Abstract
Background:
Conventional home blood glucose measurements require a sample of blood that is obtained by puncturing the skin at the fingertip. To avoid the pain associated with this procedure, there is high demand for medical products that allow glucose monitoring without blood sampling. In this review article, all such products are presented.
Methods:
In order to identify such products, four different sources were used: (1) PubMed, (2) Google Patents, (3) Diabetes Technology Meeting Startup Showcase participants, and (4) experts in the field of glucose monitoring. The information obtained were filtered by using two inclusion criteria: (1) regulatory clearance, and/or (2) significant coverage in Google News starting in the year 2016, unless the article indicated that the product had been discontinued. The identified bloodless monitoring products were classified into three categories: (1) noninvasive optical, (2) noninvasive fluid sampling, and (3) minimally invasive devices.
Results:
In total, 28 noninvasive optical, 6 noninvasive fluid sampling, and 31 minimally invasive glucose monitoring products were identified. Subsequently, these products were characterized according to their regulatory, technological, and consumer features. Products with regulatory clearance are described in greater detail according to their advantages and disadvantages, and with design images.
Conclusions:
Based on favorable technological features, consumer features, and other advantages, several bloodless products are commercially available and promise to enhance diabetes management. Paths for future products are discussed with an emphasis on understanding existing barriers related to both technical and non-technical issues.
Keywords: invasive, noninvasive, glucose, fluid sampling, minimally invasive, optical
Introduction
Optimal diabetes management requires glucose monitoring at regular intervals or continuously. 1 In the 1970s, the commercial sector responded by developing analytical systems that provided accurate readings of glucose concentrations in capillary blood samples. This technology is commonly referred to as either self-monitoring of blood glucose (SMBG) or assisted monitoring of blood glucose (AMBG) and is recognized as a major advance in managing diabetes. This technology permitted, for the first time, the ability for people with diabetes to monitor their individual glycemia on a daily basis. This technology also enabled the clinical studies that established the benefits of tight glycemic control in delaying the onset of diabetes complications.2,3
Despite improvements in sample volume requirements, analysis times, and measurement accuracy, the pain, cost, and inconvenience of SMBG technologies are driving the commercial sector to develop new analytical devices that are not based on individual blood measurements, but rather on continuous, or near continuous, glucose measurements in non-blood samples. Non-blood glucose sensing technologies have been under development since the mid-1980s, yet relatively few have realized commercialization and received regulatory approval.
This review reports the results of a literature search to uncover a listing of established and nascent commercial products that are based on non-blood glucose sensing technologies. Each product is classified as either of the following:
(1) Noninvasive optical glucose monitor (NIO-GM),
(2) Noninvasive fluid sampling glucose monitor (NIFS-GM), and
(3) Minimally invasive glucose monitor (MI-GM).
These technologies are generally characterized as providing continuous or intermittent glucose measurements with minimal or no pain.
Definitions of Glucose Monitoring Devices
The discovered products are classified according to the following set of definitions.
Invasive glucose monitors
The most recent published definition of an invasive procedure, based on an analysis of almost 400 articles from the medical literature, is “Where purposeful/deliberate access to the body is gained via an incision, percutaneous puncture, where instrumentation is used in addition to the puncture needle, or instrumentation.” 4 By this definition, when a product requires puncturing of the skin with a lancing device, this product is defined as an invasive glucose monitor (IGM) device.
Home IGM devices involve collecting a sample of capillary blood by breaking the skin barrier at the subject’s finger with a sharp lancing device.5,6 Such IGM systems have been marketed for almost half a century and have undergone continual improvements to require less blood volume and shorter measurement times. Unfortunately, the cost, pain, blood waste, and finger calluses related to this method reduce patient enthusiasm for frequent glucose testing. 7
Noninvasive optical glucose monitors
Using the above definition of an invasive procedure, a technology where the concentration of glucose is measured without inserting a device into the body is considered noninvasive. When a noninvasive measurement involves passing a type of radiation into a vascular region of the body, then the instrumentation is defined as a noninvasive optical glucose monitor (NIO-GM) . Typically, the analytical information associated with such measurements is derived from the chemical composition of the interstitial fluid (ISF) contained within the skin matrix. Additional tissue components, such as vascular and intracellular compartments, can also contribute to the spectroscopic response. 8 Most often, measurement sites correspond to accessible body compartments, such as fingers, extremities, the abdominal wall, or earlobes. Such devices are painless and free of medical waste.
Noninvasive fluid sampling glucose monitoring
A second type of noninvasive measurement involves analysis of a fluid sample that is collected without an invasive procedure. In this case, the body fluid, such as tears, sweat, saliva, and urine, is available without puncturing skin and the analytical information is determined ex vivo. Once the processes involved in collecting and analyzing a sample are packaged into an integrated product, the system is defined as a noninvasive fluid sampling glucose monitor (NIFS-GM) .
The above definition differs from those used in previous reviews that categorize glucose sensing technologies.9-11 In other reviews, analysis of fluids other than blood are grouped under either the terms minimally invasive or noninvasive, as illustrated in Figure 1A and 1B. The rationale for treating these methods as minimally invasive is based on the fact that these NIFS-GM methods require collection of a body fluid and the analytical measurement does not involve passing radiation through the body. On the other hand, other reviews place an emphasis on the lack of puncturing skin for NIFS-GM systems, thereby categorized these devices as noninvasive. Developers of such products prefer the term noninvasive over minimally invasive because of marketing advantages.
Figure 1.
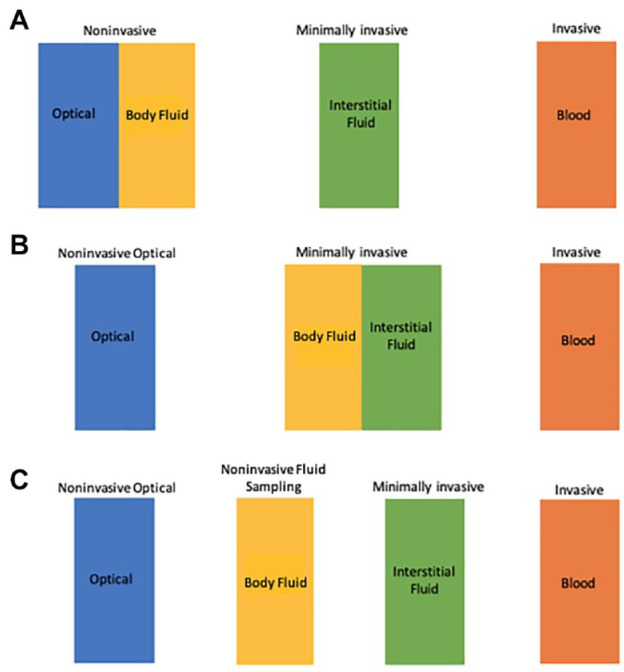
How NIFS-GM technologies have been classified. (A) Previously classified by some experts as a noninvasive technology; (B) Previously classified by other experts as a minimally invasive technology; (C) Classified per our definition in this article as a distinct technology separate from NIO-GM and MI-GM.
In our assessment, NIFS-GM systems are distinctive and require a separate designation, as illustrated in Figure 1C. These technologies are uniquely characterized by a lack of puncturing skin, instrumentation applied to the body, a potential for skin trauma, and the need for an established clinically valid correlation between the concentrations of glucose in the fluid and in blood.
Minimally invasive glucose monitoring
The insertion of a sensor into the subcutaneous tissue is an invasive procedure, but this procedure is characterized as involving minimal pain and providing glucose concentrations over an extended period with a single insertion. For these reasons, such an approach is defined as a minimally invasive glucose monitor (MI-GM). These technologies are capable of staying in place for periods of days, weeks, or even months while performing glucose measurements repeatedly.
MI-GM devices have been marketed worldwide for the past two decades and serve as the basis for state-of-the-art wearable continuous glucose monitor (CGM) systems. The commercial success of these devices is illustrated by the data presented in Figure 2. For the industry, total revenues exceeded $1.5 billion USD as of the fourth quarter of 2020. 12
Figure 2.
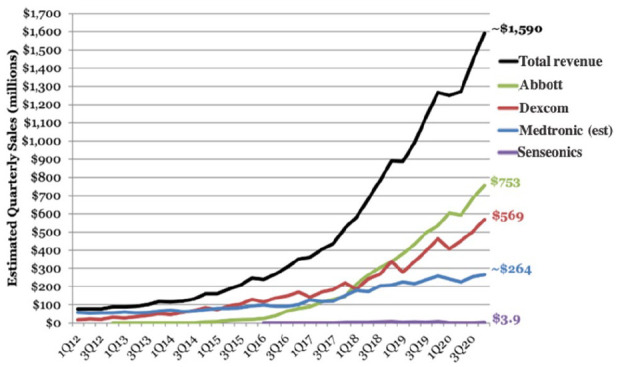
The total quarterly sales for MI-GMs produced by the world’s four largest manufacturers as of the fourth quarter of 2020.
Figure adapted from Cai et al. 12
Technological Approaches for Glucose Monitoring Products
The various glucose monitoring products rely on different technological approaches, as described below.
Technology for noninvasive optical glucose monitoring
NIO-GM involves sending harmless, low-energy radiation through a vascular body site and extracting information about the glucose concentration from the collected signal. In many cases, a selected band of electromagnetic radiation is applied to skin and the diffusely scattered photons are collected. Glucose concentrations are estimated by a multivariate analysis of the resulting spectrum. Several recent review articles provide detailed descriptions of the different NIO-GM technologies currently under development.5,13-15 These technologies include near-infrared spectroscopy, mid-infrared spectroscopy, Raman scattering spectroscopy, optical polarimetry, approaches based on microwave and radio wave sensing, and others.5,13-15
Technical hurdles for success of NIO-GM products include issues related to detection limits and selectivity of glucose measurements. Glucose concentrations in blood and ISF are at milli-molar concentrations, which places them at an intermediate level – meaning they are lower than the principal components of skin and higher than many clinical biomarkers. Still, spectroscopic signals originating from glucose molecules are weak, which challenges the signal-to-noise ratio (SNR) of the instrumentation.16-19 Fundamentally, the SNR for the instrumentation must be sufficient to enable measurement of the weak signals from glucose over background noise and other sources of instrumental variation. In addition, the measurement signal must be selective relative to all other components of skin, including membranes, glycosylated structures, and soluble compounds within the ISF matrix, such as albumin, urea, amino acids, and ascorbic acid. Ideally, such a selectivity is derived from the chemical structure of glucose, thereby providing a robust basis for measurement accuracy. Depending on the radiation used, a viable NIO-GM has to take into account skin pigmentation, surface roughness, skin thickness, breathing artifacts, blood flow, body movements, and ambient temperature. 20 Individual calibration can reduce the impact of the skin’s contribution to the results of measurement.
NIO-GM devices have been proposed based on primary or direct glucose sensing as well as secondary, or indirect glucose sensing. Primary measurements involve collecting a signal derived directly from the glucose molecule, while secondary measurements involve measuring a parameter impacted by the concentration of glucose, such as (1) heart rate changes with electrocardiography, 21 (2) rate of red blood cell aggregation with ultrasound, 22 (3) blood volume dynamics with photoplethysmographic measurement of blood,23,24 (4) dielectric properties of the skin matrix with diffuse scattering or temperature-modulated localized reflectance, 25 or (5) sudomotor dysfunction with electrochemical skin conductance and sweating asymmetry. 26
No NIO-GM product has received clearance by the United States Food and Drug Administration (FDA). Although both direct and indirect noninvasive glucose measurements bear the burden of establishing measurement accuracy, this burden can be greater for indirect methods owing to the lack of a selective signature originating from the glucose molecule. Indirect methods are based on correlations of physiological signals that may be impacted by parameters other than glucose, thereby confounding selectivity and measurement accuracy to a considerable extent. However, machine learning and neural network approaches might provide a successful path for selective indirect glucose measurements. 21 Successful approaches might require a combination of direct and indirect sources of analytical information. It is possible to quantify glucose in standard solutions and tissue phantoms under idealized conditions by such approaches; however, in the real world, the accuracy and precision of glucose measurements could be inadequate for clinical use. Measurement accuracy over time represents the major analytical challenge of all NIO-GM technologies.
The FDA recently distributed a warning concerning the impact of skin pigmentation on the accuracy of clinical pulse oximetry measurements. 27 NIO-GM are potentially subject to the same concerns depending on the wavelength of the probing radiation. Absorption of the incident radiation by melanin, or other skin pigments, will certainly impact signal measurements. 28 Substances that may affect skin pigmentation, skin structure, and reflectance properties of the probing radiation include topical medications, cosmetics, cosmeceuticals, and estrogen, as well as tobacco, and alcohol. 29 Radio waves and microwaves are not expected to be impacted and infrared wavelengths are also unlikely to be affected, although visible and near infrared wavelengths near the visible spectrum (ie, less than 1 micron can be impacted). The impact of skin pigmentation must be experimentally assessed for each system.
The only approach to developing an NIO-GM product that does not involve measurements in skin, involves direct measurements in the aqueous humor of the eye. 30 This approach eliminates the confounding effects presented by the skin barrier, however, none of the products covered in this review is based on this approach.
Although no NIO-GM device has been approved by the FDA, interest in noninvasive measurements is high. In 2020, 343 articles were referenced in PubMed with the search term “noninvasive glucose.” Also, the number of such articles in PubMed has increased progressively over the past 45 years. 31 Initially, interest in this field was mostly academic, but since the 1990s, a number of companies have been attempting to develop NIO-GM products.32-35
Technology for noninvasive fluid sampling glucose monitoring
NIFS-GM products center on technologies capable of collecting and analyzing samples of ISF, tears, sweat, or saliva. Electrochemical glucose oxidase biosensors are available to determine the concentration of glucose in the collected fluid. Sample collection technologies are specific for the measurement fluid. Reverse iontophoresis enables the collection of a representative sample of ISF without puncturing the skin. Other technologies are reported for measurements in lacrimal, 36 and tear fluids. 37
Two NIFS-GM technologies have either received clearance from the FDA or have been granted a Conformité Européenne (CE) Mark. The first is the measurement of glucose in ISF samples extracted through the skin surface by reverse iontophoresis. 38 This is an active transport system stimulated by an electrical current applied to the skin. The concentration of glucose in the resulting fluid is measured with a sensor patch placed on the skin surface. Glucose concentrations in such samples are two to three orders of magnitude below those in the original ISF, which presents an analytical challenge. 39 The SugarBEAT from Nemaura (Loughborough, England) is a NIFS-GM device that uses this method and has received a CE Mark. A comparable approach received FDA clearance in 2001 and was marketed as the GlucoWatch Biographer by Cygnus, Inc. (Redwood City, California).40,41 The resulting glucose concentration measurements lacked sufficient clinical accuracy and the device suffered from poor user experience. 42 A skin permeation process using ultrasound was briefly used more than ten years ago to collect ISF with a sensor placed over the site of skin permeation for an investigational needle-free product, 43 and this type of product is still under development. 44
Technically, urine test strips fall under the definition of a NIFS-GM device. These test strips are ineffective in estimating real-time blood glucose concentrations and are rarely used for making treatment decisions. Urine glucose testing is characterized by the following three characteristics:
(1) a long and unpredictable lag time between dynamic blood glucose concentrations and urine glucose concentrations,
(2) a high blood glucose concentration threshold for urine glucose excretion, below which glucose does not appear in the urine, and
(3) a wide person-to-person variability in this blood glucose threshold concentration where glucose spills into the urine. 45
For NIFS-GM technologies designed to measure glucose in samples of sweat, 37 saliva,46,47 and tears, 48 the actual analytical measurements might be accurate; however, the correlation is poor for glucose levels in these fluids compared to those in blood, and this correlation might be influenced by regionalized physiological stressors or stimuli. 49 Such correlations have proven difficult to control, thereby raising the possibility that physiology will render such an approach to be impractical for commercialization.
Technology for minimally invasive glucose monitoring
The majority of MI-GM products currently on the market are devices that require the user to insert the sensing element in the subcutaneous space. The sensing element is an electrochemical glucose biosensor which reports a glucose concentration every 1-15 minutes to a receiver located outside the body. The sensor is limited to 10-14 days of operation.
Three generations of biosensor technology are reported for MI-GM devices. Each generation differs by the internal path of electrons associated with the enzyme-catalyzed reaction and the electrochemical detection. In all generations, the enzyme catalyzes the oxidation of glucose to form gluconic acid, thereby producing the reduced form of the enzyme cofactor. The electron generated from the oxidation of glucose is ultimately transferred to either oxygen (first generation), an electron mediator (second generation), or the electrode directly (third generation). 50 Accuracy of glucose measurements and their resistance to interferences by drugs or other substances have improved with each successive generation of glucose sensors. Third generation sensors are not yet commercially available. 51
As an alternative to electrochemical MI-GM devices, the principles of reversible affinity sensing are available. For this purpose, two molecules, one of which is a glucose binding protein (GBP), are brought together with a dextran derivative. At low glucose concentrations, the GBP forms a macromolecular complex with the dextran-derivative. As the concentration of glucose increases, the GBP will prefer glucose, thereby dissociating the macromolecular complex. The measurement of the glucose concentration is carried out by determining the change in a physical parameter related to this dissociation of the macromolecular complex. Examples of measurements include a change in viscosity, 51 osmotic pressure against a membrane, 52 or fluorescence.53,54 Depending on the measuring principle, the change in the measured quantity is detected with either pressure or optical tranducers and the signal is correlated to a change in glucose concentration by a calibration curve.
The Eversense CGM, offered by Senseonics (Germantown, Maryland) is an FDA approved MI-GM device that measures the concentration of glucose by a fluorescence signal generated from a reversible affinity sensing mechanism involving a selective glucose binding protein. 53 This device has demonstrated clinical sufficient glucose measurement accuracy for 180 days,55,56 although it was initially cleared by the FDA for 90 days of use.57,58 This device must be implanted under the skin and removed by a healthcare professional and requires twice daily calibration.
Technological Challenges across Platforms
Regardless of the sensing technology, NIO-GM, NIFS-GM, or MI-GM, the following technical challenges must be considered for each clinically viable product.
Time delays in glucose concentrations between blood and measurement fluids
When blood glucose concentrations change, glucose levels in other physiological fluids, such as ISF, do not show similar changes instantaneously. As a result, differences in glucose concentrations reported from NIO-GM, NIFS-GM and MI-GM devices compared to blood measurements may not reflect inaccuracy in the measurement technology but may have a physiological basis. The rate of transporting glucose between the different fluids is not instantaneous and time is required for glucose to diffuse between compartments during periods of disequilibrium. Relevant body fluids include ISF within the subcutaneous matrix,59-61 extracted samples of sweat, 62 saliva, 63 tears, 64 or ISF harvested using reverse iontophoresis, 10 and aqueous humor measured from outside the body. 65
Time lags for glucose concentration measurements have three components: First, a physiological lag occurs as glucose equilibrates by diffusion (a passive transport to or from blood and the body fluid). The impact of this source of time delay depends on the rate of change in blood glucose levels. This physiological lag time can vary between individuals; but tends to be consistent over time for a specific individual for a given fluctuation in the blood glucose pattern. Second, a measurement lag can occur if the sensor’s response is slow. Third, a delay can occur owing to the data-smoothing algorithm. Such algorithms can introduce a time delay between the current glucose sensor reading and previous readings to smooth fluctuations, which leads to a delay in the measurement output. This third element of lag time is only relevant for CGM technology and not for spot glucose measurements.66,67
Reported total lag times vary depending on the measurement conditions; however, they generally range between 5 and 20 minutes. Lag times tend to be shorter with NIO-GM and MI-GM sensor methods and tend to be longer with NIFS-GM. In the latter case external glucose sensors measure fluids that must be extracted before the analytical measurement. If a manufacturer provides a single number to represent the lag time in minutes for ISF glucose compared to blood glucose for a glucose sensor, then this number cannot necessarily be relied upon because much of the lag time is dependent on the individual whose lag is being measured. 60 Furthermore, the lag time can vary depending on activities being performed 68 or whether the blood glucose concentration is rising or falling. 69
Skin irritation
SMBG and AMBG capillary blood measurements can lead to calluses and scarring on the punctured finger tips. 70 MI-GMs and the two categories of noninvasive glucose monitors (of the skin only), NIO-GMs and NIFS-GMs, have shown varying levels of skin irritation that could cause discomfort, scarring, or local skin infections. These skin reactions can be induced by the adhesives used to affix the glucose sensing element to the skin. Such adhesives have been reported to cause skin irritation and even allergic reactions. 71 Stronger adhesives used to facilitate longer wear times can lead to even more pronounced skin problems. 72 Acrylates being in the adhesives (and also in the plastic material of the sensor housing) are known to induce skin reactions.73,74
Short term studies with MI-GMs that use transcutaneous microneedles to sample ISF have demonstrated minor edema and erythema skin irritation that resolve without treatment.75,76 The use of a powerful laser for NIO-GM measurements poses a risk of pronounced skin damage. NIFS-GM methods sampling from the skin using no needles may cause dermatitis after prolonged collection (e.g., by reverse iontophoresis, 10 skin permeation, or sweat collection 77 ). NIFS-GMs that collect fluids from sites other than the skin as well as optical noninvasive glucose monitors have not been reported to cause skin irritation.
Toxicity
The issue of toxicity is more of an issue for MI-GM technologies compared to the other less invasive approaches. 51 For IGM, NIFS-GM, and MI-GM products, there is direct contact between the sensing element and a physiological fluid containing glucose. However, operation of MI-GM devices calls for the sensing element to be inserted into the body for periods of multiple days, which demands safety assurance. For IGM and NIFS-GM devices, the glucose measurements occur outside the body, thereby permitting the use of materials that would be unsuitable for indwelling MI-GM sensors. NIO-GM devices might involve direct contact between the physical glucose sensing element and skin. If so, then toxicity effects of the sensing materials must be evaluated. Manufacturers of NIO-GM systems must also be concerned with adverse effects caused by the energy and power of the probing radiation applied to the skin.
User involvement
Glucose monitoring methods that rely on user action are not well suited for automatic and continuous glucose monitoring. User involvement can be extensive for IGM and some NIFS-GM devices as well as for any bulky NIO-GM devices. Either a minimally invasive implanted sensor or a wearable noninvasive sensor would be suitable for measuring continuous and automatic glucose measurements. In comparison to intermittent measurements, CGM devices can provide the following important information and functions with minimal user inputs or adjustments:
(1) real-time glucose concentrations including the timing and magnitude of peak responses to food intake, physical activities, and medications, 78
(2) a 24-hour recording of changes of glucose levels over time, 79
(3) short-term and long-term glycemic trends, 80
(4) warnings of impending or surpassed clinically relevant low or high glucose concentrations, 81
(5) control of an automated insulin delivery (AID) system, 82 and
(6) interface with advanced digital systems for diabetes management. 83
Methods
Overview
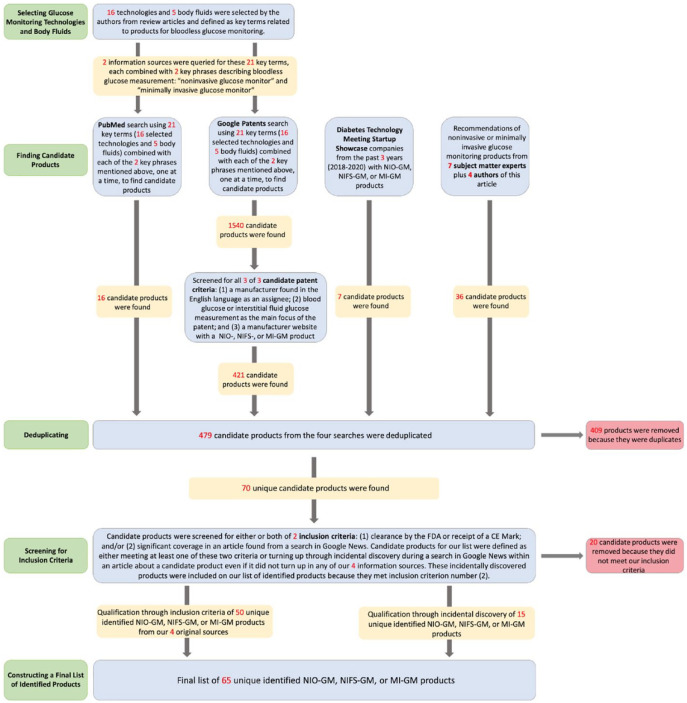
We conducted a database review to survey products for monitoring glucose that did not use invasive blood glucose sampling. We searched for products using optical noninvasive, fluid sampling noninvasive, and minimally invasive glucose monitoring technologies. We performed four types of searches to find candidate NIO-GM, NIFS-GM, and MI-GM products and their manufacturing companies to be included in our list for this article, and these searches are updated as of December 17, 2020. We listed an NIO-GM, NIFS-GM, or MI-GM product if the product met at least one of two inclusion criteria: (1) clearance or approval by FDA or CE, and/or (2) significant coverage in a news story or press release from a search of the product (or, if the product did not turn up, then a search of the manufacturer) on Google News, indicating the product was reported as viable since 2016. When we discovered a product that would possibly qualify for our list from one of our four sources of information and designated these as “candidate products.” We then performed two types of, what we called, “Inclusion Searches.” The product was defined to meet our first criterion if its manufacturer’s website or a Google search indicated that the product was cleared by FDA or had a CE Mark. The product was defined to meet our second criterion if a search of Google News for an article published since 2016 provided significant coverage, which we defined as at least one paragraph of this product. In summary, we searched for information about candidate products for NIO-GM, NIFS-GM and MI-GM technologies through four information sources. For each discovered candidate product, we applied the two inclusion criteria to determine whether the product would be included on our “identified products” list. Figure 3 presents a PRISMA diagram of how we performed our database reviews to create a list of identified NIO-GM, NIFS-GM, and MI-GM products.
