exposure to allergen(s) rather than complete avoidance, often in combination with other management strategies (Figure 2).
Figure 2.
Overview of potential control measures currently available for managing allergy to cats, and the level at which the intervention works
Reducing cat allergen exposure
Allergen avoidance or exposure reduction, where possible, is regarded as fundamental to managing allergies,7,23,88,95,97-99 as avoiding the inciting allergen is the most effective means of preventing reactions in sensitised individuals. Interestingly, while many control measures have been proven to reduce the burden of Fel d 1 in the environment, high quality evidence of clinical benefits from field trials isoften lacking.7,60,87,88,100-102 This may, at least inpart, reflect the challenges inherent in trials with a heterogeneous population of cat-allergic individuals (who may frequently have other allergies) and heterogeneous living environments, making dose-response relationships with allergens complex.23,98 Furthermore, it is widely accepted that combining several environmental control measures is likely to give superior results and thus greater clinical efficacy.7,97,98,100,101
Removing the cat from the home
Management guidelines for cat-allergic individuals frequently place emphasis on removal of the cat from the home, wherever possible, as this reliably reduces direct (cat) and indirect (environmental) Fel d 1 expo-sure.7,25,60,95,102 However, it is recognised that the strength of the human-cat bond makes this difficult for many owners and this strategy may damage the patient-physician relationship.7,25 Homes with pet cats have the highest burden of environmental Fel d 1 (typically 80-300 times higher than homeswithout cats13,24,50,74,79,83) and, while eliminating cats from the house may dramatically reduce Fel d 1 concentrations, 99 it may take 4-6 months or more to achieve levels seen in homes without cats, although aggressive cleaning measures including washing or removal of carpets may speed this up.82,103-105
The strength of the human-cat bond means that few owners are willing to part with their cat (4-35% in different studies), even when recommended to do so,104,106-108 and in one publication 70% of owners replaced the pet once it had died, despite the presence of a diagnosed allergy. 107 In an online survey of over 2000 cat owners in the USA, 84% reported they would ignore advice to give up their cat even if told to do so to help manage aller-gies. 109 This emphasises the importance of alternative measures to help control environmental Fel d 1 and, as noted, a combination of different approaches is recommended foroptimal results.7,25,87,95,97,101,102,110
Restricting access of the cat within the home environment
Limiting access so the cat is not allowed in bedrooms, only has access to certain rooms, and/or is restricted to uncarpeted rooms, has been advocated.7,25,95,97,101 The physical characteristics of dander mean these measures alone are unlikely to be sufficient to control signs of allergy. 97 In some situations environmental restriction might have important welfare implications for cats, potentially resulting in chronic frustration and behavioural issues that are risk factors for relinquishment.111,112 Limiting physical contact with the cat may also negatively impact the human-cat bond,113,114 and thus it would seem prudent to involve a veterinarian when such measures are being contemplated (with additional advice from a veterinary behaviourist if needed).
Onmental modification and cleaning
Many environmental modifications and/or cleaning regimens have been advocated to control Fel d 1 in the air and in dust. These measures and their rationale are summarised in Table 2. There is evidence that using multiple such measures is effective and may help to improve clinical disease, but continuous implementation is required.110,115,118,119
Table 2.
Environmental controls commonly utilised in catallergen management programmes
| Recommendation | Rationale |
|---|---|
| Remove carpeting from bedrooms ± other rooms and replace with hard
flooring59,60, 9 7102,110,115 |
Carpets are known to harbour large quantities of Fel d 1-containing dust |
| Remove upholstered furniture from bedrooms ± other rooms59,102,115 | Soft furnishings are known to harbour large quantities of Fel d 1-containing dust |
| Use mattress and pillow covers with a mean pore size ≤4-6 µm7,59,95,110,115 | Mattresses are known to harbour large quantities of Fel d 1-containing dust |
| Vacuum floors, carpets and furniture at least weekly with a cleaner that incorporates a high-efficiency particulate air (HEPA) filter7,60,95,102,110 | High-efficiency vacuum cleaners will not leak and disperse allergens |
| Wash bedding and curtains
regularly59,60,101,115 |
Washing will help to remove Fel d 1 |
| Wipe down walls and wash hard floors regularly95,115 | Wiping/washing physically removes dust particles that may harbour Fel d 1 antigen |
| Increase natural ventilation (eg, by keeping windows open)60,95,115 | Increasing ventilation will help to reduce airborne Fel d 1 concentrations |
| Use HEPA filters in the
home7,25,95,101,102,110,116,117 |
HEPA filters are capable of reducing airborne Fel d 1 concentrations and may be helpful when used with other measures |
| Use night-time laminar airflow system over beds7,110 | Filtered laminar airflow may displace aeroallergens from the owner’s breathing area |
In addition to the measures shown in Table 2, some authors have also recommended:
Selecting female or neutered male cats, as they generally produce less Fel d 1 (see earlier);59,101
Washing hands after touching a cat;25,101 andChanging and washing clothesregularly.7,60,95,101,102
Evidence from a study in Sweden showed that when children changed into school clothes (that were kept at school) on arrival, the reduction in airborne Fel d 1 concentrations in the classrooms was four- to six-fold, which was equivalent to banning pet ownership for all children. 120
Bathing the cat
Bathing cats, usually at weekly or twice-weekly intervals, to reduce Fel d 1 shedding is a common management recommenda-tion. 7 ’59,60’ 95 ’ 97 ’100,115 Studies of the efficacy of cat bathing have yielded inconsistent results. One study demonstrated a mean reduction of 79% in airborne Fel d 1 levels 3 h after immersing a cat in plain water, compared with 44% for a 60 s shampoo, 53 suggesting the former technique may be superior. Although consistent reductions in airborne Fel d 1 were seen 3 h after bathing, concentrations had returned to pre-bathing levels when measured a week later, even with weekly bathing. 53 Two other studies have shown a reduction in airborne Fel d 1 after bathing cats in water;54,121 although one showed a progressive decline with weekly bathing of a single cat, 121 the other showed a four- to five-fold reduction 3 h after bathing 12 cats, but that levels returned to baseline within 24 h. 54 One study failed to show any benefits from weekly bathing, 122 and one other showed that concentrations of Fel d 1 on shaved skin returned to baseline levels 2 days after a water wash. 43
Collectively, although the methodology of these studies varied, the results suggest bathing cats may reduce environmental shedding of Fel d 1, but that any reduction is short-lived. While this could form part of a combined strategy to manage environmental Fel d 1, it would seem bathing two or three times weekly would be required, and any benefits would need to be carefully assessed against the potential stress this would cause for the cat and damage to the human-cat bond.
Hypoallergenic cats
There are many anecdotal reports about certain breeds of cats being less allergenic, but no scientific data to substantiate any claims.123,124 Fel d 1 production varies between and within individual cats, and is partially influenced by testosterone levels.45-49 ,52-54 Although some cats appear to produce more Fel d 1 and may thus provoke stronger reactions in allergic individuals, 52 all cats studied thus far produce Fel d 1. 95 A study of Siberian and domestic cats revealed numerous sequence variations in Fel d 1 genes, but whether these translate to differences in Fel d 1 production or Fel d 1 allergenicity is unknown. 125 In the future, gene editing and deletion of the Fel d 1 gene using ‘CRISPR’ (clustered regularly interspaced short palindromic repeats) technology might become available, 126 but the biological implications of this have yet to be explored.
