Abstract
Introduction
Early in-home care is increasingly being used in Scandinavian countries for clinically stable premature infants. Due to challenges with travel and hospital resources, alternative ways to support parents during early in-home care are being considered. The aim of this study was to test whether the proportion of mothers exclusively breastfeeding, parental confidence and mother–infant interaction increased after early in-home care with premature infants, and to compare the outcomes of in-home care involving the use of video communication and a mobile application with those of in-home care involving in-hospital consultations.
Methods
This study was conducted in four neonatal wards offering premature infant in-home care in Denmark. Premature infants were randomised using 1:1 block randomisation. During early in-home care, families had planned consultations two to three times a week, during which they received support from nurses: the intervention group had video consultations, while the control group had in-hospital consultations.
Results
The proportion of exclusively breastfeeding mothers at discharge was 66.7% in the intervention group vs 66% in the control group and decreased to 49.4% vs 55%, respectively, 1 month after discharge. No significant improvements were found in the intervention group compared with the control group. In the intervention group, some video consultations were changed to telephone consultations due to problems with the video function, or to in-hospital consultations due to infants’ requirement for medical services. No significant differences in secondary outcomes were observed.
Discussion
The study showed similar breastfeeding proportions at discharge. No unfavourable effects of video consultation compared with in-hospital consultation were found, indicating that video consultation could be a viable option and an important supplement during early in-home care.
Trial registration
ClinicalTrials.gov ID: NCT02581800.
Keywords: Telehealth, RCT, early in-home care, breastfeeding, premature infant, discharge, video consultations
Introduction
Early in-home care is increasingly being used in Scandinavian countries. Clinically stable premature infants and parents are supported by either home visits or hospital consultation in the transition from hospital to home.1–7 This transition is difficult for many parents in the first weeks or months after leaving hospital.8,9 Parents may struggle with anxiety, depression, decreased parenting confidence and self-efficacy, and impaired parent–child interactions related to feelings caused by the premature infant’s prior health condition. 9 The literature suggests a connection between parental confidence and the ability to buffer reactions such as depression and relationship difficulties, 10 and problems in mother–infant interaction can be a risk factor for the development of cognitive dysfunction and psychopathology in the child. 11
In the transition phase, preterm infants are moved from tube feeding to full breastfeeding or bottle feeding. In Scandinavia, the initiation of breastfeeding is high among mothers giving premature birth, 12 but they stop exclusive breastfeeding earlier than mothers of term infants. 13 Factors affecting breastfeeding after coming home include infants’ difficulty latching, mothers’ unfulfilled information needs, a lack of breastfeeding skills, and poor support.12,14 A Cochrane review reported tube feeding during early in-home care to be safe and to shorten hospitalisation but did not report a difference in breastfeeding rates, 15 and test weighing has been shown to help achieve exclusive breastfeeding at an earlier postmenstrual age. 16 A Cochrane review investigating support for breastfeeding mothers with healthy term infants showed that tailored support decreased the risk of early cessation of breastfeeding, 17 and Ericson et al. 14 found a link between a lack of support and cessation of breastfeeding of preterm infants, indicating that support could be crucial to breastfeeding mothers of preterm infants.
Until now, early in-home care has been offered with support in the form of in-hospital consultations or home visits. In early in-home care, parents often describe experiencing improved early relationship-building with the infant.1,2 The use of in-hospital consultations is demanding because families must travel to and from the hospital, and home visits require a large amount of resources, as hospitals also covers rural areas. Therefore, the increasing desire to offer in-home care to all families of premature infants, regardless of distance to the hospital, has generated a need to use an alternative option – telecommunication – as a possible way to support such families. A randomised study using a smartphone application showed promising results in increasing the sense of parenting competence (self-efficacy) during early in-home care. 18 Further studies are needed to determine whether the use of telecommunication supports mothers to continue breastfeeding and increases mother-infant interaction and parents’ feelings of confidence.
Aim
The aim of this study was to test whether the proportion of mothers exclusively breastfeeding, parental confidence and mother–infant interaction increased after early in-home care with premature infants and to compare the outcomes of in-home care involving the use of video communication and a mobile application with those of in-home care involving in-hospital consultations.
Methods
This study was a randomised controlled intervention study with two parallel arms. The inclusion of participants in the study began in November 2015 and ended in September 2018 (Figure 1). The study was approved by the Regional Ethics Committee (Region Zealand, Denmark) and the Danish Data Protection Agency. Parents provided informed consent before participation. The study was carried out according to the CONSORT guidelines, 19 and was registered at ClinicalTrials.gov, ID: NCT02581800.
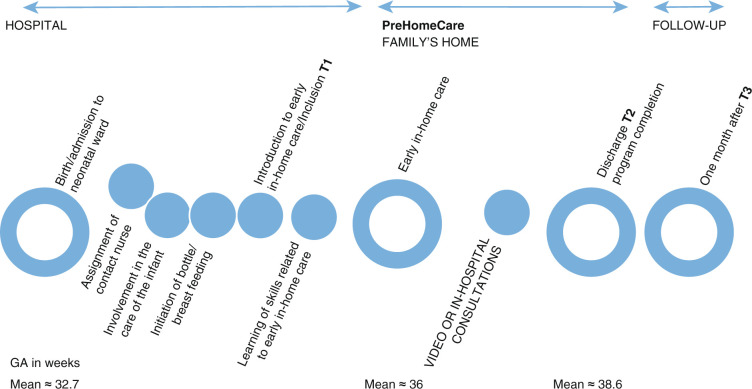
Figure 1.
Timeline from admission to discharge of the premature infants and families and study milestones. T2 = discharge, i.e., program completion.
GA, gestational age.