Figure 3.
A PRISMA diagram of how we performed our database reviews to create a list of identified NIO-GM, NIFS-GM, and MI-GM products.
Abbreviations: CE, Conformité Européenne; FDA, Food and Drug Administration; NIFS-GM, noninvasive fluid sampling glucose monitor; MI-GM, minimally invasive glucose monitor; NI-GM, noninvasive glucose monitor; NIO-GM, noninvasive optical glucose monitor; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses.
Information Sources for Finding Candidate Products
PubMed
Our first source of information for finding candidate products came from two types of searches in PubMed-referenced journals for articles describing technologies for measuring glucose noninvasively (either optically or via fluid sampling) and minimally invasively. We selected 16 technologies that have been reported to be used for NIO-GM, NIFS-GM, or MI-GM products in review articles about bloodless glucose monitors.5,13-15 These 16 technologies included: (1) spectroscopy, (2) Raman scattering spectroscopy, (3) fluorescence, (4) iontophoresis, (5) polarimetry (optical rotation of polarized light), (6) impedance, (7) osmotic pressure, (8) electrochemistry, (9) absorption, (10) scattering, (11) photoacoustics, (12) quantum cascade laser, (13) photothermal detection, (14) photoplethysmography, (15) quartz crystal microbalance, and (16) DNAzyme.5,13-15 We performed a set of 16 PubMed searches of technologies with two search terms: one of these 16 technologies + “glucose monitor.” From the articles that we found, we searched each article for a glucose monitoring product and its manufacturer. We also searched for glucose monitoring products that measured any of five body fluids that we selected from review articles about bloodless glucose monitors.5,13-15,37 These body fluids included: (1) ISF, (2) sweat, (3) tears, (4) saliva, and (5) breath vapor. We performed a set of five PubMed searches of body fluids with two search terms: one of these five body fluids + “glucose monitor.” We sorted searches by “best match” and terminated each search after finding no additional relevant articles about glucose monitoring after ten consecutive additional articles. From the articles that we found, we searched each article for a glucose monitoring product and its manufacturer. We upgraded a candidate product to our identified products list if it met at least one of the two inclusion criteria.
Google Patents
Our second source of information for finding candidate products came from a series of 42 searches in the Google Patents database, each using one of the same 16 key technology terms and five body fluid terms we used in our PubMed search plus either the phrase “diabetes minimally invasive glucose monitor” or the phrase “diabetes noninvasive glucose monitor.” We limited this search to active or pending patents published starting from January 1, 2010 using the Google Patents “Date” filter. The Google Patents search engine automatically generated the most relevant patents first and progressively less relevant patents afterward. Each page contained ten patent applications. We elevated a patent to the stage of assessing whether it met our two inclusion criteria for our list of identified products if it turned up in one of our 38 searches and also met three criteria for a candidate patent that we established for our patent search. These criteria specified that a candidate patent had: (1) a manufacturer named in the English language as an assignee, (2) a main focus on measuring glucose in blood or a body fluid, and (3) a manufacturer’s website with an NIO-GM, NIFS-GM, or MI-GM listed as a product. For each of our 42 searches, we reviewed successive pages for candidate patents. We continued reviewing page after page as long as we found at least one new candidate patent per page. If a page contained no such patents, then we reviewed one subsequent page and if that page also contained no candidate patents, then this search was terminated. If a candidate patent was found on one of those two pages, then we resumed searching page after page. For the purpose of termination, if a manufacturer generated more than one patent application during a search, then each subsequent patent after the first candidate patent was not counted as a new candidate patent. However, subsequent patents to a first patent were counted for the total number of candidate patents found, and they were later deduplicated if they were the same product. We upgraded a candidate product to our identified products list if it met at least one of the two inclusion criteria.
Diabetes technology meeting startup showcase
Our third source of information for finding candidate products came from a review of any NIO-GM, NIFS-GM, or MI-GM product presented by startup companies that participated in Diabetes Technology Society’s three Startup Showcases during the 2018, 2019, and 2020 Diabetes Technology Meetings. We used a proprietary database provided by Diabetes Technology Society to find the companies. We upgraded a candidate product to our identified products list if it met at least one of the two inclusion criteria.
Experts in glucose monitoring
Our fourth source of information for finding candidate products came from seven experts in glucose monitoring: (1) Guido Freckmann, MD (Institute for Diabetes Technology GmbH, Ulm, Germany), (2) Avner Gal, MBA, MSCEE, MSc (Iridium Consultancy and Technologies, Ltd., Herzliya, Israel), (3) H. Michael Heise, PhD (South-Westphalia University of Applied Science, Iserlohn, Germany), (4) Jeffrey La Belle, PhD, MS (Grand Canyon University, Phoenix, Arizona, United States), (5) Jeffrey Joseph, DO (Thomas Jefferson University, Philadelphia, Pennsylvania, United States), (6) John Pickup, MD, PhD (King’s College of London), and (7) Mark Prausnitz, PhD (Georgia Institute of Technology, Atlanta, Georgia, United States) and asked them to name a product and/or a manufacturer developing novel NIO-GM, NIFS-GM and MI-GM technology. We also used information provided by five of the authors (AT, MA, BV, LH, DK) to name similar products and/or manufacturers. We searched every named manufacturer’s website for a product name. We upgraded a candidate product to our identified products list if it met at least one of the two inclusion criteria.
Inclusion Criteria for Upgrading a Candidate Product to an Identified Product
FDA clearance or CE Mark
Our first inclusion criterion for upgrading a candidate product to an identified product was regulatory clearance of a candidate product. We searched the product name in the “Device Name” search box, or the manufacturer’s name in the “Applicant Name” search box of the 510(k) Pre-Market Notification Database of the FDA 84 to determine FDA clearance. If we were unable to find information on this database, then we also searched the manufacturer’s website for a claim of FDA clearance and/or a CE mark. If a manufacturer’s website did not indicate regulatory clearance, then we also searched the first page on Google using the search terms of the product name or manufacturer’s name + “glucose monitor” + “FDA” and the product name or manufacturer’s name + “glucose monitor” + “CE Mark.” If a product had FDA clearance and/or a CE mark, then we included this information in Table 1 in a column entitled “Which Inclusion Criteria Were Met.”
Table 1.
Regulatory Features of NIO-GM, NIFS-GM, or MI-GM Identified Products (in Alphabetical Order Within Each Category of Invasiveness).
| Entry number | Product and manufacturer | Degree of invasiveness | Headquarter country | Which inclusion criteria were met | PubMedarticle | Regulatory status | Adjunctive or non-adjunctive (if cleared by the FDA) |
|---|---|---|---|---|---|---|---|
| 1 | No product name; Afon Technology | NIO-GM | Wales | Google News 85 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.85,86 | N/A |
| 2 | Alertgy NI-GM; Alertgy | NIO-GM | USA | Google News 87 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 88 | N/A |
| 3 | No product name; AnnNIGM | NIO-GM | Russia | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 4 | Add on to the Apple Watch; Apple | NIO-GM | USA | Google News 90 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.90,91 | N/A |
| 5 | BioMKR; Prediktor Medical | NIO-GM | Norway | Google News 92 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 93 | N/A |
| 6 | Blood Analysis Sensor; Brolis Sensor Technology | NIO-GM | Lithuania | Google News 94 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 95 | N/A |
| 7 | CompanionCM; Socrates Health Solutions | NIO-GM | USA | Google News 96 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.97,98 | N/A |
| 8 | CompanionSR; Socrates Health Solutions | NIO-GM | USA | Google News 96 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.97,98 | N/A |
| 9 | D-Band; DiaMonTech | NIO-GM | Germany | Google News 99 | Lubinski 100 | Not cleared by FDA but the manufacturer has submitted a pre-submission to the FDA for the D-Base to the FDA. 101 Has not received CE Mark of approval. Patented technology.102,103 | N/A |
| 10 | D-Base; DiaMonTech | NIO-GM | Germany | Google News 99 | Lubinski 100 | Not cleared by FDA and has not received CE Mark of approval. Patented technology.102,103 | N/A |
| 11 | D-Pocket; DiaMonTech | NIO-GM | Germany | Google News 99 | Lubinski 100 | Not cleared by FDA and has not received CE Mark of approval. Patented technology.102,103 | N/A |
| 12 | GlucoBeam; RSP Systems | NIO-GM | Denmark | Google News 104 | Pleus 105 | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 106 | N/A |
| 13 | GlucoFit; GlucoActive | NIO-GM | Poland | Google News 107 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 14 | Gluco Quantum; Genki Vantage Ltd | NIO-GM | China | Google News 108 | None | Not cleared by FDA and has not received CE Mark of approval. Has not received CE Mark of approval but has initiated ISO 13485 certification process. Patented technology.109,110 | N/A |
| 15 | Glucosense; Glucosense Diagnostics Ltd | NIO-GM | England | Google News 111 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 112 | N/A |
| 16 | GlucoStation; GlucoActive | NIO-GM | Poland | Google News 113 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 17 | GlucoTrack; Integrity Applications Ltd | NIO-GM | Israel | Google News, 114 CE Mark 115 | Lin 116 | Not cleared by FDA. Received the CE Mark of approval June 2013 for DF-F model. Received final CE Mark of approval in March 2014. 115 | N/A |
| 18 | GlucoWear; GlucoActive | NIO-GM | Poland | Google News 107 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 19 | GlucoWise; MediWiSe | NIO-GM | England | Google News 111 | Gonzales 5 | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 117 | N/A |
| 20 | Glutrac; Add Care Ltd | NIO-GM | China | Google News 118 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 119 | N/A |
| 21 | HELO Extense; Wor(l)d Master Distributors | NIO-GM | USA | Google News 120 | Gonzales 5 | Not cleared by FDA and has not received CE Mark of approval. Patent pending.121,122 | N/A |
| 22 | HELO LX PRO; Wor(l)d Master Distributors | NIO-GM | USA | Google News 120 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending.121,122 | N/A |
| 23 | LIFELEAF; LifePlus | NIO-GM | USA | Google News 123 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 124 | N/A |
| 24 | Movano Wearable CGM; Movano | NIO-GM | USA | Google News 125 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 126 | N/A |
| 25 | No product name; Omni Sciences, Inc. | NIO-GM | USA | Google News 127 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.90,128 | N/A |
| 26 | Sanmina; Sanmina Corporation | NIO-GM | USA | Google News 129 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 130 | N/A |
| 27 | TensorTip Combo Glucometer; Cnoga Medical Ltd | NIO-GM | Israel | Google News, 131 CE Mark 132 | Pfützner 133 | Not cleared by FDA. Received the CE Mark of Approval. 132 | N/A |
| 28 | Uband; Know Labs, Inc. | NIO-GM | USA | Google News 134 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 135 | N/A |
| 29 | Bios; GraphWear Technologies Inc. | NIFS-GM | USA | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 136 | N/A |
| 30 | gSense; Nutrix | NIFS-GM | Switzerland | Google News 137 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 31 | NextGen CGM; Echo Therapeutics | NIFS-GM | USA | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 138 | N/A |
| 32 | Saliva Glucose Biosensor; Gbs Inc. | NIFS-GM | USA | Google News 139 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 140 | N/A |
| 33 | sugarBEAT; Nemaura Medical | NIFS-GM | USA | Google News, 141 CE Mark 142 | Gonzales 5 | Has received CE Mark of Approval. 142 Submitted a premarket approval medical device application to the FDA for its SugarBEAT glucose monitor. 143 | N/A |
| 34 | Tear Glucose Sensor; NovioSense | NIFS-GM | Netherlands | Google News 144 | Geelhoed-Duijvestijn 145 | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 36 | N/A |
| 35 | AiDex CGM; GlucoRx (Rebranding of MicroTech Medical)146,147 | MI-GM | England | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 36 | Biolinq CGM; Biolinq | MI-GM | USA | Google News 148 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 149 | N/A |
| 37 | Care Sense Air; i-SENS | MI-GM | South Korea | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 150 | N/A |
| 38 | Cascade CGM system; Waveform | MI-GM | USA | Google News, CE Mark 151 | Rebec 152 | Not cleared by FDA. Received CE Mark of approval in 2019 and usable for people who are over 2 years of age 151 | N/A |
| 39 | CT-100; POCTech X Ascensia | MI-GM | POCTech: China Ascensia: Switzerland | Google News, 153 CE Mark 154 | None | Not cleared by FDA. Received CE Mark of approval. 152 | N/A |
| 40 | Dexcom G6; Dexcom | MI-GM | USA | Google News, 155 FDA cleared, 156 CE Mark 157 | Isaacson 158 | Cleared by the FDA in October, 2019 for use in adults and children over 2 years of age. 156 Received CE Mark of approval in 2020. 157 | Non-adjunctive 159 |
| 41 | Dexcom G7; Dexcom | MI-GM | USA | Google News 160 | None | Not cleared by FDA and has not received CE Mark of approval. No patent identified. | N/A |
| 42 | Eclipse 3; iCGM GlySens | Implanted MI-GM | USA | Google News 161 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 162 | N/A |
| 43 | Eversense; Senseonics | Implanted MI-GM | USA | Google News, 163 FDA cleared, 164 CE Mark 165 | Fokkert 166 | Cleared by the FDA for people who are over 18 years of age. 164 Received CE Mark of approval in 2016. 165 | Non-adjunctive 167 |
| 44 | FiberSense Technology CGM; EyeSense | MI-GM | Switzerland | Google News 90 (manufacturer is mentioned) | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 168 | N/A |
| 45 | FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM | USA | Google News, 169 FDA cleared, 170 CE Mark 171 | Kudva 172 | Cleared by the FDA. 170 Received CE Mark of approval in 2014. 171 | Non-adjunctive 173 |
| 46 | FreeStyle Libre 2; Abbott Diabetes Care | MI-GM | USA | Google News, 174 FDA cleared, 175 CE Mark 176 | Denham 177 | Cleared by the FDA for adults and children with diabetes ages 4 years and above. 175 Received CE Mark of approval in 2018. 176 | Non-adjunctive 178 |
| 47 | FreeStyle Libre 3; Abbott Diabetes Care | MI-GM | USA | Google News, 179 CE Mark 179 | O’Neill 180 | Not cleared by the FDA. Has received CE Mark of approval for adults and children with diabetes ages 4 years and above. 179 | N/A |
| 48 | Glucomen Day CGM; A. Menarini Diagnostics | MI-GM | Italy | Google News, 181 CE Mark 182 | O’Neill 180 | Not cleared by the FDA. Waveform partner received CE Mark of approval to release Glucomen day. 182 | N/A |
| 49 | Glunovo i3 CGM; Infinovo Medical Co Ltd. | MI-GM | China | Google News, 183 CE Mark 184 | None | No news articles about Infinovo CE Mark. However, LinkedIn page of Infinovo indicates CE Mark has been received as of June 2019. 184 | N/A |
| 50 | Glyde CGM; GluSense | Implanted MI-GM | Israel | Google News 185 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 186 | N/A |
| 51 | Indigo CGM; Indigo | Implanted MI-GM | Belgium | Google News 187 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 188 | N/A |
| 52 | No product name; Integrated Medical Sensors | Implanted MI-GM | USA | Google News 189 | Mujeeb-U-Rahman 190 | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 191 | N/A |
| 53 | No product name; One Drop (acquired Sano Intelligence, Inc., which was developing patch biosensor glucose monitor) 192 | MI-GM | USA | Google News 193 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 194 | N/A |
| 54 | K’Watch; PKvitality | MI-GM | France | Google News 195 | Gonzales 5 | Not cleared by FDA and has not received CE Mark of approval. Patented technology.196,197 | N/A |
| 55 | Lumee; Profusa | Implanted MI-GM | USA | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.198,199 | N/A |
| 56 | Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM | USA | Google News, 200 FDA cleared, 201 CE Mark 202 | Sadhu 203 | FDA has approved the Android version of its guardian connect system. 201 Received CE Mark of approval in 2016. 202 | Adjunctive 204 |
| 57 | Medtronic Synergy; Medtronic MiniMed | MI-GM | USA | Google News 205 | None | Not cleared by FDA and has not received CE Mark of approval. No patents identified. | N/A |
| 58 | Medtronic Zeus; Medtronic MiniMed | MI-GM | USA | Google News 205 | None | Not cleared by FDA and has not received CE Mark of approval. No patents identified. | N/A |
| 59 | No product name; Metronom Health | MI-GM | USA and Belgium | Google News 89 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology.206,207 | N/A |
| 60 | PercuSense CGM; PercuSense | MI-GM | USA | Google News 208 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 209 | N/A |
| 61 | Sanvita CGM; Sanvita Medical, LLC and LifeScan | MI-GM | Sanvita Medical, LLC: USA Lifescan: USA | Google News 210 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 211 | N/A |
| 62 | Sencell; Lifecare AS | MI-GM | Norway | Google News 212 | None | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 213 | N/A |
| 63 | SugarSenz; Glucovation | MI-GM | USA | Google News 214 | None | Not cleared by FDA and has not received CE Mark of approval. Patent pending. 215 | N/A |
| 64 | SynerG; Pacific Diabetes Technologies | MI-GM | USA | Google News 216 | Jacobs 217 | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 218 | N/A |
| 65 | TouchCare System A6; Medtrum | MI-GM | China | Google News 89 | Zhou 219 | Not cleared by FDA and has not received CE Mark of approval. Patented technology. 220 | N/A |
PubMed article refers to the first author of an article indexed in the PubMed database. Abbreviations: CE, Conformité Européenne; FDA, United States Food and Drug Administration; MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sampling glucose monitor; NIO-GM, noninvasive optical glucose monitor; USA, United States of America.
Google News
Our second inclusion criterion for upgrading a candidate product to an identified product was a mention in a news article from a search on Google News of a candidate product. Such a search followed a termination process similar to the Google Patents search under the “Information Sources for Finding Candidate Products” section. The Google News search generated the most relevant articles first and progressively less relevant articles afterward. In the Google search box, we searched for the product name + “glucose monitor,” then clicked the “News” tab on Google to find news articles. Each page contained ten articles. For each candidate product we discovered from our four sources, we reviewed Google News pages for candidate articles that were published any time on or after January 1, 2016. If the first Google News page did not contain any articles that described the product in at least one paragraph, then we would continue searching the next four pages for an article containing at least one paragraph of information. If the product name + “glucose monitor” did not show any candidate articles, then we would also search the manufacturer’s name + “glucose monitor.” If a news article was found about a product, then we included the reference in Table 1 in a column entitled “Which Inclusion Criteria Were Met.” We included no more than one news article per product in this column as an example, even if more than one article was found on Google News. If no article was found about a product that lacked regulatory clearance, then we did not include this candidate product on our identified product list.
If an article found on Google News described the product we were searching for within a longer list of products, then we read through the article to see if any of the other products mentioned were already included in our list of candidate products. If any products were not included in the list of candidate products, then we added them to our candidate products. We then passed these incidentally discovered products (not originally found within our four original sources) through our inclusion criteria.
An Additional Scenario for Finding a Candidate Product and Upgrading it to an Identified Product
We upgraded one candidate product to an identified product using different inclusion criteria than the other identified products. An expert suggested the EyeSense (Basel, Switzerland) MI-GM called “FiberSense CGM,” so we then passed the product name + “glucose monitor” and manufacturer’s name + “glucose monitor” through Google News to determine if it fit one of our inclusion criteria. We found an article on Google News describing an NIFS-GM using tears being developed by EyeSense, 221 but we were unable to find specific mention of the MI-GM “FiberSense CGM.” The manufacturer’s website did not mention the NIFS-GM product using tears; however, the manufacturer’s website did mention the MI-GM “FiberSense CGM.” We decided to include this MI-GM “FiberSense CGM” in our identified products list because of the article we found in Google News mentioning the manufacturer.
Product Classification
We classified every product on our identified product list of glucose monitors, that did not use invasive sampling, into one of three categories: (1) noninvasive optical (measuring glucose with electromagnetic radiation from a blend of tissue sources including blood, ISF, and cells); (2) noninvasive fluid sampling (measuring a body fluid other than blood or urine extracted without the use of an implanted needle employing a technology requiring contact of the sensor with the fluid, including sweat, and ISF); and (3) minimally invasive (measuring ISF with a sensor inserted or implanted into the skin requiring contact of the sensor with the ISF). All three of these methods are distinct from traditional invasive blood glucose testing, which refers to measuring glucose with a needle that is inserted into the body to puncture a blood vessel so that blood can be in contact with a sensor.
Product Features
We considered 11 features for each NIO-GM, NIFS-GM, or MI-GM product, which were: (1) which inclusion criteria were met, (2) PubMed article, (3) regulatory status, (4) adjunctive/non-adjunctive status (if cleared by FDA), (5) mechanism of glucose sensing, (6) matrix of glucose sensing, (7) degree of accuracy, (8) interferences, (9) measurement cycle, that is, continuous or intermittent, (10) pricing model, and (11) size/shape. We divided these features into regulatory features (Feature 1-4), technological features (Features 5-8), and consumer features (Features 9-11). We then summarized the glucose sensing technologies and the products that belonged to each category of glucose sensing.
Finding information for product features
Information about product features was obtained from: (1) manufacturer websites, (2) articles found in a PubMed search for identified products, (3) patents found in a Google Patents search, (4) news stories found in a Google News search, (5) results from a Google search. Not every product was described on all five of these outlets (ie, a manufacturer’s website, PubMed, Google Patents, Google News, the first page of results on Google) and in many cases, no information was available about specific features of the listed glucose monitors. For products that have been cleared by the FDA or have a CE Mark, we asked an official from the manufacturer about what were their interfering substances. While we considered lag time between fluctuations in concentrations of blood glucose and NIO-GM, NIFS-GM, or MI-GM system glucose measurements, as well as the presence of skin irritation from contact by the sensor or adhesive around the sensor with the body, to be important technological features, there was not enough reliable published data on the lag times or the status of the skin problems of chronic users for a high percentage of the various products to include more than general statements for all of these products.
Manufacturer websites
To fill information about product features, we first looked through manufacturer websites. In order to find a manufacturer’s website, we performed a search on Google using the search terms: (product name OR manufacturer’s name) + “glucose monitor.” We would look through the first page of Google results to find the manufacturer’s website, which featured details about the product.
PubMed articles
When we discovered a product using the second (Google Patents), third (Startup Showcase), or fourth (experts in the field) initial information sources and the product met our inclusion criteria, we then conducted a PubMed search for articles mentioning identified products. We used the search terms product name + “glucose.” If we found no relevant product-related articles, then we conducted a second PubMed search of the manufacturer’s name + “glucose.” If a PubMed article was found about a product, then we included the reference in Table 1 in a column entitled “PubMed Article.” We included no more than one article per product in this column as an example, even if more than one article was found on PubMed. This search was not an inclusion search, but rather was conducted to find published references to be used if the product met at least one of our two inclusion criteria. Any PubMed article we found through both the information source search and this search may also have been used to provide information for columns in other tables.
Patents on Google Patents
We searched Google Patents by putting the manufacturer’s name under the “Assignee” and using the term “glucose monitor” in the search, which was a different type of search than we initially conducted using Google Patents as a source of candidate products (as described in the third paragraph of the “Methods” section). We used the information found on these patents to fill out information about product features.
News stories found in Google News
We used the same method, as was used under the “Google News” subsection of the “Inclusion Criteria” section, to find news stories in Google News to fill out details regarding product features. We summarized the most important product features in tables.
Results from a Google Search
We performed a Google Search with the (product name OR manufacturer’s name) + “glucose monitor” + “the product feature we are looking for (e.g., matrix)” on Google and looked through the first page of results for mention of a patent. We looked through each result to find details about the product feature.
Detailed Explanation for Specific Columns in Tables 1 and 2
Table 2.