Inducing autoimmunity to Fel d 1 in cats
Two studies have reported preliminary results of attempted immunisation of cats to induce anti-Fel d 1 autoantibodies.127,128 The vaccine, which includes recombinant Fel d 1 and cucumber mosaic virus-like particles, induced a strong serum IgG response after a course of three injections, and resulted in reduced tear secretion of Fel d 1. 127 In an open label, uncontrolled field trial, 10 cat-allergic humans were enrolled with their 13 cats which received the vaccine. 128 Seven of nine peoplethat completed the study reported improvement in their symptom score with provocation (petting the cat), and 8/9 reported longer duration of cat interaction before symptom development at 24 weeks post-vaccination. Although initial results suggest vaccination of cats could help in reducing Fel d 1 secretion, at the time of writing this review the vaccine is not commercially available and, although no serious adverse events were reported, the long-term safety of vaccine-induced autoimmunity in cats would need to be assured.
Using a feline diet supplemented with anti-Fel d 1 IgY immunoglobulins
A novel approach to reducing Fel d 1 environmental contamination has focused on neutralising Fel d 1 after it has been produced (rendering it non-immunogenic) by using polyclonal chicken egg anti-Fel d 1 antibodies (IgY antibodies).129-133 This approach blocks the human IgE-binding epitopes on Fel d 1, 130 but does not alter total Fel d 1 production by the cat, and thus may not interfere with any natural biological role for Fel d 1.
Chickens produce IgY antibodies against environmental antigens, which are transferred into eggs to provide passive immunity for the developing and newly hatched chicks.134-136 Large quantities of ethically produced IgY can be purified from chicken eggs and used for passive immunoprophylaxis and, unlike mammalian immunoglobulins, IgY does not stimulate mammalian complement proteins, react with rheumatoid factors, or bind to mammalian Fc receptors.136-140 Orally administered IgY antibodies have been safely used for decades in the prevention or treatment of a wide variety of conditions in both animals and humans, including bacterial or viral diar-rhoea,140-144 bacterial-induced dental caries and periodontal disease,140,144 gastrointestinal parasitic disease140,145 and bacterial or viral respiratory diseases. 146
Initial in vitro and ex vivo research confirmed that specific anti-Fel d 1 polyclonal IgY antibodies successfully blocked the binding of Fel d 1-specific IgE from cat-allergic individuals, and reduced the release of inflammatory mediators.130,133 Subsequently, studies were conducted in cats using a control diet or a test diet (same kibble but coated with anti-Fel d 1 IgY). In a pilot study, the test diet was able to neutralise an average of almost 30% of active (allergenic) Fel d 1 in cat saliva, with reductions seen as early as 2 weeks after starting the diet. In a subsequent controlled trial with 20 adult cats, the test diet produced a significant (24%) decrease in mean salivary active Fel d 1, with >80% of the cats showing ≥ 20% reduction (Figure 3). 129
Figure 3.
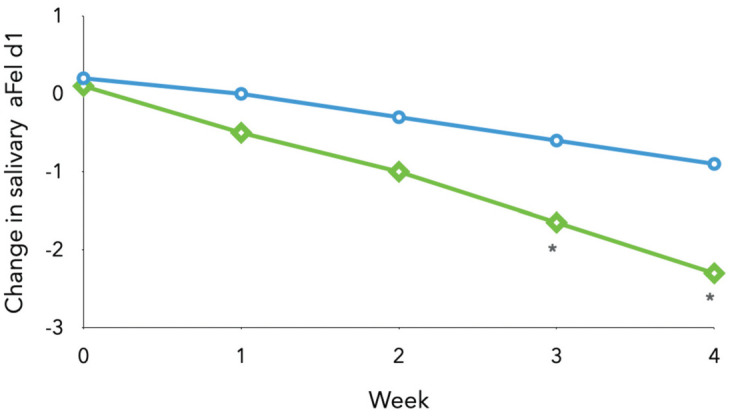
Mean change from baseline in salivary active Fel d 1 (aFel d 1; ug/mr) in control cats (blue circles) and those fed the specific polyclonal immunoglobulin (sIgY)-supplemented diet (green diamonds). Asterisks indicate measurements significantly different from baseline (P <0.05). Adapted from Satyaraj et al (2019) 129
These studies demonstrated that the IgY-supplemented diet was able to neutralise Fel d 1 present in saliva, but whether additional neutralisation of cutaneous-origin Fel d 1 occurs as a result of saliva distribution of the IgY during grooming and licking remains to be determined. However, in a 12-week study of multiple hair samples collected from 105 cats that consumed the control diet for 2 weeks and then the test diet for 10 weeks, significant reductions in active Fel d 1 on hair samples were seen by week 3 of consuming the test diet, and by week 10 there was a mean 47% reduction in active Fel d 1 present (Figure 4).131,133 Individual reductions ranged from33% to 71%,131,133 with 86% of cats showing atleast a 30% reduction and half of the cats showing a reduction of at least 50%. 131
Figure 4.
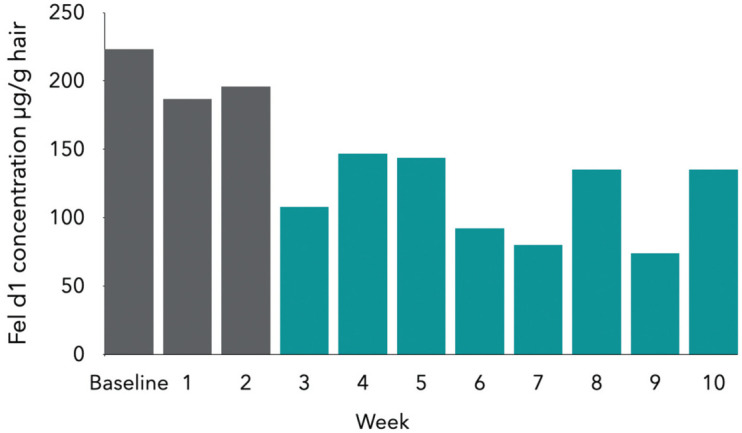
Mean active Fel d 1 concentration from hair samples of 105 cats consuming an anti-Fel d 1 IgY-supplemented diet for 10 weeks. Concentrations in weeks 3-10 are all significantly (P <0.05) lower than baseline. Adapted from Satyaraj et al (2019) 131
In a further study, eight cats were fed either the control or IgY-supplemented diet for 8 weeks and, during the last 4 weeks of feeding, blankets used by the cats as bedding were collected. Subsequently, 11 cat-allergic people(asthma sufferers excluded) were exposed to the blankets in a controlled chamber, serving as a provocation test, in a crossover design. Total nasal symptom scores were significantly lower with exposure to blankets from cats fed the IgY-supplemented diet, with significantly reduced nasal congestion, and some aspects of ocular scores were also lower, suggesting significant clinical improvements in cat-allergic individuals as a result of lower active Fel d 1 exposure.130,133
Although further studies are needed to fully understand the potential impact of an anti-Fel d 1 IgY-supplemented diet, published data are very encouraging and the long-term safety of the diet has been established. 147 This new diet, which is a complete and balanced dry food coated with anti-Fel d 1 IgY, is now available commercially (Pro Plan LiveClear; Purina) and has been given the ‘Allergy Friendly Product Award’ by Allergy UK (allergyuk.org) in recognition that it may be of value in helping to manage cat allergies. The availability of this diet gives an opportunity for the veterinary healthcare team to be better involved in discussions with both owners and human healthcare professionals about combinations of measures that can be taken to help reduce the environmental burden of Fel d 1 while addressing important issues around cat welfare and the human-cat bond.