Setting
The study was implemented in four Danish neonatal wards that receive premature infants above the gestational age (GA) of 27–28 weeks and are level IIIa wards. 20 Three of the included wards had offered early in-home care (usual care) with in-hospital consultations prior to the study. Of these three wards, one stopped including participants in the study a year before the other two wards due to staff challenges, and one stopped including participants half a year before the end of the study period due to the start of another study that interfered with the present study. The fourth ward began early in-home care when the present study started. This ward joined the study in June 2016 to ensure timely finalisation of the study. Care in the participating neonatal wards was provided by nurses (RNs) who were bedside trained in caring for premature infants. Each ward had a nurse with an International Board Certified Lactation Consultant (IBCLC) education. All nurses participating in the early in-home care program were familiar with and trained in early in-home care. The wards assigned the families a contact nurse at birth who followed the family through admission and early in-home care, except in one ward in which four responsible nurses handled care in the early in-home care program. Hospital discharge occurred when early in-home care support had been completed. The parents were informed about the early in-home care program upon admission to the neonatal ward. At the time when the infant started breastfeeding or bottle feeding and the family wished to go home and fulfilled the criteria for early in-home care, the family was offered the early in-home care program. The criteria are listed in Appendix 1.
Participants, and inclusion and exclusion criteria
The participants in the study were mothers/fathers with premature infants who were admitted to the neonatal wards. Parents with hospitalised infants born before 37 weeks of gestation were invited to participate if they fulfilled the criteria for early in-home care, spoke Danish or English, could read the Danish text in the application, and had Wi-Fi/LTE/HSDPA in their homes. In case of twins, only one twin was randomly included. The exclusion criteria were infants who did not meet the criteria for early in-home care or parents who required additional parent–infant observations or had low parenting skills based on nurses’ or doctors’ discretion.
Inclusion, randomisation and power calculation
Three to four nurses in each ward who had special interests in early in-home care served as the responsible nurses for the project. If the parents were interested in early in-home care and fulfilled the inclusion criteria for early in-home care, they were informed verbally and in writing about the study.
After receiving written consent from parents, the responsible nurse accessed a website to obtain the randomisation result that was generated through a website randomisation procedure. 21 Families were randomised to either the intervention group or control group using fixed block randomisation (block size of 4) in a 1:1 ratio per ward at the individual level. We aimed to include 160 infants/families based on the power calculations for the primary outcome. 22 We hypothesised that a two-sided, two-sample proportion test would detect an increase in the percentage of breastfeeding women between the two groups of 55% in the intervention group vs 41.5% in the control group 1 month after discharge, assuming that 68% of mothers would be breastfeeding at discharge. 22 Most families were included before knowing the duration of breastfeeding establishment; therefore, participant inclusion continued until there was a minimum of 80 infants in both groups.
Early in-home care, the content of the intervention (PreHomeCare) and implementation
Both groups received the PreHomeCare program, which involved early in-home care as usual, in which the parents received training in first aid skills, borrowed breast pumps if needed, received a leaflet and verbal information concerning the care of the infant, and were instructed on how to insert the feeding tube. In addition, parents had the opportunity to call the neonatal ward 24 h a day. If an infant required medical and/or other services during early in-home care, these services were offered. Additionally, families had two to three planned consultations a week, primarily with the contact nurse at the hospital. During the consultations, the nurse and parent had a dialogue and exchanged information concerning the nutrition plan, the infant’s current weight, bottle/breastfeeding progression, family life, the infant’s general well-being, the expression of breastmilk, the use of nipple shields and tube feeding, among other topics. Between in-hospital consultations, parents recorded their infants’ nutrition on a blank piece of paper or registration paper. When an infant had begun to receive full nutrition from breastfeeding or bottle feeding and gain weight (minimum 20–25 g/day), the family was discharged (see Figure 1).
In addition to usual care, the intervention group received an offer to have their consultations by video from their homes. Families in the intervention group received a smartphone with an application and a manual with instructions on how to use the application upon their inclusion in the study and training in how to use the application after their inclusion. 23 The application consisted of three components: (a) advice and recommendations concerning breastfeeding, breastfeeding positions, infant signals, skin-to-skin contact, physiotherapy, etc.; (b) data registration for nutrition, vitamins and weight; and (c) a link to the video consultation system through which parents could contact the ward. Parents could record infants’ nutrition in the application, and the mobile application had the ability to send reminders for planned infant meals. The family could access the infant’s weight history and share a report with notes and the infant’s nutrition and weight with the hospital. The application provided information through search options and information icons. Families could use the application from inclusion until discharge. Video consultations were planned two to three times a week. Additionally, parents borrowed a scale to weigh their infants at home. The study smartphones had LTE/HSDPA. All equipment (the phone and weighing scale) was provided by the neonatal wards throughout the study. The mobile application (beta version) was developed prior to this study through clinical and parental evaluation and was intended to provide the parents with a secure and safe experience. Both the mobile application and video consultation system were available from Viewcare A/S Herlev. 24
All responsible nurses received training in the use of the smartphone application, video consultations and the manual in two to three 2-h meetings. The remaining staff at the neonatal wards received training in staff meetings and at bedside from the responsible nurses. In addition to training, the responsible nurses could call the first author at any time for technical assistance. All the responsible nurses in the wards had quarterly meetings to discuss study challenges, study progress, program delivery and data collection.
Measures
The primary outcome was the proportion of exclusively breastfeeding mothers; exclusive breastfeeding was defined as infants breastfeeding or receiving the mother’s expressed milk in a bottle based on the definition provided by the World Health Organisation. 25 Secondary outcomes were the scores of the 15-item Karitane Parenting Confidence Scale (KPCS),10,26 and the 10-item Mother and Baby Interaction Scale (MABISC), 11 measuring parental confidence and parent–infant interaction, respectively. Validation of the KPCS in a Danish context showed an acceptable internal consistency (Cronbach’s alpha = 0.72–0.79).27,28 A Danish version of the MABISC was developed and has been used previously,29,30 showing a Cronbach’s alpha >0.70. In addition, we collected infant characteristics, birth data, parental sociodemographic data, breastfeeding experiences and information on contact during the intervention, all of which appear in Table 1.