Technological Features of NIO-GM, NIFS-GM, or MI-GM Identified Products (in Alphabetical Order Within Each Category of Invasiveness).
| Entry number | Product and manufacturer | Degree of invasiveness | Mechanism of glucose sensing | Matrix | Degree of accuracy | Interferences |
|---|---|---|---|---|---|---|
| 1 | No product name; Afon Technology | NIO-GM | Uses microwave spectroscopy. Sensor analyzes resonance shifts based on microwave signal to detect changes in glucose. Results reported for a clinical trial. 222 | Blood/ISF 222 | MARD: 21 ± 9% surveillance error grid: 48.9% in “no risk” zone, 47.1% in “slight risk” zone, 4% in “moderate risk” zone 4% 222 | No information available. |
| 2 | Alertgy NI-GM; Alertgy | NIO-GM | Uses dielectric spectroscopy. Wristband with integrated Microchip (dielectric sensor). The apparatus senses the electrical membrane permeability of cells and detects if the permeability varies from the norm. It then uses the difference between the measured permeability with the known permeability to sense glucose levels. Frequency range: 100 kiloherz–220 megaherz. Radiowave frequencies. 88 | Blood/ISF 88 | No information available. | No information available. |
| 3 | No product name; AnnNIGM | NIO-GM | Measuring principle not reported so far. Earlobe clip sensor. No description of their technology provided on their website. 223 | No information available. | No information available. | No information available. |
| 4 | Add on to the Apple Watch; Apple | NIO-GM | Uses absorption spectroscopy with light of certain wavelength to determine the concentration of glucose 224 | Blood/ISF 224 | No information available. | No information available |
| 5 | BioMKR; Prediktor Medical | NIO-GM | Uses absorption spectroscopy. An near infrared laser is used to generate an absorption spectrum for a certain measurement location of the user. A calibration model is then used to estimate blood glucose values from the infrared spectrum. 225 | Blood/ISF 225 | No information available. | None specified so far, but the device is intended for use in people age 18 or older. It is also only appropriate for use in certain cases. 226 |
| 6 | Blood Analysis Sensor; Brolis Sensor Technology |
NIO-GM | Based on integrated photonic package designed to measure glucose, lactate and ethanol. Wavelengths included at 1.7–2.5 microns. Uses laser-based sources. 227 | Blood/ISF 227 | MARD: 5.7% Clarke error grid: greater than 97% in zone A 227 | No specific information – claims that ethanol and lactate are not significant 227 |
| 7 | CompanionCM; Socrates Health Solutions | NIO-GM | Rotation of plane polarized radiation at a non-defined wavelength through a tissue phantom (mimics ear tissue). Preliminary data presented for several human subject experiments. Technology similar to CompanionSR. 228 | Tissue 228 | Resolution for the measurement of micro-radians: ±10 mg/dl. 228 | No information available. |
| 8 | CompanionSR; Socrates Health Solutions | NIO-GM | Rotation of plane polarized radiation at a non-defined wavelength through a tissue phantom (mimics ear tissue). Preliminary data presented for several human subject experiments. Technology similar to CompanionCM. 228 | Tissue 228 | Resolution for the measurement of micro-radians: ±10 mg/dl 228 | No information available. |
| 9 | D-Band; DiaMonTech | NIO-GM | Use of photothermal detection of the molecules after excitation with a mid-infrared laser (quantum cascade laser). A mid-infrared laser is used to detect glucose molecules in the ISF. The reflected wavelength is analyzed to gather data on the amount of glucose molecules. Data analysis involves a leave-one data set-out machine learning system. Technology similar to D-Base and D-Pocket. 100 | ISF 100 | No information available. | No information available. |
| 10 | D-Base; DiaMonTech | NIO-GM | Use of photothermal detection of the molecules after excitation with a mid-infrared laser (quantum cascade laser). A mid-infrared laser is used to detect glucose molecules in the ISF. The reflected wavelength is analyzed to gather data on the amount of glucose molecules. Data analysis involves a leave-one data set-out machine learning system. Technology similar to D-Band and D-Pocket. 100 | ISF 100 | MARD: 11.3%-12.1% medARD: 6.4%-6.5% Consensus error grid: 98.8%-99.1% in zones A+B 100 | Temperatures outside of the range of 10-30℃ may cause inaccurate readings. Temperature or residue on skin can interfere with accuracy. 102 |
| 11 | D-Pocket; DiaMonTech | NIO-GM | Use of photothermal detection of the molecules after excitation with a mid-infrared laser (quantum cascade laser). A mid-infrared laser is used to detect glucose molecules in the ISF. The reflected wavelength is analyzed to gather data on the amount of glucose molecules. Data analysis involves a leave-one data set-out machine learning system. Technology similar to D-Band and D-Base. 100 | ISF 100 | No information available. | No information available. |
| 12 | GlucoBeam; RSP Systems | NIO-GM | This device uses Raman scattering spectroscopy to detect the amount of glucose in ISF. An excitation laser is shined into the finger and the Raman scatter from glucose molecules is analyzed. This technology used confocal methods to focus on a specific depth into the skin matrix. 106 | ISF 106 | medARD: 18.9%. consensus error grid: 93.1% in zones A+B. 229 | Substances that have similar structures to glucose (such as ethanol) could interfere with the accuracy of the results. |
| 13 | GlucoFit; GlucoActive | NIO-GM | Using spectrophotometry to measure the scattering of light by molecules such as glucose. Irradiates the skin with wavelength of light to determine glucose concentration. Technology similar to GlucoStation and GlucoWear. 230 | Skin 230 | No information available. | No information available. |
| 14 | Gluco Quantum; Genki Vantage Ltd | NIO-GM | Uses metabolic heat (in the form of radiation, convection, and evaporation) from the finger’s skin to detect blood flow velocity and make an extrapolation of glucose levels. Infrared light is shone onto the skin to measure the skin’s temperature. 109 | Skin 109 | MARD: 13.12% 231 | No information available. |
| 15 | Glucosense; Glucosense Diagnostics Ltd. | NIO-GM | Application of fluorescence measurement after excitation with a low-energy laser. Photonic chip with fluorescent ions. Uses infrared light and measures level of ion fluorescence to determine glucose concentration. The fluorescence ions are embedded with a silica glass photonic chip. These ions fluoresce in the infrared region of the spectrum (this could be near infrared close to the visible spectral range). 232 | Skin 232 | No information available. | No information available. |
| 16 | GlucoStation; GlucoActive | NIO-GM | Using spectrophotometry to measure the scattering of light by molecules such as glucose. Irradiates the skin with wavelength of light to determine glucose concentration. Technology similar to GlucoFit and GlucoWear. 233 | Skin 233 | No information available. | No information available. |
| 17 | GlucoTrack; Integrity Applications Ltd | NIO-GM | Ultrasonic, electromagnetic, and thermal parameters in earlobe tissue are measured to estimate blood glucose levels. 234 | Skin 234 | MARD: 17.5%- 19.7% consensus error grid: 62.4% in zone A, 37.6% in zone B 235 | Device can operate in an environmental temperature of +15°C to +35°C/ +59°F to +95°F. However, If the ambient sensor detects an environmental temperature that is beyond these temperatures, the device will present an error message 236 |
| 18 | GlucoWear; GlucoActive | NIO-GM | Using spectrophotometry to measure the scattering of light by molecules such as glucose. Irradiates the skin with wavelength of light to determine glucose concentration. Technology similar to GlucoFit and GlucoStation. 237 | Skin 237 | No information available. | No information available. |
| 19 | GlucoWise; MediWiSe | NIO-GM | Using low-power radio waves scattering. Radio waves (40 GHz) are transmitted through a thin layer of skin with adequate blood supply. Radiation passes through the skin layer (transflectance experiment). Uses a film technology that makes the skin transparent to the incident radiation, thereby giving consistent readings across different types of skin. 238 | Blood 238 | No information available. | No information available. |
| 20 | Glutrac; Add Care Ltd. |
NIO-GM | Employs absorption spectroscopy, electrocardiography, photoplethysmography, and dynamic metabolic heat monitoring to collect data. Machine learning and artificial intelligence is then used to estimate the user’s blood glucose. 119 | Blood 119 | No information available. | No information available. |
| 21 | HELO Extense; Wor(l)d Master Distributors | NIO-GM | Photoplethysmography to measure glucose concentrations. 239 | Blood122,239 | Instead of blood glucose numbers, the device provides a color coded scale for glucose. 121 No information on accuracy. | No information available. |
| 22 | HELO LX PRO; Wor(l)d Master Distributors | NIO-GM | Photoplethysmography to measure glucose concentrations. 122 | Blood 122 | No information available. | No information available. |
| 23 | LIFELEAF; LifePlus | NIO-GM | Optical Sensor using photoplethysmography (detection of the reflection of infrared light) to detect glucose. 240 | Blood 240 | No information available. | No information available. |
| 24 | Movano Wearable; CGM Movano | NIO-GM | Scattering of high-frequency radio waves (in mm range). One antenna transmits a radio frequency below the skin surface. The amplitude and phase data (impedance) of the reflected waves are then processed by processing circuits and outputs a relevant value for blood glucose levels in the wrist. The sensor uses a frequency of around 60 GHz to penetrate deeper past the skin and illuminate a wider range. Uses a 122-126 GHz spectral range for measurement of glucose, blood pressure, and heart rate. Also uses Doppler measurements to isolate signals corresponding to relative movements. 241 | Blood 241 | No information available. | No information available. |
| 25 | No product name; Omni Sciences, Inc. | NIO-GM | Near infrared spectroscopy after excitation with fiber lasers. 242 | No information available | No information available. | No information available. |
| 26 | Sanmina; Sanmina Corporation | NIO-GM | Optical measurement: detect several photoplethysmography signals (detection of the reflection of infrared light) in interstitial tissue 243 | Blood volume and vascular wall 243 | MARD: 8%. Consensus error grid: 98% in zone A, 1.9% in zone B 243 | No information available. |
| 27 | TensorTip Combo Glucometer; Cnoga Medical Ltd | NIO-GM | Measurement of infra-red light (600-1000 nm) passing through the fingertip (after partial absorption in the finger). Real-time color images related to the blood glucose level in the capillaries are translated into a vector that can be used to identify patterns of glucose concentration 244 | Blood/ISF 244 | MARD: 14-18.1%. Consensus error grid: 91.1% in zone A and 7.8% in zone B133,245 | The finger would have to be adequately warmed up to ensure blood flow to the capillaries. Results might also be affected if the skin or device screen is dirty. 244 |
| 28 | Uband; Know Labs, Inc. | NIO-GM | Spectroscopy techniques combined with radio waves are used to detect glucose concentrations in the body. 246 | Blood 246 | No information available. | No information available. |
| 29 | Bios; GraphWear Technologies Inc. | NIFS-GM | Detection of biomolecules from the surface of skin: Nanotechnology to measure glucose that comes out of the skin surface through sweat89,247 | Sweat 89 | No information available. | Tattoos or other skin alterations 248 |
| 30 | gSense; Nutrix | NIFS-GM | Detection of biomolecules. Uses nanotechnology to detect glucose changes (concentration of molecules) in saliva. 249 | Saliva 249 | No information available. | No information available. |
| 31 | NextGen CGM; Echo Therapeutics | NIFS-GM | Uses technology that enhances skin permeation (using ultrasound) to measure analytes. Sensor includes a hydrogel component and electrodes. The hydrogel contains glucose oxidase to measure glucose. 250 | ISF 250 | MARD: 12.4%-20.4% Clarke error grid: 70.7%-89.6% in zone A, 9.6%-26.2% in zone B 250 | No information available. |
| 32 | Saliva Glucose Biosensor; Gbs Inc. | NIFS-GM | Glucose oxidase is used to detect glucose in a saliva sample through an electrochemical method. 251 | Saliva 251 | No information available. | No information available. |
| 33 | sugarBEAT; Nemaura Medical | NIFS-GM | Reverse iontophoresis for sampling and electrochemical sensing with glucose oxidase. Adhesive skin patch. Electrochemical signal of ISF glucose via glucose oxidase reaction using reverse iontophoresis by stimulating the migration of glucose from the ISF through an electrical current. 252 | ISF 252 | MARD: 11.92%-12.4% 252 | No information available. |
| 34 | Tear Glucose Sensor; NovioSense | NIFS-GM | Electrochemical sensing with glucose oxidase as a receptor for the biomarker, glucose. The amount of H2O2 generated is an indicator for the amount of glucose in the lacrimal fluid.36,253 | Lacrimal Fluid36,253 | medARD: 12.5% Consensus error grid: 90% in zones A+B 253 | Ascorbic acid, acetaminophen, citric acid, lactic acid, pyruvic acid, and urea are all interfering substances 253 |
| 35 | AiDex CGM; GlucoRx (Rebranding of MicroTech Medical)146,147 | MI-GM | Electrochemical sensing with glucose oxidase. 254 | ISF 254 | Consensus error grid: 89.96% in zone A 255 | No information available. |
| 36 | Biolinq CGM; Biolinq | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. Using patch with microneedles 149 | ISF 149 | No information available. | No information available. |
| 37 | Care Sense Air; i-SENS | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. 256 | ISF 256 | No information available. | No information available. |
| 38 | Cascade CGM System; Waveform | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. 257 | ISF 257 | MARD: 9.9%, MAD: 14.5 mg/dL Consensus error grid: 86.5% of the data pairs in zone A, greater than 98.6% in zones A+B 152 | Limited interference concerns. Does not interfere with acetaminophen. 151 |
| 39 | CT-100; POCTech X Ascensia | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. 258 | ISF 258 | MARD: 8.67%-10.22% Clarke error grid: 86.7%-91.6% in zone A, 8.4%-12.7% in zone B259,260 | No information available. |
| 40 | Dexcom G6; Dexcom | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. GO+Perm-selective membrane coating 261 | ISF 261 | MARD: 9% 159 | Hydroxyurea |
| 41 | Dexcom G7; Dexcom | MI-GM | No information available. | No information available. | No information available. | No information available. |
| 42 | Eclipse 3 iCGM; GlySens | Implanted MI-GM | Glucose oxidase and a potentiostatic oxygen sensor work together in the sensor to sense glucose and allow for long-term implanting of the sensor. 262 | ISF 262 | MARD: 8.2%-28.1%. Consensus error grid: 75.2% in zone A, 23.7% in zone B, 1.1% in zone C 262 | No information available. |
| 43 | Eversense; Senseonics | Implanted MI-GM | Nonenzymatic electrochemical fluorescent-based polymer 263 | ISF 263 | MARD: 8.5%-9.6% 263 | Mannitol, Tetracycline 264 |
| 44 | FiberSense Technology CGM; EyeSense | MI-GM | Optical fiber fluorescence photometer measures concentration of glucose. Uses a receptor molecule that binds glucose and also binds a competitor molecule. 265 | ISF 265 | MARD: 8%-9% 265 | No information available. |
| 45 | FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase. GO+Redox sensing, use of a mediator of osmium oxide 266 | ISF 266 | MARD: 9.4% 267 | Ascorbic acid, salicylic acid267,268 |
| 46 | FreeStyle Libre 2; Abbott Diabetes Care | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase. GO+Redox sensing, use of a mediator of osmium oxide 269 | ISF 269 | Adult MARD: 9.2%, pediatric MARD: 9.7% 269 | Ascorbic acid 269 |
| 47 | Freestyle Libre 3; Abbott Diabetes Care | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase. GO+Redox sensing, use of a mediator of osmium oxide 270 | ISF 270 | Adult MARD: 9.2% 270 | No information available. |
| 48 | Glucomen Day CGM; Menarini Diagnostics | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase. Electrochemical enzymatic sensor. 271 | ISF 271 | MARD: 9.7%. Consensus error grid: 84.9% in zone A, 12.9% in zone B 272 | No information available. |
| 49 | Glunovo i3 CGM; Infinovo Medical Co Ltd. | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase electrochemical sensor. 273 | ISF 273 | No information available. | No information available. |
| 50 | Glyde CGM; GluSense | Implanted MI-GM | Glucose detection is performed using a proprietary fluorescent. Biosensor protein. Biosensor uses the fluorescent resonant energy transfer effect. 186 When glucose is bound to the biosensor, it changes its fluorescence emission. | ISF 186 | No information available. | No information available. |
| 51 | Indigo CGM; Indigo | Implanted MI-GM | Glucose measurement with near infrared spectroscopy in a small subcutaneously implanted spectrometer, measure glucose and ketones up to 2 years 274 | ISF 274 | (Measured in swine model) MARD: 6.4%-6.5%. Consensus error grid 99.3%-99.4% in zone A, 0.6% in zone B, 0%-0.1% in zone C 275 | No information available. |
| 52 | No product name; Integrated Medical Sensors | Implanted MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction 190 | ISF 190 | (Measured in swine model) MARD: Better than 12%. Clarke error grid: 96% in Zones A+B 190 | No information available. |
| 53 | No product name; One Drop (acquired Sano Intelligence, Inc., which was developing patch biosensor glucose monitor) 192 | MI-GM | Biosensor patch with microneedles that measures ISF for glucose concentration.192,194,276 Glucose forecast by using artificial intelligence. | ISF 194 | No information available. | No information available. |
| 54 | K’Watch; PKvitality | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. Measured through microneedles277,278 | ISF277,278 | No information available. | No information available. |
| 55 | Lumee; Profusa | Implanted MI-GM | Biosensor inserted into the body will have porous smart gel and will emit fluorescent signal in response to certain analytes like glucose 279 | ISF 279 | No information available. | No information available. |
| 56 | Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction 280 | ISF 280 | MARD: 8.7% 281 | Acetaminophen hydroxyurea 280 |
| 57 | Medtronic Synergy; Medtronic MiniMed | MI-GM | No information available. | ISF 282 | No information available. | No information available. |
| 58 | Medtronic Zeus; Medtronic MiniMed | MI-GM | No information available. | ISF 282 | Pivotal results indicate possibility of iCGM standard 12 | No information available. |
| 59 | No product name; Metronom Health | MI-GM | Electrochemical opto-enzymatic sensor. 283 | ISF 283 | No information available. | No interferences with commonly taken substances 283 |
| 60 | PercuSense CGM; PercuSense | MI-GM | Electrochemical enzymatic sensor combining measurement of glucose and ketone on a single sensor. Multi-analyte function. 284 | ISF 284 | No information available. | No information available. |
| 61 | Sanvita; Sanvita Medical, LLC and LifeScan | MI-GM | No information available. | ISF 285 | No information available. | No information available. |
| 62 | Sencell; LifeCare AS | Implanted MI-GM | Implantable 3D printed nano-sensor with an osmotic pressure sensing core. Cantilever based glucose sensing. 286 Utilization of the reversible affinity sensing principle of ConA and dextran with glucose (change of binding in the presence of glucose and therefore change of osmotic pressure at a membrane). | ISF 286 | No information available. | No information available. |
| 63 | SugarSenz; Glucovation | MI-GM | Electrochemical non-enzymatic sensor 287 | ISF 287 | No information available. | No information available. |
| 64 | SynerG; Pacific Diabetes Technologies | MI-GM | Insulin infusion set and CGM sensor combined into one integrated device. CGM sensor is hollow. Uses redox mediator technology. 216 | ISF 216 | MARD:10-14%216,217 | No information available. |
| 65 | TouchCare System A6; Medtrum | MI-GM | Electrochemical signal of ISF glucose via glucose oxidase reaction. 219 | ISF 219 | MARD: 9% 288 | May be affected by strong radiation, such as MRI, X-ray, or CT scans. 118 |
Abbreviations: CGM, continuous glucose monitor; ConA, Concanavalin A; CT, computed tomography; ISF, interstitial fluid; MAD, mean absolute difference; MARD, mean absolute relative difference, medARD, median absolute relative difference; MI-GM, minimally invasive glucose monitor; MRI, magnetic resonance imaging; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
Patents under the “Regulatory Status” column in Table 1
The Table 1 column on “Regulatory Status” specifies either clearance by the FDA, clearance by CE, or (if there was no clearance) whether we found a patent or any mention of having a patent. If a product was cleared by a regulatory agency, then we assumed that the product had a patent and we did not specifically search for one. If a product was not cleared by a regulatory agency, then we attempted to identify whether the product had a patent by using the five methods described under the “Finding Information for Product Features” section to identify a patent or mention of a patent. For the “First Page of Google Results” method, we used “patent” as our term for “the product feature we were looking for.” If we did not identify a patent through these five methods, then we stated “No patent identified.” If a manufacturer reported only patent(s) that could pertain to both a cleared product and a non-cleared product, then for the non-cleared product, we stated “No patent identified.”
“Mechanism of glucose sensing” column in Table 2
Just as with the other product features, the mechanism of glucose sensing presented in Table 2 was found using the five methods described under the “Finding Information for Product Features” section. Some manufacturers have developed both cleared and non-cleared products intended for bloodless glucose monitoring, but we were unable to find details about the mechanism of glucose sensing for their non-cleared products in manufacturer websites, articles on PubMed, articles on Google News, or results on the first page of a Google search. In this case, our source of information would theoretically be the patent if it was available. However, if the patents we reviewed did not call out the product by name, then it was unclear which product the patent was associated with. Therefore, we stated “No information available.”
Product Designs
We included examples of product design if the product website included an image and if a manufacturer’s official approved our request to publish an image of their product design. We reached out to every manufacturer that had a product design on their website if contact information was available. Representatives from manufacturers of the following products provided a separate image, that was not on the manufacturer’s website, for us to use for this article: Dexcom G6, Freestyle Libre 14-day, Freestyle Libre 2, Freestyle Libre 3, GlucoBeam, Gluco Quantum, LifeLeaf, SynerG, and UBand. Every other product image came from the product website.
Advantages and Disadvantages
From the manufacturer’s website and the first page of Google results (as per the search method defined in the “Overview”), we determined which identified product had FDA and/or CE clearance. For each cleared product, we evaluated its features and created a list of advantages and disadvantages based on our experience with glucose monitoring technologies and treating people with diabetes. Advantages and disadvantages were not presented for any product which has not received regulatory clearance, because that product might eventually contain different features than what we are currently able to determine.
Results
Summary of Identified Products
We listed 65 identified products based on three categories for the degrees of invasiveness of the sensor relative to the body: (1) NIO-GM, (2) NIFS-GM, and (3) MI-GM technology. The products included 28 NIO-GMs, 6 NIFS-GMs, and 31 MI-GMs. We recorded available information on 12 different features for each glucose monitoring product. We classified these identified products according to (1) headquarter country, (2) which inclusion criteria were met, (3) PubMed article, (4) regulatory status, (5) adjunctive/non-adjunctive status (if cleared by FDA), (6) mechanism of glucose sensing, (7) matrix, (8) degree of accuracy, (9) interferences, (10) measurement cycle, that is, continuous or intermittent, (11) pricing model, and (12) size/form factor. We divided these features of the 65 identified products along with their manufacturer and degree of invasiveness into (1) regulatory features (Feature 1-5, Table 1), (2) technological features (Features 6-9, Table 2), and (3) consumer features (Features 10-12, Table 3). We presented a summary of the underlying technologies used by each identified product in Table 4. We presented images of product designs for selected identified products in Table 5A (FDA cleared and/or CE marked) and Table 5B (not FDA cleared or CE marked). We presented advantages and disadvantages of regulatory cleared products in Table 6.
Table 3.