Other dietary strategies
Pezzali and others have speculated on specific dietary manipulations that might reduce sebum (and hence Fel d 1) production. 148 Theysuggested that polyphenols, phytoestrogenisoflavones and certain carotenoids could potentially influence testosterone concentrations or testosterone metabolism in entire male cats, thus influencing sebum and Fel d 1 production. Based on studies in other species, they also suggested that a diet with enhanced omega-3 fatty acids and a low glycaemic index might have the potential to reduce sebum concentrations. They noted that none of these dietary manipulations had been investigated in cats for the purpose of altering sebum (and Fel d 1) production.
At present, the role of manipulating the nutritional profile of the diet remains speculative, although one abstract published in 2005 149 suggested that cats fed one of two different diets might have experienced differences in long-term Fel d 1 production, although details of the cats, diets and study design were lacking. Based on these data it is at least possible that manipulation of certain nutrients might have a role to play in helping to reduce Fel d 1 production, and further studies are warranted.
Medications to manage cat allergy
Although largely beyond the scope of this review, Table 3 outlines the major pharmaco-therapeutic agents used for symptomatic control of cat and other respiratory allergies. Medication is adjusted in a stepwise approach according to the severity of symptoms and the response to therapy.
Table 3.
Commonly recommended therapeutic optionsfor human allergic rhinitis and asthma
| Allergic rhinitis 87,88,100,101,150 | Asthma 97,151-154 | |
|---|---|---|
| ✜ First-line/major treatment options | ✜ Intranasal corticosteroids (eg, fluticasone, beclomethasone, mometasone, budesonide)
✜ Intranasal antihistamines (eg, azelastine, olopatadine) ✜ Oral second-generation antihistamines (eg, cetirizine, loratidine, desloratadine, bilastine, fexofenadine, levocetrizine) |
✜ Inhaled corticosteroids (eg, fluticasone, budesonide, mometasone)
✜ Inhaled long-acting β-2 agonists (eg, salmeterol, formoterol) ✜ Inhaled short-acting β-2 agonists (eg, salbutamol, terbutaline) |
| ✜ Second-line or adjuvant options | ✜ Intranasal cromolyn
✜ Intranasal anticholinergics (eg, ipratropium) ✜ Leukotriene receptor antagonists |
✜ Long-acting inhaled anticholinergics (eg, tiotropium)
✜ Antihistamines prior to exposure ✜ Leukotriene receptor antagonists ✜ Theophylline ✜ Anti-IgE antibodies ✜ Anti-IL-5 antibodies ✜ Anti-IL-5 receptor antibodies ✜ Anti-IL-4 antibodies |
Immunotherapy for cat allergy
Allergen immunotherapy, while not always successful, is recommended for people suffering moderate to severe allergic rhinitis or asthma that is inadequately controlled with pharmacotherapy.150,155 Both subcutaneous (SCIT) and sublingual immunotherapy (SLIT) have been recommended for the management of cat allergies,7,87,88,100,101,155,156 but there are relatively few good trials of allergen immunotherapy in cats and results have been mixed.7,156-158 It has been suggested that some cat-allergic patients are likely to benefit from allergen immunotherapy, but that larger studies are required for developing definitive advice.7,157
Although the mechanism(s) by which allergen-specific immunotherapy benefit the patient are still poorly understood, the induction of allergen-specific IgG molecules (especially IgG4) that compete with IgE for epitope-binding is one possibility. 159 Studies of recombinant-blocking monoclonal IgG4 against Fel d 1 have demonstrated that administration in cat-allergic humans may block IgE binding to Fel d 1, reduce clinical symptoms and act much more rapidly than allergen-specific immunotherapy, 160 and thus may have a role in clinical management.
Key points
✜ A combination of different control measures is likely to be most successful in reducing Fel d 1 concentrations in the home (and elsewhere), but not all recommendations are necessarily easy to follow and some may damage the human-cat bond.
✜ There is an important role for the veterinary healthcare team in contributing to discussions on how Fel d 1 exposure can be reduced, in ways that maintain cat welfare.
✜ The recent introduction of a cat diet that is capable of reducing active Fel d 1 in saliva, hair and bedding has the potential to contribute appreciably to JM environmental control.
Conclusions
Human sensitisation to cat allergens is a common problem and, although cat-allergic owners may spend less time in close contact with their cat in an attempt to avoid provoking symptoms, 161 it is clear that contamination of the environment with Fel d 1 is a major source of exposure and risk. Recommendations to remove the cat from the home are rarely followed, and thus efforts to reduce the environmental allergen load, combined with pharmacotherapy and allergen immunotherapy where necessary, are important.
Footnotes
The author provides consultancy services to the Purina Institute (www.purinainstitute.com) but neither the Purina Institute nor Nestlé Purina exerted any editorial control over the content of this review.