Table 1.
Data collection for the outcome measures and basic variables.
| T1 | During the intervention | T2 | T3 | Source | |
|---|---|---|---|---|---|
| Proportion of exclusively breastfeeding mothers | X | X | HR | ||
| Duration (days) of exclusive breastfeeding from T2 to T3 |
X | HR | |||
| Mother-infant interaction (MABISC) | X | X | X | SRQ | |
| Parental confidence (KPCS) | |||||
| Infant characteristics | X | X | X | HR | |
| Infant weight and nutrition, bottle feeding and/or partial breastfeeding | |||||
| Birth data (GA, weight, length, diagnosis, date of birth, sex, treatment received in the hospital) |
X | HR | |||
| Parents’ sociodemographic data (Age, education, parity, income, marital and cohabitation status, smoking, mother’s height and weight, distance to hospital) |
X | SRQ | |||
| Breastfeeding experience Breastfeeding self-efficacy (BSES-SF) 31 |
X | SRQ | |||
| Use of nipple shields | X | X | HR | ||
| Contact with hospital during the intervention Planned and unplanned consultations and consul tation method (hospital, telephone, video) |
X | HR |
BSES-SF: breastfeeding self-efficacy scale-short form; GA: gestational age; HR: hospital record; KPCS: Karitane parenting confidence scale; MABISC: Mother and baby interaction scale SRQ: self-reported questionnaire; T1: inclusion; T2: discharge; T3: 1 month after discharge.
Data collection
Data were collected from hospital records by the responsible nurses and from mothers’ self-reported questionnaires at inclusion (T1), discharge (T2) and 1 month after discharge (T3) (see Figure 1). Data on exclusive breastfeeding, characteristics of the infant, birth and planned and unplanned consultations with the hospital were collected from hospital records and entered manually into the Easytrial AsP database by the responsible nurses. Parents’ sociodemographic characteristics, breastfeeding experiences, breastfeeding self-efficacy, mother–infant interaction and parental confidence were collected from self-reported questionnaires. The variables, sources and data collection times appear in Table 1. At all time points, the questionnaires were sent via email through SurveyXact, and reminders were sent up to three times. The first questionnaire was completed while the families were still in the wards so the nurses could remind the families.
Statistical analysis
Descriptive statistics are used to present the characteristics. Categorical data are presented as percentages, normally distributed continuous data are presented as the means with standard deviations, and skewed data are presented as the medians and interquartile ranges (IQRs). We used independent t tests or the Mann-Whitney test to test differences in continuous variables and Chi-square tests for categorical variables.
The primary outcome was assessed using chi-square tests and a two-sample proportions test. As mothers who started bottle feeding had a lower probability of breastfeeding their infants, infants who initially were bottle fed at inclusion were excluded from the proportion test. We performed intention-to-treat analyses for the primary and secondary outcomes.
Last, the dataset was formatted as longitudinal data, and the primary outcome was included in a multilevel mixed-effects logistic regression with the data coded as dichotomous or categorical. To be faithful to the randomisation result, the data were not adjusted; however, to investigate the potential confounding of the results, in a supplementary analysis, the data were adjusted for the use of nipple shields, parity and a weight deviation <–22%, which has previously been shown to affect exclusive breastfeeding. 13 The secondary outcomes were also included in a multilevel mixed-effects linear regression or quantile regression with fixed effects. Finally, a per-protocol analysis of participants who complied with the protocol was conducted. Values of p < 0.05 were considered statistically significant. Data were analysed using STATA/IC 14.0 (Stata Corp CP, Texas, USA) software.
Results
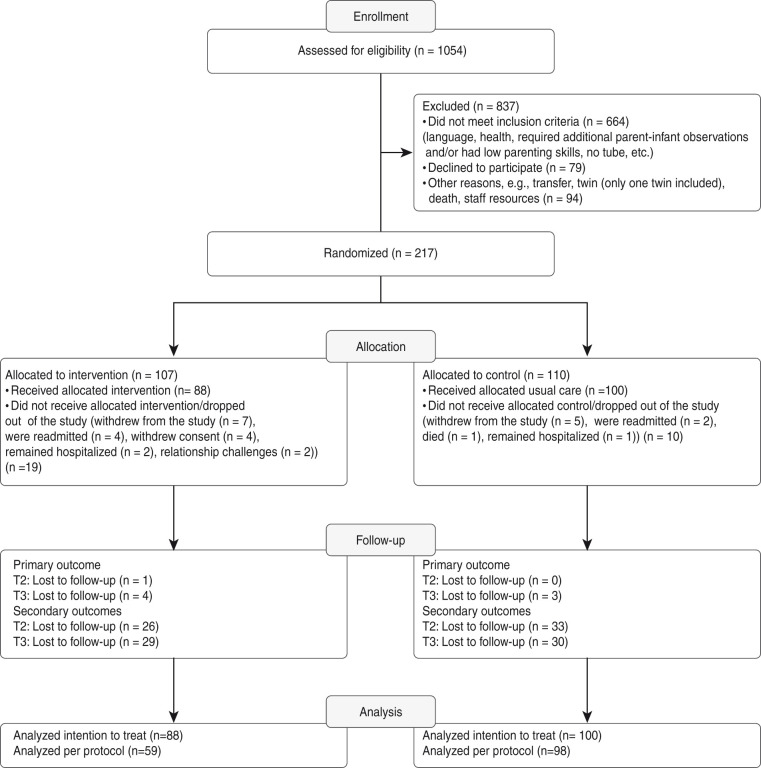
Participant selection is shown in Figure 2, which presents the flow diagram of the participants in the study. During the study, 1054 infants were born prematurely and admitted to the participating wards. Of these infants, 837 were excluded due to not meeting inclusion criteria (n = 664), their parents declining to participate (n = 79) and other reasons, including death, transfer to another ward and lack of staff resources (n = 94). Of the 217 randomised families, 19 in the intervention group dropped out, and 10 in the control group dropped out for various reasons; 88 participants remained in the intervention group, and 100 participants remained in the control group. There was a significant number of protocol deviations in the intervention group (26 cases compared with 2 cases in the control group). In the intervention group, the deviations were due primarily to problems with video function (see Figure 3).