Consumer features of NIO-GM, NIFS-GM, or MI-GM Identified Products (in Alphabetical Order Within Each Category of Invasiveness).
| Entry number | Product and manufacturer | Degree of invasiveness | Measurement cycle | Pricing model | Size/Form factor |
|---|---|---|---|---|---|
| 1 | No product name; Afon Technology | NIO-GM | Continuous 86 | One purchase of a wristwatch 86 | Wearable wristwatch 86 |
| 2 | Alertgy-NI-GM; Alertgy | NIO-GM | Continuous 88 | One purchase of a wristwatch – no disposables announced 289 | Wearable wristwatch 289 |
| 3 | No product name; AnnNIGM | NIO-GMk | Continuous 223 | One purchase of an earlobe clip. No disposables announced. 223 | Earlobe clip with a wraparound wire. 223 |
| 4 | Add on to the Apple Watch; Apple | NIO-GM | Continuous | Add on to the Apple Watch 90 | Wearable wristwatch 90 |
| 5 | BioMKR; Prediktor Medical |
NIO-GM | Continuous 225 | One purchase of a device – no disposables announced 225 | Wearable wristwatch 225 |
| 6 | Blood Analysis Sensor; Brolis Sensor Technology | NIO-GM | Continuous (based on sensor description that measurements are real-time and gathered in a fraction of a second) 227 | No information available. | Working on a wearable on-the-chip system 227 |
| 7 | CompanionCM; Socrates Health Solutions | NIO-GM | Continuous 290 | Purchase of earpiece sensor as indicated in image on product website. 290 No other information available. | Earpiece 98 |
| 8 | CompanionSR; Socrates Health Solutions | NIO-GM | Intermittent 290 | Purchase of a glucose monitor as indicated in image on product website. 290 No other information available. | Device image can be found on product website. 290 No other information available. |
| 9 | D-Band; DiaMonTech | NIO-GM | Continuous 102 | One purchase of a device – no disposables announced 102 | Wearable wristwatch 102 |
| 10 | D-Base; DiaMonTech | NIO-GM | Intermittent 102 | One purchase of a device – no disposables announced 102 | The D-Base is the size of a shoebox. 102 |
| 11 | D-Pocket; DiaMonTech | NIO-GM | Intermittent 102 | One purchase of a device – no disposables announced 102 | Handheld pocket-sized device. 102 |
| 12 | GlucoBeam; RSP Systems | NIO-GM | Intermittent 104 | One purchase of a device – no disposables announced 104 | Portable, handheld device 104 |
| 13 | GlucoFit; GlucoActive | NIO-GM | Intermittent 291 | One purchase of a device 291 | Wearable armband 291 |
| 14 | Gluco Quantum; Genki Vantage Ltd | NIO-GM | Intermittent 109 | One purchase of device- no disposables announced 109 | Portable, handheld device 109 |
| 15 | Glucosense; Glucosense Diagnostics Ltd | NIO-GM | Intermittent but working to make continuous wearable forms. 292 | One purchase of device- no disposables announced 232 | Portable, handheld device 232 |
| 16 | GlucoStation; GlucoActive | NIO-GM | Intermittent 291 | One purchase of a device 291 | Stationary device, around the size of a lunchbox. 291 |
| 17 | GlucoTrack; Integrity Applications Ltd | NIO-GM | Intermittent 293 | One purchase of a device. Each personal ear cuff must be replaced every 6 months. 294 | Portable, handheld device, with cuff that clips onto earlobe 293 |
| 18 | GlucoWear; GlucoActive | NIO-GM | Intermittent 291 | One purchase of a device 291 | Wearable wristwatch 291 |
| 19 | GlucoWise; MediWiSe | NIO-GM | Intermittent 238 | One purchase of a device – no disposables announced 238 | Small, portable device that fits between the thumb and forefinger 238 |
| 20 | Glutrac; Add Care Ltd | NIO-GM | Continuous 119 | One purchase of a device – no disposables announced 119 | Wearable wristwatch 119 |
| 21 | HELO Extense; Wor(l)d Master Distributors | NIO-GM | Intermittent 239 | Purchase of a HELO Extense glucose sensor and either HELO LX wristband ($199+ USD) or HELO LX+ ($399+ USD). No disposables announced. 295 Manufacturer is described as using a multi-level marketing practice. 296 | Fingertip clamp sensor 239 |
| 22 | HELO LX PRO; Wor(l)d Master Distributors | NIO-GM | Continuous 295 | One purchase of a device, no disposables announced. 295 Manufacturer is described as using a multi-level marketing practice. 296 | Wearable wristband 295 |
| 23 | LIFELEAF; LifePlus | NIO-GM | Intermittent 297 | One purchase of a device- no disposables announced 297 | Wearable wristwatch 297 |
| 24 | Movano Wearable CGM; Movano | NIO-GM | Continuous 126 | One purchase of a device – no disposables announced 126 | Wearable wristwatch 126 |
| 25 | No product name; Omni Sciences, Inc. | NIO-GM | No information available. | No information available. | Developing a wearable 242 |
| 26 | Sanmina; Sanmina Corporation | NIO-GM | Intermittent | No information available. | Image provided by company representative presented in Table 5 shows form factor similar to a standard pulse oximeter. 298 |
| 27 | TensorTip Combo Glucometer; Cnoga Medical Ltd | NIO-GM | Intermittent 245 | A purchased device also comes with glucose test strips kit for calibration purposes 245 | 42.8 mm × 48 mm × 82 mm 245 |
| 28 | Uband; Know Labs, Inc. | NIO-GM | Continuous 246 | One purchase of a device – no disposables announced 246 | Wearable wristwatch 246 |
| 29 | Bios; GraphWear Technologies Inc. | NIFS-GM | Continuous 247 | Patch sensor 89 | No information available. |
| 30 | gSense; Nutrix | NIFS-GM | Intermittent 249 | One purchase of a sensor 249 | Toothbrush-sized 249 |
| 31 | NextGen; CGM Echo Therapeutics | NIFS-GM | Continuous 250 | Includes self-use exfoliator, exfoliator tip, sensor, reusable transmitter, battery 44 | No information available. |
| 32 | Saliva Glucose Biosensor; Gbs Inc. | NIFS-GM | Intermittent 299 | Disposable strips 140 | No dimensions mentioned on website. In images, looks to be a small one- to 2-inch-long thin strip 299 |
| 33 | sugarBEAT; Nemaura Medical | NIFS-GM | Continuous 141 | Non-insulin users: $30/month annual subscription. 8 patches/month, transmitter, and recharger. 252 Insulin users: $55/month annual subscription. 16 patches/month, transmitter, and recharger. 252 | No information available. |
| 34 | Tear Glucose Sensor; NovioSense | NIFS-GM | Continuous 144 | One purchase of a device – no information on whether or not the device needs to be replaced 144 | Very small, flexible coil that is placed in the bottom eyelid; 15 mm long and 1.3 mm in diameter 258 |
| 35 | AiDex CGM; GlucoRx (Rebranding of MicroTech Medical) | MI-GM | Continuous | The sensor must be replaced every 14 days. The transmitter is designed to last 4 years. 255 | No dimensions. The sensor is described as small and flexible. The transmitter is compact. 255 |
| 36 | Biolinq CGM; Biolinq | MI-GM | Continuous 149 | Purchase of silicon enclosure with battery and sensor, replaceable adhesives 149 | 1 mm length 148 |
| 37 | Care Sense Air; i-SENS | MI-GM | Continuous 89 | No information available. | No information available. |
| 38 | Cascade CGM System; Waveform | MI-GM | Continuous 300 | Purchase of disposable sensors 301 | Size of United States nickel, 302 which is 21.21 mm in diameter. 303 |
| 39 | CT-100; POCTech X Ascensia | MI-GM | Continuous 258 | Purchase of disposable sensors 258 | 10 mm × 0.3 mm (L × W, approximate) 258 |
| 40 | Dexcom G6; Dexcom | MI-GM | Continuous 159 | Transmitter: $237 G6 receiver: $365 Box of G6 sensors (3 pack): $349 304 | Transmitter/sensor size: 1.8 in × 1.2 in × 0.6 in 0.42 oz. with sensor. Receiver size: 4.02 in × 2.44 in × 0.46 in. 3.3 oz. 305 |
| 41 | Dexcom G7; Dexcom | MI-GM | Continuous | No information available. | Sensor size smaller than Dexcom G6 306 |
| 42 | Eclipse 3 iCGM; GlySens | Implanted MI-GM | Continuous 307 | Sensor has expected 2-year life 307 | 3.4 cm diameter, 1.5 cm thick for previous prototype but making the implant smaller 262 |
| 43 | Eversense; Senseonics | Implanted MI-GM | Continuous 58 | $99 308 | Transmitter/sensor size: 1.48 in × 1.89 in × 0.35 in. 0.39 oz. 305 |
| 44 | FiberSense Technology CGM; EyeSense | MI-GM | Continuous 265 | Disposable sensors. No pricing information. 265 | No information available. |
| 45 | FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM | Continuous 309 | Most commercially insured patients pay between $10 and $75 per month for FreeStyle Libre 14 day sensors. One purchase of reader. 309 | Transmitter/sensor size: 5 mm height 35 mm diameter 267 |
| 46 | FreeStyle Libre 2; Abbott Diabetes Care | MI-GM | Continuous 309 | Covered by Medicare in the USA. Considered to be 70% lower cost than most CGMs depending on insurance coverage 310 | Transmitter/sensor size: 5 mm height, 35 mm diameter 269 |
| 47 | FreeStyle Libre 3; Abbott Diabetes Care | MI-GM | Continuous 311 | The FreeStyle Libre 3 will be the same price as previous FreeStyle Libre CGM systems, at $109 per month. 312 | About the size of 2 United States pennies stacked. 313 |
| 48 | Glucomen Day CGM; Menarini Diagnostics | MI-GM | Continuous 271 | £139 per month if you subscribe. Rechargeable transmitter, disposable sensors. 180 | Sensor + transmitter size: 3.5 cm × 2.5 cm × 0.9 cm 271 |
| 49 | Glunovo i3 CGM; Infinovo | MI-GM | Continuous 273 | - Starter Kit: 1 Glunovo Transmitter and 2 Glunovo Sensors. - Monthly Package: 2 Glunovo Sensors. - Annual Package: 25 Glunovo Sensors and 6 sensors shipped every 3 months. 314 |
Transmitter size: 33 mm × 19 mm × 4 mm 273 |
| 50 | Glyde CGM; GluSense | Implanted MI-GM | Continuous 185 | The sensor has to be replaced every year. 186 | No information available. |
| 51 | Indigo CGM; Indigo | Implanted MI-GM | Continuous 315 | No information available. | No information available. |
| 52 | No product name; Integrated Medical Sensors | Implanted MI-GM | Continuous 190 | It is estimated that the sensor must be replaced every 6 months. 189 | Smaller than a sesame seed. 190 |
| 53 | No product name; One Drop (acquired Sano Intelligence, Inc., which was developing patch biosensor glucose monitor) 192 | MI-GM | Continuous 192 | Biosensor patch192,194,276 | No information available. |
| 54 | K’Watch; PKvitality | MI-GM | Continuous 277 | Expected to be set at $199 for the K’Watch. K’apsul Sensor that is replaceable on the watch expected to be set at $99 per month. 316 | Watch size. 277 |
| 55 | Lumee; Profusa | Implanted MI-GM | Continuous 279 | Will have a transmitter and sensor that is inserted under the skin. 279 | Approx 5 mm in length and 500 microns in diameter. 279 |
| 56 | Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM | Continuous 317 | Starts at $50/month based on typical 20% copay with insurance 318 | 1.41 in × 1.13 in × 0.38 in. 0.04 oz. with sensor 305 |
| 57 | Medtronic Synergy; Medtronic MiniMed | MI-GM | Continuous 282 | No information available. | Sensor 50% smaller than Guardian Sensor 3. 319 |
| 58 | Medtronic Zeus; Medtronic MiniMed | MI-GM | Continuous 282 | No information available. | Same size and shape as Guardian Sensor 3. 320 |
| 59 | No product name; Metronom Health | MI-GM | Continuous 283 | Transmitter with sensor. 283 | As thin as 2 human hairs in width 321 |
| 60 | PercuSense CGM; PercuSense | MI-GM | Continuous 284 | Transmitter with disposable sensor. 284 | No information available. |
| 61 | Sanvita; Sanvita Medical, LLC and LifeScan | MI-GM | Continuous 210 | No information available. | No information available. |
| 62 | Sencell; LifeCare AS | Implanted MI-GM | Continuous 322 | Implant sensor and wristwatch. No disposables announced. 323 | Sensor size: 2 mm × 3 mm × 6 mm 323 |
| 63 | SugarSenz; Glucovation | MI-GM | Continuous 287 | The sensor must be replaced every 7-10 days. The transmitter is designed to last longer than a year. 214 | The sensor is a thin adhesive that sticks to the skin. The transmitter is the size of a smartphone. 214 |
| 64 | SynerG; Pacific Diabetes Technologies | MI-GM | Continuous 324 | One purchase of a transmitter. Disposable sensors. 324 | Dimensions unavailable. Sensor worn on the abdomen as indicated by manufacturer’s website picture. 324 |
| 65 | TouchCare System A6; Medtrum | MI-GM | Continuous 325 | One purchase of a transmitter. Disposable sensors. 325 | 76.2 mm × 48.4 mm × 9.375 mm 325 |
Abbreviations: CGM, continuous glucose monitor; MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
Table 4.
Classification of Underlying Technologies of NIO-GM, NIFS-GM and MI-GM Identified Products.
| Technology | Table entry |
|---|---|
| Noninvasive optical | |
| Short wavelength near infrared | 27 |
| Near infrared spectroscopy | 4, 5, 6 |
| Mid infrared spectroscopy | 9, 10, 11, 14, 25 |
| Raman scattering spectroscopy | 12 |
| Radio or microwave | 1, 2, 19, 24, 28 |
| Polarimetry | 7, 8 |
| Fluorescence | 15 |
| Scattering | 13, 16, 18 |
| Multiplex signals | 17, 20 |
| Photoplethysmography | 21, 22, 23, 26 |
| No information available | 3 |
| Noninvasive fluid sampling | |
| ISF extraction | 31, 33 |
| Tear fluid | 34 |
| Sweat | 29 |
| Saliva | 30, 32 |
| Minimally invasive | |
| Electrochemical glucose oxidase biosensor | 35-40, 42, 45-49, 52-54, 56, 60, 64, 65 |
| Non-enzymatic fluorescence | 43, 44, 50, 55 |
| Other | 51, 59, 62, 63 |
| No information available | 41, 57, 58, 61 |
Table 5A.
Designs of NIO-GM, NIFS-GM, and MI-GM Identified Products with Regulatory Clearance (in Alphabetical Order Within Each Category of Invasiveness).
| Product and manufacturer | Degree of invasiveness | Product design |
|---|---|---|
| GlucoTrack; Integrity Applications Ltd | NIO-GM |
 293
293
|
| sugarBEAT; Nemaura Medical | NIFS-GM |
 326
326
|
| Cascade CGM System; Waveform | MI-GM |
 327
327
|
| Dexcom G6; Dexcom | MI-GM |
 328
328
|
| Eversense; Senseonics | Implanted MI-GM |
 329
329
|
| FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM |
 330
330
|
| FreeStyle Libre 2; Abbott Diabetes Care | MI-GM |
 331
331
|
| FreeStyle Libre 3; Abbott Diabetes Care | MI-GM |
 332
332
|
| Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM |
 333
333
|
| PercuSense CGM; PercuSense | MI-GM |
 284
284
|
| Sencell; Lifecare AS | MI-GM |
 323
323
|
Abbreviations: MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
Table 5B.
Designs of NIO-GM, NIFS-GM, and MI-GM Identified Products without Regulatory Clearance (in Alphabetical Order Within Each Category of Invasiveness).
| Product; manufacturer | Degree of invasiveness | Product design |
|---|---|---|
| No product name; Afon Technology | NIO-GM |
 86
86
RCD No: 008213961-0001 |
| D-Band; DiaMonTech | NIO-GM |
 334
334
|
| D-Base; DiaMonTech | NIO-GM |
 335
335
|
| D-Pocket; DiaMonTech | NIO-GM |
 336
336
|
| GlucoBeam; RSP Systems | NIO-GM |
 337
337
|
| GlucoFit; GlucoActive | ONI-GM |
 230
230
|
| Gluco Quantum; Genki Vantage Ltd | NIO-GM |
 338
338
|
| GlucoStation; GlucoActive | NIO-GM |
 233
233
|
| GlucoWear; GlucoActive | NIO-GM |
 237
237
|
| LifeLeaf; LifePlus | NIO-GM |
 339
339
|
| Movano Wearable; CGM Movano | NIO-GM |
 340
340
|
| Sanmina; Sanmina Corporation | NIO-GM |
 298
298
|
| Uband; Know Labs, Inc. | NIO-GM |
 341
341
|
| Tear Glucose; Sensor NovioSense | NIFS-GM |
 144
144
|
| K’Watch; PKvitality | MI-GM |
 277
277
|
| SynerG; Pacific Diabetes Technologies | MI-GM |
 342
342
|
Abbreviations: MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
Table 6.
Advantages and Disadvantages of NIO-GM, NIFS-GM, or MI-GM Identified Products that were Cleared by the FDA or CE Marked (in Alphabetical Order Within Each Category of Invasiveness).
| Product and manufacturer | Degree of invasiveness | Advantages | Disadvantages |
|---|---|---|---|
| GlucoTrack; Integrity Applications Ltd | NIO-GM | - Uses a blend of 3 optical technologies. | - Ear measurement site can be inconvenient |
| TensorTip Combo Glucometer; Cnoga Medical Ltd | NIO-GM | - Portable | - Not wearable |
| - Requires regular recalibration | |||
| sugarBEAT; Nemaura Medical | NIFS-GM | - Uses a well-tolerated adhesive | - No PubMed published data. |
| - Skin ISF is measured without puncturing the skin. - You can wear the device on any single day and not have to wear it on multiple consecutive days. |
- Reverse iontophoresis irritated the skin in a similar product from a different manufacturer: GlucoWatch | ||
| - The accuracy has not been established if a patient is sweating, or if there are rapid changes in the blood glucose concentration. | |||
| - There should be good correlation between glucose level in the ISF and in the blood under stable conditions. | |||
| - Glucose measurement is based on the well-known enzymatic method. | |||
| Cascade CGM System; Waveform | MI-GM | - Accurate
152
- 14-day duration - Automatic data uploading - Inserts without a needle - Audible glucose alarms - Software identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation. | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| CT-100; POCTech X Ascensia | MI-GM | - CT-100 features a 4-electrode system. In addition to a glucose sensing electrode, it features a dedicated blank electrode that monitors background and electrochemical interferences. It enables real time correction of any background drift and interference. | - Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. - Some patients do not want to carry an inserted or implanted device on their body at all times. - No CGM is labeled to allow for exposure to radiation. |
| - CT-100 sensor is built on a flexible polyimide matrix. It is one of the most flexible sensors available. This provides higher level comfort for 24/7 wearing. | |||
| - CT-100 sensor features an all planar design and align electrodes on both sides of the matrix. It can fully utilize currently available industrial manufacturing techniques for mass production, and therefore provides a potential for a favorable cost effectiveness and easier quality control. | |||
| Dexcom G6; Dexcom | MI-GM | - Accurate
159
- 10-day duration - Automatic data uploading with sharing feature - Factory calibration - Audible glucose alarms - Software identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation. | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| Eversense; Senseonics | Implanted (MI-GM) | - Accurate
263
- 90-180 day duration- Automatic data uploading with sharing feature - Removable transmitter - Audible and vibratory glucose alarms - Software identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation. | |||
| - Possible difficulty removing a chronically implanted sensor. | |||
| FreeStyle Libre 14 day; Abbott Diabetes Care | MI-GM | - Accurate267,343,344 - 14-day duration 343 - No separate transmitter; on demand data with sharing feature - Factory calibration - Software identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| - Patient must actively perform an action to determine glucose levels | |||
| - System cannot be calibrated - no optional user calibration in cases of sensor inaccuracy | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| - No hypoglycemia alarm | |||
| FreeStyle Libre 2; Abbott Diabetes Care | MI-GM | - Accurate
269
- 14-day duration 269 - No separate transmitter; on demand data with sharing feature - Factory calibration - Audible glucose alarms - Software identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| - Patient must actively perform an action to determine glucose levels | |||
| - System cannot be calibrated - no optional user calibration in cases of sensor inaccuracy | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| FreeStyle Libre 3; Abbott Diabetes Care | MI-GM | - The FreeStyle Libre 3 system is designed to automatically deliver accurate real-time, up-to-the-minute glucose readings,
345
and optional glucose alarms directly to smartphones - Designed to be the smallest and thinnest wearable glucose sensor. - Users can view their glucose levels anytime - Designed to have the same performance as the FreeStyle Libre 2 sensor, but with a new sensor design and at the same price as previous versions |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| - System cannot be calibrated - no optional user calibration in cases of sensor inaccuracy | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| Glucomen Day CGM; Menarini Diagnostics | MI-GM | - Does not require any needles for insertion - Promoted as eco-friendly 180 - Transmitter can last 3 years 346 |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| Glunovo i3 CGM; Infinovo | MI-GM | - Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. | |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| Medtronic Guardian Connect (Powered by Medtronic Guardian Sensor 3); Medtronic MiniMed | MI-GM | - Accurate
281
- 7-day duration - Automatic data uploading - Audible glucose alarms - Software developed with IBM identifies glucose patterns |
- Point accuracy of MI-GMs that measure ISF is less than point accuracy of blood glucose monitors that use capillary blood as a comparator matrix. |
| - Some patients do not want to carry an inserted or implanted device on their body at all times. | |||
| - No CGM is labeled to allow for exposure to radiation | |||
| - Sometimes allergic dermatitis due to adhesive | |||
| - There is a need to calibrate twice a day with a blood glucose measurement | |||
| - Wear time (7 days) shorter than some other products |
Abbreviations: AID, automated insulin delivery; CGM, continuous glucose monitor; IBM, International Business Machines Corporation; iCGM, integrated continuous glucose monitor; ISF, interstitial fluid; MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sensing glucose monitor; NIO-GM, noninvasive glucose monitor.
Regulatory Features
Table 1 presents five regulatory features of the 65 identified products, including whether they received FDA clearance or a CE mark. Obtaining a CE mark requires proof of safety of the device, however, obtaining FDA clearance, requires proof of both the safety and efficacy of the device. 347 Although FDA clearance is more difficult to obtain, it is a more powerful certification. 348
Technological Features
Table 2 presents four technological features of the 65 identified products. The next four paragraphs discuss these features.
The first technological feature we considered is the mechanism of glucose sensing, which is the process through which the sensor detects and interacts with glucose. These processes were based on a variety of chemical and physical properties of glucose.
The second technological feature we considered is the matrix where sensing occurs. For each glucose monitor, the sensing of glucose is carried out within a specific matrix. The matrix can be ISF with MI-GMs, ISF, sweat, tears, or saliva with NIFS-GMs, or a combination of ISF, cells, and blood with NIO-GMs.
The third technological feature we considered is accuracy. The accuracy of glucose monitors that are not invasive is typically reported in one of two ways. First, accuracy can be reported as the percentage of data pairs of glucose monitor values compared to reference values falling within various accuracy ranges, such as within 5%, 10%, 15%, 20%, 30%, and 40%. Second, accuracy can be presented as the mean absolute relative difference (MARD) or median absolute relative difference (medARD) of the measured glucose value compared to the reference glucose value. The two values for normally distributed datasets are linked by an approximate conversion factor of 2.5. For example, if a glucose monitor has a MARD of 10% and a set of glucose values is distributed normally, then approximately 95% of its measurements will fall within +/−25% of the reference glucose value because 2.5 × 10% equals 25%. Clinical accuracy is typically reported using an error grid analysis which presents the risk to a user based on actions taken from a measured glucose value compared to the correct action that would have been based on a reference glucose value. Three widely used error grids for blood glucose have been proposed, and over time they have reflected increasingly more modern expectations of analytical accuracy, understanding of risks of hypoglycemia, and prescribing patterns for modern insulins. The most recent error grid and that which reflects input from the greatest number of diabetes clinicians (compared to earlier iterations of error grids known as the Clarke Error Grid 349 and the Parkes or Consensus Error Grid 350 ) is the Surveillance Error Grid. 351 Each grid presents data pairs in five zones of risk, although the Surveillance Error Grid is constructed so that it is possible to also generate more than five risk zones. Neither analytical accuracy data nor clinical accuracy data was available for every product because not every product has conducted clinical trials or published performance data.
The fourth technological feature we considered was the device’s possible interferences or interfering substances that might affect the glucose monitor’s accuracy. The accuracy of a glucose monitoring device might be affected by interferences or interfering substances, which are molecules that can interfere with sensing, often because of a similar structure as glucose. These substances are important to identify and characterize to ensure that users understand why or when their readings might be inaccurate.
Consumer Features
Table 3 presents three consumer features of the 65 identified products. The first consumer feature we considered is the measurement cycle, or whether the device makes automatic continuous or intermittent measurements. Any automatic measurement device must be engineered to be small enough to be a wearable device, but an intermittent measurement device does not necessarily need to be built small. The second consumer feature we considered is the pricing model. There are two main models that are used. In the first model, the user purchases the sensor and can make as many measurements as desired during the lifetime of the sensor. In the second model, the patient is charged for each measurement. Combinations of these two models are also possible. One device might require a single large payment for a device that could last for years, while another device might require periodic purchases of consumable parts or else payment of a toll charge each time the monitor is used. The third consumer feature we considered is the size/form factor of the product. A small sized sensor, compared to a large sized sensor, is usually more convenient and preferred by most users. However, a small minimally invasive implanted sensor could break off from its transmitter and be lost under the skin. A small, wearable noninvasive sensor with a streamlined form factor, compared to a large, bulky, free-standing noninvasive sensor, will usually be more costly because component miniaturization and assembly are expensive.
Underlying Glucose Sensing Technologies used in NIO-GM, NIFS-GM, or MI-GM Identified Products
Table 4 classifies the underlying glucose sensing technologies of the 65 identified products. Under NIO-GM, the glucose sensing technologies included: short wavelength near infrared, near infrared spectroscopy, mid infrared spectroscopy, Raman spectroscopy, radio or microwave devices, polarimetry, fluorescence, scattering, multiplex signals, and photoplethysmography. A few products were NIO-GM, but their exact glucose sensing technologies were not specified because this information was not available. Under NIFS-GM, the glucose sensing technologies included: saliva, tear fluid, sweat, and ISF extraction. Finally, under MI-GM, the glucose sensing technologies included: electrochemical glucose oxidase biosensor and non-enzymatic fluorescence sensors. Some MI-GM did not belong to either of these technologies, and some do not have publicly available information on what technology they use.
Images of Product Designs of Selected Identified Products
Table 5 presents images of product designs of identified products. We included images only if we were able to receive permission from manufacturer representatives. Images are not to scale relative to each other. Table 5A is for products that are FDA cleared and/or CE marked, and Table 5B is for products that are neither FDA cleared nor CE marked.