Funding: The author received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and therefore informed consent was not required. No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. Baldacci S, Maio S, Cerrai S, et al. Allergy and asthma: effects of the exposureto particulate matter and biological allergens. Respir Med 2015; 109:1089–1104. [DOI] [PubMed] [Google Scholar]
- 2. Bonnet B, Messaoudi K, Jacomet F, et al. An update on molecular cat allergens:Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen.Allergy Asthma Clin Immunol 2018; 14. DOI: 10.1186/s13223-018-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Custovic A, Simpson A, Woodcock A. Importance of indoor allergensin the induction of allergy and elicitation of allergic disease. Allergy 1998;53: 115–120. [DOI] [PubMed] [Google Scholar]
- 4. Galant S, Berger W, Gillman S, et al. Prevalence of sensitization to aero -allergens in California patients with respiratory allergy. Allergy Skin TestProject Team. Ann Allergy Asthma Immunol 1998; 81: 203–210. [DOI] [PubMed] [Google Scholar]
- 5. Sly RM. Changing prevalence of allergic rhinitis and asthma. Ann AllergyAsthma Immunol 1999; 82: 233–248; quiz 248–252. [DOI] [PubMed] [Google Scholar]
- 6. Grönlund H, Saarne T, Gafvelin G, et al. The major cat allergen, Fel d 1,in diagnosis and therapy. Int Arch Allergy Immunol 2010; 151: 265–274. [DOI] [PubMed] [Google Scholar]
- 7. Dávila I, Domínguez-Ortega J, Navarro-Pulido A, et al. Consensus documenton dog and cat allergy. Allergy 2018; 73: 1206–1222. [DOI] [PubMed] [Google Scholar]
- 8. Bousquet P-J, Chinn S, Janson C, et al. Geographical variation in the prevalenceof positive skin tests to environmental aeroallergens in theEuropean Community Respiratory Health Survey I. Allergy 2007; 62:301–309. [DOI] [PubMed] [Google Scholar]
- 9. Konradsen JR, Fujisawa T, van Hage M, et al. Allergy to furry animals:new insights, diagnostic approaches, and challenges. J Allergy ClinImmunol 2015; 135: 616–625. [DOI] [PubMed] [Google Scholar]
- 10. Kim KH, Jahan SA, Kabir E. A review on human health perspective ofair pollution with respect to allergies and asthma. Environ Int 2013; 59:41–52. [DOI] [PubMed] [Google Scholar]
- 11. Chan SK, Leung DYM. Dog and cat allergies: current state of diagnosticapproaches and challenges. Allergy Asthma Immunol Res 2018; 10:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Ahmad M, Jusufovic E, Arifhodzic N, et al. Sensitization to cat: when isnasal challenge needed? Int Arch Allergy Immunol 2019; 179: 108–113. [DOI] [PubMed] [Google Scholar]
- 13. Zahradnik E, Raulf M. Animal allergens and their presence in theenvironment. Front Immunol 2014; 5: 76. DOI: 10.3389/fimmu.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eder K, Becker S, San Nicoló M, et al. Usefulness of component resolvedanalysis of cat allergy in routine clinical practice. Allergy Asthma ClinImmunol 2016; 12: 58. DOI: 10.1186/s13223-016-0163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murray AB, Ferguson AC, Morrison BJ. The frequency and severity ofcat allergy vs. Dog allergy in atopic children. J Allergy Clin Immunol 1983;72: 145–149. [DOI] [PubMed] [Google Scholar]
- 16. Al-Mousawi MS, Lovel H, Behbehani N, et al. Asthma andsensitization in a community with low indoor allergen levelsand low pet-keeping frequency. J Allergy Clin Immunol2004; 114: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 17. Asarnoj A, Hamsten C, Wadén K, et al. Sensitization tocat and dog allergen molecules in childhood and predictionof symptoms of cat and dog allergy in adolescence:a BAMSE/MeDALL study. J Allergy Clin Immunol 2016; 137:813–821.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nwaru BI, Suzuki S, Ekerljung L, et al. Furry animal allergencomponent sensitization and clinical outcomes in adultasthma and rhinitis. J Allergy Clin Immunol Pract 2019; 7:1230–1238.e4. [DOI] [PubMed] [Google Scholar]
- 19. Vachová M, Panzner P, Vlas T, et al. Analysis of sensitizationprofiles in Central European allergy patients focused on animalallergen molecules. Int Arch Allergy Immunol 2020; 181: 278–284. [DOI] [PubMed] [Google Scholar]
- 20. Park HJ, Lim HS, Park KH, et al. Changes in allergen sensitizationover the last 30 years in Korea respiratory allergicpatients: a single-center. Allergy Asthma Immunol Res 2014; 6:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki S, Nwaru BI, Ekerljung L, et al. Characterization ofsensitization to furry animal allergen components in anadult population. Clin Exp Allergy 2019; 49: 495–505. [DOI] [PubMed] [Google Scholar]
- 22. Custovic A, Green R, Fletcher A, et al. Aerodynamic propertiesof the major dog allergen Can f 1: distribution in homes,concentration, and particle size of allergen in the air. Am JRespir Crit Care Med 1997; 155: 94–98. [DOI] [PubMed] [Google Scholar]
- 23. Custovic A, Chapman MD. Indoor allergens as a riskfactor for asthma. In: Barnes PJ, Grunstein MM, Leff A, et al. (eds). Asthma. Philadelphia: Lippincott-Raven, 1997,pp 83–103. [Google Scholar]
- 24. Custovic A, Simpson A, Pahdi H, et al. Distribution, aero -dynamic characteristics, and removal of the major cat allergenFel d 1 in British homes. Thorax 1998; 53: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reisacher WR. Allergy treatment: environmental controlstrategies. Otolaryngol Clin North Am 2011; 44: 711–725. [DOI] [PubMed] [Google Scholar]
- 26. Bousquet J, et al. ; Aria Workshop Group; World HealthOrganization. Allergic rhinitis and its effect on asthma.J Allergy Clin Immunol 2001; 108: S147–S334. [DOI] [PubMed] [Google Scholar]
- 27. Smith W, O’Neil SE, Hales BJ, et al. Two newly identified catallergens: the von Ebner gland protein Fel d 7 and thelatherin-like protein Fel d 8. Int Arch Allergy Immunol 2011;156: 159–170. [DOI] [PubMed] [Google Scholar]
- 28. de Groot H, van Swieten P, Aalberse RC. Evidence for aFel d I-like molecule in the “big cats” (Felidae species).J Allergy Clin Immunol 1990; 86: 107–116. [DOI] [PubMed] [Google Scholar]
- 29. Dolgova AS, Sudina AE, Cherkashina AS, et al. Componentresolvedmicroarray analysis of IgE sensitization profiles toFelis catus major allergen molecules in Russian cat-allergicpatients. Scand J Clin Lab Invest 2018; 78: 81–86. [DOI] [PubMed] [Google Scholar]