Figure 2.
CONSORT participant flow diagram with response rates.
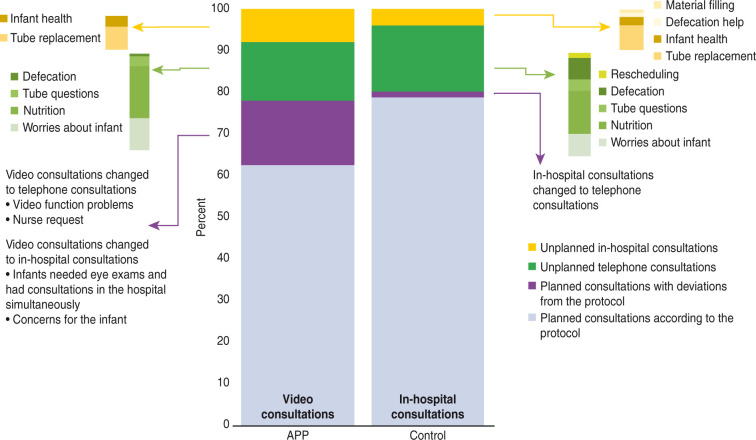
Figure 3.
Total percentage of planned and unplanned consultations, including the reasons for unplanned consultations and changes to planned consultations.
For the primary outcome, the data from 100% of the participants at T1, 99% of the participants at T2 and 96% of the participants at T3 were available for analysis. The questionnaire response rates were as follows: T1, 82% (Q1); T2, 69% (Q2); and T3, 71% (Q3). The distribution of the missing data was tested with the complete dataset of GA of the infant, distance to the hospital, exclusive breastfeeding and ward to look for a skewed distribution between the groups. There were no differences between the two groups in relation to the missing values.
The participant characteristics presented in Table 2 showed that the two groups were not significantly different in terms of basic characteristics. Infants were born primarily after 32 weeks; in the intervention group, infants were born at a mean GA of 33 + 0(±3) weeks, and in the control group, infants were born at a mean GA of 32 + 5(±4) weeks. There were no significant differences between wards. Marital status differed between the groups, as six of the parents in the intervention group and one in the control group were single/living alone. However, the total numbers were low and not considered significant for the analysis. The mean number of days between T1 and T2 was 24 days (95% confidence interval (CI) 21.4; 26.5) for the intervention group and 22.3 days (95% CI 19.6; 25) for the control group. The median distance in kilometres to the hospital was significantly different between the groups, as the intervention group had a median distance of 32 km, and the control group had a median distance of 22.5 km (see Table 2).
Table 2.
Basic characteristics of the infants and parents, including educational level, age, home and birth-related data.
|
Intervention group |
Control group |
p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Parents | |||
| Household income | |||
| Low | 5 (7.4) | 3 (4.2) | 0.73 |
| Medium | 29 (42.7) | 31 (43.7) | |
| High | 34 (50) | 37 (52.1) | |
| Geographic distance of hospital–home, kilometresb | 32 (18–39) | 22.5 (5–37.5) | 0.02 |
| Mother’s age, years a,c | 30.4 (5.8) | 30.0 (4.4) | 0.6 |
| Mother’s body mass index a,c | 25.7 (5.7) | 24.5 (5.9) | 0.2 |
| Mother’s educational level | |||
| High school level or lower | 16 (21.3) | 18 (22.5) | |
| Short-cycle education | 21 (28) | 17 (21.3) | 0.7 |
| Medium-cycle education | 28 (37.3) | 35 (43.8) | |
| Long-cycle education | 10 (13.3) | 10 (12.5) | |
| Ethnicity (DK) | 86 (97.7) | 99 (99) | 0.37 |
| Marital statusa | |||
| Single/living alone | 6 (7.9) | 1 (1.3) | 0.05 |
| Number of infantsa | |||
| Two or more | 38 (50) | 42 (53.2) | 0.69 |
| Parity | |||
| Twin % | 17 (20) | 14 (14) | 0.3 |
| BSES-SF score (range 13–65)b | 61 (57–64) | 61 (55–63) | 0.6 |
| Mode of delivery n% | |||
| Caesarian section | 46 (53) | 46 (47) | 0.5 |
| Father’s age, years a,c | 31.2 (1.2) | 31.5 (1.2) | 0.9 |
| Father’s educational level | |||
| High school level or lower | 31 (41.3) | 40 (50) | |
| Short-cycle education | 10 (13.3) | 7 (8.8) | 0.9 |
| Medium-cycle education | 18 (24) | 21 (26.6) | |
| Long-cycle education | 14 (18.7) | 11 (13.8) | |
| Infant | |||
| GA at birth | |||
| <28 weeks | 6 (6.8) | 7 (7.0) | |
| 28–32 weeks | 18 (20.5) | 26 (26.0) | 0.75 |
| >32 weeks | 64 (72.7) | 67 (67.0) | |
| Sex | |||
| Boys | 47 (53.4) | 54 (54) | 1 |
| Birthweight, grams c | 1912.1 (571) | 1899.4 (614) | 0.9 |
| Small for GA – Percentage deviation from expected birthweight32,c |
–12.2 (16.7) | –11.4 (17.7) | 0.73 |
| <–22% (2 SDs) of expected birthweight | 25 (28.4) | 26 (26.0) | 0.71 |
aMissing data: intervention group, 13.6%; control group, 20%.
bItalic = median IQR.
cMean SD.