Advantages and Disadvantages of Identified Products
Table 6 presents advantages and disadvantages of the identified products that were cleared by the FDA or CE marked. The features that we present in Table 6 for specific products are important highlights of each product, rather than a comprehensive list of advantages and disadvantages. In general, there are advantages and disadvantages for each of the three categories of glucose monitors employing bloodless sampling. All three categories of glucose monitors are less accurate than IGMs. (1) As a class, NIO-GM products are painless and do not generate biological waste, but they are less accurate than NIFS-GM, MI-GM, and IGM products. (2) As a class, NIFS-GM products require the sensor to be in direct contact with a body fluid that is not blood, and these systems generally produce less pain and less biological waste than IGMs. However, there can be a significant lag-time in their measurements, thereby rendering them less accurate than IGMs or MI-GMs. (3) As a class, MI-GM products have been studied the most of the three types of bloodless glucose monitors. More products in this category, compared to the other two categories, have received regulatory clearance. Currently, many MI-GMs are more accurate, more robust, and more conveniently calibrated than most NIO-GM and NIFS-GM products. The Abbott (Chicago, Illinois) and Dexcom (San Diego, California) MI-GMs are factory calibrated. All MI-GMs, however, require insertion or implantation of the glucose sensor and must be worn, which in rare cases creates an unpleasant sense of being tethered to a machine. 352 Many MI-GMs and some NIO-GMs and NIFS-GMs require adhesives for stabilization and allergic dermatitis has been reported with some adhesives. 73 The convenience, performance, and connectivity of MI-GMs have been advancing rapidly over the past few years and product manufacturers have predicted further advances in these areas. 353 The worldwide market for CGMs was valued at $1.8 billion in 2019 and with a projected compound annual growth rate of 22%, the market is expected to reach $8.8 billion by 2027. 354 This market has likely already surpassed four million CGM users. 355
Discussion
Evolution of Bloodless Glucose Monitoring Products
In the past, one NIFS-GM 356 and one NIO-GM 357 were introduced to the market but were eventually discontinued, and two NIO-GMs358,359 were cleared but never introduced to the market.356-359 These products were all highly anticipated before their release but failed to live up to expectations. A particular problem was that such devices were touted in the lay press, raising expectations among people with diabetes and their families. Unfortunately, their performance repeatedly failed to meet these expectations. The NIFS-GM GlucoWatch Biographer from Cygnus Inc. was cleared by the FDA, marketed, and then discontinued. 360 Three NIO-GM products were cleared with a CE Mark and then discontinued either soon after entering the market (Pendra Device from Pendragon Medical, Zürich, Switzerland), or without entering the market (NBM-200G from OrSense, Tel Aviv-Yafo, Israel and Optical Glucose Monitor from C8 MediSensors, San Jose, California). Two NIO-GM products that were cleared with a CE mark are currently on the market. They are the GlucoTrack (Integrity Applications Ltd, Ashdod, Israel) and TensorTip Combo Glucometer (Cnoga Medical Ltd, Caesarea, Israel).
The original GlucoWatch Biographer 41 was an NIFS-GM device. This device was cleared by the FDA in 2001 and marketed. 40 An updated version (the GlucoWatch G2 Biographer 42 ) was also cleared in 2002 for use by individuals under 18 years of age. 356 Because these devices used reverse iontophoresis, a low-level current was passed through the skin as part of the glucose sensing method. These products were discontinued after users complained of a burning sensation while using the device. 360
The Pendra Device, which measured the change in the frequency-dependent resistance (impedance spectroscopy) in the interstitial flow when the glucose concentration changed, 361 was initially cleared with a CE Mark of Approval in 2003. However, this device was discontinued because it had readings that were considered dangerously inaccurate and it was incompatible with 30% of people considered for using the device. In 2005, Pendragon Medical went out of business. 357
The NBM-200G, which used occlusion spectroscopy, received the CE Mark of Approval in 2007 358 but was discontinued by OrSense for unspecified reasons. The C8 Optical Glucose Monitor received the CE Mark of Approval in 2012, 359 but is no longer on the market officially because of a lack of funding. 362 Other NIO-GM products have been announced to be in development and then have subsequently disappeared from the public eye. These products include the ALIRA Infrared Biosensing sensor (Princeton University, Princeton, New Jersey), Glucoband (Calisto Medical, Plano, Texas), and a Glucose-Sensing Contact Lens (Novartis, Basel, Switzerland working with Verily, South San Francisco, California).
These shortcomings are discussed to emphasize the importance of recognizing, addressing, and avoiding past mistakes. These previous failures to create a usable product also highlight the barriers that must be overcome before a viable NIO-GM can be widely distributed, including (1) large device size, (2) potential skin reactions, (3) poor accuracy, (4) high power requirements, (5) interfering substances, and (6) the lack of a clear regulatory pathway. Although the majority of the NIO-GM products discovered in the search presented in the article are neither cleared nor publicly available, these products have potential, but one must guard against being overly optimistic for the sake of potential users.
Trends in Technology – A Path for Better Products
Many technologies are now being pursued to achieve the objective of noninvasive glucose measurements in people with diabetes. At this time, few NIO-GM or NIFS-GM systems are sufficiently mature for commercialization. The MI-GM market, however, is strong in many countries and growing worldwide. Many of these technologies not using IGM that are in development appear to have poor accuracy. Still, noninvasive approaches are attracting more attention by both researchers and investors owing to the tremendous need for managing diabetes optimally in an ever-growing number of patients and also because of commercial applications beyond medicine.
The commercial potential differs for the products identified in this article. The NIO-GMs are becoming more of a priority for medical device researchers. NIFS-GM sensors seem to be the least likely to satisfy user needs because of the requirement to harvest a representative fluid. Such approaches are prone to issues related to skin irritation as well as to the complexities of collecting, handling, and disposing of a representative clinical fluid. These issues render the noninvasive fluid sampling approach inconvenient in comparison to both the noninvasive optical and minimally invasive approaches. The former eliminates fluid handling and the later provides continuous measurements. The commercial attractiveness of NIO-GM technologies is driven by the potential to achieve either continuous glucose measurements or repeated spot-measurements, both with minimal user interactions. Commercial success has already been realized for MI-GM technologies and will likely grow as future innovations lead to longer periods of operation and the quantitation of multiple analytes.
Success of NIO-GM, NIFS-GM, and MI-GM devices has started to decrease the market size of invasive blood glucose measurement systems considerably. Just as urine glucose testing was replaced by blood glucose testing, eventually these three bloodless technologies will replace blood glucose monitoring. This trend will continue within the next 10-20 years as new sensing strategies that do not require blood become increasingly accurate, practical, and affordable. Today many of the leading blood glucose monitor manufacturers are developing NIO-GM, NIFS-GM, or MI-GM technologies or partnering with companies with these types of products. The need for invasive blood glucose measurements will also decrease as factory-calibrated MI-GM devices become more accepted by people with type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM). A CE marked MI-GM was recently introduced for athletes without diabetes. 363 As the accuracy and reliability of factory-calibrated MI-GM devices improve, the need to check an IGM reading will decrease.
Commercial success of any CGM system, regardless of whether it is noninvasive or minimally invasive, must recognize the demands placed on the user. Proper training is paramount for CGM users, particularly as it relates to the continuous information provided by these devices. 364 The users must gain confidence in viewing the large amount of data, interpreting the recorded glucose profiles, and using the data to guide therapeutic decisions. Trained users value the added analytical information provided by CGM and find that it benefits their therapy. 365 However, improved data management software will be helpful for the users who are overwhelmed by the data output.
Noninvasive Technology for Diagnosing Diabetes
The current standard for diagnosing diabetes specifies invasive blood sampling, followed by measuring either plasma glucose concentration or hemoglobin A1c concentration. 366 While several noninvasive and minimally invasive methods have been developed for monitoring glucose levels, as outlined throughout this paper, there have been few attempts to validate the use of such devices for making a diagnosis of diabetes. There is, however, an incipient technology using photoplethysmography that is intended to make this diagnosis.
Photoplethysmography measures the interaction of light with the vascular bed to determine the changes in blood volume with each heartbeat. 367 This technique is currently used in four NIO-GM products under development, including Wor(l)d Global Network’s (Miami, Florida) HELO Extense 239 and HELO LX Pro, 122 LifePlus’ (San Jose, California) LifeLeaf, 240 and Sanmina’s (San Jose, California) NIO-GM. 243 Furthermore, Avram et al. have reported using a smartphone-based photoplethysmography technology with a deep neural network scoring system to prospectively evaluate people with diabetes, by correlating the score with plasma glucose levels. 368 This study’s results suggest that this method could be potentially useful as a digital biomarker for diabetes diagnosis, 368 but further work is needed. 369
Miniaturization and Nanotechnology
Miniaturization is critical for future applications and user adoption of the glucose sensing technologies under development worldwide. Wearable devices demand small sensing packages that can be worn for prolonged periods with minimal user discomfort. Miniaturization will also facilitate comfortable systems that combine the sensing element with an insulin infusion system, perhaps in a single package, for novel AID systems. 82 As the sensing technology advances to the stage of reliable, calibration-free operation for extended periods, miniature solid-state sensing systems will be necessary to serve as the glucose sensing element of an implantable autonomous AID systems.
Nanotechnology is a materials approach with the potential to facilitate device miniaturization while enhancing sensor performance. 370 Nanostructures are capable of unique properties not available in bulk materials. The surface area to volume ratio is large for nano-dimensional materials which offers unique physical and chemical effects, such as novel interfacial phenomena, altered reactivity, and charge carrier and quantum mechanical effects. 371 Such nanoscale properties can advance miniaturization and sensor operation through higher surface areas, thereby yielding larger sensitivity, quicker responses, and higher catalytic loadings. These enhancements promise superior overall analytical performance.
Nanofabrication techniques can also generate glucose sensors with small dimensions. 372 Such small dimensions can facilitate implantation or injection of glucose sensing “tattoos.”373,374 Nanofabricated devices could potentially diminish or avoid troubling foreign body responses, resulting in simpler calibration processes and longer operational lifetimes. Solid-state photonic systems hold promise for miniaturizing spectroscopic sensors for application as NIO-GM devices. The system under development by Brolis (Vilnius, Lithuania), for example, uses a novel integrated photonic package for solid-state measurements of multiple analytes, including glucose, lactate, and ethanol. Finally, micro- and nano-electronic technologies offer the possibility of low-cost mass production, which can drive down user’s costs and expand adoption.
Overcoming Technical Barriers to Widespread Adoption
Adoption of any bloodless glucose sensing technology will depend on a host of technical and nontechnical issues. First, and foremost, the technology must be accurate and robust in operation while adding value to the quality of life for the user. Advances in engineering, physics, clinical chemistry, and medicine will also be required to overcome the technical barriers that impede widespread adoption of NIO-GM, NIFS-GM, and MI-GM products.
From the engineering perspective, more sensitive sensing technologies are needed, particularly for ex-vivo NIO-GM and NIFS-GM devices. The ability to identify and detect a robust selective signature for glucose is paramount along with instrumentation able to collect such a signature reliably under non-laboratory conditions. Measurement accuracy in the low glucose range is critical to achieve tight glycemic control while avoiding potentially dangerous hypoglycemic episodes.
From the physics perspective, algorithms are needed to convert raw sensor signals into correct glucose concentration values. For NIO-GM technologies, a principal challenge is to effectively filter out variations in the measured signal, both the noise of the instrumentation as well as uncontrolled changes in the background matrix against which the glucose signature is determined. Success calls for understanding the source of such background variances and developing multivariate data analysis methods to overcome the adverse impact of the background variance. For NIFS-GM devices, the major barrier is to find a correlation between the concentration of glucose in blood and the sampled fluid. For MI-GM systems, reliable analytical measurements are needed for periods longer than 14 days.
From the clinical chemistry perspective, a better understanding is needed about how to assure the accuracy of bloodless measurements by establishing traceability of their readings to a reference standard. 375 This challenge is currently unresolved because of the difficulty of obtaining comparator ISF samples for testing against blood. Also, the controversy about whether capillary blood or venous blood is the best comparator matrix for ISF measurements must be resolved. 375 Overall, a convenient and reliable method for collecting representative samples of ISF would have a large positive impact on the advancements of these and future NIO-GM, NIFS-GM, and MI-GM products.
From the medicine perspective, physicians need a better understanding of the clinical values of glucose measurements in fluids other than blood, that is, how to adjust the insulin dose of a patient in case of exercise, is the glucose signal from blood the more reliable signal or that from ISF? The utility of ISF glucose concentrations must be established and might actually be preferable over blood glucose concentrations for treatment decisions because the glucose concentration in ISF compared to that in blood might more closely track the glucose concentration in the brain. 54 Others have suggested that ISF glucose concentrations can be superior to blood glucose concentrations during periods of rapidly fluctuating glucose concentrations or hypoglycemia. Another clinical dimension for physicians to determine is the level of accuracy needed for various types of patients, such as those with: T1DM on multiple dose insulin therapy, T1DM on sensor augmented pump therapy, T1DM on AID therapy, T2DM using insulin, T2DM using other diabetes drugs known to cause hypoglycemia, T2DM using diabetes drugs not known to cause hypoglycemia, and T2DM using only diet and exercise for control.
Overcoming Non-Technical Barriers to Widespread Adoption
Overcoming nontechnical barriers will be required for the widespread adoption of bloodless glucose sensing technologies:
(1) The cost of these products must be reduced to make adoption possible for large populations, especially in resource-poor regions of the world.
(2) The interface between the user and instrumentation and the way in which the user interacts with the instrumentation must translate into an easily deployed wearable device that does not cause embarrassment or inconvenience, particularly in social settings.
(3) Patients must accept the concept of wearing a continuously functioning device without concern that they are losing their humanity by being dependent on a mechanical device. 376
(4) A motivational factor for many potential users can be an understanding of the present and future environmental risks caused by the large amounts of medical and plastic waste generated by today’s invasive blood glucose monitoring technologies.
The overall strategy is to provide a bloodless glucose sensing technology that simultaneously reduces waste, increases convenience, and saves money.
Regulatory agencies guided by solid clinical evidence will play an important role in determining the level of accuracy that each of these new bloodless devices must provide to achieve non-adjunctive status. This is the level of clearance whereby manufacturers can indicate on the product label that their glucose monitoring product can be used to make a treatment decision without the need for an accompanying blood glucose reading as a confirmatory test. Accuracy and robustness of the sensing product are required for non-adjunctive clearance, which will undoubtedly boost the appeal of these products. As shown in Table 3, four MI-GM products have a non-adjunctive indication per the FDA and it is likely that many future bloodless products will demonstrate adequate accuracy to also gain this distinction.
Conclusions
Commercial products for bloodless monitoring of glucose were identified and reviewed. These products are based on three categories of emerging technologies: (1) NIO-GM, (2) NIFS-GM, and (3) MI-GM. Many of these products are based on spectroscopic, electrochemical, and affinity sensing strategies for quantifying glucose molecules in ISF. A multiple database search was used to identify a total of 65 bloodless glucose monitoring products, of which 13 have received regulatory clearance and are currently on the market. The remaining products are under development. Further refinement of these technologies is anticipated over the upcoming decade to enhance both analytical performance and adoption of these products by people with diabetes. Future developments in bloodless glucose monitoring will require technical and nontechnical advances related to (1) superior analytical performance, (2) robust algorithm development, (3) traceability testing for glucose-containing matrices besides blood, such as ISF, and (4) appreciation of the clinical value of ISF glucose measurements. Bloodless glucose monitoring products, such as the ones discussed in this article, are expected to become key components of novel wearable digital health tools for monitoring glucose concentrations in the diabetes market and the fitness market.
Addendum
In May 2021, after this article was in press, Rockley Photonics disclosed a program to develop a chip size laser-based technology suitable for implementation in a smartwatch format. Health monitoring is its principal application and Apple is featured as a major partner in the development of this technology. Rockley stated that Apple is the largest of six customers with which it has entered into contracts with or engaged with, developing health and wellness devices. These companies are developing smartwatches and medical devices for biomarker detection. 377 Rockley’s sensors are designed to quantify glucose, alcohol, and oxygen levels non-invasively with infrared spectroscopy. Rockley will deliver its chipsets in 2022. 378
Acknowledgments
We thank Annamarie Sucher-Jones for her expert editorial assistance. We thank Guido Freckmann, MD, Avner Gal, MBA, MSCEE, MSc, H. Michael Heise, PhD, Jeffrey La Belle, PhD, MS, Jeffrey Joseph, DO, John Pickup, MD, PhD, and Mark Prausnitz, PhD for naming candidate products and manufacturers.
Footnotes
Abbreviations: AID, Automated insulin delivery; AMBG, assisted monitoring of blood glucose; CE, Conformité Européenne; CGM, continuous glucose monitor; ConA, Concanavalin A; DF, fluorescently labeled dextran; FDA, United States Food and Drug Administration; GBP, glucose-binding protein; IGM, invasive glucose monitor; ISF, interstitial fluid; MARD, mean absolute relative difference; medARD, median absolute relative difference; MI-GM, minimally invasive glucose monitor; NIFS-GM, noninvasive fluid sampling glucose monitor; NIO-GM, noninvasive optical glucose monitor; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SMBG, self-monitoring of blood glucose; SNR, signal-to-noise ratio; USA, United States of America
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TS, JYZ, and BNV have nothing to disclose. AT was Scientific Manager at Medtronic until April 2020. Currently he has nothing to disclose. MAA discloses a consulting arrangement with LifePlus, Inc. LH is a partner of Profil Institut für Stoffwechselforschung in Neuss, Germany. He is a member of advisory boards for Roche Diagnostics, Zense, and Medtronic. He is also on the Board of Directors for Lifecare. DCK is a consultant for EOFlow, Fractyl, Lifecare, Novo, Roche Diagnostics, Samsung, and Thirdwayv.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Foundation for Innovative New Diagnostics through support from the Swiss Agency for Development and Cooperation (SDC).
ORCID iDs: Trisha Shang  https://orcid.org/0000-0001-9687-9336
https://orcid.org/0000-0001-9687-9336
Jennifer Y. Zhang  https://orcid.org/0000-0002-3374-9777
https://orcid.org/0000-0002-3374-9777
Andreas Thomas  https://orcid.org/0000-0002-6549-2793
https://orcid.org/0000-0002-6549-2793
Mark A. Arnold  https://orcid.org/0000-0001-9375-1863
https://orcid.org/0000-0001-9375-1863
Beatrice N. Vetter  https://orcid.org/0000-0002-7898-472X
https://orcid.org/0000-0002-7898-472X
Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S85-S99. https://care.diabetesjournals.org/content/44/Supplement_1/S85 [DOI] [PubMed] [Google Scholar]
- 2. Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;(2):CD009122. doi: 10.1002/14651858.CD009122.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodriguez-Gutierrez R, Gonzalez-Gonzalez JG, Zuñiga-Hernandez JA, McCoy RG. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ. 2019;367:l5887. doi: 10.1136/bmj.l5887 [DOI] [PubMed] [Google Scholar]
- 4. Cousins S, Blencowe NS, Blazeby JM. What is an invasive procedure? A definition to inform study design, evidence synthesis and research tracking. BMJ Open. 2019;9(7):e028576. doi: 10.1136/bmjopen-2018-028576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villena Gonzales W, Mobashsher AT, Abbosh A. The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors. 2019;19(4):800. doi: 10.3390/s19040800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klonoff DC, Perz JF. Assisted monitoring of blood glucose: special safety needs for a new paradigm in testing glucose. J Diabetes Sci Technol. 2010;4(5):1027-1031. doi: 10.1177/193229681000400501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moström P, Ahlén E, Imberg H, Hansson P-O, Lind M. Adherence of self-monitoring of blood glucose in persons with type 1 diabetes in Sweden. BMJ Open Diabetes Res Care. 2017;5(1):e000342. doi: 10.1136/bmjdrc-2016-000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heise HM, Delbeck S, Marbach R. Noninvasive monitoring of glucose using near-infrared reflection spectroscopy of skin—constraints and effective novel strategy in multivariate calibration. Biosensors. 2021;11(3):64. doi: 10.3390/bios11030064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H-C, Lee A-R. Recent developments in blood glucose sensors. J Food Drug Anal. 2015;23(2):191-200. doi: 10.1016/j.jfda.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamada JA, Garg S, Jovanovic L, Pitzer KR, Fermi S, Potts RO. Noninvasive glucose monitoring: comprehensive clinical results. Cygnus Research Team. JAMA. 1999;282(19):1839-1844. doi: 10.1001/jama.282.19.1839 [DOI] [PubMed] [Google Scholar]
- 11. Nemaura Medical. Nemaura Medical. Accessed December 14, 2020. https://nemauramedical.com/