- 30. Zahradnik E, Raulf M. Respiratory allergens from furredmammals: environmental and occupational exposure. VetSci 2017; 4: 38. DOI: 10.3390/vetsci4030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ukleja-Sokołowska N, Gawrońska-Ukleja E, Żbikowska-Gotz M, et al. Analysis of feline and canine allergen componentsin patients sensitized to pets. Allergy Asthma Clin Immunol 2016; 12: 61. DOI: 10.1186/s13223-016-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamamoto K, Ishibashi O, Sugiura K, et al. Crystal structureof the dog allergen Can f 6 and structure-based implicationsof its cross-reactivity with the cat allergen Fel d 4. Sci Rep 2019; 9: 1503. DOI: 10.1038/s41598-018-38134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apostolovic D, Sánchez-Vidaurre S, Waden K, et al. The catlipocalin Fel d 7 and its cross-reactivity with the doglipocalin Can f 1. Allergy 2016; 71: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 34. Charpin C, Mata P, Charpin D, et al. Fel d I allergen distributionin cat fur and skin. J Allergy Clin Immunol 1991; 88: 77–82. [DOI] [PubMed] [Google Scholar]
- 35. Dabrowski AJ, Van der Brempt X, Soler M, et al. Cat skin asan important source of Fel d I allergen. J Allergy Clin Immunol1990; 86: 462–465. [DOI] [PubMed] [Google Scholar]
- 36. Anderson MC, Baer H, Ohman JL. A comparative studyof the allergens of cat urine, serum, saliva, and pelt. J AllergyClin Immunol 1985; 76: 563–569. [DOI] [PubMed] [Google Scholar]
- 37. Brown PR, Leitermann K, Ohman JL, Jr. Distribution ofcat allergen 1 in cat tissues and fluids. Int Arch Allergy ApplImmunol 1984; 74: 67–70. [DOI] [PubMed] [Google Scholar]
- 38. Mata P, Charpin D, Charpin C, et al. Fel d I allergen: skin andor saliva? Ann Allergy 1992; 69: 321–322. [PubMed] [Google Scholar]
- 39. Bartholome K, Kissler W, Baer H, et al. Where does cat allergen1 come from? J Allergy Clin Immunol 1985; 76: 503 –506. [DOI] [PubMed] [Google Scholar]
- 40. Kelly SM, Karsh J, Marcelo J, et al. Fel d 1 and Fel d 4 levelsin cat fur, saliva, and urine [Letter]. J Allergy Clin Immunol 2018; 142: 1990; –1992.e3. [DOI] [PubMed] [Google Scholar]
- 41. van Milligen FJ, Vroom TM, Aalberse RC. Presence of Felis domesticus allergen I in the cat’s salivary and lacrimalglands. Int Arch Allergy Appl Immunol 1990; 92: 375–378. [DOI] [PubMed] [Google Scholar]
- 42. De Andrade AD, Birnbaum J, Magalon C, et al. Fel d I levelsin cat anal glands. Clin Exp Allergy 1996; 26: 178–180. [DOI] [PubMed] [Google Scholar]
- 43. Carayol N, Birnbaum J, Magnan A, et al. Fel d 1 productionin the cat skin varies according to anatomical sites. Allergy2000; 55: 570–573. [DOI] [PubMed] [Google Scholar]
- 44. Noel AC, Hu DL. Cats use hollow papillae to wick salivainto fur. Proc Natl Acad Sci USA 2018; 115: 12377–12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bienboire-Frosini C, Cozzi A, Lafont-Lecuelle C, et al.Immunological differences in the global release of themajor cat allergen Fel d 1 are influenced by sex andbehaviour. Vet J 2012; 193: 162–167. [DOI] [PubMed] [Google Scholar]
- 46. Jalil-Colome J, de Andrade AD, Birnbaum J, et al. Sex differencein Fel d 1 allergen production. J Allergy Clin Immunol1996; 98: 165–168. [DOI] [PubMed] [Google Scholar]
- 47. Ramadour M, Birnbaum J, Magalon C, et al. Cat sex differencesin major allergen production (Fel d 1). J Allergy ClinImmunol 1998; 101: 282–284. [DOI] [PubMed] [Google Scholar]
- 48. Zielonka TM, Charpin D, Berbis P, et al. Effects of castrationand testosterone on Fel dI production by sebaceous glandsof male cats: I – immunological assessment. Clin Exp Allergy1994; 24: 1169–1173. [DOI] [PubMed] [Google Scholar]
- 49. Bastien BC, Gardner C, Satyaraj E. Influence of time andphenotype on salivary Fel d1 in domestic shorthair cats.J Feline Med Surg 2019; 21: 867–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicholas C, Wegienka G, Havstad S, et al. Influence of catcharacteristics on Fel d 1 levels in the home. Ann AllergyAsthma Immunol 2008; 101: 47–50. [DOI] [PubMed] [Google Scholar]
- 51. Siebers R, Healy B, Holt S, et al. Fel d 1 levels in domestic livingrooms are not related to cat color or hair length [Letter].J Allergy Clin Immunol 2001; 108: 652–653. [DOI] [PubMed] [Google Scholar]
- 52. Wentz PE, Swanson MC, Reed CE. Variability of catallergenshedding. J Allergy Clin Immunol 1990; 85: 94–98. [DOI] [PubMed] [Google Scholar]
- 53. Avner DB, Perzanowski MS, Platts-Millsi TAE, et al.Evaluation of different techniques for washing cats:quantitation of allergen removed from the cat and the effecton airborne Fel d 1. J Allergy Clin Immunol 1997; 100: 307–312. [DOI] [PubMed] [Google Scholar]
- 54. Nageotte C, Park M, Havstad S, et al. Duration of airborneFel d 1 reduction after cat washing. J Allergy Clin Immunol 2006; 118: 521 –;522. [DOI] [PubMed] [Google Scholar]
- 55. Kaiser L, Velickovic TC, Badia-Martinez D, et al. Structuralcharacterization of the tetrameric form of the major cat allergenFel d 1. J Mol Biol 2007; 370: 714–727. [DOI] [PubMed] [Google Scholar]
- 56. Ligabue-Braun R, Sachett LG, Pol-Fachin L, et al. The calciumgoes meow: effects of ions and glycosylation on Fel d 1,the major cat allergen. PLoS One 2015; 10: e0132311.DOI: 10.1371/journal.pone.0132311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bienboire-Frosini C, Durairaj R, Pelosi P, et al. The major catallergen Fel d 1 binds steroid and fatty acid semiochemicals:a combined in silico and in vitro study. Int J Mol Sci 2020; 21:1365. DOI: 10.3390/ijms21041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Durairaj R, Pageat P, Bienboire-Frosini C. Another cat andmouse game: deciphering the evolution of the SCGB super-familyand exploring the molecular similarity of major catallergen Fel d 1 and mouse ABP using computationalapproaches. PLoS One 2018; 13: e0197618. DOI: 10.1371/journal.pone.0197618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Erwin EA, Woodfolk JA, Custis N, et al. Animal danders.Immunol Allergy Clin North Am 2003; 23: 469–481. [DOI] [PubMed] [Google Scholar]
- 60. Liccardi G, D’Amato G, Russo M, et al. Focus on cat allergen(Fel d 1): immunological and aerodynamic characteristics,modality of airway sensitization and avoidance strategies.Int Arch Allergy Immunol 2003; 132: 1–12. [DOI] [PubMed] [Google Scholar]
- 61. Pomés A, Chapman MD, Wünschmann S. Indoor allergensand allergic respiratory disease. Curr Allergy AsthmaRep 2016; 16: 43. DOI: 10.1007/s11882-016-0622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Platts-Mills TA, Ward GW, Sporik R, et al. Epidemiology ofthe relationship between exposure to indoor allergens andasthma. Int Arch Allergy Appl Immunol 1991; 94: 339–345. [DOI] [PubMed] [Google Scholar]
- 63. Liccardi G, Barber D, Russo M, et al. Human hair: anunexpected source of cat allergen exposure. Int Arch AllergyImmunol 2005; 137: 141–144. [DOI] [PubMed] [Google Scholar]