BSES-SF: breastfeeding self-efficacy scale-short form; DK: Danish; GA: gestational age; SD: standard deviation.
Effect evaluation
Table 3 shows the raw data for the proportions of mothers exclusively breastfeeding in the two groups. Table 4 shows the results of the two-sample proportions test of exclusive breastfeeding 1 month after discharge, with no significant improvement between T2 and T3. The test was performed both with and without excluding infants who were bottle-fed at T1, with no significant improvement in either case. There was a slightly larger decrease in exclusive breastfeeding in the intervention group at T3 compared with that in the control group. There were no differences in the proportion of exclusively breastfeeding mothers between the wards at any of the three time points. The proportions of mothers exclusively bottle-feeding at T2 were 21.8% for the intervention group and 25% for the control group, respectively, and, at T3, the proportions increased to 32.2% and 31%, respectively. The proportion of mothers engaging in partial breastfeeding changed from 11.5% to 9% at T2 and to 14.9% in the intervention group and 11% in the control group at T3.
Table 3.
Proportions of mothers exclusively breastfeeding at inclusion (T1), discharge (T2) and 1 month after discharge (T3).
| T1 a n (%) | p-value | T2 n (%) | p-value | T3 n (%) | p-value | |
|---|---|---|---|---|---|---|
| Intervention group | 78 (88.6) | 58 (66.7) | 43 (49.4) | |||
| Control group | 83 (83) | 0.54 | 66 (66) | 0.78 | 55 (55) | 0.65 |
aT1 – Initially started breastfeeding (tube feeding implied).
Table 4.
Two-sample proportion test of exclusive breastfeeding. Mean proportion difference between discharge (T2) and 1 month after discharge (T3).
| T2% (95% CI) | T3% (95% CI) | Δ % | p-value | |
|---|---|---|---|---|
| Intervention group n = 84 | 66.6 (56.8; 76.6) | 51.1 (40.5; 61.9) | 15.5 | 0.2 |
| Control group n = 97 | 66.0 (57.7; 75.3) | 56.7 (46.8; 66.6) | 9.3 | |
| Intervention group n = 75 a | 74.4 (64.7; 84.0) | 57.3 (46.1; 68.5) | 17.0 | 0.1 |
| Control group n = 83 a | 76.5 (67.5; 85.5) | 65.1 (54.8; 75.3) | 11.4 |
Δ Difference in proportion from T2–T3.
aExcluded infants who were bottle-fed at T1.
When exclusive breastfeeding was included in a mixed-effects model, the model showed that the odds ratio (OR) of breastfeeding was 0.49 (95% CI: 0.05–4.87) in the intervention group, but there was no significant difference compared with that in the control group. Adjusting for parity, weight deviation <–22% and use of nipple shields showed that the intervention group had an OR of 0.67 (95% CI: 0.08; 5.55) for exclusive breastfeeding, but there was no significant difference compared with that of the control group.
Table 5 shows the medians/means of the secondary outcomes, i.e., the KPCS and MABISC scores. There was a small but significant difference in the MABISC scores at T1, with the intervention group scoring 9.8 and the control group scoring 10.9 (p-value = 0.03). Except for this difference, there were no significant differences between the scores or between the groups at T2 and T3. The mixed-effects model showed a difference in the total KPSC score, with intervention group scoring 0.9 points higher than the control group. Analysis of the MABISC scores showed that the intervention group had a –0.9 lower total score than the control group, but the difference was not significant.
Table 5.
Means and medians of the KPCS and MABISC scores at T1, T2 and T3 for the intervention group and control group.
|
Intervention |
Control |
|||||
|---|---|---|---|---|---|---|
| T1; n = 75 | T2; n = 62 | T3; n = 59 | T1; n = 80 | T2; n = 67 | T3; n = 70 | |
| Total KPCS a Median (IQR) | 43 (41–44) | 43 (41–44) | 43 (41–45) | 43 (41–44) | 42 (41–44) | 43 (40–44) |
| Total MABISCb Mean (SD) | 9.8 (3.4) | 10.4 (3.2) | 10.5 (3.1) | 10.9 (3.0) | 10.5 (2.9) | 11.3 (3.4) |
aRange 0-45 - High scores are preferable bRange 0-40 - Low scores are preferable.
IQR: interquartile range; KPCS: Karitane parenting confidence scale; MABISC: Mother and baby interaction scale; SD: standard deviation; T1: inclusion; T2: discharge; T3: 1 month after discharge.
A per-protocol analysis was performed and showed similar results as the intention-to-treat analysis regarding differences in the proportion of exclusively breastfeeding mothers, KPCS scores and MABISC scores between the groups.
Intervention fidelity
During the intervention, families in both groups received planned and unplanned consultations, as shown in Figure 3. Approximately 80% of the total consultations were planned consultations, either in the form of video consultations for the intervention group or in-hospital consultations for the control group. There was a significant difference in the median number of planned consultations, with four video consultations (from three to six) in the intervention group and four in-hospital consultations (from two to five) in the control group (p-value = 0.03). The length of early in-home care was not significantly different between the two groups, with a median of 18 days (12–26) in the intervention group and 16 days (10.4–21) in the control group. There were some protocol deviations in the planned consultations, as shown in Figure 3. In particular, the intervention group changed video consultation to telephone consultations, primarily due to problems with video function, or to in-hospital consultations, due to infants’ requirement of medical services. Medical services were delivered regardless of the randomisation group. Approximately 20% of the consultations were unplanned. Unplanned consultations were consultations that occurred between the planned consultations. Figure 3 shows the various reasons for the unplanned telephone and in-hospital consultations. The total and median numbers of unplanned consultations were not different between the groups. The number of unplanned in-hospital consultations regarding tube replacement was higher for the intervention group than for the control group. In a few cases (4.5% in the intervention group and 2% in the control group), unplanned consultations resulted in short, 1–2 day admissions due to jaundice/phototherapy, concern for the infant, gastrointestinal problems and respiratory nasal mucus. All families resumed early in-home care subsequently.