- 12. Cai A, Gutow H, Mahoney K, et al. CGM 4Q20 industry roundup – record CGM sales of $1.6 billion, rising 26% YOY – March 9, 2021. Close Concerns Knowledgebase. March 9, 2021. Accessed March 15, 2021. https://www.closeconcerns.com/knowledgebase/r/be9b1ac7
- 13. Shokrekhodaei M, Quinones S. Review of non-invasive glucose sensing techniques: optical, electrical and breath acetone. Sensors. 2020;20(5):1251. doi: 10.3390/s20051251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang L, Chang SJ, Chen C-J, Liu J-T. Non-invasive blood glucose monitoring technology: a review. Sensors. 2020;20(23):6925. doi: 10.3390/s20236925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee I, Probst D, Klonoff D, Sode K. Continuous glucose monitoring systems - current status and future perspectives of the flagship technologies in biosensor research. In press. [DOI] [PubMed] [Google Scholar]
- 16. Vahlsing T, Delbeck S, Leonhardt S, Heise HM. Noninvasive monitoring of blood glucose using color-coded photoplethysmographic images of the illuminated fingertip within the visible and near-infrared range: opportunities and questions. J Diabetes Sci Technol. 2018;12(6):1169-1177. doi: 10.1177/1932296818798347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jernelv IL, Milenko K, Fuglerud SS, Hjelme DR, Ellingsen R, Aksnes A. A review of optical methods for continuous glucose monitoring. Appl Spectrosc Rev. 2019;54(7):543-572. doi: 10.1080/05704928.2018.1486324 [DOI] [Google Scholar]
- 18. Delbeck S, Vahlsing T, Leonhardt S, Steiner G, Heise HM. Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy-opportunities and recent advances. Anal Bioanal Chem. 2019;411(1):63-77. doi: 10.1007/s00216-018-1395-x [DOI] [PubMed] [Google Scholar]
- 19. Delbeck S, Heise HM. Evaluation of opportunities and limitations of mid-infrared skin spectroscopy for noninvasive blood glucose monitoring. J Diabetes Sci Technol. 2021;15(1):19-27. doi: 10.1177/1932296820936224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrante do Amaral CE, Wolf B. Current development in non-invasive glucose monitoring. Med Eng Phys. 2008;30(5):541-549. doi: 10.1016/j.medengphy.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 21. Gusev M, Poposka L, Spasevski G, et al. Noninvasive glucose measurement using machine learning and neural network methods and correlation with heart rate variability. J Sens. Published online January 6, 2020. doi: 10.1155/2020/9628281 [DOI] [Google Scholar]
- 22. Sakaki H, Arakawa M, Yashiro S, Todate Y, Ishigaki Y, Kanai H. Ultrasound scattering by aggregated red blood cells in patients with diabetes. J Med Ultrason 2001. 2019;46(1):3-14. doi: 10.1007/s10396-018-0892-z [DOI] [PubMed] [Google Scholar]
- 23. Habbu S, Dale M, Ghongade R. Estimation of blood glucose by non-invasive method using photoplethysmography. Sādhanā. 2019;44(6):135. doi: 10.1007/s12046-019-1118-9 [DOI] [Google Scholar]
- 24. Gupta SS, Hossain S, Haque CA, Kim K-D. In-vivo estimation of glucose level using PPG signal. In: 10th 2020 International Conference on Information and Communication Technology Convergence (ICTC), Jeju Island, Korea, 21-23 October, 2020. ICTC; 2020:733-736. doi: 10.1109/ICTC49870.2020.9289629 [DOI] [Google Scholar]
- 25. Yeh S-J, Hanna CF, Khalil OS. Monitoring blood glucose changes in cutaneous tissue by temperature-modulated localized reflectance measurements. Clin Chem. 2003;49(6):924-934. doi: 10.1373/49.6.924 [DOI] [PubMed] [Google Scholar]
- 26. Guo Q-Y, Lu B, Guo Z-H, et al. Continuous glucose monitoring defined time-in-range is associated with sudomotor dysfunction in type 2 diabetes. World J Diabetes. 2020;11(11):489-500. doi: 10.4239/wjd.v11.i11.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U.S. Food and Drug Administration. Pulse oximeter accuracy and limitations: FDA safety communication. FDA. February 19, 2021. Accessed February 26, 2021. https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication
- 28. Wassenaar EB, Van den Brand JGH. Reliability of near-infrared spectroscopy in people with dark skin pigmentation. J Clin Monit Comput. 2005;19(3):195-199. doi: 10.1007/s10877-005-1655-0 [DOI] [PubMed] [Google Scholar]
- 29. Tsai J, Chien AL, Kang JU, Leung S, Kang S, Garza LA. Hyperspectral measurement of skin reflectance detects differences in the visible and near-infrared regions according to race, gender and body site. J Eur Acad Dermatol Venereol. Published online December 8, 2020. doi: 10.1111/jdv.17076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malik BH, Pirnstill CW, Coté GL. Dual-wavelength polarimetric glucose sensing in the presence of birefringence and motion artifact using anterior chamber of the eye phantoms. J Biomed Opt. 2013;18(1):17007. doi: 10.1117/1.JBO.18.1.017007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. PubMed. PubMed “Noninvasive Glucose” search. Accessed February 10, 2021. https://pubmed.ncbi.nlm.nih.gov/?term=noninvasive+glucose&sort=date&size=200
- 32. Heise HM. Non-invasive monitoring of metabolites using near infrared spectroscopy: state of the art. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 1996;28(10):527-534. doi: 10.1055/s-2007-979846 [DOI] [PubMed] [Google Scholar]
- 33. Coté GL. Noninvasive optical glucose sensing — an overview. J Clin Eng. 1997;22(4):253-259. [Google Scholar]
- 34. Klonoff DC. Noninvasive blood glucose monitoring. Diabetes Care. 1997;20(3):433-437. doi: 10.2337/diacare.20.3.433 [DOI] [PubMed] [Google Scholar]
- 35. Khalil OS. Spectroscopic and clinical aspects of noninvasive glucose measurements. Clin Chem. 1999;45(2):165-177. [PubMed] [Google Scholar]
- 36. Hanssen JHL, Biosensor Tweehuysen R. Published online April 28, 2020. Accessed December 14, 2020. https://patents.google.com/patent/US10631769B2/en?assignee=noviosense&oq=noviosense
- 37. Bruen D, Delaney C, Florea L, Diamond D. Glucose sensing for diabetes monitoring: recent developments. Sensors. 2017;17(8):1866. doi: 10.3390/s17081866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams C. FDA approves device to help diabetics measure sugar levels. Wall Street Journal. March 23, 2001. Accessed February 9, 2021. https://www.wsj.com/articles/SB985288166651518746
- 39. Tierney MJ, Tamada JA, Potts RO, et al. The GlucoWatch biographer: a frequent automatic and noninvasive glucose monitor. Ann Med. 2000;32(9):632-641. doi: 10.3109/07853890009002034 [DOI] [PubMed] [Google Scholar]
- 40. Statland BE. Glucowatch automatic glucose biographer approval order statement. March 22, 2001. Accessed March 1, 2021. https://www.accessdata.fda.gov/cdrh_docs/pdf/P990026A.pdf
- 41. Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002;18(suppl 1):S49-S53. doi: 10.1002/dmrr.210 [DOI] [PubMed] [Google Scholar]
- 42. Diabetes Research in Children Network (DirecNet) Study Group. Youth and parent satisfaction with clinical use of the GlucoWatch G2 Biographer in the management of pediatric type 1 diabetes. Diabetes Care. 2005;28(8):1929-1935. doi: 10.2337/diacare.28.8.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saur NM, England MR, Menzie W, et al. Accuracy of a novel noninvasive transdermal continuous glucose monitor in critically ill patients. J Diabetes Sci Technol. 2014;8(5):945-950. doi: 10.1177/1932296814536138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Echo Therapeutics, Inc. Echo Therapeutics Begins Testing of NextGen CGM System Components. Cision PR Newswire. June 29, 2016. Accessed December 17, 2020. https://www.prnewswire.com/news-releases/echo-therapeutics-begins-testing-of-nextgen-cgm-system-components-300291837.html
- 45. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med J Br Diabet Assoc. 2010;27(2):136-142. doi: 10.1111/j.1464-5491.2009.02894.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soares M-S-M, Batista-Filho M-M-V, Pimentel M-J, Passos I-A, Chimenos-Küstner E. Determination of salivary glucose in healthy adults. Med Oral Patol Oral Cirugia Bucal. 2009;14(10):e510-e513. doi: 10.4317/medoral.14.e510 [DOI] [PubMed] [Google Scholar]
- 47. Jurysta C, Bulur N, Oguzhan B, et al. Salivary glucose concentration and excretion in normal and diabetic subjects. J Biomed Biotechnol. 2009;2009:430426. doi: 10.1155/2009/430426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. March WF, Mueller A, Herbrechtsmeier P. Clinical trial of a noninvasive contact lens glucose sensor. Diabetes Technol Ther. 2004;6(6):782-789. doi: 10.1089/dia.2004.6.782 [DOI] [PubMed] [Google Scholar]
- 49. Leblanc JM, Haas CE, Vicente G, Colon LA. Evaluation of lacrimal fluid as an alternative for monitoring glucose in critically ill patients. Intensive Care Med. 2005;31(10):1442-1445. doi: 10.1007/s00134-005-2747-5 [DOI] [PubMed] [Google Scholar]
- 50. Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011;5(5):1068-1076. doi: 10.1177/193229681100500507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teymourian H, Barfidokht A, Wang J. Electrochemical glucose sensors in diabetes management: an updated review (2010-2020). Chem Soc Rev. 2020;49(21):7671-7709. doi: 10.1039/d0cs00304b [DOI] [PubMed] [Google Scholar]
- 52. Johannessen E, Krushinitskaya O, Sokolov A, et al. Toward an injectable continuous osmotic glucose sensor. J Diabetes Sci Technol. 2010;4(4):882-892. doi: 10.1177/193229681000400417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Klonoff DC. Overview of fluorescence glucose sensing: a technology with a bright future. J Diabetes Sci Technol. 2012;6(6):1242-1250. doi: 10.1177/193229681200600602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nielsen JK, Christiansen JS, Kristensen JS, et al. Clinical evaluation of a transcutaneous interrogated fluorescence lifetime-based microsensor for continuous glucose reading. J Diabetes Sci Technol. 2009;3(1):98-109. doi: 10.1177/193229680900300111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kropff J, Choudhary P, Neupane S, et al. Accuracy and longevity of an implantable continuous glucose sensor in the PRECISE study: a 180-day, prospective, multicenter, pivotal trial. Diabetes Care. 2017;40(1):63-68. doi: 10.2337/dc16-1525 [DOI] [PubMed] [Google Scholar]
- 56. Eversense XL. user guide: a guide for using the Eversense XL continuous glucose monitoring system. Accessed February 9, 2021. https://global.eversensediabetes.com/sites/default/files/2019-09/LBL-1402-28-001_Rev_B_Eversense_User_Guide_mgdL_UAE_Web.pdf
- 57. Senseonics submits PMA application for 180-day Eversense system to the FDA. BioSpace. October 5, 2020. Accessed February 9, 2021. https://www.biospace.com/article/senseonics-submits-pma-application-for-180-day-eversense-system-to-the-fda/
- 58. Eversense Continuous Glucose Monitoring. Long-term continuous glucose monitor. 2019. Accessed July 15, 2020. https://www.eversensediabetes.com/
- 59. Basu A, Dube S, Veettil S, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol. 2015;9(1):63-68. doi: 10.1177/1932296814554797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmelzeisen-Redeker G, Schoemaker M, Kirchsteiger H, Freckmann G, Heinemann L, Del Re L. Time delay of CGM sensors: relevance, causes, and countermeasures. J Diabetes Sci Technol. 2015;9(5):1006-1015. doi: 10.1177/1932296815590154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cobelli C, Schiavon M, Dalla Man C, Basu A, Basu R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: insight from an in silico study. Diabetes Technol Ther. 2016;18(8):505-511. doi: 10.1089/dia.2016.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moyer J, Wilson D, Finkelshtein I, Wong B, Potts R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol Ther. 2012;14(5):398-402. doi: 10.1089/dia.2011.0262 [DOI] [PubMed] [Google Scholar]
- 63. Yamaguchi M, Mitsumori M, Kano Y. Noninvasively measuring blood glucose using saliva. IEEE Eng Med Biol Mag Q Mag Eng Med Biol Soc. 1998;17(3):59-63. doi: 10.1109/51.677170 [DOI] [PubMed] [Google Scholar]
- 64. La Belle JT, Adams A, Lin C-E, Engelschall E, Pratt B, Cook CB. Self-monitoring of tear glucose: the development of a tear based glucose sensor as an alternative to self-monitoring of blood glucose. Chem Commun Camb Engl. 2016;52(59):9197-9204. doi: 10.1039/c6cc03609k [DOI] [PubMed] [Google Scholar]
- 65. Cameron BD, Baba JS, Coté GL. Measurement of the glucose transport time delay between the blood and aqueous humor of the eye for the eventual development of a noninvasive glucose sensor. Diabetes Technol Ther. 2001;3(2):201-207. doi: 10.1089/152091501300209552 [DOI] [PubMed] [Google Scholar]
- 66. POCT05Ed2. Performance Metrics for Continuous Interstitial Glucose Monitoring. 2nd ed. Clinical & Laboratory Standards Institute. Accessed February 9, 2021. https://clsi.org/standards/products/point-of-care-testing/documents/poct05/ [Google Scholar]
- 67. Sinha M, McKeon KM, Parker S, et al. A comparison of time delay in three continuous glucose monitors for adolescents and adults. J Diabetes Sci Technol. 2017;11(6):1132-1137. doi: 10.1177/1932296817704443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21(6):313-321. doi: 10.1089/dia.2018.0364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405-2409. doi: 10.2337/diacare.26.8.2405 [DOI] [PubMed] [Google Scholar]
- 70. Heinemann L. Finger pricking and pain: a never ending story. J Diabetes Sci Technol. 2008;2(5):919-921. doi: 10.1177/193229680800200526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Paret M, Barash G, Rachmiel M. “Out of the box” solution for skin problems due to glucose-monitoring technology in youth with type 1 diabetes: real-life experience with fluticasone spray. Acta Diabetol. 2020;57(4):419-424. doi: 10.1007/s00592-019-01446-y [DOI] [PubMed] [Google Scholar]
- 72. Pleus S, Ulbrich S, Zschornack E, Kamann S, Haug C, Freckmann G. Documentation of skin-related issues associated with continuous glucose monitoring use in the scientific literature. Diabetes Technol Ther. 2019;21(10):538-545. doi: 10.1089/dia.2019.0171 [DOI] [PubMed] [Google Scholar]
- 73. Kamann S, Aerts O, Heinemann L. Further evidence of severe allergic contact dermatitis from isobornyl acrylate while using a continuous glucose monitoring system. J Diabetes Sci Technol. 2018;12(3):630-633. doi: 10.1177/1932296818762946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Svedman C, Ulriksdotter J, Lejding T, Bruze M, Mowitz M. Changes in adhesive ingredients in continuous glucose monitoring systems may induce new contact allergy pattern. Contact Dermatitis. Published online January 9, 2021. doi: 10.1111/cod.13781 [DOI] [PubMed] [Google Scholar]
- 75. Jina A, Tierney MJ, Tamada JA, et al. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J Diabetes Sci Technol. 2014;8(3):483-487. doi: 10.1177/1932296814526191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wermeling DP, Banks SL, Hudson DA, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058-2063. doi: 10.1073/pnas.0710355105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Murota H, Yamaga K, Ono E, Murayama N, Yokozeki H, Katayama I. Why does sweat lead to the development of itch in atopic dermatitis? Exp Dermatol. 2019;28(12):1416-1421. doi: 10.1111/exd.13981 [DOI] [PubMed] [Google Scholar]
- 78. Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Res Clin Pract. 2020;170:108502. doi: 10.1016/j.diabres.2020.108502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol. 2014;8(5):930-936. doi: 10.1177/1932296814546025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11(6):1063-1069. doi: 10.1177/1932296817723260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin YK, Groat D, Chan O, et al. Alarm settings of continuous glucose monitoring systems and associations to glucose outcomes in type 1 diabetes. J Endocr Soc. 2020;4(1):bvz005. doi: 10.1210/jendso/bvz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klonoff DC, Shang T, Zhang J. Automated insulin dosing systems or automated insulin delivery systems? It is time for consistency. J Diabetes Sci Technol. 2021;15(2):211-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol. 2020;14(6):1035-1064. doi: 10.1177/1932296820954163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. 510(k) Premarket Notification. Accessed March 5, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm
- 85. Welsh Company Makes Breakthrough in Diabetes Management. Business News Wales. March 8, 2020. Accessed December 14, 2020. https://businessnewswales.com/welsh-company-makes-breakthrough-in-diabetes-management/
- 86. Afon Technology. Glucose monitoring. Accessed December 17, 2020. https://afontechnology.com/
- 87. Neale R. No more “prick and stick:” Alertgy working on noninvasive glucometer for diabetes patients. Florida Today. Accessed December 14, 2020. https://www.floridatoday.com/story/news/2018/12/18/melbourne-diabetes-startup-alertgy-named-one-30-fastest-growing-companies-watch/2312300002/
- 88. Technology. AlertgyTM. Accessed December 14, 2020. https://www.alertgy.com/technology/
- 89. 39 Potential Continuous Glucose Monitors Coming Soon. Healthline. February 20, 2020. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/39-new-cgms-for-diabetes
- 90. Apple patent hints at non-invasive glucose monitoring tech for Apple Watch. AppleInsider. Accessed December 14, 2020. https://appleinsider.com/articles/18/08/23/apple-patent-suggests-work-on-non-invasive-glucose-monitoring-tech
- 91. Kangas MM, Arbore MA, Simon DI, Bishop MJ, Hillendahl JW, Chen R. Reference switch architectures for noncontact sensing of substances. August 23, 2018. Accessed February 5, 2021. https://patents.google.com/patent/US20180238794A1/en?oq=20180238794
- 92. Non-Invasive Blood Glucose Monitoring Devices Market: New Study Offers Insights for 2017-2027. PharmiWeb.com. Accessed December 14, 2020. https://www.pharmiweb.com/press-release/2019-03-20/non-invasive-blood-glucose-monitoring-devices-market-new-study-offers-insights-for-2017-2027
- 93. Karstang T, Schoenfelder S. Wearable blood glucose sensor. April 15, 2020. Accessed February 5, 2021. https://patents.google.com/patent/EP3636141A1/en?q=glucose+monitor&assignee=prediktor+medical
- 94. Brolis develops laser sensor for non-invasive blood analysis - news. Compound Semiconductor. Accessed December 14, 2020. /article/109183/Brolis_develops_laser_sensor_for_non-invasive_blood_analysis [Google Scholar]
- 95. Vizbaras A, Vizbaras K, Simonyte leva, Roelkens G. Tunable hybrid II-V IV laser sensor system-on-a chip for real-time monitoring of a blood constituent concentration level. March 5, 2020. Accessed February 5, 2021. https://patents.google.com/patent/US20200069225A1/en?q=glucose&assignee=brolis&oq=brolis+glucose
- 96. 15 Healthcare Companies in Dallas Pioneering Innovations Across the Industry. Built In. Accessed January 4, 2021. https://builtin.com/dallas/healthcare-companies-in-dallas
- 97. Socrates Announces Patent for New Technique for Their Noninvasive Blood Glucose Monitor – Socrates Health Solutions. Accessed February 5, 2021. https://socrateshealthsolutions.com/socrates-announces-patent-for-new-technique-for-their-noninvasive-blood-glucose-monitor/
- 98. Bordelon M. Methods and apparatus for optical non-invasive blood glucose change indication. July 21, 2016. Accessed January 4, 2021. https://patents.google.com/patent/US20160206232A1/en?q=socrates+health+glucose+monitor&oq=socrates+health+glucose+monitor
- 99. Watch: This device uses light to help diabetics test glucose levels. BusinessInsider. Accessed December 14, 2020. https://www.businessinsider.co.za/this-company-has-developed-a-non-invasive-glucose-monitoring-watch-2018-12
- 100. Lubinski T, Plotka B, Janik S, Canini L, Mäntele W. Evaluation of a novel noninvasive blood glucose monitor based on mid-infrared quantum cascade laser technology and photothermal detection. J Diabetes Sci Technol. Published online July 5. doi: 10.1177/1932296820936634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Diamontech submits to FDA pre-submission application for non-invasive blood glucose meter D-base. DiaMonTech. September 9, 2020. Accessed January 20, 2021. https://www.diamontech.de/pressemeldungen-en/diamontech-submits-to-fda-pre-submission-application-for-non-invasive-blood-glucose-meter-d-base
- 102. DiaMonTech: non-invasive glucose measurement. Accessed December 15, 2020. https://www.diamontech.de/home
- 103. Mäntele W, Rafael MAP, Lieblein T, et al. Nicht-invasive stoffanalyse. October 30, 2019. Accessed February 5, 2021. https://patents.google.com/patent/EP3155401B1/en?q=glucose&assignee=diamontech&oq=diamontech+glucose
- 104. Danish RSP systems raises $5m series A for GlucoBeam light-based glucometer. MassDevice. July 8, 2016. Accessed December 14, 2020. https://www.massdevice.com/danish-rsp-systems-raises-5m-series-glucobeam-light-based-glucometer/
- 105. Pleus S, Schauer S, Jendrike N, et al. Proof of concept for a new Raman-based prototype for noninvasive glucose monitoring. J Diabetes Sci Technol. 2021;15(1):11-18. doi: 10.1177/1932296820947112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Patented Raman Spectroscopy. Accessed December 15, 2020. https://www.rspsystems.com/technology/
- 107. Ferreira R. Glucose monitoring startup GlucoActive from Poland wins pitch competition at Collision from Home. EU-Startups. June 26, 2020. Accessed December 14, 2020. https://www.eu-startups.com/2020/06/glucose-monitoring-startup-glucoactive-from-poland-wins-pitch-competition-at-collision-from-home/
- 108. Start-up Genki Vantage takes the sting out of blood glucose testing. PRUnderground. Accessed December 14, 2020. https://www.prunderground.com/start-up-genki-vantage-takes-the-sting-out-of-blood-glucose-testing/00182194/
- 109. About Gluco Quantum. Accessed December 14, 2020. https://www.glucoquantum.com/about
- 110. Quantum ® G Certification. Gluco Quantum. June 13, 2020. Accessed December 14, 2020. https://www.glucoquantum.com/post/certification
- 111. Diabetes CRF-20/11/2019 5 mins-Tops. Needle-free diabetes care: 8 devices that painlessly measure blood glucose. Labiotech.eu. November 20, 2019. Accessed December 14, 2020. https://www.labiotech.eu/diabetes/needle-free-glucose-monitoring-for-diabetes-medtech/
- 112. Key patent granted for Glucosense’s technology. NetScientific. July 15, 2015. Accessed February 8, 2021. https://netscientific.net/2015/07/15/key-patent-granted-for-glucosenses-technology/
- 113. Device that uses forearm to measure glucose developed. Latest news for the medical device industry. Med-Tech Innovation. December 3, 2019. Accessed December 14, 2020. https://www.med-technews.com/api/content/ce20b8e6-15c5-11ea-aed5-1244d5f7c7c6/
- 114. Inc IA. Integrity applications names Shalom Shushan as chief technology officer. GlobeNewswire News Room. November 5, 2020. Accessed December 14, 2020. http://www.globenewswire.com/news-release/2020/11/05/2121139/0/en/Integrity-Applications-Names-Shalom-Shushan-as-Chief-Technology-Officer.html
- 115. Integrity applications receives CE mark approval for multiple improvements to GlucoTrack® model DF-F. Integrity Applications. Accessed December 14, 2020. http://www.integrity-app.com/pressreleases/integrity-applications-receives-ce-mark-approval-for-multiple-improvements-to-glucotrack-model-df-f/
- 116. Lin T, Mayzel Y, Bahartan K. The accuracy of a non-invasive glucose monitoring device does not depend on clinical characteristics of people with type 2 diabetes mellitus. J Drug Assess. 2018;7(1):1-7. doi: 10.1080/21556660.2018.1423987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. 乔治·帕里卡洛斯, 塞莫斯·卡洛斯, 西姆·钱德拉·萨哈, 陈德才, 黄湘玉. 一种无创血糖检测装置及方法. September 7, 2016. Accessed February 8, 2021. https://patents.google.com/patent/CN105919601A/en?q=glucose+sensor&assignee=mediwise&oq=mediwise+glucose+sensor
- 118. Two Innovative Wearables Took Diabetes Control to the Next Level at CES 2020. Accessed December 14, 2020. https://www.healthtechzone.com/topics/healthcare/articles/2020/01/24/444307-two-innovative-wearables-took-diabetes-control-the-next.htm
- 119. Glutrac. Accessed December 15, 2020. https://add-care.net/
- 120. Corp WM& T. Launch of world’s first wearable, non-invasive, continuous, blood glucose estimation technology using WRMT’s smart wristband, Helo, will generate recurring revenues for WRMT. Accessed January 25, 2021. https://www.prnewswire.com/news-releases/launch-of-worlds-first-wearable-non-invasive-continuous-blood-glucose-estimation-technology-using-wrmts-smart-wristband-helo-will-generate-recurring-revenues-for-wrmt-300388644.html
- 121. HELO Extense FAQ. Accessed February 8, 2021. https://website.worldgn.com/wp-content/uploads/2019/02/Extense-FAQ-v1.1.pdf
- 122. Balajadia LF, Galdi F. Personal healthcare device. December 13, 2018. Accessed February 8, 2021. https://patents.google.com/patent/US20180353137A1/en?assignee=helo+corp
- 123. LifePlus announces world’s first non-invasive continous blood glucose monitoring wearable. May 16, 2018. Accessed December 14, 2020. https://www.businesswire.com/news/home/20180516005422/en/LifePlus-Announces-World%E2%80%99s-First-Non-Invasive-Continous-Blood-Glucose-Monitoring-Wearable
- 124. Startup LifePlus announces noninvasive CGM wearable currently in testing. MobiHealthNews. May 17, 2018. Accessed February 8, 2021. https://www.mobihealthnews.com/content/startup-lifeplus-announces-noninvasive-cgm-wearable-currently-testing