- 64. Esty B, Permaul P, DeLoreto K, et al. Asthma and allergies inthe school environment. Clin Rev Allergy Immunol 2019; 57:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Custovic A, Taggart SC, Woodcock A. House dust miteand cat allergen in different indoor environments. Clin ExpAllergy 1994; 24: 1164–1168. [DOI] [PubMed] [Google Scholar]
- 66. Siebers R, Jones B, Bailey L, et al. Indoor allergen exposure inprimary school classrooms in New Zealand. N Z Med J 2019;132: 42 –47. [PubMed] [Google Scholar]
- 67. Möhrenschlager M, Ring J, Lauener R. Possible in-cabinexposure to cat allergen: a 2010 airline survey on live animaltransport and a review of literature. Allergy 2010; 86:1496–1498. [DOI] [PubMed] [Google Scholar]
- 68. Martin IR, Wickens K, Patchett K, et al. Cat allergen levels inpublic places in New Zealand. N Z Med J 1998; 111: 356–358. [PubMed] [Google Scholar]
- 69. Custovic A, Fletcher A, Pickering CA, et al. Domesticallergens in public places III: house dust mite, cat, dog andcockroach allergens in British hospitals. Clin Exp Allergy1998; 28: 53–59. [DOI] [PubMed] [Google Scholar]
- 70. Gulbahar O, Sin A, Mete N, et al. Sensitization to cat allergensin non-cat owner patients with respiratory allergy.Ann Allergy Asthma Immunol 2003; 90: 635–639. [DOI] [PubMed] [Google Scholar]
- 71. Heinrich J, Bedada GB, Zock JP, et al. Cat allergen level:its determinants and relationship to specific IgE to catacross European centers. J Allergy Clin Immunol 2006; 118:674–681. [DOI] [PubMed] [Google Scholar]
- 72. Ichikawa K, Iwasaki E, Baba M, et al. High prevalence ofsensitization to cat allergen among Japanese childrenwith asthma, living without cats. Clin Exp Allergy 1999; 29:754–761. [DOI] [PubMed] [Google Scholar]
- 73. Ingram JM, Sporik R, Rose G, et al. Quantitative assessmentof exposure to dog (Can f 1) and cat (Fel d 1) allergens:relation to sensitization and asthma among children livingin Los Alamos, New Mexico. J Allergy Clin Immunol 1995; 96:449–456. [DOI] [PubMed] [Google Scholar]
- 74. Niesler A, Ścigała G, Łudzeń-Izbińska B. Cat (Fel d 1) anddog (Can f 1) allergen levels in cars, dwellings and schools.Aerobiologia (Bologna) 2016; 32: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Norbäck D, Gui-Hong C, Kreft I, et al. Cat, dog and horseallergens in Swedish day care centres – associations withfractional exhaled nitric oxide (FeNO) among day carecentre staff. Glob J Health Sci 2016; 8: 24–35. [Google Scholar]
- 76. Salo PM, Arbes SJ, Crockett PW, et al. Exposure to multipleindoor allergens in US homes and its relationship toasthma. J Allergy Clin Immunol 2008; 121: 678 –684.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Salo PM, Wilkerson J, Rose KM, et al. Bedroom allergen exposuresin US households. J Allergy Clin Immunol 2018; 141:1870–1879.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sander I, Neumann HD, Lotz A, et al. Allergen quantificationin surface dust samples from German day care centers.J Toxicol Environ Health A 2016; 79: 1094–1105. [DOI] [PubMed] [Google Scholar]
- 79. Woodcock A, Addo-Yobo EO, Taggart SC, et al. Pet allergenlevels in homes in Ghana and the United Kingdom. J AllergyClin Immunol 2001; 108: 463–465. [DOI] [PubMed] [Google Scholar]
- 80. Zhang L, Chew FT, Soh SY, et al. Prevalence and distributionof indoor allergens in Singapore. Clin Exp Allergy 1997; 27:876–885. [PubMed] [Google Scholar]
- 81. Almqvist C, Larsson PH, Egmar A-C, et al. School as a riskenvironment for children allergic to cats and a site for transferof cat allergen to homes. J Allergy Clin Immunol 1999; 103:1012–1017. [DOI] [PubMed] [Google Scholar]
- 82. van der Brempt X, Charpin D, Haddi E, et al. Cat removal andFel d I levels in mattresses. J Allergy Clin Immunol 1991; 87:595 –596. [DOI] [PubMed] [Google Scholar]
- 83. Arbes SJ, Cohn RD, Yin M, et al. Dog allergen (Can f 1) andcat allergen (Fel d 1) in US homes: results from the NationalSurvey of Lead and Allergens in Housing. J Allergy ClinImmunol 2004; 114: 111–117. [DOI] [PubMed] [Google Scholar]
- 84. Singh M, Hays A. Indoor and outdoor allergies. Prim Care 2016; 43: 451–463. [DOI] [PubMed] [Google Scholar]
- 85. Cid BJ, Perez-Mateluna G, Iturriaga C, et al. Is there an associationbetween indoor allergens and the severity of atopicdermatitis? Int J Dermatol 2019; 58: 433–439. [DOI] [PubMed] [Google Scholar]
- 86. Siah KTH, Santosa A, Cheung CKY, et al. Atopic patientswho fulfilled Rome III criteria for irritable bowel syndromehad higher animal danders sensitization. J NeurogastroenterolMotil 2020; 26: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wise SK, Lin SY, Toskala E, et al. International consensusstatement on allergy and rhinology: allergic rhinitis. IntForum Allergy Rhinol 2018; 8: 108–352. [DOI] [PubMed] [Google Scholar]
- 88. Scadding GK, Kariyawasam HH, Scadding G, et al. BSACIguideline for the diagnosis and management of allergic andnon-allergic rhinitis (revised edition 2017; first edition2007). Clin Exp Allergy 2017; 47: 856–889. [DOI] [PubMed] [Google Scholar]
- 89. Ukleja-Sokołowska N, Gawrońska-Ukleja E, Żbikowska-Gotz M, et al. Analysis of feline and canine allergen componentsin patients sensitized to pets. Allergy Asthma Clin Immunol 2016; 12: 61. DOI: 10.1186/s13223-016-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ahlstedt S, Murray CS. In vitro diagnosis of allergy:how to interpret IgE antibody results in clinical practice.Prim Care Respir J 2006; 15: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Smoldovskaya O, Feyzkhanova G, Arefieva A, et al. Allergenextracts and recombinant proteins: comparison of efficiencyof in vitro allergy diagnostics using multiplex assay on abiological microchip. Allergy Asthma Clin Immunol 2016; 12: 9.DOI: 10.1186/s13223-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Klepeis NE, Nelson WC, Ott WR, et al. The National HumanActivity Pattern Survey (NHAPS): a resource for assessingexposure to environmental pollutants. J Expo Anal EnvironEpidemiol 2001; 11: 231–252. [DOI] [PubMed] [Google Scholar]
- 93. Galvão CES, Graudenz GS, Kalil J, et al. Sensitization to catallergen and its association with respiratory allergies: crosssectionalstudy. Sao Paulo Med J 2017; 135: 488–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dharmage SC, Lodge CL, Matheson MC, et al. Exposure tocats: update on risks for sensitization and allergic diseases.Curr Allergy Asthma Rep 2012; 12: 413–423. [DOI] [PubMed] [Google Scholar]
- 95. Portnoy J, Kennedy K, Sublett J, et al. Environmentalassessment and exposure control: a practice parameter –furry animals. Ann Allergy Asthma Immunol 2012; 108:223.E1–223.E15. DOI: 10.1016/j.anai.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Salo PM, Arbes SJ, Jaramillo R, et al. Prevalence of allergicsensitization in the United States: results from the NationalHealth and Nutrition Examination Survey (NHANES)2005–2006. J Allergy Clin Immunol 2014; 134: 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ling M, Long AA. Pet dander and difficult-to-controlasthma: therapeutic options. Allergy Asthma Proc 2010; 31:385–391. [DOI] [PubMed] [Google Scholar]