Discussion
This study found no significant differences between the outcomes of support providing usual care and the outcomes of the alternative option offering video consultations, showing that video consultations can be used in the same way as usual care.
The use of video consultation, the use of the mobile application and baby weighing were not associated with a statically significant improvement or reduction in the proportion of mothers who were exclusively breastfeeding. This finding corresponds with those of Holm et al., 33 who used video consultation during early in-home care and showed comparable proportions of mothers who were exclusively breastfeeding at discharge, indicating that, compared with in-hospital consultations, the communication method via video consultation did not influence breastfeeding rates. In addition, Ortenstrand et al. found comparable results using home visits.4,5 In the present study, we gave a baby weighing scale to the families in the intervention group because Funkquist et al. found that test weighing helped achieve exclusive breastfeeding at an earlier postmenstrual age. 16 We do not know if the provision of the scale had any influence on the proportion of mothers exclusively breastfeeding, as it was part of the intervention. The present study found that 66–67% of mothers were exclusively breastfeeding at discharge. A new Danish annual report of exclusive breastfeeding from 2018 showed that only 45% of infants admitted to neonatal wards were exclusively breastfed at discharge, and that, in only one-fifth of the wards, 60% of the infants were exclusively breastfed at discharge, 34 indicating that the proportion of mothers exclusively breastfeeding at discharge found in the present study is among the higher frequencies of exclusive breastfeeding at neonatal ward discharge in Denmark.
There was a tendency for increased cessation of exclusive breastfeeding in the intervention group at 1 month after discharge, with approximately 10% of mothers in the control group and 16% in the intervention group stopping exclusive breastfeeding. Most Danish studies that have reported breastfeeding proportions among premature infants after birth have shown similar increased rates of cessation of exclusive breastfeeding.35–37 This supports the observation that the first months after discharge from the neonatal ward are an especially vulnerable period for the breastfeeding mother. The support delivered through video, the application and the weighing scale during the intervention may not have been sufficiently supportive for some mothers, who may have needed more complex supportive interventions after discharge to reduce cessation of exclusive breastfeeding. Nevertheless, there were no significant differences in cessation between those receiving video consultation and those receiving in-hospital consultation. After early in-home care and discharge from the hospital, breastfeeding support is handed over to the community health visitor. Further investigation is needed to examine mothers’ needs in the first period following discharge and the long-term effect of video communication during early in-home care.
The participants had low MABISC and high KPCS scores at all three time points, with no differences between the two groups. The scales were affected by the floor/ceiling effect as discussed by Pontoppidan et al. 27 As some mothers who have given premature birth initially experience difficulties in becoming mothers and are at risk for experiencing less positive interactions and attachment, 38 mothers of preterm infants would be expected to have lower KPCS and higher MABISC scores than mothers of term infants. Both instruments were originally designed to be used with parents of infants from 0 to 12 months.10,11 Studies on mothers of newborns using the KPCS have shown similar results but have been able to measure development over time,27,30 which was not accomplished in this study, indicating that the scales were less usable in this setting or that the scales may not capture the worries of parenting premature infants. Both scales require validation within the neonatal setting and with parents of premature infants.
The intervention group had more unplanned consultations, including medical services, than the control group. This may be interpreted to indicate that the intervention did not fully meet the prerequisites of the families and nurses on all levels. As the families could choose if they wanted to learn to replace/insert the tube and/or express if they felt insecure about it, unplanned consultations regarding tube replacement were unavoidable. The unplanned consultations may be due partly to the dysfunction of the application/video, when a video consultation was sometimes exchanged for a telephone consultation. Other problems, such as frozen screens and sound delays in relation to video, have been widely discussed elsewhere and were also an issue for the nurses and families in this study. 39 As discussed by Donaghy et al. and Hammersley et al. reliable technology is essential for the widespread implementation of video consultations.40,41 It is likely that changing the consultation type was convenient for the nurses, as solving technical challenges required extra work, as also described by Østervang et al. 42 The implementation of this study identified potential challenges in trying to create easy-to-use instructions and constant support through the application and video use, and indicated that the use of technology could have been challenging for the nurses.
For the families, the distance from the hospital to home tended to influence compliance with the protocol. In the intervention group, those living closer to the hospital had more protocol deviations in the in-hospital consultations, and those in the control group who lived farther from hospital did not receive the planned in-hospital consultations. Access to health professionals by video consultation may be suitable for simple problems but not those requiring physical examination, 41 and may not be perceived as supportive by families in all cases. 39 Further studies must determine the needs for contact depending on distance and preferences as well as how nurses use video consultations to engage in a dialogue and exchange information with families during early in-home care.
This study was strengthened by the design and the effective randomisation, which led to two homogeneous groups. The wards were geographically spread over Denmark and represented a variety of care cultures. There were no differences between the groups and wards. Therefore, the block randomisation by ward achieved its objective.
The study had several limitations. First, there were missing data, as some questionnaires were not submitted despite reminders and text messages. It is possible that the email should have been addressed to only one parent. Furthermore, the application was a beta version, resulting in less attention from the provider than had been agreed upon. Second, the families included in the study generally had higher educational levels, with 50–55% of the women having up to middle and higher education, which is a higher range than that in the general Danish population in the study regions, where 35–40% have up to middle and higher education. 43 Nevertheless, there were no differences in the distribution of parents’ educational levels between the two groups, and the proportions of mothers exclusively breastfeeding at discharge are similar to those in prior studies.12,44
In conclusion, the results of this study did not indicate any unfavourable effects of video consultation compared with in-hospital consultation during early in-home care, indicating that video consultation could be a viable option during early in-home care and a very important supplement for families during early in-home care. The findings will make it possible for parents living in remote areas or long distances from a hospital to come home earlier without having to travel long distances two to three times a week. According to the results, future use of the video consultations will be a matter of personal preference, meaning that some parents will prefer in-hospital consultations if they live close to the hospital or need supplemental support, while other parents will prefer the use of video consultations to avoid unnecessary travel to and from the hospital. There will be even better possibilities for video consultations with improvements in Internet networks in outer areas and improvements in mobile devices and applications.