- 125. Inc M. UPDATE: Movano Inc. exits stealth mode and secures $10M in additional funding to transform glucose monitoring with non-invasive technology. GlobeNewswire News Room. July 14, 2020. Accessed December 14, 2020. http://www.globenewswire.com/news-release/2020/07/14/2062224/0/en/UPDATE-Movano-Inc-Exits-Stealth-Mode-and-Secures-10M-in-Additional-Funding-to-Transform-Glucose-Monitoring-with-Non-Invasive-Technology.html
- 126. Movano Inc. Accessed December 15, 2020. https://movano.com/solution
- 127. One of the patents that the “Secret Apple Team” was working on for diabetes testing for Apple Watch surfaces. Patently Apple. Accessed January 4, 2021. https://www.patentlyapple.com/patently-apple/2018/08/one-of-the-patents-that-the-secret-apple-team-was-working-on-for-diabetes-testing-for-apple-watch-surfaces.html
- 128. Islam MN. Near-infrared lasers for non-invasive monitoring of glucose, ketones, HBA1C, and other blood constituents. October 29, 2015. Accessed February 8, 2021. https://patents.google.com/patent/US20150305658A1/en?q=glucose+monitor&assignee=omni+medsci&oq=omni+medsci+glucose+monitor
- 129. A closer look at biosensor technology at sensors USA 2019. IDTechEx. November 1, 2019. Accessed January 4, 2021. https://www.idtechex.com/en/research-article/a-closer-look-at-biosensor-technology-at-sensors-usa-2019/18604
- 130. Newberry RS. System and method for health monitoring using a non-invasive, multi-band biosensor. May 9, 2017. Accessed February 8, 2021. https://patents.google.com/patent/US9642578B2/en?q=glucose+monitor&assignee=sanmina&oq=sanmina+glucose+monitor
- 131. Ltd CM. CNOGA medical appointed ARTECH to distribute their non-invasive medical devices in Italy. Accessed December 14, 2020. https://www.prnewswire.com/news-releases/cnoga-medical-appointed-artech-to-distribute-their-non-invasive-medical-devices-in-italy-581493111.html
- 132. CNOGA medical appointed ARTECH to distribute their non-invasive medical devices in Italy - Cnoga medical. Accessed December 14, 2020. https://cnogacare.co/artech-distribute-non-invasive-devices-italy/
- 133. Pfützner A, Demircik F, Pfützner J, et al. System accuracy assessment of a combined invasive and noninvasive glucometer. J Diabetes Sci Technol. 2020;14(3):575-581. doi: 10.1177/1932296819883306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Know Labs signs research agreement with Mayo Clinic. MassDevice. July 21, 2020. Accessed December 15, 2020. https://www.massdevice.com/know-labs-signs-research-agreement-with-mayo-clinic/
- 135. Know Labs Granted Patent for its Bio-RFIDTM Technology. April 7, 2020. Accessed December 15, 2020. https://www.businesswire.com/news/home/20200407005221/en/Know-Labs-Granted-Patent-for-its-Bio-RFID%E2%84%A2-Technology
- 136. Gudibande RR, Radhakrishnan S. Replaceable sensor systems and methods. September 26, 2019. Accessed February 8, 2021. https://patents.google.com/patent/WO2019183279A1/en?q=glucose+monitor&assignee=graphwear
- 137. Health care innovators strive to make a difference. MIT News. Massachusetts Institute of Technology. Accessed December 15, 2020. https://news.mit.edu/2020/health-care-innovators-strive-to-make-difference-0123
- 138. Inc ET. Echo therapeutics announces new patent for its non-invasive sensing technology. Accessed February 8, 2021. https://www.prnewswire.com/news-releases/echo-therapeutics-announces-new-patent-for-its-non-invasive-sensing-technology-300288439.html
- 139. Gbs looking to raise $20M in Nasdaq IPO to develop noninvasive SARS-CoV-2 diagnostic. Accessed December 15, 2020. https://www.bioworld.com/articles/499955-gbs-looking-to-raise-20m-in-nasdaq-ipo-to-develop-noninvasive-sars-cov-2-diagnostic
- 140. FAQs. GBS Inc. Accessed December 15, 2020. https://gbs.inc/faqs/
- 141. Nemaura Medical expands painless sugarBEAT glucose monitor footprint in Europe, and eyes entry into US market. Proactiveinvestors NA. August 4, 2020. Accessed December 15, 2020. https://www.proactiveinvestors.com/companies/news/925727/nemaura-medical-expands-painless-sugarbeat-glucose-monitor-footprint-in-europe-and-eyes-entry-into-us-market-925727.html
- 142. Nemaura announces CE mark approval of SugarBEAT®. Nemaura Medical. May 29, 2019. Accessed December 15, 2020. https://nemauramedical.com/nemaura-announces-ce-mark-approval-of-sugarbeat/
- 143. Nemaura medical submits PMA application for sugarBEAT® to U.S. FDA. BioSpace. Published July 7, 2020. Accessed January 12, 2021. https://www.biospace.com/article/nemaura-medical-submits-pma-application-for-sugarbeat-to-u-s-fda/
- 144. NovioSense announces positive clinical trial results of tear glucose measurement technology. Medgadget. Accessed December 15, 2020. https://www.medgadget.com/2018/10/noviosense-announces-positive-clinical-trial-results-of-tear-glucose-measurement-technology.html
- 145. Geelhoed-Duijvestijn P, Vegelyte D, Kownacka A, Anton N, Joosse M, Wilson C. Performance of the prototype noviosense noninvasive biosensor for tear glucose in type 1 diabetes. J Diabetes Sci Technol. Published online October 23, 2020. doi: 10.1177/1932296820964844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. admin. CGM for 2020 – will new entrants reduce costs? Diabettech - Diabetes and Technology. Published December 10, 2019. Accessed December 16, 2020. https://www.diabettech.com/cgm/cgm-for-2020-will-new-entrants-reduce-costs/
- 147. Continuous Glucose Monitoring System-MicroTech Medical Inc. Accessed December 16, 2020. http://www.microtechmd.com/en/Products/CGMS
- 148. Inc B. Biolinq announces results from first-in-man clinical studies showing feasibility of a novel, minimally invasive approach to continuous glucose monitoring in the dermis. Accessed December 15, 2020. https://www.prnewswire.com/news-releases/biolinq-announces-results-from-first-in-man-clinical-studies-showing-feasibility-of-a-novel-minimally-invasive-approach-to-continuous-glucose-monitoring-in-the-dermis-301076992.html
- 149. Biolinq. Intelligent continuous glucose monitoring system. Accessed December 15, 2020. https://www.biolinq.me/
- 150. Lee MH, Kim MH, Lee UK, et al. Electrochemical biosensor with improved accuracy. September 5, 2017. Accessed February 8, 2021. https://patents.google.com/patent/US9753004B2/en?q=glucose+monitor&assignee=i-sens
- 151. Inc WT. WaveForm Technologies Inc. Awarded CE mark approval for their continuous glucose monitoring system. Accessed December 15, 2020. https://www.prnewswire.com/news-releases/waveform-technologies-inc-awarded-ce-mark-approval-for-their-continuous-glucose-monitoring-system-300952002.html
- 152. Rebec M, Cai K, Dutt-Ballerstadt R, Anderson E. A prospective multicenter clinical performance evaluation of the C-CGM system. J Diabetes Sci Technol. Published online October 21, 2020. doi: 10.1177/1932296820964574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ascensia diabetes care announces partnership; new CGM products. dLife. January 24, 2019. Accessed December 15, 2020. https://dlife.com/ascensia-diabetes-care-announces-partnership-new-cgm-products/
- 154. Chinese CGM maker POCTech raises $15m in Series B. MassDevice. April 17, 2017. Accessed December 15, 2020. https://www.massdevice.com/chinese-cgm-maker-poctech-raises-15m-series-b/
- 155. Why Dexcom diabetes CGM technology is so hot. Healthline. September 16, 2020. Accessed December 14, 2020. https://www.healthline.com/diabetesmine/dexcom-technology-overview
- 156. U.S. Food and Drug Administration. FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices. FDA. March 24, 2020. Accessed December 15, 2020. https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review
- 157. Dexcom lands CE Mark for G6 glucose monitor. MassDevice. Published June 9, 2020. Accessed December 15, 2020. https://www.massdevice.com/dexcom-lands-ce-mark-for-g6-glucose-monitor/
- 158. Isaacson B, Kaufusi S, Sorensen J, et al. Demonstrating the clinical impact of continuous glucose monitoring within an integrated healthcare delivery system. J Diabetes Sci Technol. Published online September 16, 2020. doi: 10.1177/1932296820955228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Discover Dexcom continuous glucose monitoring (CGM) technology. Dexcom Provider. Accessed December 15, 2020. https://provider.dexcom.com/dexcom-cgm
- 160. Crumly J. DexCom has a lot to prove in 2021. The Motley Fool. December 9, 2020. Accessed January 4, 2021. https://www.fool.com/investing/2020/12/09/dexcom-has-a-lot-to-prove-in-2021/
- 161. Incorporated G. GlySens incorporated closes incremental $15 million financing supporting on-going clinical evaluations and first-in-man study of the third generation Eclipse® 3 ICGM® system. Accessed January 4, 2021. https://www.prnewswire.com/news-releases/glysens-incorporated-closes-incremental-15-million-financing-supporting-on-going-clinical-evaluations-and-first-in-man-study-of-the-third-generation-eclipse-3-icgm-system-301074892.html
- 162. Routh T, Lucisano J, Markle W, Perkins M. Analyte sensor receiver apparatus and methods. June 7, 2018. Accessed February 8, 2021. https://patents.google.com/patent/US20180153450A1/en?q=glucose+monitor&assignee=glysens&oq=glysens+glucose+monitor
- 163. Struggling Senseonics allies with blood glucose monitoring giant Ascensia, taps much-needed $80M. MedTech Dive. Accessed December 15, 2020. https://www.medtechdive.com/news/struggling-senseonics-allies-with-blood-glucose-monitoring-giant-ascensia/583270/
- 164. Premarket Approval (PMA). Accessed February 9, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P160048
- 165. Senseonics announces CE mark approval of the Eversense® CGM system. Accessed December 15, 2020. https://www.senseonics.com/investor-relations/news-releases/2016/05-10-2016-224122137
- 166. Fokkert M, van Dijk PR, Edens MA, et al. Performance of the Eversense versus the Free Style Libre Flash glucose monitor during exercise and normal daily activities in subjects with type 1 diabetes mellitus. BMJ Open Diabetes Res Care. 2020;8(1):e001193. doi: 10.1136/bmjdrc-2020-001193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. FDA approves nonadjunctive indication for Eversense CGM. Accessed February 9, 2021. https://www.healio.com/news/endocrinology/20190607/fda-approves-nonadjunctive-indication-for-eversense-cgm
- 168. Invest in the future of blood glucose monitoring • EyeSense GmbH [EN]. EyeSense GmbH [EN]. Accessed February 8, 2021. https://en.eyesense.com/invest-in-the-future-of-blood-glucose-monitoring/
- 169. Abbott’s FreeStyle® Libre 14 day system now available in U.S. for hospitalized patients with diabetes during COVID-19 pandemic. Accessed December 15, 2020. https://www.prnewswire.com/news-releases/abbotts-freestyle-libre-14-day-system-now-available-in-us-for-hospitalized-patients-with-diabetes-during-covid-19-pandemic-301037529.html
- 170. Health C for D and R. Freestyle Libre 14 day flash glucose monitoring system - P160030/S017. FDA. March 29, 2019. Accessed December 15, 2020. https://www.fda.gov/medical-devices/recently-approved-devices/freestyle-libre-14-day-flash-glucose-monitoring-system-p160030s017
- 171. Abbott receives CE mark for FreeStyle® Libre, a revolutionary glucose monitoring system for people with diabetes. Abbott MediaRoom. Accessed December 15, 2020. https://abbott.mediaroom.com/2014-09-03-Abbott-Receives-CE-Mark-for-FreeStyle-Libre-a-Revolutionary-Glucose-Monitoring-System-for-People-with-Diabetes
- 172. Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre flash glucose monitoring systems in adults. J Endocr Soc. 2018;2(12):1320-1337. doi: 10.1210/js.2018-00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Abbott’s Freestyle Libre system becomes first CGM to be FDA cleared for use without fingersticks. MobiHealthNews. September 27, 2017. Accessed December 15, 2020. https://www.mobihealthnews.com/content/abbotts-freestyle-libre-system-becomes-first-cgm-be-fda-cleared-use-without-fingersticks
- 174. Writer PS. FreeStyle Libre 2 sensors added to drug tariff and DND list. Latest Pharmacy News. Business Magazine - Pharmacy Business. October 28, 2020. Accessed December 15, 2020. https://www.pharmacy.biz/freestyle-libre-2-sensors-added-to-drug-tariff-and-dnd-list/
- 175. 510(k) Premarket notification FreeStyle Libre 2 flash glucose monitoring system. Accessed December 15, 2020. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K193371
- 176. Abbott’s FreeStyle® Libre 2, with optional real-time alarms, secures CE mark for use in Europe. Abbott MediaRoom. Accessed December 15, 2020. https://abbott.mediaroom.com/2018-10-01-Abbott-s-FreeStyle-R-Libre-2-with-Optional-Real-Time-Alarms-Secures-CE-Mark-for-Use-in-Europe
- 177. Denham D. A head-to-head comparison study of the first-day performance of two factory-calibrated CGM systems. J Diabetes Sci Technol. 2020;14(2):493-495. doi: 10.1177/1932296819895505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. NEWS: FDA OKs FreeStyle Libre 2 with real-time glucose alerts. Healthline. June 16, 2020. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/news-freestyle-libre-2-gets-approved
- 179. Abbott scores European CE mark for its FreeStyle Libre 3. MobiHealthNews. September 28, 2020. Accessed December 15, 2020. https://www.mobihealthnews.com/news/abbott-scores-european-ce-mark-its-freestyle-libre-3
- 180. O’Neill S. News and views: update on technologies, medicines and treatments including Libre 3, Minimed 780G and Glucomen Day continuous glucose monitoring. Diabet Med. 2021;38(1):e14451. doi: 10.1111/dme.14451 [DOI] [Google Scholar]
- 181. Menarini IFR. Continuous blood glucose monitoring: the Menarini diagnostics digital “patch” now available. PRNewswire. June 25, 2020. Accessed December 15, 2020. https://www.prnewswire.co.uk/news-releases/continuous-blood-glucose-monitoring-the-menarini-diagnostics-digital-patch-now-available-880146146.html
- 182. WaveForm Diabetes (formerly Agamatrix) and Bayer CGM. Desang Diabetes Services. June 16, 2020. Accessed December 15, 2020. https://www.desang.net/2020/06/waveform-diabetes-formerly-agamatrix-and-bayer-cgm/
- 183. China Money AI. Vertex Ventures Leads Series B Round In Chinese Glucose Monitoring Device Developer Infinovo. China Money Network. July 29, 2020. Accessed December 15, 2020. https://www.chinamoneynetwork.com/2020/07/29/vertex-ventures-leads-series-b-round-in-chinese-glucose-monitoring-device-developer-infinovo
- 184. (1)Infinovo Medical Co Limited: overview. LinkedIn. Accessed December 15, 2020. https://www.linkedin.com/company/infinovo-medical-co-limited/
- 185. GluSense gets funding for implantable biosensor it says could be a year-long CGM. MobiHealthNews. February 15, 2017. Accessed December 16, 2020. https://www.mobihealthnews.com/content/glusense-gets-funding-implantable-biosensor-it-says-could-be-year-long-cgm
- 186. Glyde CGM. GluSense. June 8, 2020. Accessed December 16, 2020. https://glusensemedical.com/glyde-cgm-2/
- 187. Belgian startup Indigo Diabetes scores €38 million to develop its under the skin CGM sensor. MobiHealthNews. July 27, 2020. Accessed December 15, 2020. https://www.mobihealthnews.com/news/belgian-startup-indigo-diabetes-scores-38-million-develop-its-under-skin-cgm-sensor
- 188. Delbeke D, Baets R, Bogaerts W, Ryckeboer EMP. Implantable sensor. October 23, 2018. Accessed February 8, 2021. https://patents.google.com/patent/US10105081B2/en?q=glucose+monitor&assignee=indigo+
- 189. Seed-sized sensor could redefine glucose monitoring. Healthline. Published January 18, 2018. Accessed December 16, 2020. https://www.healthline.com/diabetesmine/implantable-sesame-seed-sized-cgm
- 190. Mujeeb-U-Rahman M, Nazari MH, Sencan M, Antwerp WV. A novel needle-injectable millimeter scale wireless electrochemical glucose sensing platform for artificial pancreas applications. Sci Rep. 2019;9(1):17421. doi: 10.1038/s41598-019-53680-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Nazari MH, Mujeeb-U-Rahman M, Sencan M. Wireless sensing platform for multi-analyte sensing. June 11, 2020. Accessed February 8, 2021. https://patents.google.com/patent/US20200178801A1/en?q=glucose+monitor&assignee=integrated+medical+sensors
- 192. One Drop Acquires Sano. One Drop. Accessed December 16, 2020. https://onedrop.today/blogs/press-releases/one-drop-acquires-sano
- 193. Drop O. One Drop Acquires Sano. Accessed December 17, 2020. https://www.prnewswire.com/news-releases/one-drop-acquires-sano-301038963.html
- 194. Pushpala A, Szmodis A, Chapman M, Hall W, Miller S, Hafezi H. On-body microsensor for biomonitoring. September 18, 2014. Accessed December 16, 2020. https://patents.google.com/patent/US20140259652A1/en
- 195. Continuous Glucose Measuring Smartwatch, K’Watch glucose, expected soon. Medgadget. Accessed December 15, 2020. https://www.medgadget.com/2018/11/continuous-glucose-measuring-smartwatch-kwatch-glucose-expected-soon.html
- 196. Pierart L. Système de surveillance corporelle par électrophorèse. November 25, 2020. Accessed February 8, 2021. https://patents.google.com/patent/EP3740118A1/en?q=glucose+monitor&assignee=pkvitality&oq=pkvitality+glucose+monitor
- 197. Pierart L. Système de surveillance corporelle avec adhésif. July 25, 2019. Accessed February 8, 2021. https://patents.google.com/patent/WO2019141743A1/en?q=glucose+monitor&assignee=pkvitality&oq=pkvitality+glucose+monitor
- 198. Helton K, Mcmillan WA, Wisniewski N. Tissue-integrating sensors. January 23, 2020. Accessed February 8, 2021. https://patents.google.com/patent/AU2017264987B2/en?q=glucose+monitor&assignee=profusa&oq=profusa+glucose+monitor
- 199. McMillan WA, Wisniewski N. Method and system for directing a localized biological response to an implant. March 10, 2020. Accessed February 8, 2021. https://patents.google.com/patent/US10583308B2/en?q=glucose+monitor&assignee=profusa&oq=profusa+glucose+monitor
- 200. plc M. Medtronic GuardianTM connect continuous glucose monitoring (CGM) system for diabetes now compatible with android devices. GlobeNewswire News Room. Published May 22, 2020. Accessed December 15, 2020. http://www.globenewswire.com/news-release/2020/05/22/2037605/0/en/Medtronic-Guardian-Connect-Continuous-Glucose-Monitoring-CGM-System-for-Diabetes-Now-Compatible-with-Android-Devices.html
- 201. Health C for D and R. Guardian connect system – P160007. FDA. February 9, 2019. Accessed December 15, 2020. https://www.fda.gov/medical-devices/recently-approved-devices/guardian-connect-system-p160007
- 202. Medtronic wins CE Mark for guardian connect mobile CGM. MassDevice. July 19, 2016. Accessed December 15, 2020. https://www.massdevice.com/medtronic-wins-ce-mark-guardian-connect-mobile-cgm/
- 203. Sadhu AR, Serrano IA, Xu J, et al. Continuous glucose monitoring in critically ill patients with COVID-19: results of an emergent pilot study. J Diabetes Sci Technol. 2020;14(6):1065-1073. doi: 10.1177/1932296820964264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Important Safety Information. Medtronic Diabetes. December 14, 2012. Accessed February 9, 2021. https://www.medtronicdiabetes.com/important-safety-information
- 205. Hot New Technology from Medtronic Diabetes. Healthline. July 1, 2020. Accessed January 4, 2021. https://www.healthline.com/diabetesmine/medtronic-diabetes-technology
- 206. Botvinick E, Bremer TM. Implantable biosensor and methods of use thereof. June 7, 2016. Accessed February 8, 2021. https://patents.google.com/patent/US9357952B2/en?q=glucose+monitor&assignee=Metronom+Health+Inc
- 207. Bremer TM, Bartholomeusz DA. Analyte sensors and methods of manufacturing analyte sensors. January 9, 2020. Accessed February 8, 2021. https://patents.google.com/patent/US20200008719A1/en?q=glucose+monitor&assignee=Metronom+Health+Inc
- 208. New diabetes tech will monitor both glucose and ketones. Healthline. November 23, 2020. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/new-diabetes-sensor-glucose-ketones
- 209. Shah R, Liang BC, Bowman E, Wolfe K. Multi-analyte continuous glucose monitoring. August 15, 2019. Accessed February 8, 2021. https://patents.google.com/patent/US20190246962A1/en?q=glucose+monitor&assignee=percusense&oq=percusense+glucose+monitor
- 210. Inc L. LifeScan enters into agreement with Sanvita medical to market and distribute continuous glucose monitoring (CGM) devices. Accessed December 15, 2020. https://www.prnewswire.com/news-releases/lifescan-enters-into-agreement-with-sanvita-medical-to-market-and-distribute-continuous-glucose-monitoring-cgm-devices-300853566.html
- 211. Peterson TH, Winarta H, Florindi A. Continuous glucose monitoring system and method. January 16, 2020. Accessed February 8, 2021. https://patents.google.com/patent/US20200015720A1/en?q=glucose+monitor&assignee=sanvita&oq=sanvita+glucose+monitor
- 212. AG B. Lifecare floats on Oslo stock exchange. Accessed December 15, 2020. https://european-biotechnology.com/up-to-date/latest-news/news/lifecare-floats-on-oslo-stock-exchange.html
- 213. Lifecare - about. Accessed February 8, 2021. https://www.lifecare.no/about-lifecare
- 214. Glucovation: A CGM sensor for the non-diabetes mainstream? Healthline. May 21, 2014. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/glucovation-cgm
- 215. Our Technology. Glucovation. Accessed February 8, 2021. http://glucovation.com/index.php/our-technology/
- 216. New for diabetes: combined insulin infusion & CGM sensor! Healthline. December 12, 2019. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/combined-infusion-set-and-cgm-sensor
- 217. Jacobs PG, Tyler NS, Vanderwerf SM, et al. Measuring glucose at the site of insulin delivery with a redox-mediated sensor. Biosens Bioelectron. 2020;165:112221. doi: 10.1016/j.bios.2020.112221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Patents. Pacific diabetes technologies. Accessed February 8, 2021. https://www.pacificdt.com/patents
- 219. Zhou J, Zhang S, Li L, et al. Performance of a new real-time continuous glucose monitoring system: A multicenter pilot study. J Diabetes Investig. 2018;9(2):286-293. doi: 10.1111/jdi.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Yang C. Analyte sensing system. September 17, 2019. Accessed February 8, 2021. https://patents.google.com/patent/US10413224B2/en?q=glucose+monitor&assignee=medtrum
- 221. Apple is testing a noninvasive blood glucose monitor: CNBC. FierceBiotech. Accessed December 15, 2020. https://www.fiercebiotech.com/medical-devices/apple-testing-a-noninvasive-blood-glucose-monitor-cnbc
- 222. Quereshi MRA, Shillingford J, Handy CM, Chaudhry MS. Novel microwave based non-invasive glucose device. Unified Citation Journals. April 12, 2020. Accessed December 15, 2020. https://www.ucjournals.com/diabetes/novel-microwave-based-non-invasive-glucose-device/
- 223. AnnNIGM en. Accessed December 16, 2020. https://annnigm.com/en
- 224. United States Patent Application: 0180238794. Accessed December 15, 2020. http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.html&r=16&p=1&f=G&l=50&d=PG01&S1=(apple.AANM.+AND+20180823.PD.)&OS=aanm/apple+and+pd/8/23/2018&RS=(AANM/apple+AND+PD/20180823)
- 225. Prediktor Medical. BioMKR: Non Invasive CGM, a glucose smartwatch. prediktormedical. Accessed December 15, 2020. https://www.prediktormedical.com
- 226. FAQ. prediktormedical. Accessed December 15, 2020. https://www.prediktormedical.com/faq
- 227. BROLIS. Blood analysis sensor. Accessed December 15, 2020. https://brolis-sensor.com/
- 228. Science & Tech – Socrates Health Solutions. Accessed January 4, 2021. https://socrateshealthsolutions.com/science-and-tech/
- 229. Pleus S, Wintergerst P, Waldenmaier D, et al. Measurement accuracy of a newly developed prototype system for non-invasive glucose monitoring. DTM 2019 Abstracts. J Diabetes Sci Technol. 2020;14(2):361-492, A90. [Google Scholar]
- 230. GlucoFit. GlucoActive. Accessed December 15, 2020. https://gluco-active.com/glucofit/
- 231. Shenzhen Carelife Health Technology Co., Ltd. Clinical data study report of a non-invasive glucose monitor. March 10, 2020. Accessed December 15, 2020. https://cc65bd7d-0a22-48ec-98d6-1ba1faa1dead.filesusr.com/ugd/5f45ff_2dd728ae6a3f4399aaf8ced2089f4a51.pdf?fbclid=IwAR1Zat_kgNFPew8waQqaniCGBsWtkzpMZuh9ovM4w_Npe1EsPCud-CI5wUI
- 232. University of Leeds. Glucosense. Accessed December 15, 2020. https://www.leeds.ac.uk/site/custom_scripts/profile-single.php?profileTypeID=&categoryID=2000&profileID=116
- 233. GlucoStation. GlucoActive. Accessed December 15, 2020. https://gluco-active.com/glucostation/
- 234. Bahartan K, Horman K, Gal A, Drexler A, Mayzel Y, Lin T. Assessing the performance of a noninvasive glucose monitor in people with type 2 diabetes with different demographic profiles. J Diabetes Res. 2017;2017:4393497. doi: 10.1155/2017/4393497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Pfützner A, Strobl S, Sachsenheimer D, Lier A, Ramljak S, Demircik F. Evaluation of the non invasive glucose monitoring device Glucotrack in patients with type 2 diabetes and subjects with prediabetes. J Diabetes Treat. December 8, 2019. Accessed January 27, 2021. https://www.gavinpublishers.com/articles/research-article/Journal-of-Diabetes-and-Treatment/evaluation-of-the-non-invasive-glucose-monitoring-device-glucotrack-in-patients-with-type-2-diabetes-and-subjects-with-prediabetes