- 98. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy forasthma management and prevention: GINA executivesummary. Eur Respir J 2008; 31: 143–178. [DOI] [PubMed] [Google Scholar]
- 99. Shirai T, Matsui T, Suzuki K, et al. Effect of pet removal onpet allergic asthma. Chest 2005; 127: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 100. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practiceguideline: allergic rhinitis. Otolaryngol Head Neck Surg 2015;152: S1–43. [DOI] [PubMed] [Google Scholar]
- 101. Wallace DV. Pet dander and perennial allergic rhinitis:therapeutic options. Allergy Asthma Proc 2009; 30: 573–583. [DOI] [PubMed] [Google Scholar]
- 102. Cosme-Blanco W, Arce-Ayala Y, Malinow I, et al. Primary andsecondary environmental control measures for allergicdiseases. In: Mahmoudi M, Craig T, Ledford D. (eds). Allergyand asthma. Cham, Switzerland: Springer, 2018, pp 1–36. [Google Scholar]
- 103. Wood RA, Chapman MD, Adkinson NF, et al. The effect ofcat removal on allergen content in household-dust samples.J Allergy Clin Immunol 1989; 83: 730–734. [DOI] [PubMed] [Google Scholar]
- 104. Chan-Yeung M, Ferguson A, Dimich-Ward H, et al.Effectiveness of and compliance to intervention measuresin reducing house dust and cat allergen levels. Ann AllergyAsthma Immunol 2002; 88: 52–58. [DOI] [PubMed] [Google Scholar]
- 105. Poole TB, King SP, Suphioglu C. Effectiveness of vacuumingand carpet washing in the removal of the major catallergen, Fel d 1 [Letter]. Allergy 2020; 75: 2694–2695. [DOI] [PubMed] [Google Scholar]
- 106. Busse PJ, Wang JJ, Halm EA. Allergen sensitization evaluationand allergen avoidance education in an inner-cityadult cohort with persistent asthma. J Allergy Clin Immunol2005; 116: 146–152. [DOI] [PubMed] [Google Scholar]
- 107. Coren S. Allergic patients do not comply with doctors’advice to stop owning pets [Letter]. BMJ 1997; 314: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sánchez J, Díez S, Cardona R. Pet avoidance in allergycases: is it possible to implement it? Biomedica 2015; 35:357–362. [DOI] [PubMed] [Google Scholar]
- 109. Human Animal Bond Research Institute. New survey showscat owners with cat allergen sensitivities go to extraordinarylengths to manage cat allergens. https://habri.org/pressroom/20200514 (2020, accessed 17 September 2021). [Google Scholar]
- 110. Custovic A, Simpson A. The role of inhalant allergens inallergic airways disease. J Investig Allergol Clin Immunol 2012;22: 393–401. [PubMed] [Google Scholar]
- 111. Mills D, Karagiannis C, Zulch H. Stress – its effects onhealth and behavior: a guide for practitioners. Vet Clin NorthAm Small Anim Pract 2014; 44: 525–541. [DOI] [PubMed] [Google Scholar]
- 112. Rioja-Lang F, Bacon H, Connor M, et al. Determining prioritywelfare issues for cats in the United Kingdom using expertconsensus. Vet Rec Open 2019; 6: e000365. DOI: 10.1136/vetreco-2019-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Adamelli S, Marinelli L, Normando S, et al. Owner and catfeatures influence the quality of life of the cat. Appl AnimBehav Sci 2005; 94: 89–98. [Google Scholar]
- 114. Vitale Shreve KR, Mehrkam LR, Udell MAR. Social interaction,food, scent or toys? A formal assessment of domesticpet and shelter cat (Felis silvestris catus) preferences.Behav Processes 2017; 141: 322–328. [DOI] [PubMed] [Google Scholar]
- 115. Björnsdottir US, Jakobinudottir S, Runarsdottir V, et al. Theeffect of reducing levels of cat allergen (Fel d 1) on clinicalsymptoms in patients with cat allergy. Ann Allergy AsthmaImmunol 2003; 91: 189–194. [DOI] [PubMed] [Google Scholar]
- 116. Gherasim A, de Blay F. Does air filtration work for catallergen exposure? Curr Allergy Asthma Rep 2020; 20: 18.DOI: 10.1007/s11882-020-00912-w. [DOI] [PubMed] [Google Scholar]
- 117. Gherasim A, Jacob A, Schoettel F, et al. Efficacy of air cleanersin asthmatics allergic to cat in ALYATEC® environmentalexposure chamber. Clin Exp Allergy 2020; 50: 160–169. [DOI] [PubMed] [Google Scholar]
- 118. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a homebasedenvironmental intervention among urban childrenwith asthma. N Engl J Med 2004; 351: 1068. –1080. [DOI] [PubMed] [Google Scholar]
- 119. Stillerman A, Nachtsheim C, Li W, et al. Efficacy of a novel airfiltration pillow for avoidance of perennial allergens insymptomatic adults. Ann Allergy Asthma Immunol 2010; 104:440–449. [DOI] [PubMed] [Google Scholar]
- 120. Karlsson AS, Andersson B, Renström A, et al. Airborne catallergen reduction in classrooms that use special schoolclothing or ban pet ownership. J Allergy Clin Immunol 2004;113: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 121. de Blay F, Chapman MD, Platts-Mills TA. Airborne catallergen (Fel d I). Environmental control with the cat in situ.Am Rev Respir Dis 1991; 143: 1334–1339. [DOI] [PubMed] [Google Scholar]
- 122. Klucka CV, Ownby DR, Green J, et al. Cat shedding of Fel d I is not reduced by washings, Allerpet-C spray, or acepromazine. J Allergy Clin Immunol 1995; 95: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 123. Butt A, Rashid D, Lockey RF. Do hypoallergenic catsand dogs exist? Ann Allergy Asthma Immunol 2012; 108:74–76. [DOI] [PubMed] [Google Scholar]
- 124. Lockey RF. The myth of hypoallergenic dogs (and cats)[Editorial]. J Allergy Clin Immunol 2012; 130: 910–911. [DOI] [PubMed] [Google Scholar]
- 125. Sartore S, Landoni E, Maione S, et al. Polymorphism analysisof Ch1 and Ch2 genes in the Siberian cat. Vet Sci 2017; 4: 63.DOI: 10.3390/vetsci4040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brackett N, Riedy J, Adli M, et al. Gene editing the major catallergen, Fel d 1, using CRISPR-Cas9 [Abstract]. J AllergyClin Immunol 2020; 145: AB156. [Google Scholar]
- 127. Thoms F, Jennings GT, Maudrich M, et al. Immunization ofcats to induce neutralizing antibodies against Fel d 1, themajor feline allergen in human subjects. J Allergy ClinImmunol 2019; 144: 193–203. [DOI] [PubMed] [Google Scholar]
- 128. Thoms F, Haas S, Erhart A, et al. Immunization of catsagainst Fel d 1 results in reduced allergic symptoms ofowners. Viruses 2020; 12. 288. DOI: 10.3390/v12030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Satyaraj E, Li Q, Sun P, et al. Anti-Fel d1 immunoglobulin Yantibody-containing egg ingredient lowers allergen levelsin cat saliva. J Feline Med Surg 2019; 21: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Satyaraj E, Sun P. Fel d1 blocking antibodies against themajor cat allergen Fel d1 [Abstract]. Allergy 2019; 74: 324. [Google Scholar]