Supplemental Material
Supplemental material, JTT913411 Supplemental Material for Comparison of video and in-hospital consultations during early in-home care for premature infants and their families: A randomised trial by Mai-Britt Hägi-Pedersen, Ram B Dessau, Annelise Norlyk, Hristo Stanchev and Hanne Kronborg in Journal of Telemedicine and Telecare
Acknowledgements
First, we would like to extend warm acknowledgments to all families who participated in the study and express our deep gratitude to the responsible nurses for the project, namely, Birgit Hagelskær Dam (International Board of Lactation Consultant Examiners (IBCLC)), Catarina Krogh Andersen, Charlotte Maria Melgaard, Dorte Lissi Steen Hansen, Hanne Dalsgaard Loberg, Inge Nedergaard Henriksen, Inger Norup, Astrid Jespersen, Anna-Lisse Ingemann, Lilli Boel, Marie Rønn, Irene Dahlstrøm Larsen, Kirstine H Rotvig Erichsen, Merete van Deurs Petersen, Sille Nymann, Tina Thaulov Stoltenborg and Vibeke Fris Kyndesen (IBCLC), and all other staff at the Naestved, Roskilde, Herning and Viborg neonatal wards for their daily work, engagement and involvement in the study. Furthermore, we would like to thank the nurse managers, Hanne Schjøning, Lis Dueholm, Birthe Kruuse, Karin Hallum (IBCLC) and Annemi Frandsen; the doctors, Gholamreza Krog Dayani, Hristo Stanchev and Jens Peter Nielsen; and other staff for making the study possible and ensuring the organisational measures to support the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This PhD study was financed by Aarhus University, the Danish Foundation TrygFonden, the Health Foundation, the Danish Nurses’ Organisation, the Region Zealand Health Scientific Research Foundation and the local research foundation of NSR hospital.
ORCID iDs: Mai-Britt Hägi-Pedersen https://orcid.org/0000-0002-4349-4755
Annelise Norlyk https://orcid.org/0000-0002-8512-228X
Supplemental material
Supplemental material for this article is available online.
References
- 1.Dellenmark-Blom M, Wigert H. Parents’ experiences with neonatal home care following initial care in the neonatal intensive care unit: a phenomenological hermeneutical interview study. J Adv Nurs 2014; 70: 575–586. [DOI] [PubMed] [Google Scholar]
- 2.Sturm LD. Implementation and evaluation of a home gavage program for preterm infants. Neonatal Netw 2005; 24: 21–25. [DOI] [PubMed] [Google Scholar]
- 3.Holm KG, Brødsgaard A, Zachariassen G, et al. Parent perspectives of neonatal tele-homecare: a qualitative study. J Telemed Telecare 2019; 25: 221–229. [DOI] [PubMed] [Google Scholar]
- 4.Örtenstrand A, Waldenstrom U, Winbladh B. Early discharge of preterm infants needing limited special care, followed by domiciliary nursing care. Acta Paediatr 1999; 88: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 5.Örtenstrand A, Winbladh B, Nordström G, et al. Early discharge of preterm infants followed by domiciliary nursing care: parents’ anxiety, assessment of infant health and breastfeeding. Acta Paediatr 2001; 90: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg B, Lindgren C, Palme-Kilander C, et al. Hospital-assisted home care after early discharge from a Swedish neonatal intensive care unit was safe and readmissions were rare. Acta Paediatr 2016; 105: 895–901. [DOI] [PubMed] [Google Scholar]
- 7.Brødsgaard A, Zimmermann R, Petersen M. A preterm lifeline: early discharge programme based on family-centred care. J Spec Pediatr Nurs 2015; 20: 232–243. [DOI] [PubMed] [Google Scholar]
- 8.Broedsgaard A, Wagner L. How to facilitate parents and their premature infant for the transition home. Int Nurs Rev 2005; 52: 196–203. [DOI] [PubMed] [Google Scholar]
- 9.Boykova M, Kenner C. Transition from hospital to home for parents of preterm infants. J Perinat Neonatal Nurs 2012; 26: 81–87. [DOI] [PubMed] [Google Scholar]
- 10.Crncec R, Barnett B, Matthey S. Development of an instrument to assess perceived self-efficacy in the parents of infants. Res Nurs Health 2008; 31: 442–453. [DOI] [PubMed] [Google Scholar]
- 11.Høivik MS, Burkeland NA, Linaker OM, et al. The mother and baby interaction scale: a valid broadband instrument for efficient screening of postpartum interaction? A preliminary validation in a Norwegian community sample. Scand J Caring Sci 2013; 27: 733–739. [DOI] [PubMed] [Google Scholar]
- 12.Måstrup R. Breastfeeding of preterm infants. Associated factors in infants, mothers and clinical practice. PhD Thesis, Lunds Universitet, Lund, Sweden, 2014.
- 13.Maastrup R, Hansen BM, Kronborg H, et al. Factors associated with exclusive breastfeeding of preterm infants. Results from a prospective national cohort study. PLoS One 2014; 9: e89077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ericson J, Eriksson M, Hoddinott P, et al. Breastfeeding and risk for ceasing in mothers of preterm infants-long-term follow-up. Matern Child Nutr 2018; 14: e12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database Syst Rev 2015; 7: CD003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funkquist EL, Tuvemo T, Jonsson B, et al. Influence of test weighing before/after nursing on breastfeeding in preterm infants. Adv Neonatal Care 2010; 10: 33–39. [DOI] [PubMed] [Google Scholar]
- 17.McFadden A, Gavine A, Renfrew MJ, et al. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2017; 2: CD001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garfield CF, Lee YS, Kim HN, et al. Supporting parents of premature infants transitioning from the NICU to home: a pilot randomized control trial of a smartphone application. Internet Interv 2016; 4: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG, Moher D. Declaration of transparency for each research article. BMJ 2013; 347: f4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark AR. and American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics 2004; 114: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 21.Easytrial_AsP. Clinical trial management software, https://regionsjaelland.easytrial.net/login.aspx?ReturnUrl=%2fFrontPage.aspx&cookies=1 (2009–2020, accessed 24 January 2020).