- 236. GlucoTrack product benefits leaflet. Accessed January 27, 2021. http://glucotrack.com.au/development/images/content/GlucoTrackApp-Leaflet.pdf
- 237. GlucoWear. GlucoActive. Accessed December 15, 2020. https://gluco-active.com/glucowear/
- 238. Get involved. Accessed December 15, 2020. https://gluco-wise.com/
- 239. HELO Extense Brochure. Accessed February 9, 2021. https://website.worldgn.com/wp-content/uploads/2018/10/HELO-EXTENSE-BROCHURE-EN-web.pdf
- 240. How LifeLeaf works. LifePlus. Accessed December 15, 2020. https://www.lifeplus.ai/how-lifeleaf-works/
- 241. Leabman MA. Systems for radio wave based health monitoring that utilize data derived from amplitude and/or phase data. June 18, 2020. Accessed December 15, 2020. https://patents.google.com/patent/US20200187793A1/en?assignee=movano&oq=movano
- 242. Omni MedSci Inc.: Tech innovator likes to “play where the puck is going to be.” Crain’s Detroit Business. July 11, 2015. Accessed January 4, 2021. https://www.crainsdetroit.com/article/20150711/AWARDS1315/150719987/omni-medsci-inc-tech-innovator-likes-to-play-where-the-puck-is
- 243. The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes Conference Madrid, Spain—February 19–22, 2020. Diabetes Technol Ther. 2020;22(suppl 1):A1-A250. doi: 10.1089/dia.2020.2525.abstracts [DOI] [PubMed] [Google Scholar]
- 244. Pfützner A, Strobl S, Demircik F, et al. Evaluation of a new noninvasive glucose monitoring device by means of standardized meal experiments. J Diabetes Sci Technol. 2018;12(6):1178-1183. doi: 10.1177/1932296818758769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. CoG - Hybrid Glucometer. Cnoga Medical. Accessed December 15, 2020. https://cnogacare.co/hybridglucometer/
- 246. FAQ — Know Labs. Accessed December 15, 2020. https://www.knowlabs.co/faq
- 247. Inc GT. GraphWear. Accessed December 17, 2020. https://www.graphwear.co
- 248. GraphWear Technologies Inc. A clinical feasibility study of the bios device for continuous glucose monitoring in subjects with type 1 or type 2 diabetes mellitus. clinicaltrials.gov. 2020. Accessed December 30, 2020. https://clinicaltrials.gov/ct2/show/NCT04226846
- 249. gSense. Nutrix. Accessed December 15, 2020. https://nutrix.tech/gsense/
- 250. Chuang H, Hurley JP, Kost J. Transdermal analyte monitoring systems and methods for analyte detection. September 12, 2008. Accessed December 17, 2020. https://patents.google.com/patent/CA2680213A1/zh
- 251. The biosensor platform. GBS Inc. Accessed December 15, 2020. https://gbs.inc/the-biosensor-platform/
- 252. SugarBEAT: first no-needles glucose monitor. Healthline. October 25, 2019. Accessed December 15, 2020. https://www.healthline.com/diabetesmine/non-invasive-sugarbeat-cgm-diabetes
- 253. Kownacka AE, Vegelyte D, Joosse M, et al. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing a biopolymer coating. Biomacromolecules. 2018;19(11):4504-4511. doi: 10.1021/acs.biomac.8b01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. ID F. 005 Transmitter User Manual Microtech Medical (Hangzhou). FCC ID. Accessed December 16, 2020. https://fccid.io/2ATOV-005/User-Manual/UM-4349146
- 255. CGM. GlucoRx. Accessed December 16, 2020. https://www.glucorx.co.uk/cgm/
- 256. i-sens. i-SENS Company Presentation_Q3 2019. November 6, 2019. Accessed January 4, 2021. https://i-sens.com/?kboard_content_redirect=60
- 257. Rebec M, Anderson EM, Dutt-Ballerstadt R, Haidar A, Janez A. Accuracy evaluation of the waveform cascade CGM system vs. Dexcom G5 Sensors. Diabetes. 2018;67(suppl 1). doi: 10.2337/db18-81-LB [DOI] [Google Scholar]
- 258. User manual- POC Tech. Accessed December 15, 2020. http://www.poctechcorp.com/upload/files/CT100B%20System%20User%20Manual.pdf
- 259. About Us, Zhejiang POCTech Co., Ltd. Accessed December 15, 2020. http://www.poctechcorp.com/en/channels/273.html
- 260. Cai L, Ge W, Zhu Z, Zhao X, Li Z. Data Analysis and accuracy evaluation of a continuous glucose-monitoring device. J Sens. 2019;2019:1-8. doi: 10.1155/2019/4896862 [DOI] [Google Scholar]
- 261. U.S. Food and Drug Administration. Evaluation of automatic class III designation for Dexcom G6 continuous glucose monitoring system: Decision Summary DEN170088. Accessed December 15, 2020. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170088.pdf
- 262. Lucisano JY, Routh TL, Lin JT, Gough DA. Glucose monitoring in individuals with diabetes using a long-term implanted sensor/telemetry system and model. IEEE Trans Biomed Eng. 2017;64(9):1982-1993. doi: 10.1109/TBME.2016.2619333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Christiansen MP, Klaff LJ, Bailey TS, Brazg R, Carlson G, Tweden KS. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the PRECISION study. Diabetes Technol Ther. 2019;21(5):231-237. doi: 10.1089/dia.2019.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Lorenz C, Sandoval W, Mortellaro M. Interference assessment of various endogenous and exogenous substances on the performance of the eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2018;20(5):344-352. doi: 10.1089/dia.2018.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. FiberSense technology: continuous glucose monitoring • EyeSense GmbH [EN]. EyeSense GmbH [EN]. Accessed December 15, 2020. https://en.eyesense.com/fibersense-technology-continuous-glucose-monitoring/
- 266. Hoss U, Budiman ES. Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther. 2017;19(suppl 2):S44-S50. doi: 10.1089/dia.2017.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Abbott Diabetes Care. Freestyle Libre 14 Day user manual. 2018. Accessed January 28, 2021. https://freestyleserver.com/Payloads/IFU/2018/ART39764-001_rev-A-Web.pdf
- 268. Abbott Laboratories. FreeStyle Libre Safety Information. FreeStyle. Accessed April 12, 2021. https://provider.myfreestyle.com/safety-information.html
- 269. FreeStyle Libre 2 user manual. Accessed January 4, 2021. https://www.manualslib.com/manual/1976109/Abbott-Freestyle-Libre-2.html
- 270. FreeStyle Libre 3: world’s smallest sensor is here. Abbott Newsroom. Accessed January 4, 2021. https://www.abbott.com/corpnewsroom/strategy-and-strength/freeStyle-libre-3-worlds-smallest-sensor-is-here.html
- 271. Continuous Glucose Monitoring system. GlucoMen Day. Menarini Diagnostics. Accessed December 15, 2020. https://glucomenday.com/continuous-glucose-monitoring-system/
- 272. Simic A, Taucher M, Hochfellner DA, et al. Accuracy assessment of the new GlucoMen® DayCGM system in individuals with type 1 diabetes. DTM 2020 Abstracts. J Diabetes Sci Technol. 2021;15(2):A67. [Google Scholar]
- 273. Glunovo i3. Infinovo CGM. Accessed December 17, 2020. https://en.infinovo.com/glunovo-i3/
- 274. Indigo. ATTD 2020 - Video. Indigo. March 10, 2020. Accessed December 15, 2020. https://indigomed.com/attd-2020-video/
- 275. Accuracy of a subcutaneously inserted NIR spectrometer sensor for continuous glucose, ketone and lactate measurement in interstitial fluid: proof-of-concept in pig model - Virtual Meeting. EASD. Accessed December 15, 2020. https://www.easd.org/virtualmeeting/home.html#!resources/accuracy-of-a-subcutaneously-inserted-nir-spectrometer-sensor-for-continuous-glucose-ketone-and-lactate-measurement-in-interstitial-fluid-proof-of-concept-in-pig-model
- 276. Best J. Blood, sweat, tears and big data: The new wave of innovation in managing diabetes. ZDNet. Accessed December 16, 2020. https://www.zdnet.com/article/blood-sweat-tears-and-big-data-the-new-wave-of-innovation-in-managing-diabetes/
- 277. K’Watch Glucose. PKvitality. Accessed December 15, 2020. https://www.pkvitality.com/ktrack-glucose/
- 278. PKvitality. Press Release PKvitality, November 28, 2018. Accessed December 15, 2020. https://www.pkvitality.com/wp-content/uploads/2018/11/PKVITALITY-KWatch-CGM-21112018-EN.pdf
- 279. Our Vision. Profusa, Inc. Accessed December 17, 2020. https://profusa.com/our-vision/
- 280. Guardian Sensor 3 user guide. 2018. Accessed July 16, 2020. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Sensor-3-user-guide.pdf
- 281. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446-456. doi: 10.1089/dia.2017.0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Medtronic Synergy and Zeus. Accessed January 4, 2021. https://investorrelations.medtronic.com/static-files/f579d35c-220f-40e1-bf5d-49a268c091bb
- 283. Health M. Cutting edge CGM. Our technology. Metronom Health. December 16, 2020. Accessed December 16, 2020. http://metronomhealth.com/our-technology
- 284. Continuous Monitoring. PercuSense, United States. PercuSense. Accessed December 15, 2020. https://www.percusense.com
- 285. Sanvita Medical, LLC. Pilot Clinical Evaluation: Accuracy and Precision of the Sanvita OneTouch Real Time Continuous Glucose Monitoring System. clinicaltrials.gov; 2021. Accessed January 11, 2021. https://clinicaltrials.gov/ct2/show/NCT04205084
- 286. Pfützner A, Ramljak S, Kloppstech K, et al. Miniaturization of an osmotic pressure-based glucose sensor by means of nanosensing technology. 1. [Google Scholar]
- 287. Suri JT. Non-enzymatic electrochemical sensor for measuring analytes. August 3, 2017. Accessed December 15, 2020. https://patents.google.com/patent/US20170219521A1/en?assignee=glucovation&oq=glucovation
- 288. Medtrum. Accessed December 15, 2020. https://www.medtrum.com/A6.html
- 289. For diabetics. AlertgyTM. Accessed December 17, 2020. https://www.alertgy.com/for-diabetics/
- 290. Meet companion – Socrates health solutions. Accessed January 4, 2021. https://socrateshealthsolutions.com/meet-companion/
- 291. Home. GlucoActive. Accessed December 17, 2020. https://gluco-active.com/
- 292. GlucoSense Team. GlucoSense Presentation. Accessed December 17, 2020. https://netscientific.net/wp-content/uploads/2017/03/NetScientific-CMD-Glucosense-May2015.pdf
- 293. GlucoTrack. GlucoTrack. Accessed December 17, 2020. http://www.glucotrack.com/about-glucotrack/
- 294. Glucose testing via earlobe, not stressful fingersticks. Healthline. April 15, 2014. Accessed December 17, 2020. https://www.healthline.com/diabetesmine/glucose-testing-via-earlobe
- 295. HELO LX: most advanced health tracking wearable by wor(l)d. Accessed February 9, 2021. https://marketbolt.com/worldglobalnetwork/helo/
- 296. Helo LX: the story of multi-level marketing and one overpriced health band. Wareable. July 27, 2017. Accessed January 5, 2021. https://www.wareable.com/fitness-trackers/helo-lx-band-multi-level-marketing-9876
- 297. Home. LifePlus. Accessed December 17, 2020. https://www.lifeplus.ai/
- 298. Sanmina Image. Provided by Robert Newberry of Sanmina Corporation. Accessed January 15, 2021. [Google Scholar]
- 299. GBS Inc. Replacing Finger-Pricks. GBS Inc. Accessed December 17, 2020. https://gbs.inc/
- 300. Waveform Technologies. Continuous glucose monitoring solutions. Accessed December 17, 2020. https://waveformdiabetes.com/
- 301. How It Works. Waveform. Accessed December 17, 2020. https://waveformdiabetes.com/how-it-works/
- 302. New Continuous Glucose Monitors in the Works: Ascensia and AgaMatrix. Healthline. January 16, 2019. Accessed December 17, 2020. https://www.healthline.com/diabetesmine/new-cgms-ascensia-agamatrix
- 303. Coin Specifications. U.S. Mint. Accessed December 17, 2020. https://www.usmint.gov/learn/coin-and-medal-programs/coin-specifications
- 304. Can You Buy CGM Supplies at the Pharmacy? Healthline. February 19, 2020. Accessed December 17, 2020. https://www.healthline.com/diabetesmine/cgm-access-pharmacies
- 305. Diabetes forecast consumer guide continuous glucose monitors. Accessed December 17, 2020. http://main.diabetes.org/dforg/pdfs/2020/2020-cg-continuous-glucose-monitors.pdf?utm_source=Offline&utm_medium=Print&utm_content=cgms&utm_campaign=DF&s_src=vanity&s_subsrc=cgms
- 306. The Dex Factor – coming in 2021, Dexcom’s G7 CGM sensor. Desang Diabetes Services. July 8, 2020. Accessed January 4, 2021. https://www.desang.net/2020/07/the-dex-factor-coming-in-2021-dexcoms-g7-cgm-sensor/
- 307. Technology - GlySens Incorporated. Accessed December 17, 2020. http://glysens.com/?page_id=40
- 308. Senseonics launches program for low-cost Eversense CGM systems. MobiHealthNews. March 25, 2019. Accessed December 17, 2020. https://www.mobihealthnews.com/content/senseonics-launches-program-low-cost-eversense-cgm-systems
- 309. Continuous Glucose Monitoring System- Freestyle Libre 14 Day. Accessed December 17, 2020. https://www.freestyle.abbott/us-en/
- 310. Freestyle Libre 2: now available in U.S. Abbott Newsroom. Accessed December 17, 2020. https://www.abbott.com/corpnewsroom/diabetes-care/freestyle-libre-2-now-available-in-us.html
- 311. FreeStyle Libre 3 sets new peak in diabetes care. https://www.abbott.com/corpnewsroom/diabetes-care/freeStyle-libre-3-sets-new-peak-in-diabetes-care.html
- 312. FreeStyle Libre 3 cleared in Europe – smaller, thinner, and no more scanning! diaTribe. September 29, 2020. Accessed December 17, 2020. https://diatribe.org/freestyle-libre-3-cleared-europe-smaller-thinner-and-no-more-scanning
- 313. Abbott’s FreeStyle® Libre 3 system receives CE mark - features world’s smallest, thinnest sensor with best-in-class performance at the same low cost for people with diabetes. Abbott MediaRoom. Accessed January 28, 2021. https://abbott.mediaroom.com/2020-09-28-Abbotts-FreeStyle-R-Libre-3-System-Receives-CE-Mark-Features-Worlds-Smallest-Thinnest-Sensor-with-Best-in-Class-Performance-at-the-Same-Low-Cost-for-People-with-Diabetes
- 314. Pricing. Infinovo CGM. Accessed January 5, 2021. https://en.infinovo.com/pricing/
- 315. Hassle-free glucose monitoring with our next-gen sensor - Indigo. Accessed December 17, 2020. https://indigomed.com/
- 316. K’Watch Glucose Review smart health review. AB Smart Health Promo. April 8, 2020. Accessed December 17, 2020. https://www.absmarthealth.com/kwatch-glucose/
- 317. Guardian Connect CGM System. World’s First Smart CGM - Medtronic. Accessed December 17, 2020. https://www.medtronicdiabetes.com/products/guardian-connect-continuous-glucose-monitoring-system
- 318. Medtronic Launches Stand-Alone CGM: Guardian Connect. Healthline. March 13, 2018. Accessed December 17, 2020. https://www.healthline.com/diabetesmine/new-medtronic-stand-alone-cgm-guardian-connect
- 319. NEWS: medtronic embraces interoperable diabetes devices. Healthline. June 10, 2019. Accessed January 4, 2021. https://www.healthline.com/diabetesmine/news-tidepool-loop-medtronic-and-dexcom
- 320. What’s coming and what’s delayed in continuous glucose monitoring? diaTribe. May 20, 2020. Accessed February 10, 2021. https://diatribe.org/whats-coming-and-whats-delayed-continuous-glucose-monitoring
- 321. Metronom: building a better continuous glucose monitor. Healthline. March 27, 2018. Accessed December 17, 2020. https://www.healthline.com/diabetesmine/metronom-health-cgm
- 322. Lifecare. Sencell - continous glucose monitoring. Accessed December 17, 2020. https://www.lifecare.no/
- 323. Sencell - driven by nature. Accessed December 17, 2020. https://www.lifecare.no/about-sencell
- 324. Learn more. 19OCT2020. Accessed December 17, 2020. https://www.pacificdt.com/learn-more
- 325. A6 Touchcare user manual. Accessed December 15, 2020. https://www.medtrum.com/pdf/0911/UG882111GB%C2%A0A6%C2%A0TouchCare%C2%AE%C2%A0System%C2%A0Touchscreen%C2%A0User%C2%A0Guide%C2%A0V2.23-20190513.pdf
- 326. Meet sugarBEAT - sugarBEAT. Accessed January 4, 2021. https://sugarbeat.com/
- 327. Continuous Glucose Monitoring System - Waveform Technologies. Waveform. Accessed January 4, 2021. https://waveformdiabetes.com/continuous-glucose-monitoring/
- 328. Dexcom G6 Set Image. Provided by David Price of Dexcom. Accessed January 5, 2021. [Google Scholar]
- 329. Eversense Products - Senseonics. Accessed January 4, 2021. https://global.eversensediabetes.com/eversense-products
- 330. FreeStyle Libre 14-Day Image. Provided by Naunihal Virdi of Abbott Diabetes Care. Accessed January 5, 2021. [Google Scholar]
- 331. FreeStyle Libre 2 Image. Provided by Naunihal Virdi of Abbott Diabetes Care. Accessed January 5, 2021. [Google Scholar]
- 332. FreeStyle Libre 3 Image. Provided by Naunihal Virdi of Abbott Diabetes Care. Accessed January 5, 2021. [Google Scholar]
- 333. Guardian Connect System Support. Medtronic Diabetes. September 4, 2018. Accessed January 4, 2021. https://www.medtronicdiabetes.com/customer-support/guardian-connect-system-support
- 334. D-Band Image. Provided by DiaMonTech AG. Accessed January 5, 2021. [Google Scholar]
- 335. D-Base Image. Provided by DiaMonTech AG. Accessed January 5, 2021. [Google Scholar]
- 336. D-Pocket Image. Provided by DiaMonTech AG. Accessed January 5, 2021. [Google Scholar]
- 337. GlucoBeam. Provided by Dorte Pamperin of RSP Systems. Accessed January 5, 2021. [Google Scholar]
- 338. GlucoQuantum Image. Provided by William Chee of Genki Vantage Ltd. Accessed January 5, 2021. [Google Scholar]
- 339. LifeLeaf Image. Provided by Sweta Moitra of LifePlus. Accessed January 5, 2021. [Google Scholar]
- 340. Movano Inc. Accessed January 4, 2021. https://movano.com/
- 341. About. Know LABS. Accessed January 5, 2021. https://www.knowlabs.co/about
- 342. SynerG Image. Provided by Thomas Seidl of Pacific Diabetes Technologies. Accessed January 5, 2021. [Google Scholar]
- 343. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. doi: 10.1016/S0140-6736(16)31535-5 [DOI] [PubMed] [Google Scholar]
- 344. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. doi: 10.1007/s13300-016-0223-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. Published online September 19, 2020. doi: 10.1177/1932296820958754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. About Us – Infinovo CGM. Accessed February 9, 2021. https://www.infinovo.co.uk/about-us/
- 347. Conley D. Two paths for medical device approval: FDA vs. CE. HealthManagement. 2015;15(2). Accessed February 12, 2021. https://healthmanagement.org/c/healthmanagement/issuearticle/two-paths-for-medical-device-approval-fda-vs-ce [Google Scholar]
- 348. CE Mark or FDA Approval? Guided Solutions. November 5, 2018. Accessed February 12, 2021. https://www.guidedsolutions.co.uk/blog/ce-mark-or-fda-approval/
- 349. Clarke WL. The original Clarke Error Grid Analysis (EGA). Diabetes Technol Ther. 2005;7(5):776-779. doi: 10.1089/dia.2005.7.776 [DOI] [PubMed] [Google Scholar]
- 350. Pfützner A, Klonoff DC, Pardo S, Parkes JL. Technical aspects of the Parkes Error Grid. J Diabetes Sci Technol. 2013;7(5):1275-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Klonoff DC, Lias C, Vigersky R, et al. The surveillance error grid. J Diabetes Sci Technol. 2014;8(4):658-672. doi: 10.1177/1932296814539589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Messer LH, Tanenbaum ML, Cook PF, et al. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020;22(10):760-767. doi: 10.1089/dia.2019.0509 [DOI] [PubMed] [Google Scholar]
- 353. Advanced diabetes technology poised to play larger role in care, management. Accessed February 12, 2021. https://www.healio.com/news/endocrinology/20201210/advanced-diabetes-technology-poised-to-play-larger-role-in-care-management
- 354. Continuous Glucose Monitoring Systems (CGMS) Market 2020-2027 - ResearchAndMarkets.com. July 22, 2020. Accessed February 12, 2021. https://www.businesswire.com/news/home/20200722005453/en/Continuous-Glucose-Monitoring-Systems-CGMS-Market-2020-2027—ResearchAndMarkets.com
- 355. Close K. Dexcom 4Q20 – Record FY20 revenue of $1.93 billion (+31% YOY); “greater than 900,000” Dexcom users globally; G7 ready for CE submission, but more data needed for FDA; newly launched Dexcom Ventures fund. Close Concerns Knowledgebase. February 11, 2021. Accessed February 12, 2021. https://www.closeconcerns.com/knowledgebase/r/61daec87?utm_source=Closer+Look+Subscribers+2018&utm_campaign=b5ab6397b2-2021-02-11_Dexcom_and_AZ_4Q2002_11_2021&utm_medium=email&utm_term=0_c55d924bf1-b5ab6397b2-412261953
- 356. U.S. Food and Drug Administration. GlucoWatch G2 Biographer. August 26, 2002. Accessed December 17, 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf/P990026S008b.pdf
- 357. Wentholt IME, Hoekstra JBL, Zwart A, DeVries JH. Pendra goes Dutch: lessons for the CE mark in Europe. Diabetologia. 2005;48(6):1055-1058. doi: 10.1007/s00125-005-1754-y [DOI] [PubMed] [Google Scholar]
- 358. OrSense Receives European CE Mark Approval for Continuous Non-Invasive Glucose Monitoring System. June 20, 2007. Accessed December 17, 2020. https://www.businesswire.com/news/home/20070620005465/en/OrSense-Receives-European-CE-Mark-Approval-for-Continuous-Non-Invasive-Glucose-Monitoring-System
- 359. C8 MediSensors. C8 MediSensors gains CE mark approval for the C8 MediSensors optical glucose monitor(TM) system for people with diabetes. Accessed December 17, 2020. https://www.prnewswire.com/news-releases/c8-medisensors-gains-ce-mark-approval-for-the-c8-medisensors-optical-glucose-monitortm-system-for-people-with-diabetes-175821951.html
- 360. Dolan B. The promise of wireless, non-invasive CGM. MobiHealthNews. Published April 7, 2010. Accessed December 17, 2020. https://www.mobihealthnews.com/7184/the-promise-of-wireless-non-invasive-cgm
- 361. Forst T, Pfützner A, Forst S, et al. Accuracy of the non-invasive glucose monitoring device Pendra® compared to alternate site glucose measurements at the lower forearm during dynamic blood glucose changes in type 1 diabetic patients. Diabetes. 2004;53(suppl 2):A102. [Google Scholar]
- 362. Lin T, Gal A, Mayzel Y, Horman K, Bahartan K. Non-invasive glucose monitoring: a review of challenges and recent advances. Curr Trends Biomed Eng Biosci. 2017;6(5):1-8. doi: 10.19080/CTBEB.2017.06.555696 [DOI] [Google Scholar]
- 363. Abbott launches first glucose sport biosensor for athletes. Abbott. Accessed February 26, 2021. https://www.abbott.com/corpnewsroom/strategy-and-strength/abbott-launches-first-glucose-sport-biosensor-for-athletes.html
- 364. Gehr B, Holder M, Kulzer B, et al. Spectrum. J Diabetes Sci Technol. 2017;11(2):284-289. doi: 10.1177/1932296816661735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Schlüter S, Freckmann G, Heinemann L, Wintergerst P, Lange K. Evaluation of the SPECTRUM training programme for real-time continuous glucose monitoring: A real-world multicentre prospective study in 120 adults with type 1 diabetes. Diabet Med J Br Diabet Assoc. 2021;38(2):e14467. doi: 10.1111/dme.14467 [DOI] [PubMed] [Google Scholar]
- 366. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- 367. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1-39. doi: 10.1088/0967-3334/28/3/R01 [DOI] [PubMed] [Google Scholar]
- 368. Avram R, Olgin JE, Kuhar P, et al. A digital biomarker of diabetes from smartphone-based vascular signals. Nat Med. 2020;26(10):1576-1582. doi: 10.1038/s41591-020-1010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Klonoff DC. Diagnosing diabetes mellitus from smartphone-based vascular signals. Nat Rev Endocrinol. 2020;16(12):681-682. doi: 10.1038/s41574-020-00433-6 [DOI] [PubMed] [Google Scholar]
- 370. Thomas A, Heinemann L, Ramírez A, Zehe A. Options for the development of noninvasive glucose monitoring: is nanotechnology an option to break the boundaries? J Diabetes Sci Technol. 2016;10(3):782-789. doi: 10.1177/1932296815616133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. The Basics of Nanotechnology. What Is Nanotechnology and Why Does It Matter? John Wiley & Sons, Ltd; 2010:1-19. doi: 10.1002/9781444317992.ch1 [DOI] [Google Scholar]
- 372. Thomas A, Torres Tapia E, Ramirez A, Zehe A. Las nanopartículas – Nanomateriales de tantas aplicaciones asombrosas en nanomedicina y nanotecnología biomédica. Internet Electron J Nanociencia Moletrónica. 2015;13:2315-2326. [Google Scholar]
- 373. Brown JQ, McShane MJ. Modeling of spherical fluorescent glucose microsensor systems: design of enzymatic smart tattoos. Biosens Bioelectron. 2006;21(9):1760-1769. doi: 10.1016/j.bios.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 374. Stein EW, Grant PS, Zhu H, McShane MJ. Microscale enzymatic optical biosensors using mass transport limiting nanofilms. 1. Fabrication and characterization using glucose as a model analyte. Anal Chem. 2007;79(4):1339-1348. doi: 10.1021/ac061414z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Freckmann G, Nichols JH, Hinzmann R, et al. Standardization process of continuous glucose monitoring: traceability and performance. Clin Chim Acta Int J Clin Chem. 2021;515:5-12. doi: 10.1016/j.cca.2020.12.025 [DOI] [PubMed] [Google Scholar]
- 376. Schermer M. The mind and the machine. On the conceptual and moral implications of brain-machine interaction. Nanoethics. doi: 10.1007/s11569-009-0076-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Dahad N. Rockley Photonics to Deliver Glucose Monitoring for Apple Smartwatches. EETimes. Published May 4, 2021. Accessed May 11, 2021. https://www.eetimes.com/rockley-photonics-to-deliver-glucose-monitoring-for-apple-smartwatches/
- 378. Avery D. Apple may be adding blood-sugar monitor to upcoming Apple Watch. Daily Mail Science and Tech. Published May 3, 2021. Accessed May 11, 2021. https://www.dailymail.co.uk/sciencetech/article-9539021/Apple-add-sensors-monitor-blood-sugar-alcohol-levels-upcoming-Apple-Watch.html