- 131. Satyaraj E, Gardner C, Filipi I, et al. Reduction of active Feld1 from cats using an antiFel d1 egg IgY antibody. ImmunInflamm Dis 2019; 7: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wedner JH, Mantia T, Satyaraj E, et al. Feeding cats eggproduct with polyclonal-anti-Fel d1 antibodies decreasesenvironmental Fel d1 and allergic response: a proof ofconcept study. J Allergy Infect Dis 2021; 2: 1–8. [Google Scholar]
- 133. Satyaraj E, Wedner HJ, Bousquet J. Keep the cat, changethe care pathway: a transformational approach to managingFel d 1, the major cat allergen. Allergy 2019; 74 Suppl 107: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bedrani L, Helloin E, Guyot N, et al. Passive maternalexposure to environmental microbes selectively modulatesthe innate defences of chicken egg white by increasingsome of its antibacterial activities. BMC Microbiol 2013; 13:128. DOI: 10.1186/1471-2180-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hamal KR, Burgess SC, Pevzner IY, et al. Maternal antibodytransfer from dams to their egg yolks, egg whites, andchicks in meat lines of chickens. Poult Sci 2006; 85:1364–1372. [DOI] [PubMed] [Google Scholar]
- 136. Pereira EPV, van Tilburg MF, Florean EOPT, et al. Egg yolkantibodies (IgY) and their applications in human andveterinary health: a review. Int Immunopharmacol 2019; 73:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Larsson A, Sjöquist J. Chicken IgY: utilizing the evolutionarydifference. Comp Immunol Microbiol Infect Dis 1990; 13:199–201. [DOI] [PubMed] [Google Scholar]
- 138. Vega CG, Bok M, Vlasova AN, et al. IgY antibodies protectagainst human rotavirus induced diarrhea in the neonatalgnotobiotic piglet disease model. PLoS One 2012; 7: e42788.DOI: 10.1371/journal.pone.0042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang X, Calvert RA, Sutton BJ, et al. IgY: a key isotype inantibody evolution. Biol Rev Camb Philos Soc 2017; 92:2144–2156. [DOI] [PubMed] [Google Scholar]
- 140. Schade R, Calzado EG, Sarmiento R, et al. Chicken egg yolkantibodies (IgY-technology): a review of progress in productionand use in research and human and veterinarymedicine. Altern Lab Anim 2005; 33: 129–154. [DOI] [PubMed] [Google Scholar]
- 141. Diraviyam T, Zhao B, Wang Y, et al. Effect of chicken eggyolk antibodies (IgY) against diarrhea in domesticatedanimals: a systematic review and meta-analysis. PLoS One 2014; 9: e97716. DOI: 10.1371/journal.pone.0097716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li X, Wang L, Zhen Y, et al. Chicken egg yolk antibodies(IgY) as non-antibiotic production enhancers for use inswine production: a review. J Anim Sci Biotechnol 2015; 6: 40.DOI: 10.1186/s40104-015-0038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Spillner E, Braren I, Greunke K, et al. Avian IgY antibodiesand their recombinant equivalents in research, diagnosticsand therapy. Biologicals 2012; 40: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Leiva CL, Gallardo MJ, Casanova N, et al. IgY-technology(egg yolk antibodies) in human medicine: a review ofpatents and clinical trials. Int Immunopharmacol 2020; 81:106269. DOI: 10.1016/j.intimp.2020.106269. [DOI] [PubMed] [Google Scholar]
- 145. Thirumalai D, Visaga Ambi S, Vieira-Pires RS, et al. Chickenegg yolk antibody (IgY) as diagnostics and therapeutics inparasitic infections – a review. Int J Biol Macromol 2019; 136:755–763. [DOI] [PubMed] [Google Scholar]
- 146. Abbas AT, El-Kafrawy SA, Sohrab SS, et al. IgY antibodies forthe immunoprophylaxis and therapy of respiratory infections.Hum Vaccin Immunother 2019; 15: 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Matulka RA, Thompson L, Corley D. Multi-level safetystudies of anti Fel d 1 IgY ingredient in cat food. Front Vet Sci 2019; 6: 477. DOI: 10.3389/fvets.2019.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pezzali JG, Smith SC, Aldrich CG. An overview of the effectof diet on the allergenicity of cats to susceptible humans.SOJ Vet Sci 2018; 4: 1–9. DOI: 10.15226/2381-2907/4/2/00151. [DOI] [Google Scholar]
- 149. Arlian LG, Morgan MS, Neal JS, et al. Influence of diet on Feld 1 production by cats [Abstract]. J Allergy Clinical Immunol 2005; 115: S236. [Google Scholar]
- 150. Bousquet J, Pfaar O, Togias A, et al. 2019 ARIA Care pathwaysfor allergen immunotherapy. Allergy 2019; 74:2087–2102. [DOI] [PubMed] [Google Scholar]
- 151. Chung LP, Paton JY. Two sides of the same coin? –Treatment of chronic asthma in children and adults. FrontPediatr 2019; 7: 62. DOI: 10.3389/fped.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Côté A, Godbout K, Boulet L-P. The management ofsevere asthma in 2020. Biochem Pharmacol 2020; 179: 114112.DOI: 10.1016/j.bcp.2020.114112. [DOI] [PubMed] [Google Scholar]
- 153. Asthma GIF. Pocket guide for asthma management and prevention.Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2020/04/Main-pocket-guide_2020_04_03-final-wms.pdf (2020. accessed 17 September 2021). [Google Scholar]
- 154. Nakamura Y, Tamaoki J, Nagase H, et al. Japanese guidelinesfor adult asthma 2020. Allergol Int 2020; 69: 519–548. [DOI] [PubMed] [Google Scholar]
- 155. Jutel M, Agache I, Bonini S, et al. International consensus onallergy immunotherapy. J Allergy Clin Immunol 2015; 136:556–568. [DOI] [PubMed] [Google Scholar]
- 156. Alvaro-Lozano M, Akdis CA, Akdis M, et al. EAACI allergenimmunotherapy user’s guide. Pediatr Allergy Immunol 2020;31 Suppl 25: 1–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dhami S, Agarwal A. Does evidence support the use ofcat allergen immunotherapy? Curr Opin Allergy Clin Immunol2018; 18: 350–355. [DOI] [PubMed] [Google Scholar]
- 158. Dhami S, Kakourou A, Asamoah F, et al. Allergenimmunotherapy for allergic asthma: a systematic reviewand meta-analysis. Allergy 2017; 72: 1825–1848. [DOI] [PubMed] [Google Scholar]
- 159. Wachholz PA, Durham SR. Mechanisms of immunotherapy:IgG revisited. Curr Opin Allergy Clin Immunol 2004; 4: 313–318. [DOI] [PubMed] [Google Scholar]
- 160. Orengo JM, Radin AR, Kamat V, et al. Treating cat allergywith monoclonal IgG antibodies that bind allergen andprevent IgE engagement. Nat Commun 2018; 9: 1421. DOI: 10.1038/s41467-018-03636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yang MS, Lee SP, Kwon YJ, et al. Dog and cat allergies andallergen avoidance measures in Korean adult pet ownerswho participated in a pet exhibition. Allergy Asthma ImmunolRes 2018; 10: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]