- 22.Hägi-Pedersen MB, Norlyk A, Dessau R, et al. Multicentre randomised study of the effect and experience of an early inhome programme (PreHomeCare) for preterm infants using video consultation and smartphone applications compared with inhospital consultations: protocol of the PreHomeCare study. BMJ Open 2017; 7: e013024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hägi-Pedersen M-B. Præmatur appen prehomecare brugervejledning. Næstved: Childrens Ward, 2015. [Google Scholar]
- 24. Sundhedsinnovation Sjælland. Børne apps på rundtur , http://sundhedsinnovationsjaelland.dk/content/borne-apps-pa-rundtur (2014, accessed 3 October 2014).
- 25.World Health Organization. Exclusive breastfeeding, http://www.who.int/nutrition/topics/exclusive_breastfeeding/en/ (2016, accessed 11 May 2016).
- 26.Crncec R, Barnett B, Matthey S. Review of scales of parenting confidence. J Nurs Meas 2010; 18: 210–240. [DOI] [PubMed] [Google Scholar]
- 27.Pontoppidan M, Andrade SB, Kristensen IH, et al. Maternal confidence after birth in at-risk and not-at-risk mothers: internal and external validity of the Danish version of the Karitane Parenting Confidence Scale (KPCS). J Patient Rep Outcomes 2019; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen IH, Simonsen M, Trillingsgaard T, et al. First-time mothers’ confidence mood and stress in the first months postpartum. A cohort study. Sex Reprod Healthc 2018; 17: 43–49. [DOI] [PubMed] [Google Scholar]
- 29.Pontoppidan M. The effectiveness of the incredible years parents and babies program as a universal prevention intervention for parents of infants in Denmark: study protocol for a pilot randomized controlled trial. Trials 2015; 16: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pontoppidan M, Klest SK, Sandoy TM. The incredible years parents and babies program: a pilot randomized controlled trial. PLoS One 2016; 11: e0167592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennis CL. The breastfeeding self-efficacy scale: psychometric assessment of the short form. J Obstet Gynecol Neonatal Nurs 2003; 32: 734–744. [DOI] [PubMed] [Google Scholar]
- 32.Marsál K, Persson PH, Larsen T, et al. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996; 85: 843–848. [DOI] [PubMed] [Google Scholar]
- 33.Holm KG, Clemensen J, Brødsgaard A, et al. Growth and breastfeeding of preterm infants receiving neonatal tele-homecare compared to hospital-based care. J Neonatal Perinatal Med 2019; 12: 277–284. [DOI] [PubMed] [Google Scholar]
- 34.(DKN) Danish Quality Database for Newborn on Neonatal Wards. National annual report 2018, https://www.sundhed.dk/sundhedsfaglig/kvalitet/kliniske-kvalitetsdatabaser/graviditet-og-foedsel/nyfoedte/ (2018, accessed 5 November 2019).
- 35.Maastrup R, Hansen BM, Kronborg H, et al. Breastfeeding progression in preterm infants is influenced by factors in infants, mothers and clinical practice: the results of a national cohort study with high breastfeeding initiation rates. PLoS One 2014; 9: e108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ericson J, Eriksson M, Hellström-Westas L, et al. Proactive telephone support provided to breastfeeding mothers of preterm infants after discharge: a randomised controlled trial. Acta Paediatr 2018; 107: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meerlo-Habing ZE, Kosters-Boes EA, Klip H, et al. Early discharge with tube feeding at home for preterm infants is associated with longer duration of breast feeding. Arch Dis Child Fetal Neonatal Ed 2009; 94: F294–F297. [DOI] [PubMed] [Google Scholar]
- 38.Flacking R, Ewald U, Nyqvist KH, et al. Trustful bonds: a key to “becoming a mother” and to reciprocal breastfeeding. Stories of mothers of very preterm infants at a neonatal unit. Soc Sci Med 2006; 62: 70–80. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg B, Axelsson K, Ohrling K. Experience with videoconferencing between a neonatal unit and the families’ home from the perspective of certified paediatric nurses. J Telemed Telecare 2009; 15: 275–280. [DOI] [PubMed] [Google Scholar]
- 40.Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract 2019; 69: e586–e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammersley V, Donaghy E, Parker R, et al. Comparing the content and quality of video, telephone, and face-to-face consultations: a non-randomised, quasi-experimental, exploratory study in UK primary care. Br J Gen Pract 2019; 69: e595–e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Østervang C, Vestergaard LV, Dieperink KB, et al. Patient rounds with video-consulted relatives: qualitative study on possibilities and barriers from the perspective of healthcare providers. J Med Internet Res 2019; 21: e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.StatisticsDenmark. HFUDD10: educational attainmant(15-67 years) by region, ancestry, highest education completed, age and sex, https://www.statistikbanken.dk/statbank5a/default.asp?w=1920 (2019, accessed October 2019).
- 44.Ericson J. Breastfeeding in mothers of preterm infants: prevalence and effects of support. Uppsala, Sweden: Uppsala Universitet, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JTT913411 Supplemental Material for Comparison of video and in-hospital consultations during early in-home care for premature infants and their families: A randomised trial by Mai-Britt Hägi-Pedersen, Ram B Dessau, Annelise Norlyk, Hristo Stanchev and Hanne Kronborg in Journal of Telemedicine and Telecare